Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel synthesis of benzotriazolyl alkyl esters: an unprecedented CH2 insertion†
Mohamed Elagawanyabc,
Lingaiah Maramab and
Bahaa Elgendy *abd
*abd
aDepartment of Pharmaceutical and Administrative Sciences, University of Health Sciences and Pharmacy, St. Louis, MO 63110, USA. E-mail: belgendy@wustl.edu
bCenter for Clinical Pharmacology, Washington University School of Medicine, St. Louis College of Pharmacy, St. Louis, MO 63110, USA
cDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, Damanhour University, Damanhour, Egypt
dChemistry Department, Faculty of Science, Benha University, Benha 13518, Egypt
First published on 17th February 2021
Abstract
We have developed a novel method for the synthesis of benzotriazolyl alkyl esters (BAEs) from N-acylbenzotriazoles and dichloromethane (DCM) under mild conditions. This reaction is one of few examples to show the use of DCM as a C-1 surrogate in carbon–heteroatom bond formation and to highlight the versatility of using DCM as a methylene building block.
Introduction
Benzotriazolyl alkyl esters (BAEs) are bifunctional building blocks that can be used for the construction of a myriad of multifunctional chemical compounds. The benzotriazolyl (Bt) and acyloxy groups are good leaving groups and can be selectively eliminated to provide useful scaffolds for the synthesis of important chemical compounds. For example, upon treatment of benzotriazolyl alkyl esters with organozinc reagents, one can substitute the benzotriazolyl moiety with several groups (e.g. alkyl, aryl, alkenyl,…etc) to synthesize esters.1 Alternatively, chemoselective reduction of benzotriazolyl alkyl esters with SmI2 leads to the cleavage of the acyloxy group and formation of a Bt bearing radical that undergoes cross-coupling with carbonyl compounds to form a variety of β-(benzotriazole-1-yl)alcohols.2Multifunctional ligands based on the BAE moiety (i.e. I and II, Fig. 1) were developed as nitrogen-donor ligands, and were shown to have the ability to coordinate to transition metals such as Rh(I), Ir(I), and Co(II). These ligands stabilized their metal ions in multiple oxidation states and geometries, and improved their activity in the carbonylation of methanol. These ligands were synthesized by condensation of the hydroxymethyl-benzotriazole and carboxylic acids in DCM in the presence of DCC, DMAP, and 4-pyrrolidinopyridine.3
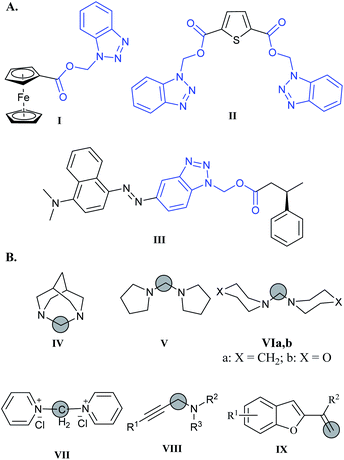 | ||
| Fig. 1 (A) Examples of multifunctional ligands based on BAE moiety (I and II) and aryl azo BAE enzyme substrates (III). (B) Compounds IV–IX synthesized using DCM as a C-1 surrogate. | ||
Aryl azo benzotriazolyl alkyl esters (III, Fig. 1) are used as masked enzyme substrates that can be detected by surface-enhanced resonance Raman scattering (SERRS) only after hydrolysis by the enzyme of interest. This enzymatic hydrolysis generates the dye that has strong affinity to the surface and can be detected by SERRS at very low levels and consequently allow for an enzyme assay with ultra-sensitivity for enzyme turnover monitoring.4–6
The most common synthetic approaches to synthesize BAEs are condensation of the hydroxymethylbenzotriazole and carboxylic acids,3 and the reaction of the appropriate sodium benzotriazolate with chloromethyl esters.4
Dichloromethane (DCM) is reported to react with strong nucleophiles,7–9 and bases.10 For example, DCM is reported to degrade at low temperature in bispidine and give 1,3-diazaadamantane hydrochloride (IV) as the major product (Fig. 1).11 Methylene bridged cyclic amines such as dipyrrolidylmethane (V), dipiperidylmethane (VIa), and dimorpholinylmethane (VIb) are useful chelating ligands (Fig. 1). These ligands can be synthesized easily at room temperature by stirring the corresponding bases with DCM in absence of light.12 Similarly, methylenebispyridinium dichloride (VII) was formed when pyridine was dissolved in DCM and left for long time.13 DCM was used as a C-1 surrogate in several synthetic reactions. For example, Zhang et al. used DCM as a C-1 building block in the synthesis of propargylamines VIII via CuCl catalysis.14 Another example was the synthesis of substituted benzofurane IX via a three component reaction involving DCM as a source of methylene in a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation.15
C bond formation.15
In continuation of our work developing new synthetic approaches for the preparation of biologically active compounds,16–26 we discovered and developed a new methodology for the synthesis of BAEs via CH2 insertion using DCM. The new method is general, convenient and has a broad scope of applicability to various substrates.
Results and discussion
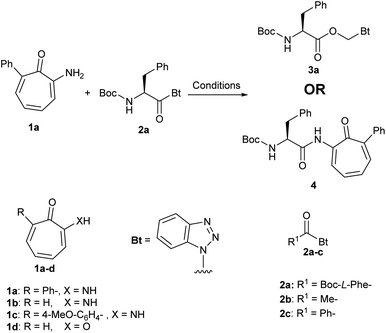
One of our ongoing research programs is to develop tropolones as antibacterial agents.27,28 While developing aminoacid conjugates of aminotropones 1a, we used acyl benzotriazole as an acylating reagent. Aminotropones were reported to couple with similar acylating reagents such as acid chlorides under conventional reaction conditions.29,30 When we reacted aminotropone 1a with acyl benzotriazole 2a in acetonitrile (ACN) in presence of Hunig's base (Scheme 1) according to standard protocol of coupling using N-acyl benzotriazole as an acylating reagent,16,22 we observed no progress after one hour of stirring at room temperature, and the starting materials were largely insoluble. Therefore, we added few drops of dichloromethane (DCM) as a co-solvent to enhance the solubility of the starting materials. The reaction mixture became homogeneous, but no reaction took place. We repeated the reaction using one equivalent of DMAP, but no change was observed at room temperature. Heating the reaction mixture at 60 °C for 12 h led to consumption of the acyl benzotriazole and formation of a new product 3a, which was isolated and purified (Scheme 1). Aminotropone 1a was isolated as a side product. The molecular weight of the expected product 4 is 444, but LC/MS of 3a showed a molecular weight of 397 (M + H) (i.e. 48 mass unit difference from 4 and 31 mass unit difference from 2a). Comparing 1H NMR spectrum of 3a to spectrum of 2a showed two additional protons at 6.50 and 7.70 ppm. The two protons appeared as douplets with the same coupling constant (i.e. J = 11.3 Hz). The 13C NMR spectrum showed an extra aliphatic carbon at 68.2 ppm. We ran a series of 2D NMR to identify the product and we found that the two unexpected protons at 6.50 and 7.70 ppm are attached to the same aliphatic carbon at 68.2 ppm and the new product has the Boc-L-Phe moiety.When we tried the coupling of aminotropone 1a with either acyl benzotriazole (2b) and/or benzoyl benzotriazole (2c) (Scheme 1) under the same reaction conditions (ACN/DCM, DMAP/60 °C), we obtained products that has the same mass differences from substrates and expected products that was observed in the case of Boc-L-Phe–Bt (i.e. 31 and 48). Coupling of other aminotropones (i.e. 1b and 1c), and tropone 1d led to the formation of similar unexpected products with 48 mass unit difference than the expected products. In both cases aminotropones (1b and 1c) and tropone (1d) were isolated as side products. We repeated the reaction under same conditions but avoided using DCM as a co-solvent and we obtained the expected amide and benzotriazole as a side product.
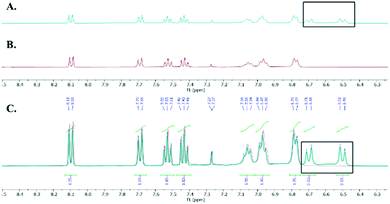
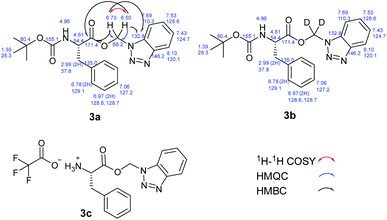
We hypothesized that the extra CH2 was generated from DCM and to test this hypothesis, we ran the reaction in deuterated DCM. We obtained product 3b and by analyzing and comparing the 1H NMR spectra of 3a and 3b, we found that the two spectra were superimposed with the extra two protons disappeared in case of deuterated DCM, which suggests that these protons are generated from DCM (Fig. 2). The molecular formula of 3a (C21H24N4O4Na+) was determined by HRESIMS data (m/z 419.1687 [M + Na]+, calcd 419.1690). Similarly, the molecular formula of 3b (C21H22D2N4O4Na+) was determined by HRESIMS data (m/z 421.1812 [M + Na]+, calcd 421.1815).
Finally, we were able to determine the structure of the product to be benzotriazolyl alkyl ester using X-ray crystallography. Deprotection of the Boc group of 3a using TFA in DCM led to the formation of 3c that was suitable to obtain a single crystal X-ray structure (Fig. 3). The molecular formula of 3c (C16H16N4O2Na+) was determined by HRESIMS data (m/z 319.1165 [M + Na]+, calcd 319.1165).
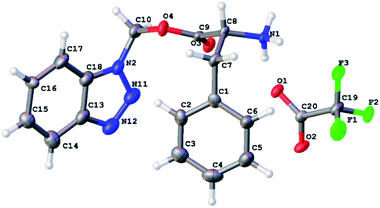 | ||
| Fig. 3 An ORTEP representation of the X-ray crystallography structure of BAE 3c with the ellipsoid shown at 50% contour % probability level. | ||
The structures of 3a and 3b were further confirmed following a thorough analysis of 2D NMR data. All the protons and carbons were assigned based on COSY, HSQC, and HMBC correlations (Fig. 4).
To determine if aminotropone derivatives contribute to the observed transformation, we carried out the reaction in absence of aminotropone and obtained the same product in the same reaction time and with the same yield, which indicates that aminotropone did not play a role in this transformation.
We hypothesize that the mechanism of this reaction takes place via: (i) base-catalysed hydrolysis of N-acyl benzotriazole.31 (ii). In situ formation of chloromethyl benzotriazole through reaction of benzotriazole with DCM in presence of base (benzotriazole was reported to react with DCM in DMF in presence of sodium hydride at reflux conditions).32 (iii) The base facilitates the formation of acyloxy anion that reacted with chloromethyl benzotriazole to form the desired BAE.33
To explore the scope of this reaction, we started first by optimizing the reaction conditions using synthesis of (1H-benzo[d][1,2,3]triazol-1-yl)methyl benzoate (5a) as a template reaction (Table 1). Screening numerous solvents revealed that ACN and DCM were the best solvents and gave the desired product 5a in 93% yield (Table 1, entries 1 and 2). Although using DCM only as a solvent and reagent afforded the product in high yield (93%), ACN is preferred to minimize the hazardous of using larger quantity of DCM and to design a reaction that is green in nature. Replacing ACN with DMF or THF led to the formation of the product in lower yields (i.e. 55 and 63%, respectively) (Table 1, entries 3–4). No product was observed when ACN was replaced by 1,4-dioxane, toluene, water, or ethanol (Table 1, entries 5–8).
| Entry | Solvent | Base | Yield (%) |
|---|---|---|---|
| a Conditions: 2c (0.3 mmol), base (0.3 mmol), in solvent (4 mL) and DCM (1 mL) at 60 °C for 5 h.b Anhydrous solvents and inert argon conditions.c H2O (2.0 equiv.).d H2O (5.0 equiv.).e H2O (10.0 equiv.).f H2O (20.0 equiv.). | |||
| 1 | Acetonitrile | DMAP (1 equiv.) | 93 |
| 2 | DCM | DMAP (1 equiv.) | 93 |
| 3 | DMF | DMAP (1 equiv.) | 55 |
| 4 | THF | DMAP (1 equiv.) | 63 |
| 5 | 1,4-Dioxane | DMAP (1 equiv.) | N.R. |
| 6 | Toluene | DMAP (1 equiv.) | N.R. |
| 7 | Water | DMAP (1 equiv.) | N.R. |
| 8 | Ethanol | DMAP (1 equiv.) | N.R. |
| 9 | Acetonitrile | DIPEA (1–3 equiv.) | N.R. |
| 10 | Acetonitrile | TEA (1–3 equiv.) | N.R. |
| 11 | Acetonitrile | DBU (1 equiv.) | 65 |
| 12 | Acetonitrile | KOH (1 equiv.) | N.R. |
| 13 | Acetonitrile | t-BuOK (1 equiv.) | N.R. |
| 14b | Acetonitrile | DMAP (1 equiv.) | N.R. |
| 15b,c | Acetonitrile | DMAP (1 equiv.) | 20 |
| 16b,d | Acetonitrile | DMAP (1 equiv.) | 50 |
| 17b,e | Acetonitrile | DMAP (1 equiv.) | 95 |
| 18b,f | Acetonitrile | DMAP (1 equiv.) | 95 |
To identify the optimum base for the reaction, we screened several other bases in addition to DMAP in the best identified solvent (i.e. ACN) (Table 1, entries 9–13). DMAP was found to give the best result (Table 1, entry 1). No reaction was observed when DIPEA or TEA were used, and the starting material remained intact. When DBU was used, the product 5a was obtained in 65% (entry 11). The use of KOH or potassium-tert-butoxide led to hydrolysis of the starting N-acyl benzotriazole 2c (entries 12 and 13).
To further confirm the proposed mechanism and verify that water is the source of oxygen in the formed product, we have conducted a series of controlled experiments with anhydrous solvents under inert conditions and using water as a reagent to test if water is the source of oxygen and if this is the case to determine how many equivalents of water are needed to ensure sufficient reactivity (Table 1, entries 14–18). Compound 5a was not formed using anhydrous ACN and DCM in absence of water under argon and starting materials remained intact (entry 14). When we added H2O to the reaction, compound 5a was formed and the best yield was achieved using 10.0 equivalents of H2O. Increasing H2O to 20 equivalents did not improve the yield further and no hydrolysis of starting materials was observed. Therefore, H2O is the source of oxygen and 10 equiv. is the optimum ratio to obtain the desired product in the model reaction.
To investigate if this kind of CH2 insertion could take place with other N-acyl derivatives, we used N-phenylbenzamide and N,N-diphenylbenzamide as substrates (Scheme 2A). No reaction was observed in both cases under the optimized reaction conditions. Finally, we ran a competition experiment in the presence of piperidine as a strong nucleophile. The amide product was obtained exclusively with no insertion taking place at all (Scheme 2B). This observation illustrates that in presence of strong nucleophile, the coupling is the preferred pathway, which is usually the case in coupling reactions involving N-acyl benzotriazoles. Insertion of CH2 was the favored pathway in case of aminotropones (Scheme 1) due to their weak nucleophilicity.
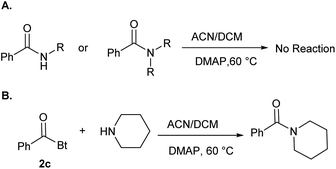 | ||
| Scheme 2 (A) Reaction of N-phenylbenzamide and N,N-diphenylbenzamide with DCM. (B) Competition experiment using piperidine as a nucleophile. | ||
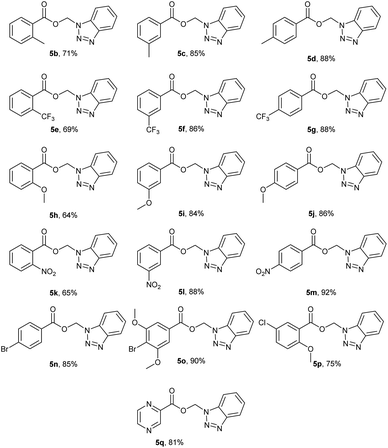
To further explore the generality of this reaction, we screened various aromatic acyl benzotriazoles (2d–s) bearing electron donating groups, electron withdrawing groups, and substituted at o-, m-, and p- positions of the phenyl ring. We have also used 1H-benzotriazol-1-yl-2-pyrazinyl methanone as a representative of heterocyclic acyl benzotriazoles. The desired BAEs (5a–n) were obtained in all cases in moderate to excellent yields (63–93%) (Fig. 5). It is worth mentioning that compounds 5b,e,h,k were obtained in low yields (i.e. <10%) using our standard reaction conditions. Yields were improved significantly when we used DBU as a base under the same reaction conditions (i.e. 64–71%). Compounds 5b,e,h,k possess ortho-substituents at the phenyl ring and we hypothesize that these compounds have better steric complementarity with DBU over DMAP. Compound 5p was the only exception because it was obtained in good yield (75%) using DMAP. Although 5p is analogous to 5h (both 5p and 5h possess the same ortho-methoxy substituent), the meta-chloro substituent in 5p most likely contributed to the observed difference in reactivity.
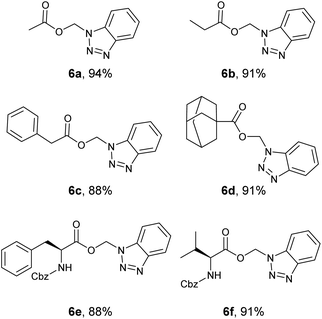
Aliphatic BAEs (6a–d) were synthesized from their corresponding N-acylbenzotriazoles (Fig. 6). Acetate, propionate, and phenylacetate derivatives were synthesized in 88–93% yield. The adamantane-1-carboxylate benzotriazolyl alkyl ester (6d) was synthesized in 91% yield. Moreover, we extended our new methodology to synthesize amino acid derivatives of benzotriazolyl alkyl ester. Boc-L-Phe- benzotriazolyl alkyl ester (3a, Fig. 4) was synthesized in 94% yield. This compound was deprotected in TFA/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and gave the free aminoacid derivative 3c in quantitative yield (Fig. 4). Using deuterated DCM afforded compound 3b in 91% yield (Fig. 4). This clearly showed that our method was compatible with Boc protecting group. The method was also compatible with Cbz protecting group and we synthesized both Cbz-L-Phe- and Cbz-L-Val- benzotriazolyl alkyl ester (6e and 6f) in 88 and 91% yield, respectively.
1) and gave the free aminoacid derivative 3c in quantitative yield (Fig. 4). Using deuterated DCM afforded compound 3b in 91% yield (Fig. 4). This clearly showed that our method was compatible with Boc protecting group. The method was also compatible with Cbz protecting group and we synthesized both Cbz-L-Phe- and Cbz-L-Val- benzotriazolyl alkyl ester (6e and 6f) in 88 and 91% yield, respectively.
Conclusions
In summary, we have developed a simple and practical method for the synthesis of benzotriazolyl alkyl esters (BAEs), which are bi-functional building blocks useful for the synthesis of numerous multifunctional compounds. The structure of BAE 3c was confirmed using X-ray crystallography. We used DCM as a C-1 surrogate in a carbon–heteroatom bond formation under metal-free conditions that highlights the versatility of using DCM as a building block. The new method is convenient and requires simple operation, applicable to broad scope of substrates, and products are obtained in high yields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Center for Clinical Pharmacology, Washington University School of Medicine and St. Louis College of Pharmacy, St. Louis, MO 63110, USA.Notes and references
- A. R. Katritzky, S. Rachwal and B. Rachwal, A Novel Synthesis of Esters via Substitution of the Benzotriazolyl Group in 1-(Benzotriazol-1-yl)alkyl Esters with Organozinc Reagents, Synthesis, 1991, 1991, 69–73 CrossRef.
- X. Wang, H. Mao, G. Xie and J. Du, Chemoselective Removal of Acyloxy in 1-(Benzotriazole-1-yl)alkyl Esters and Its Application in the Preparation of β-(Benzotriazole-1-yl)alcohols, Synth. Commun., 2008, 38, 2908–2918 CrossRef CAS.
- C. M. Thomas, B. Therrien, A. Neels, H. Stœckli-Evans and G. Süss-Fink, New benzotriazole derivatives as multifunctional ligands, J. Organomet. Chem., 2002, 658, 251–258 CrossRef CAS.
- N. P. Power, D. Bethell, L. Proctor, E. Latham and P. Dawson, Chloromethyl chlorosulfate: a new, catalytic method of preparation and reactions with some nucleophiles, Org. Biomol. Chem., 2004, 2, 1554–1562 RSC.
- A. M. Ingram, K. Stirling, K. Faulds, B. D. Moore and D. Graham, Investigation of enzyme activity by SERRS using poly-functionalised benzotriazole derivatives as enzyme substrates, Org. Biomol. Chem., 2006, 4, 2869–2873 RSC.
- B. D. Moore, L. Stevenson, A. Watt, S. Flitsch, N. J. Turner, C. Cassidy and D. Graham, Rapid and ultra-sensitive determination of enzyme activities using surface-enhanced resonance Raman scattering, Nat. Biotechnol., 2004, 22, 1133–1138 CrossRef CAS.
- S. Bekkevoll, I. Svorstøl, H. Høiland and J. Songstad, Solvent Properties of Dichloromethane. 1. The Reactivity of Dichloromethane toward Some Ionic Nucleophiles. Dichloromethane as Solvent for Finkelstein Reactions, Acta Chem. Scand., 1983, 37b, 935–945 CrossRef.
- G. O. Nevstad, J. Songstad, B. Rodriguez, L. Mörch and T. Norin, Solvent Properties of Dichloromethane. II. The Reactivity of Dichloromethane Toward Amines, Acta Chem. Scand., 1984, 38b, 469–477 CrossRef.
- J. E. Mills, C. A. Maryanoff, R. M. Cosgrove, L. Scott and D. F. McComsey, The Reaction of Amines with Methylene Chloride. A BRIEF REVIEW, Org. Prep. Proced. Int., 1984, 16, 97–114 CrossRef CAS.
- G. L. Closs and L. E. Closs, Carbenes from Alkyl Halides and Organolithium Compounds. I. Synthesis of Chlorocyclopropanes1, J. Am. Chem. Soc., 1960, 82, 5723–5728 CrossRef CAS.
- H. Cui, R. Goddard and K.-R. Pörschke, Degradation of dichloromethane by bispidine, J. Phys. Org. Chem., 2012, 25, 814–827 CrossRef CAS.
- S. J. Kyran, S. G. Sanchez, C. J. Arp and D. J. Darensbourg, Syntheses and Structures of [CH2(NCnH2n)2]Mo(CO)4 (n = 4,5) Complexes with Bis(cycloamine) Ligands Easily Prepared from CH2Cl2, Organometallics, 2015, 34, 3598–3602 CrossRef CAS.
- A. B. Rudine, M. G. Walter and C. C. Wamser, Reaction of Dichloromethane with Pyridine Derivatives under Ambient Conditions, J. Org. Chem., 2010, 75, 4292–4295 CrossRef CAS.
- D. Yu and Y. Zhang, Copper-Catalyzed Three-Component Coupling of Terminal Alkyne, Dihalomethane and Amine to Propargylic Amines, Adv. Synth. Catal., 2011, 353, 163–169 CrossRef CAS.
- J.-P. Wan, H. Wang, Y. Liu and H. Ding, Synthesis of 2-Vinylbenzofurans via the Copper-Catalyzed Multicomponent Reactions Involving an Oxa-Michael/Arylation/Vinylation Cascade, Org. Lett., 2014, 16, 5160–5163 CrossRef CAS.
- A. R. Katritzky, B. E.-D. M. El-Gendy, E. Todadze and A. A. A. Abdel-Fattah, (α-Aminoacyl)amino-Substituted Heterocycles and Related Compounds, J. Org. Chem., 2008, 73, 5442–5445 CrossRef CAS.
- A. R. Katritzky, M. Yoshioka-Tarver, B. E.-D. M. El-Gendy and C. D. Hall, Synthesis and photochemistry of pH-sensitive GFP chromophore analogs, Tetrahedron Lett., 2011, 52, 2224–2227 CrossRef CAS.
- B. Draghici, B. E.-D. M. El-Gendy and A. R. Katritzky, Synthesis of Benzoxazines, Quinazolines and 4H-Benzo[e][1,3]thiazine by ANRORC Rearrangements of 1,2,4-Oxadiazoles, Synthesis, 2012, 44, 547–550 CAS.
- E. H. Ghazvini Zadeh, B. E.-D. M. El-Gendy, A. G. Pop and A. R. Katritzky, Synthesis of chiral α-amino acid-derived 1H-1,2,4-triazoles and 1,2,4-triazines, Med. Chem. Commun., 2012, 3, 52–55 RSC.
- M. El Khatib, M. Elagawany, F. Jabeen, E. Todadze, O. Bol'Shakov, A. Oliferenko, L. Khelashvili, S. A. El-Feky, A. Asiri and A. R. Katritzky, Traceless chemical ligations from O-acyl serine sites, Org. Biomol. Chem., 2012, 10, 4836–4838 RSC.
- M. El Khatib, M. Elagawany, E. Todadze, L. Khelashvili, S. A. El-Feky and A. R. Katritzky, Microwave-assisted regiospecific synthesis of pseudohalohydrin esters, Synlett, 2012, 23, 1384–1388 CrossRef CAS.
- M. El Khatib, M. Elagawany, E. Çalişkan, E. F. Davis, H. M. Faidallah, S. A. El-Feky and A. R. Katritzky, Total synthesis of cyclic heptapeptide Rolloamide B, Chem. Commun., 2013, 49, 2631–2633 RSC.
- M. Elagawany and M. A. Ibrahim, An improved route for the synthesis of Rolloamide B, Tetrahedron Lett., 2016, 57, 3837–3840 CrossRef CAS.
- M. A. Ibrahim, M. Elagawany and T. S. Ibrahime, Green and catalyst-free synthesis of olsalazine analogs, Green Chem. Lett. Rev., 2016, 9, 91–95 CrossRef CAS.
- T. S. Ibrahim, I. A. Seliem, S. S. Panda, A. M. M. Al-Mahmoudy, Z. K. M. Abdel-Samii, N. A. Alhakamy, H. Z. Asfour and M. Elagawany, An Efficient Greener Approach for N-acylation of Amines in Water Using Benzotriazole Chemistry, Molecules, 2020, 25, 2501 CrossRef CAS.
- W. Saaed, M. Elagawany, M. M. Azab, A. S. Amin, N. P. Rath, L. Hegazy and B. Elgendy, Catalyst-and organic solvent-free synthesis, structural, and theoretical studies of 1-arylidenamino-2,4-disubstituted-2-imidazoline-5-ones, Results Chem., 2020, 2, 100042 CrossRef.
- M. Elagawany, L. Hegazy, F. Cao, M. J. Donlin, N. Rath, J. Tavis and B. Elgendy, Identification of 4-isopropyl-thiotropolone as a novel anti-microbial: regioselective synthesis, NMR characterization, and biological evaluation, RSC Adv., 2018, 8, 29967–29975 RSC.
- F. Cao, C. Orth, M. J. Donlin, P. Adegboyega, M. J. Meyers, R. P. Murelli, M. Elagawany, B. Elgendy and J. E. Tavis, Synthesis and Evaluation of Troponoids as a New Class of Antibiotics, ACS Omega, 2018, 3, 15125–15133 CrossRef CAS.
- A. Mori, T. Itoh, H. Taya, K. Kubo, S. Ujiie, U. Baumeister, S. Diele and C. Tschierske, Mesomorphic property of 2,5-dibenzoyloxy-, 5-benzoylamino-2-benzoyloxy-, and 2,5-dibenzoylamino-tropones with mono-, di-, and tri-alkoxyl groups on the benzoyl groups and their benzenoid derivatives, Liq. Cryst., 2010, 37, 355–368 CrossRef CAS.
- M. Richter, V. Boldescu, D. Graf, F. Streicher, A. Dimoglo, R. Bartenschlager and C. D. Klein, Synthesis, Biological Evaluation, and Molecular Docking of Combretastatin and Colchicine Derivatives and their hCE1-Activated Prodrugs as Antiviral Agents, ChemMedChem, 2019, 14, 469–483 CrossRef CAS.
- M. Reboud-Ravaux, N-Acetylbenzotriazole hydrolysis and aminolysis. Broensted relationships and rate-determining steps in the uncatalyzed and catalyzed aminolysis, J. Am. Chem. Soc., 1980, 102, 1039–1046 CrossRef CAS.
- A. A. Joshi and C. L. Viswanathan, Docking studies and development of novel 5-heteroarylamino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as potential antimalarials, Bioorg. Med. Chem. Lett., 2006, 16, 2613–2617 CrossRef CAS.
- A. R. Katritzky, W. Kuzmierkiewicz, B. Rachwal, S. Rachwal and J. Thomson, The chemistry of N-substituted benzotriazoles. Part 5. Reactions of benzotriazole with aldehydes and thionyl chloride—formation of (benzotriazol-1-yl)-1-chloroalkanes and bis(benzotriazolyl)alkanes, J. Chem. Soc., Perkin Trans. 1, 1987, 811–817 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1998062. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra10413b |
| This journal is © The Royal Society of Chemistry 2021 |