Open Access Article
Open Access ArticleEnhancement of exchangeable Cd and Pb immobilization in contaminated soil using Mg/Al LDH-zeolite as an effective adsorbent†
Van Minh Danga,
Huu Tap Van *b,
N. D. Vinhc,
Thi Minh Hoa Duongd,
Thi Bich Hanh Nguyenb,
Thị Tuyet Nguyenb,
Thi Ngoc Ha Tranb,
Trung Kien Hoangb,
Thị Pha Trand,
Lan Huong Nguyene and
Manh Nhuong Chuf
*b,
N. D. Vinhc,
Thi Minh Hoa Duongd,
Thi Bich Hanh Nguyenb,
Thị Tuyet Nguyenb,
Thi Ngoc Ha Tranb,
Trung Kien Hoangb,
Thị Pha Trand,
Lan Huong Nguyene and
Manh Nhuong Chuf
aThai Nguyen University, Tan Thinh ward, Thai Nguyen city, Vietnam
bFaculty of Natural Resources and Environment, TNU – University of Sciences, Tan Thinh ward, Thai Nguyen city, Vietnam. E-mail: tapvh@tnus.edu.vn
cFaculty of Chemistry, TNU – University of Sciences, Tan Thinh ward, Thai Nguyen city, Vietnam
dFaculty of Environment, TNU – University of Agriculture and Forestry, Quyet Thang ward, Thai Nguyen city, Vietnam
eFaculty of Environment – Natural Resources and Climate Change, Ho Chi Minh City University of Food Industry (HUFI), Ho Chi Minh City, Vietnam
fFaculty of chemistry, TNU – University of Education, No. 20, Luong Ngoc Quyen Road, Thai Nguyen City, Vietnam
First published on 10th May 2021
Abstract
In the present study, experiments using zeolite and Mg/Al LDH-zeolite for immobilization of Cd and Pb ions in artificial soil were conducted. The conditions which affect Cd and Pb ion immobilization in soil were evaluated, namely soil pH (5–7), the mass ratio of adsorbents (1%, 3% and 5%), incubation time (15 days, 30 days and 45 days) and soil moisture (30%, 50% and 70%). The results indicated that the optimal soil pH, mass ratio of adsorbents, incubation time and soil moisture for immobilization of Cd and Pb ions by the adsorbent were, respectively, 7.0, 3%, 30 days and 70%. The exchangeable Cd ion content in the contaminated soil dropped from 22.17 mg kg−1 (87.65%) to 11.03 mg kg−1 (43.48%) and 6.47 mg kg−1 (26.36%) on incubation with zeolite and Mg/Al LDH-zeolite, respectively, while the exchangeable Pb content fell from 23.28 mg kg−1 (90.02%) to 14.12 mg kg−1 (54.04%) and 9.47 mg kg−1 (35.24%) using zeolite and Mg/Al LDH-zeolite as absorbents in contaminated soil, respectively. Fe–Mn oxide occluded (F2), carbonate bound (F3) and organically complexed (F4) were the main forms for immobilization of the exchangeable Cd and Pb when the zeolite and Mg/Al LDH-zeolite absorbents were separately cultivated into soil. Precipitation, co-precipitation and electrostatic attraction were the main mechanisms of exchangeable Cd and Pb immobilization onto the Mg/Al LDH-zeolite to form carbonate metals (CdCO3 and PbCO3). This was due to the surface functional groups of the adsorbent and the presence of Fe and Al oxyhydroxides, Mn oxides, and Si and O elements in the Mg/Al LDH-zeolite's constituents. The efficiency of Cd and Pb immobilization by the Mg/Al LDH-zeolite was higher than that by zeolite from 1.5 to 1.6 times. The Mg/Al LDH-zeolite showed an enhanced ability of exchangeable Cd and Pb immobilization in contaminated soil.
1. Introduction
Heavy metal pollution in agricultural soil is a major threat to the food chain and human health around the world due to its penetration into the environment from various anthropogenic sources, such as ore extraction, the metallurgical industry, residual fertilizer and pesticide in agriculture, vehicle exhaust, tire wear and weathering of buildings.1 The negative impacts of heavy metal pollution towards the environment and human health occur through soil–plant–food chain interaction resulting in a decrease in biological activities and the quality of agricultural products.2 Among the heavy metals, both lead (Pb) and cadmium (Cd) have high toxicities towards human health, animals, and plants. The previous studies reported the content of both Cd and Pb was from 3.5 mg kg−1 to 55.1 mg kg−1 and 173.4 mg kg−1 to 2696.3 mg kg−1, respectively, in soils from purple moor-grass (Molinia caerulea L.) which is located near the zinc and lead tailings ore landfill of Mining & Metallurgy Enterprise “Bolesław” SA in Bukowno.3 Besides, the presence of both Cd and Pb, respectively, in contaminated soil was from 12.7 mg kg−1 to 41.9 mg kg−1 and less than 100 mg kg−1 due to the influence of emission from Kovohutě Příbram stack in the Czech Republic.4 The average Pb content was between 10 and 67 mg kg−1 in surface soils around the world.5 The Cd and Pb content of garden soil in Silesia Province southern Poland was <2.0–69.9 mg kg−1 and <20.0–2823.9 mg kg−1, respectively.6 In Vietnam, several areas have been also polluted by both Pb and Cd, such as heavy metal (Pb, Cd) pollution in agricultural soil in Dong Mai lead recycling craft village in Hung Yen province7 and a suburban areas of Hanoi city.8 Consequently, the human health was threated by food contaminants due to both Cd and Pb.7 The Pb and Cd content, respectively, was 34.77 mg kg−1 and 0.47 mg kg−1 in Chau Khe craft village, Tu Son town, Bac Ninh province, Vietnam.9 Another report also indicated that the content of Pb and Cd in outdoor soil samples at Bui Village, Northern of Vietnam was 76.94 mg kg−1 and 0.35 mg kg−1, respectively.10 The Cd content was higher in carbonate rock derived-soil than that in non-karst derived-soil.11 In addition, Cd and Pb contents in soil samples taken from land nearby Lang Hich lead and zinc mine in Vietnam were 34 and 2472 mg kg−1, respectively.12The Cd is easily accumulated in the food chain because of its high soluble ability in comparison with other heavy metals.13 Also, the Pb is considered one of the most popular heavy metals existing in soils, plants and waters with high accumulation ability. Both heavy metals exist very popular in many soils, particular in tropical soils.12 Hence, the Cd and Pb immobilization study in soil is extremely necessary.
The previous studies showed that there have been three main mechanisms, comprising adsorption, precipitation/co-precipitation, and complexion can be existed for the immobilization of Pb and Cd in soil.14 The Cd and Pb-polluted soil has often been remediated by various methods such as soil washing, soil replacement, phytoremediation, water management and physical methods.2 Feng et al. (2020) applied the soil washing method as efficient remediation technique using ethylene diamine tetra (methylene phosphonic acid) (EDTMP) and polyacrylic acid (PAA) for removal of Cd, Pb, and Zn-contaminated soil.15 Synthetic hydroxyapatite and natural phosphate rock were investigated to immobilize Cd, Cu, Pb, and Zn from mine waste soils16 with removal efficiencies from about 84% to 99%. The trace metals mobility in soil was often minimized through precipitation thanks to the fall in solubility, adsorption and complexes.17 There was an increase in CEC (48.0%), pH (0.08), and EC (59.4%) and a decrease in soil extractable Cd (42.1%) and Pb (47.1%) after adding biocharto soil.18 However, the limitations of these methods are high treatment cost and difficult in control of soil effect factors. The in situ stabilization by absorbents can be considered as the low-cost and eco-friendly method to immobilize the exchangeable Cd and Pb ions in the contaminated soil. Therefore, adsorption has been used in many studies of heavy metals stabilization in soil.
Recently, the adsorption was used as inexpensive method for immobilization of heavy metals in soil. For instance, He et al. (2013)19 reported that immobilization efficiency of Pb and Cd in polluted soil by applying nano-hydroxyapatite achieved 72% and 90%, respectively. Oxalic acid-activated phosphate rocks was also used to decrease the Pb, Cd and Zn content in polluted soil.20 Several literatures were also reported that the biochars were effective adsorbents for immobilization of Pb and Cd in contaminated soils, such as bamboo and rice straw-derived biochars,21 non-magnetized and magnetized-originated biochars22 and commercial activated carbon.23 The adsorbents derived from egg shell and banana stem were applied to immobilize Pb, Cd, and Zn in alkaline soil, which showed significantly improvement in removal of heavy metals in soil.14 Besides, Pb and Cd in the contaminated soil were also removed by application of natural materials, such as limestone,24 lignin, carboxymethyl cellulose, and sodium alginate,25 modified magnesium silicate stabilizer.1 Nitrilotriacetic acid anhydride-modified ligno-cellulosic material was also used to remove Cd(II) and Pb(II) from aqueous solutions with the adsorption capacities of 143.4 and 303.5 mg g−1,26 respectively. Cd(II) content in the rice production area of Southern of China was controlled by wheat straw biochar at a mass ratio of 40 t per ha during two-year period between 2010 and 2011. The report showed that biochar can immobilize Cd(II) and meet the allowable limitation standard of FAO/WHO in rice.27 Heavy metal-polluted soil was controlled to reduce their phytoavailability, transfer and accumulate in crops by biochar.28,29 Blast furnace slag, fly ash, corncob biochar and phosphate fertilizer were used to control Pb (400 mg kg−1) in simulated soil. The report indicated that blast furnace slag and phosphate fertilizer were factors that could significantly control Pb in soil.30 Report of Xu et al. (2018) indicated that 49% of Cd in soil could be controlled by immobilization in nut-shell biochar.31 Bagasse biochar was also applied to immobilize 85% and 63% of Cd and Cr, respectively, in contaminated soil.32 The control of Cd, Pb and As in the soil from the vegetable field near the Dangping tungsten mining area in Ganzhou City, China was observed by considerably immobilization onto zeolite and biochar.33 Cd(II) from waterlogged paddy soil in Changsha (Hunan Province, China) was also controlled by adsorption in wheat straw biochar. The report indicated that wheat straw biochar could immobilize 96% of Cd(II) at pH of 7.0 and initial Cd(II) concentration of 50 mg L−1.34 Moreover, hydroxyapatite, bentonite and biochar were applied to control Cd and Pb in the soil for the crops of pepper and cabbage.35 The report showed that there was a significant decrease in bioaccessible Cd and Pb in pepper and cabbage after remediation.
Natural zeolites which belong to aluminosilicate mineral family have been considered the effective adsorbents to immobilize the contaminants in water and soil due to their high CEC, abundant existence in nature.36 Many literatures indicated the potential applications of zeolite for immobilization of heavy metals in soil. For example, natural zeolite from Gunungkidul in Indonesia was used to immobilize Cu, Pb, Zn and Cd in soil with the high stability efficiency. Bentonite, dolomite, natural zeolite, and manure were applied for the immobilization of Cd, Pb and Zn in the polluted soil with the drop in Cd, Pb and Zn availability in soil compared to the control after two years of incubation.37 Shi et al., (2013) also reported that exchangeable Pb content fell in contaminated soil owing to adding zeolite. Moreover, zeolite was considered as alkaline porous alumino-silicate with a negative charge corresponding to cation exchangeable ability.38
In recent, layered double hydroxides (LDHs) have increasingly been attracted in application as an effective adsorbent to remove pollutants in water due to possessing hydrotalcite-like structure which contains brucite-like layers. Specially, the LDHs which are composited with zeolite have been become the highly attractive adsorbents to remove the pollutants, including both cationic and anionic pollutants from water and wastewater.39 Despite of its advantages, up to now, there have been no studies which have used Mg/Al layered double hydroxides composited zeolite (Mg/Al LDH-zeolite) as an adsorbent for enhancing the immobilization of exchangeable Pb and Cd in contaminated soil. Therefore, the novelty of this study was applying Mg/Al layered double hydroxides-composited zeolite (Mg/Al LDH-zeolite) as the adsorbent for immobilization of both exchangeable Pb and Cd in soil.
The purpose of this research, therefore, was to investigate the influence of various soil conditions for Cd and Pb immobilization using the incubation experiments with natural zeolite and Mg/Al LDH-zeolite adsorbents. The experiments using the natural zeolite and the Mg/Al LDH-zeolite were conducted separately to compare the removal efficiency of Cd and Pb as well as to choose the best incubation condition for immobilization of Pb and Cd in contaminated soil. Besides, the effect of various immobilization conditions was also evaluated, including the content ratios of zeolite or Mg/Al LDH-zeolite and soil; soil pH, incubation time and soil moisture.
2. Materials and methods
2.1. Materials
The Mg/Al LDH-zeolite was synthesized using the co-precipitation method.40 In a 400 mL beaker, 100 mL of solution containing 0.01 mol of Al(NO3)3 and 0.02 mol of Mg(NO3)2 was mixed with a certain amount of zeolite which was calculated according to the LDH/zeolite ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and solution pH was adjusted to 11 using the 1 M NaOH and 0.5 M Na2CO3 solution. The mixture was then heated to 80 °C and kept at this temperature for 4 h. Mg/Al LDHs was formed by the reaction of Mg2+, Al3+, OH−, and CO32− ions. Afterwards, the obtained solid was separated from the liquid using filter membrane, washed, dried and stored in plastic bags. This solid was the Mg/Al LDH-zeolite adsorbent.
7 and solution pH was adjusted to 11 using the 1 M NaOH and 0.5 M Na2CO3 solution. The mixture was then heated to 80 °C and kept at this temperature for 4 h. Mg/Al LDHs was formed by the reaction of Mg2+, Al3+, OH−, and CO32− ions. Afterwards, the obtained solid was separated from the liquid using filter membrane, washed, dried and stored in plastic bags. This solid was the Mg/Al LDH-zeolite adsorbent.
2.2. Immobilization experiments of Cd and Pb in soil
The immobilization experiments of Cd and Pb were carried out separately and parallel using both natural zeolite and Mg/Al LDH-zeolite to compare the removal effectiveness of Cd and Pb from contaminated soils. Four experiment series were conducted to investigate the influence of soil pH, the mass ratio of absorbents (zeolite or Mg/Al LDH-zeolite) with Cd and Pb in contaminated soil, incubation time and soil moisture onto immobilization of the exchangeable Cd and Pb in soil. All treatments were done in triplicate. The controls (artificial Cd and Pb contaminated soil without treating with absorbents) were parallel used in all experiments to compare the adsorption efficiency of both applied adsorbents between artificial contaminated and pristine soil samples. Each treatment with 50 g soil which was contaminated with the mixture of Cd and Pb and zeolite or Mg/Al LDH-zeolite was separately put in plastic pot with inner diameter, height and wide of 5.0 cm, 18.0 cm, and 5.0 cm, respectively. The experiments are described in detail as in Table 1.| Experiments | Experimental conditions | ||||
|---|---|---|---|---|---|
| pH | Mass ratios of zeolite or Mg/Al LDH-zeolite (% w/w) | Soil moisture (%) | Incubation time (days) | Room temperature (°C) | |
| a In all experiments, the soil samples were collected at the endpoint of incubation period. The samples then were dried at 100 °C for 24 h before analyzing. | |||||
| Experiment 1: effect of soil pH | 5–7 | 3 | 70 | 30 | 25 ± 2 |
| Experiment 2: effect of the mass ratio between absorbents (zeolite or Mg/Al LDH-zeolite) and contaminated soil on immobilization of exchangeable Cd and Pb | 5 | 1, 3, 5 | 70 | 30 | 25 ± 2 |
| Experiment 3: effect of incubation time | 5 | 3 | 70 | 15, 30, 45 | 25 ± 2 |
| Experiment 4: effect of soil moisture | 5 | 3 | 30, 50, 70 | 30 | 25 ± 2 |
2.3. Analysis
The soil pH and electrical conductivity (EC) were determined using method developed by Bian et al. (2013)27 and Bian et al. (2014).41 Organic carbon (OC) in soil and amendments were measured using the Walkley–Black titration method in which the OC was oxidized by K2Cr2O7–H2SO4 mixture followed by back titration of the excessive dichromate content by (Fe(NH4)2(SO4)2−·6H2O). Soil texture was analyzed using the pipette method which was applied to identify proportion of sand, limon and clay content.42Total Cd and Pb content contained in the soil and materials was determined by digestion of soil samples with concentrated HNO3 and HCl (ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Then the filtered suspension was used for quantification of exchangeable Cd and Pb content using atomic absorption spectrophotometer (Hitachi Model Z-2000, Japan).43,44 Five fraction analyses of Cd and Pb in soil were conducted by sequential extraction procedure which was developed by Tessier et al. (1979)45 and modified by Nguyen et al. (2009).46 This method was also used by Dang et al. (2019).12 Binding and exchangeable forms of heavy metals were determined using a five-fold fractionation. Two grams of soil were placed in a polycarbonate centrifuge tube and the next extraction procedures were performed sequentially as follows:
3). Then the filtered suspension was used for quantification of exchangeable Cd and Pb content using atomic absorption spectrophotometer (Hitachi Model Z-2000, Japan).43,44 Five fraction analyses of Cd and Pb in soil were conducted by sequential extraction procedure which was developed by Tessier et al. (1979)45 and modified by Nguyen et al. (2009).46 This method was also used by Dang et al. (2019).12 Binding and exchangeable forms of heavy metals were determined using a five-fold fractionation. Two grams of soil were placed in a polycarbonate centrifuge tube and the next extraction procedures were performed sequentially as follows:
- Fraction 1 – F1 (exchangeable Cd and Pb): extraction with 20 mL of 1 M NH4OAc at pH 7 for 2 h at room temperature (25 ± 2 °C).
- Fraction 2 – F2 (Fe–Mn oxides occluded Cd and Pb): extraction of the residue from F1 with 20 mL of 1 M NH4OAc at pH 5 for 2 h at room temperature (25 ± 2 °C).
- Fraction 3 – F3 (carbonate-bound Cd and Pb): extraction of the residue from F2 with 20 mL of 0.04 M NH2OH–HCl in 25% HOAc for 6 h in a water bath at 60 °C.
- Fraction 4 – F4 (organically complexed Cd and Pb): extraction of the residue from F3 with 15 mL of 30% H2O2 at pH 2 for 5.5 h in a water bath at 80 °C.
- Fraction 5 – F5 (residual Cd and Pb): after cooling, 5 mL of 3.2 M NH4OAc in 20% HNO3 was added to the residue of F4. Sample was shaken for 0.5 h, and finally diluted to 20 mL with distilled water.
Specifically, the extraction procedure was summarized in Table 2.
| Fraction | Phase | Reagent | Shaking time |
|---|---|---|---|
| F1 | Exchangeable | 1 M NH4OAc at pH 7 | 2 h at 25 °C |
| F2 | Fe–Mn oxide occlude | 1 M NH4OAc at pH 5 | 2 h at 25 °C |
| F3 | Carbonate bound | 0.04 M NH2OH–HCl in 25% HOAc | 6 h at 60 °C |
| F4 | Organically complexed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
30% H2O2 at pH 2 in a water bath | 5.5 h at 80 °C |
| F5 | Residual | 3.2 M NH4OAc in 20% HNO3 | 0.5 h at 25 °C |
The content of Pb and Cd in each fraction was measured using ICP-OMS (Model ULTIMA EXPERT, Horiba, Japan). Total content of Pb and Cd in the fresh soil was also determined using ICP-OMS after the soil samples were digested with the mixture of concentrated HNO3 and HCl at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.12
3.12
The morphologies of zeolite and Mg/Al LDH-zeolite were examined using an energy dispersive X-ray spectroscopy equipped with EDS and SEM system (HITACHI S-4800). The surface area and the porous structure were determined using Brunauer–Emmett–Teller (BET – BET, Builder, SSA-4300). The chemical functional groups were determined by Fourier-transform infrared spectroscopy (FT-IR, Spectrum Two).
All data were treated using Excel 2020 and SPSS 19.0 software. Analysis of variance was used to determine the standard deviation and the significant difference between the treatments. The significant level was defined at p < 0.05.
3. Results and discussion
3.1. Characteristics of the fresh soil and adsorbents
| Fresh soil | |||||||
|---|---|---|---|---|---|---|---|
| Sand (%) | 55.16 ± 1.51 | pH | 4.71 ± 0.3 | Cr (mg kg−1) | 0.42 ± 0.006 | Ca (mg kg−1) | 2.72 ± 0.03 |
| Limon (%) | 23.82 ± 1.25 | OC (%) | 2.03 ± 0.01 | Cd (mg kg−1) | 0.50 ± 0.002 | Mn (mg kg−1) | 1.26 ± 0.01 |
| Clay (%) | 21.02 ± 1.50 | EC (µS cm−1) | 27.2 ± 6.5 | Pb (mg kg−1) | 1.92 ± 0.004 | Fe (mg kg−1) | 4.35 ± 0.04 |
| Zeolite | Mg/Al LDH-zeolite | ||||||
| pH | 8.2 ± 0.3 | Cd (mg kg−1) | 0.052 ± 0.01 | pH | 8.13 ± 0.08 | Cd (mg kg−1) | 0.056 ± 0.01 |
| EC (µS cm−1) | 180 ± 1.05 | Pb (mg kg−1) | 0.42 ± 0.03 | EC (µS cm−1) | 172 ± 1.03 | Pb (mg kg−1) | 0.44 ± 0.02 |
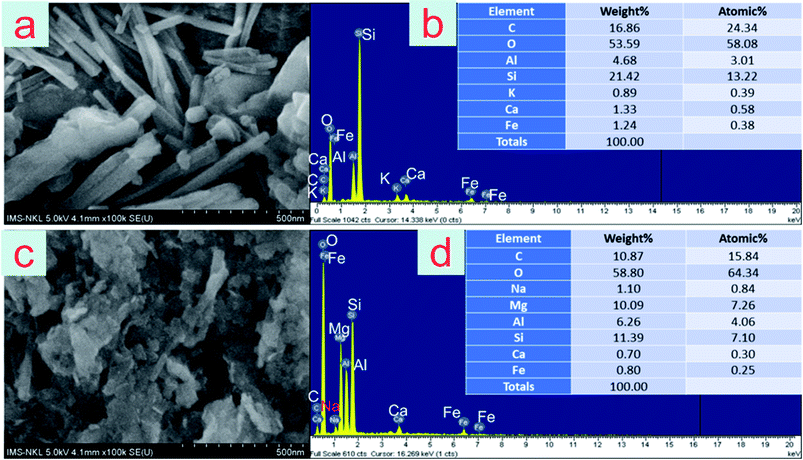 | ||
| Fig. 1 SEM images of zeolite (a), Mg/Al LDH-zeolite (c); EDS spectra of zeolite (b) and Mg/Al LDH-zeolite (d). | ||
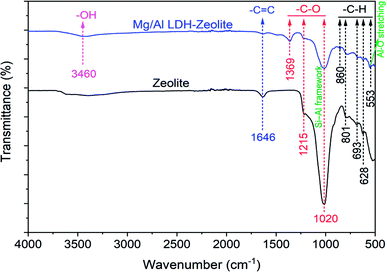
The chemical functional groups which were presence on both zeolite and Mg/Al LDH-zeolite's surface are illustrated in Fig. 2. From the Fig. 2, it is clear that there was the appearance of –CH functional groups at peaks of 628, 693, 801 and 860 cm−1 in both zeolite and Mg/Al LDH-zeolite, and the new peak of 553 cm−1 which was appeared in Mg/Al LDH-zeolite indicated the Al–O stretching mode. The peaks of 1020 and 1215 cm−1 were represented the C–O groups in both zeolite and Mg/Al LDH-zeolite. The peak at 1369 cm−1 appeared in Mg/Al LDH-zeolite was also indexed to the C–O group corresponding to the interlayer carbonate group in the carbonate layered double hydroxides. Moreover, the peak at 1020 cm−1 can be demonstrated to bending modes of Si–Al framework. Besides, there was presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups at the peak at 1640 cm−1 in both zeolite and Mg/Al LDH-zeolite. While the spectrum of Mg/Al LDH-zeolite illustrated the presence of the hydroxyl group at peak of 3460 cm−1.
C groups at the peak at 1640 cm−1 in both zeolite and Mg/Al LDH-zeolite. While the spectrum of Mg/Al LDH-zeolite illustrated the presence of the hydroxyl group at peak of 3460 cm−1.
3.2. Effect of soil pH onto immobilization of the exchangeable Cd and Pb
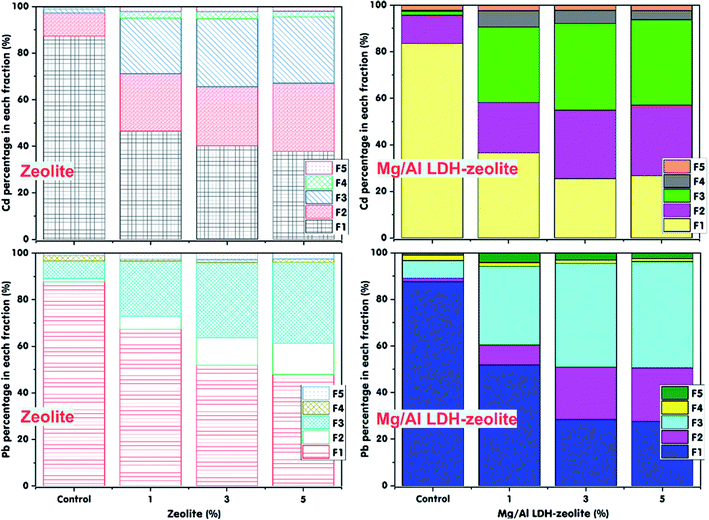
The soil pH is an important factor that decides the existence forms of heavy metals in soil. The data in Fig. 3, Tables S1a and S1b† illustrate the effect of soil pH onto the Cd and Pb immobilization in the contaminated soil after a 30 days incubation period with a mixture ratio of 3% of zeolite or Mg/Al LDH-zeolite. From the data, it can be seen that the content of the exchangeable Cd and Pb decreased after 30 days of incubation with both zeolite or Mg/Al LDH-zeolite at mixture ratio of 3% at all soil pH values in range from 5 to 7. Conversely, in control treatment, there was an insignificant change in Cd and Pb content during the incubation period. The exchangeable Cd and Pb contents (F1) in the control treatment, respectively, made up 21.96 mg kg−1 (85.36%) and 22.03 mg kg−1 (84.05%) (p ≤ 0.05), respectively, compared to five left fraction forms (F2–F5). However, the F1 forms of Cd and Pb fell with a rise in soil pH from 5 to 7. Overall, the lowest remain contents of the exchangeable Cd was 12.33 mg kg−1 (48.63%) and 8.57 mg kg−1 (33.11%) (p < 0.05) in incubation with zeolite and Mg/Al LDH-zeolite, respectively, at soil pH of 7. The exchangeable Pb content also possessed a similar trend in incubation treatment by zeolite and Mg/Al LDH-zeolite. The content of exchangeable Pb reached the lowest at soil pH of 7 with 13.42 mg kg−1 (51.49%) and 8.84 mg kg−1 (33.41%) (p ≤ 0.05) in incubation with zeolite and Mg/Al LDH-zeolite, respectively. It can easily be observed that the contents of exchangeable Cd and Pb went down while the forms of the Fe–Mn oxide occlude (F2), carbonate bound (F3), organically complexed (F4) went up after a 30 days incubation period with both zeolite and Mg/Al LDH-zeolite. Among these forms, almost Pb content was changed to carbonate bound (F3) form for the incubation with both zeolite and Mg/Al LDH-zeolite while almost Cd element existed in the forms of Fe–Mn oxide occlude (F2) for incubation with both zeolite and Mg/Al LDH-zeolite. And the second-high existence form of Cd in soil was organically complexed (F4).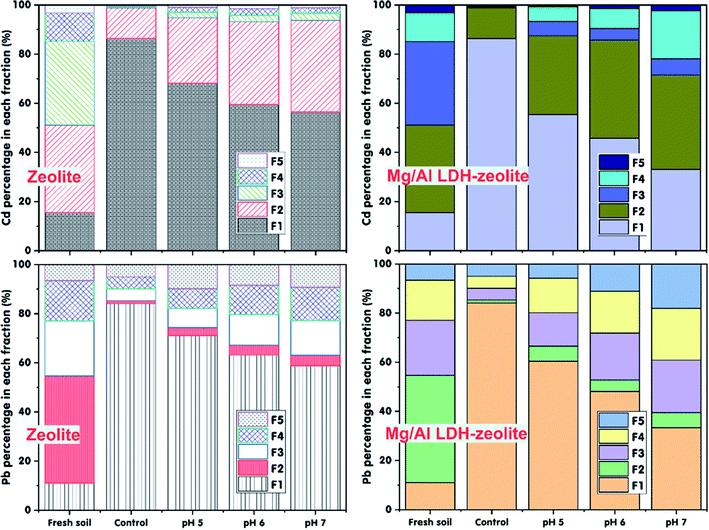 | ||
| Fig. 3 Effect of soil pH on the immobilization of exchangeable Cd and Pb into various forms in soil after 30 days incubation period at soil moisture of 70% and 50 mg kg−1 of Cd and Pb content. | ||
These results showed that nearly all exchangeable Cd and Pb ions were immobilized in the forms of Fe–Mn oxide, carbonate bound and organic matters when the zeolite and Mg/Al LDH-zeolite were supplemented into the contaminated soil. This proved that the exchangeable Cd and Pb in contaminated soils can be immobilized well by both zeolite and Mg/Al LDH-zeolite. However, the Mg/Al LDH-zeolite had higher capacity in immobilization of both Cd and Pb compared to zeolite. It is may be due to the higher electrostatic attraction of heavy metal of Mg/Al LDH-zeolite when was modified from zeolite. There was a slight growth in soil pH and EC values after 30 days of incubation with zeolite and Mg/Al LDH-zeolite compared to the control sample (Table 4). It might be because of the reduction of the solubility of Zn and Cd in soil. Besides, the soil organic matters might enhance the immobilization of Pb and Cd thanks to supplemented adsorbents by stimulating organic acid production that bound heavy metals to the organic matter fraction47 leading to the transfer of Cd and Pb exchangeable forms to the carbonate bounds in soil corresponding to the increase in soil pH values48 after added of zeolite and Mg/Al LDH-zeolite. Moreover, the rise in soil pH also triggered the drop the content of exchangeable Cu owing to addition of adsorbents.48 Also, the increase in soil pH also was because of the metal adsorption onto the surfaces of zeolite resulting in the formation of insoluble metal hydroxides.49 Moeen et al, (2020)50 indicated that there was a high heavy metal immobilization in soil due to a significant growth in the soil pH after the soil was added the zeolite. The addition of zeolite and Mg/Al LDH-zeolite not only caused the growth in soil pH but also promoted carbonate precipitation and oxide formation.51 These results illustrate that both zeolite and Mg/Al LDH-zeolite can be used to immobilize heavy metals and improved the pH for the acidic soils as well as the soil quality. These findings were agreed with the previous report of Ok et al., (2011) for immobilization of Pb in contaminated soil using waste oyster shells and improvement of soil quality.52
| Treatment | Zeolite | Mg/Al LDH-zeolite | ||
|---|---|---|---|---|
| pH of day 30 | EC of day 30 (µS cm−1) | pH of day 30 | EC of day 30 (µS cm−1) | |
| a Treated soils were contaminated soils of exchangeable Cd and Pb which were incubated with zeolite or Mg/Al LDH-zeolite for 30 days. Mean ± SD, n = 3. | ||||
| Control | 5.38 ± 0.249 | 59.00 ± 7.81 | 5.38 ± 0.249 | 59.00 ± 7.81 |
| pH 5 | 5.50 ± 0.27 | 99.33 ± 8.50 | 5.69 ± 0.38 | 103.33 ± 5.507 |
| pH 6 | 6.74 ± 0.36 | 116.00 ± 23.43 | 6.83 ± 0.38 | 124.67 ± 12.09 |
| pH 7 | 7.84 ± 0.12 | 132.00 ± 7.03 | 7.96 ± 0.242 | 152.33 ± 15.69 |
The complexion of exchangeable heavy metals with functional groups on the surface of adsorbents can achieve more highly due to the electrostatic attraction of physical adsorption. The soil pH was higher, the fraction of Pb in the soil which was associated with –CO group of zeolite or Mg/Al LDH-zeolite to form the Pb carbonate bound (F3) was larger.53 Moreover, soluble Cd and Pb (exchangeable forms) might grow the precipitation of insoluble minerals in zeolite and Mg/Al LDH-zeolite triggering the enhancement of Cd and Pb immobilization when increasing soil pH.53,54 The forms of Cd and Pb precipitation as CdCO3 and PbCO3 rose corresponding to a growth in the soil pH. The Cd and Pb were also changed from acid-soluble fraction to reducible, oxidizable, and more stabilized residual forms due to their dissolution and precipitation.36 Therefore, the Cd and Pb immobilization was higher at higher soil pH value. In this study, the soil pH of 7 was used for further experiments.
3.3. Effect of mass ratio of soil and adsorbent on immobilization of the exchangeable Cd and Pb
The influence of mixture mass ratios between zeolite or Mg/Al LDH-zeolite and soil on Cd and Pb immobilization was investigated with varying ratios in range of 1%, 3% and 5% (w/w) at soil moisture of 70%, soil pH of 7.0 and a 30 days incubation period with 50 mg kg−1 of both exchangeable Cd and Pb content. The results are presented in Fig. 4, Tables S2a and S2b.† As is illustrated by data in the Fig. 4, Tables S2a and S2b,† in comparison with the control treatment, the exchangeable Cd and Pb contents (F1) plummeted after a 30 days incubation period with the mass ratios of zeolite or Mg/Al LDH-zeolite from 1% to 5%. The lowest left exchangeable Cd and Pb contents were achieved at ratio of 3% and there was a insignificant drop in exchangeable Cd and Pb contents when the mixture ratio rose to 5%.The exchangeable Cd content and percentage fell from 21.41 mg kg−1 (87.31%) (in control sample) to 11.33 mg kg−1 (46.53%), 9.67 mg kg−1 (40.21%) and 9.47 mg kg−1 (37.80%) (p ≤ 0.05) for the mass ratio of zeolite at 1%, 3% and 5%, respectively. Meanwhile, with the mass ratio of Mg/Al LDH-zeolite of 1%, 3% and 5%, the exchangeable Cd content and percentage dropped to 9.56 mg kg−1 (36.55%), 6.32 mg kg−1 (25.50%) and 6.56 mg kg−1 (26.70%), respectively. The similar trends were also achieved with the exchangeable Pb content and percentage at the various mixture mass ratios of both zeolite and Mg/Al LDH-zeolite. The exchangeable Pb percentage reached 51.86% (12.91 mg kg−1) and 28.47% (7.11 mg kg−1) after incubation at 3% of zeolite and Mg/Al LDH-zeolite, respectively. These findings indicated that there was no significant different at the mixture mass ratios between 3% and 5% of adsorbents on immobilization of Cd and Pb in contaminated soil. However, the Mg/Al LDH-zeolite had higher efficiency in Cd and Pb immobilization compared with zeolite from 15% (Cd) to 23% (Pb) at the mass ratio of 3%. Most of exchangeable Cd and Pb forms were transferred to the forms of Fe–Mn oxide occlude (F2) and Carbonate bound (F3) after incubation periods. The rests were in the forms of organically complexed (F4) and residual (F5). Similar results were found in previous researches. Specifically, the Cd, Pb and Zn immobilization in soil reached at optimal biochar mass ratio of 3% by Dang et al. (2019).12 Ok et al. (2011)52 also used the 1% and 5% ratios of waste oyster shells for exchangeable Cd and Pb immobilization in contaminated soil with a significant reduction in Pb (98.9%) and Cd (69.5%). The similar trend of drop in the exchangeable forms in soil by using hydrothermal biochar was observer by Zuo et al. (2016).55 The result showed the lower exchangeable Pb and Cd forms corresponding with the higher mass ratios of adsorbents. Besides, the findings in this study also agreed with the previous investigation about using the biochars derived from pine cone and vegetable waste at the application rates of 2.5% and 5% to immobilize the exchangeable Pb in contaminated soil.56 Zhu et al. (2020)57 indicated the remain Cd proportion of 29.71%, 31.54%, 30.08%, and 32.57% with supplement of 1%, 2%, 4%, and 8% of thiourea-modified biochar into the soil for 30 days incubation period, respectively.
In the present study, there was a significant decrease in the exchangeable Cd and Pb forms when supplementing zeolite or Mg/Al LDH-zeolite into the contaminated soil for 30 days of incubation but there was no the significant different at the mixture mass ratios between 3% and 5% on immobilization of Cd and Pb in contaminated soil. This can be due to the increase in active sites on the surface of zeolite or Mg/Al LDH-zeolite when they were mixed with contaminated soil leading to a growth in the binding sites for immobilization of Cd and Pb in soil. Finally, these caused the drop in exchangeable forms and the rise in the binding sites for formation of Fe–Mn oxide occlude (F2) and carbonate bound (F3) forms. However, when the mass ratios of both zeolite and Mg/Al LDH-zeolite were went up to 5%, the capacity of Cd and Pb immobilization remained steady. Thus, it can draw conclusion that the absorbents achieved a critical level for immobilization of metals at the mixture mass ratio of 3% in the tested soil.
3.4. Effect of incubation time on immobilization of the exchangeable Cd and Pb
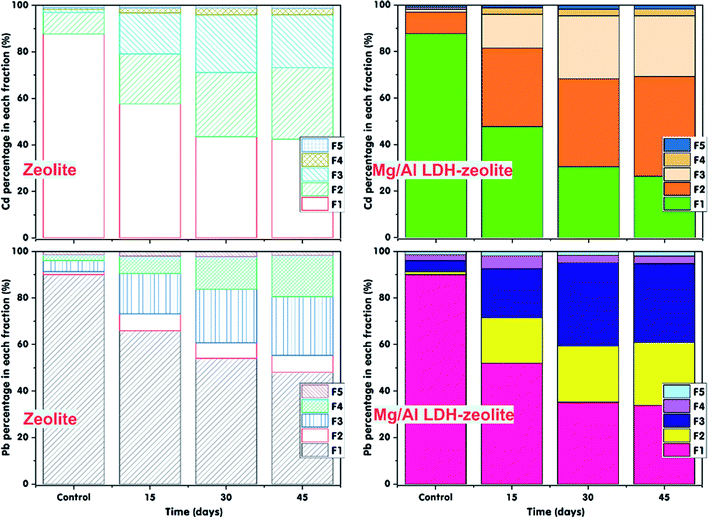
To investigate the effect of incubation time onto immobilization of exchangeable Cd and Pb ions in soil, the absorbents, consisting of both zeolite and Mg/Al LDH-zeolite were incubated, in separate, with the Cd and Pb contaminated soil over a 15, 30 and 45 days period. The control treatment was incubated over 45 days and used to compare to the treatments which were added zeolite or Mg/Al LDH-zeolite, separately. The artificial Cd and Pb contaminated soils were used at mixture mass ratio of 3% between zeolite or Mg/Al LDH-zeolite and soil (w/w). Tables S3a, 3b† and Fig. 5 illustrate the remaining Cd and Pb contents in contaminated soil after 15, 30 and 45 days of incubation at soil pH of 7.0, soil moisture of 70% and 50 mg kg−1 of both exchangeable Cd and Pb. The results indicate that the left exchangeable Cd and Pb contents in treatments for 15, 30, and 45 days were lower than those of control treatment. The proportion of exchangeable Cd forms in soil dropped from 87.65% (22.17 mg kg−1) in control treatment to 57.67% (14.18 mg kg−1), 43.48% (11.03 mg kg−1) and 42.51% (10.68 mg kg−1) in treatments incubated with zeolite and 47.69% (11.17 mg kg−1), 30.43% (7.47 mg kg−1) and 26.36% (6.74 mg kg−1) in treatments incubated with Mg/Al LDH-zeolite after 15, 30 and 45 days, respectively. The proportion of Pb also possessed the similar trend after 15, 30 and 45 days of incubation with both zeolite and Mg/Al LDH-zeolite. After 30 days of incubation, the remaining proportion of Pb was 54.04% (14.12 mg kg−1) and 35.24% (9.47 mg kg−1) in treatment by zeolite and Mg/Al LDH-zeolite, respectively. There was no the significant different in exchangeable Pb and Cd contents in soil between incubation time of 30 days and 45 days towards both applied absorbents. However, the immobilization efficiency of Cd and Pb using Mg/Al LDH-zeolite was higher than that of the pristine zeolite.Furthermore, from the Fig. 5, it is clear that the exchangeable Cd form (F1) was transferred to Fe–Mn oxide occlude (F2) and carbonate bound (F3) forms after treated with both zeolite and Mg/Al LDH-zeolite. Meanwhile, the exchangeable Pb form (F1) was transferred to the forms of carbonate bound (F3) and organically complexed (F4) in treatment by zeolite and Fe–Mn oxide occlude (F2) and carbonate bound (F3) forms in treatment by Mg/Al LDH-zeolite. The rests were existed in the residual form (F5). The changes from exchangeable form into more immobilized forms of metals were much higher during the first 30 days of incubation. This occurred because there were abundant active sites on the surfaces of zeolite and Mg/Al LDH-zeolite during the incubation period of 15 and 30 days. After that, the active sites might be saturated due to the adsorption of exchangeable Cd and Pb on the surfaces of zeolite and Mg/Al LDH-zeolite. Therefore, there was no significant drop when the incubation time continued rise to 45 days. Igalavithana et al. (2019)56 also reported that the optimal incubation time of 30 days and 45 days was applied for Pb immobilization in contaminated soil by biochars derived from pine cone and vegetable waste. Iqbal et al. (2016)58 indicated the exchangeable Pb in contaminated soil decreased after 28 days of incubation with farm manure. Incubation time of 30 days was also applied to reduce the exchangeable Cd content in contaminated soil using thiourea-modified biochar.57 The results highlighted that the remaining proportion of exchangeable Cd was 31.54% after incubation of 30 days with 3% of thiourea-modified biochar. Al-Wabel et al. (2015)59 showed there was no significant fall in heavy metals (Fe, Mn, Zn, Cd, Cu and Pb) between the various rates of biochar at 1.0, 3.0 and 5.0% after the 30 days incubation period.
3.5. Effect of soil moisture on immobilization of the exchangeable Cd and Pb
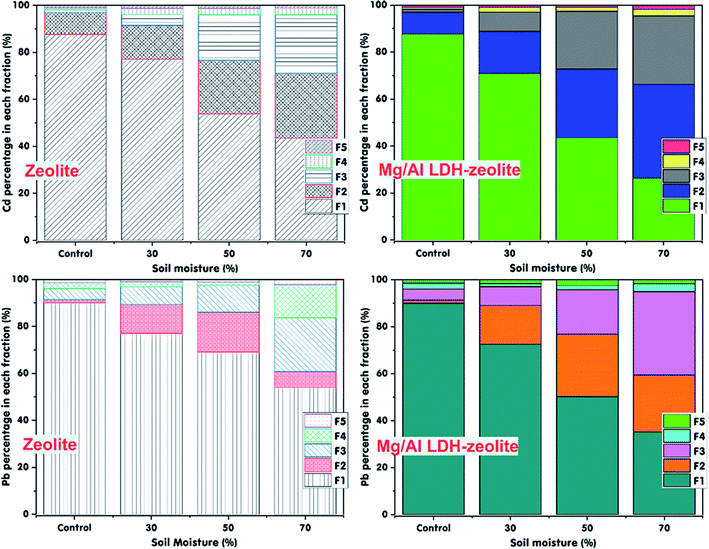
Soil moisture is one of the most important factors affected the mobility of heavy metals in soil. In this study, the experiments with varying of soil moistures of 30%, 50% and 70% were conducted to evaluate the effect of soil moisture on the Cd and Pb immobilization in the contaminated soil after the 30 days incubation period with zeolite or Mg/Al LDH-zeolite at soil pH of 7. The control treatment was performed in separate to compare the immobilization efficiency of both exchangeable Cd and Pb ions in soil. The obtained data are presented in Tables S4a, 4b† and Fig. 6. The results show that after 30 days incubation period, the proportion of exchangeable Cd and Pb fell corresponding to the growth in soil moisture from 30% to 50% and 70% in both contaminated soils treated with zeolite or Mg/Al LDH-zeolite. There was a slight downward trend in content of exchangeable Cd and Pb in the treatments with 30% of soil moisture during incubation period, reached 19.93 mg kg−1 (77.12% Cd) and 20.23 mg kg−1 (77% Pb) (for zeolite) and 18.53 mg kg−1 (70.86% Cd) and 19.15 mg kg−1 (72.57% Pb) (for Mg/Al LDH-zeolite). Meanwhile, the exchangeable Cd and Pb content in soil saw a deep drop corresponding with the increase in soil moisture of 50% and 70%. The remaining content of exchangeable Cd was 13.45 mg kg−1 (53.80%) and 11.03 mg kg−1 (43.48%) (for zeolite) and 10.76 mg kg−1 (43.56%) and 6.47 mg kg−1 (26.36%) (for Mg/Al LDH-zeolite) at soil moisture of 50% and 70%, respectively. The contents of exchangeable Pb also dropped to 17.06 mg kg−1 (69.01%) and 14.12 mg kg−1 (54.04%) (for zeolite) and 12.45 mg kg−1 (50.31%) and 9.47 mg kg−1 (35.24%) (for Mg/Al LDH-zeolite) at soil moisture of 50% and 70%, respectively, after 30 days of incubation time.Besides, Fig. 6 also shows the proportion of exchangeable Cd and Pb forms in contaminated soil at control and treated with adsorbents. What stands out from data in Fig. 6 is that there was a corresponding downward trend in proportions of exchangeable Cd and Pb forms with the growth in the soil moisture. The proportion of Cd and Pb in forms of Fe–Mn oxide occlude (F2) and carbonate bound (F3) rose with a growth in the soil moisture of 30%, 50% and 70% after 30 days incubation period and these transferred proportions were higher in the treated soils with both adsorbents compared to that in the control samples. The highest drop in the exchangeable Cd and Pb content occurred at soil moisture of 70%, reached 43.48% for Cd and 54.04% for Pb with using zeolite and 26.36% for Cd and 35.24% for Pb with using Mg/Al LDH-zeolite. However, the Mg/Al LDH-zeolite possessed the immobilization efficiency of both Cd and Pb ions more highly than that of nature zeolite from 1.5 to 1.6 times. These results can be explained that the immobilization of exchangeable Cd and Pb increased corresponding to the rise in the soil moisture during the incubation period leading to more crystallization of Fe–Mn oxides forms in higher soil moisture.60 Therefore, the Cd and Pb immobilization rose corresponding to the growth in the soil moisture from 30% to 70%. Besides, it might has more negative charges on the adsorbents surfaces at higher soil moisture triggering the drop in the exchangeable heavy metals.60 Under high soil moisture condition, there was the re-precipitation of hydrous Fe–Mn oxides with Cd and Pb and transferring into a crystalline form. Therefore, the proportion of Cd and Pb in forms of Fe–Mn oxide occlude (F2) increased from the transferred forms and the exchangeable forms. In summary, the findings indicate that the modified zeolite (Mg/Al LDH-zeolite) was the attractive adsorbent for exchangeable Cd and Pb immobilization in contaminated soil.
3.6. Plausible mechanism discussion of the exchangeable Cd and Pb immobilization by adsorbents
As can be seen from aforementioned data, almost exchangeable Cd and Pb forms in the contaminated soil were transferred to Fe–Mn oxide occlude (F2), carbonate bound (F3) and organically complexed (F4) forms after the zeolite or Mg/Al LDH-zeolite was incubated into soil at the certain time. The heavy metal immobilization in soil can relate to several main mechanisms. Firstly, values of pH and EC affected the adsorption capacities of Cd and Pb ions in contaminated soil. The pH and CEC can be good indicators to evaluate the adsorption capacities of heavy metals. In this study, the pH and EC values, respectively, were 8.2 and 180 (for zeolite) and 8.13 and 172 (for Mg/Al LDH-zeolite) which had significantly contribution in enhancement of adsorption capacities of Cd and Pb ions. Secondly, the co-precipitation process occurred thanks to the presence of both Fe and Al oxyhydroxides in adsorbents (EDS analysis data shown in Fig. 1b and d)14,61 which triggered the co-precipitation of Cd and Pb ions in contaminated soil with Fe and Mn oxides in zeolite and Mg/Al LDH-zeolite. However, the proportion of Al in Mg/Al LDH-zeolite was higher than that in zeolite, thus the immobilization capacity of exchangeable Cd and Pb in contaminated soil by Mg/Al LDH-zeolite reached more highly (Fig. 2). The obtained results in this study were analogue with the previous studies. Specifically, the co-precipitation of metals with Fe and Mn oxides occurring in acid soil due to the mobility of Fe, Al and Mn.61 The immobilization mechanism of exchangeable Cd and Pb onto Mg/Al LDH-zeolite also related to the precipitation in form of carbonate in the interlayer region of LDH. Moreover, the Mg/Al LDH-zeolite possessed the higher BET surface area by about 10 times in comparison with the natural zeolite (26.15 m2 g−1 for zeolite and 252.66 m2 g−1 for Mg/Al LDH-zeolite) which triggered the much higher immobilization capacity of Mg/Al LDH-zeolite by pore filling mechanism, compared to the zeolite. Besides, the higher BET surface area was, more active adsorption sites on the surface of Mg/Al LDH-zeolite for attack of Cd and Pb in these sites. Moreover, the structure of Mg/Al LDH-zeolite was more heterogamous than that of zeolite leading to higher immobilization efficiency of Cd and Pb ions in contaminated soil. This finding was in well agreement with the previous report of important role in complexion on surface of natural zeolite due to its high specific surface.38Besides, surface oxygen-containing functional groups, including carboxyl and hydroxyl groups on materials surface were responsible for the immobilization of metal ions.62 The formation of organic matters might be due to combination with the functional groups of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –CH on the zeolite and Mg/Al LDH-zeolite's surfaces (Fig. 2). While the Cd might combined with –C
O and –CH on the zeolite and Mg/Al LDH-zeolite's surfaces (Fig. 2). While the Cd might combined with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group to form carbonate bound. This can lead to higher binding ability for Cd and Pb ions based on the complexation processes. The electrostatic attraction between carboxylate groups (–C
O group to form carbonate bound. This can lead to higher binding ability for Cd and Pb ions based on the complexation processes. The electrostatic attraction between carboxylate groups (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and exchangeable Cd and Pb might enhance the immobilization capacity of both zeolite and Mg/Al LDH-zeolite. The carboxylate groups (–C
O) and exchangeable Cd and Pb might enhance the immobilization capacity of both zeolite and Mg/Al LDH-zeolite. The carboxylate groups (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) can form the complexion of Mg–Al–CO3–LDH-zeolite which led to the enhancement of the Cd and Pb immobilization in form of CdCO3 and PbCO3 precipitations contributing to the increase in Fe–Mn oxide-bound Pb and Cd in this study. Therefore, Mg/Al LDH-zeolite had higher efficiency in exchangeable Cd and Pb immobilization compared to the pristine zeolite. A similar phenomenon has also been reported by Sneddon et al. (2006).63 The formation of Pb carbonate was the main controlling phase in the contaminated soil. The report of Albert et al. (2021)18 also illustrated that the functional groups of –COOH, –NH2, PO4−, and –C
O) can form the complexion of Mg–Al–CO3–LDH-zeolite which led to the enhancement of the Cd and Pb immobilization in form of CdCO3 and PbCO3 precipitations contributing to the increase in Fe–Mn oxide-bound Pb and Cd in this study. Therefore, Mg/Al LDH-zeolite had higher efficiency in exchangeable Cd and Pb immobilization compared to the pristine zeolite. A similar phenomenon has also been reported by Sneddon et al. (2006).63 The formation of Pb carbonate was the main controlling phase in the contaminated soil. The report of Albert et al. (2021)18 also illustrated that the functional groups of –COOH, –NH2, PO4−, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the biochar's surface complexed with metal ions in soil corresponding with a decrease in the bioavailability of potentially toxic elements.
O on the biochar's surface complexed with metal ions in soil corresponding with a decrease in the bioavailability of potentially toxic elements.
Another mechanism was that the reaction of metal ions with SiO2 constituent presence in adsorbents might form both metal silicides and metal oxides.64 Si and O elements were appeared in both zeolite and Mg/Al LDH-zeolite (data in Fig. 1b and d). Thus, they also contributed to the exchangeable Cd and Pb immobilization after the addition of zeolite and Mg/Al LDH-zeolite into the contaminated soil. A high reactivity of MgO might be better for Pb immobilization in the short term because of its rapidly dissolved and hydrated potential.65 Besides, as can be seen from data in the Fig. 1b and d, the presence of Mg and O elements in the Mg/Al LDH-zeolite contributed in Cd and Pb immobilization in contaminated soil that was not happen in the pristine zeolite.
In summary, the precipitation, co-precipitation, electrostatic attraction and pore filling were the main mechanisms of exchangeable Cd and Pb immobilization in contaminated soil in forms of Fe–Mn oxide occlude (F2), carbonate bound (F3) and organically complexed (F4) when both zeolite and Mg/Al LDH-zeolite adsorbents were incubated into contaminated soil. The Mg/Al LDH-zeolite can be used to enhance the exchangeable Cd and Pb ions immobilization in the contaminated soil due to possessing much higher BET surface area and the amount of Mg, Al, Fe, Si elements compared the pristine zeolite. Furthermore, under suitable soil environmental conditions, including optimal pH, moisture, incubation time and mixture mass ration of soil and absorbents, the absorbents' surface functional groups became effective in immobilization of heavy metals in soil.
4. Conclusions
The present study was successful in conducting experiments to investigate the effect of various factors on the exchangeable Cd and Pb immobilization by incubation of natural zeolite and Mg/Al LDH-zeolite into the contaminated soil. The results show that the optimal soil pH, the mixture mass ratio between soil and adsorbents, incubation time and soil moisture were 7.0, 3%, 30 days and 70%, respectively, for exchangeable Cd and Pb immobilization using both zeolite and Mg/Al LDH-zeolite. At these conditions, the highest decrease in the exchangeable heavy metal forms was 43.48% for Cd and 54.04% for Pb with using zeolite and 26.36% for Cd and 35.24% for Pb with using Mg/Al LDH-zeolite. Almost exchangeable Cd and Pb were transferred into the forms of Fe–Mn oxide occlude (F2), carbonate bound (F3) and organically complexed (F4) thanks to the precipitation, co-precipitation and electrostatic attraction mechanisms. Besides, due to possessing extremely high area surface, the Mg/Al LDH-zeolite had the much higher Cd and Pb immobilization efficiency than that of the pristine zeolite by 1.5 to 1.6 times. In conclusion, the Mg/Al LDH-zeolite was low-cost, high effective and eco-friendly adsorbent which had the potential in immobilization of the exchangeable heavy metals in contaminated soil and improved the soil quality, especially acid soil.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 105.08-2019.01.References
- X. Yuan, T. Xiong, H. Wang, Z. Wu, L. Jiang, G. Zeng and Y. Li, Environ. Sci. Pollut. Res., 2018, 25, 32562–32571 CrossRef CAS PubMed.
- Y. Hamid, L. Tang, B. Hussain, M. Usman, L. Liu, A. Sher and X. Yang, Ecotoxicol. Environ. Saf., 2020, 196, 110539–110548 CrossRef CAS PubMed.
- M. Pietrzykowski, J. Antonkiewicz, P. Gruba and M. Pajak, Open Chem., 2018, 16, 1143–1152 CAS.
- K. Šichorová, P. Tlustoš, J. Száková, K. Kořínek and J. Balík, Plant Soil Environ., 2004, 50, 525–534 CrossRef.
- R. A. Wuana and F. E. Okieimen, ISRN Ecol., 2011, 2011, 402647–402666 Search PubMed.
- M. Ćwieląg-Drabek, A. Piekut, K. Gut and M. Grabowski, Sci. Rep., 2020, 10, 3363–3370 CrossRef PubMed.
- V. Radovanovic, I. Djekic and B. Zarkovic, Sustain, 2020, 12, 1–11 Search PubMed.
- N. T. L. Huong, M. Ohtsubo, L. Li, T. Higashi and M. Kanayama, Commun. Soil Sci. Plant Anal., 2010, 41, 390–407 CrossRef.
- H. T. L. Tra, C. T. Son, N. T. Ha and N. K. Tan, Int. J. Agric. Environ. Res., 2017, 2698–2711 Search PubMed.
- H. T. T. Ngo, L. Liang, D. B. Nguyen, H. N. Doan and P. Watchalayann, Appl. Ecol. Environ. Res., 2020, 42, 71–84 Search PubMed.
- C. Li, Z. Yang, T. Yu, Q. Hou, X. Liu and J. Wang, Ecotoxicol. Environ. Saf., 2021, 208, 111505–111604 CrossRef CAS PubMed.
- V. M. Dang, S. Joseph, H. T. Van, T. L. A. Mai, T. M. H. Duong, S. Weldon, P. Munroe, D. Mitchell and S. Taherymoosavi, Environ. Technol., 2019, 40, 3200–3215 CrossRef CAS PubMed.
- G. Farid, Adv. Crop Sci. Technol., 2015, 04, 1–7 Search PubMed.
- M. Ashrafi, S. Mohamad, I. Yusoff and F. S. Hamid, Environ. Sci. Pollut. Res., 2015, 22, 223–230 CrossRef CAS PubMed.
- W. Feng, S. Zhang, Q. Zhong, G. Wang, X. Pan, X. Xu, W. Zhou, T. Li, L. Luo and Y. Zhang, J. Hazard. Mater., 2020, 381, 120997–121015 CrossRef CAS PubMed.
- J. Bech, P. Duran, N. Roca, W. Poma, I. Sánchez, L. Roca-Pérez, R. Boluda, J. Barceló and C. Poschenrieder, J. Geochem. Explor., 2012, 123, 109–113 CrossRef CAS.
- H. Han, H. Cai, X. Wang, X. Hu, Z. Chen and L. Yao, Ecotoxicol. Environ. Saf., 2020, 195, 110375–110382 CrossRef CAS PubMed.
- H. A. Albert, X. Li, P. Jeyakumar, L. Wei, L. Huang, Q. Huang, M. Kamran, M. Sabry, D. Hou, J. Rinklebe and Z. Liu, Sci. Total Environ., 2021, 755, 142582–142596 CrossRef CAS PubMed.
- M. He, H. Shi, X. Zhao, Y. Yu and B. Qu, Procedia Environ. Sci., 2013, 18, 657–665 CrossRef CAS.
- Z. Zhang, G. Guo, M. Wang, J. Zhang, Z. Wang, F. Li and H. Chen, Environ. Sci. Pollut. Res., 2018, 25, 2861–2868 CrossRef CAS PubMed.
- X. Yang, J. Liu, K. McGrouther, H. Huang, K. Lu, X. Guo, L. He, X. Lin, L. Che, Z. Ye and H. Wang, Environ. Sci. Pollut. Res., 2016, 23, 974–984 CrossRef CAS PubMed.
- Z. Tan, Y. Wang, L. Zhang and Q. Huang, Environ. Sci. Pollut. Res., 2017, 24, 24844–24855 CrossRef CAS PubMed.
- S. jia Liu, Y. guo Liu, X. fei Tan, G. ming Zeng, Y. hui Zhou, S. bo Liu, Z. hong Yin, L. hua Jiang, M. fang Li and J. Wen, Chemosphere, 2018, 208, 655–664 CrossRef PubMed.
- S. W. Yun and C. Yu, J. Chem., 2015, 1–8 Search PubMed.
- X. Tao, A. Li and H. Yang, Environ. Pollut., 2017, 222, 348–355 CrossRef CAS PubMed.
- Y. Huang, C. Yang, Z. Sun, G. Zeng and H. He, RSC Adv., 2015, 5, 11475–11484 RSC.
- R. Bian, D. Chen, X. Liu, L. Cui, L. Li, G. Pan, D. Xie, J. Zheng, X. Zhang, J. Zheng and A. Chang, Ecol. Eng., 2013, 58, 378–383 CrossRef.
- F. Moreno-Barriga, V. Díaz, J. A. Acosta, M. Á. Muñoz, Á. Faz and R. Zornoza, Geoderma, 2017, 301, 19–29 CrossRef CAS.
- Z. Shen, Y. Zhang, F. Jin, O. McMillan and A. Al-Tabbaa, Sci. Total Environ., 2017, 609, 1401–1410 CrossRef CAS PubMed.
- S. X. Liang, X. C. Xi and Y. R. Li, Open Chem., 2020, 18, 911–917 CAS.
- Y. Xu, B. Seshadri, B. Sarkar, H. Wang, C. Rumpel, D. Sparks, M. Farrell, T. Hall, X. Yang and N. Bolan, Sci. Total Environ., 2018, 621, 148–159 CrossRef CAS PubMed.
- S. Bashir, Q. Hussain, M. Akmal, M. Riaz, H. Hu, S. S. Ijaz, M. Iqbal, S. Abro, S. Mehmood and M. Ahmad, J. Soils Sediments, 2018, 18, 874–886 CrossRef CAS.
- X. J. Zheng, M. Chen, J. F. Wang, Y. Liu, Y. Q. Liao and Y. C. Liu, ACS Omega, 2020, 5, 27374–27382 CrossRef CAS PubMed.
- M. Wu, H. Liu and C. Yang, Int. J. Environ. Res. Publ. Health, 2019, 16, 205–225 CrossRef PubMed.
- D. Zhang, A. Ding, T. Li, X. Wu, Y. Liu and R. Naidu, J. Soils Sediments, 2021 DOI:10.1007/s11368-021-02928-9.
- S. Bashir, M. Shaaban, Q. Hussain, S. Mehmood, J. Zhu, Q. Fu, O. Aziz and H. Hu, J. Soils Sediments, 2018, 18, 2948–2959 CrossRef CAS.
- N. O. Vrînceanu, D. M. Motelică, M. Dumitru, I. Calciu, V. Tănase and M. Preda, Catena, 2019, 176, 336–342 CrossRef.
- W. yu Shi, H. Li, S. Du, K. bo Wang and H. bo Shao, Appl. Clay Sci., 2013, 85, 103–108 CrossRef.
- Y. Yuan, X. Zhang, Y. Lei, Y. Jiang, Z. Xu, S. Zhang, J. Gao and S. Zhao, J. Taiwan Inst. Chem. Eng., 2018, 87, 73–82 CrossRef CAS.
- L. Guo, X. Zhang and Q. Chen, Environ. Sci. Pollut. Res., 2016, 23, 6749–6757 CrossRef CAS PubMed.
- R. Bian, S. Joseph, L. Cui, G. Pan, L. Li, X. Liu, A. Zhang, H. Rutlidge, S. Wong, C. Chia, C. Marjo, B. Gong, P. Munroe and S. Donne, J. Hazard. Mater., 2014, 272, 121–128 CrossRef CAS PubMed.
- V. M. Dang, S. Joseph, H. T. Van, T. L. A. Mai, T. M. H. Duong, S. Weldon, P. Munroe, D. Mitchell and S. Taherymoosavi, Environ. Technol., 2018, 40, 3200–3215 CrossRef PubMed.
- V. M. Dang, S. Joseph, H. T. Van, T. L. A. Mai, T. M. H. Duong, S. Weldon, P. Munroe, D. Mitchell and S. Taherymoosavi, Environ. Technol., 2019, 40, 3200–3215 CrossRef CAS PubMed.
- G. E. Rayment and D. J. Lyons, Soil chemical methods – Australasia, CSIRO Publishing, 2010 Search PubMed.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844–851 CrossRef CAS.
- M. Nguyen Ngoc, S. Dultz and J. Kasbohm, Agric. Ecosyst. Environ., 2009, 129, 8–16 CrossRef.
- C. Nie, X. Yang, N. K. Niazi, X. Xu, Y. Wen, J. Rinklebe, Y. S. Ok, S. Xu and H. Wang, Chemosphere, 2018, 200, 274–282 CrossRef CAS PubMed.
- X. Yang, K. Lu, K. McGrouther, L. Che, G. Hu, Q. Wang, X. Liu, L. Shen, H. Huang, Z. Ye and H. Wang, J. Soils Sediments, 2017, 17, 751–762 CrossRef CAS.
- V. P. Gadepalle, S. K. Ouki, R. Van Herwijnen and T. Hutchings, Soil Sediment Contam., 2007, 16, 233–251 CrossRef CAS.
- M. Moeen, T. Qi, Z. Hussain, Q. Ge, Z. Maqbool, X. Jianjie and F. Kaiqing, Ecol. Eng., 2020, 21, 186–196 CrossRef.
- M. Radziemska, Catena, 2018, 163, 123–129 CrossRef CAS.
- Y. S. Ok, J. E. Lim and D. H. Moon, Environ. Geochem. Health, 2011, 33, 83–91 CrossRef CAS PubMed.
- J. K. Yoon, X. Cao and L. Q. Ma, J. Environ. Qual., 2007, 36, 373–378 CrossRef CAS.
- V. Laperche, S. J. Traina, P. Gaddam and T. J. Logan, Environ. Sci. Technol., 1996, 30, 3321–3326 CrossRef CAS.
- X. Zuo, Z. Liu and M. Chen, Bioresour. Technol., 2016, 207, 262–267 CrossRef CAS PubMed.
- A. D. Igalavithana, E. E. Kwon, M. Vithanage, J. Rinklebe, D. H. Moon, E. Meers, D. C. W. Tsang and Y. S. Ok, Environ. Int., 2019, 127, 190–198 CrossRef CAS PubMed.
- Y. Zhu, J. Ma, F. Chen, R. Yu, G. Hu and S. Zhang, Int. J. Environ. Res. Publ. Health, 2020, 17, 1–14 Search PubMed.
- M. M. Iqbal, H. U. Rehman, G. Murtaza, T. Naz, W. Javed, S. M. Mehdi, S. Javed and A. A. Sheikh, J. Agric. Environ. Sci., 2016, 1, 140–145 Search PubMed.
- M. I. Al-Wabel, A. R. A. Usman, A. H. El-Naggar, A. A. Aly, H. M. Ibrahim, S. Elmaghraby and A. Al-Omran, Saudi J. Biol. Sci., 2015, 22, 503–511 CrossRef CAS PubMed.
- S. Zheng and M. Zhang, J. Environ. Sci., 2011, 23, 434–443 CrossRef CAS.
- A. Ruttens, K. Adriaensen, E. Meers, A. De Vocht, W. Geebelen, R. Carleer, M. Mench and J. Vangronsveld, Environ. Pollut., 2010, 158, 1428–1434 CrossRef CAS PubMed.
- Y. Xia, H. Liu, Y. Guo, Z. Liu and W. Jiao, Sci. Total Environ., 2019, 685, 1201–1208 CrossRef CAS.
- I. R. Sneddon, M. Orueetxebarria, M. E. Hodson, P. F. Schofield and E. Valsami-Jones, Environ. Pollut., 2006, 144, 816–825 CrossRef CAS PubMed.
- P. Ricou-Hoeffer, V. Hequet, I. Lecuyer and P. Le Cloirec, Water Sci. Technol., 2000, 42, 79–85 CrossRef CAS.
- Z. Shen, S. Pan, D. Hou, D. O'Connor, F. Jin, L. Mo, D. Xu, Z. Zhang and D. S. Alessi, Environ. Int., 2019, 131, 104990–104996 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10530a |
| This journal is © The Royal Society of Chemistry 2021 |