Open Access Article
Open Access ArticleNovel sustainable materials from waste plastics: compatibilized blend from discarded bale wrap and plastic bottles
Arvind Gupta a,
Manjusri Misra
a,
Manjusri Misra *ab and
Amar K. Mohanty
*ab and
Amar K. Mohanty *ab
*ab
aBioproduct Discovery and Development Centre, Department of Plant Agriculture, University of Guelph, Crop Science Building, Guelph, Ontario (ON) N1G 2W1, Canada. E-mail: mmisra@uoguelph.ca; mohanty@uoguelph.ca
bSchool of Engineering, University of Guelph, Thornbrough Building, Guelph, Ontario (ON) N1G 2W1, Canada
First published on 26th February 2021
Abstract
This work studies a novel sustainable polymeric material made from a reactive blend of two agri-food waste plastics, with the new material showing strong promise for value-added industrial uses. Discarded bale wrap destined for landfill that was originally made from linear low density polyethylene (LLDPE) and used polyethylene terephthalate (PET)-based plastic bottles were melt mixed in a twin-screw extruder. The miscibility of such recycled LLDPE (rLLDPE) in recycled PET (rPET) is enhanced by the incorporation of a compatibilizer and the PET molecular architecture is maintained using a chain extender, which governs its melt strength. Microscopic analysis of the blends with the compatibilizer and chain extender confirms the enhanced interaction of rPET and rLLDPE chains and the formation of co-continuous morphologies. The efficient interaction of a soft phase (rLLDPE) with a hard phase (rPET) leads to prolonged fracture propagation by an appropriate impact energy transfer mechanism, which ultimately enhances the impact resistance and elongation at break of the resulting blend. The incorporation of a compatibilizer and chain extender in the rPET/rLLDPE blend makes it a toughened blend (with 60 J m−1 notched Izod impact strength) with ∼80% elongation at break in comparison to ∼3% for the blend without a compatibilizer or chain extender. Around ∼36% enhancement is observed in the tensile strength without affecting the tensile and flexural modulus in comparison to the blend without a compatibilizer or chain extender. Applications of the developed materials can extend from rigid packaging applications to the production of filaments for 3D printing.
Introduction
There has been a consensus among researchers and scientists regarding the reutilization of polymers having equal importance to that of finding plastics from renewable resources. It is predicted from the statistical extrapolation of data that if plastic waste generation continues at the same pace, ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 million metric tonnes of plastics will be amassed in landfills or our surrounding environment by 2050.1 Data analysis of waste plastic (up to 2015) revealed that ∼79% of generated plastic waste mainly ended up in landfill and only about 9% was actually recycled.1 An initiative has been undertaken by several governments and industries to implement the concept of a circular economy for the years ahead aiming at the long-term utilization of resources. This implies minimum extraction along with recycling and regeneration of materials after their current life, unlike linear economics that relies on the take-use-waste system.2 A shift towards a circular economy provides us with the opportunity to reuse and recycle polymers and transform them into value-added products.3
000 million metric tonnes of plastics will be amassed in landfills or our surrounding environment by 2050.1 Data analysis of waste plastic (up to 2015) revealed that ∼79% of generated plastic waste mainly ended up in landfill and only about 9% was actually recycled.1 An initiative has been undertaken by several governments and industries to implement the concept of a circular economy for the years ahead aiming at the long-term utilization of resources. This implies minimum extraction along with recycling and regeneration of materials after their current life, unlike linear economics that relies on the take-use-waste system.2 A shift towards a circular economy provides us with the opportunity to reuse and recycle polymers and transform them into value-added products.3
There are several methods for recycling these waste plastics (i.e. polyethylene terephthalate, PET) such as mechano-thermal processing, chemical recycling processes,4 recycling using gas phase treatment,5 enzymatic recycling,6 and upcycling to higher value chemicals.7 The employment of the recycling process for plastic recycling is based entirely on ease of processing and investment; therefore, recycling processes which are economically feasible, cost effective and environmentally friendly have to be considered. In the current scenario, mechano-thermal and chemical recycling are the most promising methods with high industrial potential for the recycling of PET. Chemical recycling mainly targets the depolymerization of PET macromolecules to their constituents using relatively high temperature and/or pressure along with the use of harmful chemicals.8–10 Although mechano-thermal processing of the polymers is relatively cost effective and environmentally friendly, it may lead to degradation and produce relatively lower valued materials after recycling several times. Mechano-thermal processing requires efficient pre-processing: i.e. washing, grinding, melt-filtration etc. Efficient washing and segregation pre-processing may result in a significantly improved product from mechano-thermal processing.11 Also, the use of recycled materials in value-added applications where virgin plastics are currently used reduces petroleum-use and these recycled wastes are diverted from landfill to reuse, which helps towards a reduced-carbon economy and provides better environmental benefit.
Its competitive properties, such as relatively high modulus and melting point, resistance to chemicals, durability, transparency, and high impact strength, have over time resulted in the widespread use of polyethylene terephthalate (PET) in the beverage packaging industry and other consumer products.12 These beverage bottles have transformed consumer behaviour in many ways, which has led to the generation of a large amount of waste from single-use PET bottles. These excessive discarded plastic wastes have led to several problems, such as the clogging of waterways and environmental and ocean pollution. The recycling and reuse of these generated plastic bottles could lead to reduced dependence on resins, with enhanced cost recovery and environmental cleanup. The reprocessing of PET could lead to a reduction in the property of the end product due to chain scission and thermal, mechanical, oxidative or hydrolytic degradation. Therefore, the scientific community is continuously searching for a solution. The incorporation of plasticizers, additives, crosslinkers or chain extenders in the polymer blends during the extrusion process may alter the rheological properties of the material and ultimately affect the melt viscosity of the polymer or blend.13 The use of chain extenders, such as diisocyanate compounds,14 caprolactam or trimellitic anhydride,15 a polymeric molecule based on epoxy, acrylic acid ester and styrene comonomers (Joncryl),16 or polyepoxides,17 can enhance the parameters for processing and properties.18 Among other additives, a multifunctional additive like styrene–acrylic–glycidyl methacrylate is found to be very effective for the chain extension of condensation polymers like PET.19 It protects the post-consumer PET chains from thermal degradation during reprocessing and increases the molecular weight via a chain extension mechanism.20,21 Reprocessing PET to a toughened material with balanced mechanical properties for selective applications may be achieved by blending it with other olefinic polymers such as polypropylene or polyethylene.22
According to the Ontario Federation of Agriculture (OFA), around 3500 tonnes of agricultural plastic is discarded as waste in Ontario every year.23 Due to limited recycling options, bale wraps used for agriculture purposes or animal feed wrap ultimately ends up in landfill or incinerators. Therefore, recycling of these used bale wraps to value-added products could be beneficial for farmers as well as for dairy and cattle industries for the time being. Several research groups have attempted to utilize LLDPE to fabricate PET based blends24–30 or multilayer films. Incorporation of LLDPE in PET may enhance the engineering aspect of the extrusion process, such as mold releasability,31 melt processability, reducing bulk density as well as improving toughness etc.32
The aromatic functionality of PET chains makes this polymer thermodynamically immiscible and incompatible with aliphatic LLDPE, exhibiting poor extrusion mixing and resulting in undesirable mechanical properties of the produced blend.33 One approach, to improve the miscibility of PET with LLDPE, is the grafting of LLDPE itself with maleic anhydride (LLDPE-g-MA) and utilizing that for blend preparation. The limited reaction of the anhydride groups with the hydroxyl end group of the PET chains may form a random copolymer and induce interfacial adhesion. Conversely, the dispersed phase begins to detach when the LLDPE-g-MA content increases.34 However, the integration of a compatibilizer could achieve optimized properties and good processing ability of a PET and polyolefin blend.35 The composition and phase morphology mainly govern the mechanical properties of blends. The compatibility of PET and LLDPE can be enhanced by improving the interfacial adhesion and chain entanglement in the blend via the addition of a third component, such as a sodium ionomer of poly(ethylene-co-methacrylic acid),24 ethylene–ethylene acrylic acid ester-glycidyl methacrylate terpolymers, ethylene/glycidyl methacrylate copolymer,32 diethyl-maleate grafted polyethylene,26 or poly(ethylene-co-methacrylic acid)-lithium ionomer.27 Recycling of PET and its compatibilization with LLDPE using poly(styrene-ethylene/butyldiene-styrene) (SEBS)36 or maleic anhydride-grafted SEBS (SEBS-g-MA) is found to be effective.37 The presence of a maleic anhydride moiety in SEBS molecules makes it an effective compatibilizer for PET and LLDPE polymers.38 An increased content (20%) of SEBS-g-MA leads to superior interfacial adhesion between PET and LLDPE phases, which results in improved toughness and elongation at break.25 However, the use of a large amount of compatibilizer reduces the sustainable content in the polymer system and may affect the cost of its processing.
Using a chain extender along with the compatibilizer can alter the blend properties significantly. As mentioned previously, reprocessing of PET encounters thermal degradation whereas the incorporation of a chain extender inhibits the thermal degradation of PET and therefore does not lower its molecular weight. Using PMDI and SEBS-g-MA as chain extender and compatibilizer, respectively, helps to enhance the impact strength of a PET and LLDPE blend.39 However, the hyperactivity of the isocyanate-terminated moiety can lead to different undesirable side reactions, which may negatively affect the miscibility and ultimately the mechanical properties of the blend. The incorporation of the chain extender and compatibilizer in a one-step melt process can be effective and improve the properties along with the use of a lower amount of compatibilizer. Therefore, the present work has focused on the fabrication of a polymer blend with more than 90% sustainable content by utilizing post-consumer PET bottles and waste LLDPE bale wraps followed by a determination of their properties. In order to improve the compatibility of rPET and rLLDPE, a linear triblock copolymer of styrene (30%) and ethylene/butylene (SEBS) with 1.0–1.7% maleic anhydride grafted onto the rubber midblock (Kraton™) is used as a compatibilizer whereas styrene–acrylic–glycidyl methacrylate (Joncryl) is used as a chain extender.
Materials and methods
Materials
Water bottles made of polyethylene terephthalate (PET) (Nestle® Pure Life®, 500 mL) were obtained from a grocery shop in Guelph, Ontario, Canada. PET bottles were cleared of paper wrap, caps and ring before drying for 24 h in a hot air oven at 80 °C followed by grinding and sieving with 250 μm mesh. Such PET is considered as one time-use PET and named recycled PET (rPET) in this work. Nott Farms, Clinton, ON, Canada supplied used and cleaned bale wrap made up of linear low density polyethylene (LLDPE). The used bale wrap was wiped with water-soaked tissue in order to remove dirt followed by drying for 24 h in a hot air oven at 80 °C. The bale wrap was pelletized using a Leistritz Micro27 twin-screw extruder at 130 °C. The linear triblock copolymer of styrene (30%) and ethylene/butylene (SEBS) with 1.0–1.7% maleic anhydride grafted onto the rubber midblock was supplied by Kraton Corporation under the trade name Kraton FG1901X and used as the compatibilizer. Joncryl, a multifunctional reactive styrene–acrylic based polymer, a product of BASF, was used as the chain extender containing glycidyl methacrylate.Blend fabrication
Pellets of recycled PET (rPET) and recycled bale wraps (rLLDPE) were mixed together manually in different ratios at room temperature and kept in a hot air oven at 80 °C overnight before processing. Fabrication of the blend was performed at 260 °C using benchtop twin screw co-rotating extruder cum injection molding (DSM Xplorer®, The Netherlands, L/D ratio of 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 mm) equipped with a melt recycling facility. The screw speed was kept to 100 rpm with two-minute residence time for proper mixing. Blend fabrication was also undertaken with a predetermined content of compatibilizer (Kraton) and chain extender (Joncryl) using the same processing conditions. The samples were also prepared for rPET using the same processing conditions while rLLDPE was processed at 150 °C.
18 mm) equipped with a melt recycling facility. The screw speed was kept to 100 rpm with two-minute residence time for proper mixing. Blend fabrication was also undertaken with a predetermined content of compatibilizer (Kraton) and chain extender (Joncryl) using the same processing conditions. The samples were also prepared for rPET using the same processing conditions while rLLDPE was processed at 150 °C.
Characterization
The surface morphology of the produced materials was observed using a scanning electron microscope (SEM). The samples were positioned on carbon tape before gold coating. Further, the samples were gold coated using a sputtering unit (Cressington sputter coater 108) for 15 s under inert conditions and characterized using an SEM Phenom ProX (Phenom World BV, the Netherlands) at an acceleration voltage of 10 kV. A Leica microtome (Germany) was used to prepare the samples for atomic force microscopy (AFM) analysis. The surfaces of the specimens were polished at room temperature, and subjected to an AFM cantilever probe in peak force tapping mode using a multimode-8 AFM instrument (Bruker Nano Inc. CA, USA).A universal testing machine (UTM) was employed to examine the mechanical characteristics of the produced blend samples. Tensile and flexural properties of the samples were evaluated using an Instron Instrument Model 3382. The tensile properties were evaluated at a test rate of 5 mm min−1 following ASTM 638 with type IV samples whereas the flexural test was conducted at a crosshead speed of 14 mm min−1 following ASTM D790 standard procedure B. The specimens were preconditioned under typical laboratory conditions for 48 hours at 23 °C and 50% relative humidity using ASTM D618-08 before testing. An impact tester (Zwick/Roell HP25, Ulm, Germany) was used to measure the notched impact resistance as per the ASTM D256 standard. The notched samples were tested using a hammer with a capacity of 2.75 J.
The thermal transitions of the developed blends were determined using differential scanning calorimetry (DSC). The samples were heated from −50 °C to 260 °C at a heating rate of 10 °C min−1 followed by a 2 min isotherm step in order to remove the thermal history. The sample was cooled to −50 °C at a cooling rate of 10 °C min−1 followed by heating to 260 °C at the same heating rate. The percent crystallinity was measured using eqn (1)
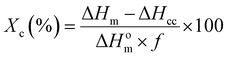 | (1) |
The heat deflection temperatures (HDT) of the samples were assessed via DMA Q800 from TA Instruments equipped with a 3-point bending clamp in controlled force mode according to ASTM-648-07. The samples were fixed and stressed with 0.455 MPa stress and heated with a ramp of 2 °C min−1 and the deflection was measured at 250 μm sample deformation. Dynamic mechanical analysis of the samples was also conducted using the same instrument equipped with a dual cantilever clamp in the temperature ramp/frequency sweep mode at a frequency of 1 Hz and oscillation amplitude of 15 μm. The samples were tested in the temperature range from −50 °C to 150 °C at a rate of 3 °C min−1.
The coefficients of linear thermal expansion (CLTE) of the prepared blends were measured using a thermomechanical analyzer (TMA, Q400, TA Instruments) in accordance with ASTM E831. The tests were conducted by heating the sample (from −40 °C to 120 °C at a rate of 3 °C min−1) forced with an expansion probe with 0.1 N force fitted normal to the melt injection flow direction. CLTE was calculated in the linear range of expansion.
The melt flow index (MFI) of the developed blends was measured using a Melt Flow Indexer from Qualitest (2000A) at 260 °C with a load of 2.16 kg according to ASTM D1238.
A rheometer (Anton Paar MCR302, GmbH, Graz, Austria) was employed to illustrate the rheological properties of the materials. The materials were analyzed using a parallel plate (25 mm diameter) arrangement with a 1 mm gap distance. The test was conducted at a shear rate between 0.01 and 1000 s−1 at 260 °C under an inert atmosphere.
Results and discussion
Effect of compatibilizer on rPET and rLLDPE blend properties
The reprocessing of post-consumer PET bottles is found to be a critical issue due to loss of melt strength, molecular weight, etc. In order to develop a toughened recycled PET based blend, we blended rPET with waste LLDPE bale wrap. Blending rLLDPE with rPET in different ratios was conducted and the mechanical properties were evaluated. Table 1 shows the mechanical properties of rPET/rLLDPE blends with different contents of rLLDPE and compatibilizer. With an increase in content of rLLDPE from 20 to 50%, a decrease in tensile strength was observed from 33 MPa to 17.1 MPa. A similar phenomenon was found in the case of the tensile modulus, whereas the elongation at break was found to be constant at around 2–3%. There was no enhancement in toughness, even after the addition of 50% rLLDPE, suggesting the incompatibility of LLDPE with PET chains, leading to a reduction in mechanical properties. As per the data for tensile strength, the rPET blend with 20% rLLDPE was further examined.| rPET/rLLDPE | Compatibilizer | Tensile strength | Tensile modulus | Elongation at break | Impact strength |
|---|---|---|---|---|---|
| 100/0 | 0 | 54 ± 1 | 2960 ± 52 | 1.6 ± 0.5 | 11 ± 1.1 |
| 0/100 | 0 | 26 ± 0.7 | 182 ± 9 | 313 ± 5 | No break |
| 50/50 | 0 | 17 ± 1.7 | 1023 ± 137 | 3.0 ± 0.9 | 32 ± 2.5 |
| 70/30 | 0 | 29 ± 1.4 | 1452 ± 105 | 3.9 ± 0.7 | 14 ± 0.3 |
| 80/20 | 0 | 33 ± 2.0 | 1778 ± 55 | 2.9 ± 0.5 | 11 ± 1.9 |
| 80/20 | 5% | 43 ± 0.5 | 1799 ± 100 | 18 ± 0.5 | 25 ± 1.5 |
Because of the immiscibility of LLDPE chains in the rPET matrix, a globular interface is formed, which may be responsible for early breakage upon tensile pull. Incorporation of a compatibilizer in different contents initiates an interaction between PET and LLDPE chains. The addition of 5% compatibilizer leads to an enhancement in the tensile strength to 42 MPa against the blend without a compatibilizer. The presence of maleic anhydride content in the compatibilizer provides reactive functionality to the SEBS chains. It reacts with the PET chain's functional group and forms a covalent bond42 and other rubbery SEBS chains form a miscible phase with LLDPE chains.36 The reaction of the maleic anhydride group with the hydroxyl and carboxylic group works as an anchor which binds PET chains with LLDPE chains. Basically, the anchored PET chains are not miscible, but it enhances the compatibility with LLDPE.43 Enhanced compatibility leads to an increased molecular surface area of contact in the blend ultimately resisting chain pulling and in turn requiring more energy to break during tensile pull. Further, an increase in the compatibilizer results in reduced tensile strength due to the rubbery nature of the compatibilizer working as a plasticizer which increases chain flexibility. In terms of tensile modulus, a slight enhancement was found. However, a significant enhancement in elongation at break was noticed due to the presence of the compatibilizer, which provides chain sliding, which eventually ends up prolonging breakage.22 Elongation at break was reduced to around 49% after the addition of 5% compatibilizer. The incorporation of compatibilizer and its anchoring effect on PET chains results in a significant enhancement in the impact resistance. Around a 200% improvement was recorded in impact resistance in comparison to the blend (rPET and rLLDPE). On the basis of tensile strength and impact strength data, the rPET blend with 20% LLDPE and 5% compatibilizer was further inspected.
Effect of chain extender on mechanical properties of rPET/rLLDPE blend
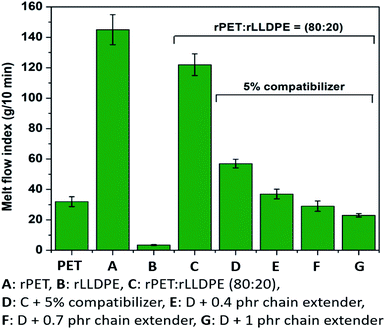
From the previous section, it was found that an optimized rPET with 20% rLLDPE and 5% compatibilizer has comparatively superior mechanical properties in terms of tensile strength, elongation at break, modulus and impact strength, as shown in Table 1. In order to process a thermoplastic, it is desirable to have a polymer with a specific melt strength.44 In the context of industry, melt strength is indirectly measured using a melt flow indexer as melt flow index (MFI) at a particular temperature. In the process of recycling of rPET, deterioration of its melt strength via molecular weight reduction, thermal degradation etc., is most likely to happen.45 It was noted that the MFI of recycled PET was found to be around 145 g/10 min in comparison to the MFI of flakes of post-consumer bottles (32 g/10 min). Further, the addition of 20% rLLDPE to rPET had no significant effect on MFI (122 g/10 min) in comparison to rPET. However, the incorporation of a compatibilizer significantly reduces the MFI to 57 g/10 min due to the anchoring effect of the SEBS based compatibilizer. The reaction of hydroxyl or carboxyl end groups of rPET chains with the maleic anhydride functionality of the compatibilizer leads to the formation of blocks of chains in the blend, eventually recovering the MFI.46 This formation of a polymer chain block leads to enhanced miscibility of rLLDPE in the rPET matrix and overall chain mobility is restricted. In order to further prevent the reduction in molecular weight of rPET chains, the chain extender was used in different fractions. The use of a chain extender further leads to a reduction in MFI to 23 g/10 min (Fig. 1) which is considered as suitable for melt processing of the polymers in industry.47 A gradual reduction in MFI was found with a slight increase in the content of chain extender.It is known that the chain extender used (Joncryl) is a multifunctional oligomeric compound and after the reaction it forms a crosslinked structure in the matrix which is responsible for the reduction in MFI. The crosslinked chains act as a hindrance to flow at a particular temperature. Crosslinking of PET chains using a chain extender retains its molecular weight intact.21,48 Further, the addition of a chain extender affects mechanical and other properties.
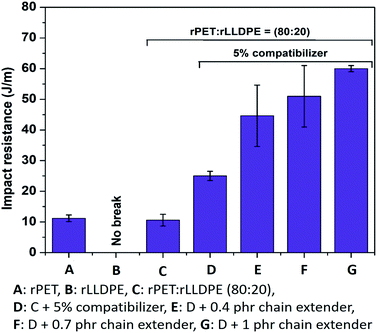
Crosslinking of PET chains using a chain extender and compatibilization with rLLDPE chains by incorporating a compatibilizer further enhances the impact strength of the prepared blend. A significant enhancement (140%) in impact strength was recorded after the addition of a 1 phr chain extender in comparison with the blend without a chain extender (Fig. 2). Impact strength is entirely related to the ability of a material to absorb applied energy.49 Enhanced impact strength of the prepared materials suggests that the incorporation of a chain extender not only retains the molecular weight of the rPET chains, it also helps in the compatibilization of rLLDPE. Due to the presence of a chain extender and compatibilizer, rLLDPE works as a soft phase (a material with a relatively low glass transition and melting temperature) and rPET as a hard phase (a material with relatively high glass transition and melting temperature). With an applied impact at a constant force, the sample tries to absorb the applied energy, but cracks propagate through the PET phase and the energy is efficiently transferred to the soft phase LLDPE. As a soft phase, LLDPE stretches itself and absorbs the maximum amount of energy applied and prolongs the crack propagation, ultimately resulting in higher impact resistance.50
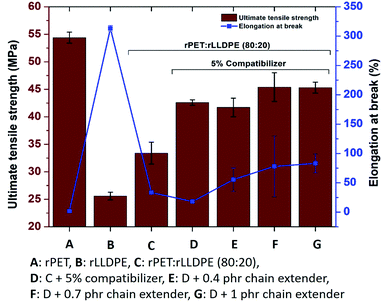
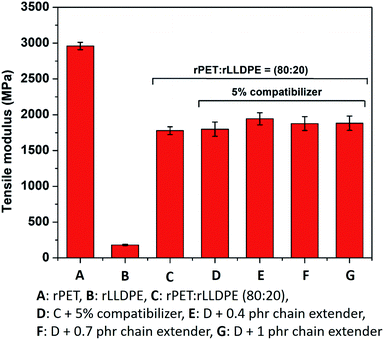
The efficient formation of soft and hard phases in the prepared blend also affects the elongation at break and ultimate tensile strength.51 Around a 60% improvement in elongation at break was observed in comparison to the blend without a chain extender, whereas the tensile modulus and strength were found to increase partially after the addition of a chain extender (Fig. 3 and 4).
As the flexural strength and modulus are a combination of properties derived through tensile and compression simultaneously, it is a highly realistic property to use in understanding the behaviours of polymeric materials. The incorporation of a chain extender in the polymer blend has a low effect on the flexural strength and modulus. The results suggest that the flexural properties are affected by the molecular weight and melt strength of the developed blend. An improvement in the compatibility of LLDPE with rPET or enhancing the melt strength using a chain extender does not affect the flexural properties. Flexural strength and modulus are unaffected by the presence of a crosslinking point (Fig. 5).
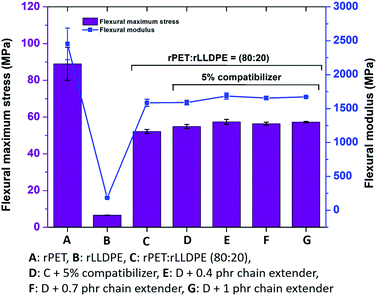 | ||
| Fig. 5 Effect of compatibilizer and chain extender on the flexural properties of rPET, rLLDPE blends. | ||
Effect of chain extender on thermal properties of rPET/rLLDPE blends
The thermal transitions of the developed blend are summarized in Table 2. The presence of two distinct melting transitions of the blend at around 245 °C and 124 °C suggests that the blends have two different types of crystallites corresponding to rPET and rLLDPE. The addition of a compatibilizer and chain extender has no significant effect on the melting temperature of the polymeric entities. However, a significant reduction in melting enthalpies was observed. The incorporation of 20% rLLDPE chains in the rPET matrix leads to a reduction in melt enthalpy from 34.4 J g−1 to 29.8 J g−1, which was further reduced to 24.1 J g−1 and 16.9 J g−1 after the addition of a compatibilizer and chain extender, respectively. In the case of rLLDPE in the blend, the melt enthalpy was reduced to 4.6 J g−1. This reduction in melt enthalpy is an indicator that the compatibilizer and chain extender work as a plasticizer and prevent the chains forming crystallites.52 The presence of an anchoring effect due to the incorporation of a compatibilizer prevents the rPET chains aligning, which was further prevented after the addition of the chain extender. The low melt enthalpy of the blends results in a lower degree of crystallinity (8.3% for rLLDPE and 15.9% for rPET), as shown in Table 2.| Sample | Cooling cycle | Second heating cycle | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J g−1) | Tm (°C) | ΔHm (J g−1) | Xc (%) | ||||||
| LLDPE | PET | LLDPE | PET | LLDPE | PET | LLDPE | PET | LLDPE | PET | |
| rPET | — | 223.3 | — | 46.2 | — | 248.5 | — | 34.4 | — | 24.6 |
| rLLDPE | 110.6 | — | 32.2 | — | 124.4 | — | 57.3 | — | 19.6 | — |
rPET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rLLDPE (80 rLLDPE (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
114.4 | 220.5 | 10.5 | 58 | 124.5 | 247.0 | 13.1 | 29.8 | 22.4 | 26.6 |
| 5% compatibilizer | 109.9 | 221.4 | 7.5 | 48.9 | 122.6 | 249.9 | 5.5 | 24.1 | 9.9 | 22.7 |
| 1 phr chain extender | 109.7 | 209.5 | 7.3 | 46.4 | 122.9 | 245.2 | 4.6 | 16.9 | 8.3 | 15.9 |
The suitability of polymeric materials for high heat applications can be measured using the heat deflection temperature (HDT). As per the data, no adverse effect was found on HDT after the addition of Joncryl as a chain extender (Fig. 6). HDT was reduced to around 65 °C after the addition of a 1 phr chain extender in comparison to 74 °C for the blend without a chain extender. Incorporation of the compatibilizer and chain extender enhances the crosslinking nodes in the polymer matrix, which in turn enhances the free volume of the chains.53 Due to the increased free volume, chains will have more entropy to move upon increased heat54 which may lead to a reduction in the heat deflection temperature at a particular load.
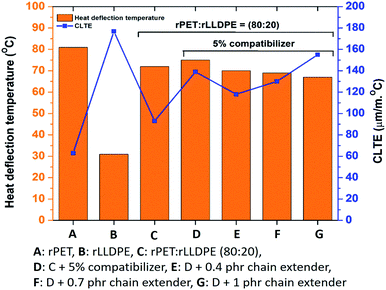 | ||
| Fig. 6 Heat deflection temperature and coefficient of linear thermal expansion of rPET and rLLDPE blends. | ||
The increased free volume in the system also affects the coefficient of linear thermal expansion (CLTE). Application of heat to the polymer system instigates the swelling of the material due to the volume expansion of the polymer chains resulting from the enhanced free volume.55 The increased vibration of the polymeric chain ends in a developed free volume due to the increased heat, which leads to an increase in CLTE (Fig. 6).
Effect of chain extender on viscoelastic properties of rPET/rLLDPE blends
It is understood from the preceding sections that the addition of a compatibilizer and chain extender significantly affects the properties of an rPET/rLLDPE blend as it affects the melt flow index of the materials, which is also related to the viscosity of the system. The crosslinking and chain extension of the polymers lead to enhanced complex viscosity, which is shown in Fig. 7. The complex viscosity was raised after the addition of a compatibilizer and further increased subsequently when incorporated with a chain extender. Similar behaviour of recycled PET was also observed by Incarnato et al. after the addition of pyromellitic dianhydride (PMDA).47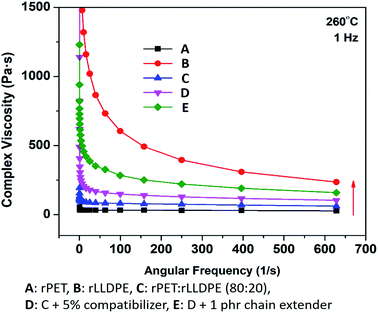 | ||
| Fig. 7 Effect of compatibilizer and chain extender on the complex viscosity of rPET and rLLDPE blends against angular frequency. | ||
Plausible mechanism for increased toughness of rPET/rLLDPE blends
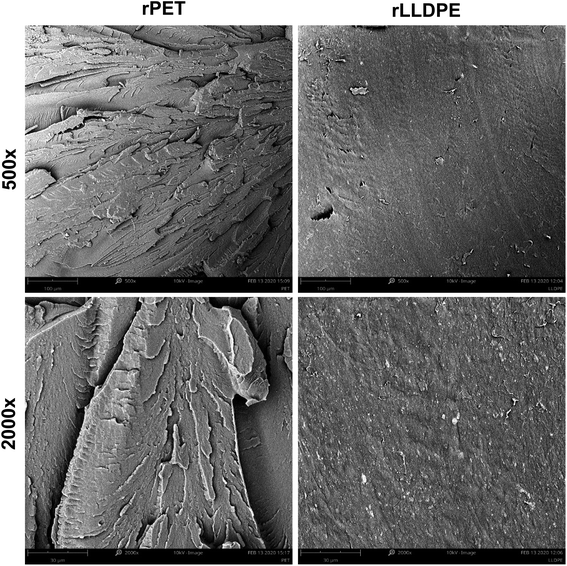
It is understood from the previous sections that the incorporation of a compatibilizer and chain extender in one-step melt processing enhances the properties of the blend. It is known that the molecular weight of PET is reduced during melt reprocessing, which affects its several properties.45 The incorporation of a compatibilizer and chain extender in a one-step process leads to enhanced mechanical properties with a minimum of processing steps. The maleic anhydride ring on the SEBS chain is subjected to opening and works as an anchor for the PET chains.46 On the other hand, ethylene chains form a homogeneous phase with LLDPE chains. The reaction of hydroxyl or carboxyl end functional groups with the maleic anhydride moiety and the formation of a homogeneous phase slightly enhances the miscibility of the LLDPE chains in the PET chains. Microscopic analysis of the impact fracture surface of these samples is shown in Fig. 8 and 9. In the case of the rPET sample, the surface of the samples was found to be very rough, indicating poor toughness. The melt processing of rPET with 20% rLLDPE resulted in a phase-separated blend which showed relatively poor mechanical properties and impact strength. Due to the low interaction and higher surface energy, the blends developed an inhomogeneous two-phase system. The phases formed separated globular structures, which can be seen in the SEM micrograph of the blend (rPET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rLLDPE = 80
rLLDPE = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). The dimensions and structure of these globules were altered upon the addition of compatibilizer, which indicates the reduction in the surface energies of the respective polymer contact surfaces.56 The reduction in the surface energy was plausibly achieved by the reaction of maleic anhydride with the PET chains followed by SEBS chain miscibility in the LLDPE phase57 and showed a stranded LLDPE phase distributed in a sea of PET phase, which was also confirmed by the AFM modulus map.
20). The dimensions and structure of these globules were altered upon the addition of compatibilizer, which indicates the reduction in the surface energies of the respective polymer contact surfaces.56 The reduction in the surface energy was plausibly achieved by the reaction of maleic anhydride with the PET chains followed by SEBS chain miscibility in the LLDPE phase57 and showed a stranded LLDPE phase distributed in a sea of PET phase, which was also confirmed by the AFM modulus map.
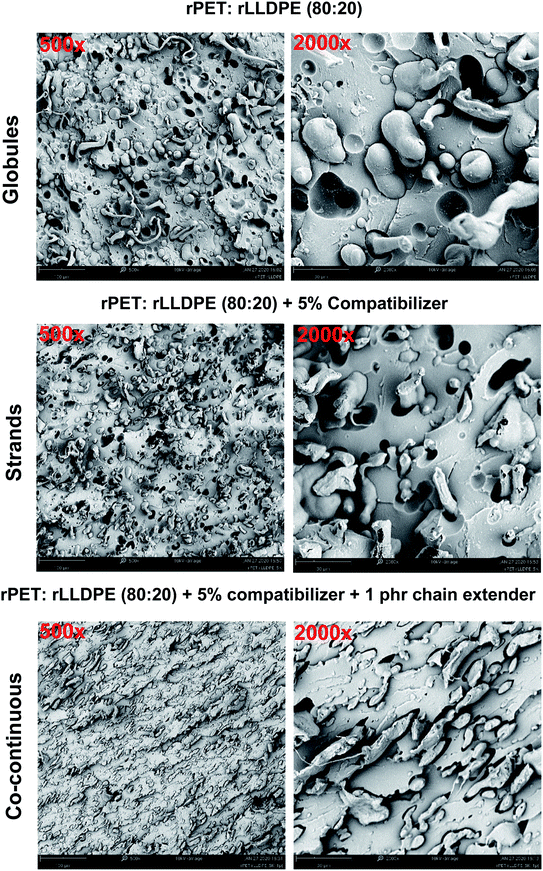 | ||
| Fig. 9 SEM images of rPET and rLLDPE blends with 5% compatibilizer and 1 phr chain extender [scale 100 μm (500×) and 30 μm (2000×)]. | ||
Atomic force microscopy was used for surface mapping based on the modulus of the samples, as mechanical analysis confirmed a large difference in the modulus between rPET (2960 MPa) and rLLDPE (182 MPa). The AFM modulus maps of rPET, rLLDPE, and compatibilized blends are shown in Fig. 10. The modulus map of the rPET surface was found to be smooth in term of modulus, as shown in Fig. 10A, whereas a circular rLLDPE phase was present in Fig. 10B, which shows the incompatibility of the LLDPE chains with the rPET chains.35 Circular phase formation shows a minimum energy architecture with the same type of molecules.58 A disruption was found in the circular phase of rLLDPE after the addition of a compatibilizer (Fig. 10C), which suggests a lowering of the surface energies against other types of molecules, which was further intensified (Fig. 10D) due to the addition of a chain extender.56
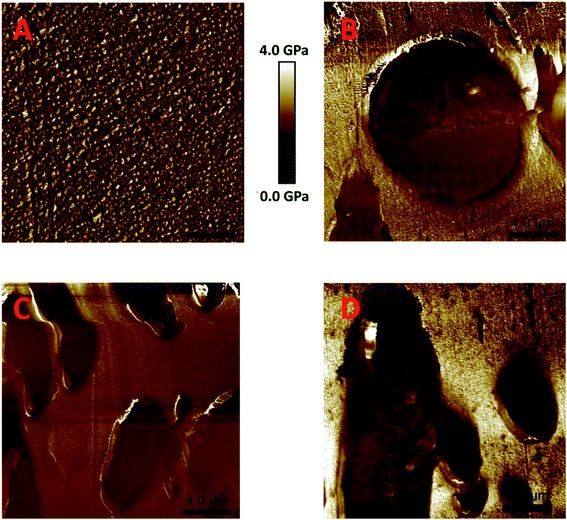 | ||
Fig. 10 AFM modulus maps for prepared microtome polished samples. (A) rPET, (B) rPET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rLLDPE (80 rLLDPE (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), (C) B + 5% compatibilizer, (D) C + 1 phr chain extender (scale: 4.0 μm). 20), (C) B + 5% compatibilizer, (D) C + 1 phr chain extender (scale: 4.0 μm). | ||
The incorporation of the chain extender during the melt processing further transformed the stranded soft LLDPE phase to a co-continuous phase with a hard PET phase. A multifunctional oligomeric chain extender works as a crosslinking node for rPET chains and may form a three-dimensional structure with PET chains as its arms. The reaction of hydroxyl and carboxylic groups of polyesters with a chain extender is responsible for the formation of crosslinked nodes.59 These extended arms interact with the compatibilizer to form a homogeneous continuous soft LLDPE phase with a hard PET phase. The distribution of the soft phase in the matrix of the hard phase and the improved interaction of both phases leads to the judicious transfer of impact energy. The efficient energy transfer contributes to the higher impact resistance which was a result of the prolonged fracture due to the impact. The same phenomenon is also responsible for the simultaneous increment in the tensile strength and elongation at break. Usually, it is considered that the tensile strength and elongation at break are inversely proportional; however, the present work shows an enhancement in both the mentioned properties. An uplift in both the tensile strength and elongation at break is possible when the system has two phases. In the current study, these two phases were achieved by the incorporation and compatibilization of soft LLDPE chains in a sea of PET chains. A plausible reaction mechanism and compatibilization of the present system are given in Scheme 1. In the scheme, it is shown that the chain extender works as a crosslinking node which maintains the molecular architecture of the PET, whereas compatibilization was achieved using a compatibilizer. Overall, the simultaneous use of a chain extender and a compatibilizer improves the overall mechanical properties, such as tensile and flexural strength, elongation at break and modulus of the rPET/rLLDPE blend (Table 3), using one-step melt processing.
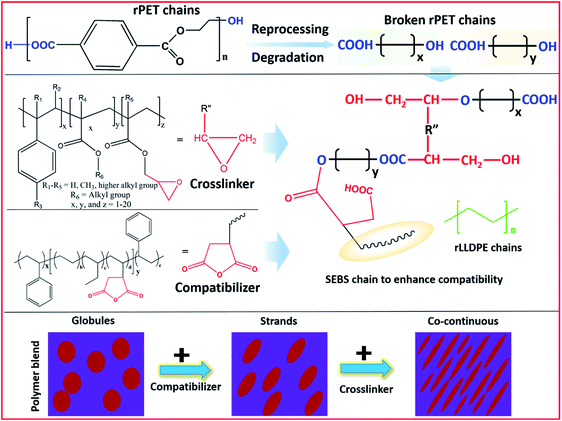 | ||
| Scheme 1 A plausible rPET and rLLDPE blend mechanism in the presence of a compatibilizer and chain extender.57,59 | ||
| Sl. no. | Blend components (%) | Impact resistance notched | Tensile modulus (MPa) | Tensile strength (MPa) | Elongation a break (%) | Flexural modulus (MPa) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PET (A) | LLDPE (B) | Compatibilizer (C) | Chain extender (D) | Ratio (%) A/B/C/D | |||||||
| a rPET: recycled PET, PEMA–Li: ethylene/methacrylic acid copolymer (15 wt% methacrylic acid partially neutralized with lithium), SEBS: poly(styrene-ethylene/butyldiene-styrene), SEBS-g-MA: maleic anhydride (1–2%)-grafted SEBS, LLDPE-g-MA: linear low density polyethylene grafted 1% maleic anhydride, PMDI: polymeric methylene diphenyl diisocyanate (30–32% isocyanate group). | |||||||||||
| 1 | Virgin | Virgin | PEMA–Li | — | 69.7/23.3/7/0 | 25 J m−1 (Izod) | ∼1650 | ∼37 | ∼260 | — | 27 |
| 2 | Scrap | Virgin | SEBS | — | 76/19/5/0 | 6.7 kJ m−2 (Charpy) | — | 31.2 | 69.2 | 1465 | 25 |
| 3 | Scrap | Virgin | SEBS-g-MA | — | 76/19/5/0 | 9.7 kJ m−2 (Charpy) | — | 32 | 113.8 | 1590 | 25 |
| 4 | Scrap | — | LLDPE-g-MA | — | 80/0/20/0 | 10.4 kJ m−2 (Charpy) | — | 35.8 | 365.2 | 1676 | 34 |
| 5 | Scrap | Virgin | SEBS | — | 80/20/5/0 | 6.6 kJ m−2 (Charpy) | — | 31.5 | ∼70 | — | 36 |
| 6 | Scrap | Virgin | SEBS-g-MA | PMDI | 72/18/10/1.1 | 32 kJ m−2 (Charpy) | — | 27.3 | 42.1 | 1298 | 39 |
| 7 | Scrap | Virgin | SEBS-g-MA | — | 72/18/10/0 | 14.7 kJ m−2 (Charpy) | — | 29.6 | 261 | 1436 | 38 |
| 8 | Recycled | Virgin | SEBS | — | 70/20/10/0 | 11.6 kJ m−2 (Charpy) | — | 34.1 | 217 | 1480 | 37 |
| 9 | Recycled | Virgin | SEBS-g-MA | — | 70/20/10/0 | 16.2 kJ m−2 (Charpy) | — | 34.8 | 263 | 1486 | 37 |
| 10 | Recycled | Recycled | SEBS-g-MA | Joncryl | 80/20/05/1 phr | 60 J m−1 | ∼2000 | ∼45 | ∼80 | ∼1700 | Current work |
Conclusions
In the present work, we successfully utilized post-consumer water bottles made of polyethylene terephthalate as a hard phase and waste bale wrap made out of low density polyethylene as a soft phase to fabricate toughened polymer blends with more than 90% sustainable content in one-step melt processing. The simultaneous incorporation of a compatibilizer and a chain extender in the polymer blend leads to a lower (only 5%) requirement for compatibilizer to achieve ∼45 MPa tensile strength, ∼60 J m−1 impact resistance and ∼80% elongation at break while maintaining the modulus unaffected. The use of Joncryl as the chain extender contributed to maintaining the PET molecular architecture which governed the melt flow index of the blend and maintained it as required for its smooth processing (∼23 g/10 min). Around ∼36% enhancement was noticed in the tensile strength in comparison to the blend without the compatibilizer or chain extender. The combined use of a compatibilizer and a chain extender effectively contributed to the formation of two continuous phases with soft and hard content in order to develop a toughened material from a relatively brittle polymer blend (only rPET and rLLDPE) with ∼2.9% elongation at break and ∼10.6 J m−1 impact resistance. The formation of soft and hard phases leads to the efficient transfer of impact energy, ultimately resulting in a prolonged fracture with enhanced impact strength and elongation at break. The developed toughened blend can be utilized for packaging applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the financial support of (i) the Ontario Research Fund, Research Excellence Program; Round 9 (ORF-RE09) Ontario Ministry of Economic Development, Job Creation and Trade (Project No. 053970 and 054345); (ii) the Agriculture and Agri-Food Canada (AAFC) and Competitive Green Technologies, Canada through AgSci Cluster Research Program (Project No. 054712); (iii) the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)/University of Guelph – Bioeconomy for Industrial Uses Research Program (Project #030578); and (iv) the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chair (CRC) program (project no. 460788). The authors would also like to thank to Don Nott, Nott Farms, Clinton, ON, Canada for donation of recycled and cleaned bale wrap samples for this research.References
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef.
- A. P. M. Velenturf, S. A. Archer, H. I. Gomes, B. Christgen, A. J. Lag-Brotons and P. Purnell, Sci. Total Environ., 2019, 689, 963–969 CrossRef CAS.
- G. W. Coates and Y. D. Y. L. Getzler, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef CAS.
- D. R. Merkel, W. Kuang, D. Malhotra, G. Petrossian, L. Zhong, K. L. Simmons, J. Zhang and L. Cosimbescu, ACS Sustainable Chem. Eng., 2020, 8, 5615–5625 CrossRef CAS.
- S. A. Kulyukhin, A. V. Gordeev and A. F. Seliverstov, J. Hazard. Mater., 2020, 400, 123268 CrossRef CAS.
- A. Carniel, A. d. C. Gomes, M. A. Z. Coelho and A. M. de Castro, Bioprocess Biosyst. Eng., 2020 DOI:10.1007/s00449-020-02461-y.
- H. T. Kim, J. K. Kim, H. G. Cha, M. J. Kang, H. S. Lee, T. U. Khang, E. J. Yun, D.-H. Lee, B. K. Song, S. J. Park, J. C. Joo and K. H. Kim, ACS Sustainable Chem. Eng., 2019, 7, 19396–19406 CrossRef CAS.
- A. B. Raheem, Z. Z. Noor, A. Hassan, M. K. Abd Hamid, S. A. Samsudin and A. H. Sabeen, J. Cleaner Prod., 2019, 225, 1052–1064 CrossRef CAS.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS.
- N. George and T. Kurian, Ind. Eng. Chem. Res., 2014, 53, 14185–14198 CrossRef CAS.
- L. K. Krehula, A. P. Siročić, M. Dukić and Z. Hrnjak-Murgić, J. Elastomers Plast., 2013, 45, 429–444 CrossRef CAS.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, Science, 2016, 351, 1196–1199 CrossRef CAS.
- C. Abeykoon, P. Pérez and A. L. Kelly, Polym. Eng. Sci., 2020, 60, 1244–1265 CrossRef CAS.
- X. Tang, W. Guo, G. Yin, B. Li and C. Wu, J. Appl. Polym. Sci., 2007, 104, 2602–2607 CrossRef CAS.
- L. Gouissem, A. Douibi and D. Benachour, Polym. Sci., Ser. A, 2014, 56, 844–855 CrossRef CAS.
- N. G. Karsli, J. Thermoplast. Compos. Mater., 2017, 30, 1157–1172 CrossRef CAS.
- M. Xanthos, M.-W. Young, G. P. Karayanndis and D. N. Bikiaris, Polym. Eng. Sci., 2001, 41, 643–655 CrossRef CAS.
- Z. Zhao, Y. Wu, K. Wang, Y. Xia, H. Gao, K. Luo, Z. Cao and J. Qi, ACS Omega, 2020, 5, 19247–19254 CrossRef CAS.
- I. S. Duarte, A. A. Tavares, P. S. Lima, D. L. A. C. S. Andrade, L. H. Carvalho, E. L. Canedo and S. M. L. Silva, Polym. Degrad. Stab., 2016, 124, 26–34 CrossRef CAS.
- A. A. Tavares, D. F. A. Silva, P. S. Lima, D. L. A. C. S. Andrade, S. M. L. Silva and E. L. Canedo, Polym. Test., 2016, 50, 26–32 CrossRef CAS.
- Z. Yang, C. Xin, W. Mughal, X. Li and Y. He, J. Appl. Polym. Sci., 2018, 135, 45805 CrossRef.
- A. D. Todd, R. J. McEneany, V. A. Topolkaraev, C. W. Macosko and M. A. Hillmyer, Macromolecules, 2016, 49, 8988–8994 CrossRef CAS.
- S. Brackenridge, Ontario innovation will help reduce on-farm plastic waste, https://ofa.on.ca/newsroom/ontario-innovation-will-help-reduce-on-farm-plastic-waste/, accessed 27 September, 2020 Search PubMed.
- N. K. Kalfoglou, D. S. Skafidas and D. D. Sotiropoulou, Polymer, 1994, 35, 3624–3630 CrossRef CAS.
- H. Zhang, W. Guo, Y. Yu, B. Li and C. Wu, Eur. Polym. J., 2007, 43, 3662–3670 CrossRef CAS.
- L. Márquez, M. A. Sabino and I. A. Rivero, Polym. Bull., 1998, 41, 191–198 CrossRef.
- A. Retolaza, J. I. Eguiazábal and J. Nazábal, J. Appl. Polym. Sci., 2003, 87, 1322–1328 CrossRef CAS.
- K. Lamnawar, F. Vion-Loisel and A. Maazouz, J. Appl. Polym. Sci., 2010, 116, 2015–2022 CAS.
- A. M. Alqaflah, M. L. Alotaibi, J. N. Aldossery, M. S. Alghamdi and F. D. Alsewailem, Polym. Adv. Technol., 2018, 29, 52–60 CrossRef CAS.
- H.-Z. He, F. Xue, P.-F. Jia, G.-J. He, Z.-X. Huang, S.-M. Liu and B. Xue, J. Appl. Polym. Sci., 2018, 135, 46489 CrossRef.
- S. Sapieha, J. Cerny, J. E. Klemberg-sapieha and L. Martinu, J. Adhes., 1993, 42, 91–102 CrossRef CAS.
- D. Tsiourvas, E. Tsartolia, A. Stassinopoulos, M. Barrell and J. Bontemps, Adv. Polym. Technol., 1995, 14, 227–236 CrossRef CAS.
- J. Maris, S. Bourdon, J.-M. Brossard, L. Cauret, L. Fontaine and V. Montembault, Polym. Degrad. Stab., 2018, 147, 245–266 CrossRef CAS.
- H. Zhang, Y. Zhang, W. Guo, D. Xu and C. Wu, J. Appl. Polym. Sci., 2008, 109, 3546–3553 CrossRef CAS.
- T. D. Traugott, J. W. Barlow and D. R. Paul, J. Appl. Polym. Sci., 1983, 28, 2947–2959 CrossRef CAS.
- Y. Zhang, H. Zhang, Y. Yu, W. Guo and C. Wu, J. Appl. Polym. Sci., 2009, 114, 1187–1194 CrossRef CAS.
- Y. Liu, H. Xu, G. Liu and S. Pu, Polym. Bull., 2017, 74, 4223–4233 CrossRef CAS.
- Y. Zhang, H. Zhang, W. Guo and C. Wu, Polym. Adv. Technol., 2011, 22, 1851–1858 CrossRef CAS.
- Y. Zhang, W. Guo, H. Zhang and C. Wu, Polym. Degrad. Stab., 2009, 94, 1135–1141 CrossRef CAS.
- D. Li, L. Zhou, X. Wang, L. He and X. Yang, Materials, 2019, 12, 1746 CrossRef CAS.
- X. You, M. R. Snowdon, M. Misra and A. K. Mohanty, ACS Omega, 2018, 3, 11759–11769 CrossRef CAS.
- N. P. Birch, K. Liu, S. C. Mun, G. Ghazaryan, C. T. Senger, C. J. Ellison, C. W. Macosko, T. H. Peterson, S. Mukhopadhyay and C. M. Thurber, Macromolecules, 2019, 52, 8359–8366 CrossRef CAS.
- C. W. Macosko, H. K. Jeon and T. R. Hoye, Prog. Polym. Sci., 2005, 30, 939–947 CrossRef CAS.
- F. P. La Mantia and D. Acierno, Polym. Eng. Sci., 1985, 25, 279–283 CrossRef CAS.
- X. Colin and J. Verdu, C. R. Chim., 2006, 9, 1380–1395 CrossRef CAS.
- B. C. Trivedi and B. M. Culbertson, in Maleic Anhydride, Springer US, Boston, MA, 1982, pp. 479–517, DOI:10.1007/978-1-4757-0940-7_12.
- L. Incarnato, P. Scarfato, L. Di Maio and D. Acierno, Polymer, 2000, 41, 6825–6831 CrossRef CAS.
- M. Härth, A. Dörnhöfer, J. Kaschta, H. Münstedt and D. W. Schubert, J. Appl. Polym. Sci., 2021, 138, 50110 CrossRef.
- P. S. Leevers, in Mechanical Properties and Testing of Polymers: An A–Z Reference, ed. G. M. Swallowe, Springer Netherlands, Dordrecht, 1999, pp. 71–74, DOI:10.1007/978-94-015-9231-4_18.
- G. A. Buxton and A. C. Balazs, Macromolecules, 2005, 38, 488–500 CrossRef CAS.
- R. O. Ritchie, Nat. Mater., 2011, 10, 817–822 CrossRef CAS.
- E. H. Immergut and H. F. Mark, in Plasticization and Plasticizer Processes, American Chemical Society, 1965, ch. 1, vol. 48, pp. 1–26 Search PubMed.
- M. Bocqué, C. Voirin, V. Lapinte, S. Caillol and J.-J. Robin, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 11–33 CrossRef.
- R. P. White and J. E. G. Lipson, Macromolecules, 2016, 49, 3987–4007 CrossRef CAS.
- B. A. Mlekusch, Mech. Time-Depend. Mater., 1998, 2, 129–169 CrossRef CAS.
- V. O. Bulatović, A. Mihaljević and E. Govorčin Bajsić, Polym. Eng. Sci., 2018, 58, 1911–1922 CrossRef.
- N. A. Jamaludin, I. M. Inuwa, A. Hassan, N. Othman and M. Jawaid, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/APP.42608.
- S. Guttman, Z. Sapir, M. Schultz, A. V. Butenko, B. M. Ocko, M. Deutsch and E. Sloutskin, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 493–496 CrossRef CAS.
- M. A. Abdelwahab, S. Taylor, M. Misra and A. K. Mohanty, Macromol. Mater. Eng., 2015, 300, 299–311 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |