 Open Access Article
Open Access ArticleApplication of nanomaterials in the treatment of rheumatoid arthritis
Miaomiao Zhengab,
Huiju Jiaab,
Huangwei Wangab,
Linhong Liua,
Zhesheng Head,
Zhiyong Zhanga,
Wenzhi Yang*b,
Liang Gaoc,
Xueyun Gao *c and
Fuping Gao
*c and
Fuping Gao *a
*a
aCAS Key Laboratory for the Biological Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. E-mail: gaofp@ihep.ac.cn
bSchool of Pharmacy, Hebei University, Baoding 071002, China. E-mail: yangwz@hbu.edu.cn
cDepartment of Chemistry and Biology, Beijing University of Technology, Beijing 100124, China. E-mail: gaoxy@ihep.ac.cn
dUniversity of Chinese Academy of Science, Beijing 100049, China
First published on 10th February 2021
Abstract
Rheumatoid Arthritis (RA) is a chronic autoimmune disease, which mainly causes inflammation of the synovial joints and destruction of cartilage and bone tissue. At present, a variety of clinical drugs have been applied in the treatment of rheumatoid arthritis. With the development of nanotechnology, more and more nano-drugs have been applied in the treatment of rheumatoid arthritis due to the unique physical and chemical properties of nanomaterials. Treatment of RA with nanomaterials can improve bioavailability and selectively target damaged joint tissue. In this review, we summarized the progress of the application of nanomaterials in the treatment of rheumatoid arthritis and also proposed challenges faced by nanomaterials in the treatment of rheumatoid arthritis.
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by synovial joint inflammation, and irreversible destruction of cartilage and bone tissue, which can lead to disability, inability to participate in work and social activities, and increased mortality, and seriously affects the patient's quality of life.1,2 Rheumatoid arthritis affects males to females in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. It can develop at any age, but it most commonly starts at the age of 40–60.3 This is a multi-joint, symmetrical, and aggressive joint inflammation that usually occurs in the small joints of the hands and feet, such as wrists, fingers, and toes.4 The main clinical pathological features are as follows: inflammatory cell infiltration in the joint causes synovial hyperplasia, inflammatory factors increase, and there is invasion of adjacent cartilage, resulting in bone erosion and cartilage tissue damage.
3. It can develop at any age, but it most commonly starts at the age of 40–60.3 This is a multi-joint, symmetrical, and aggressive joint inflammation that usually occurs in the small joints of the hands and feet, such as wrists, fingers, and toes.4 The main clinical pathological features are as follows: inflammatory cell infiltration in the joint causes synovial hyperplasia, inflammatory factors increase, and there is invasion of adjacent cartilage, resulting in bone erosion and cartilage tissue damage.
The drugs currently used to treat rheumatoid arthritis include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), disease-modifying anti-rheumatic drugs (DMARDs) and biological agents.5 Although these drugs can alleviate the progress of the disease, they can cause serious adverse reactions such as tuberculosis, fungal infection, liver damage and heart failure.6–8
Tumor necrosis factor (TNF-α) plays an important role in the pathogenesis of rheumatoid arthritis, and it has been shown to be a new therapeutic target. TNF-α inhibitors, such as adalimumab and etanercept, are the mainstream of biological agents in the treatment of rheumatoid arthritis.9 Baricitinib is an oral JAK inhibitor that has been studied in extensive clinical trials and is approved for the treatment of patients with moderate to severe rheumatoid arthritis.10 Studies have shown that these clinical drugs can produce serious neurological and blood side effects and cause harm to the human body.11,12 Most of the current treatments for rheumatoid arthritis are combined therapy of methotrexate and biological agents, which is significantly better than monotherapy, but expensive biological drugs may bring unbearable economic burden to many patients and their families.
Therefore, the development of drugs that can treat joint injuries and have good biocompatibility is our goal, and nanotechnology shows great promise in improving traditional drug treatments.13–15 This review summarizes the current progress in the application of various nanomaterials in the treatment of rheumatoid arthritis.
2. Different types of nanomaterials used in the treatment of rheumatoid arthritis
The nanotechnology has been applied in many fields such as chemistry, biology, materials science, engineering and medicine.16–18 In medicine field, its main advantage is that it can increase the solubility of water-insoluble drugs, increase the surface area and surface interaction, thereby increasing the dissolution rate. It can also prolong the circulating half-life of the drug in the body, sustain to release the drug, target drug delivery, and reduce systemic side effects.19–21 Nanomaterials can be used as a drug delivery system as a specific carrier for disease treatment. These techniques are currently used for cancer, diabetes, pain, asthma, allergies and infections.22–25 There are different types of nanoparticles, including polymer nanoparticles, liposomes, magnetic nanoparticles, metal nanoparticles, polymer micelles, etc.26–30 The current research on nanomaterials for the treatment of rheumatoid arthritis can be roughly divided into the following three types: (1) traditional nanomaterials: the drug is encapsulated in a nanocarrier and selectively delivered to the predetermined site of action through a targeted method; (2) using cell membranes to coat nanomaterials to form biomimetic nanomaterials to treat rheumatoid arthritis; (3) nanomaterials have their own inherent characteristics as drugs for the treatment of rheumatoid arthritis (Fig. 1).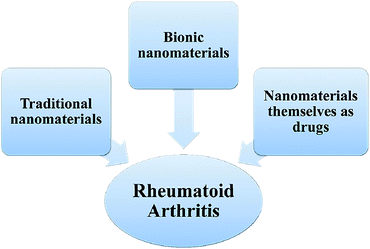 | ||
| Fig. 1 The current research on nanomaterials for the treatment of rheumatoid arthritis in this review. | ||
3. Traditional nanomaterials for rheumatoid arthritis treatment
The application of traditional nanomaterials in the treatment of rheumatoid arthritis is mainly as the carrier of anti-inflammatory drugs. Through different surface modification with the carrier, the drugs can be released to the joint site to improve the accumulation of drugs in the joint site and enhance the effect of drugs. Organic polymer nanocarriers, liposomes and inorganic nanomaterials can be used as carriers of anti-inflammatory drugs.31,323.1 Polymer nanoparticles
Polymer nanoparticles have good biodegradability and biocompatibility, can also target specific organs and tissues, and have been widely used as drug carriers. Anti-rheumatoid arthritis drugs can be adsorbed or encapsulated in polymer nanoparticles and delivered to arthritic sites. At present, the most commonly method is to modify the surface of nanoparticles with polyethylene glycol (PEG). On the one hand, this method can increase the stability of nano-drugs; on the other hand, it can also reduce immunogenicity, making it difficult for the immune system to recognize and clear it. Wang et al.33 used PCL–PEG micelles targeting to deliver low doses of dexamethasone for the treatment of arthritis. The micelles existed for a long time in the systemic circulation, and preferentially accumulated at the joint inflammation site. The results showed that micellar delivery of dexamethasone can effectively reduce joint swelling, bone erosion, and the expression of inflammatory factors in serum and joint tissues. The adverse effects of PCL–PEG micelles on body weight, lymphocyte count and blood glucose concentration were much lower than that of dexamethasone alone, and the activation effect on the complement system is relatively weak. Jain et al.34 prepared an alginate nanoparticle containing IL-10 plasmid DNA and modified the surface of the nanoparticle with tuftsin peptide to achieve macrophage targeting. Near-infrared imaging could observe that the tuftsin-modified alginate nanoparticles accumulated in the inflamed paws of arthritic rats. Magnetic resonance imaging and histology showed that treatment with the nanoparticles significantly reduced the expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in joint tissues, and prevented inflammation progression and joint injury. Importantly, the nanoparticles can re-polarize macrophages from M1 to M2. This research provides a new idea for the treatment of arthritis. Qindeel et al.35 synthesized polycaprolactone–polyethylene glycol–polycaprolactone (PCL–PEG–PCL) triblock copolymer by a ring-opening copolymerization reaction and used it as a carrier for the fabrication of MTX-loaded nanomicelle. Nanoparticles are loaded into a hydrogel with eucalyptus oil as a penetrant for use on the skin, which can avoid the first pass effect, improve patient compliance, and reduce liver toxicity. When the nanoparticle is applied to the RA mouse model, compared with free MTX, the nanoparticle can significantly aggregate in the inflamed joints, reduce the expression of inflammatory factors, and restore the behavioral response ability of mice.Small interfering RNA (siRNA) silencing can block the expression of inflammation-related genes in disease treatment.36,37 However, due to its poor stability in body fluids and easy degradation, it is difficult to enter the cytoplasm,38 so the exploration of safe and effective siRNA delivery drug carriers can become a new strategy for RA treatment. Park et al.39 prepared PLGA nanoparticles coated with anti-COX2 siRNA in the surface and loaded with dexamethasone for the treatment of rheumatoid arthritis. In this study, dexamethasone and anti-COX2 siRNA act simultaneously to suppress the expression of genes and proteins related to arthritis. Combined treatment of dexamethasone and anti-COX2 siRNA significantly reduced the expression of inflammation and apoptosis-related factors in a human chondrocyte cell line (C28/I2) (Fig. 2).
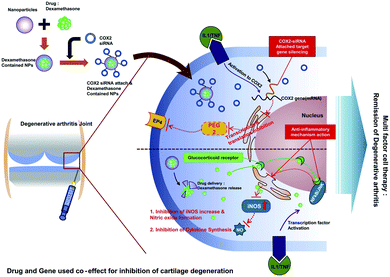 | ||
| Fig. 2 Schematic view of dexamethasone-loaded PLGA nanoparticles complexed with COX-2 siRNA for rheumatoid arthritis.39 Adapted with permission from ref. 39 (copyright 2012, American Chemical Society). | ||
A study by Lee et al.40 investigated a nano-composites (psi-tGC-NPs) of polymerized siRNA (poly-siRNA) targeting TNF-α with thiolated glycol chitosan (tGC) polymers for targeted treatment of arthritis. Psi-tGC-NPs can be quickly taken up by macrophage RAW 264.7 and can silence TNF-α gene. In vivo near-infrared fluorescence (NIRF) images showed that the nanoparticles were obviously accumulated at the joints of collagen-induced arthritis (CIA) mice. Micro-computed tomography (micro-CT) was used to evaluate the repair of the nanoparticles on the injury of the mice's feet.
Kim et al.41 studied self-assembled dextran sulfate nanoparticles for targeted treatment of arthritis. The amphiphilic copolymer (DS-b-PCL) was prepared by using dextran sulfate (DS) as hydrophilic block and polycaprolactone (PCL) as hydrophobic block. The results showed that these nanoparticles could bind to overexpressed macrophage receptors in the synovial membrane and selectively aggregate into the inflammatory synovial membrane of CIA mice. This study demonstrates that dextran sulfate can be used as an anti-inflammatory drug carrier, and also as a targeted part of macrophages to identify inflammatory sites. On this basis, Heo et al.42 studied the effect of methotrexate-loaded dextran sulfate nanoparticles (MTX-DSNPs) in the treatment of arthritis. When MTX-DSNPs was administered to CIA mice via the tail vein, the nanoparticles could effectively aggregated in the inflamed joints, which was more than 12 times more than that of normal mice. In addition, compared with free MTX, DS nanoparticles loaded with MTX have a significantly improved therapeutic effect on cartilage in CIA mice (Fig. 3).
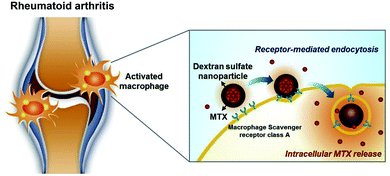 | ||
| Fig. 3 Schematic illustration of DSNPs as nanocarriers for targeted RA therapy.42 Adapted with permission from ref. 42 (copyright 2017, American Chemical Society). | ||
3.2 Liposomes
Liposomes are spherical nanoparticles composed of a double layer membrane with natural phospholipids as the outer layer and water phase as the inner cavity.43 The physical and chemical properties of liposomes enable them to serve as effective delivery systems, where drugs can be loaded in water inner cavity or in lipid double layer membranes.44 Many researches have studied the application of liposomes in rheumatoid arthritis.Polyethylene glycol (PEG) is an effective hydrophilic polymer that can reduce the recognition and absorption of liposomes by the reticuloendothelial system (RES), thereby prolonging the time of liposomes in the systemic circulation.45,46 Wang et al.47 used polymeric stealth liposomes as a carrier to improve the effect of dexamethasone in the treatment of arthritis. They used 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine (DC89PC) and 1,2-distearoylsn-glycero-3-phospho-ethanolamine–poly(ethyleneglycol) (DSPE–PEG2000) to prepare the liposome by thin film hydration method and the PEG chains provided a stealth layer. The results show that the liposomes can preferentially accumulate in inflamed joints after injected into arthritis rats, inhibit the level of pro-inflammatory factors in joint tissues, and reduce the swelling of inflamed joints. This study shows that polymeric stealth liposomes can be used as a new type of drug delivery vehicle for various therapeutic applications.
Wu et al.48 prepared iRGD peptide-functionalized echogenic liposomes (iELPs) encapsulated MTX, which contained indocyanine green (ICG) fluorescent probes for fluorescence tracking, and the drug release was triggered by low-frequency ultrasound. In RA mice, iELPs can bind to the αvβ3 integrin in the synovium, which greatly improves the therapeutic effect. Compared with untreated RA mice, inflammatory cell infiltration and angiogenesis in joint tissues were significantly reduced. This method of drug delivery controlled by ultrasound is also worth studying its application in the treatment of other diseases (Fig. 4).
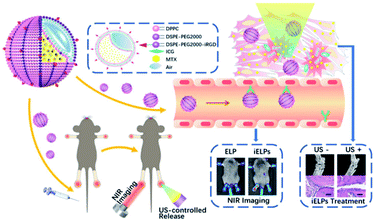 | ||
| Fig. 4 The schematic illustration of iELPs and the mechanism of NIR fluorescence imaging and treatment.48 Adapted with permission from ref. 48 (copyright 2020, American Chemical Society). | ||
Meka et al.49 used liposome targeted delivery of dexamethasone for the treatment of arthritis. The authors used a new peptide ligand (CKPFDRALC) named ART-2 to develop a liposome drug delivery system, which can accumulate in inflammation when intravenously injected into CIA rats. The results showed that ART-2 coated dexamethasone (DEX) liposomes were more effective than liposomes without ART-2 or free DEX in inhibiting the progression of arthritis.
Ren et al.50 evaluated the role of liposome size, physical and chemical properties, surface charge, and chain length and concentration of polyethylene glycol in targeted treatment of rheumatoid arthritis. In this study, the authors used near-infrared fluorescence (NIRF) imaging system to observe the targeting of liposomes of different properties in CIA mice. The uptake ability of different liposomes by synovium cells was evaluated by flow cytometry. The results showed that liposomes with a diameter of 100 nm, a slight negative charge, and a 5 kDa PEG incorporation of 10% had better circulation time and joint inflammation targeting than other liposomes.
3.3 Metallic nanoparticle
Metal nanoparticles have excellent properties, can be modified multiple functional groups, and are widely used in biomedical aspects.51 At present, gold, iron and cerium nanoparticles are widely used in treatment of RA.Lee et al.52 studied RGD-linked Au half-shell nanoparticles containing MTX for targeted chemo-photothermal therapy of RA. RGD peptides can target inflammation sites. Under near-infrared (NIR) irradiation, the Au half-shell generates heat to increase the drug release rate, and at the same time transfer heat and drugs to the inflamed joint. When combined with near-infrared irradiation, nanoparticles containing low doses of MTX showed better therapeutic effects in CIA mice than MTX alone. Meanwhile, Lee et al.53 studied the role of hyaluronate gold nanoparticle/-tocilizumab complex (HA-Au NP/-TCZ) in CIA mice. Au nanoparticles have an anti-angiogenesis effect, TCZ is an anti-interleukin-6 (IL-6) receptor inhibitor, which can interfere with the role of IL-6 in the pathogenesis of RA, and HA has a protective effect on cartilage and lubrication. The therapeutic effect of HA-Au NP/-TCZ complex on RA was confirmed by ELISA, histology and western blot.
Kim et al.54 synthesized manganese ferrite/ceria co-decorated mesoporous silica nanoparticles (MFC–MSNs) to treat RA. In the CIA rat model, injection of MFC–MSNs could relieve intra-articular hypoxia, inflammation and other pathological features. The nanoparticles have a synergistic effect on scavenging reactive oxygen species (ROS) and producing O2, and can reduce the M1 macrophages and induce M2 macrophages polarization. In addition, monodisperse silica nanospheres as a delivery carrier can continuously release methotrexate and enhance the therapeutic effect (Fig. 5).
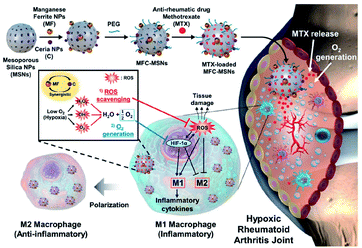 | ||
| Fig. 5 Therapeutic mechanisms of MFC–MSNs in RA treatment.54 Adapted with permission from ref. 54 (copyright 2019, American Chemical Society). | ||
Simultaneously, Kim et al.55 prepared methotrexate-loaded Au/Fe/Au plasmonic nanoparticles conjugated with RGD for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Under near-infrared (NIR) irradiation, the Au half-shell generates heat at the inflammation site and accelerates the release of MTX. The Fe half-shell can deliver the nanoparticles to the inflamed joints under the action of an external magnetic field, and can increase their residence time in the joints. The study combined the application of near-infrared radiation and external magnetic field, so that low-dose MTX can achieve a better effect of treating arthritis.
Kalashnikova et al.56 synthesized albumin-cerium oxide nanoparticles conjugated with indocyanine green (ICG). The ability of the nanoparticle to clear reactive oxygen species and to polarized M1 macrophages into M2 was verified in RAW 264.7 cells and ThP-1 cells. When the CIA mice were injected with the nanoparticles, in vivo imaging system could be used to observe that they aggregated in the inflamed joints and showed a better therapeutic effect. The targeting and therapeutic effect of this novel albumin-cerium oxide nanoparticles provide us with a new direction for the treatment of arthritis.
3.4 Inorganic non-metallic nanomaterials
Silica is an inorganic non-metallic material widely used in the treatment of rheumatoid arthritis. Li et al.57 synthesized a core–cone structure of mesoporous silica nanoparticles (MSN-CC). In a rat model of arthritis, this nanomaterial can promote the production of HA, inhibit synovitis inflammation and promote bone repair. Since mesoporous silica has good biocompatibility, degradability and high protein loading capacity, functionalized group PEI is added to its surface to load and deliver hyaluronan synthase type 2 (HAS2). After intra-articular injection of MSN-CC-PEI, HAS2 was successfully introduced into synovial cells and promoted the production of endogenous hyaluronic acid in and out of the body. Compared with the previous method of injecting HA, this method of administration is more convenient, efficient, and has a long duration and good therapeutic effect.4. Bionic nanomaterials for rheumatoid arthritis treatment
4.1 Nanoparticles coated with cell membrane
Inspired by natural biological systems, biological cell-mediated drug delivery systems have received extensive attention in recent years. This technology reduces the immunogenicity of the nanoparticles and prolongs the blood circulation time by coating the surface of the nanoparticle with the biological endogenous cell membrane (such as macrophage membrane, neutrophil membrane, red blood cell membrane, etc.) as a functional material.58,59 The nanomaterials coated with the biological cell membrane inherit the antigenic exterior and associated membrane functions of the source cells, such as chemotaxis to inflammatory sites, neutralization of cytokines and so on.Macrophages and neutrophils are important innate immune cells in the body. They are involved in the inflammatory response of the body and can cause synovium hyperplasia, secrete a variety of degrading enzymes, and cause cartilage destruction and the expression of inflammatory factors.60,61 Therefore, immune cells are widely used to construct biomimetic nano-drugs with anti-inflammatory properties.
Li et al.62 used macrophage-derived microvesicle (MMV) encapsulated nanoparticles (MNP) for targeted therapy of RA. The study used cytochalasin B (CB) to relax the interaction between the cytoskeleton of macrophages and the cell membrane, thereby stimulating the secretion of MMV. MMV membrane proteins are similar to macrophages, indicating that MMV can show similar inflammatory targeting ability as macrophages. Then the poly(lactic-co-glycolic acid) (PLGA) nanoparticles were coated with MMV and tacrolimus was encapsulated in the bionic nanomaterials. Both in vivo and in vitro experiments show that the bionic nanomaterial has more obvious targeting and anti-inflammatory effects than nanoparticles coated with red blood cell membranes. The study shows that MMV can become a promising target bionic carrier for the treatment of arthritis (Fig. 6).
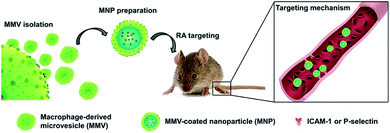 | ||
| Fig. 6 Schematic illustration of MMV-coated nanoparticle (MNP) targeting sites of RA. MNP could target sites of RA through ICAM-1 or P-selectin adhesion.62 Adapted with permission from ref. 62 (copyright 2019, American Chemical Society). | ||
Headland et al.63 found that there were a large number of neutrophil-derived microvesicles in synovial fluid of joints, so they were coupled with anti-inflammatory membrane junction protein A1 (AnxA1) to form a complex (AnxA1+ MVs), demonstrating its protective effect on joints. In vitro experiments have shown that the complex can lead to extracellular matrix accumulation and cartilage protection by reducing the expression of interleukin-8 (IL-8) and prostaglandin E2 (PGE2). Intra-articular injection of AnxA1+ MVs can reduce cartilage degradation caused by inflammatory arthritis and increase the production of transforming growth factor-β (TGF-β) in chondrocytes. Zhang et al.64 directly used neutrophil membranes coated nanoparticles to alleviate joint injury. In CIA mice and human arthritis transgenic mouse models, these nanoparticles can reduce the production of pro-inflammatory factors, inhibit synovial inflammation, protect cartilage, and show significant therapeutic effects. Hu et al.65 used red blood cell membranes to coat polymer nanoparticles to treat RA. The poly(lactic-co-glycolic acid) (PLGA) particles are extruded with vesicles derived from red blood cells to form nanoparticles disguised as red cell membranes, which show a better half-life in the body and can be used for long-term drug transport.
4.2 Exosomes encapsulated nanoparticles
Exosomes are cell vesicles containing complex RNA and proteins, which can be used as drug carriers to improve biocompatibility and provide more options for personalized treatment of inflammation.66 Nanoparticle drug delivery based on exosomes has been successfully applied to a variety of different diseases, such as cancer, Parkinson, nephropathy and encephalitis,67–70 but so far is rarely used for RA. Yan et al.71 synthesized a biomimetic exosome (Exo) encapsulated with dexamethasone sodium phosphate (Dex) nanoparticles (Exo/Dex), which was modified with folic acid (FA)–polyethylene glycol (PEG)–cholesterol (Chol) complex (FPC-Exo/Dex) to target inflammatory joints. The uptake of RAW264.7 cells with the nanoparticle and further biological distribution studies showed that FPC-Exo/Dex could aggregate at the site of inflammation. At the same time, the FPC-Exo/Dex nanoparticles can inhibit the production of pro-inflammatory cytokines and increase the expression of anti-inflammatory cytokines. The safety evaluation in vivo shows that the biomimetic system has good biocompatibility and no hepatotoxicity.Liu et al.72 found that a SPARC (secreted protein complied-based and rich in cysteine) was overexpressed in synovial membrane of patients with rheumatoid arthritis. This new finding deserves the attention of the researchers. Because SPARC has a strong affinity for albumin, Liu et al. synthesized methotrexate-loaded human serum albumin nanoparticles (MTX@HSA NMs) and used them as a biomimetic drug delivery system to treat RA (Fig. 7). Fluorescence/magnetic resonance dual-mode imaging showed that the nanoparticles were effective in aggregating at the site of joint inflammation in CIA mice compared with free methotrexate. The results of animal experiments showed that MTX@HSA NMS containing a lower dose of MTX had a significant effect on reducing arthritis. This work provides a new idea for people to treat arthritis.
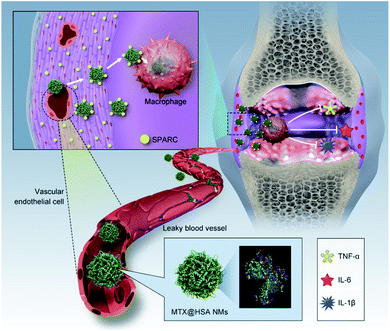 | ||
| Fig. 7 Biomimetic delivery of methotrexate-loaded human serum albumin nanomedicines (MTX@HSANMs) in rheumatoid arthritis (RA) joints for efficient blockade of RA progression.72 Adapted with permission from ref. 72 (copyright 2019, American Chemical Society). | ||
Although some research have made some achievements in using biomimetic nanomaterials to treat diseases, most of these researches are still in the preliminary stage of exploration, and there are still many problems to be solved if they are to be applied in clinical practice. First of all, the process of membrane extraction and purification is more complex and requires higher technical requirements than that of synthetic materials. In the preparation of nanomaterials, how to maintain the stability of membrane structure is a problem to be considered. Secondly, we should also consider whether membrane shedding and drug leakage will occur during the blood circulation of the nanomedicine coated by cell membrane. However, we believe that with the further development of science and technology and more and more people's attention and discussion on diseases, biomimetic nanomaterials will become another new direction for treating inflammation.
5. The material itself with anti-inflammatory properties for the treatment of rheumatoid arthritis
5.1 Au clusters
In 1890, monovalent gold drugs possessing anti-inflammatory and other properties, was discovered to be used to treat RA patients, however, its use was restricted due to its high incidence of side effects.73 At present, gold clusters are a promising material for the treatment of rheumatic diseases with low toxicity.74Our group75 synthesized gold clusters (GA) to treat arthritis and protect joint bone/cartilage. Au clusters with natural tripeptide glutathione (GSH = γ-Glu–Cys–Gly) as the thioligand, after purification, have such excellent properties as ultrafine size, good biocompatibility and high stability. As a new type of nanomaterial, they have direct biological effects instead of using drug carriers. Studies have shown that Au clusters can effectively prevent and treat CIA in rats without systemic side effects.75,76 Au clusters not only inhibited the inflammatory mediators and improved the inflammatory state of arthritic rats, but it also reversed the cartilage and bone damage caused by arthritis (Fig. 8). This is because Au clusters can directly inhibit the differentiation and function of osteoclasts induced by receptor activator of nuclear factor-κB ligand (RANKL). Our further research also shows that Au clusters can prevent bone injury by inhibiting the activation of NF-κB signaling pathway.77 Traditional anti-inflammatory treatment is usually only to inhibit the development of inflammation, and ignore the bone erosion and bone destruction caused by inflammation. Bone erosion is generally mediated by enhancing the activation of osteoclasts (OCs). Therefore, inhibiting the activation of pro-inflammatory cytokines and the differentiation and function of osteoclast may be an important method to prevent inflammatory bone destruction. Our study showed that Au clusters significantly inhibited bone damage in CIA rats. Our data firstly prove that the super-small nanoclusters, e.g. Au clusters, could be novel candidate nano-drug for RA treatment.
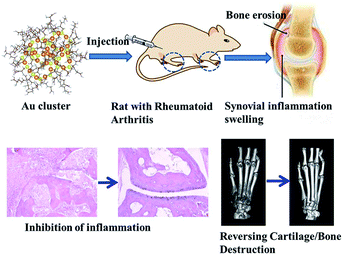 | ||
| Fig. 8 Schematic diagram of gold clusters for treating arthritis and preventing bone erosion.75 Adapted with permission from ref. 75 (copyright 2020, Wiley). | ||
5.2 Cationic nanoparticle
Cell-free DNA (cfDNA) released from damaged or dead cells can be recognized by nucleic acid (NA) sensors such as toll-like receptors (TLR), leading to immune system activation and chronic inflammation and exacerbating the onset of arthritis.78,79 Clinical studies have also shown that the content of cfDNA in serum and synovial fluid of patients with rheumatoid arthritis is increased.80 Therefore, removing cfDNA can be an effective strategy for treating arthritis.Cationic polymers have been widely used in gene therapy as non-viral vectors of nucleic acids.81 Cationic polymers can inhibit the progression of arthritis by scavenging cfDNA. However, due to the strong positive charge of cationic, it is easy to cause systemic toxicity. In order to reduce toxicity, Wu et al.82 used self-assembled poly(lactic-co-glycolic acid)-block-poly(2-(dimethylamino)ethyl methacrylate) (PLGA-b-PDMA) copolymer to prepare cationic nanoparticles that can effectively inhibit joints inflammation, repair damaged cartilage tissue, improve its pharmacokinetic properties, and avoid potential systemic toxicity. The introduction of PEG fragments on the PDMA shell can reduce the surface charge of the nanoparticles, reducing their toxicity to cells and rats while retaining their ability to inhibit joint inflammation. In addition, the nanoparticles have more accumulation and longer retention in the inflammatory joints, so the frequency of administration can be reduced. This study demonstrated that the therapeutic effect of cationic materials can be regulated by introducing surface neutral groups (Fig. 9).
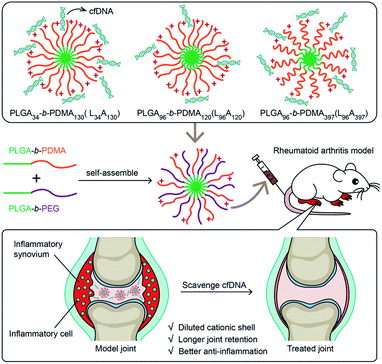 | ||
| Fig. 9 Binding cell-free DNAs that induce inflammation of rheumatoid arthritis with cationic nanoparticles may inhibit disease development.82 Adapted with permission from ref. 82 (copyright 2020, American Chemical Society). | ||
This research group83 further investigated the ability of cationic nanoparticles to remove cfDNA from synovium of patients with rheumatoid arthritis. They injected cationic nanoparticles through tail vein injection into the CpG-induced mouse models or collagen-induced arthritis rats. The results of clinical scores, micro-CT images, MRI, histology and ELISA proved that the nanoparticles could relieve ankle joint and tissue swelling, bone and cartilage damage, indeed have a good therapeutic effect on arthritis.
6. Conclusions
As one of the key technologies in the 21st century, nanotechnology plays an important role in promoting the progress of science and technology in various fields. As a new drug delivery system, nano-drug controlled-release systems have been widely studied, especially in the fields of targeted and localized drug delivery, mucosal absorption drug delivery, and protein and polypeptide controlled release. Nanomaterials have irreplaceable advantages. The nanomaterials have shown great application prospects in the treatment of rheumatoid arthritis. When nanomaterials are used as drug carriers, it changes the pharmacokinetics of traditional anti-arthritis drugs, increases their accumulation in joint tissues, and reduces the side effects of drugs. And some nanomaterials have inherent anti-inflammatory pharmacological activities through different mechanisms, when compared with traditional drugs such as DMARDs, non-steroidal anti-inflammatory drugs and corticosteroids, nanomaterials with inherent anti-inflammatory effects have lower toxicity, improved the effectiveness of treatment for rheumatoid arthritis while providing greater safety. Nanomaterials are not only easy to synthesize, but also most of the nanoparticles have good degradability in vivo. Similar to the EPR effect of tumor targeted drug therapy, nanomaterials can actively target inflammatory joints, so that drugs can directly act on specific sites.Although nanomaterials have achieved good therapeutic effects in the treatment of rheumatoid arthritis, their potential therapeutic mechanisms are still unclear, and there are still certain limitations in application. We need to further explore and research the biocompatibility of the nanometer carrier and metabolic pathways in the body. Precise control of drug release rate and retention time is the direction that we should strive for next. At present, most researches are only for single-targeted therapy. We can consider targeting multiple targets to treat arthritis, using multiple drugs to synergistically block multiple pathways in the pathogenesis of RA, alleviate the disease process, and improve the therapeutic effect. Perhaps it can become a new direction for the treatment of RA in the future. We believe that with the development of nanotechnology, advanced nanomaterials will definitely play a key role in the treatment of rheumatoid arthritis in the future.
Abbreviation index
| RA | Rheumatoid arthritis |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| GCs | Glucocorticoids |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| TNF-α | Tumor necrosis factor α |
| IL-1β | Interleukin-1β |
| PEG | Polyethylene glycol |
| PCL | Polycaprolactone |
| IL-10 | Interleukin-10 |
| MTX | Methotrexate |
| siRNA | Small interfering RNA |
| COX-2 | Cyclooxygenase-2 |
| NIRF | Near-infrared fluorescence |
| CIA | Collagen-induced arthritis |
| Micro-CT | Micro-computed tomography |
| DS | Dextran sulfate |
| RES | Reticuloendothelial system |
| DEX | Dexamethasone |
| ROS | Reactive oxygen species |
| IELPs | iRGD peptide-functionalized echogenic liposomes |
| ICG | Indocyanine green |
| HA | Hyaluronate |
| TCZ | Tocilizumab |
| HAS2 | Hyaluronan synthase type 2 |
| MMV | Macrophage-derived microvesicle |
| CB | Cytochalasin B |
| TGF-β | Transforming growth factor-β |
| PLGA | Poly(lactic-co-glycolic acid) |
| AnxA1 | Anti-inflammatory membrane junction protein A1 |
| IL-8 | Interleukin-8 |
| Exo | Exosome |
| SPARC | Secreted protein complied-based and rich in cysteine |
| GA | Gold clusters |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| NF-κB | Nuclear factor kappa-B |
| OCs | Osteoclasts |
| cfDNA | Cell-free DNA |
| NA | Nucleic acid |
| TLR | Toll-like receptors |
| EPR | Enhanced permeability and retention effect |
Author contributions
The manuscript was written through the contribution of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (31971269, 31670976, 82071958, U2067214, 11621505, 11875267 and 31771082), Natural Science Foundation of Beijing Municipality (7202238) and the Natural Science Foundation of Hebei Province (H2018201045).Notes and references
- G. Firestein, Nature, 2003, 423, 356–361 CrossRef CAS
.
- Q. Guo, Y. Wang, D. Xu, J. Nossent, N. J. Pavlos and J. Xu, Bone Res., 2018, 6, 15 CrossRef
.
- C. G. Shinde, M. P. Venkatesh, T. M. Kumar and H. G. Shivakumar, J. Pain Palliat. Care Pharmacother., 2014, 28, 351–358 CrossRef
.
- A. Marrelli, P. Cipriani, V. Liakouli, F. Carubbi, C. Perricone, R. Perricone and R. Giacomelli, Autoimmun. Rev., 2011, 10, 595–598 CrossRef CAS
.
- G. R. Burmester and J. E. Pope, Lancet, 2017, 389, 2338–2348 CrossRef
.
- L. Crofford, Arthritis Res. Ther., 2013, 15, S2 CrossRef
.
- L. Moreland and J. O'Dell, Arthritis Rheum., 2002, 46, 2553–2563 CrossRef CAS
.
- A. Sepriano, A. Kerschbaumer, J. Smolen, D. van der Heijde, M. Dougados, R. van Vollenhoven, I. McInnes, J. Bijlsma, G. Burmester, M. de Wit, L. Falzon and R. Landewé, Ann. Rheum. Dis., 2020, 79, 760–770 CrossRef CAS
.
- Y. Tanaka, Ann. Rheum. Dis., 2013, ii, 124–127 CrossRef
.
- A. Mogul, K. Corsi and L. McAuliffe, Ann. Pharmacother., 2019, 53, 947–953 CrossRef CAS
.
- G. Guidelli, O. Viapiana, N. Luciano, M. De Santis, N. Boffini, L. Quartuccio, D. Birra, E. Conticini, M. Chimenti, C. Bazzani, E. Bruschi, M. Riva, L. Canziani, G. Bianchi, M. Pozzi, M. Limonta, R. Gorla, R. Perricone, B. Frediani, P. Moscato, S. De Vita, L. Dagna, M. Rossini and C. Selmi, Clin. Exp. Rheumatol., 2020, 38 Search PubMed
.
- S. Dogra and G. Khullar, Indian J. Dermatol. Venereol. Leprol., 2013, S35–S46 CrossRef
.
- C. T. Pham, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 607–619 CAS
.
- J. A. Copp, R. H. Fang, B. T. Luk, C. M. Hu, W. Gao, K. Zhang and L. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 13481–13486 CrossRef CAS
.
- B. Peng, H. Liang, Y. Li, C. Dong, J. Shen, H. Q. Mao, K. W. Leong, Y. Chen and L. Liu, Angew. Chem., Int. Ed. Engl., 2019, 58, 4254–4258 CrossRef CAS
.
- K. Lee, K. Shameli, Y. Yew, S. Teow, H. Jahangirian, R. Rafiee-Moghaddam and T. Webster, Int. J. Nanomed., 2020, 15, 275–300 CrossRef CAS
.
- M. Madsen and K. Gothelf, Chem. Rev., 2019, 119, 6384–6458 CAS
.
- C. Deng, C. Xu, Q. Zhou and Y. Cheng, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1576 Search PubMed
.
- E. M. Merisko-Liversidge and G. G. Liversidge, Toxicol. Pathol., 2008, 36, 43–48 CrossRef CAS
.
- S. Chen, R. Li, X. Li and J. Xie, Adv. Drug Delivery Rev., 2018, 132, 188–213 CrossRef CAS
.
- C. Ng, G. Baeg, L. Yu, C. Ong and B. Bay, Curr. Med. Chem., 2018, 25, 1409–1419 CrossRef CAS
.
- Y. Zeng, R. Nixon, W. Liu and R. Wang, Biomaterials, 2020, 268, 120560 CrossRef
.
- A. Singh, A. Biswas, A. Shukla and P. Maiti, Signal Transduction Targeted Ther., 2019, 4, 33 CrossRef
.
- P. Boomi, R. Ganesan, G. Prabu Poorani, S. Jegatheeswaran, C. Balakumar, H. Gurumallesh Prabu, K. Anand, N. Marimuthu Prabhu, J. Jeyakanthan and M. Saravanan, Int. J. Nanomed., 2020, 15, 7553–7568 CrossRef CAS
.
- T. Phạm and D. Kim, Nanomedicine, 2020, 1897–1913 CrossRef
.
- S. R. Mudshinge, A. B. Deore, S. Patil and C. M. Bhalgat, Saudi Pharm. J., 2011, 19, 129–141 CrossRef CAS
.
- I. M. Oliveira, C. Gonçalves, R. L. Reis and J. M. Oliveira, Nano Res., 2018, 11, 4489–4506 CrossRef CAS
.
- B. Chen, J. Xing, M. Li, Y. Liu and M. Ji, Colloids Surf., B, 2020, 190, 110896 CrossRef CAS
.
- K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef
.
- Q. Li, W. Yao, X. Yu, B. Zhang, J. Dong and Y. Jin, Colloids Surf., B, 2017, 158, 709–716 CrossRef CAS
.
- Q. Ain, M. Zeeshan, S. Khan and H. Ali, J. Biomed. Mater. Res., Part A, 2019, 107, 2595–2600 CrossRef CAS
.
- A. Bordat, T. Boissenot, J. Nicolas and N. Tsapis, Adv. Drug Delivery Rev., 2019, 138, 167–192 CrossRef CAS
.
- Q. Wang, J. Jiang, W. Chen, H. Jiang, Z. Zhang and X. Sun, J. Controlled Release, 2016, 230, 64–72 CrossRef CAS
.
- S. Jain, T. H. Tran and M. Amiji, Biomaterials, 2015, 61, 162–177 CrossRef CAS
.
- M. Qindeel, D. Khan, N. Ahmed, S. Khan and R. Asim Ur, ACS Nano, 2020, 14, 4662–4681 CrossRef CAS
.
- T. Manaka, A. Suzuki, K. Takayama, Y. Imai, H. Nakamura and K. Takaoka, Biomaterials, 2011, 32, 9642–9648 CrossRef CAS
.
- N. Cao, D. Cheng, S. Zou, H. Ai, J. Gao and X. Shuai, Biomaterials, 2011, 32, 2222–2232 CrossRef CAS
.
- D. Bumcrot, M. Manoharan, V. Koteliansky and D. Sah, Nat. Chem. Biol., 2006, 2, 711–719 CrossRef CAS
.
- J. S. Park, H. N. Yang, S. Y. Jeon, D. G. Woo, M. S. Kim and K. H. Park, Biomaterials, 2012, 33, 8600–8612 CrossRef CAS
.
- S. J. Lee, A. Lee, S. R. Hwang, J. S. Park, J. Jang, M. S. Huh, D. G. Jo, S. Y. Yoon, Y. Byun, S. H. Kim, I. C. Kwon, I. Youn and K. Kim, Mol. Ther., 2014, 22, 397–408 CrossRef CAS
.
- S. H. Kim, J. H. Kim, D. G. You, G. Saravanakumar, H. Y. Yoon, K. Y. Choi, T. Thambi, V. G. Deepagan, D. G. Jo and J. H. Park, Chem. Commun., 2013, 49, 10349–10351 RSC
.
- R. Heo, D. G. You, W. Um, K. Y. Choi, S. Jeon, J. S. Park, Y. Choi, S. Kwon, K. Kim, I. C. Kwon, D. G. Jo, Y. M. Kang and J. H. Park, Biomaterials, 2017, 131, 15–26 CrossRef CAS
.
- W. Wang, S. Feng and C. Zheng, Int. J. Pharm., 2016, 513, 387–392 CrossRef CAS
.
- Y. Malam, M. Loizidou and A. M. Seifalian, Trends Pharmacol. Sci., 2009, 30, 592–599 CrossRef CAS
.
- F. Mastrotto, C. Brazzale, F. Bellato, S. De Martin, G. Grange, M. Mahmoudzadeh, A. Magarkar, A. Bunker, S. Salmaso and P. Caliceti, Mol. Pharm., 2020, 17, 1444 CrossRef CAS
.
- T. Shimizu, A. Abu Lila, R. Fujita, M. Awata, M. Kawanishi, Y. Hashimoto, K. Okuhira, Y. Ishima and T. Ishida, Eur. J. Pharm. Biopharm., 2018, 127, 142–149 CrossRef CAS
.
- Q. Wang, L. He, D. Fan, W. Liang and J. Fang, J. Mater. Chem. B, 2020, 8, 1841–1851 RSC
.
- H. Wu, Y. He, H. Wu, M. Zhou, Z. Xu, R. Xiong, F. Yan and H. Liu, Theranostics, 2020, 10, 10092–10105 CrossRef CAS
.
- R. Meka, S. Venkatesha, B. Acharya and K. Moudgil, Nanomedicine, 2019, 14, 1455–1469 CrossRef CAS
.
- H. Ren, Y. He, J. Liang, Z. Cheng, M. Zhang, Y. Zhu, C. Hong, J. Qin, X. Xu and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 20304–20315 CrossRef CAS
.
- M. C. Edmundson, M. Capeness and L. Horsfall, Nat. Biotechnol., 2014, 31, 572–578 CAS
.
- S. Lee, H. Kim, Y. Ha, Y. Park, S. Lee, Y. Park and K. Yoo, ACS Nano, 2013, 7, 50–57 CrossRef CAS
.
- H. Lee, M. Lee, S. Bhang, B. Kim, Y. Kim, J. Ju, K. Kim and S. Hahn, ACS Nano, 2014, 8, 4790–4798 CrossRef CAS
.
- J. Kim, H. Y. Kim, S. Y. Song, S. H. Go, H. S. Sohn, S. Baik, M. Soh, K. Kim, D. Kim, H. C. Kim, N. Lee, B. S. Kim and T. Hyeon, ACS Nano, 2019, 13, 3206–3217 CrossRef CAS
.
- H. J. Kim, S. M. Lee, K. H. Park, C. H. Mun, Y. B. Park and K. H. Yoo, Biomaterials, 2015, 61, 95–102 CrossRef CAS
.
- I. Kalashnikova, S. J. Chung, M. Nafiujjaman, M. L. Hill, M. E. Siziba, C. H. Contag and T. Kim, Theranostics, 2020, 10, 11863–11880 CrossRef CAS
.
- H. Li, H. Guo, C. Lei, L. Liu, L. Xu, Y. Feng, J. Ke, W. Fang, H. Song, C. Xu, C. Yu and X. Long, Adv. Mater., 2019, 31, e1904535 CrossRef
.
- J. Dalli, T. Montero-Melendez, L. Norling, X. Yin, C. Hinds, D. Haskard, M. Mayr and M. Perretti, Mol. Cell. Proteomics, 2013, 12, 2205–2219 CrossRef CAS
.
- J. Li, X. Zhen, Y. Lyu, Y. Jiang, J. Huang and K. Pu, ACS Nano, 2018, 12, 8520–8530 CrossRef CAS
.
- R. Berckmans, R. Nieuwland, M. Kraan, M. Schaap, D. Pots, T. Smeets, A. Sturk and P. Tak, Arthritis Res. Ther., 2005, 7, R536–R544 CrossRef CAS
.
- M. Gresnigt, L. Joosten, I. Verschueren, J. van der Meer, M. Netea, C. Dinarello and F. van de Veerdonk, J. Immunol., 2012, 189, 4806–4815 CrossRef CAS
.
- R. Li, Y. He, Y. Zhu, L. Jiang, S. Zhang, J. Qin, Q. Wu, W. Dai, S. Shen, Z. Pang and J. Wang, Nano Lett., 2019, 19, 124–134 CrossRef CAS
.
- S. Headland, H. Jones, L. Norling, A. Kim, P. Souza, E. Corsiero, C. Gil, A. Nerviani, F. Dell'Accio, C. Pitzalis, S. Oliani, L. Jan and M. Perretti, Sci. Transl. Med., 2015, 7, 315ra190 CrossRef
.
- Q. Zhang, D. Dehaini, Y. Zhang, J. Zhou, X. Chen, L. Zhang, R. H. Fang, W. Gao and L. Zhang, Nat. Nanotechnol., 2018, 13, 1182–1190 CrossRef CAS
.
- C. M. Hu, L. Zhang, S. Aryal, C. Cheung, R. H. Fang and L. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10980–10985 CrossRef CAS
.
- F. Tavasolian, A. Moghaddam, F. Rohani, E. Abdollahi, E. Janzamin, A. Momtazi-Borojeni, S. Moallem, T. Jamialahmadi and A. Sahebkar, Autoimmun. Rev., 2020, 19, 102511 CrossRef CAS
.
- M. Haney, N. Klyachko, Y. Zhao, R. Gupta, E. Plotnikova, Z. He, T. Patel, A. Piroyan, M. Sokolsky, A. Kabanov and E. Batrakova, J. Controlled Release, 2015, 207, 18–30 CrossRef CAS
.
- D. Yuan, Y. Zhao, W. Banks, K. Bullock, M. Haney, E. Batrakova and A. Kabanov, Biomaterials, 2017, 142, 1–12 CrossRef CAS
.
- Q. Lin, M. Qu, B. Zhou, H. Patra, Z. Sun, Q. Luo, W. Yang, Y. Wu, Y. Zhang, L. Li, L. Deng, L. Wang, T. Gong, Q. He, L. Zhang, X. Sun and Z. Zhang, J. Controlled Release, 2019, 104–116 CrossRef CAS
.
- T. T. Tang, L. L. Lv, B. Wang, J. Y. Cao, Y. Feng, Z.
L. Li, M. Wu, F. M. Wang, Y. Wen, L. T. Zhou, H. F. Ni, P. S. Chen, N. Gu, S. D. Crowley and B. C. Liu, Theranostics, 2019, 9, 4740–4755 CrossRef CAS
.
- F. Yan, Z. Zhong, Y. Wang, Y. Feng, Z. Mei, H. Li, X. Chen, L. Cai and C. Li, J. Nanobiotechnol., 2020, 18, 115 CrossRef CAS
.
- L. Liu, F. Hu, H. Wang, X. Wu, A. S. Eltahan, S. Stanford, N. Bottini, H. Xiao, M. Bottini, W. Guo and X. J. Liang, ACS Nano, 2019, 13, 5036–5048 CrossRef CAS
.
- F. Raeman, F. Hanssens and M. Delattre, Clin. Rheumatol., 1984, 33–38 CrossRef CAS
.
- M. F. Hornos Carneiro and F. Barbosa Jr, J. Toxicol. Environ. Health, Part B, 2016, 19, 129–148 CAS
.
- F. Gao, Q. Yuan, P. Cai, L. Gao, L. Zhao, M. Liu, Y. Yao, Z. Chai and X. Gao, Adv. Sci., 2019, 6, 1801671 CrossRef
.
- J. Yuan, K. Hou, Y. Yao, Z. Du, C. Lu, Q. Yuan and X. Gao, Nanomaterials, 2020, 10, 712 CrossRef CAS
.
- Q. Yuan, F. Gao, Y. Yao, P. Cai, X. Zhang, J. Yuan, K. Hou, L. Gao, X. Ren and X. Gao, Theranostics, 2019, 9, 1825–1836 CrossRef CAS
.
- A. Ablasser, C. Hertrich, R. Wassermann and V. Hornung, Clin. Immunol., 2013, 147, 207–215 CrossRef CAS
.
- W. Bao, H. Xia, Y. Liang, Y. Ye, Y. Lu, X. Xu, A. Duan, J. He, Z. Chen, Y. Wu, X. Wang, C. Zheng, Z. Liu and S. Shi, Sci. Rep., 2016, 6, 22579 CrossRef CAS
.
- X. Y. Zhong, I. von Muhlenen, Y. Li, A. Kang, A. K. Gupta, A. Tyndall, W. Holzgreve, S. Hahn and P. Hasler, Clin. Chem., 2007, 53, 1609–1614 CrossRef CAS
.
- H. Arima, S. Yamashita, Y. Mori, Y. Hayashi, K. Motoyama, K. Hattori, T. Takeuchi, H. Jono, Y. Ando, F. Hirayama and K. Uekama, J. Controlled Release, 2010, 146, 106–117 CrossRef CAS
.
- J. Wu, H. Liang, Y. Li, Y. Shi, M. Bottini, Y. Chen and L. Liu, Adv. Funct. Mater., 2020, 30, 2000391 CrossRef CAS
.
- H. Liang, B. Peng, C. Dong, L. Liu, J. Mao, S. Wei, X. Wang, H. Xu, J. Shen, H. Q. Mao, X. Gao, K. W. Leong and Y. Chen, Nat. Commun., 2018, 9, 4291 CrossRef
.
| This journal is © The Royal Society of Chemistry 2021 |
