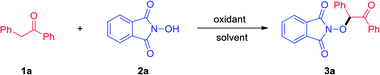Open Access Article
Open Access ArticleSynthesis of N-alkoxyphthalimide derivatives via PIDA-promoted cross dehydrogenative coupling reaction†
Rongxiang Chena,
Bing Liub,
Wenbo Lib,
Kai-Kai Wang *a,
Changqing Miaob,
Zhizhuang Lib,
Yingjie Lvc and
Lantao Liu
*a,
Changqing Miaob,
Zhizhuang Lib,
Yingjie Lvc and
Lantao Liu *d
*d
aSchool of Pharmacy, Xinxiang University, Xinxiang 453000, P. R. China. E-mail: wangkaikaii@163.com
bSchool of Chemistry and Materials Engineering, Xinxiang University, Xinxiang 453000, P. R. China
cXinxiang Tuoxin Pharmaceutical Company Limited, Xinxiang 453000, P. R. China
dCollege of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, Henan 476000, P. R. China. E-mail: liult05@iccas.ac.cn
First published on 19th February 2021
Abstract
A PIDA-promoted cross-dehydrogenative coupling reaction between N-hydroxyphthalimide (NHPI) and aryl ketones for efficient synthesis of N-alkoxyphthalimide products in moderate to good yields has been described. This methodology is distinguished by catalyst-free conditions, readily available starting materials, wide substrate scope and operational simplicity. In addition, a gram-scale reaction and synthetic transformation of the product into synthetically useful intermediates has been demonstrated.
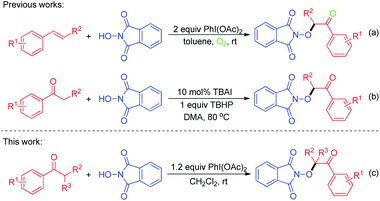
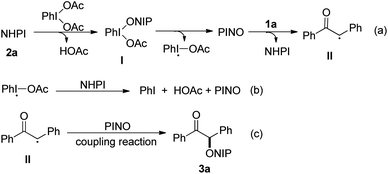
N-Alkoxyphthalimide derivatives are one of the privileged core structural frameworks in the synthesis of pharmaceuticals and functional materials.1 Consequently, the development of highly efficient methods for the synthesis of N-alkoxyphthalimides has emerged as a research topic.2 Traditionally, the method to generate N-alkoxyphthalimides mainly involved the modified Gabriel reaction of N-hydroxyphthalimide (NHPI) with alcohol.3 Recently, the direct dioxygenation of alkenes with NHPI has been developed to produce β-keto-N-alkoxyphthalimides, which was realized by copper,4 iron5 and manganese catalyst,6 separately. In 2016, the group of Adimurthy has developed an efficient method for the synthesis of α-oxygenated ketones through PIDA (phenyliodine diacetate)-mediated oxidative functionalization of styrenes with NHPI and molecular oxygen (Scheme 1a).7
In addition, due to its versatility and atom-economy potential, direct and selective C(sp3)–H bond functionalization has become one of the most powerful and efficient tool in organic synthesis in recent years, which allow direct conversion of C–H bonds to C–C and C–X bonds from simple precursors.8 Transition-metal-catalyzed C(sp3)–H functionalization has been extensively utilized for the construction of various chemical bonds. Considering the fact that these strategies are not environmentally friendly,9 therefore, great efforts have been devoted to develop metal-free free oxidative functionalization of C(sp3)–H bond. Tetrabutylammonium iodide (TBAI)/tert-butyl hydroperoxide (TBHP) has been proved as an efficient transition-metal-free system to apply in C(sp3)–H bond functionalization,10 especially through cross dehydrogenative coupling (CDC), which could assemble complicated molecules from the widely available and simple materials.11 For example, Prabhu reported a TBAI-catalyzed α-aminoxylation of ketones using TBHP as oxidant to directly construct corresponding N-alkoxyphthalimide products (Scheme 1b).12 Although many notable advances toward the synthesis of N-alkoxyphthalimide derivatives have been reported, the development of more simple, concise and efficient synthetic routes remains highly attractive. As a continuation of our previous work focused on free radical chemistry without transition metal catalyst,13 in this context, herein we disclose a novel and efficient method towards N-alkoxyphthalimides via direct cross dehydrogenative coupling of aryl ketones with NHPI without transition metal catalyst via a radical process (Scheme 1c).
As a cheap and readily available reagent, PIDA has been widely used in organic synthesis.14 For example, Zhao and co-workers described a PIDA-mediated oxygenation reaction of N,N-diaryl tertiary amines.15 Recently, a PIDA-mediated radical cyclization of o-(allyloxy)arylaldehydes with NHPI has been realized by Wang's group.16 At the outset of this investigation, we chose 1,2-diphenylethanone 1a and N-hydroxyphthalimide 2a as the model substrates to optimize the reaction conditions (Table 1). The reaction went smoothly and the desired product 3a could be obtained in 92% yield by using PIDA as the oxidant and dichloromethane as a solvent at room temperature under air atmosphere (Table 1, entry 1). Encouraged by this result, we next tested different solvents and the results indicated that dichloromethane is the optimum solvent for this transformation (entries 2–9). Meanwhile, the effect of different oxidants was also investigated; however, they significantly hampered product formation (entries 10–15). When using high valence iodine reagent 2-iodoisoindoline-1,3-dione (NIS) as an oxidant, product 3a was afforded in 61% yield (entry 16). Elevating reaction temperature to 60 °C has no perceptible effect on the reaction efficiency (entry 17). Shortening reaction time to 4 h and 1 h delivered 93% and 64% yield of the desired product, respectively (entries 18 and 19). Moreover, a control experiment suggested that the oxidant was essential for this transformation (entry 20).
| Entry | Solvent | Oxidant | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.36 mmol, 1.2 equiv.), oxidant (0.36 mmol, 1.2 equiv.), and solvent (2.0 mL) in a test tube at room temperature for 10 h.b Isolated yields.c At 60 °C.d 1 h.e 4 h. n.d. = not detected. TBHP = tert-butyl hydroperoxide (70% in water). DTBP = di-tert-butyl peroxide. BQ = 1,4-benzoquinone. NIS = N-iodosuccinimide. | |||
| 1 | CH2Cl2 | PhI(OAc)2 | 92 |
| 2 | CHCl3 | PhI(OAc)2 | 74 |
| 3 | DCE | PhI(OAc)2 | 77 |
| 4 | Toluene | PhI(OAc)2 | <10 |
| 5 | THF | PhI(OAc)2 | <5 |
| 6 | CH3CN | PhI(OAc)2 | 77 |
| 7 | DMSO | PhI(OAc)2 | 13 |
| 8 | Acetone | PhI(OAc)2 | 53 |
| 9 | EtOAc | PhI(OAc)2 | 52 |
| 10 | CH2Cl2 | TBHP | n.d. |
| 11 | CH2Cl2 | DTBP | n.d. |
| 12 | CH2Cl2 | K2S2O8 | n.d. |
| 13 | CH2Cl2 | BQ | n.d. |
| 14 | CH2Cl2 | H2O2 | n.d. |
| 15 | CH2Cl2 | I2 | <5 |
| 16 | CH2Cl2 | NIS | 61 |
| 17c | CH2Cl2 | PhI(OAc)2 | 93 |
| 18d | CH2Cl2 | PhI(OAc)2 | 93 |
| 19e | CH2Cl2 | PhI(OAc)2 | 64 |
| 20e | CH2Cl2 | n.d. | |
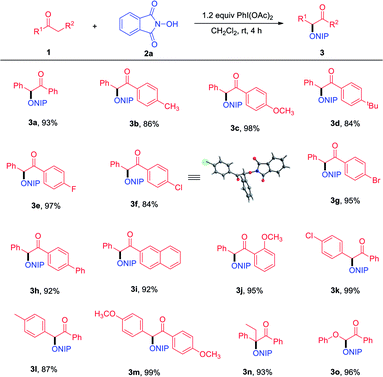
With the optimized reaction conditions in hand, the generality and limitation of the protocol were examined in Table 2. Firstly, the substrate scope of linear-chain aryl ketones was explored and we found that various substituents on aryl rings of aryl ketones were well tolerated under optimized reaction conditions, regardless of electron-donating and electron-withdrawing functional groups (3a–3j). The structure of 3f was confirmed by X-ray crystallographic analysis (see ESI†).17 1-(Naphthalen-2-yl)-2-phenylethanone and 1-(2-methoxyphenyl)-2-phenylethanone also exhibited excellent reactivity, in which the corresponding products 3i and 3j were isolated in 92% and 95% yield, respectively. Meanwhile, 2-aryl substituted acetophenones have also been shown to be suitable substrates to furnish the corresponding products 3k–3l in moderate to good yields. The efficiency of the reaction was not affected by the substituents on both benzene rings (3m). To our delight, 1,2-diphenylbutan-1-one and 2-phenoxy-1-phenylethanone were compatible with reaction conditions, which reacted smoothly with 2a to furnish the corresponding products in good yields (3n–3o).
| a Reaction conditions: 1a (0.3 mmol, 1 equiv.), 2a (1.2 equiv., 0.36 mmol), PhI(OAc)2 (1.2 equiv., 0.36 mmol) and CH2Cl2 (2 mL) at room temperature for 4 h in a sealed tube. Isolated yield. NIPO = phthalimide N-oxyl. |
|---|
 |
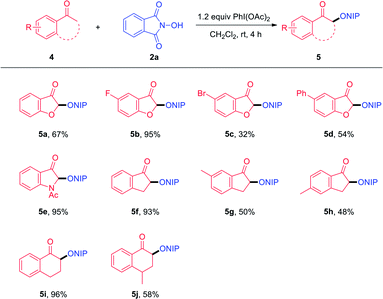
We next evaluated the scope of this C–H functionalization reaction using cyclic aryl ketones as the substrates (Table 3). Benzofuran-3(2H)-ones displayed no obvious detrimental effect on the reaction efficiency and afford the desired products 5a–5d in moderate to good yields. In addition, 1-acetylindolin-3-one was proved to be a suitable substrate to deliver product 5e in 95% yield. Moreover, 2,3-dihydro-1H-inden-1-ones also demonstrated satisfactory compatibility with the reaction (5f–5h). To our delight, 3,4-dihydronaphthalen-1(2H)-ones were shown to be slightly less efficient yet nonetheless suitable substrates, affording the desired products 5i and 5j in 96% and 58% yield, respectively.
| a Reaction conditions: 4 (0.3 mmol, 1 equiv.), 2a (1.2 equiv., 0.36 mmol), PhI(OAc)2 (1.2 equiv., 0.36 mmol), and CH2Cl2 (2 mL) at room temperature for 4 h in a sealed tube. Isolated yield. |
|---|
 |
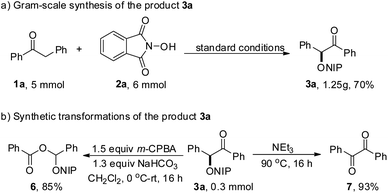
We also inspected the scalability of this PIDA-promoted C–H functionalization reaction and the current protocol could be readily executed on a gram scale by successfully reacting 5 mmol of 1a with 2a in one pot to obtain 3a in 70% yield (Scheme 2a). The further conversion of the product 3a was also conducted. Using m-chloroperbenzoic acid as the oxidant, 3a could also be readily oxidized to afford the corresponding ester 6 in 85% yield. In addition, treatment of 3a and NEt3 led to the formation of benzil 7 in 93% yield (Scheme 2b).
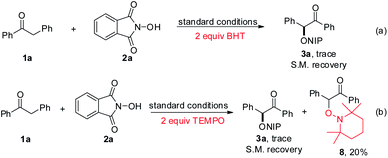
To shed light on the reaction mechanism for this transformation, control experiments were performed, as shown in Scheme 3. As expected, the addition of well-known radical-trapping reagent BHT (3,5-di-tert-butyl-4-hydroxytoluene) suppressed the reaction and the substrate was recovered, which indicated that free radical intermediate may be involved in this transformation (Scheme 3a). Furthermore, when radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) was subjected into the reaction system under standard conditions, no product was detected and radical adduct 8 was separated in 20% yield, which revealed a radical process was involved in this PIDA-promoted C–H functionalization reaction (Scheme 3b).
Based on the above experiments and previous studies from the literatures,12,18 we propose a plausible mechanism for this transformation, which is depicted in Scheme 4. Initially, a ligand exchange between PhI(OAc)2 and NHPI would generate intermediate I, which is converted into a PINO radical by thermal homolytic cleavage. Radical intermediate II was formed through H-abstraction of C(sp3)–H bonds by PINO radical and regenerated NHPI (Scheme 4a), which undergoes second ligand exchange and thermal homolytic cleavage with iodobenzene acetate radical to yield PINO radical (Scheme 4b). Then, the coupling reaction of radical intermediate II and PINO radical gave final product 3a (Scheme 4c).
Conclusions
In summary, we have implemented a novel metal-free dehydrogenation coupling of aryl ketones with N-hydroxyphthalimide through direct C(sp3)–H functionalization to furnish a range of N-alkoxyphthalimide derivatives under mild conditions. Our strategy features easily available starting materials, operational simplicity, broad substrate scope and good functionality tolerance. Moreover, we have conducted a gram-scale reaction and further synthetic transformation of the product. Further explorations on the synthetic utility of this transformation are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (21801214, 21572126), Natural Science Foundation of Henan (202300410016) and the Science and Technology Research Plan Project of Henan Province (202102210224).Notes and references
-
(a) L. E. Canne, A. R. Ferre-D'Amare, S. K. Burley and S. B. H. Kent, J. Am. Chem. Soc., 1995, 117, 2998–3007 CrossRef CAS
; (b) M. Bahta, G. T. Lountos, B. Dyas, S.-E. Kim, R. G. Ulrich, D. S. Waugh and T. R. Burke, J. Med. Chem., 2011, 54, 2933–2943 CrossRef CAS
; (c) C. M.-Z. Wang, H. Xu, T.-W. Liu, Q. Feng, S.-J. Yu, S.-H. Wang and Z.-M. Li, Eur. J. Med. Chem., 2011, 46, 1463–1472 CrossRef
; (d) Y. Li, H.-Q. Zhang, J. Liu, X.-P. Yang and Z.-J. Liu, J. Agric. Food Chem., 2006, 54, 3636–3640 CrossRef CAS
.
-
(a) Y. Lv, K. Sun, T. Wang, G. Li, W. Pu, N. Chai, H. Shen and Y. Wu, RSC Adv., 2015, 5, 72142–72145 RSC
; (b) X.-F. Xia, Z. Gu, W. Liu, H. Wang, Y. Xia, H. Gao, X. Liu and Y.-M. Liang, J. Org. Chem., 2015, 80, 290–295 CrossRef CAS
; (c) I. B. Krylov, S. A. Paveliev, M. A. Syroeshkin, A. A. Korlyukov, P. V. Dorovatovskii, Y. V. Zubavichus, G. I. Nikishin and A. O. Terent'ev, Beilstein J. Org. Chem., 2018, 14, 2146–2155 CrossRef CAS
; (d) M.-Z. Zhang, N. Luo, R.-Y. Long, X.-T. Gou, W.-B. Shi, S.-H. He, Y. Jiang, J.-Y. Chen and T. Chen, J. Org. Chem., 2018, 83, 2369–2375 CrossRef CAS
; (e) T. E. Anderson and K. A. Woerpel, Org. Lett., 2020, 22, 5690–5694 CrossRef CAS
.
- A. Alanine, A. Bourson, B. Büttelmann, R. Gill, M.-P. Heitz, V. Mutel, E. Pinard, G. Trube and R. Wyler, Bioorg. Med. Chem. Lett., 2003, 13, 3155–3159 CrossRef CAS
.
-
(a) R. Bag, D. Sar and T. Punniyamurthy, Org. Lett., 2015, 17, 2010–2013 CrossRef CAS
; (b) X.-F. Xia, S.-L. Zhu, Z. Gu, H. Wang, W. Li, X. Liu and Y.-M. Liang, J. Org. Chem., 2015, 80, 5572–5580 CrossRef CAS
; (c) A. A. Andia, M. R. Miner and K. A. Woerpel, Org. Lett., 2015, 17, 2704–2707 CrossRef CAS
.
-
(a) Y. Lv, X. Wang, H. Cui, K. Sun, W. Pu, G. Li, Y. Wu, J. He and X. Ren, RSC Adv., 2016, 6, 74917–74920 RSC
; (b) J.-z. Zhang and Y. Tang, Adv. Synth. Catal., 2016, 358, 752–764 CrossRef CAS
.
- D. Yamamoto, M. Soga, H. Ansai and K. Makino, Org. Chem. Front., 2016, 3, 1420–1424 RSC
.
- S. Samanta, R. R. Donthiri, C. Ravi and S. Adimurthy, J. Org. Chem., 2016, 81, 3457–3463 CrossRef CAS
.
-
(a) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS
; (b) S.-Y. Zhang, F.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2011, 40, 1937–1949 RSC
; (c) B. G. Hashiguchi, S. M. Bischof, M. M. Konnick and R. A. Periana, Acc. Chem. Res., 2012, 45, 885–898 CrossRef CAS
; (d) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107–1295 RSC
; (e) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900–2936 RSC
; (f) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086–9139 CrossRef CAS
; (g) Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247–9301 CrossRef CAS
; (h) W. Wang, M. M. Lorion, J. Shah, A. R. Kapdi and L. Ackermann, Angew. Chem., Int. Ed., 2018, 57, 14700–14717 CrossRef CAS
; (i) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS
.
-
(a) J. J. Mousseau and A. B. Charette, Acc. Chem. Res., 2013, 46, 412–424 CrossRef CAS
; (b) R. Narayan, K. Matcha and A. P. Antonchick, Chem.–Eur. J., 2015, 21, 14678–14693 CrossRef CAS
.
- For selected reviews:
(a) X.-F. Wu, J.-L. Gong and X. Qi, Org. Biomol. Chem., 2014, 12, 5807–5817 RSC
; (b) R. Rossi, M. Lessi, C. Manzini, G. Marianetti and F. Bellina, Adv. Synth. Catal., 2015, 357, 3777–3814 CrossRef CAS
; (c) Y. Qin, L. Zhu and S. Luo, Chem. Rev., 2017, 117, 9433–9520 CrossRef CAS
; (d) R. Chen, J. Chen, J. Zhang and X. Wan, Chem. Rec., 2018, 18, 1292–1305 CrossRef CAS
.
- For selected reviews:
(a) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS
; (b) L. Yang and H. Huang, Chem. Rev., 2015, 115, 3468–3517 CrossRef CAS
; (c) H. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh and A. Lei, Chem. Rev., 2017, 117, 9016–9085 CrossRef CAS
; (d) S.-r. Guo, P. S. Kumar and M. Yang, Adv. Synth. Catal., 2017, 359, 2–25 CrossRef CAS
; (e) X.-Q. Chu, D. Ge, Z.-L. Shen and T.-P. Loh, ACS Catal., 2018, 8, 258–271 CrossRef CAS
; (f) H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS
.
- Y. Siddaraju and K. R. Prabhu, Org. Biomol. Chem., 2015, 13, 11651–11656 RSC
.
-
(a) R. Chen, K.-K. Wang, Z.-Y. Wang, C. Miao, D. Wang, A.-a. Zhang and L. Liu, J. Org. Chem., 2019, 84, 16068–16075 CrossRef CAS
; (b) R. Chen, W. Chen, Y. Shen, Z.-Y. Wang, W. Dai, K.-K. Wang and L. Liu, Synlett, 2019, 30, 1708–1712 CrossRef CAS
.
-
(a) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328–3435 CrossRef CAS
; (b) X. Wang and A. Studer, Acc. Chem. Res., 2017, 50, 1712–1724 CrossRef CAS
; (c) S. R. Guo, P. S. Kumar and M. H. Yang, Adv. Synth. Catal., 2017, 359, 2–25 CrossRef CAS
; (d) K. Muniz, Acc. Chem. Res., 2018, 51, 1507–1519 CrossRef CAS
; (e) I. F. D. Hyatt, L. Dave, N. David, K. Kaur, M. Medard and C. Mowdawalla, Org. Biomol. Chem., 2019, 17, 7822–7848 RSC
; (f) Z. Z. Han and C. P. Zhang, Adv. Synth. Catal., 2020, 362, 4256–4292 CrossRef CAS
.
- N. Zhang, R. Cheng, D. Zhang-Negrerie, Y. Du and K. Zhao, J. Org. Chem., 2014, 79, 10581–10587 CrossRef CAS
.
- D.-M. Chen, Y.-Y. Sun, Q.-Q. Han and Z.-L. Wang, Tetrahedron Lett., 2020, 61, 152482 CrossRef CAS
.
- CCDC 2049393 contains the supplementary crystallographic data for compound 3f.†.
-
(a) N.-N. Wang, W.-J. Hao, T.-S. Zhang, G. Li, Y.-N. Wu, S.-J. Tu and B. Jiang, Chem. Commun., 2016, 52, 5144–5147 RSC
; (b) H. Ni, X. Shi, Y. Li, X. Zhang, J. Zhao and F. Zhao, Org. Biomol. Chem., 2020, 18, 6558–6563 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Data for new compounds and experimental procedures. CCDC 2049393. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra00375e |
| This journal is © The Royal Society of Chemistry 2021 |