Open Access Article
Open Access ArticleUltralight hybrid silica aerogels derived from supramolecular hydrogels self-assembled from insoluble nano building blocks†
Zongjian Liua,
Ling Liub,
Zhenggen Zhongb,
Yuanyuan Rana,
Jianing Xi*a and
Jin Wang *b
*b
aDepartment of Rehabilitation, Beijing Rehabilitation Hospital, Capital Medical University, Beijing 100144, P. R. China. E-mail: xijn999@ccmu.edu.cn
bKey Laboratory of Multifunctional Nanomaterials and Smart Systems, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, P. R. China. E-mail: jwang2014@sinano.ac.cn
First published on 12th February 2021
Abstract
Supramolecular hydrogels are a type of hydrogel cross-linked by non-chemical bonds and they have been widely applied in the field of smart systems, sensors, tissue engineering, and controlled drug delivery. Most supramolecular hydrogels are formed by soluble molecules, polymers, and metal ions. In this work, supramolecular hydrogels self-assembled between two insoluble nano building blocks (ISNBBs), graphene oxide (GO) and amino-functionalized silica nanoparticles (SiO2–NH2), have been discovered and synthesized. The gelation conditions of the two ISNBBs have been investigated. A step further, ultralight hybrid silica aerogels are obtained by supercritical drying of the physical hydrogels. No visible volume shrinkage is observed, due to the fact that the hydrogel networks are formed by rigid ISNBBs. Thus the hybrid aerogels possess ultralow density (down to 7.5 mg cm−3), high specific surface areas (178.6 m2 g−1), and extremely high porosity (99.6%). The present work shows an alternative strategy to design and synthesize supramolecular hydrogels and aerogels using predetermined building blocks, together with designable morphology and physical properties for the target aerogels.
Introduction
Supramolecular hydrogels (or physical hydrogels) are a type of network cross-linked by non-covalent interactions such as hydrogen bonding,1 hydrophobic interactions,2 van der Waals interactions,3 electrostatic interactions,4 and π–π interactions.5 Due to their non-covalent nature, the supramolecular hydrogels are normally reversible soft materials and are considered to be potential materials to construct smart systems, such as stimuli-responsiveness triggered by pH, temperature, electronic field, magnetic field, and light. Thus, they have been widely investigated as biomaterials for use in tissue engineering, controlled drug delivery, and tissue repairs.6–12Aerogels are highly porous materials that are generally synthesized by a two-step process, sol–gel transition (to obtain a porous network) and specific dying process (e.g. supercritical liquid drying, to remove liquid in gels while keep the volume and microstructure unchanged or with limited changes).13–25 Aerogels exhibit various attractive properties such as extremely low density, low thermal conductivity, high specific surface areas, high porosity, and high pore volume. Thus, they have found potential applications in the fields of aerospace, catalysis, filtration, thermal insulation, electronics, sensors, water purification, tissue engineering, and drug delivery. Recently, significant progress in aerogels have been achieved. For example, eco-friendly cellulose aerogels that were capable for oil absorption and oil/water separation,26–28 anisotropic cellulose nanofibril composite sponges that were able to be used as senor and electromagnetic interference shielding,29 fibrous polyimide sponges and aerogels with superior mechanical and thermal properties,30,31 anisotropic nanocellulose aerogels that were suitable for fast liquid transportation,32 ultrablack aerogels for solar steam generation,33,34 etc. have been prepared. Reviews on the comprehensive developments and applications of aerogels are also available.13,14,35–37 Aerogels and hydrogels could be considered as the same porous network in different stages, that hydrogels are filled with water in their pores while aerogels are filled with air in their pores. Therefore, there are plenty of similarities between hydrogels and aerogels, such as the same porous structure, and the same potential applications as catalyst support, water purification absorbent, and drug delivery system. Nevertheless, differences can be observed between these two materials. For instance, hydrogels are reversible and stimuli-responsive, but the stimuli-responsiveness in aerogels are rare because the movements of molecules are restricted in the solid state.10,11
On the other hand, supramolecular hydrogels are normally formed by soluble precursors,38–41 which are soluble in the molecular level when they form the hydrogel networks. Due to the weak connection of supramolecular hydrogels, their corresponding aerogels cannot be obtained because there are no preformed network to support their volumes and structures. Recently graphene oxide (GO) has been recognized as an attractive two dimensional building blocks for supramolecular hydrogels, which are insoluble dispersed nano plates and various GO-based supramolecular hydrogels have been prepared by different secondary interactions including hydrogen bonding, π–π stacking, electrostatic interaction, and coordination.42–45 Cross-linkers been used to prepare GO based supramolecular hydrogels including poly(vinyl alcohol) (PVA), poly(ethylene oxide) (PEO), hydroxypropylcellulose (HPC), poly(vinyl pyrrolidone) (PVP), polyethylenimine (PEI), cetyltrimethyl ammonium bromide (CTAB), tetramethylammonium chloride (TMAC), and melamine. The cross-linkers are all soluble components so that they can efficiently form non-covalent interactions with GOs. Moreover, GOs can be self-assembled between themselves via π–π stacking through reduction, and their corresponding hydrogels can be successfully transferred to GO aerogels, which showed potential applications in energy storage and water purification.46–49 Hybrid GO based hydrogels and aerogels incorporated with other insoluble nano building blocks (ISNBBs), such as MoS2, carbon nanotubes (CNTs), and boron nitride (BN),50–54 have been synthesized and they showed improved performances. Nevertheless, these ISNBBs did not act as cross-linkers for the GO to form supramolecular hydrogels, they acted as fillers that were embed in the GO matrix.
In this work, amino group (–NH2) functionalized silica nanoparticles (SiO2–NH2) are synthesized and found to form hybrid supramolecular hydrogels with GO. The SiO2–NH2 nanoparticles played as cross-linkers and hydrogen bonds were formed between SiO2–NH2 and GO as illustrated in Fig. 1. Hybrid supramolecular hydrogels can be formed in a wide range of concentration. The supramolecular hydrogels were formed solely by ISNBBs, which formed interconnected network throughout the hydrogels. Herein, the gelation behaviour, parameter, and the corresponding hybrid aerogels will the systematically investigated.
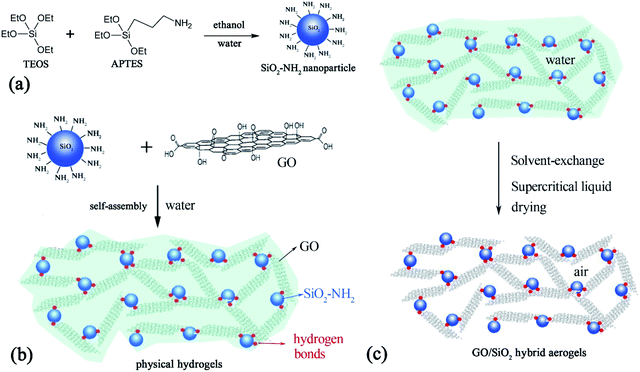 | ||
| Fig. 1 (a) Schematic description of the synthesis of SiO2–NH2; (b) GO–SiO2–NH2 hybrid supramolecular hydrogels; and (c) the corresponding GO–SiO2–NH2 hybrid aerogels. | ||
Experimental
Materials
Graphite powder was purchased from Qingdao Tianheda Graphite Co., Ltd, Qingdao, China (purity 99.8%). 3-Aminopropyltriethoxysilane (APTES) (AR, purity 99%) and tetraethoxysilane (TEOS) (GR, purity 98%) were purchased from Aladdin Industrial Corporation, Shanghai, China. GO were prepared according to literatures by a modified Hummers method42,55,56 and GO aqueous solution were diluted to various concentrations. Other reagents are of analytical purity and used as received.Synthesis of SiO2–NH2 nanoparticles
SiO2–NH2 nanoparticles were synthesized through a revised Stöber method57,58 as follows: to a mixture of 125 ml distilled water and 100 μl ammonium hydroxide, 10 ml TEOS was added under stilling for 2 h. Then, 1.5 ml APTES was added. SiO2–NH2 nanoparticles were formed as white precipitate and dried in vacuum. SiO2–OH nanoparticles were synthesized via a Stöber method without the addition of APTES for comparison.Synthesis of GO–SiO2 supramolecular hydrogels and aerogels
GO–SiO2 supramolecular hydrogels were synthesized by mixing GO aqueous solution and SiO2–NH2 nanoparticle aqueous solution, take the GO5–SiO215 for example: 1 ml fleshly prepared SiO2–NH2 aqueous solution (15 mg ml−1) was mixed with 1 ml GO aqueous solution (5 mg ml−1) and stirred for 5 min. Supramolecular hydrogels were formed in 2 h. The supramolecular hydrogels were named as GOm–SiO2n, where m and n indicate the concentration of GO and SiO2 nanoparticles, respectively. The supramolecular hydrogels were converted to aerogels by solvent-exchange with ethanol and supercritical CO2 drying.Characterization
Fourier transform infrared (FT-IR) spectra are recorded on a Nicolet iN10 spectrometer (Thermo Scientific, USA) in the reflection mode. The morphologies are observed on a field-emission scanning electron microscopy (FESEM, Quanta 400 FEG, USA), the samples are coated with Au nanoparticles at a current of 20 mA for 2 min in advance. TEM measurement was carried out on a Tecnai G2 F20 S-TWIN instrument. N2 adsorption–desorption isotherms are performed on Micromeritics (ASAP 2020, USA). The specific surface area of the samples are determined by the Brunauer–Emmett–Teller (BET) method based on the amount of N2 adsorbed at pressures 0.05 < P/P0 < 0.3. The pore volumes are measured at the point P/P0 = 0.99. The pore size distribution and the average pore diameters are analyzed by the Barrett–Joyner–Halenda (BJH) method. Densities of the aerogels are calculated by weighting the samples and measuring the volumes.Results and discussion
GO based hydrogels have attractive increasing interests and various cross-linkers can be used. Nevertheless, challenges remain for the self-assembly of GO with ISNBBs, while network formed by IsNBBs (such as metal nano spheres) show great potential for functional materials.59 On the other hand, SiO2 nanoparticles have found wide applications in catalyst, energy, environment, and biomedicine.60–63 Since there are plenty of –OH groups on the surfaces of SiO2 nanoparticles prepared by Stöber method, it could be interesting to see if GO and SiO2 nanoparticles could be self-assembled into hydrogels, as it was happened between GO and PVA. However, hydrogels could not be formed between GO and SiO2 nanoparticles. Interestingly, we found that –NH2 functionalized SiO2 nanoparticles could self-assembly with GO and form supramolecular hydrogels.The synthetic approach of SiO2–NH2 nanoparticles is illustrated in Fig. 1a, which is a revised Stöber method that a diluted NH3H2O aqueous solution is used, then TEOS is added under stirring and there are no observable changes. After the addition of APTES, white precipitates were formed. APTES acted as both co-monomers to functionalize the silica nanoparticles with –NH2 groups and as basic catalyst to promote the hydrolysis and condensation of TEOS.64,65 As depicted in Fig. 1b, supramolecular hydrogels were formed after mixed GO and SiO2–NH2 nanoparticles aqueous solution. The only differences of the silica nanoparticles were the replacement of –OH with –NH2, which may form stronger hydrogen bonds between GO and result in the formation of supramolecular hydrogels. After solvent-exchange with ethanol, hybrid aerogels (Fig. 1c) were synthesized by supercritical CO2 drying.
Fig. 2 shows the morphology and the N2 absorption–desorption isotherm of the SiO2–NH2 nanoparticles. As can be seen from the SEM image, SiO2–NH2 nanoparticles were spherical particles with diameters lower than 50 nm. The TEM image of the SiO2–NH2 nanoparticles shown in Fig. S1, ESI† further indicated that the diameter of them ranged from 20–40 nm. The specific surfaces area (SSA) of the SiO2–NH2 nanoparticles was determined to be 163 m2 g−1. The small size and large SSA value of the SiO2–NH2 nanoparticles may facilitate the formation of supramolecular hydrogels with GO, which significantly increased the contact areas between the two ISNBBs.
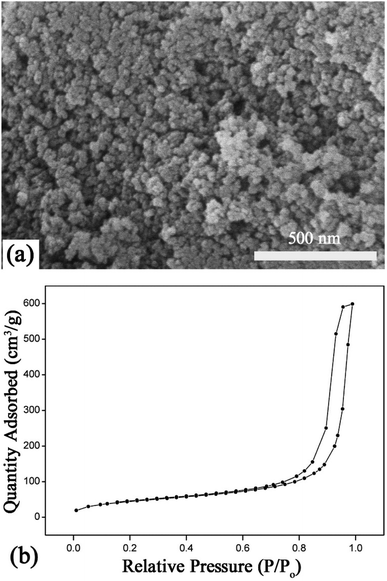 | ||
| Fig. 2 (a) SEM image of SiO2–NH2 nanoparticles; (b) nitrogen absorption–desorption isotherm of the SiO2–NH2 nanoparticles. | ||
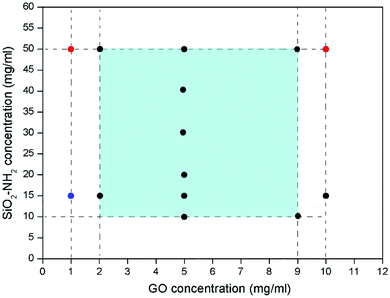
The gelation behaviour between GO and SiO2–NH2 nanoparticles are systematically investigated, and the results are presented in Fig. 3. GO were synthesized according to our previous work by a modified Hummers method,55,56 the size of graphite powder was 30–40 μm. The SEM image of the GO is shown in Fig. S2, ESI.† They were diluted into difference concentrations as shown in Fig. 3. It can be seen that supramolecular hydrogels can be formed with a wide range of GO concentration from 2 mg ml−1 to 10 mg ml−1, while the concentration of SiO2–NH2 nanoparticles ranged from 10 mg ml−1 to 50 mg ml−1 (see Fig. S3, ESI† for the photo images of the hydrogels). When the concentration of the IsNBBs were located in the light blue region, supramolecular hydrogels would possibly be formed. It should be pointed out that when the concentration of SiO2–NH2 nanoparticles reached to 50 mg ml−1, supramolecular hydrogels did not formed when the concentration of GO was 10 mg ml−1. The dispersion of GO and SiO2–NH2 nanoparticles was difficult to homogenously mixed. When the concentration of GO was down to 1 mg ml−1, hydrogels could also not be formed, instead precipitates were obtained when the concentration of SiO2–NH2 nanoparticles was 15 mg ml−1. The results indicated that diluted GO dispersion (e.g. 1 mg ml−1) cannot form continuous network throughout the mixture solution. Nevertheless, GO can still interconnected with SiO2–NH2 nanoparticles to form precipitates.
Since the supramolecular hydrogels were formed solely by ISNBBs, there must be phase separated network that could persist its porous structure to obtain the corresponding aerogels. Therefore, the supramolecular hydrogels were dried by supercritical CO2, and GO–SiO2 hybrid aerogels were successfully obtained (Fig. S4, ESI†). The density of the aerogels was down to 7.5 mg ml−1 for the GO5–SiO210 aerogels, which was equal to the theoretical density. Considering the negligible volume shrinkage of the aerogels, it could be concluded that there were no loss of GO and SiO2–NH2 nanoparticles during the solvent-exchange and supercritical liquid drying process. The nearly 100% yield, which is a superior aspect for aerogel prepared by ISNBBs. In contrast, GO aerogels that were cross-linked by π–π stacking via reduction of GO,55,56 or cross-linked by soluble polymers such as PVA, PEO, and PVP,46–49 were remarkably shrunk during both the solvent-exchange and drying processes.
According to the equation:11
| Porosity = 1 − ρb/ρs |
The FTIR spectra of GO, SiO2–NH2 nanoparticles, and the hybrid aerogels are presented in Fig. 4. Peaks located at 2982 cm−1 could be clearly observed for the SiO2–NH2 nanoparticles, which can be ascribed to the C–H vibration from APTES groups. The broad and strong peak located at 3100–3400 cm−1 suggested the presence of –NH2. The vibration peaks of Si–O–Si and Si–C can be observed at 1100 and 840 cm−1, respectively. The vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in GO of the aerogel located at 1644 cm−1 (black arrow) showed a shift from 1720 cm−1 (red arrow), which indicated the formation of hydrogen bonds between GO and the SiO2–NH2 nanoparticles that resulted in the formation of supramolecular hydrogels.66 In addition, the supramolecular hydrogel could be transferred into sol when temperature elevated up to 90 °C (Fig. S5a, ESI†), possibly due to the broken of hydrogen bonds between GO and SiO2–NH2. Besides, SiO2–OH nanoparticles without –NH2 groups were synthesized, and they could not form hydrogels with SiO2–NH2 (Fig. S5b, EIS†) and GO (Fig. S5c†). Those results indicated that the hydrogen bonds in the supramolecular hydrogels may be formed between –NH2 (in SiO2–NH2) and –COOH groups (in GO).
O stretching vibration in GO of the aerogel located at 1644 cm−1 (black arrow) showed a shift from 1720 cm−1 (red arrow), which indicated the formation of hydrogen bonds between GO and the SiO2–NH2 nanoparticles that resulted in the formation of supramolecular hydrogels.66 In addition, the supramolecular hydrogel could be transferred into sol when temperature elevated up to 90 °C (Fig. S5a, ESI†), possibly due to the broken of hydrogen bonds between GO and SiO2–NH2. Besides, SiO2–OH nanoparticles without –NH2 groups were synthesized, and they could not form hydrogels with SiO2–NH2 (Fig. S5b, EIS†) and GO (Fig. S5c†). Those results indicated that the hydrogen bonds in the supramolecular hydrogels may be formed between –NH2 (in SiO2–NH2) and –COOH groups (in GO).
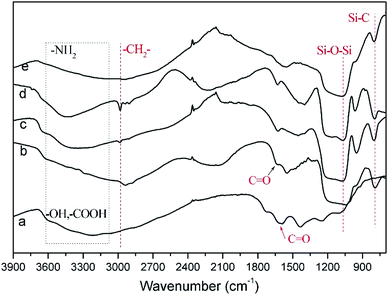 | ||
| Fig. 4 FT-IR spectra of (a) GO; (b) GO5–SiO210 hybrid aerogels; (c) GO5–SiO215 hybrid aerogels; (d) GO5–SiO220 hybrid aerogels; (e) SiO2–NH2 nanoparticles. | ||
The microstructure of the GO–SiO2 hybrid aerogels is investigated by SEM. Fig. 5 shows the SEM image of GO5–SiO215 aerogels. Pore size up to 1 μm could be observed, and the pores were homogenously distributed. From the SEM image with larger magnification times, SiO2–NH2 nanoparticles can be clearly identified and were found to be dispersed on the GO sheets without remarkable aggregation. The results confirmed that GO were gelled cross-linked by SiO2–NH2 nanoparticles, as illustrated in Fig. 1b. Combine with the highly porous structure of the aerogel and hydrogels, the hybrid aerogels might be ideal candidate for tissue engineering and drug delivery matrix.
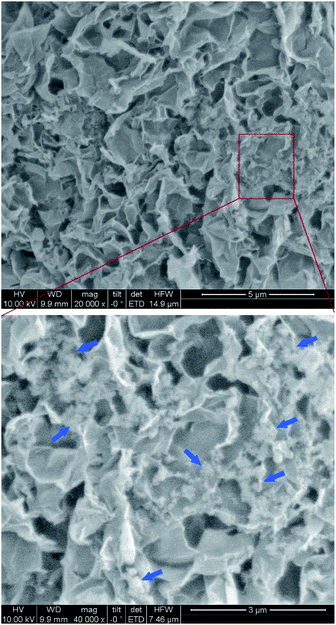 | ||
| Fig. 5 SEM images of the GO5–SiO215 hybrid aerogels, blue arrows pointed to the SiO2–NH2 nanoparticles. | ||
The N2 adsorption–desorption isotherms and the pore size distribution curves of the hybrid aerogels are shown in Fig. 6. All the aerogels exhibited hysteresis loops, which were due to the typical features of mesoporous materials (type IV isotherms). The BET surface areas of the samples were 178, 120, 150 m2 g−1 for the GO5–SiO210, GO5–SiO215, and GO5–SiO220 hybrid aerogels, respectively. All the aerogels possessed high pore volume ranged from 0.92 to 1.24 cm3 g−1. Fig. 6b indicated that the aerogels also possessed mesopores, with average diameter of 43, 37, and 36 nm for GO5–SiO210, GO5–SiO215, and GO5–SiO220 hybrid aerogels, respectively. The SEM and BET results indicated that the hybrid aerogels possessed hierarchically pores ranged from tens of nanometers to several micrometers.
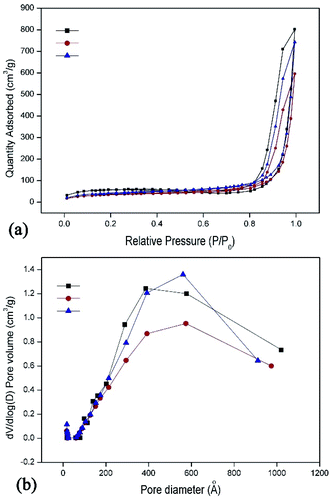 | ||
| Fig. 6 (a) Nitrogen absorption–desorption isotherm of the hybrid aerogels; (b) pore distribution of the hybrid aerogels. | ||
Conclusions
In conclusions, the gelation between GO and SiO2–NH2 nanoparticles, which are ISNBBs, have been observed. They can form supramolecular hydrogels in a wide range of concentration from 2 mg ml−1 to 10 mg ml−1 for GO and 10 mg ml−1 to 50 mg ml−1 for SiO2–NH2 nanoparticles. The framework of the supramolecular hydrogels were build up with GO and silica nanoparticles, where silica nanoparticles were dispersed on the GO nanosheet and acted as cross-linkers through the formation of hydrogen bonds between –NH2 and –COOH groups in SiO2–NH2 and GO, respectively. Interestingly, the hybrid hydrogels could be successfully transferred into hybrid aerogels without observable volume shrinkage. The aerogels exhibited extremely low density of 7.5 mg ml−1, large pore volume of 1.24 cm3 g−1, high porosity of 99.6%, and high BET SSA ranged from 120–178 m2 g−1. In addition, the aerogels possessed hierarchically porous structures from tens of nanometers to several micrometers, combined with the excellent biocompatibility of GO and SiO2, the stimuli responsive behaviours, these supramolecular hydrogels and aerogels may find useful application in biomedicine, such as drug carriers and tissue engineering. The results also indicated that hydrogels and aerogels could be synthesised from ISNBBS with predetermined structure and properties.Author contributions
Z. Liu and L. Liu completed the experiment and analyzed the data. Z. Zhong contributed to formal analysis. J. Wang designed and supervised the project. J. Xi supervised the project. Z. Liu wrote the original draft, and all the author reviewed and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (91963124, 51773225, 81671161).Notes and references
- Y. Zhao, Z. Li, Q. Li, L. Yang, H. Liu, R. Yan, L. Xiao, H. Liu, J. Wang, B. Yang and Q. Lin, Macromol. Rapid Commun., 2020, 41, 2000441 CrossRef CAS.
- H. Xiong, Y. Li, H. Ye, G. Huang, D. Zhou and Y. Huang, J. Mater. Chem. B, 2020, 8, 10309–10313 RSC.
- Y. Yan, A. Keizer, A. A. Martens, C. L. P. Oliveira, J. S. Pedersen, F. A. de Wolf, M. Drechsler, M. A. C. Stuart and N. A. M. Besseling, Langmuir, 2009, 25, 12899–12908 CrossRef CAS.
- J. Li, Z. Su, X. Ma, H. Xu, Z. Shi, J. Yin and X. Jiang, Mater. Chem. Front., 2017, 1, 310–318 RSC.
- S. K. Mandal, T. Kar and P. K. Das, Chem.–Eur. J., 2013, 19, 12486–12496 CrossRef CAS.
- A. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, 1800259 CrossRef.
- X. Dou, N. Mehwish, C. Zhao, J. Liu, C. Xing and C. Feng, Acc. Chem. Res., 2020, 53, 852–862 CrossRef CAS.
- L. Voorhaar and R. Hoogenboom, Chem. Soc. Rev., 2016, 45, 4013–4031 RSC.
- A. Rey-Rico and M. Cucchiarini, Polymers, 2019, 11, 514 CrossRef CAS.
- J. Wang, Q. Fang, L. Ye, A. Zhang and Z. G. Feng, Soft Matter, 2020, 16, 5906–5909 RSC.
- J. Wang and X. Zhang, ACS Nano, 2015, 11, 11389–11397 CrossRef.
- S. Qie, Y. Hao, Z. Liu, J. Wang and J. Xi, Acta Chim. Sin., 2020, 78, 232–244 CrossRef.
- S. Jiang, S. Agarwal and A. Greiner, Angew. Chem., Int. Ed., 2017, 56, 15520–15538 CrossRef CAS.
- J. Wang and J. Wang, Acta Chim. Sin., 2021 DOI:10.6023/A20110531.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22–45 CrossRef.
- R. Ciriminna, A. Fidalgo, V. Pandarus, F. Béland, L. M. Ilharco and M. Pagliaro, Chem. Rev., 2013, 113, 6592–6620 CrossRef CAS.
- M. Antonietti, N. Fechler and T. P. Fellinger, Chem. Mater., 2014, 26, 196–210 CrossRef CAS.
- A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243–4265 CrossRef CAS.
- J. P. Randall, M. A. B. Meador and S. C. Jana, ACS Appl. Mater. Interfaces, 2011, 3, 613–626 CrossRef CAS.
- C. Ziegler, A. Wolf, W. Liu, A. K. Herrmann, N. Gaponik and A. Eychmüller, Angew. Chem., Int. Ed., 2017, 56, 13200–13221 CrossRef CAS.
- Z. Liu, Y. Ran, J. Xi and J. Wang, Soft Matter, 2020, 16, 9160–9175 RSC.
- J. Wang, X. Wang and X. Zhang, J. Mater. Chem. A, 2017, 5, 4308–4313 RSC.
- J. Wang, Y. Zhang and X. Zhang, J. Mater. Chem. A, 2016, 4, 11408–11415 RSC.
- J. Wang, R. Du and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 1468–1473 CrossRef CAS.
- J. Wang, Y. Zhang, Y. Wei and X. Zhang, Microporous Mesoporous Mater., 2015, 218, 192–198 CrossRef CAS.
- Q. Shang, J. Chen, X. Yang, C. Liu, Y. Hu and Y. Zhou, J. For. Eng., 2019, 4, 105–111 CrossRef.
- L. Zhou, H. Zhou, J. Li, S. Tan, P. Chen and Z. Xu, J. For. Eng., 2019, 4, 67–73 Search PubMed.
- Q. Shang, Y. Hu, C. Liu, X. Yang and Y. Zhou, J. For. Eng., 2019, 4, 86–92 Search PubMed.
- Y. Chen, L. Zhang, C. Mei, Y. Li, G. Duan, S. Agarwal, A. Greiner, C. Ma and S. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 35513–35522 CrossRef CAS.
- S. Jiang, J. Y. Cheong, J. S. Nam, I. Kim, S. Agarwal and A. Greiner, ACS Appl. Mater. Interfaces, 2020, 12, 19006–19014 CrossRef CAS.
- X. Li, J. Wang, Y. Zhao and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 16901–16910 CrossRef CAS.
- Y. Chen, L. Zhou, L. Chen, G. Duan, C. Mei, C. Huang, J. Han and S. Jiang, Cellulose, 2019, 26, 6653–6667 CrossRef CAS.
- H. Wang, A. Du, X. Ji, C. Zhang, B. Zhou, Z. Zhang and J. Shen, ACS Appl. Mater. Interfaces, 2019, 11, 42057–42065 CrossRef CAS.
- M. Tan, J. Wang, W. Song, J. Fang and X. Zhang, J. Mater. Chem. A, 2019, 7, 1244–1251 RSC.
- A. Du, B. Zhou, Z. Zhang and J. Shen, Materials, 2013, 6, 941–968 CrossRef CAS.
- L. Hu, R. He, H. Lei and D. Fang, Int. J. Thermophys., 2019, 40, 39 CrossRef.
- A. Lamy-Mendes, R. F. Silva and L. Durães, J. Mater. Chem. A, 2018, 6, 1340–1369 RSC.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS.
- D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler and F. Huang, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS.
- Q. Li, J. Wang, L. Ye, A. Zhang, X. Zhang and Z. G. Feng, ChemNanoMat, 2019, 5, 838–846 CrossRef CAS.
- Z. Xu and C. Gao, ACS Nano, 2011, 5, 2908–2915 CrossRef CAS.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS.
- H. Bai, C. Li, X. Wang and G. Shi, J. Phys. Chem. C, 2011, 115, 5545–5551 CrossRef CAS.
- E. Greco, J. Shang, J. Zhu and T. Zhu, ACS Omega, 2019, 4, 20948–20954 CrossRef CAS.
- L. A. Huang, Z. S. He, J. F. Guo, S. E. Pei, H. B. Shao and J. M. Wang, ChemElectroChem, 2019, 6, 2698–2706 CrossRef CAS.
- A. Plyushch, T. L. Zhai, H. S. Xia, C. Santillo, L. Verdolotti, M. Lavorgna and P. Kuzhir, Materials, 2019, 12, 213 CrossRef CAS.
- W. K. Wang, Y. H. Wu, Z. W. Jiang, M. Z. Wang, Q. C. Wu, X. Zhou and X. W. Ge, Chin. Chem. Lett., 2018, 29, 931–934 CrossRef CAS.
- Y. Zhong, T. Shi, Y. Huang, S. Cheng, G. Liao and Z. Tang, Nanoscale Res. Lett., 2019, 14, 85 CrossRef.
- F. Jiang, H. Liu, Y. Li, Y. Kuang, X. Xu, C. Chen, H. Huang, C. Jia, X. Zhao, E. Hotz, Y. Zhou, R. Yang, L. Cui and L. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 1104–1112 CrossRef CAS.
- B. Lee, S. Lee, M. Lee, D. H. Heong, Y. Baek, J. Yoon and Y. H. Kim, Nanoscale, 2015, 7, 6782–6789 RSC.
- M. Wang, T. Zhang, D. Mao, Y. Yao, X. Zeng, L. Ren, Q. Cai, S. Mateti, L. H. Li, X. Zeng, G. Du, R. Sun, Y. Chen, J. Xu and C.-P. Wong, ACS Nano, 2019, 13, 7402–7409 CrossRef CAS.
- F. An, X. Li, P. Min, H. Li, Z. Dai and Z. Z. Yu, Carbon, 2018, 126, 119–127 CrossRef CAS.
- G. Li, X. Zhang, J. Wang and J. Fang, J. Mater. Chem. A, 2016, 4, 17042–17049 RSC.
- R. Sun, G. Li, W. He, J. Wang, Q. Song and X. Zhang, Adv. Mater. Interfaces, 2016, 3, 1600541 CrossRef.
- W. Stöber and A. Fink, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- Y. Zhang, J. Wang and X. Zhang, J. Colloid Interface Sci., 2018, 515, 1–9 CrossRef CAS.
- Y. Zhang, J. Wang, Y. Wei and X. Zhang, New J. Chem., 2017, 41, 1953–1958 RSC.
- T. Li, S. Shi, S. Goel, X. Shen, X. Xie, Z. Chen, H. Zhang, S. Li, X. Qin, H. Yang, C. Wu and Y. Liu, Acta Biomater., 2019, 89, 1–13 CrossRef CAS.
- S. Sivasankar and S. Chu, Nano Lett., 2007, 7, 3031–3034 CrossRef CAS.
- M. Xuan, Z. Wu, J. Shao, L. Dai, T. Si and Q. He, J. Am. Chem. Soc., 2016, 138, 6492–6497 CrossRef CAS.
- D. Tam, C. E. Ashley, M. Xue, E. C. Carnes, J. I. Zink and C. J. Brinker, Acc. Chem. Res., 2013, 46, 792–801 CrossRef.
- Y. Wei, J. Wang, Y. Zhang, L. Wang and X. Zhang, RSC Adv., 2015, 5, 91407–91413 RSC.
- J. Wang, Y. Wei, W. He and X. Zhang, RSC Adv., 2014, 4, 51146–51155 RSC.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of GO, photo image of supramolecular hydrogels, etc. See DOI: 10.1039/d1ra00418b |
| This journal is © The Royal Society of Chemistry 2021 |