Open Access Article
Open Access ArticleMussel-inspired in situ fabrication of a photothermal composite hydrogel for MR-guided localized tumor ablation
Lixia Xu†
ab,
Ronghua Qin†b,
Jingjing Zhangb,
Jinjin Liub,
Suwan Liub,
Feng Lib,
Aihua Gongb,
Qian Hanliang*a,
Fengyi Du *b and
Miaomiao Zhang*b
*b and
Miaomiao Zhang*b
aDepartment of Ophthalmology, Affiliated Hospital of Jiangsu University, Zhenjiang 212002, Jiangsu, P. R. China
bSchool of Medicine, Jiangsu University, Zhenjiang, 212013, People' s Republic of China. E-mail: biodfy@ujs.edu.cn
First published on 28th May 2021
Abstract
Photothermal ablation could be considered an effective treatment for tumors, but accurate administration and enrichment of photothermal agents remain a huge challenge. Herein, a mussel-inspired photothermal polymeric hydrogel (PPH) was synthesized through a ferric iron-triggered simultaneous metal–catechol coordination reaction and oxidative polymerization of covalently linked pyrrole. The PPH with rapid gelation (less than 10 s) exhibited high photothermal conversion efficiency (49.3%), which enabled effective hyperthermal therapy in situ. Besides, the introduced iron could be used as a T2-weighted contrast agent for real-time MR imaging to explore the retention and bio-degradation of PPH in vivo. Overall, our findings evidence that the resultant PPH, which possesses potential application in tumor ablation in situ, and metal–catechol coordination strategy inspired by mussel adhesion may stimulate biomedical hydrogel development.
1 Introduction
Photothermal therapy (PTT) is a promising treatment technology using hyperthermia to eradicate solid tumors.1 During the process, certain photosensitizers (PS) concentrated in the tumor area generate heat to increase the local temperature of tumor tissues under laser irradiation.2 At 40–45 °C, the biomacromolecules in tumor cells, such as proteins, nucleic acids and lipids, will suffer irreversible damage, thereby the tumors are destroyed.3 Furthermore, due to the disordered vascular network and blocked blood vessels caused by PTT, tumor cells are more sensitive than normal tissues. Due to the obvious advantages, PTT-based technology is increasingly being developed and used in cancer treatment and research. Many inorganic materials (gold nanostructures,4,5 palladium nanoflakes and metal sulfide nanoparticles) and organic materials (carbon-based nanoscale materials, near-infrared (NIR) dyes, polydopamine (PDA),6,7 and polypyrrole (PPY)) have been explored as PS for effective PTT applications.8,9 Among them, PPY has attracted great interest and attention due to its inherent excellent near infrared photothermal conversion ability, photothermal stability and good bio-compatibility.10–12 Polypyrrole (PPY) can be formed by pyrrole through coordination polymerization in the presence of an oxidant (such as Fe3+) by an electro chemical coupling reaction.13,14 It is worth noting that the therapeutic effect of PTT depends heavily on the concentration of PS based on physical method. Despite the EPR effect and targeting modification,15 it is difficult to achieve an ideal concentration in tumor sites using traditional intravenous administration of PS.16 Consequently, it is urgent to develop novel PS delivery system to achieve the desired PTT therapeutic effect.Injectable hydrogels with good biocompatibility and biodegradability have great prospect in drug delivery in situ due to their unique hydrodynamic performance.17–21 Numerous multi-functional injectable hydrogels were designed and prepared based on distinct principles, such as ion exchange,22 enzyme catalysis,23 borate reaction,24 Schiff Base reaction,25 Michael addition reaction26 and catechol-based reaction.27 It was worth noting that catechol-based hydrogel exhibited favorable advantages in gelation in situ as injectable carriers for drug delivery,28 which ascribed to hydrogen bond formation and metal–catechol coordination interaction.29,30 The mechanism of catechol-based gelation was derived from adhesion of mussels to turbulent rock surfaces due to their secretion of a protein rich in catechol groups.31–33 Due to the diversity of catechol chemical properties, a variety of bio-adhesive hydrogel were fabricated via polyphenol interaction for wearable devices, biological glue and gelation in situ,34 such as tannin-mediated hydrogel,35 dopamine-modified hydrogel.36
Inspired by those results, we intend to develop a new type of injectable photothermal polymeric hydrogel (PPH) for tumor ablation in situ. Firstly, catechol grafted PEI (Cate-PEI) was synthesized using 3,4-dihydroxy hydrocinnamic acid (caffeic acid) as precursor under amide reaction. Then, the PPH was quickly formed via ferric ion-mediated coordination reaction and oxidative polymerization. During the process, ferric ion both used as oxidant for catalyzing pyrrole to form polypyrrole (PPY) and chelating catechol groups in side chain of Cate-PEI. The photothermal conversion efficiency of PPH was calculated and its photothermal therapeutic effect was verified in vitro and vivo. In addition, iron element could be used as MRI contrast agent for real-time imaging to guide precise tumor ablation in vivo.37
2 Experimental
2.1 Chemicals and reagents
All chemicals are used directly without purification and analysis. 3,4-Dihydroxyhydrocinnamic acid (caffeic acid), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (≥98.5%), pyrrole (≥99%), iron chloride hexahydrate all were purchased from Aladdin Reagent Co. Ltd. (Shanghai, China). Poly(ethyleneimine) solution (PEI, average Mw ∼ 25 kDa by LS, 50 wt% in H2O) was obtained by Sigma-Aldrich (USA).2.2 Synthesis of Cate-PEI
Cate-PEI was synthesized via grafting caffeic acid on the side chain of PEI through EDC chemical reaction. Briefly, 3,4-dihydroxyhydro-cinnamic acid (0.05, 0.125, 0.25, 0.5, 0.75, 1.0, 1.25 mmol) and excessive EDC (0.06, 0.15, 0.3, 0.6, 0.9, 1.2, 1.5 mmol) were dissolved in 50 mL of phosphate buffer saline (PBS) solution at pH of 5.5 using 1 N HCl solution to activate carboxyl. Then, PEI (0.25 mmol) was added into the mixture solution and then stirred for 4 h at pH of 5.5. Afterward, the unreacted chemicals were removed by dialysis (MWCO: 2000). Finally, Cate-PEI power was obtained after two days of freeze drying. The different molar ratio of caffeic acid to PEI was conducted during the above process. The degree of coupling of catechol on the side chain of PEI was measured using an ultraviolet-visible spectrophotometer at a absorption wavelength of 280 nm. The chemical structure of Cate-PEI was further analyzed by Nuclear magnetic resonance (NMR) spectroscopy.2.3 Fabrication and characterization of PPH
The PPH was fabricated in situ by ferric ion-mediated coordination reaction and oxidative polymerization. Briefly, 4 mg of Cate-PEI aqueous solution (500 μL) with different grafting rates and 35 μL of pyrrole were added into a centrifuge tube to form an evenly mixed solution. Then, 500 μL of iron chloride hexahydrate (FeCl3·6H2O) aqueous solution (0.1 g mL−1) were added into the mixed solution and then let stand. The gelation time was measured by the oblique method and hydrogel performance was determined by the inversion method. In order to observe the microstructure of PPH, Scanning Electron Microscope (SEM) was carried out. In brief, the formed hydrogel was placed in a refrigerator at −80 °C and then freeze-dried for 2 days. Afterward, the frozen hydrogel was cut into half with a knife and the structure of its cross section was observed by Scanning Electron Microscopy (SEM) (JSM-7800F, Japan).2.4 Instruments and equipment
The dry solid hydrogel was obtained from Freeze dryer (Boyikang, Beijing). The composition of the Cate-PEI was analyzed by UV-Vis spectrophotometer (Aoyi, Shanghai). The chemical structure of Cate-PEI was determined by Nuclear Magnetic Resonance (1H NMR) spectrometer (AVANCE II 400 MHz, Swiss). The cross-section structure of hydrogel PPH was observed by SEM. Rheological properties of PPH hydrogels were characterized by a Thermo HAAKE MARS 60 (HAAKE, Germany). The potential photothermal effects of PPH were investigated by 808 nm NIR optical laser (Leizhiwei, Beijing). The infrared thermal images were recorded by an infrared camera (NO:HT-19) purchased from Dongguan Xintai Instrument & Meter Co., Ltd (Guangdong, china).2.5 In vitro photothermal performance of PPH
In order to evaluate the photothermal performance, 0.1 g of PPH was irradiated by the 808 nm laser for 5 minutes under different power density (0.2, 0.3, 0.5, 0.6 W cm−2) and the temperature was recorded every 20 s. Distilled water was set as a control, the corresponding infrared thermal images were photographed using an infrared camera. To simulate photothermal performance in vivo, 0.1 g of PPH in different volume of distilled water (0.1, 0.5, 0.9, 1.3, 1.5 mL) was placed in a EP tube and irradiated by the 808 nm laser (0.6 W cm−2, 5 min). To evaluate the photothermal stability, 0.1 g of PPH hydrogel was subjected into 5 cycles of laser irradiation at 0.6 W cm−2 (laser on/off: 5 min), and the temperature was recorded every 20 s.2.6 Photothermal conversion efficiency of PPH
0.1 g of PPH or 0.1 g distilled water was irradiated by the 808 nm laser at a power density of 0.6 W cm−2 for 10 min until a stable maximum temperature, and then the irradiated laser or water was allowed to cool down to room temperature for 10 min. The following formula was used to calculate the photothermal conversion efficiency (η),
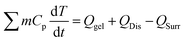 | (1) |
According to the law of conservation of energy, Qgel is the energy input of PPH. QDis represents the heat dissipated from the photoabsorption of the sample cell itself. QSurr is the lost heat energy to the surroundings.
| Qgel = I(1 − 10−Aλ)η | (2) |
| QSurr = hSΔT | (3) |
| QDis = QSurr = hSΔTMax,water | (4) |
In the whole system, the heat inputs are the heat generated by PPH hydrogel (Qgel) and the heat generated by water (QDis), which are equal to the heat output at the maximum steady-state temperature, so the equation can be
| Qgel + QDis = QSurr = hSΔTMax,mix | (5) |
According to the eqn (2), (4) and (5), the photothermal conversion efficiency (η) can be expressed as following:
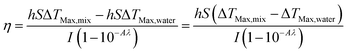 | (6) |
ΔTMax,mix is the temperature change of PPH hydrogel at the maximum steady state temperature, and ΔTMax,water is the temperature change of distilled water at the maximum steady state temperature, Only the hS remains unknown for the calculation of (η).
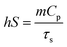 | (7) |
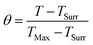 | (8) |
2.7 In vitro cytotoxicity
Hydrogel and cells were co-cultured in transwell to investigate the cytotoxicity. Normal fibroblasts (MEF) were planted in the lower culture plate, hydrogels of different quality (25, 50, 100, 200, 400 mg) were placed in the upper chamber, and then incubated with the cells for 24 hours, standard cck-8 kit was used to detect cell viability.2.8 In vitro and in vivo MR imaging of PPH
MR imaging of the synthesized PPH was obtained by Nuclear Magnetic Resonance scanner (Germany SIEMENS, Model: Magnetom Trio Tim) to investigate the possibility as a T2 MR contrast agent. Different amounts of PPH (0.8 mg, 1.6 mg, 2.4 mg, 3.2 mg, 4.0 mg) was placed in a 96-well plate respectively. The measurement parameters were as follows: TR = 3500 ms, TE = 89 ms.For in vivo MR imaging, PPH (100 μL) was injected subcutaneously into the tumor of breast tumor mouse after anesthetize mice with 1% sodium pentobarbital. The retention of PPH were observed in vivo at different durations (0 h, 2 h, 24 h, 48 h, 7 d), and the mice pre and post injection were scanned to observe the distribution and retention of the PPH in tumors.
2.9 In vivo PTT therapy
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Jiangsu University, the animal experiments were conducted according on the Animal Management Rules of the Ministry of Health of the People's Republic of China and approved by the Animal Ethics Committee of Jiangsu University. In order to establish the breast cancer mouse model, female BALB/C mice aged 6–8 weeks were all purchased from experimental Animal Center of Jiangsu University (Permit No. SYXK Su 2018-0053). 4T1 orthotopic tumor model was established by subcutaneously injecting 100 μL of 4T1 cells (1 × 106 mL−1) into the left breast pad of mice. After establishing a mouse model of breast cancer, anti-tumor photothermal therapy and imaging experiments were conducted.When the tumor volume reached about 200 mm3, the mice were randomly divided into three groups (five mice in each group) to receive PPH hydrogel injection in situ (100 μL per mouse) including (1) PPH, (2) laser (1 W cm−2, 5 min), (3) PPH + laser (1 W cm−2, 5 min). The temperature changes of the tumor site were measured after 808 nm laser irradiation, and the corresponding thermal images were obtained by the infrared digital camera. The tumor growth and body weight were recorded every 2 days. The tumor volume was calculated according to the following equation: V = L × W2/2. V represented the tumor volume, L represented tumor length, W represented tumor width.
2.10 In vivo biocompatibility of PPH
The potential toxicity of PPH hydrogel was systematically studied in vivo. Fifteen healthy female Balb/c mice (6–8 weeks old) were randomly divided into 3 groups: (1) PPH, (2) laser (1 W cm−2, 5 min), (3) PPH + laser (1 W cm−2, 5 min). Twenty days after the injection, liver, spleen, kidney, heart, lung and other major organs and tumors were sliced and stained with hematoxylin and eosin.2.11 Statistical analysis
All the experimental data were expressed by the mean ± standard deviation (SD). The statistically difference analysis was investigated by one-way of ANOVA using SPSS software. In all the statistical evaluations, P < 0.05 was considered significant difference, P < 0.01 was considered highly significant difference, and P < 0.001 was considered very highly significant difference.3 Results and discussion
3.1 Preparation and characterization of PPH hydrogel
Inspired by catechol-mediated mussel adhesion, we proposed a novel promising strategy for preparation of multifunctional hydrogel. In order to synthesize PPH, Cate-PEI was firstly synthesized through grafting catechol group to the side chain of PEI via the amide reaction. Then, PPH was rapidly formed by ferric ion mediated coordination reaction and oxidative polymerization (Scheme 1a). Specifically, ferric ion not only served as oxidant to catalyze pyrrole (py) to polypyrrole (PPY), but also as a cross-linking agent to coordinate with the catechol groups on the side chain of the Cate-PEI (Scheme 1b). In addition, the interaction between the resultant PPY and catechol groups further enhanced gelation formation. In order to form the hydrogel at tumor site in situ, a double-tube injection device was adopted to control gelation behavior, which was conducive to the adequate coverage completion of photothermal ablation at the tumor area under laser irradiation. Besides, the PPH can be used as a MR contrast agent for real time imaging-guided treatment (Scheme 1c).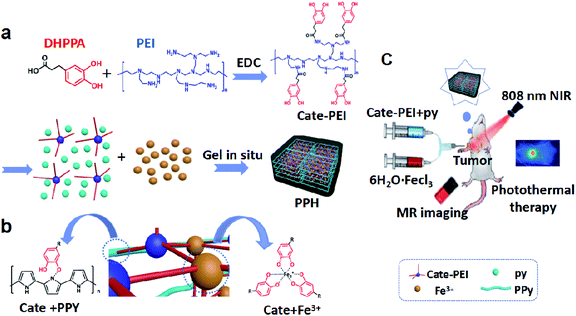 | ||
| Scheme 1 Schematic illustration of the fabrication of synthetic PPH (a). Potential molecular mechanism model of gelation (b) and its applications in vivo (c). | ||
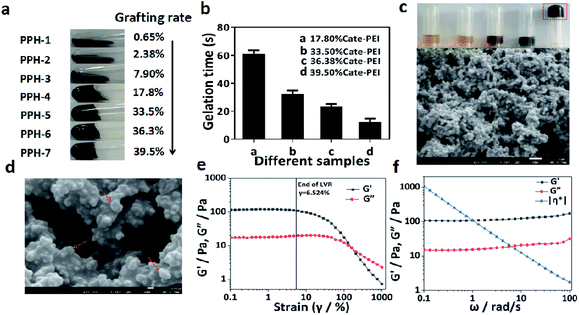
We suppose that the grafting degree of catechol on the PEI branch directly affects the formation of hydrogels. Therefore, it is important to regulate and optimize the grafting rate of catechol groups on the side chain of PEI. We choose Nuclear Magnetic Resonance spectroscopy (NMR) and UV-Vis absorption spectrum to investigate catechol grafting and measure the grafting rate. NMR results shown that the presence of the three protons peaks at about 6.5 ppm were clearly visible in the spectrum, which were corresponding with the characteristic peaks of the pure product of catechol, while the individual PEI has no peak. This data preliminarily demonstrated that catechol have been successfully grafted on PEI (Fig. 1a). Then, UV-Vis absorption spectrum further confirmed this result according to characteristic absorption peak at about 280 nm due to benzene ring (Fig. 1b).
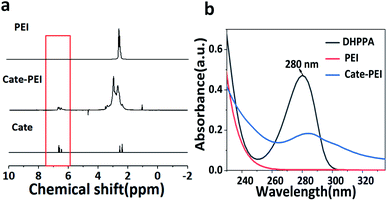 | ||
| Fig. 1 Optimization of the grafting rate of Cate-PEI. (a) 1H NMR spectra of PEI, Cate-PEI, Cate. (b) UV-Vis absorption spectrum of Cate-PEI. | ||
Next, a series of Cate-PEI with distinct grafting rate were prepared using DHPPA as catechol precursor. As Table 2 shown, the grafting rate increased gradually as the amount of DHPPA increased and the highest grafting rate reached 39.5%. The reaction products formed by Cate-PEI with different grafting rate (from 0.65%, 2.38%, 7.9%, 17.8%, 33.5%, 36.3%, 39.5%) were noted as PPH-1, PPH-2, PPH-3, PPH-4, PPH-5, PPH-6, PPH-7. The gelation time of different hydrogels was studied by vial-tilting method. Fig. 2a shown that PPH-1, PPH-2, PPH-3 could not form hydrogel at any time. Along with grafting rate increasing, PPH-4, PPH-5, PPH-6, PPH-7 exhibited solid rigidity, revealing the formation of hydrogel. The gelation time of PPH-7 with the maximum grafting rate was 10 seconds in Fig. 2b. Therefore, PPH-7 was chosen in subsequent experiments. Next, we investigated gelation performance under each ingredient (Fig. 2c). The results indicated that Cate-PEI and pyrrole, pyrrole and FeCl3, Cate-PEI and FeCl3 did not form hydrogel. In contrast, hydrogel was only formed under Cate-PEI, pyrrole and FeCl3 blending in aqueous solution. It was well known that ferric ion could easily coordinated with catechol group to form stable complex. In spite of this, ferric ion was thought to react with Cate-PEI, but the resultant complex was incapable of forming hydrogel. In view of the finding, we proposed that PPY was likely to play a critical role in metal–catechol hydrogel formation.
| ∑m | Cp | τs | ΔTMax,mix | ΔTMax,water | I (W) | 1 − 10−Aλ | η |
|---|---|---|---|---|---|---|---|
| 0.1 | 4.2 | 160.76 | 59.1 °C | 3 °C | 0.3 | 0.99 | 49.3% |
| Proportion | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.24 0.24 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) .4 .4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8 4.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
|---|---|---|---|---|---|---|---|
PEI (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) | 0.237 g (0.25 mmol) |
| DHPPA | 0.05 mmol | 0.125 mmol | 0.25 mmol | 0.5 mmol | 0.75 mmol | 1 mmol | 1.25 mmol |
| EDC | 0.06 mmol | 0.15 mmol | 0.3 mmol | 0.6 mmol | 0.9 mmol | 1.2 mmol | 1.5 mmol |
| Rate (%) | 0.65% | 2.38% | 7.9% | 17.8% | 33.5% | 36.3% | 39.5% |
After freeze-drying, the cross-sectional morphology of PPH was obtained by scanning electron microscope (SEM) analysis. PPH exhibited a three-dimensional microporous foam network similar to traditional hydrogels27 (Fig. 2c and d). A lot of spherical particles could be clearly observed in the microporous structure. This might be attributed to the formation of PPY nanoparticles in situ.
In order to study the rheological and mechanical properties of PPH, we monitored the storage (G′) and loss modulus (G′′) and complex viscosity (η*) of the hydrogel under different strains (0.1–1000%) and different angular frequencies. As shown in Fig. 2e, in the linear viscoelastic region (LVR 0.1–6.524%), the storage modulus (G′) was significantly higher than the loss modulus (G′′). When the deformation was greater than 6.524%, the storage modulus (G′) and loss modulus (G′′) both decreased, and hydrogel network structure was destroyed. In the linear viscoelastic region, as the test results of the sample modulus changing with angular frequency (Fig. 2f), it can be seen that the storage modulus (G′) of the sample was slightly higher than the loss modulus (G′′) in the entire frequency sweep range. This all confirmed the hydrogel was stable and appeared as a viscoelastic solid. The complex viscosity (η*) decreased with the increase of angular frequency. This change in mechanical behavior could be attributed to the change in crosslink density in the PPH hydrogels.
3.2 In vitro photothermal performance of PPH
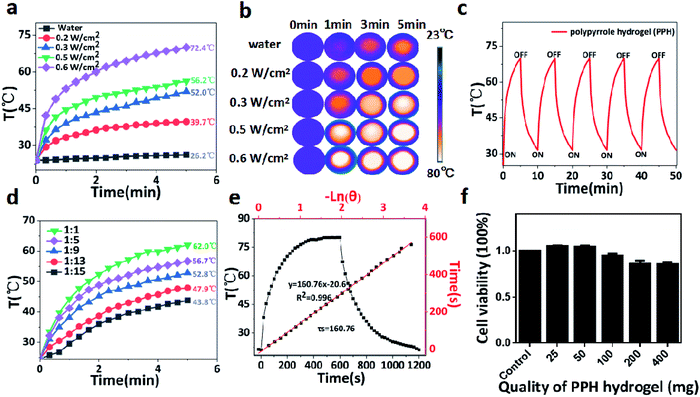
PPY has been widely used as an effective photothermal agent in tumor ablation due to its broad near infrared absorption properties. Encouraged by this property, the photothermal performance of PPH was evaluated under 808 nm laser irradiation with different output power. As shown in Fig. 3a, the PPH was exposed to 808 nm NIR irradiation for 5 minutes at different power density (0.2, 0.3, 0.5, 0.6 W cm−2). The temperature of the PPH rapidly heated above 55 °C under irradiation at a low power density of 0.5 W cm−2, but the temperature of pure water did not change significantly. The corresponding thermal images confirmed the conclusion in Fig. 3b. The result shown that the PPH as an ideal photothermal agent had a great potential in tumor ablation. In addition, the photothermal effect of PPH was relatively stable without discernible recession after five rounds of repeated laser irradiation (Fig. 3c). Then, the capability of PPH to influence the surrounding environment was further investigated to simulate the photothermal effect in tumor region after in situ injection. Briefly, 0.1 g of PPH in different proportions of distilled water was irradiated using NIR laser (808 nm, 0.6 W cm−2) for 5 min in Fig. 4d. The results indicated that PPH in each group after irradiation could lead to rapid rising of the solution temperature, even when the ratio was up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (g
15 (g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL). According to the reported method, as Table 1 shown, the photothermal conversion efficiency (η)38,39 of PPH was calculated to be around 49.3% in Fig. 4e and f, which was better than traditional photothermal preparations, including Au nanorods (21%),40 Cu9S5 nanocrystals (25.7%),41 WS2 nanosheets (32.8%).42
mL). According to the reported method, as Table 1 shown, the photothermal conversion efficiency (η)38,39 of PPH was calculated to be around 49.3% in Fig. 4e and f, which was better than traditional photothermal preparations, including Au nanorods (21%),40 Cu9S5 nanocrystals (25.7%),41 WS2 nanosheets (32.8%).42
3.3 In vitro cytotoxicity
The cytocompatibility of PPH hydrogels must be evaluated before in vivo experiments in mice. In this study, cck-8 method was used to detect cell viability. PPH hydrogel was co-incubated with MEF cells for 24 h, and cell viability was measured. As can be seen in Fig. 3f, cell viability in distinct treatment was relatively stable. Even at the highest dose of 400 mg of PPH hydrogel, the cell viability still reached 95%. These results indicated that PPH has good biocompatibility and can be applied to biomedicine.3.4 In vitro and in vivo MR imaging of PPH
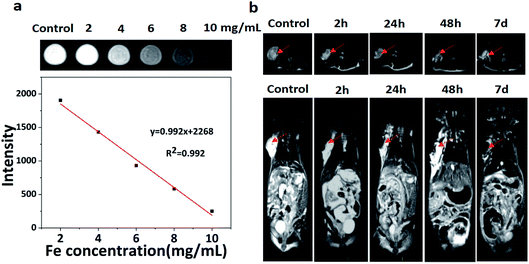
On account of the iron element doped in the PPH during gelation process, we speculated that PPH might be used as contrast agent in MRI for imaging-guided tumor ablation in vivo. PBS solution was used as a control for T2-weighted MR imaging. As shown in Fig. 4a, it can be obviously seen that the signal intensity gradually decreased with the increase of iron concentration and exhibited good concentration dependent manner. Next, we investigated whether PPH could be used as a T2-weighted contrast agent for MR imaging in breast cancer mice (Fig. 4b). PPH was injected in situ into the tumor region when the tumor volume reached 200 mm3. A black area in tumor region could be observed after PPH injection at 2 h. After 7 days, the black signal derived from PPH still remained in the tumor site, which demonstrated the excellent imaging capability of PPH in vivo. More importantly, these results indicated that PPH was conducive to persistent photothermal therapy without multiple injection. In addition, PPH could be degraded and absorbed under physical condition. Overall, PPH with excellent MR imaging performance could be used to monitor the progress of tumor treatment in real time.3.5 In vivo PTT efficacy of PPH
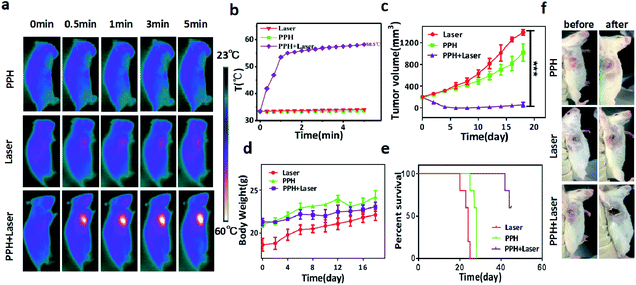
The therapeutic efficacy of PPH in vivo was evaluated in mice bearing orthotopic 4T1 tumor. When the tumor volume reached 200 mm3, 100 μL of PPH was in situ injected into the tumor and irradiated with NIR laser (808 nm, 1 W cm−2) for 5 min. The mice weight and tumor volume was measured during the following 20 days. The temperature change of the tumor was monitored and recorded by an infrared camera under the laser irradiation. In the Fig. 5a and b, it can be seen that the temperature in PPH group or laser groups hardly changed after 5 minutes, but the temperature in PPH combined laser group increased sharply up to 58.5 °C. Furthermore, the mice in PPH combined laser group showed almost complete tumor ablation in 20 days, while mice in PPH group or laser groups showed rapid tumor growth (Fig. 5c). Similarly, we can see from the mice images in Fig. 5f that tumor growth was significantly inhibited after 7 days of combined laser treatment. In addition, the mice in the three groups showed similar trends in body weight. Finally, the average life span of mice in PPH combined laser treatment could be prolonged to 45 days, but that of mice in PPH or laser group was less than 30 days (Fig. 5e). In general, these results indicated that PPH could be served as ideal PTT agent for cancer treatment. As shown in Fig. 6, there were no obvious histological abnormalities or lesions in PPH combined laser group, and the toxicity of the hydrogel in vivo is not obvious. After PPH pulse laser treatment, the tumor sections showed obvious vacuolar degeneration, indicating the presence of pathological changes associated with necrosis occurred.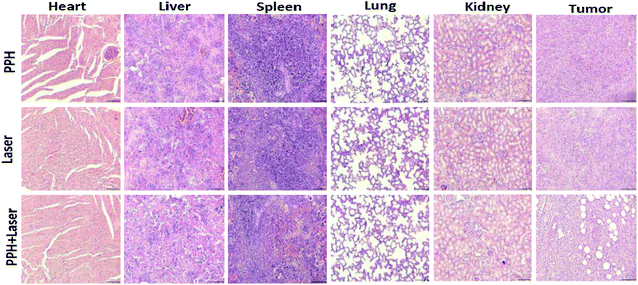 | ||
| Fig. 6 Representative micrographs of H&E stained slices after the corresponding treatment: (1) PPH; (2) laser; (3) PPH + laser. All images share the same scale bar: (200 μm) (n = 5). | ||
4 Conclusions
To sum up, we have successfully proposed a new method of injectable photothermal therapeutic PPH for MR imaging-guided tumor ablation. Inspired by mussel adhesion, catechol grafted PEI (Cate-PEI) was produced via EDC chemical reaction. Then, the PPH was in situ synthesized based on ferric ion-mediated simultaneous oxidative polymerization and metal–catechol coordination. The results demonstrated that PPH was capable of fast gelation in situ and highly photothermal conversion efficiency up to 49.3%. In addition, PPH could be used as an ideal T2-weighted contrast agent for MR imaging in vivo. Furthermore, PPH could be injected in situ into orthotopic tumor, then effectively killed tumor cells and inhibited tumor growth under NIR laser irradiation. This study provides not only a versatile photothermal therapeutic agent for tumor treatment, but also a novel strategy to design and prepare multifunctional hydrogel based on biomimetics.Conflicts of interest
All authors claim that there is no conflicts of interest.Acknowledgements
The research was supported by Natural Science Foundation of Jiangsu Province (SBK2020022937, BK20161317, BK20201418), Key Talents of Young Medicine in Jiangsu Province (QNRC2016444), Six Talent Peaks Project in Jiangsu Province (No. WSN-281), Zhenjiang Key Research and Development Plan (SH2019002, SH2019063), Major Natural Science Research Projects of Colleges and Universities in Jiangsu Province (19KJA150004).Notes and references
- G. Ou, Z. Li, D. Li, L. Chen, Z. Liu and H. Wu, Nano Res., 2016, 9, 1236–1243 CrossRef CAS.
- Y. Xu, S. Li, Y. Yang, F. Zhou, N. Dong, K. Yan, B. Wang, Y. Zeng, N. Du, X. Li and W. R. Chen, Theor. Biol. Med. Modell., 2019, 16, 1–11 CrossRef PubMed.
- Y. Guan, Hepatoma Res., 2015, 1, 159–164 CrossRef.
- A. S. Schwartz-Duval, C. J. Konopka, P. Moitra, E. A. Daza, I. Srivastava, E. V. Johnson, T. L. Kampert, S. Fayn, A. Haran, L. W. Dobrucki and D. Pan, Nat. Commun., 2020, 11, 4530 CrossRef CAS PubMed.
- K. Kumar, P. Moitra, M. Bashir, P. Kondaiah and S. Bhattacharya, Nanoscale, 2020, 12, 1067–1074 RSC.
- Z. Zhou, Y. Yan, L. Wang, Q. Zhang and Y. Cheng, Biomaterials, 2019, 203, 63–72 CrossRef CAS PubMed.
- Z. Zhou, Y. Yan, K. Hu, Y. Zou, Y. Li, R. Ma, Q. Zhang and Y. Cheng, Biomaterials, 2017, 141, 116–124 CrossRef CAS PubMed.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- X. Song, Q. Chen and Z. Liu, Nano Res., 2015, 8, 340–354 CrossRef CAS.
- Z. Zha, Z. Deng, Y. Li, C. Li, J. Wang, S. Wang, E. Qu and Z. Dai, Nanoscale, 2013, 5, 4462–4467 RSC.
- Y. Jin, Y. Li, X. Ma, Z. Zha, L. Shi, J. Tian and Z. Dai, Biomaterials, 2014, 35, 5795–5804 CrossRef CAS PubMed.
- J. Li, B. Arnal, C. W. Wei, J. Shang, T. M. Nguyen, M. O'Donnell and X. Gao, ACS Nano, 2015, 9, 1964–1976 CrossRef CAS PubMed.
- X. Chen, M. Zhang, S. Li, L. Li, L. Zhang, T. Wang, M. Yu, Z. Mou and C. Wang, J. Mater. Chem. B, 2017, 5, 1772–1778 RSC.
- Q. Wang, J. Wang, G. Lv, F. Wang, X. Zhou, J. Hu and Q. Wang, J. Mater. Sci., 2014, 49, 3484–3490 CrossRef CAS.
- Y. Yang, X. Tai, K. Shi, S. Ruan, Y. Qiu, Z. Zhang, B. Xiang and Q. He, Theranostics, 2016, 6, 2141–2160 CrossRef CAS PubMed.
- P. Gierlich, A. I. Mata, C. Donohoe, R. M. M. Brito, M. O. Senge and L. C. Gomes-da-Silva, Molecules, 2020, 25, 5317 CrossRef CAS PubMed.
- Y. Chao, L. Xu, C. Liang, L. Feng, J. Xu, Z. Dong, L. Tian, X. Yi, K. Yang and Z. Liu, Nat. Biomed. Eng., 2018, 2, 611–621 CrossRef CAS PubMed.
- Y. Li, J. Rodrigues and H. Tomas, Chem. Soc. Rev., 2012, 41, 2193–2221 RSC.
- Y. Li, D. Maciel, J. Rodrigues, X. Shi and H. Tomas, Chem. Rev., 2015, 115, 8564–8608 CrossRef CAS PubMed.
- Y. Li, D. Maciel, H. Tomas, J. Rodrigues, H. Ma and X. Shi, Soft Matter, 2011, 7, 6231–6238 RSC.
- C. Wang, J. Wang, X. Zhang, S. Yu, D. Wen, Q. Hu, Y. Ye, H. Bomba, X. Hu, Z. Liu, G. Dotti and Z. Gu, Sci. Transl. Med., 2018, 10, 3682 CrossRef PubMed.
- J. Zhao, C. Zhou, C. Wu, H. Wu, C. Zhu, C. Ye, S. Wang and D. Zou, ACS Appl. Mater. Interfaces, 2018, 10, 41947–41955 CrossRef CAS PubMed.
- L. Zhang, J. Zhou, L. Hu, X. Han, X. Zou, Q. Chen, Y. Chen, Z. Liu and C. Wang, Adv. Funct. Mater., 2020, 30, 1906922 CrossRef CAS.
- W. Shi, B. Hass, M. A. Kuss, H. Zhang, S. Ryu, D. Zhang, T. Li, Y. Li and B. Duan, Carbohydr. Polym., 2020, 233, 115803 CrossRef CAS PubMed.
- S. Bhattacharya, R. S. Phatake, S. N. Barnea, N. Zerby, J. Zhu, R. Shikler, N. G. Lemcoff and R. Jelinek, ACS Nano, 2019, 13, 1433–1442 CrossRef CAS PubMed.
- S. Liang, Y. Zhang, H. Wang, Z. Xu, J. Chen, R. Bao, B. Tan, Y. Cui, G. Fan, W. Wang, W. Wang and W. Liu, Adv. Mater., 2018, 30, 1704235 CrossRef PubMed.
- L. Zhou, L. Fan, X. Yi, Z. Zhou, C. Liu, R. Fu, C. Dai, Z. Wang, X. Chen, P. Yu, D. Chen, G. Tian, Q. Wang and C. Ning, ACS Nano, 2018, 12, 10957–10967 CrossRef CAS PubMed.
- M. Kamra, P. Moitra, D. Ponnalagu, A. A. Karande and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2019, 11, 37442–37460 CrossRef CAS PubMed.
- F. Rizzo and N. S. Kehr, Adv. Healthcare Mater., 2020, 10, 2001341 CrossRef PubMed.
- A. Andersen, M. Krogsgaard and H. Birkedal, Biomacromolecules, 2018, 19, 1402–1409 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- M. J. Sever, J. T. Weisser, J. Monahan, S. Srinivasan and J. J. Wilker, Angew. Chem., Int. Ed., 2004, 43, 448–450 CrossRef CAS PubMed.
- J. J. Wilker, Angew. Chem., Int. Ed., 2010, 49, 8076–8078 CrossRef CAS PubMed.
- Y. He, J. Chen, I. Rafique and Z. Lu, Eur. Polym. J., 2020, 139, 110025 CrossRef CAS.
- H. G. Nam, M. G. Nam and P. J. Yoo, Soft Matter, 2019, 15, 785–791 RSC.
- Y. Liu, H. Meng, S. Konst, R. Sarmiento, R. Rajachar and B. P. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 16982–16992 CrossRef CAS PubMed.
- B. R. Lin, C. H. Chen, S. Kunuku, T. Y. Chen, T. Y. Hsiao, H. Niu and C. P. Lee, Sci. Rep., 2018, 8, 7058 CrossRef PubMed.
- L. Hang, S. Gan, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef PubMed.
- W. Ren, Y. Yan, L. Zeng, Z. Shi, A. Gong, P. Schaaf, D. Wang, J. Zhao, B. Zhou, H. Yu, G. Chen, E. M. B. Brown and A. Wu, Adv. Healthcare Mater., 2015, 4, 1526–1536 CrossRef CAS PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed.
- Q. Tian, F. Jiang, R. Zou, Q. Liu, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS PubMed.
- Y. Yong, L. Zhou, Z. Gu, L. Yan, G. Tian, X. Zheng, X. Liu, X. Zhang, J. Shi, W. Cong, W. Yin and Y. Zhao, Nanoscale, 2014, 66, 10394–11040 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |