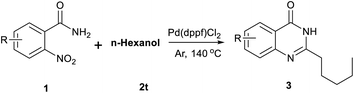
Open Access Article
Open Access ArticlePalladium-catalyzed one-pot synthesis of 2-substituted quinazolin-4(3H)-ones from o-nitrobenzamide and alcohols†
Ke Wang ,
Hao Chen,
Xinyan Dai,
Xupeng Huang and
Zhiqiang Feng*
,
Hao Chen,
Xinyan Dai,
Xupeng Huang and
Zhiqiang Feng*
Beijing Key Laboratory of Active Substance Discovery and Drugability Evaluation, Institute of Materia Medica, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 1 Xiannongtan Street, Beijing 100050, P R China. E-mail: fengzhq@imm.ac.cn
First published on 7th April 2021
Abstract
Palladium-catalyzed 2-substituted quinazolin-4(3H)-one formation from readily available o-nitrobenzamides and alcohols using hydrogen transfer is described. Various quinazolin-4(3H)-ones were obtained in good to high yields. The cascade reaction including alcohol oxidation, nitro reduction, condensation, and dehydrogenation occurs without any added reducing or oxidizing agent.
The quinazolinones and their derivatives are a family of privileged heterocyclic structures commonly found in a wide variety of natural products, biologically active molecules, and functional organic materials.1 Due to quinazolinone's important value, various conventional methods have been developed for the synthesis of these molecules in both academic and industrial settings.2 The most common approaches utilize 2-aminobenzoic acid and its derivatives as the starting materials along with aldehydes,3 acyl chlorides or their analogues4 to obtain the corresponding structural motifs. These methods usually have the disadvantages of multistep reactions,5 low yields,6 long reaction times,7 and the use of stoichiometric amounts of strong or toxic oxidants.8
In the past decade, direct coupling methods of C–N bonds9 through which o-aminobenzamides react with benzylic alcohols by metal-catalyzed cascade reactions have been recognized as an attractive methods for the synthesis of 2-substituted quinazolinone derivatives. For example, Yokoyama and coworkers reported the synthesis of quinazolin-4(3H)-ones via a palladium-catalyzed domino reaction of o-aminobenzamides with benzylic alcohols.10 Wang et al. also reported the one-pot oxidative cyclization of o-aminobenzamide with alcohols to quinazolin-4(3H)-ones catalyzed by MnO2 under hydrogen transfer conditions.11 The groups of Watson,12 Paul,13 and some others14 also independently reported the Ru-, Ni-, and Cu-catalyzed synthesis of quinazolin-4(3H)-ones via the acceptorless dehydrogenative coupling of o-aminobenzamides with alcohols. Considering that aniline is prepared from the corresponding nitroarenes,15 and that nitroarenes are cheaper and commercially available, the direct use of nitroarenes as amino sources for quinazolinone synthesis features their atom-economic advantage over anilines. However, methodologies for the synthesis quinazolinone derivatives via the acceptorless dehydrogenative coupling of nitroarenes with alcohols are relatively scarce.16
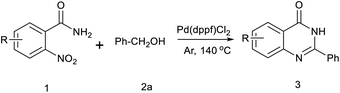
Herein, we report a new approach for directly synthesizing 2-substituted quinazolin-4(3H)-ones from o-nitrobenzamides with alcohols via a hydrogen transfer methodology.17
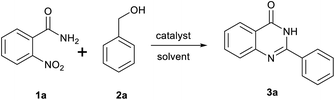
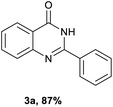
Initially, a model reaction of o-nitrobenzamide (1a) and benzyl alcohol (2a) was chosen to optimize the reaction conditions (Table 1). We carried out the reaction of o-nitrobenzamide with 2.5 equivalents alcohol in toluene at 150 °C and an argon atmosphere for 8 h. As shown in Table 1, various catalysts were screened for the reaction. No or poor yield was observed in the presence of either a copper or an iron catalyst (Table 1, entries 1–7). With regard to the palladium catalyst (Table 1, entries 8–10), Pd(dppf)Cl2 showed the best activity, and 3a was obtained in 59% yield (Table 1, entry 10). Several solvents such as DMF, DMSO, and chlorobenzene were screened, and chlorobenzene was found to be the optimal solvent (Table 1, compared entries 11–13), the yield of 3a increased to 80% (Table 1, entry 13). The impact of reaction temperatures was also investigated. The reaction conducted at 140 °C resulted in a higher yield (87%) than that conducted at 150 °C (Table 1, entry 14). But the reaction performed at 130 °C resulted in a lower 3a yield of to 80% (Table 1, entry 15). When the reaction time was reduced to 5 h, the yield of 3a was slightly reduced to 79% (Table 1, entry 17), but the yield did not increase with increasing of reaction time to 10 h. When the amount of catalyst was decreased from 0.1 to 0.05 equiv., the yield of 3a decreased to 78% (Table 1, entry 17). However, 0.15 equiv. of catalyst did not change the yield of the product (Table 1, entry 18). A slightly lower yield was obtained when the reaction was carried out in air (80%) (Table 1, entry 19).
| Entry | Catalyst | Solvent | t (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 mmol of 1a, 2.5 equiv. of 2a, catalyst and 1 mL of solvent in a 10 mL sealed tube at 150 °C under Ar for 8 h. Yield of the isolated product based on 1a.b At 140 °C under Ar for 8 h.c At 130 °C under Ar for 8 h.d At 140 °C under Ar for 5 h.e Pd (dppf) Cl2, 5%.f Pd (dppf) Cl2, 15%.g At 140 °C under air for 8 h. | ||||
| 1 | CuCl | Toluene | 8 | 0 |
| 2 | CuCl2 | Toluene | 8 | 0 |
| 3 | dppf | Toluene | 8 | 28 |
| 4 | FeCl3 | Toluene | 8 | 22 |
| 5 | Cu(OAc)2 | Toluene | 8 | 0 |
| 6 | CuBr2 | Toluene | 8 | Trace |
| 7 | CuBr | Toluene | 8 | Trace |
| 8 | PdCl2 | Toluene | 8 | 14 |
| 9 | Pd(OAc)2 | Toluene | 8 | 42 |
| 10 | Pd(dppf)Cl2 | Toluene | 8 | 59 |
| 11 | Pd(dppf)Cl2 | DMF | 8 | 77 |
| 12 | Pd(dppf)Cl2 | DMSO | 8 | 56 |
| 13 | Pd(dppf)Cl2 | PhCl | 8 | 80 |
| 14 | Pd(dppf)Cl2b | PhCl | 8 | 87 |
| 15 | Pd(dppf)Cl2c | PhCl | 8 | 80 |
| 16 | Pd(dppf)Cl2d | PhCl | 5 | 79 |
| 17 | Pd(dppf)Cl2e | PhCl | 8 | 78 |
| 18 | Pd(dppf)Cl2f | PhCl | 8 | 87 |
| 19 | Pd(dppf)Cl2g | PhCl | 8 | 80 |
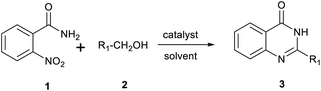
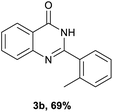
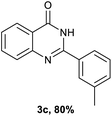
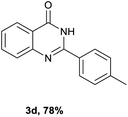
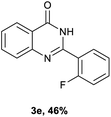
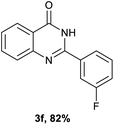
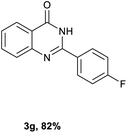
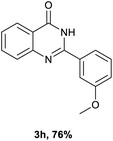
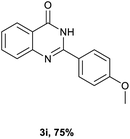
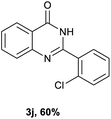
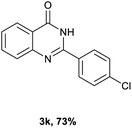
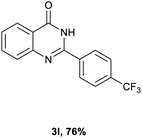
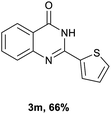
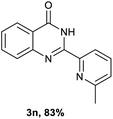
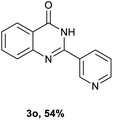
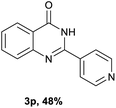
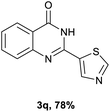
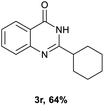
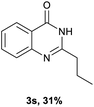
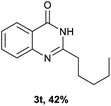
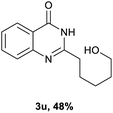
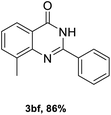
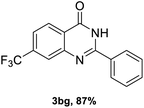
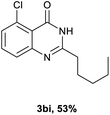
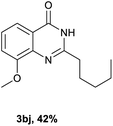
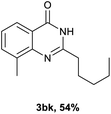
After optimized reaction conditions (Pd(dppf)Cl2, chlorobenzene, 140 °C, argon) were established, the reaction scope and generality of various primary alcohols were tested, as shown in Table 2. The position and electronic effect of substituents on the phenyl ring of benzyl alcohols was examined. A series of benzyl alcohols with electron-donating groups (Me and MeO) could be converted to the desired products in good yields. Benzyl alcohols with methyl substituents at ortho-, meta-, or para-positions (Table 2, 3b–d) produced the corresponding quinazolin-4(3H)-ones in 69%, 80% and 78% isolated yields. The lower yield of the o-methylbenzyl alcohol may be due to the steric effect of ortho groups on the substrate. Benzyl alcohols having methoxyl substituents at meta- or para-positions also produced the corresponding quinazolin-4(3H)-ones in 76% and 75% isolated yields. Halogen substituents on the benzylic alcohols were also well tolerated in the reaction. Fluorine and chlorine substituted at the meta- and para-positions of substrates were applied to the synthesis of the corresponding quinazolin-4(3H)-ones in good yields (Table 2, 3f, 3g and 3k). Similarly, lower yields were observed using o-fluorobenzyl alcohol and o-chlorobenzyl alcohol as coupling partners (46% and 60%) (Table 2, 3e and 3j). A strong electron-withdrawing group such as CF3 was also found to be compatible affording quinazolin-4(3H)-ones in good yield (76%) (Table 2, 3l). Starting from 6-methyl-2-pyridinemethanol, 2-thiophenemethanol and thiazole-5-methanol, the corresponding quinazolin-4(3H)-ones were obtained in 83%, 66% and 78% isolated yields. However, lower yields (54% and 48%) (Table 2, 3o and 3p) were observed using 3-pyridinemethanol and 4-pyridinemethanol as coupling partners. Aliphatic alcohols were also tested, providing the corresponding products in moderate to good yields. The reaction of cyclohexyl methanol with 2-nitrobenzamide afforded the corresponding quinazolin-4(3H)-ones in 64% isolated yield (Table 2, 3r). 1-Butanol and 1-hexanol afforded the corresponding quinazolin-4(3H)-ones (3s and 3t) in moderate yields of 31% and 42%. Notably, 1,6-hexanediol was also smoothly coupled with o-nitrobenzamide 1a and afforded 3u in 48% yield.
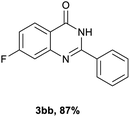
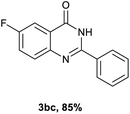
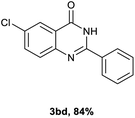
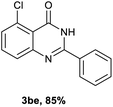
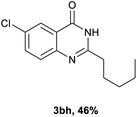
Next, substituent effects on the o-nitrobenzamide were also evaluated. Not surprisingly, o-nitrobenzamide with either electron-donating or electron-withdrawing groups could be successfully coupled with benzyl alcohol 2a to provide the corresponding products in high yields (84–87%) (Table 3, 3bb–3bg). The yield of the product was not affected by the o-nitrobenzamide with substituents at the 3 or 6 positions (Table 3, 3be–3bf). In addition to benzylic alcohols, long-chain aliphatic alcohols such as 1-hexanol also yielded the corresponding quinazolin-4(3H)-one in moderate to good yields (42–54%) (Table 4, 3bh–3bk).
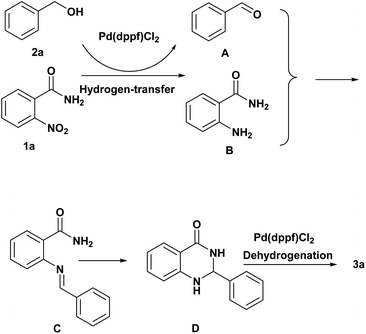
Based on the above experimental results along with the literature,17 we proposed possible mechanisms of the reaction (Scheme 1). The reaction involved the oxidation of the alcohol to aldehyde (A) and the generation of hydrogen in the presence of Pd(dppf)Cl2, and the hydrogen will reduce 2-nitrobenzamide to 2-aminobenzamide (B) via a hydrogen-transfer process. One equivalent of 2-aminobenzamide (B) condenses with the aldehyde (A) and an intermediate that easily dehydrates to yield the imine (C). Then, the nitrogen atom of the amine attacked the imine to generate dehydrogenated quinazolinone (D). Finally, dehydrogenation of D can achieve the desired product 3a via a hydrogen-transfer process.
Conclusions
We have developed a strategy for Pd(dppf)Cl2-catalyzed quinazolin-4(3H)-one formation from o-nitrobenzamides and alcohols via the hydrogen-transfer. Functional groups such as halogen, methoxy, methyl and hydroxyl groups were all well tolerated under the optimized reaction conditions. A wide variety of desired quinazolin-4(3H)-ones were obtained in moderate to high yields starting from inexpensive and easily available nitrobenzamides and alcohols. Further studies on the synthetic applications of this reaction are under investigation in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Beijing Municipal Natural Science Foundation (7192133), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT35003) and CAMS Innovation Fund for Medical Sciences (CIFMS) (2017-I2M-1-010).Notes and references
- (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787 CrossRef CAS; (b) I. Khan, A. Ibrar, N. Abbas and A. Saeed, Eur. J. Med. Chem., 2014, 76, 193–244 CrossRef CAS PubMed; (c) T. Gupta, A. Rohilla, A. Pathak, M. J. Akhtar, M. R. Haider and M. S. Yar, Synth. Commun., 2018, 48, 1099 CrossRef CAS; (d) U. A. Kshirsagar, Org. Biomol. Chem., 2015, 13, 9336 RSC.
- (a) D. J. Connolly, D. Cusack, T. P. O'Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS; (b) A. Witt and J. Bergman, Curr. Org. Chem., 2003, 7, 659 CrossRef CAS; (c) Z. Ma, T. Song, Y. Yuan and Y. Yang, Chem. Sci., 2019, 10, 10283 RSC; (d) T. B. Nguyen, J. L. Bescont, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 6218 CrossRef CAS PubMed; (e) M. Kumar, Richa, S. Sharma, V. Bhatt and N. Kumar, Adv. Synth. Catal., 2015, 357, 2862 CrossRef CAS; (f) F. Hu, X. Cui, Z. Ban, G. Lu, N. Luo and G. Huang, Org. Biomol. Chem., 2019, 17, 2356 RSC.
- (a) X. Chen, T. Chen, Y. Zhou, D. Han, L. B. Han and S. F. Yin, Org. Biomol. Chem., 2014, 23, 3802 RSC; (b) I. Yoshio, S. Sadayuki, T. Ryuichi and U. Mitsuru, Synthesis, 1981, 1, 35 Search PubMed; (c) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730 CrossRef CAS PubMed; (d) W. Ge, X. Zhu and Y. Wei, RSC Adv., 2013, 3, 10817 RSC; (e) Z. Ma, T. Song, Y. Yuan and Y. Yang, Chem. Sci., 2019, 10, 10283 RSC; (f) J. K. Laha, K. V. Patel, T. Satanarayana and N. Dayal, Chem. Commun., 2016, 52, 10245 RSC.
- (a) T. M. Potewar, R. N. Nadaf, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Synth. Commun., 2005, 35, 231 CrossRef CAS; (b) J. Li, W. Wang, X. Su, X. Zhang, Y. Zhang, X. Zhang, M. Cai, Y. Cao, J. Jin and Y. Xu, Heterocycles, 2018, 96, 2107 CrossRef CAS.
- (a) B. Q. Hu, L. X. Wang, Y. Luo, J. F. Xiang and Y. L. Yang, Eur. J. Org. Chem., 2015, 2015, 4504 CrossRef CAS; (b) W. Zhao, P. Liu and F. Li, ChemCatChem, 2016, 8, 1523 CrossRef CAS; (c) L. Long, Y. H. Wang, J. X. Zhuo, Z. C. Tu, R. Wu, M. Yan, Q. Liu and G. Liu, Eur. J. Med. Chem., 2018, 157, 1361 CrossRef CAS PubMed.
- S. M. Patel, H. Chada, S. Biswal, S. Sharma and D. S. Sharada, Synthesis, 2019, 51, 3160 CrossRef CAS.
- Y. Jang, S. B. Lee, J. Hong, S. Chun, J. Lee and S. Hong, Org. Biomol. Chem., 2020, 18, 5435 RSC.
- (a) J. Li, W. Wang, X. Su, X. Zhang, Y. Zhang, X. Zhang, M. Cai, Y. Cao, J. Jin and Y. Xu, Heterocycles, 2018, 96, 2107 CrossRef CAS; (b) D. Zhan, T. Li, X. Zhang, C. Dai, H. Wei, Y. Zhang and Q. Zeng, Synth. Commun., 2013, 43, 2493 CrossRef CAS.
- (a) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS PubMed; (b) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed.
- H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046 CrossRef CAS PubMed.
- Z. Zhang, M. Wang, C. Zhang, Z. Zhang, J. Lu and F. Wang, Chem. Commun., 2015, 51, 9205 RSC.
- A. A. Watson, A. C. Maxwell and J. M. Williams, Org. Biomol. Chem., 2012, 10, 240 RSC.
- (a) S. Parua, S. Das, R. Sikari, S. Sinha and N. D. Paul, J. Org. Chem., 2017, 82, 7165 CrossRef CAS PubMed; (b) S. Das, S. Sinha, D. Samanta, R. Mondal, G. Chakraborty, P. Brandao and N. D. Paul, J. Org. Chem., 2019, 84, 10160 CrossRef CAS PubMed.
- (a) R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475 CrossRef CAS; (b) N. Montazeri, K. Pourshamsian, S. Yosefiyan and S. S. Momeni, J. Chem. Sci., 2012, 124, 883 CrossRef CAS; (c) A. Davoodnia, S. Allameh, A. R. Fakhari and N. Tavakoli-Hoseini, Chin. Chem. Lett., 2010, 21, 550 CrossRef CAS.
- (a) H. P. Hemantha and V. V. Sureshbabu, Org. Biomol. Chem., 2011, 9, 2597 RSC; (b) C. Yu, B. Liu and L. Hu, J. Org. Chem., 2001, 66, 919 CrossRef CAS PubMed; (c) Y. Zhou, H. Zhou, S. Liu, D. Pi and G. Shen, Tetrahedron, 2017, 73, 27 Search PubMed.
- (a) A. H. Romero, J. Salazar and S. E. Lopez, Synthesis, 2013, 45, 2043 CrossRef CAS; (b) H. Wang, X. Cao, F. Xiao, S. Liu and G. J. Deng, Org. Lett., 2013, 15, 4900 CrossRef CAS PubMed.
- (a) T. Suzuki, Chem. Rev., 2011, 111, 1825 CrossRef CAS PubMed; (b) G. Guillena, D. J. Ramon and M. Yus, Chem. Rev., 2010, 110, 1611 CrossRef CAS PubMed; (c) Y. Obora, Top. Curr. Chem., 2016, 374, 11 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01755a |
| This journal is © The Royal Society of Chemistry 2021 |