Open Access Article
Open Access ArticleSolid phase extraction based on trimethylsilyloxy silica aerogel†
V. Lakshmi Prasanna ,
Igal Gozlan,
Aviv Kaplan,
Daniel Zachor-Movshovitz and
Dror Avisar
,
Igal Gozlan,
Aviv Kaplan,
Daniel Zachor-Movshovitz and
Dror Avisar *
*
The Water Research Center, The Hydro-Chemistry Laboratory, Porter School for Environment and Earth Sciences, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel Aviv University, Tel Aviv 69978, Israel. E-mail: droravi@tauex.tau.ac.il
First published on 24th May 2021
Abstract
Solid-phase extraction (SPE) based on trimethylsilyloxy-modified silica aerogel was developed for extraction of chemotherapeutic drugs from water. The developed method is easy and affordable, can be performed in separating funnel and does not require a vacuum and SPE manifold. The extraction and recovery of cyclophosphamide (CYP), dexamethasone (DEX), and paclitaxel (TAX) by the aerogel from water were investigated. The factors governing the extraction efficiency such as sample pH, sample volume, volume of eluent and concentration of analytes were studied. The LOD and LOQ of the developed method were calculated and linearity was found in the range of 4–100 μg L−1. The extraction efficiency of the aerogel was compared to that of other SPE cartridges, Oasis HLB, Strata-X-C, C18 and polymeric reversed phase, and the aerogel showed similar or better performance than the other commercial cartridges available on the market. The developed method was also used to extract chemotherapeutic drugs spiked in hospital wastewater.
1. Introduction
Due to an overall rise in cancer, there has been an upsurge in the production and consumption of chemotherapeutic drugs (CDs); 30 to 90% of the consumed anticancer drugs will not be metabolized by the body and will be excreted through the patients' urine and excreta. These chemotherapeutic drugs end up in municipal wastewater-treatment plants where the methods applied are not effective at degrading these compounds.1 The effluent from wastewater treatment plants contains CDs which are transported to surface and ground water.2 In addition to consumed drugs, expired chemotherapeutic drugs that are not properly disposed of also end up in surface and ground water via wastewater treatment plants. aus der Beek et al.3 surveyed 71 countries and found 631 different pharmaceuticals in a range of 0.003 to 520 μg L−1 in the environment. Rowney et al.4 reported that CDs such as oxaliplatin, cisplatin, carboplatin, cyclophosphamide, doxorubicin and fluorouracil are present in the Thames River, UK, at nanogram per litre concentrations. Ferrando-Climent et al.5 quantified 11 anticancer drugs in urban water cycles—from hospital effluents through urban wastewater treatment plants to surface water. CDs are mutagenic, cytotoxic and endocrine disruptors; identifying their presence is therefore essential.6Various techniques such as solid-phase extraction (SPE), liquid–liquid extraction, liquid-phase microextraction and dispersive liquid–liquid microextraction have been employed to extract pharmaceuticals from water.7–9 Compared to other techniques, SPE is widely employed to minimise the use of organic solvents, improve high throughput with automation, provide exhaustive sample extraction, and concentrate analytes at trace and ultra-trace levels quantification.8–11 Owing to their high surface area, aerogels demonstrate more efficient adsorption of analytes than their micro and nano counterparts.12 Aerogels based on carbon and silica have been used to extract pharmaceuticals, metal ions and pesticides.15–17 Due to their high porosity, surface area, mechanical strength, temperature resistance and number of active adsorption sites, silica aerogels offer a promising material for SPE.12,13 Amine-functionalized silica aerogel was used for separation and pre-concentration of Pb(II) ions in water.14 A copper wire coated with silica aerogel was used for microextraction of chlorobenzenes.15 Silica aerogel was coated on stainless-steel wires to develop an in-tube solid-phase microextraction device for extraction of polycyclic aromatic hydrocarbons.16
The surface of unmodified silica aerogel is polar on which only polar molecules are adsorbed on the aerogel. In order to adsorb non-polar molecules the hydroxyl group of the silanol group has to be modified with non-polar molecules such as trimethylsilyl, tetramethylsilylsilyl, tetraethylsilyl groups.17–21 The aerogel under study with high surface are (1113.4 m2 g−1), open pore network through which the inner pore also can be assessed for increasing the adsorption capacity. In present work trimethylsilyloxy-modified silica aerogel, was used to extract CDs from water and hospital wastewater. This aerogel configuration has already been shown to remove micropollutants from drinking water and leachates.18 In the present study, this aerogel was used as a sorbent in SPE to extract the CDs cyclophosphamide (CYP), paclitaxel (TAX) and dexamethasone (DEX) from water and wastewater samples. The aerogel's performance was compared with those of the commercial SPE cartridges Oasis HLB, and Strata-X-C, C18 and Polymeric Reversed Phase.
2. Materials and methods
2.1 Chemicals
Lumira LA1000 trimethylsilyloxy-modified (TMSO) silica aerogel was from Cabot (Boston, MA). CYP (C7H15Cl2N2O2P·H2O), 97%, standard, and DEX (C22H29FO5), 96%, were from Acros Organics (Fair Lawn, NJ), TAX (C47H51NO14), 99%, standard, was from Fermentek Ltd (Jerusalem, Israel), and methanol (CH3OH), ultrapure LC-MS, and formic acid (CHOOH), 99%, were from Bio-Lab Ltd (Jerusalem, Israel). The cartridges Oasis HLB, Strata-X-C, Strata-X 33 μm Polymeric Reversed Phase, and Strata C18 were from Phenomenex (Torrance, CA).2.2 Material characterization
Brunauer–Emmet–Teller (BET) surface area, pore volume and radius were determined by N2 adsorption–desorption isotherms collected at 77 K using a Quantachrome instrument (Anton Paar, Graz, Austria). Prior to experiments, the samples were degassed at 393.15 K for 12 h. Pore structure parameters such as specific surface area, pore volume were calculated based on nonlocal density functional theory (NLDFT) method. The Brunauer–Emmett–Teller (BET) method was applied to calculate the specific surface area using N2 adsorption data in a relative pressure range from 0.05 to 0.25. The total pore volumes were estimated according to N2 uptake at a relative pressure (P/P0) of 0.99.2.3 LC-MS analysis
CYP, DEX and TAX were analysed by high-pressure liquid chromatography (HPLC, Agilent 1100) equipped with an ACE 5 phenyl column, 250 × 2.1 mm, 5 μm particle size and column temperature of 50 °C. For the mobile phase, solution A consisted of 0.1% formic acid in deionized (DI) water and solution B was 0.1% formic acid in methanol (MeOH). The composition of the mobile phase was 95% A for 5 min, 30% A at 15 min followed by 95% A at 20 min; injection volume was 10 μL and flow rate was 0.5 mL min−1. Detection and quantification were performed in a high-resolution Q-TOF Premier (Waters) mass spectrometer (MS) via an ESI interface in positive mode. Data acquisition and evaluation were performed using Waters chromatography MassLynx 4.1 software. CDs were identified according to their retention time and exact mass [M + H]+.3. Experimental section
3.1 Extraction of CDs by TMSO modified silica aerogel
An important step in SPE is the adsorption of analytes to the adsorbent. In this step, the adsorption of CYP, DEX and TAX was investigated from DI water. The aerogel (500 ± 30 mg) was placed in a separating funnel, conditioned with 5 mL of MeOH and equilibrated with 10 mL of DI water. Then 50 μg L−1 each of CYP, DEX and TAX in 10 mL DI water was added to the aerogel and the solution was eluted after 15 min. The percentage of CDs adsorbed to the aerogel was calculated by analyzing the solution prior to and after adsorption to the aerogel using HPLC-MS. Absolute MeOH was used to extract the adsorbed CDs from the aerogel: 6 mL of absolute MeOH was added to the aerogel each time and eluted after 15 min. The protocol for extraction of drugs were detailed in Fig. S1.† The quantity of CDs eluted at each step was analyzed by HPLC-MS. Fig. S2† shows aerogel before and after conditioning with MeOH.3.2 Effect of sample volume on adsorption and recovery of CDs from TMSO modified silica aerogel
After conditioning the aerogel with MeOH and water as described in Section 3.1, 50 μg L−1 of each analyte in different sample volumes (5, 25, 50, 75 and 100 mL) was added to the aerogel in the separating funnel, and adsorption and recovery of CDs were analyzed by HPLC-MS.3.3 Reusability of TMSO modified silica aerogel for adsorption and recovery of CDs
Reusability of the aerogel was studied for adsorption and recovery of CDs from tap water. After activation with MeOH and equilibration with water, 25 mL of a 50 μg L−1 mixture of CDs was added to the aerogel and the solution was eluted after 15 min. The analytes were recovered by MeOH as described in Section 3.1. After complete recovery of the CDs, the aerogel was again conditioned with MeOH and water. An additional 25 mL of the 50 μg L−1 mixture of CDs was added to the aerogel and recovered with MeOH. The procedure was repeated 11 times with no loss of aerogel efficiency.3.4 Comparison of TMSO modified silica aerogel to commercial SPE cartridges
The adsorption and recovery efficiency of the aerogel was compared to that of the commercial SPE cartridges Oasis HLB, Strata-X-C, Strata C18 and Strata Polymeric Reversed Phase. The detailed experimental procedure is given in the ESI.†3.5 Adsorption and recovery of drugs from hospital wastewater
Hospital wastewater was collected from Tel-Hashomer hospital in Israel and centrifuged to remove suspended solids. The supernatant was spiked with CDs to achieve a concentration of 10–100 μg L−1 and adsorption and recovery of CDs were analysed as described in Section 3.1.4. Results and discussion
4.1 Material characterization
X-Ray diffraction (XRD) and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) of the aerogel are detailed in our earlier work.22 Briefly, XRD showed that the aerogel is composed of amorphous silica with a broad peak at 2Θ = 22–24 Å.22,23 FTIR showed the presence of Si–O and Si–CH3 vibrations corresponding to SiO2, and trimethylsilyl groups on the surface of the aerogel.22,24,25 This confirmed that the aerogel used in this study is a trimethylsilyloxy-modified silica aerogel. BET surface area was calculated by N2 adsorption isotherm was found to be 1113.41 m2 g−1, pore volume and radius was calculated to be 3.09 cm3 g−1 and 3.12 nm respectively.4.2 Optimization of the SPE procedure
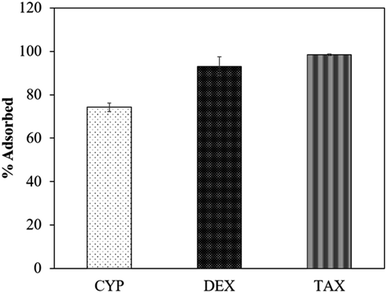
The partition time between the aerogel and the water matrix is essential in the adsorption of analytes (the kinetics of percent adsorption of TAX, 100 mL of 50 μg L−1, to the aerogel over time is shown in Fig. S3†). Time-dependent adsorption of TAX was observed for 15 min, after which no change in adsorption was seen, implying that 15 min is essential for complete adsorption of CDs on the aerogel. Hence, 15 min was kept constant throughout the experiments. The percent adsorption of CYP, DEX and TAX on the aerogel was 72.6, 93.0 and 98.4, respectively (Fig. 1). LC-MS of unadsorbed and recovered chemotherapeutic drugs are shown in Fig. S4.†This trend can be explained by the physiochemical properties of the aerogel and CDs. The aerogel used in this work is hydrophobic, owing to the trimethylsilyloxy coating on its surface. However, not all of the silica (Si) groups will be modified by trimethylsilyl groups, and unmodified silica groups will end up with –OH groups (silanols), which are polar (Fig. S5†). CYP is a relatively polar compound26 and it interacts with the Si–OH group on the silica aerogel, whereas DEX and TAX have both polar and nonpolar groups27,28 which can interact with Si–OH and trimethylsilyloxy groups on the aerogel, respectively. However, lypophilic interaction on the surface of aerogel with molecules (chemotherapeutic drugs) plays the dominant factor.
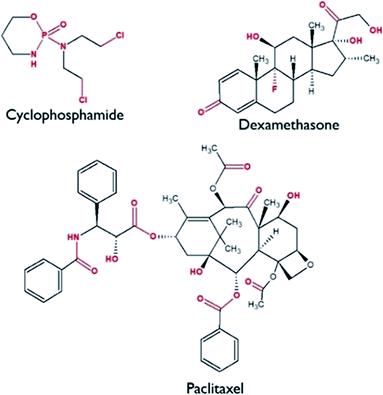
Fig. 2 shows the molecular structures of the CDs, with polar (high dipole bonds, marked in red) and non-polar groups (marked in black). Log P of CYP, DEX and TAX is 0.63, 1.92 and 3.18, respectively (ACDLABS, ACD Persepta ver.2019), and the hydrophobicity of the selected analytes follows the order TAX > DEX > CYP (Table S1†). The rate of adsorption and hydrophobicity follow the same trend, suggesting that hydrophobic interactions have a major role in adsorption of CDs by TMSO modified silica aerogel.29
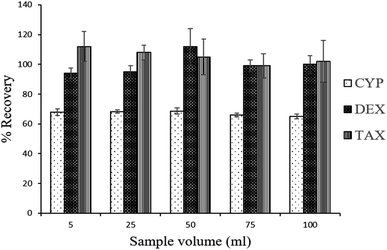
To understand the role of electrostatic interactions on the adsorption of CDs to the aerogel, sample pH was varied from 3, 7 and 8. There were no changes in the rate of adsorption (Fig. S6†), indicating that electrostatic interactions do not play an important role in the process owing to the physiochemical properties of the analytes. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D of CYP, DEX and TAX remained the same at pH 3–8 (Fig. S7†), implying that the compounds are not ionized and hence no change in adsorption or recovery is anticipated. Recoveries of CDs with different amounts of MeOH as eluent are demonstrated in Fig. 3. The highest recovery values were obtained using 18 mL MeOH to extract CDs adsorbed on the aerogel. A higher volume of MeOH as eluent did not improve recovery rates. To investigate the enrichment of trace amounts of CDs on the aerogel from large sample volumes, the adsorption and recovery of CDs with sample volumes ranging from 5 to 100 mL, keeping the concentration constant, were studied. Satisfactory recoveries were observed up to 100 mL, and an enrichment factor of up to 100 was therefore achieved by this method30 (Fig. 4).
D of CYP, DEX and TAX remained the same at pH 3–8 (Fig. S7†), implying that the compounds are not ionized and hence no change in adsorption or recovery is anticipated. Recoveries of CDs with different amounts of MeOH as eluent are demonstrated in Fig. 3. The highest recovery values were obtained using 18 mL MeOH to extract CDs adsorbed on the aerogel. A higher volume of MeOH as eluent did not improve recovery rates. To investigate the enrichment of trace amounts of CDs on the aerogel from large sample volumes, the adsorption and recovery of CDs with sample volumes ranging from 5 to 100 mL, keeping the concentration constant, were studied. Satisfactory recoveries were observed up to 100 mL, and an enrichment factor of up to 100 was therefore achieved by this method30 (Fig. 4).
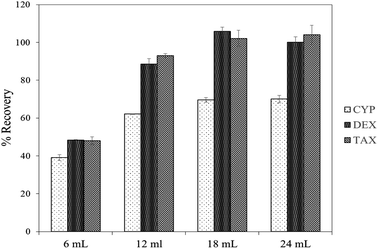 | ||
| Fig. 3 Effect of eluent volume on recovery of chemotherapeutic drugs from TMSO modified silica aerogel. | ||
4.3 Method validation for analysis of CDs
Linearity, limits of detection and quantification (LOD and LOQ, respectively), and repeatability were studied to validate the method. The linear range was calculated according to: y = ax + b, where y is the area of the peak, a is the slope, b is the point intercepting the y axis and x is the analyte concentration.The linearity range of the three CDs was studied between 4 and 100 μg L−1. LOD was determined by calculating the sum of the parameters ā (average slope) and b (interception point) for three calibration curves:31
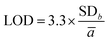 | (1) |
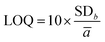 | (2) |
Calibration curves were plotted with area of the peak on the y axis against concentration of the analyte on the x axis. The parameters for validating the method are given in Table 1. A satisfactory regression coefficient (R2 of 0.992–0.998 for all analytes) indicated good linearity in the studied range. The linearity obtained for all three compounds by this method was in the range of 4 to 100 μg L−1. The precision of the method was assessed by performing each analysis in triplicate. The Relative Standard Deviation (RSD) showed the reusability of the developed method for the identification of chemotherapeutic drugs in water.
| Drug | Linear range (μg L−1) | R2 | LOD (μg L−1) | LOQ (μg L−1) | RSD (n = 3) |
|---|---|---|---|---|---|
| CYP | 4–100 | 0.998 | 2.45 | 7.42 | 4.65 |
| DEX | 4–100 | 0.998 | 0.88 | 2.664 | 4.92 |
| TAX | 4–100 | 0.999 | 3.37 | 10 | 4.18 |
4.4 Comparison of TMSO modified silica aerogel to commercial SPE cartridges
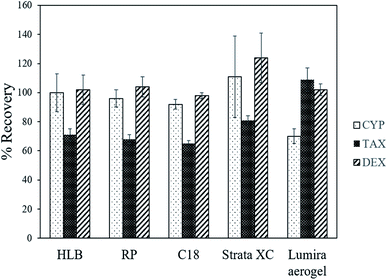
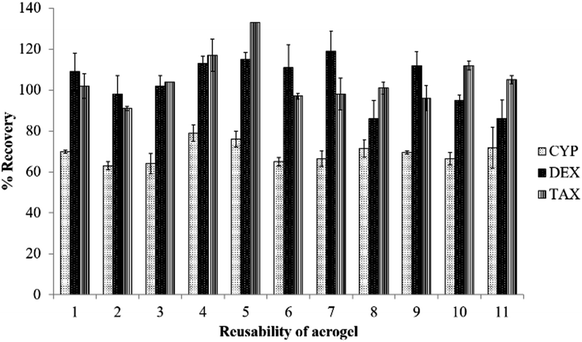
Fig. 5 compares CD recovery by the aerogel to that by some commercial SPE cartridges. Recovery of TAX and DEX by the aerogel was comparable to or better than the studied commercial cartridges (Table S2†). However, the aerogel was relatively less efficient at recovering CYP compared to these commercial cartridges. The advantage of the TMSO modified silica aerogel over commercial SPE cartridges is that no vacuum manifold or pump are required in the process, because the sample is in contact with the aerogel and later removed by gravity alone.Fig. 6 shows the recyclability of the aerogel's recovery of CDs from water. There was no loss of extraction efficiency by the aerogel, even after 11 cycles, implying good reusability of the aerogel for the recovery of CDs from water. The TMSO modified silica aerogel is thus reusable and relatively less expensive than the commercial SPE cartridges. The main disadvantage of the developed aerogel extraction method is that it is time-consuming, as it requires 15 min for adsorption on the aerogel, unlike commercial SPE, which is immediate. After extraction of drugs from the aerogel it is washed with water, air dried at room temperature and can be keep in a sealed container for further use.
4.5 Extraction of CDs from hospital wastewater by TMSO modified silica aerogel
To investigate the applicability of the developed aerogel extraction method, it was applied to extract CDs from hospital wastewater. Hospital wastewater is mixture of various organic and inorganic compounds, making it an ideal candidate to understand the matrix effect on extraction performance of an aerogel. Table 2 shows that the aerogel efficiently extracted CDs from hospital wastewater, and that non-spiked hospital wastewater contains 3 μg L−1 CYP. The percent adsorption of CYP decreased with increasing concentration. This is due to the decreased availability of polar groups on the surface of the aerogel. Because the aerogel is hydrophobic with many available non-polar groups, there was no change in the adsorption of DEX or TAX with increasing concentration. These results prove that the aerogel can be used for real-time applications.| Concentration of drug (μg L−1) | CYP | DEX | TAX |
|---|---|---|---|
| Unspiked | 3 ± 1 | 0 | 0 |
| 10 | 9.9 ± 2.1 | 9.95 ± 0.7 | 10 ± 1 |
| 25 | 21 ± 3 | 24.75 ± 1.4 | 24.5 ± 0.7 |
| 50 | 36 ± 2.82 | 49.25 ± 0.77 | 50 ± 1.05 |
| 75 | 53 ± 2.3 | 74.62 ± 0.1 | 73.87 ± 0.82 |
| 100 | 64 ± 1.2 | 97 ± 1.4 | 98 ± 1.1 |
5. Conclusions
A novel SPE method based on trimethylsilyloxy-modified silica aerogel for extraction of CDs in water and wastewater was developed. Hydrophobic–hydrophobic interactions were found to play a major role in the extraction of CDs by the aerogel. The aerogel was also found to be reusable for CD extraction with no loss in performance. The HPLC method was used to verify the analytical performance of the aerogel. Linearity, LOD, LOQ and RSD of the method were found to be reasonable. The performance efficiency of the aerogel was comparable to, or relatively higher than that of commercial SPE cartridges for the studied CDs. Aerogel is significantly less expensive, the method does not require a vacuum manifold or pump, and reusability renders it an affordable alternative to commercial cartridges. However, the main disadvantage is that it is time-consuming. The developed aerogel-based SPE-HPLC method was also found to be efficient for real-time applications.Author contributions
For this research paper, L. P., D. A., I. G. and A. K formulated the research ideas. L. P and D. Z performed the experiments. D. A, I. G, A. K and L. P analysed the results. L. P wrote the manuscript.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
The authors gratefully acknowledge the financial support of the Tel Aviv University postdoctoral Moshe Mirilashvili fellowship. We also acknowledge Cabot Corp. and Mamane's laboratory for supplying samples of Lumira aerogel. The authors thank Ms. Adi Zilbenman for providing hospital wastewater from Tel-Hashomer.References
- F. W. Rabii, P. A. Segura, P. B. Fayad and S. Sauvé, Sci. Total Environ., 2014, 487, 792–800 CrossRef CAS PubMed.
- M. Jureczko and J. Kalka, Eur. J. Pharmacol., 2020, 866, 172816 CrossRef CAS PubMed.
- T. aus der Beek, F.-A. Weber, A. Bergmann, S. Hickmann, I. Ebert, A. Hein and A. Küster, Environ. Toxicol. Chem., 2016, 35, 823–835 CrossRef CAS PubMed.
- N. C. Rowney, A. C. Johnson and R. J. Williams, Environ. Toxicol. Chem., 2011, 30, 1729 CrossRef.
- L. Ferrando-Climent, S. Rodriguez-Mozaz and D. Barceló, Environ. Pollut., 2014, 193, 216–223 CrossRef CAS PubMed.
- T. Lazarević, A. Rilak and Ž. D. Bugarčić, Eur. J. Med. Chem., 2017, 142, 8–31 CrossRef PubMed.
- D. M. Pavlović, S. Babić, A. J. M. Horvat and M. Kaštelan-Macan, TrAC Trends Anal. Chem., 2007, 26, 1062–1075 CrossRef.
- F. R. Mansour and M. A. Khairy, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1061–1062, 382–391 CrossRef CAS PubMed.
- Q. Han, Q. Liang, X. Zhang, L. Yang and M. Ding, J. Chromatogr. A, 2016, 1447, 39–46 CrossRef CAS PubMed.
- A. M. Devasurendra, D. S. W. Palagama, A. Rohanifar, D. Isailovic, J. R. Kirchhoff and J. L. Anderson, J. Chromatogr. A, 2018, 1560, 1–9 CrossRef CAS PubMed.
- M. Arabi, A. Ostovan, A. R. Bagheri, X. Guo, L. Wang, J. Li, X. Wang, B. Li and L. Chen, TrAC, Trends Anal. Chem., 2020, 128, 115923 CrossRef CAS.
- A. R. Bagheri, M. Arabi, M. Ghaedi, A. Ostovan, X. Wang, J. Li and L. Chen, Talanta, 2019, 195, 390–400 CrossRef CAS PubMed.
- X. Song, J. Li, J. Wang and L. Chen, Talanta, 2009, 80, 694–702 CrossRef CAS PubMed.
- J. Kecht, A. Schlossbauer and T. Bein, Chem. Mater., 2008, 20, 7207–7214 CrossRef CAS.
- T. Feng, X. Ye, Y. Zhao, Z. Zhao, S. Hou, N. Liang and L. Zhao, New J. Chem., 2019, 43, 5159–5166 RSC.
- Y. Tian, J. Feng, X. Wang, C. Luo and M. Sun, J. Chromatogr. A, 2019, 1583, 48–54 CrossRef CAS PubMed.
- N. Saad, M. Chaaban, D. Patra, A. Ghanem and H. El-Rassy, Microporous Mesoporous Mater., 2020, 292, 109759 CrossRef CAS.
- M. Falahnejad, H. Zavvar Mousavi, H. Shirkhanloo and A. M. Rashidi, Microchem. J., 2016, 125, 236–241 CrossRef CAS.
- M. Y. Baktash and H. Bagheri, J. Chromatogr. A., 2017, 1500, 69–75 CrossRef CAS PubMed.
- X. Ji, J. Feng, C. Li, S. Han, J. Feng, W. Guo and M. Sun, Anal. Methods, 2019, 11, 5784–5792 RSC.
- W. J. Malfait, S. Zhao, R. Verel, S. Iswar, D. Rentsch, R. Fener, Y. Zhang, B. Milow and M. M. Koebel, Chem. Mater., 2015, 27, 6737–6745 CrossRef CAS.
- V. L. Prasanna, H. Mamane, V. K. Vadivel and D. Avisar, J. Hazard. Mater., 2020, 384, 121396 CrossRef CAS PubMed.
- M. V. Khedkar, S. B. Somvanshi, A. V. Humbe and K. M. Jadhav, J. Non. Cryst. Solids, 2019, 511, 140–146 CrossRef CAS.
- N. Saad, M. Al-Mawla, E. Moubarak, M. Al-Ghoul and H. El-Rassy, RSC Adv., 2015, 5, 6111–6122 RSC.
- M. L. N. Perdigoto, R. C. Martins, N. Rocha, M. J. Quina, L. Gando-Ferreira, R. Patrício and L. Durães, J. Colloid Interface Sci., 2012, 380, 134–140 CrossRef CAS PubMed.
- C. Gómez-Canela, N. Cortés-Francisco, X. Oliva, C. Pujol, F. Ventura, S. Lacorte and J. Caixach, Environ. Sci. Pollut. Res., 2012, 19, 3210–3218 CrossRef PubMed.
- Q. Chen, D. Zielinski, J. Chen, A. Koski, D. Werst and S. Nowak, J. Pharm. Biomed. Anal., 2008, 48, 732–738 CrossRef CAS PubMed.
- A. Andersen, D. J. Warren, P. F. Brunsvig, S. Aamdal, G. B. Kristensen and H. Olsen, BMC Clin. Pharmacol., 2006, 6, 2 CrossRef PubMed.
- W. Chen, L. Duan and D. Zhu, Environ. Sci. Technol., 2007, 41, 8295–8300 CrossRef CAS PubMed.
- X. Zhang, Q. Liang, Q. Han, W. Wan and M. Ding, Analyst, 2016, 141, 4219–4226 RSC.
- S. Vallejos, A. Muñoz, S. Ibeas, F. Serna, F. C. García and J. M. García, ACS Appl. Mater. Interfaces, 2015, 7, 921–928 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01803e |
| This journal is © The Royal Society of Chemistry 2021 |