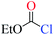Open Access Article
Open Access ArticleInsights into the nucleophilic substitution of pyridine at an unsaturated carbon center†
Pan Du‡
a,
Jiyang Zhao‡ *b,
Shanshan Liub and
Zhen Yueb
*b,
Shanshan Liub and
Zhen Yueb
aSchool of Life Science and Chemistry, Jiangsu Second Normal University, Nanjing, 210013, China
bSchool of Environmental Science, Nanjing Xiaozhuang University, Nanjing, 211171, China. E-mail: jyzhao1981@163.com
First published on 12th July 2021
Abstract
Bimolecular nucleophilic substitution (SN2) is a fundamental reaction that has been widely studied. So far, the nucleophiles are mainly anionic species in SN2 reactions. In this study, we use density functional theory calculations to assess the mechanisms of substitution of carbonyl, imidoyl, and vinyl compounds with a neutral nucleophile, pyridine. Charge decomposition analysis is performed to explore the main components of the transition state's LUMO. For reactions of imidoyl or carbonyl compounds with pyridine or Cl−, the LUMOs of the transition states are composed of mixed orbitals originating from the nucleophile and the substrate. Considering the unique mixed nature of the orbitals, the reaction mode is termed SNm (m means mix). Moreover, the main components of the transition state's LUMO are pure σ*C–Cl MO in the reactions of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl with pyridine or Cl−. Computations were also performed for RY
CHCl with pyridine or Cl−. Computations were also performed for RY![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHX substrates with different X and Y groups (X= Cl−, Br−, or F−; Y = O, N, or C).
CHX substrates with different X and Y groups (X= Cl−, Br−, or F−; Y = O, N, or C).
Introduction
Bimolecular nucleophilic substitution (SN2) is a fundamental reaction that is implicated in different chemical and biological processes.1–10 This reaction is triggered by the attack of a nucleophile (Nu−) on a central atom (A), which results in the displacement of a leaving group (Y) (Scheme 1(a)). The nucleophilic attack can occur from the backside (SN2-b) or front side (SN2-f) of the reacting molecule. For most compounds, SN2-b is preferred to SN2-f, and the central atom at which substitution occurs is sp3 hybridized. However, in some cases, nucleophilic substitution may occur at sp2 hybridized olefinic sites, and the reaction is referred to as nucleophilic vinylic substitution (SNV) (Scheme 1(b)).11–14 SNV reactions proceed via perpendicular concerted π attack with retention (SNVπ) or via in-plane σ* attack with inversion (SNVσ).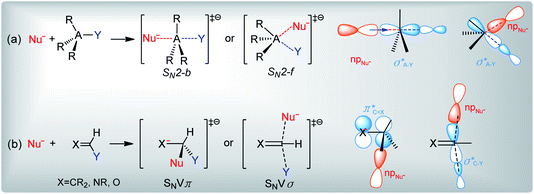 | ||
| Scheme 1 General reaction schemes and molecular orbital interactions of (a) SN2 and (b) SNV reactions. Nu− is the nucleophile, A is the central atom, and Y is the leaving group. | ||
Although the mechanisms of nucleophilic substitution are well established for many substrates, the exact reactions steps for imidoyl and carbonyl compounds are controversial.15–21 According to Bach and Lee, the nucleophilic substitution of these compounds proceeds via the out-of-plane SNVπ mechanism (Scheme 1(b)).15,16 However, Lee and Yamabe show that X− + RCOY displacement reactions cannot follow the SNVπ mechanism since the σ*C–Y molecular orbital (MO) is the main component of the transition state's LUMO, and since the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X and σ*C–Y MOs are efficiently mixed.17–21
X and σ*C–Y MOs are efficiently mixed.17–21
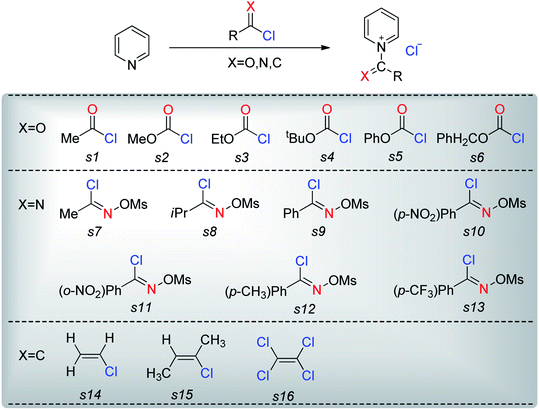
The nucleophiles involved in SN2 and SNV reactions are mainly anionic species, such as Cl−, Br−, I−, OH−, CN−, and SH−. Neutral nucleophiles are very rare, particularly the large ones.22,23 Recently, we had studied the electrophilic addition reactions of pyridine to acyl chlorides, chloroformates and chloro-oxime reagents (Scheme 2).24,25 These reactions can be described as SN2 reactions, where pyridine, a bulky neutral compound, is the nucleophile, and Cl− is the leaving group. Considering that pyridine and anionic species (ex. Cl−) exhibit significantly different electrostatic potential diagrams (Fig. 1), it is expected that their nucleophilic activity and mechanism will also be different. In this study, we investigate the SN2 reactions of pyridine with three kinds of substrates (RCOCl, RClCNR and RClCCR2, shown in Scheme 2).
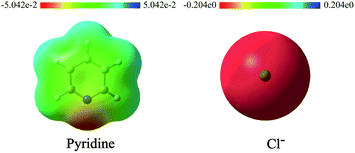 | ||
| Fig. 1 Electrostatic potential diagrams of pyridine and Cl− (isosurface = 0.05 a.u.). Red and blue regions in the ESP map represent areas of negative and positive potential, respectively. | ||
Computational details
Quantum chemistry calculations were conducted by the Gaussian16 program.26 The geometries of all stable species and transition states were optimized using the M06-2X functional with 6-311+G(d,p) basis set.27–30 All calculations were conducted in dichloromethane solution using the self-consistent reaction field (SCRF) method with the IEFPCM solvation model.31 The harmonic frequencies were calculated at the same level of theory in order to obtain the relevant thermodynamic energy corrections and to characterize the optimized stationary points as minima or saddle points. To ascertain the reasonability of our computational method, other functionals (QCISD, MP2, PBE1PBE, B3LYP, B3LYP-d3, w-b97xd) were also used to investigate the structures and energies of some important intermediates and transition states. The Gibbs free energies of all species were determined at T = 298.15 K and P = 1 atm. Intrinsic reaction coordinate (IRC) analysis was used to confirm important transition states and natural bond orbital analyses were carried out at the M062x/6-311+G** level.32–36 The 3D-optimized structures in this study were observed using the CYLview visualization program.37 As for the orbital interactions and orbital composition analysis, they were studied according to the charge decomposition analysis (CDA) method and Hirshfeld method implemented in the Multiwfn program.38–46 IBO (intrinsic bond orbital) analyses was performed to study the electronic structure changes in the reaction of pyridine with CH3COCl along the reaction pathway.47Results and discussion
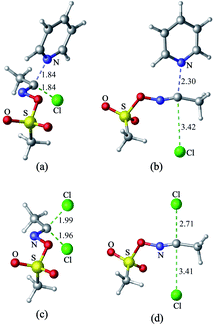
1 Nucleophilic substitution reactions of CH3COCl with pyridine or Cl−
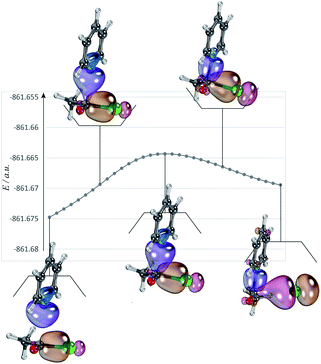
The nucleophilic substitution reactions of acyl chloride CH3COCl with pyridine or Cl− are shown in Fig. 2. Considering that pyridine is not charged, it shows no minima related to the reactant complex, unlike the anionic nucleophile. However, the Cl− eliminated by the nucleophilic attack of pyridine leads to a potential well that is associated with product complex. The transition state (TS-pyridine, illustrated in Fig. 2) shows that when pyridine attacking the substrate, it induces the formation of a C–N bond and the simultaneous cleavage of the C–Cl bond. Fig. 3 shows that the IBOs transform continuously from the electronic structure of the starting material to that of the product along the intrinsic reaction coordinate. Two IBOs show a concerted transformation of one pyridine lone pair (top) into a Cl–C σ bond, while the C–Cl σ bond (down) breaks and transforms into a lone pair. Based on the calculated energy values, the free energy barrier of the reaction is only 13.8 kcal mol−1 (in dichloromethane), which indicates that pyridine is a strong nucleophile. The distance between the carbon center on acyl chloride and the nitrogen atom of pyridine is shortened from 2.81 Å in the reactants to 1.94 Å in the transition state, and to 1.51 Å in the product complex. Meanwhile, the C–Cl bond is elongated from 1.82 Å in CH3COCl to 1.96 Å in the transition state. Using the ωb97xd and B3LYP-d3 functionals, the related reaction barriers are 13.0 and 15.5 kcal mol−1, respectively, indicating that the results obtained using the two functionals and M062X functional are in good agreement. The structures of the transition states calculated by ωb97xd and B3LYP-d3 functionals are shown in Fig. S1 in the ESI.†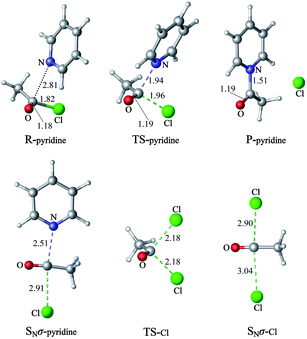 | ||
| Fig. 2 The nucleophilic substitution reactions of acyl chloride CH3COCl with pyridine or Cl− (R: reactant, TS: transition state, P: product). | ||
 | ||
| Fig. 3 IBOs of the two reactive orbitals in the reaction of pyridine with CH3COCl along the reaction pathway. | ||
Although the reaction of nucleophilic substitution of acyl chloride with Cl− has been studied extensively, we chose to investigate it as well for the purpose of direct comparison.15–21 As shown in Fig. 2, the transition state in this reaction (TS-Cl) is characterized by a C–Cl bond length of 2.18 Å, which is longer than that calculated for TS-pyridine. Also, the dihedral angle Cl–O–C–C in TS-pyridine (139.4°) is greater than that in TS-Cl (126.9°), which suggests that Cl− induces more deformation of acyl chloride than pyridine due to its more negative charge and less hindrance.
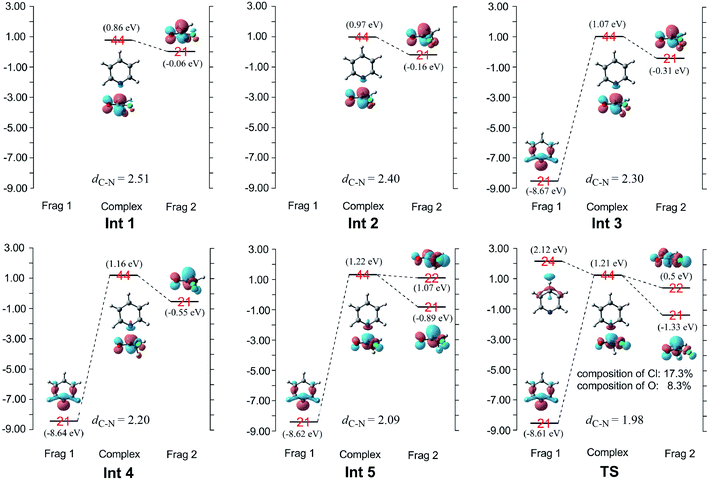
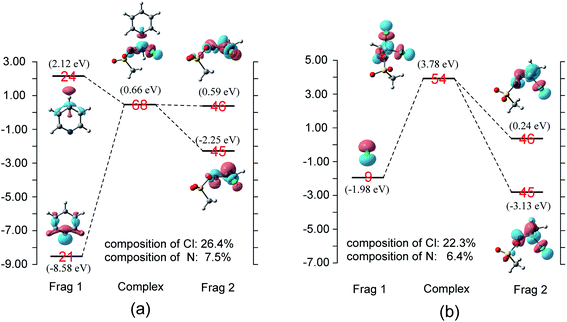
When pyridine approaches the central carbon of CH3COCl, an electron migrates from the electron-rich HOMO of the nucleophile to the LUMO of the substrate, which result in the deformation of the latter.17,18 Considering that the energy gap between the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and σ*C–Cl molecular orbitals of CH3COCl is small (1.3 eV), electron migration from pyridine induces sufficient mixing of these two MOs.15,17–19,21 Based on the results of charge decomposition analysis (CDA)38–42 illustrated in Fig. 4, the LUMO (orbital 44) of the reactant complex (Int 1) is primarily composed of acyl chloride's orbital 21 (π*C
O and σ*C–Cl molecular orbitals of CH3COCl is small (1.3 eV), electron migration from pyridine induces sufficient mixing of these two MOs.15,17–19,21 Based on the results of charge decomposition analysis (CDA)38–42 illustrated in Fig. 4, the LUMO (orbital 44) of the reactant complex (Int 1) is primarily composed of acyl chloride's orbital 21 (π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, LUMO). However, as pyridine approaches, orbitals 21 and 24 of pyridine gradually mix into the LUMO of the complex (orbital 44), along with orbital 21 of CH3COCl. In the transition state, the LUMO is formed by mixing the orbitals 21 and 24 of pyridine with orbitals 21 and 22 of CH3COCl. Based on the orbital composition analysis (Fig. 3(TS)), atom orbitals of chloride and carbonyl oxygen have 17.3% and 8.3% contributions to MO 44 in the transition state, respectively, indicating that the σ*C–Cl MO becomes the main component of the transition state's LUMO. Therefore, it may be concluded that the nucleophilic substitution reaction is initiated by the attack of pyridine's np orbital on the π*C
O, LUMO). However, as pyridine approaches, orbitals 21 and 24 of pyridine gradually mix into the LUMO of the complex (orbital 44), along with orbital 21 of CH3COCl. In the transition state, the LUMO is formed by mixing the orbitals 21 and 24 of pyridine with orbitals 21 and 22 of CH3COCl. Based on the orbital composition analysis (Fig. 3(TS)), atom orbitals of chloride and carbonyl oxygen have 17.3% and 8.3% contributions to MO 44 in the transition state, respectively, indicating that the σ*C–Cl MO becomes the main component of the transition state's LUMO. Therefore, it may be concluded that the nucleophilic substitution reaction is initiated by the attack of pyridine's np orbital on the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O MO of CH3COCl. Knowing that after charge transfer, σ*C–Cl is mixed into the LUMO of CH3COCl, eventually it becomes the major component. Considering the transition state's LUMO is a mixed orbital, the attack mode is labeled SNm (m means mix).
O MO of CH3COCl. Knowing that after charge transfer, σ*C–Cl is mixed into the LUMO of CH3COCl, eventually it becomes the major component. Considering the transition state's LUMO is a mixed orbital, the attack mode is labeled SNm (m means mix).
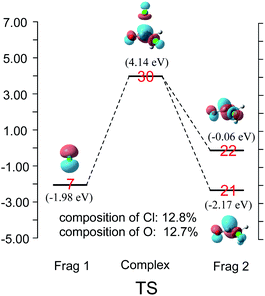
CDA was also performed on the reaction of Cl− and CH3COCl. The results shown in Fig. 5 demonstrate that the transition state's LUMO consists of orbital 7 (np) of Cl− and orbitals 21 and 22 of CH3COCl. The contributions of chloride atom orbital and carbonyl oxygen atom orbital to MO 30 in the transition state are 12.8% and 12.7%, respectively, indicating that the σ*C–Cl and π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O MOs are nearly identical in the transition state's LUMO. The energy level of orbital 22 (σ*) of CH3COCl in TSCl reduced by 0.89 eV from free CH3COCl, which is larger than that in TSpyridine (0.25 eV). Therefore, more π*C
O MOs are nearly identical in the transition state's LUMO. The energy level of orbital 22 (σ*) of CH3COCl in TSCl reduced by 0.89 eV from free CH3COCl, which is larger than that in TSpyridine (0.25 eV). Therefore, more π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O MO mix into orbital 22 in TSCl compared to TSpyridine (as shown in Fig. 3 and 4). As a result, π*C
O MO mix into orbital 22 in TSCl compared to TSpyridine (as shown in Fig. 3 and 4). As a result, π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O MO remains comparable contributions to σ*C–Cl in the transition state. This reaction mode is different from that observed in earlier studies, wherein the σ*C–Cl MO was shown to be the main component of the LUMO of the transition state in the reaction of X− (X = Cl, F) with CH3COCl.17–21
O MO remains comparable contributions to σ*C–Cl in the transition state. This reaction mode is different from that observed in earlier studies, wherein the σ*C–Cl MO was shown to be the main component of the LUMO of the transition state in the reaction of X− (X = Cl, F) with CH3COCl.17–21
An alternative mechanism of the nucleophilic substitution of CH3COCl with pyridine is the in-plane σ attack with inversion (SNσ-pyridine, Fig. 2).15,16,21 The free energy barrier of this reaction is 29.5 kcal mol−1 in dichloromethane, which is much higher than that of the SNm path (13.8 kcal mol−1). The C–N and C–Cl bonds lengths in the SNσ transition state are 2.51 and 2.91 Å, respectively. Both bonds are longer than those observed in the SNm transition state, resulting in a larger free energy barrier of the SNσ reaction. However, the C–Cl bond lengths of the SNm and SNσ transition states corresponding to the reaction of CH3COCl with Cl− are 2.18 and 2.90 Å, respectively. The smaller difference between these two values compared to that determined for the reaction of CH3COCl and pyridine indicates that the deformation energies of the two mechanisms (SNm and SNσ) are close. Therefore, the related free energy barriers of the SNm and SNσ pathways of CH3COCl + Cl− are close (16.1 and 20.1 kcal mol−1, respectively).
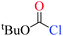
Chloroformates were also assessed. The energy values listed in Table 1 indicate that the reactions of these substrates (s2–s6; Scheme 2) with pyridine are similar to that of s1. Overall, the calculated energies suggested that the investigated chloroformates can easy react with pyridine under very low reaction temperature, which is consistent with previous experimental observations (−20 °C).24,25 The very small energy gaps between the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and σ*C–Cl MOs of all substrates (0.3 to 0.9 eV) indicates that these MOs are efficiently mixed in the transition states.17–19 Moreover, the main component of the LUMO of each transition state is the σ*C–Cl MO of the chloroformates. The optimized geometries of the transition states and the structures of the mixed orbitals are presented in Fig. S2–S3 of ESI.†
O and σ*C–Cl MOs of all substrates (0.3 to 0.9 eV) indicates that these MOs are efficiently mixed in the transition states.17–19 Moreover, the main component of the LUMO of each transition state is the σ*C–Cl MO of the chloroformates. The optimized geometries of the transition states and the structures of the mixed orbitals are presented in Fig. S2–S3 of ESI.†
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of all substrates (eV)
O of all substrates (eV)
The transition state of the reaction of acyl bromide with pyridine (Fig. 6(a)) is similar to that of the acyl chloride. However, when the leaving group is F−, no transition state is formed. Instead, the reaction of acyl fluoride with pyridine leads to a tetrahedral intermediate (Fig. 6(b)) that lies at 3.4 kcal mol−1 above the reactants (M06-2X level). The absence of a transition state for this reaction may be attributed to the greater strength of the C–F bond compared to the C–Cl bond.19 A tetrahedron intermediate is also detected for the reaction of acyl fluoride with NH3 (Fig. 6(c)). Based on M06-2X calculations, this intermediate lies at 5.4 kcal mol−1 above the reactants. The tetrahedral structure of the intermediate was verified using other theoretical methods including QCISD, MP2, PBE1PBE, B3LYP, B3LYP-d3, ωb97xd. The structures of these tetrahedral structures are illustrated in Fig. S1 in the ESI.† As for the reaction of CH3COF with Cl−, it shows only a product complex (CH3COCl…F−), with no transition state or tetrahedral intermediate. The CH3COCl…F− complex is similar to that reported previously by Yamabe et al. for the reaction of CH3COF with Cl−.17,18
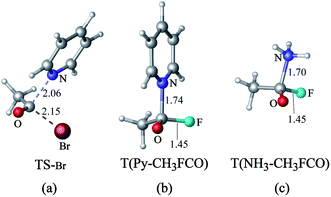 | ||
| Fig. 6 (a) Transition state of the nucleophilic substitution reaction of acyl bromide with pyridine. Tetrahedron intermediates formed by reacting acyl fluoride with (b) pyridine and (c) NH3. | ||
Fig. S4 in ESI† depicts the potential energy diagrams representing the reaction of acyl fluoride with different nucleophiles (pyridine, NH3, and Cl−). These diagrams clearly show stable intermediates for the reactions of acyl fluoride with pyridine and NH3, but not with Cl−. According to previous studies, the formation of tetrahedron intermediate in nucleophilic substitution reactions of acyl chlorides is controlled by the leaving ability of the nucleofuge and by the energy gap between the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and σ*C–Cl MOs.18–20 In the case of acyl fluoride, the energy of newly formed bond in the product is also related to the formation of an intermediate. Based on our calculations, the bond energies of the newly formed C–N (pyridine), C–N (NH3), and C–Cl in the products of the reactions of acyl fluoride with pyridine, NH3, and Cl− are 79.1, 67.7 and 65.5 kcal mol−1, respectively. With the least strong bond, the CH3COCl…F− adduct is the least stable, and so, its formation is not favored. The greater strength of the C–N bond compared to the C–Cl bond also explains why the reaction barrier of nucleophilic substitution of CH3COCl with pyridine is lower than that of substitution with Cl−.
O and σ*C–Cl MOs.18–20 In the case of acyl fluoride, the energy of newly formed bond in the product is also related to the formation of an intermediate. Based on our calculations, the bond energies of the newly formed C–N (pyridine), C–N (NH3), and C–Cl in the products of the reactions of acyl fluoride with pyridine, NH3, and Cl− are 79.1, 67.7 and 65.5 kcal mol−1, respectively. With the least strong bond, the CH3COCl…F− adduct is the least stable, and so, its formation is not favored. The greater strength of the C–N bond compared to the C–Cl bond also explains why the reaction barrier of nucleophilic substitution of CH3COCl with pyridine is lower than that of substitution with Cl−.
2 Nucleophilic substitution reactions of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) NOTf with pyridine or Cl−
NOTf with pyridine or Cl−
Pyridine may also be used as a nucleophile in substitution reactions of imidoyl substrates (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety, Scheme 2).24,25 Based on our theoretical analyses, the nucleophilic substitution reaction of CH3ClC
N moiety, Scheme 2).24,25 Based on our theoretical analyses, the nucleophilic substitution reaction of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf (s7) with pyridine proceed via two possible mechanisms, SNm and SNσ (Fig. 7(a) and (b)), which are characterized by free energy barriers of 22.8 and 49.3 kcal mol−1, respectively. The energy values indicate that the SNm pathway is relatively feasible, but the SNσ pathway is improbable under moderate reaction conditions. We recalculate the SNm reactions of substrate s7 using the ωb97xd and B3LYP-d3 functionals. Related reaction barriers of SNm mechanism are 23.4 and 24.6 kcal mol−1, respectively, in agreement with the values obtained using the M062X functional. The structures of transition states calculated by ωb97xd and B3LYP-d3 functionals are shown in Fig. S1 in the SI.† As for reaction of CH3ClC
NOTf (s7) with pyridine proceed via two possible mechanisms, SNm and SNσ (Fig. 7(a) and (b)), which are characterized by free energy barriers of 22.8 and 49.3 kcal mol−1, respectively. The energy values indicate that the SNm pathway is relatively feasible, but the SNσ pathway is improbable under moderate reaction conditions. We recalculate the SNm reactions of substrate s7 using the ωb97xd and B3LYP-d3 functionals. Related reaction barriers of SNm mechanism are 23.4 and 24.6 kcal mol−1, respectively, in agreement with the values obtained using the M062X functional. The structures of transition states calculated by ωb97xd and B3LYP-d3 functionals are shown in Fig. S1 in the SI.† As for reaction of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf with Cl−, it may occur via SNm and SNσ mechanisms (Fig. 7(c) and (d)), with free energy barriers of 26.5 and 40.0 kcal mol−1, respectively. Overall, the calculated energy values suggest that the nucleophilic substitution of CH3ClC
NOTf with Cl−, it may occur via SNm and SNσ mechanisms (Fig. 7(c) and (d)), with free energy barriers of 26.5 and 40.0 kcal mol−1, respectively. Overall, the calculated energy values suggest that the nucleophilic substitution of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf (s7) with pyridine is more facile than that of Cl−.
NOTf (s7) with pyridine is more facile than that of Cl−.
The energy gap between the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and σ*C–Cl MOs of CH3ClC
N and σ*C–Cl MOs of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf is only 1.1 eV. Therefore, electron migration induces sufficient mixing of these two orbitals when pyridine reacts with CH3ClC
NOTf is only 1.1 eV. Therefore, electron migration induces sufficient mixing of these two orbitals when pyridine reacts with CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf. The CDA presented in Fig. 8(a) shows that the LUMO (orbital 68) of the transition state consists of mixed np (orbital 21, pyridine), orbitals 45 and 46 of CH3ClC
NOTf. The CDA presented in Fig. 8(a) shows that the LUMO (orbital 68) of the transition state consists of mixed np (orbital 21, pyridine), orbitals 45 and 46 of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf, with σ* orbital being the main component because of the large composition of chloride (26.4%). For the reaction of Cl− with CH3ClC
NOTf, with σ* orbital being the main component because of the large composition of chloride (26.4%). For the reaction of Cl− with CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf, the CDA results of the transition state are similar to that observed in the reaction of pyridine with CH3ClC
NOTf, the CDA results of the transition state are similar to that observed in the reaction of pyridine with CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf (Fig. 8(b)). These reaction modes are similar to that observed in earlier studies.17–21
NOTf (Fig. 8(b)). These reaction modes are similar to that observed in earlier studies.17–21
The reactions of the imidoyl substrates s8–s13 with pyridine are similar to that of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf (s7). As shown in Table 2, these reactions exhibit free energy barriers in the narrow range of 23.4–26.3 kcal mol−1, which is higher than the energy barriers corresponding to the reaction of acyl chloride with pyridine. The free energy barriers of these reactions indicate that these reactions can occur under the condition of proper heating. In experiments, these reactions were carried out at 80 °C. Therefore, theoretical calculation results are reliable. Moreover, considering that the energy gaps between π*C
NOTf (s7). As shown in Table 2, these reactions exhibit free energy barriers in the narrow range of 23.4–26.3 kcal mol−1, which is higher than the energy barriers corresponding to the reaction of acyl chloride with pyridine. The free energy barriers of these reactions indicate that these reactions can occur under the condition of proper heating. In experiments, these reactions were carried out at 80 °C. Therefore, theoretical calculation results are reliable. Moreover, considering that the energy gaps between π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and σ*C–Cl MOs of the imidoyl substrates are low (0.5 and 2.4 eV), it may be concluded that these orbitals are efficiently mixed. Like CH3ClC
N and σ*C–Cl MOs of the imidoyl substrates are low (0.5 and 2.4 eV), it may be concluded that these orbitals are efficiently mixed. Like CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf, the main component of the transition state LUMOs of substrates (s8–s13) is the σ*C–Cl MO. The transition state and mixing orbitals of the imidoyl substrates are shown in Fig. S5 and S6 of ESI.†
NOTf, the main component of the transition state LUMOs of substrates (s8–s13) is the σ*C–Cl MO. The transition state and mixing orbitals of the imidoyl substrates are shown in Fig. S5 and S6 of ESI.†
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N MOs of all substrates (eV)
N MOs of all substrates (eV)
The relationship between the free energy barrier and the product yield of the substrate (s7–s11) reaction with pyridine is shown in Fig. 9. As can be seen from Fig. 9, there is a good linear relationship between the yields of these reactions and the free energy barriers. The lower the free energy barrier, the higher the yields. The theoretical calculation results are in good agreement with experimental observations. Thus, theoretical calculation results are reliable.
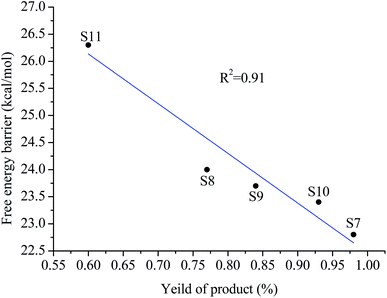 | ||
| Fig. 9 The relationship between the free energy barrier and the product yield of the substrate (s7–s11) reaction with pyridine. | ||
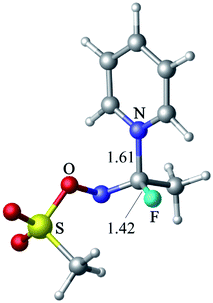
The reaction of CH3FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf with pyridine leads to the formation of a tetrahedral intermediate (Fig. 10, 19.9 kcal mol−1 above the reactants at the M06-2X level), which is characterized by C–N and C–F bond lengths of 1.61 and 1.42 Å, respectively. When Cl− is used as nucleophile, no stable adduct or transition state are evident, just like in the case of the reaction between Cl− and CH3COF. The profile scans of nucleophile approach to CH3FC
NOTf with pyridine leads to the formation of a tetrahedral intermediate (Fig. 10, 19.9 kcal mol−1 above the reactants at the M06-2X level), which is characterized by C–N and C–F bond lengths of 1.61 and 1.42 Å, respectively. When Cl− is used as nucleophile, no stable adduct or transition state are evident, just like in the case of the reaction between Cl− and CH3COF. The profile scans of nucleophile approach to CH3FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf (Fig. S7 in ESI†) confirm that a tetrahedral intermediate is formed with pyridine but not with Cl−.
NOTf (Fig. S7 in ESI†) confirm that a tetrahedral intermediate is formed with pyridine but not with Cl−.
In summary, the reaction of pyridine with CH3XC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf proceeds via a transition state when X is Cl− or forms a stable adduct when X is F−. Similarly, a transition state is discerned for the substitution of CH3ClC
NOTf proceeds via a transition state when X is Cl− or forms a stable adduct when X is F−. Similarly, a transition state is discerned for the substitution of CH3ClC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf with Cl−; however, the CH3FC
NOTf with Cl−; however, the CH3FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOTf substrate shows neither a transition state nor a stable adduct when reacting with chloride.
NOTf substrate shows neither a transition state nor a stable adduct when reacting with chloride.
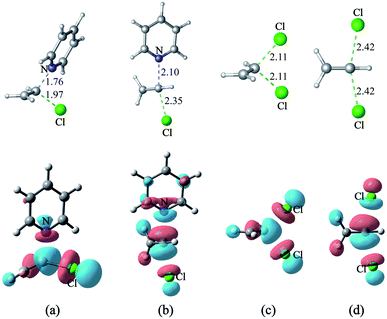
3 Nucleophilic substitution reactions of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) CHCl with pyridine or Cl−
CHCl with pyridine or Cl−
The energy gap between the π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and σ*C–Cl molecular orbitals of H2C
C and σ*C–Cl molecular orbitals of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl (s14) is only 0.6 eV, so electron migration from pyridine or Cl− induces sufficient mixing of these two MOs. As shown in Fig. 11(a) – 10(d), the main components of each transition state's LUMO of the nucleophilic substitution reaction of H2C
CHCl (s14) is only 0.6 eV, so electron migration from pyridine or Cl− induces sufficient mixing of these two MOs. As shown in Fig. 11(a) – 10(d), the main components of each transition state's LUMO of the nucleophilic substitution reaction of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl with pyridine or Cl− is the σ*C–Cl MO of H2C
CHCl with pyridine or Cl− is the σ*C–Cl MO of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl. The mechanism is different from earlier studies, wherein the SNV reactions proceed via concerted π attack (SNVπ) or via in-plane σ* attack (SNVσ).11–14 There are two ways nucleophile attack H2C
CHCl. The mechanism is different from earlier studies, wherein the SNV reactions proceed via concerted π attack (SNVπ) or via in-plane σ* attack (SNVσ).11–14 There are two ways nucleophile attack H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl. One way is for nucleophile to attack the reactive molecule vertically (the mode is labeled SNV-σv, Fig. 10(a) and (c)), and the other way is to attack it from backside (the mode is labeled SNV-σb, Fig. 11(b) and (d)).
CHCl. One way is for nucleophile to attack the reactive molecule vertically (the mode is labeled SNV-σv, Fig. 10(a) and (c)), and the other way is to attack it from backside (the mode is labeled SNV-σb, Fig. 11(b) and (d)).
The free energy barriers of the reaction of pyridine with H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl (s14) via the SNV-σv and SNV-σb mechanisms are 42.4 and 47.5 kcal mol−1, respectively. Similarly, the related free energy barriers for the reaction of H2C
CHCl (s14) via the SNV-σv and SNV-σb mechanisms are 42.4 and 47.5 kcal mol−1, respectively. Similarly, the related free energy barriers for the reaction of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl with Cl− via two modes are 47.9 kcal mol−1 and 42.3 kcal mol−1, respectively. The calculated barriers are consistent with that reported by the group of Radom.48 It can be concluded that SNV-σv is more facile than SNV-σb. CH3HC
CHCl with Cl− via two modes are 47.9 kcal mol−1 and 42.3 kcal mol−1, respectively. The calculated barriers are consistent with that reported by the group of Radom.48 It can be concluded that SNV-σv is more facile than SNV-σb. CH3HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3Cl (s15) and Cl2C
CCH3Cl (s15) and Cl2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCl2 (s16) are also tested as substrates, and the free energy barriers of their nucleophilic substitution reactions with pyridine are 46.0 and 38.2 kcal mol−1, respectively (SNV-σv mode).
CCl2 (s16) are also tested as substrates, and the free energy barriers of their nucleophilic substitution reactions with pyridine are 46.0 and 38.2 kcal mol−1, respectively (SNV-σv mode).
Conclusion
The mechanisms of nucleophilic substitution of carbonyl, imidoyl, and vinyl carbon centers with pyridine or halides are investigated using detailed density functional theory calculations. The main conclusions are summarized below.(1) The transition state LUMOs of the reactions of RCOCl with pyridine, RClCNR with pyridine and RClCNR with Cl− are composed of mixed orbitals (part from pyridine or Cl− and part from substrate), with the σ* MO of the substrate being the main component. However, for the reaction of Cl− and CH3COCl, the σ*C–Cl and π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O MOs are nearly identical in the transition state's LUMO. This type of transition state has not been reported previously for nucleophilic substitution reactions of acyl chlorides. In fact, earlier studies show that the transition state LUMOs corresponding to the reaction of CH3COCl with halides are composed primarily of the σ*C–Cl MO. The newly discovered reaction mode is called SNm (m means mix).
O MOs are nearly identical in the transition state's LUMO. This type of transition state has not been reported previously for nucleophilic substitution reactions of acyl chlorides. In fact, earlier studies show that the transition state LUMOs corresponding to the reaction of CH3COCl with halides are composed primarily of the σ*C–Cl MO. The newly discovered reaction mode is called SNm (m means mix).
(2) For the reactions of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl with pyridine and Cl−, the main components of each transition state's LUMO is the σ*C–Cl MO. The mechanism is different from earlier studies, wherein the SNV reactions proceed via concerted π attack (SNVπ) or via in-plane σ* attack (SNVσ). According to the way of attack, there are two ways nucleophile attack H2C
CHCl with pyridine and Cl−, the main components of each transition state's LUMO is the σ*C–Cl MO. The mechanism is different from earlier studies, wherein the SNV reactions proceed via concerted π attack (SNVπ) or via in-plane σ* attack (SNVσ). According to the way of attack, there are two ways nucleophile attack H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl (SNV-σv and SNV-σb).
CHCl (SNV-σv and SNV-σb).
(3) The reactions of pyridine with RCOX and RXCNR proceed via transition states when X is Cl−; however, when X is F−, a tetrahedral intermediate is formed instead. Similarly, transition states are observed for the reactions of RCOCl and RClCNR with Cl−, but no transition states or intermediate are detected for the RCOF and RFCNR substrates. Our results suggest that adduct formation is favored by stronger C–X bonds (X = pyridine, NH3, or Cl−).
SNV reactions are involved in several biological processes, and we believe that our proposed mechanism can provide inspiration for the study of these biological processes. In the future, we would like to investigate other neutral nucleophiles so as to discover new bimolecular nucleophilic substitution reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant no. 18KJB150010, 19KJB150034).Notes and references
- W. L. Hase, Science, 1994, 266, 998–1002 CrossRef CAS.
- M. L. Chabinyc, S. L. Craig, C. K. Regan and J. I. Brauman, Science, 1998, 279, 1882–1886 CrossRef CAS.
- J. Mikosch, S. Trippel, C. Eichhorn, R. Otto, U. Lourderaj, J. X. Zhang, W. L. Hase, M. Weidemüller and R. Wester, Science, 2008, 319, 183–186 CrossRef CAS PubMed.
- I. Szabó and G. Czakó, Nat. Commun., 2015, 6, 5972–5977 CrossRef PubMed.
- J. Xie, R. Otto, J. Mikosch, J. Zhang, R. Wester and W. L. Hase, Acc. Chem. Res., 2014, 47, 2960–2969 CrossRef CAS PubMed.
- J. Xie and W. L. Hase, Science, 2016, 352, 32–33 CrossRef CAS.
- T. A. Hamlin, M. Swart and F. M. Bickelhaupt, ChemPhysChem, 2018, 19, 1315–1330 CrossRef CAS.
- Y. G. Proenza, M. A. F. de Souza and R. L. Longo, Chem. – Eur. J., 2016, 22, 16220–16229 CrossRef CAS.
- T. A. Hamlin, B. van Beek, L. P. Wolters and F. M. Bickelhaupt, Chem. – Eur. J., 2018, 24, 5927–5938 CrossRef CAS PubMed.
- J. Z. A. Laloo, L. Rhyman, O. Larrañaga, P. Ramasami, F. M. Bickelhaupt and A. de Cózar, Chem.–Asian J., 2018, 13, 1138–1147 CrossRef CAS PubMed.
- V. Lucchini, G. Modena and L. Pasquato, J. Am. Chem. Soc., 1995, 117, 2297–2300 CrossRef CAS.
- C. K. Kim, K. H. Hyun, C. K. Kim and I. Lee, J. Am. Chem. Soc., 2000, 122, 2294–2299 CrossRef CAS.
- C. F. Bernasconi and Z. Rappoport, Acc. Chem. Res., 2009, 42, 993–1003 CrossRef CAS PubMed.
- I. Fernández, F. M. Bickelhaupt and E. Uggerud, J. Org. Chem., 2013, 78, 8574–8584 CrossRef.
- H. G. Li, C. K. Kim, B. Lee, C. K. Kim, S. K. Rhee and I. Lee, J. Am. Chem. Soc., 2001, 123, 2326–2333 CrossRef CAS PubMed.
- J. M. Fox, O. Dmitrenko, L. Liao and R. D. Bach, J. Org. Chem., 2004, 69, 7317–7328 CrossRef CAS PubMed.
- S. Yamabe and T. Minato, J. Org. Chem., 1983, 48, 2972–2975 CrossRef CAS.
- S. Yamabe, T. Minato and Y. Kawabata, Can. J. Chem., 1984, 62, 235–240 CrossRef CAS.
- I. Lee, D. Lee and C. K. Kim, J. Phys. Chem. A, 1997, 101, 879–885 CrossRef CAS.
- I. Lee, C. K. Kim, H. G. Li, C. K. Sohn, C. K. Kim, H. W. Lee and B.-S. Lee, J. Am. Chem. Soc., 2000, 122, 11162–11172 CrossRef CAS.
- C. K. Kim, H. G. Li, H. W. Lee, C. K. Sohn, Y. I. Chun and I. Lee, J. Phys. Chem. A, 2000, 104, 4069–4076 CrossRef CAS.
- D. D. Sung, T. J. Kim and I. Lee, J. Phys. Chem. A, 2009, 113, 7073–7079 CrossRef CAS.
- I. S. Han, C. K. Kim, C. K. Sohn, E. K. Ma, H. W. Lee and C. K. Kim, J. Phys. Chem. A, 2011, 115, 1364–1370 CrossRef CAS PubMed.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642–2713 CrossRef CAS PubMed.
- P. S. Fier, J. Am. Chem. Soc., 2017, 139, 9499–9502 CrossRef CAS PubMed.
- M. J. Frisch; G. W. Trucks; H. B. Schlegel; G. E. Scuseria; M. A. Robb; J. R. Cheeseman; G. Scalmani; V. Barone; B. Mennucci; G. A. Petersson; H. Nakatsuji; M. Caricato; X. Li; H. P. Hratchian; A. F. Izmaylov; J. Bloino; G. Zheng; J. L. Sonnenberg; M. Hada; M. Ehara; K. Toyota; R. Fukuda; J. Hasegawa; M. Ishida; T. Nakajima; Y. Honda; O. Kitao; H. Nakai; T. Vreven; J. A. Montgomery Jr.; J. E. Peralta; F. Ogliaro; M. Bearpark; J. J. Heyd; E. Brothers; K. N. Kudin; V. N. Staroverov; R. Kobayashi; J. Normand; K. Raghavachari; A. Rendell; J. C. Burant; S. S. Iyengar; J. Tomasi; M. Cossi; N. Rega; J. M. Millam; M. Klene; J. E. Knox; J. B. Cross; V. Bakken; C. Adamo; J. Jaramillo; R. Gomperts; R. E. Stratmann; O. Yazyev; A. J. Austin; R. Cammi; C. Pomelli; J. W. Ochterski; R. L. Martin; K. Morokuma; V. G. Zakrzewski; G. A. Voth; P. Salvador; J. J. Dannenberg; S. Dapprich; A. D. Daniels; O. Farkas; J. B. Foresman; J. V. Ortiz; J. Cioslowski and D. J. FoxGaussian 16, Revision A.03, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS.
- J. Miao, S. Hua and S. Li, Chem. Phys. Lett., 2012, 541, 7–11 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS.
- K. Fukui, Acc. Chem. Res., 1981, 14, 363–368 CrossRef CAS.
- H.-P. Hratchian and H.-B. Schlegel, Theory and Applications of Computational Chemistry: The First 40 Years, ed. C. E. Dykstra, Elsevier, Amsterdam, 2005, pp. 195−249 Search PubMed.
- K.-B. Wiberg, Tetrahedron, 1968, 24, 1083–1096 CrossRef CAS.
- A.-E. Reed, L.-A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- F. Weinhold, J. Comput. Chem., 2012, 33, 2363–2379 CrossRef CAS PubMed.
- C.-Y. Legault, CYLview, 1.0b, Université de Sherbrooke, 2009, http://www.cylview.org Search PubMed.
- S. Dapprich and G. Frenking, J. Phys. Chem., 1995, 99, 9352–9362 CrossRef CAS.
- W. Li, L. Chen, Z. Lin, S. Man, X. Qin, Y. Lyu, C. Li and G. Leng, Inorg. Chem., 2020, 59(14), 9667–9682 CrossRef CAS PubMed.
- Y. Yang, X. Hou, T. Zhang, J. Ma, W. Zhang, S. Tang, H. Sun and J. Zhang, J. Org. Chem., 2018, 83(19), 11905–11916 CrossRef CAS PubMed.
- L. Liu, B. Sun, R. Ding and Y. Mao, J. Phys. Chem. A, 2020, 124(52), 11093–11101 CrossRef CAS PubMed.
- L. M. Loftus, A. Li, K. L. Fillman, P. D. Martin, J. J. Kodanko and C. Turro, J. Am. Chem. Soc., 2017, 139(50), 18295–18306 CrossRef CAS PubMed.
- F. L. Hirshfeld, Theor. Chim. Acta, 1977, 44, 129–138 CrossRef CAS.
- P. Bultinck, C. V. Alsenoy, P. W. Ayers and R. Carbó-Dorca, J. Chem. Phys., 2007, 126, 144111 CrossRef PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Mol. Graphics Modell., 2012, 38, 314–323 CrossRef CAS PubMed.
- G. Knizia and J. E. M. N. Klein, Angew. Chem., Int. Ed., 2015, 54, 5518–5522 CrossRef CAS PubMed.
- M. N. Glukhovtsev, A. Pross and L. Radom, J. Am. Chem. Soc., 1994, 116, 5961–5962 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The transition states and mixing orbitals of the carbonyl and imidoyl substrates, the profile scans of nucleophile (pyridine or Cl−) approach to substrates, corrected free energies, imaginary frequency and Cartesian coordinates are included in the ESI. See DOI: 10.1039/d1ra03019a |
| ‡ P. Du and J. Zhao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |