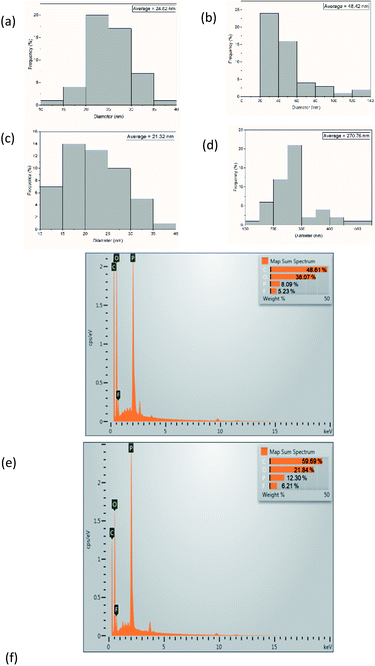
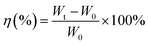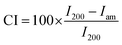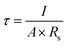Open Access Article
Open Access ArticlePreparation and characterization of nanofibrous cellulose as solid polymer electrolyte for lithium-ion battery applications
Qolby Sabrina *ab,
Christin Rina Ratri
*ab,
Christin Rina Ratri a,
Andri Hardiansyaha,
Titik Lestariningsiha,
Achmad Subhana,
Abdulloh Rifaia,
Rike Yudianti
a,
Andri Hardiansyaha,
Titik Lestariningsiha,
Achmad Subhana,
Abdulloh Rifaia,
Rike Yudianti *a and
Hiroshi Uyama
*a and
Hiroshi Uyama *b
*b
aResearch Center for Physics, Indonesian Institute of Sciences, Kawasan Puspiptek Serpong Gd. 442 Tangerang Selatan, Banten 15314, Indonesia. E-mail: qolby89@gmail.com; qolb001@lipi.go.id
bDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
First published on 29th June 2021
Abstract
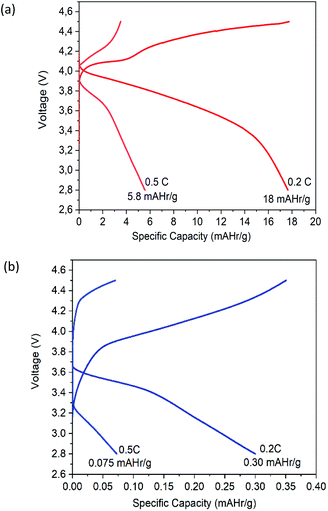
A novel bacterial cellulose (BC)-based nanofiber material has been utilized as an ionic template for the battery system solid polymer electrolyte (SPE). The effect of drying techniques such as oven and freeze-drying on the gel-like material indicate differences in both visual and porous structures. The morphological structure of BC after oven and freeze-drying observed by field-emission scanning electron microscopy indicates that a more compact porous structure is found in freeze-dried BC than oven-dried BC. After the BC-based nanofiber immersion process into lithium hexafluorophosphate solution (1.0 M), the porous structure becomes a host for Li-ions, demonstrated by significant interactions between Li-ions from the salt and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of freeze-dried BC as shown in the infrared spectra. X-ray diffraction analysis of freeze-dried BC after immersion in electrolyte solution shows a lower degree of crystallinity, thus allowing an increase in Li-ion movement. As a result, freeze-dried BC has a better ionic conductivity of 2.71 × 10−2 S cm−1 than oven-dried BC, 6.00 × 10−3 S cm−1. Freeze-dried BC as SPE also shows a larger electrochemical stability window around 3.5 V, reversible oxidation/reduction peaks at 3.29/3.64 V, and an initial capacity of 18 mAHr g−1 at 0.2C. The high tensile strength of the freeze-dried BC membrane of 334 MPa with thermal stability up to 250 °C indicates the potential usage of freeze-dried BC as flexible SPE to dampen ionic leakage transfer.
O groups of freeze-dried BC as shown in the infrared spectra. X-ray diffraction analysis of freeze-dried BC after immersion in electrolyte solution shows a lower degree of crystallinity, thus allowing an increase in Li-ion movement. As a result, freeze-dried BC has a better ionic conductivity of 2.71 × 10−2 S cm−1 than oven-dried BC, 6.00 × 10−3 S cm−1. Freeze-dried BC as SPE also shows a larger electrochemical stability window around 3.5 V, reversible oxidation/reduction peaks at 3.29/3.64 V, and an initial capacity of 18 mAHr g−1 at 0.2C. The high tensile strength of the freeze-dried BC membrane of 334 MPa with thermal stability up to 250 °C indicates the potential usage of freeze-dried BC as flexible SPE to dampen ionic leakage transfer.
Introduction
Lithium-ion batteries (LiBs) have been widely used in portable electronics and developed as power sources in electric vehicles and stationary energy storage in renewable energies. With the rapid development of LIBs for high power applications, safety becomes a significant concern for batteries due to flammable organic liquid electrolytes. Solid polymer electrolyte (SPE) is considered to tackle the safety issue. Due to the non-flammable properties of solid electrolytes, LiBs are expected to exhibit fewer side reactions and higher safety. The use of SPE promises solutions to the leakage, evaporation, and degradation of LiBs containing liquid electrolytes that are still widely used today. SPE also helps to improve dendrite puncture resistance during the recharge process, which enhance its shelf life. SPE could promote dendrite-free stable lithium deposition which opens up new possibilities for research.1,2SPE plays a critical role in facilitating ion transport and functions as the separation membrane between the cathode and the anode in the battery system.3 Batteries with SPE provide a long charge–discharge life cycle. However, SPE has a small current density because of low ionic conductivity compared with aqueous-based electrolyte. In recent years, several polymeric materials as electrolyte matrices have been developed.4 Poly(ethylene oxide) (PEO) produced commercially has been used as an electrolyte membrane. A good SPE requires high ionic conductivity, low toxicity, good mechanical properties, and high thermal stability. In PEO, at high temperatures, its ionic conductivity tends to decrease due to reducing membrane moisture.5,6 In addition, PEO has poor mechanical properties resulting in easy ion leakage, where as the flexible polymer electrolyte is advantageous for good contact between electrode and electrolyte. Currently, SPE research focuses on providing the right environment for the dissolution of lithium salts and suitable cations because the interaction between lithium ions and electron donors of the polymer chains leads to low ionic mobility. The formation of pores across the membrane may facilitate the process of lithium-ion intercalation on LiB.7 Ion transport will be easier on the polymer nanoporous structure.
Bacterial cellulose (BC) is an environmentally friendly material that possesses a nanofibrous cellulosic structure. Strong intermolecular hydrogen bonds provide superior mechanical performance, thermal stability, and dielectric properties.8 The nanofibrous structure of BC also has excellent porosity, flexibility in surface functionalities, and heteroatoms (oxygen atoms) with free pair of electrons which facilitate ionic conduction. The layered BC with the compact porous structure in the composite provides efficiency for the wetting of the electrolyte and the uniformity of the ion flux, so that guarantee the electrolyte absorbent capacity and provide abundant ionic passageways.9,10
In recent work, we reported the utilization of BC as SPE of LiB. BC is preferred to be used as a flexible membrane and endows Li+ transport between electrodes. BC-based nanofibers materials were prepared with two dry treatments to allow cross-linking reactions to take place. Two drying techniques, such as oven and freeze-drying, were subjected to BC to develop its dissociation, adsorption, retaining, and accelerating the migration of lithium ions from the electrolyte solution added to BC. Several material characterizations were performed to examine the potential application of the BC as SPE in LiB. Structure and mechanical properties were investigated by using field-emission scanning electron microscopy (FESEM), Fourier-transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), thermo gravimetric analysis (TGA), tensile test, and Brunauer–Emmet–Teller (BET) analysis. Electrochemical performances were examined by using electrochemical impedance spectroscopy (EIS), linear sweep, cyclic voltammetry, and charge–discharge (CD) measurements.
Experimental
Preparation of oven- and freeze-dried BC
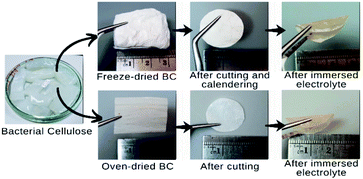
BC gel was purchased from small enterprise coconut local farmers. BCs with an area size of 3 × 3 cm2 underwent a neutralization process by boiling the BCs in deionized water for 1 h. Subsequently, the samples were soaked in ethanol for two days to improve the porous structure, including pore size and morphology.11,12 Two sets of samples were prepared, including oven- and freeze-dried BC. For the oven-dried BCs, the samples were oven-dried in 60 °C for one day. Freeze-drying treatment of the samples for freeze-dried BCs was conducted using a freeze dryer (BUCHI L-300) at −77.8 °C until an estimated moisture content below 5% was obtained. The preparation of BC-based nanofibers samples is shown in Fig. 1.Fabrication of SPE membranes
BCs with a diameter of 19 mm and thickness of around 100 μm were transferred to an argon-filled glovebox with moisture level below 0.1 ppm. It was then immersed in a solution of 50 μL lithium hexafluorophosphate (LiFP6) 1 M in ethylene carbonate and diethyl carbonate with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature. Then the samples were dried at room temperature to obtain BC solvent-free or dry solid polymer electrolyte.
1 at room temperature. Then the samples were dried at room temperature to obtain BC solvent-free or dry solid polymer electrolyte.
Structure and mechanical properties characterization
The major functional groups of BC were characterized by using FTIR spectroscopy, Thermo Fisher Scientific Nicolet iS-10. The spectra were collected using ambient air as a background over the wavelength range from 4000 to 400 cm−1 at room temperature. The crystallinity of the resultant materials was evaluated by using a D2 Phase BRUKER X-ray powder diffractometer with Cu Kα1 (1.5406 Å) radiation. The tensile strength of BC membranes was tested at 25 °C with a universal testing machine, Yasuda 216-10k Universal Material Tester, which was conducted according to the ASTM D 882 method for a thin film with a tensile test speed of 5 mm min−1. The length and width of the samples were 10 and 5 mm, respectively. The specimen was attached to chucks on both ends. Then, the elongation force is applied continuously through the load cell until breakage occurred. The thermal stability of the membranes was examined by using TGA PT 1600 Linseis, with a heating rate of 10 °C min−1 from 50° to 500° under flowing nitrogen atmosphere. The morphology of the dried BC membranes were characterized by FESEM JEOL JIB 4160F and the calculation of diameter fiber with software analysis image j. BET Surface Area and Pore Size Analyzer Quantachrome Nova 4200e is measurement based for determination of specific surface area of porous materials. The specific surface area can be estimated from the total pore volume and pore diameters. The electrolyte uptake of the membrane is the main factor for ionic conductivity.13 The dried BC was exposed in an argon-filled glove box for 90 min to measure the electrolyte retention. The weights of BC before (W0) and after (Wt) absorption were measured to show the liquid electrolyte uptake (η) as calculated using eqn (2)where η is the uptake of liquid electrolyte.
Electrochemical characterization
The electrochemical stability window was characterized by linear sweep voltammetry (LSV) with a two-electrode configuration using stainless steel (SS) electrode as a working electrode and Li metal as a reference and counter electrodes. The assembly process of SS/BC-liPF6/Li metal was conducted in a glove box filled with argon gas. LSV cyclic voltammetry (CV) and galvanostatic charge/discharge (CD) measurements were performed using WBCS3000 Automatic Battery Cycler with a constant scan rate of 5 mV s−1 in a potential range of 0 to 5 V at 27 °C. CR2032 coin cells were assembled using Li metal as a reference and counter electrode and LiMnPO4 as a positive electrode to perform the ionic conductivity, CV, and CD measurements. Ionic conductivity properties and Li+ transfer number of the BC membrane was measured by using EIS HIOKI 9263 SMD Test Fixture LCR with a frequency of 0.6–20 kHz.Results and discussion
Structure and mechanical properties characterization
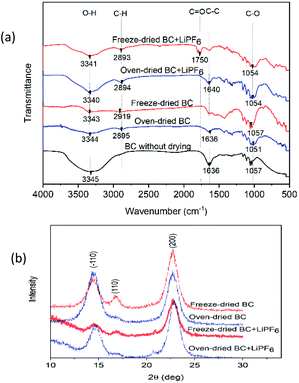
The FTIR studies of solid polymer electrolytes focus on the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) region at 1750 cm−1. In the spectra, oxygen atoms (C
O) region at 1750 cm−1. In the spectra, oxygen atoms (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) were found on the IR spectrum of freeze-dried BC after immersion in the LIPF6 electrolyte, while the IR spectrum of oven-dried BC as SPE does not show any carbonyl (C
O) were found on the IR spectrum of freeze-dried BC after immersion in the LIPF6 electrolyte, while the IR spectrum of oven-dried BC as SPE does not show any carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) region. The C
O) region. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O acts as electron donors and forms a coordinate bond with Li ions from electrolyte LiPF6, thereby forming polymer–salt complexes. The presence of hydroxyl groups also helps BC affinity towards electrolyte, and high electrolyte uptake builds a strong pathway for the migration of lithium ions, which enhances the ionic conductivity of the membrane electrolyte.16
O acts as electron donors and forms a coordinate bond with Li ions from electrolyte LiPF6, thereby forming polymer–salt complexes. The presence of hydroxyl groups also helps BC affinity towards electrolyte, and high electrolyte uptake builds a strong pathway for the migration of lithium ions, which enhances the ionic conductivity of the membrane electrolyte.16
where I200 is the maximum intensity of the principal peak (200) lattice diffraction, and Iam is the intensity of amorphous cellulose. The highest diffraction peak represents the amount of the crystalline phase, while the minimum intensity between the major peaks represents the amount of the amorphous phase.
The values of crystallinity indices of BC are 87.6% and 89.9% for freeze- and oven-dried BC, respectively. After immersion in the LiPF6 electrolyte, the freeze- and oven-dried BC crystallinity index values were 86.36% and 90.43%, respectively. Freeze-dried BC before and after immersion of electrolyte solution registers a slight reduction of crystallinity than oven-dried BC. A lower degree of crystallinity value on the polymer allows the increased ionic mobility of the polymer chain as the result of a large number of Li+ ion movements.18,19 Celgard separator has sharp and distinct peaks around 2θ = 14.0°, 17.0°, and 18.5°, which is characteristic of isotactic polypropylene with the crystallinity degree of 31%.20 Many researchers believe the high crystallinity could cause low ionic conductivity and low stability due to the reduced migration rate of lithium ions in the crystalline phase.21
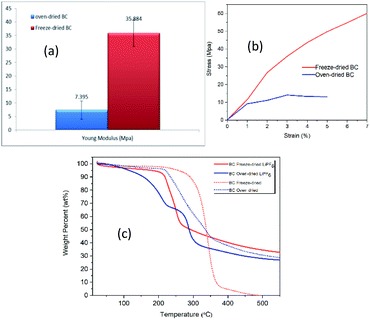 | ||
| Fig. 3 (a) Young's modulus (b) Tensile stress–strain curves (c) TGA curves of freeze- and oven-dried BC membranes. | ||
Manufacturing procedures battery with liquid electrolyte requires separators able to sustain a stress of about 13 MPa without being damaged.22 BC has satisfied the mechanical requirement of a separator in the LIBs system and is suitable for this application. Fan and Maier have reported the tensile study on PEO-based polymer electrolyte is 1.8 MPa, which is inferior to BC's value.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and porous structure of freezed-dried BC Fig. 4(d), makes easily thermal decomposition of LiPF6. The decomposition rate depends on the temperature and the structure such as crystallinity and orientation.23 In freeze-dried bc, there is no fiber network visible in the structure Fig. 4(c), this causes the crystallinity of freeze-dried to be lower than oven-dried. Several factors can influence thermogravimetric measurements such as pore morphology and homogeneity, which may affect heat transfer within the samples and thus influence the diffusion rate of reaction.24,25 This result reveals that the freeze-dried BC with homogeneous porous structure can be used as a host for SPE at ambient and moderate temperatures below 250 °C, which is suitable for application in electrochemical devices, such as batteries.
O) and porous structure of freezed-dried BC Fig. 4(d), makes easily thermal decomposition of LiPF6. The decomposition rate depends on the temperature and the structure such as crystallinity and orientation.23 In freeze-dried bc, there is no fiber network visible in the structure Fig. 4(c), this causes the crystallinity of freeze-dried to be lower than oven-dried. Several factors can influence thermogravimetric measurements such as pore morphology and homogeneity, which may affect heat transfer within the samples and thus influence the diffusion rate of reaction.24,25 This result reveals that the freeze-dried BC with homogeneous porous structure can be used as a host for SPE at ambient and moderate temperatures below 250 °C, which is suitable for application in electrochemical devices, such as batteries.
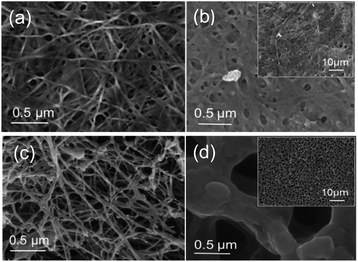 | ||
| Fig. 4 Morphology of (a) oven-dried BC, (b) oven-dried BC after immersed in LiPF6, (c) freeze-dried BC, and (d) freeze-dried BC after immersed in LiPF6. | ||
Thermal stability studies of PEO polymer electrolytes exhibit minimal weight loss until the samples 200 °C; this indicates that the PEO–LiPF6 is stable up to 200 °C before decomposition. At 200 °C, the LiPF6 starts to melt, and the system is no longer stable.26 SPE, which is assembled in a lithium-ion battery system, is generally operated at a temperature higher than room temperature, typically cycled at 50–80 °C to achieve good performance.27 Thermal stability is vital for solid polymer electrolytes in Li-ion battery applications.28 PP separator shows large thermal shrinkage; the weight loss was seen at around 50–100 °C is attributed to the decomposition.29 Resultantly, compared to the commercial PP separator and PEO polymer electrolytes, the BC membrane presents higher thermal stability.
| Sample | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Thickness (μm) |
|---|---|---|---|
| BC oven-dried | 122 | 0.685 | 80 |
| BC freeze-dried | 439 | 0.535 | 34 |
Electrochemical characterization
where τ, l, A, and Rs represent ionic conductivity, SPE thickness, SPE-electrode contact area, and SPE resistance, respectively. The ionic conductivity is shown in Table 2. The ionic conductivity value of freeze-dried BC is higher than that of oven-dried BC, which is 2.71 × 10−2 S cm−1. This value is better than the ionic conductivity of biopolymer membranes. The ionic conductivity of biopolymeric SPEs at room temperature such as lignin (10−7 to 10−5 S cm−1), chitosan (0.066 × 10−3 S cm−1) and algae (1.15 × 10−3 S cm−1).35 Despite this, the presence of a large number of polar groups in biopolymer help dissolves a significant concentration of salts via Lewis acid–base interactions and electrostatic forces, which is necessary for achieving a significant ionic conductivity.35 The ionic conductivity is thus not only directly linked to the polymer dynamics, where high conductivity is possible under the low-temperature drying condition of the BC freeze-dried polymer. The higher freeze-dried BC conductivity value is obtained due to higher amounts of hydrogen bonds with anions of lithium salts, to promote the dissociation of lithium salts and facilitate the migration of lithium ions. We take Li/BC LiPF6/Li to measure Li+ transfer number. Freeze dried bc have better value Li+ transfer number than oven dried bc which is 0.48 and 0.2. The polar functional hydroxyl on the freeze-dried BC can not only enhance the salt dissociation beneficial to lithium ionic conductivity but also be used as a trapper for the anion of most lithium salt mostly owing to the hydrogen bond between them. The ionic conductivity of a polymer electrolyte is influenced by the mobility and concentration of the conducting species (cation and anion).36 Lower crystallinity index promotes the transportation of ions on freeze-dried BC and increases the ionic conductivity.37 These properties appear to be better demonstrated by the BC prepared by the freeze-drying.
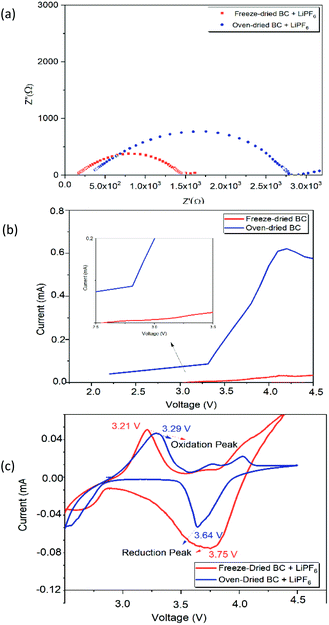 | ||
| Fig. 6 (a) Nyquist plot of freeze- and oven-dried BC obtained from EIS measurement. (b) LSV of freeze- and oven-dried BC as SPE (c) CV of the freeze-and oven- BC as SPE. | ||
| Sample | Ionic conductivity (S cm−1) |
|---|---|
| BC freeze dry + LIPF6 | 2.71 × 10−2 |
| BC oven dry + LIPF6 | 6.00 × 10−3 |
The electrochemical stability (i.e., working cell potential range) of SPE is an essential criterion for its application of electrochemical devices.2 In linear sweep voltammetry (LSV), a potential which varies linearly with time is applied between an initial potential and a final potential (LSV) or cycled between two extreme (or switching) potential values at a given potential scan rate v (usually expressed in mV s−1). The optimum electrochemical stability window of BC is an essential requirement for choosing suitable electrolytes for the application of Li-ion batteries.
During the charge–discharge cycling process of Li-ion batteries, anions such as PF6 in the electrolyte are also movable besides lithium-ion transfer. Those anions will probably concentrate and be absorbed or decomposed on the surface of electrodes during the cycles, thus will affect the performance of the battery. The significant capacity decrease may be attributed to the degradation of the electrolytes.41 These different performances are given because, during the discharge, the low porosity of the oven-dried BC as SPE acts as a bridge for the migration of ions, limiting their diffusion towards the positive electrode. The better specific capacity of freeze-dried BC as SPE is related to the ionic conductivity of SPE.42 When freeze-dried BC is in contact with the electrode, its porous structure allows a quick diffusion during the discharge process, which effectively improves lithium ions' mass transport. The concentration polarization resistance increases and consumes Li+, caused by partly irreversible electrode reaction and thickened solid-electrolyte interphase film during the cycle process.43
Conclusions
This study showed that different drying methods affect the structure, mechanical properties, and electrochemical performance of BC as SPE. Freeze-dried BC showed significant interactions between Li+ ions from the salt and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. It has a crystallinity index of 86.36%, an excellent tensile strength of 60.17 MPa, the thermal stability of up to 250 °C, and a homogeneous porous structure with a high surface area. This BC was achieved by the frozen substance when sublimation occurred. The electrochemical performances of freeze-dried BC as SPE is better than those of oven-dried BC, such as higher ionic conductivity of 2.71 × 10−2 S cm−1, higher stability window beyond 3.5 V, oxidation/reduction peaks of 3.29/3.64 V, and specific capacity of 18 mA h g−1 at 0.2C and 5.6 mA h g−1 at 0.5C. The structure and morphology of freeze-dried BC still need to be optimized to get SPE with better electrochemical performance. Despite the current results, the freeze-dried BC can be applied as SPE in lithium-ion batteries since BC is environmentally friendly and can be produced by using a low-cost method.
O groups. It has a crystallinity index of 86.36%, an excellent tensile strength of 60.17 MPa, the thermal stability of up to 250 °C, and a homogeneous porous structure with a high surface area. This BC was achieved by the frozen substance when sublimation occurred. The electrochemical performances of freeze-dried BC as SPE is better than those of oven-dried BC, such as higher ionic conductivity of 2.71 × 10−2 S cm−1, higher stability window beyond 3.5 V, oxidation/reduction peaks of 3.29/3.64 V, and specific capacity of 18 mA h g−1 at 0.2C and 5.6 mA h g−1 at 0.5C. The structure and morphology of freeze-dried BC still need to be optimized to get SPE with better electrochemical performance. Despite the current results, the freeze-dried BC can be applied as SPE in lithium-ion batteries since BC is environmentally friendly and can be produced by using a low-cost method.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to gratefully acknowledge all kind of supports from the Japan Society for The Promotion of Science in the form of the RONPAKU Program, Department of Applied Chemistry, Graduate School of Engineering Osaka University, Research Center for Physics – Indonesian Institute of Sciences and its Advanced Characterization Laboratories Serpong through E-Layanan Sains for the facilities, scientific, and technical support. Financial support for this publication was provided by RISPRO PRN “Litbang Pembesaran Skala Produksi Bahan Baku Baterai Lithium Merah Putih 83/E1/PRN/2020”. The authors would also thank Dr Rizna Triana Dewi and Mr Muhammad Ghozali for the immense help on the sample preparations of freeze-dried and tensile strength measurement.References
- P. Barai, K. Higa and V. Srinivasan, Phys. Chem. Chem. Phys., 2017, 19, 20493–20505 RSC.
- L. Long, S. Wang, M. Xiao and Y. Meng, J. Mater. Chem. A, 2016, 4, 10038–10039 RSC.
- Z. Gadjourova, Y. G. Andreev, D. P. Tunstall and P. G. Bruce, Nature, 2001, 412, 520–523 CrossRef CAS PubMed.
- B. S. Kumar Jr and G. Lawrence, US pat., 6190806, USPTO, 2000 Search PubMed.
- S. Cho, S. Kim, W. Kim, S. Kim and S. Ahn, Polymers, 2018, 10, 1–16 Search PubMed.
- Z. Wang, H. Gu, Z. Wei, J. Wang, X. Yao and S. Chen, Ionics, 2019, 25, 907–916 CrossRef CAS.
- Y. S. Wu, C. C. Yang, S. P. Luo, Y. L. Chen, C. N. Wei and S. J. Lue, Int. J. Hydrogen Energy, 2017, 42, 6862–6875 CrossRef CAS.
- D. Lasrado, S. Ahankari and K. Kar, J. Appl. Polym. Sci., 2020, 137, 1–14 CrossRef.
- K. Y. Chua, A. D. Azzahari, C. N. Abouloula, F. Sonsudin, N. Shahabudin and R. Yahya, J. Polym. Res., 2020, 27(5), 115 CrossRef CAS.
- R. Pan, Z. Wang, R. Sun, J. Lindh, K. Edström, M. Strømme and L. Nyholm, Cellulose, 2017, 24, 2903–2911 CrossRef CAS.
- C. Campano, A. Balea, A. Blanco and C. Negro, Cellulose, 2016, 23, 57–91 CrossRef CAS.
- Y. Hu and J. M. Catchmark, Am. Soc. Agric. Biol. Eng. Annu. Int. Meet., 2009, 8, 5274–5288 Search PubMed.
- G. Yang, H. Cai, X. Li, M. Wu, X. Yin, H. Zhang and H. Tang, RSC Adv., 2020, 10, 5077–5087 RSC.
- L. Indrarti, Indriyati, A. Syampurwadi and S. Pujiastuti, AIP Conf. Proc., 2016, 1711 DOI:10.1063/1.4941633.
- R. Yudianti, A. Syampurwadi, H. Onggo, M. Karina, H. Uyama and J. Azuma, Polym. Adv. Technol., 2016, 27, 1102–1107 CrossRef CAS.
- M. R. Asghar, M. T. Anwar, A. Naveed and J. Zhang, Membranes, 2019 DOI:10.3390/membranes9070078.
- L. Segal, J. J. Creely, A. E. Martin and C. M. Conrad, Text. Res. J., 1959, 29, 786–794 CrossRef CAS.
- D. Zhao, Y. Zhu, W. Cheng, W. Chen, Y. Wu and H. Yu, Adv. Mater., 2020, 2000619, 1–18 Search PubMed.
- S. Jafirin, I. Ahmad and A. Ahmad, BioResources, 2013, 8, 5947–5964 CrossRef.
- C. T. Love, J. Power Sources, 2011, 196, 2905–2912 CrossRef CAS.
- J. A. Matthews, Encycl. Environ. Chang., 2014 DOI:10.4135/9781446247501.n1321.
- Q. Fu, G. Lin, X. Chen, Z. Yu, R. Yang, M. Li, X. Zeng and J. Chen, Energy Technol., 2018, 6, 144–152 CrossRef CAS.
- Z. Cai and J. Kim, Cellulose, 2010, 17, 83–91 CrossRef CAS.
- B. Ravdel, K. M. Abraham, R. Gitzendanner, J. DiCarlo, B. Lucht and C. Campion, J. Power Sources, 2003, 119–121, 805–810 CrossRef CAS.
- D. Kotatha, K. Morishima, S. Uchida, M. Ogino, M. Ishikawa, T. Furuike and H. Tamura, Res. Chem. Intermed., 2018, 44, 4971–4987 CrossRef CAS.
- S. Ibrahim and M. R. Johan, Int. J. Electrochem. Sci., 2012, 7, 2596–2615 CAS.
- S. T. Hsu, B. T. Tran, R. Subramani, H. T. T. Nguyen, A. Rajamani, M. Y. Lee, S. S. Hou, Y. L. Lee and H. Teng, J. Power Sources, 2020, 449, 227518 CrossRef CAS.
- M. Li, X. Wang, Y. Wang, B. Chen, Y. Wu and R. Holze, RSC Adv., 2015, 5, 52382–52387 RSC.
- M. Raja and A. M. Stephan, RSC Adv., 2014, 4, 58546–58552 RSC.
- N. Li, J. Zheng, P. Hadi, M. Yang, X. Huang, H. Ma, H. W. Walke and B. S. Hsiao, Membranes, 2019, 9(6) DOI:10.3390/membranes9060070.
- J. Weidner, ECS Trans., 2009, 19, 30 Search PubMed.
- H. Ben Youcef, B. Orayech, J. M. L. Del Amo, F. Bonilla, D. Shanmukaraj and M. Armand, Solid State Ionics, 2020, 345, 115168 CrossRef.
- Y. Xia, W. S. Xiong, Y. Jiang, W. Sun, H. Q. Sang, R. X. He, Q. Tai, B. Chen, Y. Liu and X. Z. Zhao, RSC Adv., 2017, 7, 21988–21996 RSC.
- H. Raj, S. Singh and A. Sil, Electrochim. Acta, 2019, 326 DOI:10.1016/j.electacta.2019.134981.
- E. Lizundia and D. Kundu, Adv. Funct. Mater., 2021, 31, 1–29 CrossRef.
- T. K. Lee, N. F. M. Zaini, N. N. Mobarak, N. H. Hassan, S. A. M. Noor, S. Mamat, K. S. Loh, K. H. KuBulat, M. S. Su’ait and A. Ahmad, Electrochim. Acta, 2019, 316, 283–291 CrossRef CAS.
- M. Rayung, M. M. Aung, S. C. Azhar, L. C. Abdullah, M. S. Su’ait, A. Ahmad and S. N. A. M. Jamil, Materials, 2020, 13(4) DOI:10.3390/ma13040838.
- L. Singheiser, P. Huczkowski, T. Markus and W. J. Quadakkers, Shreir’s Corrosion, 2010, pp. 482–517 Search PubMed.
- A. Arya and A. L. Sharma, Polymer electrolytes for lithium ion batteries: a critical study, Ionics, 2017, 23(3), 497–540 CrossRef CAS.
- N. M. J. Rasali, M. A. Saadiah, N. K. Zainuddin, Y. Nagao and A. S. Samsudin, Express Polym. Lett., 2020, 14, 619–637 CrossRef CAS.
- S. B. Aziz, T. J. Woo, M. F. Z. Kadir and H. M. Ahmed, J. Sci. Adv. Mater. Devices, 2018, 3, 1–17 CrossRef.
- C. M. Costa, Y. H. Lee, J. H. Kim, S. Y. Lee and S. Lanceros-Méndez, Energy Storage Mater., 2019, 22, 346–375 CrossRef.
- Y. Liu, J. Chen, Z. Liu, H. Xu, Y. Zheng, J. Zhong, Q. Yang, H. Tian, Z. Shi, J. Yao and C. Xiong, J. Alloys Compd., 2020, 829, 154541 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |