Open Access Article
Open Access ArticleIlliciumlignans G–O from the leaves of Illicium dunnianum and their anti-inflammatory activities†
Sen-Ju Ma‡
 a,
Hai-Bo Li‡
a,
Hai-Bo Li‡ b,
Ting Li
b,
Ting Li a,
Zhen-Zhen Su
a,
Zhen-Zhen Su b,
Zhen-Zhong Wang
b,
Zhen-Zhong Wang b,
Xin-Sheng Yao
b,
Xin-Sheng Yao *a,
Wei Xiao
*a,
Wei Xiao *b and
Yang Yu
*b and
Yang Yu *a
*a
aInstitute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou 510632, P. R. China. E-mail: 1018yuyang@163.com; tyaoxs@jnu.edu.cn; Fax: +86-20-85221559; Tel: +86-20-85221559
bJiangsu Kanion Pharmaceutical Co., Ltd., State Key Laboratory of New-tech for Chinese Medicine Pharmaceutical Process, Lianyungang, Jiangsu 222001, China. E-mail: xw_kanion@163.com
First published on 15th September 2021
Abstract
Phytochemical investigations on the dry leaves of Illicium dunnianum have led to the isolation of 24 lignans. Illiciumlignans G–K (1–5) were five undescribed benzofuran lignans, illiciumlignan L (6) was one undescribed ditetrahydrofuran lignan, illiciumlignans M–O (7–9) were three new sesquilignans, and compounds 10, 12, 13, 15, and 18–21 were firstly isolated from the genus Illicium. Their structures were elucidated by detailed spectroscopic analyses (UV, IR, HR-ESI-MS, and NMR) and CD experiments. All isolates were evaluated by measuring their inhibitory effects on PGE2, and NO production in LPS-stimulated RAW 264.7 macrophages.
1 Introduction
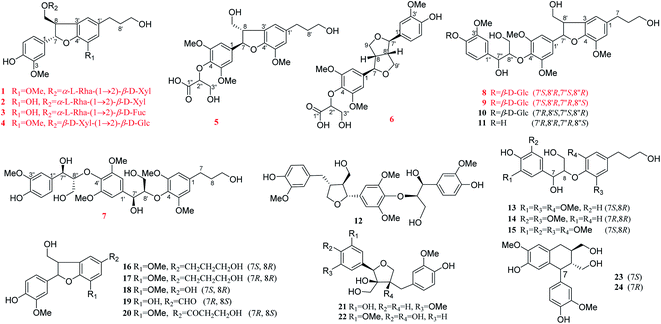
The genus Illicium L. belongs to the family Magnoliaceae. It is commonly distributed in the southwest and east of China, with a total of 28 species (including 2 varieties).1 Illicium plants have a long medical history, which was recorded as early as the Ming Dynasty in Compendium of Materia Medica. Illicium dunnianum is a folk plant found throughout Southern China and used as a medicine for relieving pain and treating rheumatism.2 Modern pharmacological studies have shown that it possesses multiple biological activities, such as anti-inflammatory,3,4 central and peripheral analgesic effects,5,6 relieving gastrointestinal smooth muscle spasm, and regulating immune activities.4 Previous phytochemical investigations of I. dunnianum indicated the presence of sesquiterpenes,7–11 phenylpropanoids,7,12–15 phenol glycosides,16 flavonoids,2,17,18 triterpenes,2,14 and others.2,7,17 Up to now, research mainly focused on the fruit and roots of I. dunnianum and there is a lack of chemical studies of its leaves. To explore the bioactive constituents, the chemical constituents of the leaves of I. dunnianum were investigated, and 24 lignans were isolated (Fig. 1), of which illiciumlignans G–O (1–9) were new lignans, and compounds 10, 12, 13, 15, 18–21 were isolated from the genus Illicium for the first time. In addition, all isolates were evaluated for their inhibitory effects on PGE2 and NO. Herein, the isolation, structure elucidation, and anti-inflammatory activities assay of these compounds are reported.2 Results and discussion
2.1 Structural elucidation
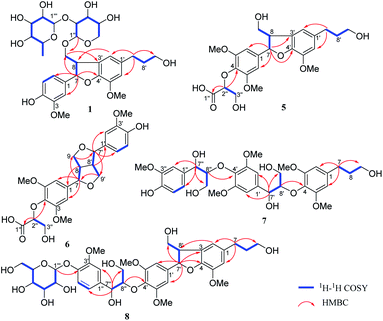
Compound 1 was obtained as a brown amorphous powder with an optical rotation of [α]25D −35.2 (c 0.75, MeOH). Its molecular formula was determined as C31H42O14 by analysing the HRESIMS peak at m/z 661.2471 [M + Na]+ (calcd for 661.2472). The 1H NMR (Table 1) spectrum displayed the presence of one 1,2,4-trisubstituted aromatic ring [δH 6.96 (1H, d, J = 1.8 Hz, H-2), 6.83 (1H, dd, J = 8.2, 1.8 Hz, H-6), 6.75 (1H, d, J = 8.2 Hz, H-5)] and one 1,2,3,5-tetrasubstituted aromatic ring [δH 6.73 (2H, br s, H-2′, H-6′)]. Signals for one oxymethine [δH 5.57 (1H, d, J = 5.8 Hz, H-7)], two oxymethylenes [δH 3.92 (2H, m, H-9), 3.57 (2H, t, J = 6.5 Hz, H-9′)], one methine [δH 3.62 (1H, m, H-8)], two methylenes [δH 2.62 (2H, t, J = 7.7 Hz, H-7′), 1.82 (2H, m, H-8′)] and two methoxys [δH 3.85 (3H, s, 5′-OCH3), 3.83 (3H, s, 3-OCH3)], along with two anomeric proton signals [δH 4.35 (1H, d, J = 6.9 Hz, H-1′′), 5.18 (1H, d, J = 1.3 Hz, H-1′′′)] were also observed. The 13C NMR data with assistance of HSQC spectrum displayed 31 carbon resonances, including two aromatic rings [δC 149.0, 147.5, 147.4, 145.2, 136.9, 134.8, 129.0, 119.7, 118.0, 116.1, 114.2, 110.8], together with a rhamnosyl group [δC 102.3, 72.3, 72.2, 74.0, 69.9, 17.9] and a xylopyranose group [δC 103.6, 79.2, 78.9, 71.5, 66.9], as well as two methines [δC 89.8, 53.1], four methylenes [δC 72.6, 62.2, 35.8, 32.9] and two methoxys [δC 56.8, 56.4]. The NMR spectroscopic data of 1 were highly similar to those of (−)-(7S,8R)-4,9,9′-trihydroxy-3′,5-dimethoxy-4′,7-epoxy-8,3′-neoligan-9-O-[α-L-rhamnopyranosyl (1→6)]-β-D-glucopyranoside, except that the β-D-glucopyranoside moiety was replaced by a β-D-xylopyranosyl moiety.19 This was further confirmed by HPLC analysis after acid hydrolysis and glycosyl derivatization (Fig. S82†), as well as the J value of the anomeric proton mentioned above. The β-D-xylopyranosyl moiety was located at C-9 and the α-L-rhamnopyranosyl unit was linked to C-2′′ of the β-D-xylopyranosyl moiety, according to the key HMBC correlations from H-1′′ to C-9 and from H-1′′′ to C-2′′ (Fig. 2).| Pos. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 134.8 | 135.1 | 135.2 | 134.7 | ||||
| 2 | 110.8 | 6.96, d (1.8) | 110.7 | 6.99, d (1.5) | 110.9 | 7.02, d (1.3) | 110.9 | 6.98, d (1.5) |
| 3 | 149.0 | 148.9 | 148.9 | 149.1 | ||||
| 4 | 147.4 | 147.2 | 147.2 | 147.5 | ||||
| 5 | 116.1 | 6.75, d (8.2) | 116.1 | 6.75, d (8.1) | 116.0 | 6.74, d (8.1) | 116.2 | 6.78, d (8.1) |
| 6 | 119.7 | 6.83, dd (8.2, 1.8) | 119.7 | 6.86, dd (8.1, 1.5) | 119.7 | 6.87, dd (8.1, 1.3) | 120.1 | 6.86, dd (8.1, 1.5) |
| 7 | 89.8 | 5.57, d (5.8) | 89.5 | 5.57, d (5.6) | 89.3 | 5.63, d (5.5) | 89.4 | 5.62, d (6.8) |
| 8 | 53.1 | 3.62, m | 53.3 | 3.60, m | 53.4 | 3.60, m | 53.0 | 3.64, dd (12.2, 6.9) |
| 9 | 72.6 | 3.92, m | 72.8 | 3.91, m | 72.9 | 3.92, m | 71.9 | 4.06, dd (9.1, 7.9), 3.88, m |
| 1′ | 136.9 | 136.7 | 136.7 | 137.0 | ||||
| 2′ | 118.0 | 6.73, brs | 116.7 | 6.61, brs | 116.8 | 6.63, brs | 117.9 | 6.74, brs |
| 3′ | 129.0 | 129.0 | 129.2 | 129.5 | ||||
| 4′ | 147.5 | 146.5 | 146.5 | 147.5 | ||||
| 5′ | 145.2 | 141.9 | 141.9 | 145.2 | ||||
| 6′ | 114.2 | 6.73, brs | 117.1 | 6.57, brs | 117.1 | 6.56, brs | 114.2 | 6.73, brs |
| 7′ | 32.9 | 2.62, t (7.7) | 32.7 | 2.56, t (7.7) | 32.7 | 2.56, t (7.6) | 32.9 | 2.63, t (7.6) |
| 8′ | 35.8 | 1.82, m | 35.8 | 1.79, m | 35.8 | 1.80, m | 35.9 | 1.82, m |
| 9′ | 62.2 | 3.57, t (6.5) | 62.3 | 3.56, t (6.5) | 62.3 | 3.56, t (6.6) | 62.3 | 3.58, t (6.5) |
| 1′′ | 103.6 | 4.35, d (6.9) | 103.6 | 4.36, d (6.6) | 103.4 | 4.33, d (7.6) | 103.6 | 4.48, d (6.9) |
| 2′′ | 79.2 | 3.40, d (7.1) | 79.3 | 3.41, d (6.1) | 77.1 | 3.67, m | 83.0 | 3.45, m |
| 3′′ | 78.9 | 3.44, d (8.8) | 78.9 | 3.45, d (8.8) | 76.3 | 3.60, m | 77.0 | 3.53, m |
| 4′′ | 71.5 | 3.50, dd (9.3, 5.2) | 71.5 | 3.50, dd (9.1, 5.7) | 73.6 | 3.56, m | 71.0 | 3.53, m |
| 5′′ | 66.9 | 3.18, dd (11.4, 9.9) | 66.9 | 3.19, dd (10.5, 9.8) | 71.9 | 3.60, m | 66.5 | 3.22, m |
| 3.87, d (6.6) | 3.86, d (5.4) | 3.88, m | ||||||
| 6′′ | 16.7 | 1.27, d (6.4) | ||||||
| 1′′′ | 102.3 | 5.18, d (1.3) | 102.3 | 5.18, brs | 102.2 | 5.18, brs | 105.4 | 4.43, d (7.6) |
| 2′′′ | 72.3 | 3.92, m | 72.3 | 3.91, m | 72.4 | 3.92, m | 76.2 | 3.22, m |
| 3′′′ | 72.2 | 3.68, dd (9.5, 3.3) | 72.2 | 3.68, dd (9.5, 2.9) | 72.2 | 3.70, dd (6.1, 3.4) | 77.6 | 3.27, m |
| 4′′′ | 74.0 | 3.36, m | 74.0 | 3.36, m | 74.1 | 3.35, d (9.6) | 71.4 | 3.27, m |
| 5′′′ | 69.9 | 3.90, m | 69.9 | 3.90, m | 69.8 | 3.92, m | 78.2 | 3.03, m |
| 6′′′ | 17.9 | 0.99, d (6.2) | 17.9 | 1.01, d (6.1) | 17.9 | 1.0, d (6.2) | 62.6 | 3.70, dd (12.0, 2.1), 3.58, m |
| 3-OCH3 | 56.4 | 3.83, s | 56.4 | 3.83, s | 56.5 | 3.84, s | 56.5 | 3.83, s |
| 5′-OCH3 | 56.8 | 3.85, s | 56.7 | 3.86, s | ||||
NOESY correlations of H-8/H-2, H-8/H-6 and H-7/H-9 combining with coupling constant (J7,8 = 5.8 Hz) indicated that H-7 and H-8 were in relative-trans form. The absolute configuration of 1 was assessed to be 7S and 8R, respectively, based on the positive Cotton effect at 242 and 291 nm and the negative Cotton effect at 226 nm(ref. 19 and 20) (Fig. S9†). Therefore, the structure of 1 was assigned as (−)-(7S,8R)-4,9,9′-trihydroxy-3,5′-dimethoxy-4′,7-epoxy-8,3′-neoligan-9-O-[α-L-rhamnopyranosyl (1→2)]-β-D-xylopyranoside, and given a trivial name of illiciumlignan G.
Compound 2 was isolated as a yellow amorphous powder. The HRESIMS data showed a sodium adduct molecular ion at m/z 647.2333 [M + Na]+ (calcd for 647.2316), corresponding to a molecular formula of C30H40O14 with eleven degrees of unsaturation. The NMR spectra (Table 1) of 2 were highly similar to 1, except for the substitution at C-5′. Further analysis indicated that the 5′-OCH3 in 1 was replaced by a hydroxyl group. The relative-trans configuration of 2 was confirmed by NOESY correlations and the coupling value J7,8 = 5.6 Hz. The (7S,8R) absolute configuration of 2 was deduced from the CD data (positive Cotton effects at 239 and 293 nm, and negative Cotton effect at 226 nm) (Fig. S18†). Thus, the structure of 2 was elucidated and named as illiciumlignan H.
Compound 3 was yielded as a brown amorphous powder. Its molecular formula was shown to be C31H42O14 based on its [M + Na]+ ion at m/z 661.2477 in the HRESIMS (calcd for 661.2472). The 1H and 13C NMR spectra of 3 were very similar to 2 (Table 1), except for the substitution of glycosyl groups at C-9. Acid hydrolysis and subsequent HPLC analysis of hydrolysate of 3 showed that retention time of the saccharide derivative peaks were consistent to D-fucose and L-rhamnose derivatives, respectively. The relative configuration of fucosyl unit were determined to be β, on the basis of coupling constant value [δH 4.33 (1H, d, J = 7.6 Hz, H-1′′)], while rhamnosyl unit for α [δH 5.18 (1H, brs, H-1′′′)]. The rhamnosyl group was located at C-2′′, according to the HMBC correlation from H-1′′′ to C-2′′. The absolute configuration was assessed to be 7S and 8R, respectively, based on the NOESY data and CD spectrum (Fig. S27†) using the same protocol as previously described. Therefore, the structure of 3 was elucidated and named as illiciumlignan I.
Compound 4 was obtained as a brown amorphous powder. The sodium adduct ion at m/z 677.2423 [M + Na]+ by HRESIMS demonstrated that the molecular formula of 4 was C31H42O15. Comparison of 1H and 13C NMR data of 4 and 1 (Table 1) indicated that they have the same aglycone except for the substitution of glycosyl groups at C-9. Using the same method as above, it was determined that the two sugar groups were β-D-xylopyranoside and β-D-glucopyranoside. The glucosyl group was located at C-2′′, according to the HMBC correlation from H-1′′′ to C-2′′. In addition, the absolute configuration was also assessed to be 7S and 8R (Fig. S36†), respectively. Thus, the structure of 4 was defined and named as illiciumlignan J.
Illiciumlignan K (5), a yellow oil, gave the molecular formula of C24H30O10 based on HRESIMS (m/z 479.1923 [M + H]+, calcd for 479.1917). The 1H NMR (Table 2) spectrum displayed the presence of a symmetrical 1,2,3,5-tetrasubstituted aromatic ring [δH 6.73 (2H, s, H-2 and H-6)] and an asymmetrical 1,2,3,5-tetrasubstituted aromatic ring [δH 6.74 (1H, brs, H-6′), 6.71 (1H, brs, H-2′)]. Signals for three methoxys [δH 3.81 (6H, s, 3,5-OCH3), 3.87 (3H, s, 5′-OCH3)], three oxymethylenes [δH 3.90, (1H, m, H-9a), 3.75, (1H, m, H-9b); 3.56 (2H, t, J = 6.4 Hz, H-9′); 3.86, (2H, m, H-3′′)], two methylenes [δH 2.62 (2H, t, J = 7.7 Hz, H-7′), 1.81 (2H, m, H-8′)] and three methines [δH 5.56 (1H, d, J = 5.7 Hz, H-7), 3.46 (1H, dd, J = 12.6, 5.7 Hz, H-8), 4.50 (1H, t, J = 3.1 Hz, H-2′′)]. The 13C NMR spectrum displayed 24 carbon resonances including a carbonyl [δC 174.1], twelve sp2 aromatic carbons [δC 154.1 × 2, 147.4, 145.3, 140.2, 137.2, 136.1, 129.5, 117.9, 114.3, 103.8 × 2], three methoxys [δC 56.8, 56.7 × 2], five methylenes [δC 65.1, 63.5, 62.2, 35.8, 32.9] and three methines [δC 88.5, 83.9, 55.8]. The NMR spectroscopic data of 5 were very similar to those of dunnianeolignan A,7 except that the group connected at C-4. Carbon signals at δ 174.1, 83.9 and 63.5, corresponding to a carbonyl, an oxymethine [δH 4.50 (1H, t, J = 3.1 Hz, H-2′′)] and an oxymethylene [δH 3.86 (2H, m, H-3′′)]. HMBC correlation from H-2′′ to C-4, C-1′′ and H-3′′ to C-1′′, suggested that it was an a glyceric acid moiety located at C-4 (Fig. 2).
| Pos. | 5 | 6 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| a Measured at 400 MHz for 1H and 100 MHz for 13C in CD3OD. | ||||
| 1 | 140.2 | 139.5 | ||
| 2 | 103.8 | 6.73, s | 104.2 | 6.71, s |
| 3 | 154.1 | 154.1 | ||
| 4 | 136.1 | 135.9 | ||
| 5 | 154.1 | 154.1 | ||
| 6 | 103.8 | 6.73, s | 104.2 | 6.71, s |
| 7 | 88.5 | 5.56, d (5.7) | 87.2 | 4.76, d (3.8) |
| 8 | 55.8 | 3.46, dd (12.6,5.7) | 55.3 | 3.13, m |
| 9 | 65.1 | 3.75, m; 3.90, m | 72.7 | 3.90, m |
| 1′ | 137.2 | 133.7 | ||
| 2′ | 117.9 | 6.71, s | 111.0 | 6.95, d (1.5) |
| 3′ | 129.5 | 149.1 | ||
| 4′ | 147.4 | 147.3 | ||
| 5′ | 145.3 | 116.1 | 6.77, d (8.1) | |
| 6′ | 114.3 | 6.74, s | 120.0 | 6.81, dd (8.1, 1.5) |
| 7′ | 32.9 | 2.62, t (7.7) | 87.4 | 4.71, d (4.1) |
| 8′ | 35.8 | 1.81, m | 55.8 | 3.13, m |
| 9′ | 62.2 | 3.56, t (6.4) | 72.9 | 4.26, dt (6.1, 5.2) |
| 1′′ | 174.1 | 174.2 | ||
| 2′′ | 83.9 | 4.50, t (3.1) | 84 | 4.50, t (3.8) |
| 3′′ | 63.5 | 3.86, m | 63.6 | 3.85, m |
| 3-OCH3 | 56.7 | 3.81, s | 56.7 | 3.85, s |
| 5-OCH3 | 56.7 | 3.81, s | 56.7 | 3.85, s |
| 3′-OCH3 | 56.4 | 3.85, s | ||
| 5′-OCH3 | 56.8 | 3.87, s | ||
A trans configuration of H-7 and H-8 of 5 was determined by the J7,8 value (5.7 Hz) and the NOESY cross peaks from H-8 to H-2/H-6 and from H-7 to H-9. The optical rotation values of 5 were closed to zero, combined with the results of chiral column analysis, indicating that 5 was racemic mixtures. The peaks of the of 5 (5a and 5b) were observed at tR 20.0 (5a)/23.5 (5b) min, respectively, and their relative peak area ratio in the HPLC chromatogram was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S79†). Therefore, structure of 5 was elucidated.
1 (Fig. S79†). Therefore, structure of 5 was elucidated.
Compound 6 was obtained as a yellow oil with molecular formula C24H28O10 by HRESIMS (m/z 477.1762 [M + H]+, calcd for 477.1761). The NMR spectroscopic data (Table 2) of 6 was similar to that of medioresinol,21 expect that 6 has an additional carbon signals at δ 174.2, 84.0 and 63.6. Compared with compound 5, it was identified as a glyceric acid moiety. HMBC (Fig. 2) correlation from H-2′′ to C-4, 1′′, 3′′; H-3′′ to C-1′′, suggested that glyceric acid moiety located at C-4. Glyceric acid moiety was degraded from sesquilignan and the absolute configuration of C-2′′ are not stereospecificity.22 On the basis of the coupling constants of the oxymethine protons [δ 4.76 (1H, d, J = 3.8 Hz, H-7) and 4.71 (1H, d, J = 4.1 Hz, H-7′)] of 6, two sets of protons (H-7/H-8 and H-7′/H-8′) were indicated as being trans oriented. The NOESY correlations between H-8 and H-2, H-6 and between H-8′ and H-2′, H-6′ also confirmed the postulated arrangement. Compound 6 were found to be enantiomers by chiral chromatographic column analysis, and the relative content ratio of the two enantiomers was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 (Fig. S80†). Therefore, the structure of 6 was elucidated, and named as illiciumlignan L.
80 (Fig. S80†). Therefore, the structure of 6 was elucidated, and named as illiciumlignan L.
Compound 7 was isolated as a brown amorphous powder. Its molecular formula was C32H42O13 indicated by HRESIMS m/z 657.2526 [M + Na]+, calcd for 657.2523.
The 1H NMR spectrum (Table 3) showed three aromatic proton signals at [δH 7.02 (1H, brs, H-2′′), 6.75 (1H, m, H-5′′), 6.87 (1H, dd, J = 8.1, 1.3 Hz, H-6′′)], indicating the presence of an ABX-coupled benzene ring. Two symmetrical 1,2,3,5-tetrasubstituted aromatic ring at [δH 6.76 (2H, s, H-2′, 6′); 6.54 (2H, s, H-2, 6)] revealed the other two benzenes. Five methoxy proton signals at [δH 3.81 (6H, s, 3,5-OCH3), 3.84 (3H, s, 3′′-OCH3), and 3.86 (6H, s, 3′,5′-OCH3)], four methines signals at [δH 4.99 (1H, d, J = 7.2 Hz, H-7′′), 4.95 (1H, d, J = 5.2 Hz, H-7′), 4.20 (1H, dd, J = 9.0, 4.8 Hz, H-8′), 4.01 (1H, m, H-8′′)] and five methylenes signals at [δH 3.90 (2H, m, H-9′), 3.76 (1H, m, H-9′′a), 3.56 (2H, t, J = 6.4 Hz, H-9), 3.29 (1H, m, H-9′′b), 2.64 (2H, t, J = 7.7 Hz, H-7), 1.83 (2H, m, H-8). The 13C NMR spectrum revealed 25 peaks for 32 carbons, including 18 aromatic carbons for three aromatic rings, four oxymethylene carbon signals, five methines and five methoxy carbon signals. The 1H–1H COSY correlations between H-7/H-8 and H-9 and HMBC correlations from H-7 to C-1,2,6,8,9 confirmed the presence of a propanolguaiacol unit. Similarly, the presence of two guaiacylglycerol units were determined based on the COSY correlations of H-7′/H-8′/H-9′, H-7′′/H-8′′/H-9′′, and the HMBC correlations from H-7′ to C-1′,2′,6′,8′,9′ and from H-7′′ to C-1′′,2′′,6′′,8′′,9′′. In addition, the HMBC correlations between H-8′′ and C-4′, between H-8′ and C-4 confirmed the oxygen bridge between C-8′′ and C-4′ as well as C-8′ and C-4, respectively. The positions of the methoxy groups were also confirmed by HMBC correlations (Fig. 2). These above assignments suggested that the basic skeleton of 7 is an 8-O-4′ system sesquineolignan. The 7′,8′-erythro and 7′′, 8′′-threo configurations for 7 were substantiated by the J7′,8′ value (5.2 Hz) and J7′′,8′′ value (7.2 Hz).23,24 Similar to 6, compound 7 was determined to be a pair of enantiomers by chiral column analysis, and the results showed that the relative peak area ratio of 7a and 7b was 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (Fig. S81†). Based on the above data, structure of 7 was elucidated, and it was named as illiciumlignan M.
30 (Fig. S81†). Based on the above data, structure of 7 was elucidated, and it was named as illiciumlignan M.
| Pos. | 7 | 8 | 9 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 140.0 | 137.2 | 137.2 | |||
| 2 | 106.9 | 6.54, s | 117.9 | 6.72, brs | 118.0 | 6.72, s |
| 3 | 154.3 | 129.6 | 129.5 | |||
| 4 | 134.7 | 147.5 | 147.5 | |||
| 5 | 154.3 | 145.3 | 145.3 | |||
| 6 | 106.9 | 6.54, s | 114.2 | 6.74, brs | 114.2 | 6.74, s |
| 7 | 33.4 | 2.64, t (7.7) | 32.9 | 2.63, t (7.6) | 32.9 | 2.63, t (7.6) |
| 8 | 35.4 | 1.83, m | 35.8 | 1.82, m | 35.8 | 1.82, m |
| 9 | 62.2 | 3.56, t (6.4) | 62.2 | 3.57, t (6.4) | 62.2 | 3.57, t (6.4) |
| 1′ | 139.0 | 139.6 | 139.8 | |||
| 2′ | 105.3 | 6.76, s | 104.0 | 6.70, s | 103.9 | 6.72, s |
| 3′ | 153.9 | 154.5 | 154.6 | |||
| 4′ | 136.4 | 136.2 | 136.2 | |||
| 5′ | 153.9 | 154.5 | 154.6 | |||
| 6′ | 105.3 | 6.76, s | 104.0 | 6.70, s | 103.9 | 6.72, s |
| 7′ | 74.0 | 4.95, d (5.2) | 88.7 | 5.53, d (6.1) | 88.6 | 5.55, d (5.9) |
| 8′ | 87.2 | 4.20, dd (9.0, 4.8) | 55.7 | 3.47, m | 55.7 | 3.46, m |
| 9′ | 61.4 | 3.90, m | 65.0 | 3.75, m | 65.1 | 3.75, m |
| 3.85, m | 3.85, d (2.2) | |||||
| 1′′ | 133.4 | 137.4 | 137.4 | |||
| 2′′ | 111.8 | 7.02, brs | 112.3 | 7.05, d (1.1) | 112.4 | 7.06, d (1.2) |
| 3′′ | 148.8 | 150.4 | 150.5 | |||
| 4′′ | 147.2 | 147.2 | 147.2 | |||
| 5′′ | 115.9 | 6.75, m | 117.4 | 7.09, d (8.2) | 117.6 | 7.12, d (8.3) |
| 6′′ | 121.0 | 6.87, dd (8.1, 1.3) | 120.9 | 6.89, dd (8.2, 1.1) | 120.7 | 6.92, dd (8.3, 1.2) |
| 7′′ | 74.6 | 4.99, d, (7.2) | 73.8 | 4.92, d (5.4) | 73.8 | 4.94, d (5.2) |
| 8′′ | 89.2 | 4.01, m | 87.0 | 4.27, dd (8.9, 5.1) | 87.2 | 4.24, dd (8.8, 5.0) |
| 9′′ | 61.7 | 3.29, m | 61.6 | 3.61, m | 61.5 | 3.57, m |
| 3.76, m | 3.90, m | 3.90, d (4.9) | ||||
| 1′′′ | 102.8 | 4.87, d (7.4) | 102.8 | 4.88, d (7.2) | ||
| 2′′′ | 74.9 | 3.50, m | 74.9 | 3.49, m | ||
| 3′′′ | 78.2 | 3.39, m | 78.2 | 3.40, m | ||
| 4′′′ | 71.4 | 3.36, m | 71.4 | 3.40, m | ||
| 5′′′ | 77.8 | 3.45, m | 77.8 | 3.46, m | ||
| 6′′′ | 62.6 | 3.65, m | 62.5 | 3.69, dd (12.1, 2.7) | ||
| 3.84, m | 3.85, m | |||||
| 3-OCH3 | 56.6 | 3.81, s | ||||
| 5-OCH3 | 56.6 | 3.81, s | 56.8 | 3.87, s | 56.8 | 3.87, s |
| 3′,5′-OCH3 | 56.6 | 3.86, s | 56.6 | 3.78, s | 56.7 | 3.78, s |
| 3′′-OCH3 | 56.4 | 3.84, s | 56.7 | 3.83, s | 56.7 | 3.83, s |
Compound 8 was obtained as a yellowish oil and its molecular formula was confirmed as C37H48O16 by HRESIMS at m/z 771.2847 [M + Na]+ (C37H48O16Na, calcd for 771.2840), possessing 14 degrees of unsaturation. The 1H and 13C NMR (Table 3) of 8 showed signals attributed to a 1,2,4-trisubstituted aromatic rings at [δH 7.05 (1H, d, J = 1.1 Hz, H-2′′), 7.09 (1H, d, J = 8.2 Hz, H-5′′), and 6.89 (1H, dd, J = 8.2, 1.1 Hz, H-6′′)], a symmetrical 1,2,3,5-tetrasubstituted aromatic ring at [δH 6.70 (2H, s, H-2′/6′)], an asymmetrical 1,2,3,5-tetrasubstituted aromatic ring at [δH 6.74 (1H, brs, H-6), 6.72 (1H, brs, H-2)], and four methoxy groups at [δH 3.87 (3H, s, 5-OCH3), 3.78 (6H, s, 3′,5′-OCH3), 3.83 (3H, s, 3′′-OCH3)]. Additionally, an anomeric proton [δH 4.87 (1H, d, J = 7.4 Hz, H-1′′′)] was indicative of a monosaccharide moiety in 8, which was identified as β-D-glucosyl residue by HPLC analysis after acid hydrolysis and glycosyl derivatization. The 13C NMR gave 37 carbon signals, except for eighteen aromatic carbons, six sugar carbons and four methoxy carbons, the remaining carbons were four oxymethines [(δC 88.7, 87.0, 73.8, and 55.7)] and five methylenes [(δC 65.0, 62.2, 61.6, 35.8, and 32.9)], attributing to three C3 moieties, which were confirmed by 1H–1H COSY and HMBC correlations. These NMR spectroscopic data supposed 8 to be a sesquilignan glycoside and were in good agreement with those of acernikol-4′′-O-β-D-glucopyranoside.25 Subsequently, the 1H–1H COSY, HSQC and HMBC data (Fig. 2) confirm that they shared the same planar structure. The absolute configuration of the dihydrofuran ring was determined to be 7′S,8′R, based on the NOESY correlations of H-8′/H-2′, H-6′ and H-7′/H-9′, coupling constant (J7′,8′ = 6.1 Hz), and the negative Cotton effect at 225 nm. The relative configuration of H-7′′ and H-8′′ was determined to be erythro based on the small coupling constant (J7′′,8′′ = 5.4 Hz) and the absolute configuration was assigned as (7′′S,8′′R) based on the negative Cotton effect at 242 nm (Fig. S69†). Thus, structure of 8 was elucidated and named as illiciumlignan N.
Compound 9 was obtained as white amorphous powder with an optical rotation of [α]25D −15.43 (c 0.7, MeOH). The HRESIMS data of 9 indicated that it possessed the same molecular formula as 8. A comparison of the 1H, 13C, and 2D NMR data (Table 3) of 9 and 8 suggested that the two compounds had the same planar structures. The 7′,8′-trans and 7′′,8′′-erythro configuration of 9 was identical to 8, which was confirmed by NOSEY correlations of H-8′/H-2′, H-6′, H-7′/H-9′ and coupling constant values of J7′,8′ = 5.9 Hz, and J7′′,8′′ = 5.2 Hz. The absolute configuration was assigned as (7′S,8′R,7′′R,8′′S) on the basis of a negative Cotton effect at 228 nm and a positive Cotton effect at 243 nm observed in the CD spectrum (Fig. S78†). Based on the above data, structure of 9 was elucidated, and named as illiciumlignan O.
The other fifteen known compounds were identified as acernikol-4′′-O-β-D-glucopyranoside25 (10), acernikol26 (11), seslignanoccidentaliol A27 (12), erythro-4,7,9,9′-tetrahydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan28 (13), threo-4,7,9,9′-tetra-hydroxy-3,3′-dimethoxy-8-O-4′-neolignan29(14), erythro-4,7,9,9′-tetrahydroxy-3,5,3′,5′-tetramethoxy-8,4′-oxyneolignan30 (15), (7S*,8R*)-dihydrodehydrodiconiferyl alcohol31 (16), (7R*,8R*)-dihydrodehydrodiconiferyl alcohol32 (17), samwirin A33 (18), hierochin C34 (19), prunustosanan AI35 (20), (7′R*,8S*,8′S*)-3,5′-dimethoxy-3′,4,8′,9′-tetrahydroxy-7′,9-epoxy-8,8′-lignan36 (21), massoniresinol37 (22), isolariciresinol28 (23), and burselignan28 (24).
2.2 The activity of anti-inflammatory
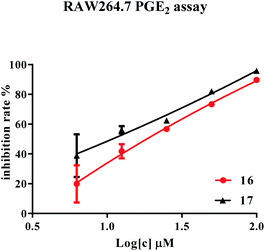
PGE2 and NO levels of LPS-stimulated RAW264.7 cells were tested to evaluate the anti-inflammatory effects of the isolated compounds. The microscopic observation showed that all compounds performed no obvious cytotoxicity to RAW264.7 cells at the maximum concentrations of 100 μM, indicated that the anti-inflammatory effects of the tested compounds was not caused by cytotoxicity. Results showed that compounds 16 and 17 exhibited inhibitory effects on the production of PGE2 with the IC50 values of 18.41 μM and 10.84 μM, respectively (Fig. 3). Compound 20 had a moderate inhibitory effect with an IC50 value of 50.58 μM (Fig. S83†), while other compounds showed no effect at dosage up to 100 μM. Besides, compounds 16 and 17 can decrease amount of NO release of the cells with an IC50 values of 53.09 μM and 53.70 μM, respectively (Fig. S84†).The observed PGE2 and NO inhibitory activities appear to be somewhat correlated with their structures. For example, with regard to the results for 5, 16 and 17, it appeared that the 4-OH might be important for higher activities.38 Comparing the structures and inhibitory activities of 16–20, it appeared that the carbonyl group at C-7′ and shortening of the side chain may cause a reduction in the inhibition of PGE2 and NO production.39 Interestingly, consideration of the structures of 1–4 versus 16-17 suggested that 9-OH was replaced by glycosyl groups, resulting in a decrease in the inhibitory activities of those dihydrobenzofuran neolignans.40,41 In addition, the inflammatory activity of one pair of diastereoisomers, 16 and 17, was similar, which led us to conclude that the absolute configuration of the compounds might have little or no inhibitory effect on PGE2 and NO production.42 These were not sufficient to clarify the accurate structure–activity relationship between the lignan derivatives and/or other components. More research may be required to clarify their potential selective NO and PGE2 inhibitory activity.
3 Conclusions
In the present research, nine undescribed compounds (1–9), including five benzofuran lignans, one ditetrahydrofuran lignan and three sesquilignans, together with fifteen know analogues (10–24) were isolated from the leaves of I. dunnianum. Their structures were established on the basis of extensive spectroscopic analysis (MS, UV, IR, NMR), in combination with CD spectrum and chemical methods (acidic hydrolysis and sugar analysis). Anti-inflammatory evaluation of the isolates suggested that compounds 16 and 17 had a moderate inhibitory effect on NO and PGE2 production in LPS-stimulated RAW264.7 cells. This study not only enriched the chemical diversity of ligans in I. dunnianum, but also provided an experimental basic for its anti-inflammatory activity.4 Experimental
4.1 General experimental procedures
Optical rotations were recorded using a JASCO P1020 digital polarimeter. Circular dichroism (CD) spectra were tested by JASCO J-810 circular dichroism spectrometer. Ultraviolet spectra (UV) were recorded on a JASCO V550 UV spectrometer. Infrared spectra (IR) were measured using a JASCO FT/IR-480 plus spectrometer. NMR spectra were acquired on Bruker AV 600 MHz or 400 MHz using solvent signal (CD3OD: δH 3.30/δC 49.0) as internal reference. Deuterated solvents were purchased from Cambridge Isotope Laboratories, Inc. (Saint Louis, MO, US). High-resolution electrospray ionization mass (HR-ESI-MS) spectra were obtained using a Waters Synapt G2 mass spectrometer.Analytical HPLC was conducted on a Shimadzu HPLC system with an LC-20AB solvent delivery system and an SPD-20A UV/vis detector using a Phenomenex Gemini C18 column (5 μm, Φ 4.6 × 250 mm; Phenomenex Inc., Los Angeles, USA). Semi-preparative HPLC was carried out on a Shimadzu LC-6AD liquid chromatography system equipped with a SPD-20A detector on a Phenomenex Gemini C18 column (5 μm, Φ 10.0 × 250 mm; Phenomenex Inc., Los Angeles, USA) and preparative HPLC using a Cosmosil Packed C18 column (5 μm, Φ 20.0 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan). Diaion HP-20 (Mitsubishi Chemical Co., Tokyo, Japan), silica gel 200–300 mesh and polyamide 50–100 mesh (Qingdao Haiyang Chemical Co., Ltd., Shandong, China), octadecyl silane (ODS) silica gel (12 nm, S-50 μm, YMC Ltd., Tokyo, Japan) were used for column chromatography (CC). Precoated silica gel GF254 plates for thin-layer chromatography (TLC) were from Qingdao Haiyang Chemical Co., Ltd. HPLC-grade methanol and acetonitrile were bought from Oceanpack Alexative Chemicals Co. Ltd. (Gothenburg, Sweden). All analytical grade reagents were from Concord Chemicals Co. Ltd., (Tianjin, China).
High glucose Dulbecco's modified Eagle's medium (DMEM) and 0.25% trypsin-EDTA were purchased from Gibco BRL Co. (New York, US). Fetal bovine serum (FBS) was purchased from Lonsera Bio. Tech. (Shanghai, China). Lipopolysaccharide (LPS) was purchased from Nanjing Da Zhi Biological Technology Co., Ltd. (Nanjing, China). DMSO was purchased from Aladdin Reagent (Shanghai, China). PGE2 ELISA kit was purchased from Enzo Life Sciences (Farmingdale, US). The 24-well plates were purchased from JET company. Murine macrophage cell line RAW264.7 was obtained from Chinese Academy of Traditional Chinese Medicines (Beijing, China).
5 Plant material
The leaves of I. dunnianum were purchased from Ji'an County, Jiangxi, China, in 2018, and were identified by Prof. Zhou Wu (Jiangsu Kanion Pharmaceutical Co. Ltd.). A sample (2018ID101) was deposited in Institute of Traditional Chinese Medicine & Natural Products, college of pharmacy, Jinan University, Guangzhou, China.5.1 Extraction and isolation
The air-dried leaves of I. dunnianum (ID, 15.5 kg) were extracted with 50% EtOH by heat reflux for 3 times (2 h each). Total extracts (IDEs, 2 kg) were yielded by evaporation under reduced pressure. IDEs were separated by HP-20 resin column chromatography (CC) eluted with EtOH–H2O (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 30
100, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 50
70, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 95
50, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) gradient to afford 4 fractions (ID-1 to ID-4). Fr. ID-3 (180 g) was separated over a silica gel column eluting with a CH2Cl2–MeOH gradient (100
5) gradient to afford 4 fractions (ID-1 to ID-4). Fr. ID-3 (180 g) was separated over a silica gel column eluting with a CH2Cl2–MeOH gradient (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 98
0, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 95
2, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 85
10, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 80
15, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 50
40, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 0
50, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to afford 15 fractions (Fr. 3A to 3O). Fr. 3L (17.4 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (30
100) to afford 15 fractions (Fr. 3A to 3O). Fr. 3L (17.4 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70–70
70–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 100
30, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to give 9 subfractions (Fr. 3L1–3L9). Fr. 3L4 was further chromatographed by polyamide CC using a EtOH–H2O gradient elution (10
0) to give 9 subfractions (Fr. 3L1–3L9). Fr. 3L4 was further chromatographed by polyamide CC using a EtOH–H2O gradient elution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90–40
90–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 95
60, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to give 7 subfractions (Fr. 3L4A–3L4G). Fr. 3 L4A was isolated using preparative HPLC [35% CH3OH–H2O (containing 0.1% HCOOH), 8 mL min−1] to yield 2 (10 mg) and fractions 3L4A2–3L4A9. Fr. 3L4A5 was isolated using semipreparative HPLC [22% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 3 (5.5 mg) and 8 (13 mg). Fr. 3L4A6 was isolated using semipreparative HPLC [16% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 9 (7.9 mg) and 10 (15.3 mg). Fr. 3L4A7 was isolated using semipreparative HPLC [18% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 4 (4.3 mg). Fr. 3L4A8 was isolated using semipreparative HPLC [22% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 5 (11.8 mg). Fr. 3L4A9 was isolated using semipreparative HPLC [23% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 1 (45.4 mg). Fr. 3 G (7.1 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (25
5) to give 7 subfractions (Fr. 3L4A–3L4G). Fr. 3 L4A was isolated using preparative HPLC [35% CH3OH–H2O (containing 0.1% HCOOH), 8 mL min−1] to yield 2 (10 mg) and fractions 3L4A2–3L4A9. Fr. 3L4A5 was isolated using semipreparative HPLC [22% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 3 (5.5 mg) and 8 (13 mg). Fr. 3L4A6 was isolated using semipreparative HPLC [16% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 9 (7.9 mg) and 10 (15.3 mg). Fr. 3L4A7 was isolated using semipreparative HPLC [18% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 4 (4.3 mg). Fr. 3L4A8 was isolated using semipreparative HPLC [22% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 5 (11.8 mg). Fr. 3L4A9 was isolated using semipreparative HPLC [23% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 1 (45.4 mg). Fr. 3 G (7.1 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75–65
75–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, 100
35, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to give 16 subfractions (Fr. 3G1–3G16). Fr.3G8 was further purified by semipreparative HPLC [40% CH3OH–H2O (0.1% HCOOH), 3 mL min−1] to yield 6 (5.6 mg). Fr. 3G9 was further purified by semipreparative HPLC [20% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 11 (21 mg). Fr. 3F (5.7 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (30
0) to give 16 subfractions (Fr. 3G1–3G16). Fr.3G8 was further purified by semipreparative HPLC [40% CH3OH–H2O (0.1% HCOOH), 3 mL min−1] to yield 6 (5.6 mg). Fr. 3G9 was further purified by semipreparative HPLC [20% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 11 (21 mg). Fr. 3F (5.7 g) was chromatographed by ODS CC using a CH3OH–H2O gradient elution (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70–60
70–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 100
40, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to give 16 subfractions (Fr. 3F1–3F15). Compound 13 (9.3 mg) was obtained from Fr. 3F6 by semipreparative HPLC with 14% CH3CN–H2O (0.1% HCOOH, 3.0 mL min−1). Compound 22 (3.5 mg) was obtained from Fr. 3F6B by semipreparative HPLC with 30% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1). Fr. 3F6D was further purified by semipreparative HPLC [30% CH3OH–H2O (0.1% HCOOH), 3 mL min−1] to yield 24 (3.2 mg). Compounds 14 (6.8 mg), 18 (1.1 mg) and 19 (1.1 mg) were obtained from Fr. 3F6E by semipreparative HPLC with 25% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1). Compound 15 (2.6 mg), compound 20 (3 mg), compound 21 (3.8 mg) and compound 23 (22 mg) were obtained from Fr. 3F by semipreparative HPLC with 20% CH3CN–H2O, 30% CH3OH–H2O, 25% CH3OH–H2O and 30% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1), respectively. Compound 16 (1.1 g) was obtained from Fr. 3F8 by silica gel column eluting with a cyclohexane–ethyl acetate gradient. Compound 12 (6.4 mg) was obtained from Fr. 3F9 by semipreparative HPLC with 19% CH3CN–H2O (0.1% HCOOH, 3.0 mL min−1). Fr. 3F9E and Fr. 3F9F was further purified by semipreparative HPLC [40% CH3OH–H2O, 25% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 17 (9.6 mg) and 7 (1.6 mg), respectively. The chiral HPLC analysis of compounds 5 and 6 were using EnantioPak OD column (4.6 × 250 mm) with 20% CH3CN–H2O and 30% CH3CN–H2O (0.1% HCOOH, 1 mL min−1, 35 °C), respectively. Compound 7 was using EnantioPak OZ-3 column (4.6 × 250 mm) with 35% CH3CN–H2O.
0) to give 16 subfractions (Fr. 3F1–3F15). Compound 13 (9.3 mg) was obtained from Fr. 3F6 by semipreparative HPLC with 14% CH3CN–H2O (0.1% HCOOH, 3.0 mL min−1). Compound 22 (3.5 mg) was obtained from Fr. 3F6B by semipreparative HPLC with 30% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1). Fr. 3F6D was further purified by semipreparative HPLC [30% CH3OH–H2O (0.1% HCOOH), 3 mL min−1] to yield 24 (3.2 mg). Compounds 14 (6.8 mg), 18 (1.1 mg) and 19 (1.1 mg) were obtained from Fr. 3F6E by semipreparative HPLC with 25% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1). Compound 15 (2.6 mg), compound 20 (3 mg), compound 21 (3.8 mg) and compound 23 (22 mg) were obtained from Fr. 3F by semipreparative HPLC with 20% CH3CN–H2O, 30% CH3OH–H2O, 25% CH3OH–H2O and 30% CH3OH–H2O (0.1% HCOOH, 3.0 mL min−1), respectively. Compound 16 (1.1 g) was obtained from Fr. 3F8 by silica gel column eluting with a cyclohexane–ethyl acetate gradient. Compound 12 (6.4 mg) was obtained from Fr. 3F9 by semipreparative HPLC with 19% CH3CN–H2O (0.1% HCOOH, 3.0 mL min−1). Fr. 3F9E and Fr. 3F9F was further purified by semipreparative HPLC [40% CH3OH–H2O, 25% CH3CN–H2O (0.1% HCOOH), 3 mL min−1] to yield 17 (9.6 mg) and 7 (1.6 mg), respectively. The chiral HPLC analysis of compounds 5 and 6 were using EnantioPak OD column (4.6 × 250 mm) with 20% CH3CN–H2O and 30% CH3CN–H2O (0.1% HCOOH, 1 mL min−1, 35 °C), respectively. Compound 7 was using EnantioPak OZ-3 column (4.6 × 250 mm) with 35% CH3CN–H2O.
5.2 Structural characterization of undescribed compounds
Illiciumlignan G (1): brown amorphous powder; [α]25D −35.2 (c 0.75, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 207 (4.90), 236 (4.34), 284 (4.04) nm; IR (KBr) νmax 3340, 2930, 2882, 1611, 1506, 1455, 1362, 1274, 1212, 1138, 1042 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 661.2471 [M + Na]+ (calcd for C31H42O14Na, 661.2472).
ε) 207 (4.90), 236 (4.34), 284 (4.04) nm; IR (KBr) νmax 3340, 2930, 2882, 1611, 1506, 1455, 1362, 1274, 1212, 1138, 1042 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 661.2471 [M + Na]+ (calcd for C31H42O14Na, 661.2472).
Illiciumlignan H (2): yellow amorphous powder; [α]25D −23.8 (c 0.55, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (4.66), 236 (4.10), 285 (3.88) nm; IR (KBr) νmax 3436, 2946, 2873, 1602, 1514, 1450, 1390, 1356, 1271, 1132, 1045 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 647.2333 [M + Na]+ (calcd for C30H40O14Na, 647.2316).
ε): 206 (4.66), 236 (4.10), 285 (3.88) nm; IR (KBr) νmax 3436, 2946, 2873, 1602, 1514, 1450, 1390, 1356, 1271, 1132, 1045 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 647.2333 [M + Na]+ (calcd for C30H40O14Na, 647.2316).
Illiciumlignan I (3): brown amorphous powder; [α]25D −18 (c 0.55, MeOH); UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 205 (4.65), 235 (4.10), 285 (3.86); IR (KBr) νmax 3371, 2927, 2879, 1614, 1511, 1450, 1378, 1333, 1277, 1130, 1056 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 661.2477 [M + Na]+ (calcd for C31H42O14Na, 661.2472).
ε): 205 (4.65), 235 (4.10), 285 (3.86); IR (KBr) νmax 3371, 2927, 2879, 1614, 1511, 1450, 1378, 1333, 1277, 1130, 1056 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 661.2477 [M + Na]+ (calcd for C31H42O14Na, 661.2472).
Illiciumlignan J (4): brown amorphous powder; [α]25D −14.75 (c 0.4, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (4.7), 236 (4.16), 284 (3.89) nm; IR (KBr) νmax 3289, 2927, 2876, 1735, 1611, 1504, 1455, 1424, 1271, 1212, 1127, 1073, 1039 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 677.2423 [M + Na]+ (calcd for C31H42O15Na, 677.2421).
ε): 206 (4.7), 236 (4.16), 284 (3.89) nm; IR (KBr) νmax 3289, 2927, 2876, 1735, 1611, 1504, 1455, 1424, 1271, 1212, 1127, 1073, 1039 cm−1; the 1H and 13C NMR spectra data see Table 1; HR-ESI-MS m/z: 677.2423 [M + Na]+ (calcd for C31H42O15Na, 677.2421).
Illiciumlignan K (5): yellow oil; [α]25D −6.7 (c 1.0, MeOH); UV (MeOH) λmax (log ε): 209 (4.77), 236 (4.19), 285 (3.71) nm; IR (KBr) νmax 3462, 3408, 2933, 2867, 1738, 1608, 1501, 1458, 1424, 1331, 1214, 1127, 1053 cm−1; the 1H and 13C NMR spectra data see Table 2; HR-ESI-MS m/z: 479.1923 [M + H]+ (calcd for C24H31O10, 479.1917).
Illiciumlignan L (6): yellow oil; [α]25D −38.24 (c 0.51, MeOH); UV(MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 207 (5.26), 233 (4.63), 280 (4.01) nm; IR (KBr) νmax 3420, 2938, 2861, 1750, 1599, 1511, 1461, 1424, 1371, 1336, 1271, 1232, 1124, 1053 cm−1; the 1H and 13C NMR spectra data see Table 2; HR-ESI-MS m/z: 477.1762 [M + H]+ (calcd for C24H29O10, 477.1761).
ε): 207 (5.26), 233 (4.63), 280 (4.01) nm; IR (KBr) νmax 3420, 2938, 2861, 1750, 1599, 1511, 1461, 1424, 1371, 1336, 1271, 1232, 1124, 1053 cm−1; the 1H and 13C NMR spectra data see Table 2; HR-ESI-MS m/z: 477.1762 [M + H]+ (calcd for C24H29O10, 477.1761).
Illiciumlignan M (7): brown amorphous powder; [α]25D +10.13 (c 0.8, MeOH); UV(MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (4.81), 236 (4.23), 275 (3.79) nm; IR (KBr) νmax 3369, 2944, 2854, 1599, 1509, 1458, 1419, 1381, 1333, 1235, 1127, 1036 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 657.2526 [M + Na]+ (calcd for C32H42O13Na, 657.2523).
ε): 206 (4.81), 236 (4.23), 275 (3.79) nm; IR (KBr) νmax 3369, 2944, 2854, 1599, 1509, 1458, 1419, 1381, 1333, 1235, 1127, 1036 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 657.2526 [M + Na]+ (calcd for C32H42O13Na, 657.2523).
Illiciumlignan N (8): yellowish oil; [α]25D −52.89 (c 0.45, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (5.18), 236 (4.64), 277 (4.23) nm; IR (KBr) νmax 3303, 2933, 2873, 1602, 1506, 1458, 1424, 1375, 1331, 1266, 1223, 1130, 1068 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 771.2847 [M + Na]+ (calcd for C37H48O16Na, 771.2840).
ε): 206 (5.18), 236 (4.64), 277 (4.23) nm; IR (KBr) νmax 3303, 2933, 2873, 1602, 1506, 1458, 1424, 1375, 1331, 1266, 1223, 1130, 1068 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 771.2847 [M + Na]+ (calcd for C37H48O16Na, 771.2840).
Illiciumlignan O (9): white amorphous powder; [α]25D −15.43 (c 0.7, MeOH); UV(MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (5.03), 235 (4.51), 277 (4.14) nm; IR (KBr) νmax 3374, 2936, 2884, 1722, 1614, 1504, 1461, 1427, 1328, 1212, 1127, 1036 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 771.2833 [M + Na]+ (calcd for C37H48O16Na, 771.2840).
ε): 206 (5.03), 235 (4.51), 277 (4.14) nm; IR (KBr) νmax 3374, 2936, 2884, 1722, 1614, 1504, 1461, 1427, 1328, 1212, 1127, 1036 cm−1; the 1H and 13C NMR spectra data see Table 3; HR-ESI-MS m/z: 771.2833 [M + Na]+ (calcd for C37H48O16Na, 771.2840).
5.3 Acid hydrolysis and sugar analysis
The compounds (1.0 mg) were hydrolyzed with 2 mL of 2 M HCl for 2 h at 90 °C. The hydrolysates were extracted with equal volume of ethyl acetate twice. The aqueous layer was dried, and then reacted with 2.5 mg L-cysteine methyl ester hydrochloride in 1 mL of pyridine for 1 h at 60 °C. After 1 h, a total of 5 μL of o-tolyl isothiocyanate was added to the reaction mixture and further reacted at 60 °C for 1 h. The reaction products were filtered by a 0.45 μm filter membrane for HPLC analysis, detected by a UV detector at 250 nm. Authentic samples of D-Glc, L-Glc, D-Xyl, L-Xyl, D-Rha, L-Rha and D-Fuc, L-Fuc were treated following same procedure.5.4 Anti-inflammatory activity assays
Inhibition of PGE2 production RAW264.7 cells were plated in 24-well plates at 1 × 105 cells per mL (400 μL per well) and cultured for 24 h in a 37 °C 5% CO2 incubator, then the supernatants were remove. Compounds were first dissolved in DMSO to prepare the stock solution at a concentration of 100 mM, and then the dilution of the compounds in DMEM (0.1% DMSO) were added in the sample wells (495 μL per well). After one hour later, 5 μL of LPS at 100 μg mL−1 was added into each well, the control/model wells was given 5 μL 0.1% DMSO in DMEM. Then the cells were cultured under normal condition for 18 h. Then the supernatants were collected and PGE2 levels were determined.NO suppression RAW264.7 cells were plated in 96-well plates at 2 × 106 cells per mL (100 μL per well). Then the cells were treated by LPS stimulation following the above method. After the cells had been treated with a series of compounds for 24 h, the production of NO in each supernatant was determined based on the Griess reaction, and the absorbance was measured at 540 nm in a microplate reader.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (program No. 81630097). The authors are grateful to State Key Laboratory of New-Tech for Chinese Medicine Pharmaceutical Process, Jiangsu Kanion Pharmaceutical Co. Ltd. for their assistance of activity test.Notes and references
- H. P. Deng, L. Sun, Z. X. Xi, X. Li, C. Chen and L. N. Sun, J. Pharm. Pract., 2012, 30, 8–13 CAS.
- J. W. Zhang, Zhongguo Zhongyao Zazhi, 1989, 14, 36–37 CAS.
- L. F. Zhang, K. Z. Lin, W. D. Yang, J. L. Wei, G. H. Yang and X. W. Lu, Zhongguo Zhongyao Zazhi, 1995, 717–718 Search PubMed.
- W. L. Zeng, J. Y. Song, Y. F. Cen, Y. Z. Fang, L. S. Qu and S. Z. Li, Journal of Guiyang College of Traditional Chinese Medicine, 1992, 60–62 Search PubMed.
- Q. Lin, Chin. Tradit. Herb. Drugs, 2002, 33, 654–657 Search PubMed.
- Y. Z. Fang, J. Y. Song, Y. F. Cen, W. L. Zeng and D. S. Xie, Journal of Guiyang College of Traditional Chinese Medicine, 1989, 1, 59–62 Search PubMed.
- J. Bai, H. Chen, Z. F. Fang, S. S. Yu, S. G. Ma, Y. Li, J. Qu, S. Xu, J. H. Ren, H. N. Lu and X. Chen, J. Asian Nat. Prod. Res., 2012, 14, 940–949 CrossRef CAS PubMed.
- J. Bai, H. Chen, Z. F. Fang, S. S. Yu, W. J. Wang, Y. Liu, S. G. Ma, Y. Li, J. Qu, S. Xu, J. H. Liu, F. Zhao and N. Zhao, Phytochemistry, 2012, 80, 137–147 CrossRef CAS PubMed.
- L. Sy and G. Brown, Phytochemistry, 1998, 47, 301–302 CrossRef CAS.
- J. Huang, J. Wang and C. Yang, Phytochemistry, 1997, 46, 777–780 CrossRef CAS.
- I. Kouno, N. Kawano and C. S. Yang, J. Chem. Soc., Perkin Trans. 1, 1988, 1537–1539 RSC.
- J. Li, D. Geng, J. Xu, L. J. Weng, Q. Liu and L. T. Yi, Eur. J. Pharmacol., 2013, 707, 112–119 CrossRef CAS PubMed.
- L. Sy and G. Brown, Phytochemistry, 1996, 43, 1417–1419 CrossRef CAS.
- L. Sy, R. Saunders and G. Brown, Phytochemistry, 1997, 44, 1099–1108 CrossRef CAS.
- C. Y. IsaoKouno, Chem. Pharm. Bull., 1991, 39, 2606–2608 CrossRef.
- J. Bai, Z. F. Fang, H. Chen, S. S. Yu, D. Zhang, H. L. Wei, S. G. Ma, Y. Li, J. Qu, S. Xu, J. H. Ren, F. Zhao, N. Zhao and J. H. Liu, Carbohydr. Res., 2012, 361, 206–211 CrossRef CAS PubMed.
- D. Geng, L. J. Weng, Y. Y. Han and X. Yang, Zhongguo Zhongyao Zazhi, 2012, 1914, 1586–1589 Search PubMed.
- D. Geng, L. J. Weng, Y. Y. Han and X. Yang, Zhongguo Zhongyao Zazhi, 2013, 2115, 3375–3377 Search PubMed.
- L. Y. Wang, M. H. Chen, J. Wu, H. Sun, W. Liu, Y. H. Qu, Y. C. Li, Y. Z. Wu, R. Li, D. Zhang, S. Wang and S. Lin, J. Nat. Prod., 2017, 80, 1808–1818 CrossRef CAS PubMed.
- C. S. Kim, L. Subedi, S. Y. Kim, S. U. Choi, K. H. Kim and K. R. Lee, J. Nat. Prod., 2015, 78, 1174–1178 CrossRef CAS PubMed.
- H. H. Xie, J. Su, X. L. Ge, T. T. Dong, X. Li, H. M. Wen and B. H. Sun, Nat. Prod. Res., 2018, 32, 418–424 CrossRef CAS PubMed.
- Y. Kamaya, F. Nakatsubo and T. Higuchi, Agric. Biol. Chem., 1983, 47, 299–308 CAS.
- L. Xiong, C. Zhu, Y. Li, Y. Tian, S. Lin, S. Yuan, J. F. Hu, Q. Hou, N. H. Chen, Y. C. Yang and J. Shi, J. Nat. Prod., 2011, 74, 1188–1200 CrossRef CAS PubMed.
- S. S. Li, Z. L. Hou, G. D. Yao, R. Guo, Y. X. Wang, B. Lin, X. X. Huang and S. J. Song, Phytochemistry, 2020, 178, 112461 CrossRef CAS PubMed.
- P. Y. Gao, L. Z. Li, Y. Peng, F. F. Li, C. Niu, X. X. Huang, M. Ming and S. J. Song, Biochem. Syst. Ecol., 2010, 38, 988–992 CrossRef CAS.
- T. Morikawa, J. Tao, K. Ueda, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2003, 51, 62–67 CrossRef CAS PubMed.
- S. F. Li, Y. T. Di, Y. H. Wang, C. J. Tan, X. Fang, Y. Z. Zhang, Y. T. Zheng, L. Li, H. P. He, L. S. Li and X. J. Hao, Helv. Chim. Acta, 2010, 93, 1795–1802 CrossRef CAS.
- A. Jutiviboonsuk, H. Zhang, G. T. Tan, C. Ma, N. Van Hung, N. M. Cuong, N. Bunyapraphatsara, D. D. Soejarto and H. H. Fong, Phytochemistry, 2005, 66, 2745–2751 CrossRef CAS.
- A. Ahmed, W. Li, J. S. Zhang, P. H. Sam, Y. H. Zou, G. H. Tang and S. Yin, Nat. Prod. Res., 2018, 32, 175–181 CrossRef CAS PubMed.
- W. H. Cai, K. Matsunami, H. Otsuka and Y. Takeda, Am. J. Plant Sci., 2011, 2, 609 CrossRef CAS.
- H. X. Kuang, Y. G. Xia, B. Y. Yang, Q. H. Wang and S. W. Lü, Arch. Pharmacal Res., 2009, 32, 329–334 CrossRef CAS PubMed.
- R. X. Tan, J. Jakupovic and Z. J. Jia, Planta Med., 1990, 56, 475–477 CrossRef CAS PubMed.
- H. H. Xiao, Y. Dai, M. S. Wong and X. S. Yao, J. Asian Nat. Prod. Res., 2015, 17, 625–632 CrossRef CAS.
- M. Yoshikawa, T. Morikawa and F. Xu, Heterocycles, 2003, 60, 1787–1792 CrossRef CAS.
- Q. B. Liu, X. X. Huang, M. Bai, X. B. Chang, X. J. Yan, T. Zhu, W. Zhao, Y. Peng and S. J. Song, J. Agric. Food Chem., 2014, 62, 7796–7803 CrossRef CAS PubMed.
- S. J. In, K. H. Seo, N. Y. Song, D. S. Lee, Y. C. Kim and N. I. Baek, Arch. Pharmacal Res., 2015, 38, 26–34 CrossRef CAS PubMed.
- Z. Shen and O. Theander, Phytochemistry, 1985, 24, 364–365 CrossRef CAS.
- X. X. Huang, M. Bai, L. Zhou, L. L. Lou, Q. B. Liu, Y. Zhang, L. L. Li and S. J. Song, J. Agric. Food Chem., 2015, 63, 7252–7260 CrossRef CAS PubMed.
- L. Xiao, Y. Huang, Y. Wang, J. Xu and X. He, Fitoterapia, 2020, 140, 104440 CrossRef CAS PubMed.
- N. Cho, H. Yang, J. W. Kim, Y. C. Kim and S. H. Sung, Bioorg. Med. Chem. Lett., 2014, 24, 5675–5678 CrossRef CAS PubMed.
- B. T. Zhao, N. D. Hung, J. H. Lee, B. S. Min and M. H. Woo, Bioorg. Med. Chem. Lett., 2017, 27, 524–529 CrossRef.
- Q. B. Liu, X. X. Huang, M. Bai, X. B. Chang, X. J. Yan, T. Zhu, W. Zhao, Y. Peng and S. J. Song, J. Agric. Food Chem., 2014, 62, 7796–7803 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of all new compounds. See DOI: 10.1039/d1ra03520g |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |