Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly porous copper-supported magnetic nanocatalysts: made of volcanic pumice textured by cellulose and applied for the reduction of nitrobenzene derivatives†
Reza Taheri-Ledari a,
Mahdi Saeidirada,
Fateme Sadat Qazia,
Atefeh Fazelia,
Ali Maleki
a,
Mahdi Saeidirada,
Fateme Sadat Qazia,
Atefeh Fazelia,
Ali Maleki *a and
Ahmed Esmail Shalan‡
*a and
Ahmed Esmail Shalan‡
 *bc
*bc
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir
bBC Materials, Basque Center for Materials, Applications and Nanostructures, Martina Casiano, UPV/EHU Science Park, Barrio Sarriena s/n, Leioa 48940, Spain. E-mail: a.shalan133@gmail.com; ahmed.shalan@bcmaterials.net
cCentral Metallurgical Research and Development Institute (CMRDI), P. O. Box 87, Helwan, Cairo 11421, Egypt
First published on 21st July 2021
Abstract
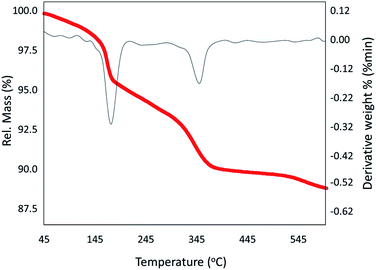
Herein, a novel designed heterogeneous catalytic system constructed of volcanic pumice magnetic particles (VPMPs), cellulose (CLS) as a natural polymeric matrix, and copper nanoparticles (Cu NPs) is presented. Also, to enhance the inherent magnetic property of VPMP, iron oxide (Fe3O4) nanoparticles have been prepared and incorporated in the structure via an in situ process. As its first and foremost excellent property, the designed composite is in great accordance with green chemistry principles because it contains natural ingredients. Another brilliant point in the architecture of the designed composite is the noticeable porosity of VPMP as the core of the composite structure (surface area: 84.473 m2 g−1). This great porosity leads to the use of a small amount (0.05 g) of the particles for catalytic purposes. However, the main characterization methods, such as Fourier-transform infrared and energy-dispersive X-ray spectroscopy, thermogravimetric analysis, and electron microscopy, revealed that the spherical metallic particles (Fe and Cu oxides) were successfully distributed onto the surface of the VPMP and CLS matrices. Further, vibrating-sample magnetometer analysis confirmed the enhancement of the magnetic property (1.5 emu g−1) of the composite through the addition of Fe3O4 nanoparticles. Further, the prepared (Fe3O4@VPMP/CLS–Cu) nanocomposite has been applied to facilitate the reduction reaction of hazardous nitrobenzene derivatives (NBDs) to their aniline analogs, with 98% conversion efficiency in eight minutes under mild conditions. Moreover, the good reusability of the catalytic system has been verified after recycling it ten times without any significant decrease in the performance.
1. Introduction
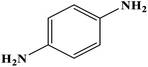
As time goes on, microscale and nanoscale heterogeneous catalytic systems are gaining increasing attention for a variety of reasons, of which one of the most important is that they are able to create a high surface area for chemical reactions between the involved reactants and the catalytic substrate.1–3 In this regard, heterogeneous catalytic systems based on iron oxide nanoparticles have become more important due to the possibility of their easy separation by only holding an external magnet at the bottom of the reaction flask and decanting the content.4–11 Heterogeneous catalytic composites are modified by various components, such as organic structures, polymers, inorganic particles, biological structures, copper nanoparticles, and palladium nanoparticles, so that the majority of the catalytic sites are available.12–20 In recent years, more attention has been paid to green chemistry due to environmental concerns.20,21 That is why researchers are constantly trying to design novel natural heterogeneous catalytic systems with a high degree of biocompatibility. Undoubtedly, volcanic pumice benefits from a very porous structure that can lead to a high surface area, and it possesses properties such as high magnetic property, biocompatibility, and great surface functionalization capability; thus, it can inherently act as an eco-friendly substrate to create a heterogeneous catalytic composite.21–25The use of cellulose (CLS) in this work, because it is natural, has been a strong indication of how important green chemistry was to us in this work.26,27 There are many reasons why cellulose, as a substantial polymeric substrate with a biocompatible origin, is used in this work; one of the most important of these is that cellulose, due to its large number of hydroxyl (–OH) functional groups, can form physicochemical hydrogen bonds with the –OH groups of pumice, which can lead to integration in the composite. On the other hand, the rest of the hydroxyl functional groups of cellulose, which are free of hydrogen bonds with the hydroxyl functional groups of pumice, are suitable for chelation by cationic metals such as copper and palladium and can form heterogeneous catalytic composites from natural components; this is one of the most important properties of this composite.28 The synthesized composite is notable even in terms of mechanical properties because it is a stable hybrid structure.29,30 As mentioned earlier, the synthetic heterogeneous catalytic composite in this work is suitable for scaling up and industrial applications because of its high biocompatibility and because it is made from components found in abundance in nature. In this work, after pumice, as an inorganic base with –OH groups functions as a suitable host for cellulose, as an organic base, Cu2+ ions are added to the system to act as catalytic sites to reduce the nitro functional groups of different compounds to amines after their conversion to Cu.31,32
Herein, an attempt was made to introduce a novel and convenient method for the synthesis of amine derivatives from the corresponding nitro compounds using Fe3O4@VPMP/CLS–Cu catalyst, which was synthesized with natural VPMP, CLS, and also Cu NPs. Then, the magnetic behavior and other critical characteristic properties of the prepared catalytic system, such as its average size, porosity, present chemical state, metallic elements, and thermal stability, were carefully investigated using different analytical methods. Then, the ability of this catalyst to reduce nitrobenzene derivatives (NBDs) was carefully examined. In summary, it was found that using the Fe3O4@VPMP/CLS–Cu catalytic system at 70 °C led to a 98% reaction yield in just 8 minutes. Also, the catalytic system under study can be reused for 10 successive runs due to its magnetic property, which leads to easy magnetic separation of this catalyst from the reaction medium. Also, in this study, water solvent was used as an eco-friendly solvent to evaluate the performance of the synthesized catalytic composite; this easily confirms the importance of green chemistry in this study.
2. Results and discussion
2.1. Preparation of the Fe3O4@VPMP/CLS–Cu nanocatalyst
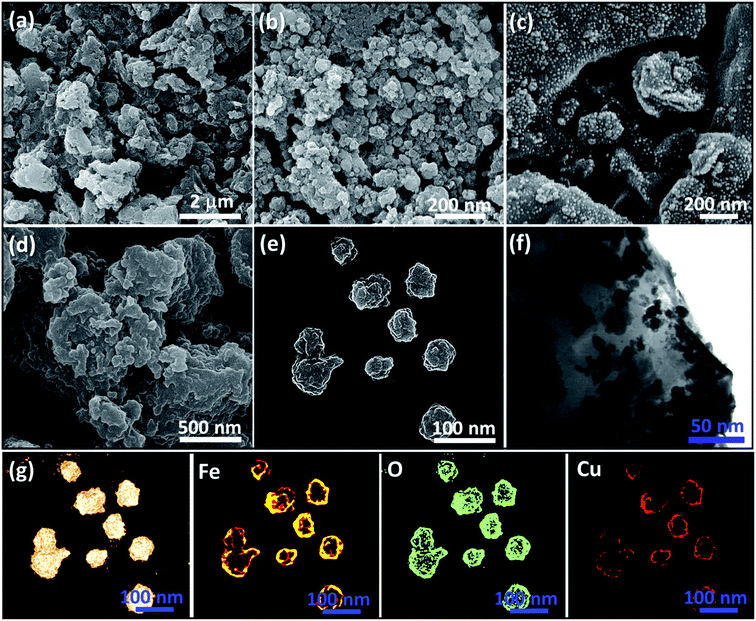
As shown in Scheme 1, several steps are taken to prepare the Fe3O4@VPMP/CLS–Cu composite. Initially, the pumice is ground by a ball-mill to form uniform particles.33 After that, because the pumice is very porous and there may be useless fillers in its pores, it should be calcinated at a high temperature in a furnace.34 Then, if electron microscopy (EM) confirms that the calcinated pumice has uniformity and the useless fillers have been successfully removed from its pores, the magnetic property of the pumice is increased by Fe3O4 in order to separate it from the solvent on a laboratory scale, although the pumice itself has an inherent magnetic property. Next, it is it is dispersed and mixed in a concentrated solution of CLS under mild conditions. At the end of this step, the formed composite is magnetically separated and washed to remove the unbound CLS. Herein, a composite called Fe3O4@VPMP/CLS was prepared that contains a large number of hydroxyl functional groups in its VPMP and CLS structures and can be used several times. In another flask, an aqueous solution of CuCl2·H2O was prepared, which was added to a solution of dispersed Fe3O4@VPMP/CLS in which the hydroxyl functional groups were activated by an alkaline aqueous solution. After completion of the reaction, the resulting composite, Fe3O4@VPMP/CLS–Cu(II), which was now brown and no longer black like magnetic pumice, was magnetically separated and washed to remove excess salts and ions trapped inside the pores and silicate network of the pumice. Ultimately, Cu(II) in the composite of Fe3O4@VPMP/CLS–Cu(II) was reduced to Cu(0) by sodium borohydride under alkaline conditions.35 Herein, we intend to monitor the catalytic ability of the Fe3O4@VPMP/CLS–Cu nanocatalyst in the reduction reactions of NBDs, and the results obtained from the mentioned experiments are reported in Table 1, in the optimization section.| Entry | Cat. | Cat. (mg) | Cat. (mmol%) | NaBH4 (mmol) | Temp. (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| a Optimum condition using the Fe3O4@VPMP/CLS–Cu nanocomposite (50.0 mg), nitrobenzene (1.0 mmol), NaBH4 (2.0 mol), and at 70 °C. Calculations related to mmol% of catalyst has been given in the ESI file. R.t. stands for room temperature. | |||||||
| 1 | — | — | — | — | 80 | 180 | N.R. |
| 2 | — | — | — | 2 | 80 | 180 | Trace |
| 3 | Fe3O4@VPMP/CLS | 30 | 12 | 2 | 80 | 90 | Trace |
| 4 | Fe3O4@VPMP/CLS–Cu | 10 | 4 | 2 | 70 | 8 | 85 |
| 5 | Fe3O4@VPMP/CLS–Cu | 30 | 12 | 2 | 70 | 8 | 90 |
| 6 | Fe3O4@VPMP/CLS–Cu | 50 | 20 | 2 | 70 | 8 | 98a |
| 7 | Fe3O4@VPMP/CLS–Cu | 60 | 24 | 2 | 70 | 8 | 98 |
| 8 | Fe3O4@VPMP/CLS–Cu | 50 | 20 | 3 | 70 | 8 | 94 |
| 9 | Fe3O4@VPMP/CLS–Cu | 50 | 20 | 1 | 70 | 8 | 90 |
| 10 | CLS–Cu | 50 | 20 | 2 | 70 | 8 | 92 |
| 11 | Fe3O4@VPMP/CLS–Cu | 50 | 20 | 2 | R.t. | 8 | 81 |
| 12 | Fe3O4@VPMP/CLS–Cu | 50 | 20 | 2 | R.t. | 30 | 97 |
2.2. Characterization of Fe3O4@VPMP/CLS–Cu nanocatalyst
2.3. Application of Fe3O4@VPMP/CLS–Cu nanocatalyst in the reduction reactions of nitrobenzene derivatives (NBDs)
| Entry | NB structure | Product | Time (min) | Yield (%) | Mp (°C) | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 1 |  |
 |
8 | 98 | Liquid sample | (Ref. 46) |
| 2 |  |
 |
13 | 97 | 145–146 | 144–146 (ref. 47) |
| 3 |  |
 |
7 | 96 | 135–138 | 136–138 (ref. 48) |
| 4 |  |
 |
3 | 98 | 172–175 | 171–173 (ref. 49) |
| 5 |  |
 |
4 | 96 | 187–189 | 186–188 (ref. 46) |
| 6 | 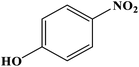 |
 |
8 | 95 | 184–186 | 185–187 (ref. 49) |
| 7 |  |
 |
13 | 89 | 65–66 | 65–66 (ref. 50) |
| 8 |  |
 |
12 | 86 | 51–53 | 52–53 (ref. 51) |
| 9 |  |
 |
7 | 97 | 42–44 | 42–43 (ref. 52) |
| 10 |  |
 |
9 | 97 | 70–71 | 70–72 (ref. 53) |
| Entry | Catalyst | Conditions | Cat. (mg) | Time (min) | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|
| a Poly-triallylamine (MPTA-1).b SBA-15 is a mesoporous silica.c Cobalt nanoparticles.d Reduced graphene oxide.e Copper nanoparticles.f The present work. | ||||||
| 1 | Ag–MPTA-1a | 80 °C/NaOH/N2 | 250 | 600 | 97 | 56 |
| 2 | Ag–SBA-15b | R.t./NaBH4 | 10 | 6 | 95 | 57 |
| 3 | Nano Coc | R.t./N2H4·H2O | 5.6 | 30 | 50 | 58 |
| 4 | PhNO2,Pd NPs/RGOd | 50 °C/NaBH4 | 6.0 | 90 | 98 | 59 |
| 5 | Cu NPse | 50 °C | 6.0 | 120 | 95 | 60 |
| 6 | Fe3O4@VPMP/CLS–Cuf | 70 °C | 5.0 | 8 | 98 | — |
3. Experimental
3.1. Chemicals and instruments
All the chemicals, reagents, and equipment used in this study are listed in Table 4.| Materials and equipment | Purity and brand |
|---|---|
| Pumice stone | Granulated – Sigma Aldrich |
| Cellulose | Microcrystalline – Sigma Aldrich |
| FeCl2·4H2O | Sigma Aldrich (98%) |
| FeCl3·6H2O | Sigma Aldrich (≥98%) |
| Ammonia | Merck (25%) |
| Sodium hydroxide | Merck, 97% |
| CuCl2·2H2O | Sigma Aldrich (≥99%) |
| Ethanol | Sigma Aldrich – 97% |
| Sodium borohydride | Sigma Aldrich (≥96%) |
| FT-IR analysis | Shimadzu IR-470 spectrometer |
| EDX analysis | Numerix DXP-X10P |
| SEM analysis | Sigma-Zeiss microscope |
| TEM analysis | Philips Cm 12 Instrument |
| VSM analysis | Lakeshore 7407 |
| TGA/DTA analysis | STA504 device |
| XRD analysis | JEOL JDX-8030 (30 kV, 20 mA) |
| BET analysis | Micromeritics ASAP 2010 |
| XPS analysis | K-Alpha+ |
| ICP-OES analysis | DV 5300 |
| ICP-MS analysis | ELAN 6100 DRC-e |
| NMR analysis | Varian Unity Inova 500 MHz |
| Ultrasound cleaning bath | KQ-250 DE (40 kHz, 250 W) |
| Melting point measurement | Electrothermal 9100, made in UK |
3.2. Preparation methods
3.3. A general procedure for reduction of nitroarenes with the Fe3O4@VPMP/CLS–Cu(0) system
To a round-bottom flask (25 mL) containing deionized water (5 mL), nitrobenzene (1 mmol, 0.123 g) and Fe3O4@VPMP/CLS–Cu(II) (0.05 g) were added. Then, the pH of the mixture was tuned to ca. 8.0 by addition of K2CO3 (0.04 g). Next, NaBH4 (2 mmol, 0.075 g) was added to the mixture, and the resulting mixture was stirred at 70 °C. After completion of the reaction, the catalyst was separated by an external magnet and the product was extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure afforded the pure aniline in 98% yield.3.4. Large scale experiment on the reduction of nitrobenzene by the Fe3O4@VPMP/CLS–Cu catalytic system
To evaluate the efficiency of the prepared Fe3O4@VPMP/CLS–Cu catalytic system in industrial applications, a gram scale model experiment was carried out. For this purpose, in a round bottom flask (500 mL), 40 mmol (4.9 g) of nitrobenzene was dissolved in 160 mL of water. Then, the pH of the mixture was tuned to ca. 8.0 by addition of K2CO3 (1.6 g), and the temperature of the flask was controlled with an ice bath. In a separate flask (100 mL), 2.0 g of Fe3O4@VPMP/CLS–Cu powder was well dispersed in 40 mL of water via ultrasonication by a cleaner bath (50 KHz, 100 W L−1) and finally added to the main flask (500 mL) containing the nitrobenzene solution. Then, NaBH4 (80 mmol, 3.0 g) was added to the mixture, and the resulting mixture was stirred in an ice bath. At this stage, the reaction temperature was maintained at around 20 °C. After completion of the reaction, the magnetic particles were separated with an external magnet several times. Next, the residue was divided into five 40 mL portions, and liquid–liquid extraction was performed using EtOAc (20 mL) (three times for each portion). Finally, the organic phase was dried over anhydrous Na2SO4 (5.0 g), and the solvent was evaporated under reduced pressure (pure aniline in 98% yield).4. Conclusion
In this study, a magnetic catalytic system using natural-based materials was presented that has the ability to reduce various derivatives of nitrobenzene (NB) in a very short time of 8 minutes. This efficient catalytic system was characterized by FT-IR spectroscopy, SEM, EDX spectroscopy, XRD spectroscopy, TGA, and VSM. This catalytic system has many advantages, such as (1) convenient separation, which is due to the superparamagnetic behavior of the nanoscale catalytic system, which was also proven by VSM analysis; (2) due to the high porosity of the pumice according to SEM, this catalytic system is able to provide a large active surface area; and (3) the use of low amounts of the heterogeneous catalyst (0.05 g) and many other advantages can introduce this composite as a suitable candidate for scaling up and industrial applications.Author contributions
R. T. L. designed the project and led the hypothesis. M. S. and F. S. Q. carried out the experimental sections and provided the required analyses. R. T. L. and M. S. prepared the first draft of manuscript. All the authors helped in interpreting the obtained results, writing, editing and revising the manuscript. A. M. and A. E. S. managed and supervised all the work sections.Conflicts of interest
The authors listed in this article have no conflict of interests.Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST). Furthermore, AES thanks the National Research grants from MINECO, Spain, “Juan de la Cierva” [FJCI-2018-037717]. Also, the authors appreciate the kind accompaniment of Mr Seyed Masoud Seyed Ali Routeh from IAUSR.References
- S. Bao, Y. Wang, Z. Wei, W. Yang, Y. Yu and Y. Sun, J. Hazard. Mater., 2021, 416, 125825 CrossRef CAS.
- R. Taheri-Ledari and A. Maleki, New J. Chem., 2021, 45, 4135–4146 RSC.
- B. Reddy, R. Dadigala, R. Bandi, K. Seku, G. Koteswararao, K. Mangatayaru and A. E. Shalan, RSC Adv., 2021, 11, 5139–5148 RSC.
- A. Maleki, R. Taheri-Ledari, J. Rahimi, M. Soroushnejad and Z. Hajizadeh, ACS omega, 2019, 4, 10629–10639 CrossRef CAS PubMed.
- V. Soltaninejad, M. R. Ahghari, R. Taheri-Ledari and A. Maleki, Langmuir, 2021, 37(15), 4700–4713 CrossRef CAS PubMed.
- R. Taheri-Ledari, A. Maleki, E. Zolfaghari, M. Radmanesh, H. Rabbani, A. Salimi and R. Fazel, Ultrason. Sonochem., 2020, 61, 104824 CrossRef CAS PubMed.
- X. Meng, Y. Liu, G. Han, W. Yang and Y. Yu, Carbon, 2020, 162, 356–364 CrossRef CAS.
- S. Bao, W. Yang, Y. Wang, Y. Yu and Y. Sun, J. Hazard. Mater., 2021, 409, 124470 CrossRef CAS PubMed.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Mater. Res. Express, 2020, 7(1), 015067 CrossRef CAS.
- S. Nabih, A. E. Shalan, E. S. A. Serea, M. A. Goda and M. F. Sanad, J. Mater. Sci.: Mater. Electron., 2019, 30, 9623–9633 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari, R. Ghalavand and R. Firouzi-Haji, J. Phys. Chem. Solids, 2020, 136, 109200 CrossRef CAS.
- R. Taheri-Ledari, J. Rahimi, A. Maleki and A. E. Shalan, New J. Chem., 2020, 44, 19827–19835 RSC.
- A. Maleki, R. Taheri-Ledari and M. Soroushnejad, ChemistrySelect, 2018, 3, 13057–13062 CrossRef CAS.
- R. Taheri-Ledari, M. S. Esmaeili, Z. Varzi, R. Eivazzadeh-Keihan, A. Maleki and A. E. Shalan, RSC Adv., 2020, 10(66), 40055–40067 RSC.
- S. M. Abdellatif Soliman, M. F. Sanad and A. E. Shalan, RSC Adv., 2021, 11, 11541–11548 RSC.
- M. S. Esmaeili, Z. Varzi, R. Taheri-Ledari and A. Maleki, Res. Chem. Intermed., 2021, 47, 973–996 CrossRef CAS.
- R. Taheri-Ledari, S. S. Mirmohammadi, K. Valadi, A. Maleki and A. E. Shalan, RSC Adv., 2020, 10, 43670–43681 RSC.
- R. Taheri-Ledari and A. Maleki, J. Pept. Sci., 2020, 26, e3277 CrossRef CAS PubMed.
- A. E. Shalan, M. Afifi, M. M. El-Desoky and M. K. Ahmed, New J. Chem., 2021, 45, 9212–9220 RSC.
- S. Parvaz, R. Taheri-Ledari, M. S. Esmaeili, M. Rabbani and A. Maleki, Life Sci., 2020, 240, 117099 CrossRef CAS PubMed.
- Z. Varzi, M. S. Esmaeili, R. Taheri-Ledari and A. Maleki, Inorg. Chem. Commun., 2021, 125, 108465 CrossRef CAS.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- R. Taheri-Ledari, K. Valadi, S. Gharibi and A. Maleki, Mater. Res. Bull., 2020, 130, 110946 CrossRef CAS.
- A. Maleki, S. Gharibi, K. Valadi and R. Taheri-Ledari, Phys. Chem. Solids., 2020, 142, 109443 CrossRef CAS.
- X. Meng, W. Lei, W. Yang, Y. Liu and Y. Yu, J. Colloid Inter. Sci., 2021, 600, 382–389 CrossRef CAS PubMed.
- M. H. Abu Elella, E. S. Goda, H. M. Abdallah, A. E. Shalan, H. Gamal and K. R. Yoon, Int. J. Biol. Macromol., 2021, 167, 1113–1125 CrossRef CAS PubMed.
- S. Karami, B. Zeynizadeh and Z. Shokri, Cellulose, 2018, 25, 3295–3305 CrossRef CAS.
- S. S. Soltani, R. Taheri-Ledari, S. M. F. Farnia, A. Maleki and A. Foroumadi, RSC Adv., 2020, 10, 23359–23371 RSC.
- X. Zhang, C. Shi, E. Liu, N. Zhao and C. He, ACS Appl. Mater. Interfaces, 2018, 10, 37586–37601 CrossRef CAS PubMed.
- K. Valadi, S. Gharibi, R. Taheri-Ledari and A. Maleki, Solid State Sci., 2020, 101, 106141 CrossRef CAS.
- T. B. Devi and M. Ahmaruzzaman, Mater. Today: proceedings, 2017, 5, 2098–2104 Search PubMed.
- M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartoonia, M. Bagherzadeh and R. Safari, J. Mol. Catal. A Chem., 2015, 400, 22–30 CrossRef CAS.
- S. P. K. Kohobhange, C. H. Manoratne, H. M. T. G. A. Pitawala and R. M. G. Rajapakse, Powder Technol., 2018, 330, 266–274 CrossRef CAS.
- B. Demirel and O. Keleştemur, Fire Saf. J., 2010, 45, 385–391 CrossRef CAS.
- K. Naseem, Z. H. Farooqi, R. Begum and A. Irfan, J. Clean. Prod., 2018, 187, 296–307 CrossRef CAS.
- Z. Hajizadeh, K. Valadi, R. Taheri-Ledari and A. Maleki, ChemistrySelect, 2020, 5, 2441–2448 CrossRef CAS.
- R. Taheri-Ledari, S. M. Hashemi and A. Maleki, RSC Adv., 2019, 9, 40348–40356 RSC.
- A. M. Youssef, F. M. Assem, H. S. El-Sayed, S. M. El-Sayed, M. Elaaser and M. H. Abd El-Salam, RSC Adv., 2020, 10(62), 37857–37870 RSC.
- R. Taheri-Ledari, K. Valadi and A. Maleki, Prog. Photovolt. Res. Appl., 2020, 28, 956–970 CrossRef CAS.
- Y. Sun, Y. Zhou, C. Zhu, W. Tu, H. Wang, H. Huang, Y. Liu, M. Shao, J. Zhong, S. T. Lee and Z. Kang, Appl. Catal. B, 2019, 244, 795–801 CrossRef CAS.
- P. Chang, H. Mei, Y. Zhao, W. Huang, S. Zhou and L. Cheng, Adv. Funct. Mater., 2019, 29(34), 1903588 CrossRef.
- R. Taheri-Ledari, W. Zhang, M. Radmanesh, S. S. Mirmohammadi, A. Maleki, N. Cathcart and V. Kitaev, Small, 2020, 16, 2002733 CrossRef CAS PubMed.
- S. Suresh, S. Karthikeyan and K. Jayamoorthy, J. Sci. Adv. Mater. Dev., 2016, 1, 343–350 Search PubMed.
- D. Ferrah, A. R. Haines, R. P. Galhenage, J. P. Bruce, A. D. Babore, A. Hunt, I. Waluyo and J. C. Hemminger, ACS Catal., 2019, 9(8), 6783–6802 CrossRef CAS.
- L. Guo, J. Cao, J. Zhang, Y. Hao and K. Bi, J. Mater. Sci., 2019, 54(14), 10379–10388 CrossRef CAS.
- V. Kandathil, T. S. Koley, K. Manjunatha, R. B. Dateer, R. S. Keri, B. S. Sasidhar and S. A. Patil, Inorg. Chim. Acta, 2018, 478, 195–210 CrossRef CAS.
- S. L. Arora, J. L. Fergason and A. Saupe, Mol. Cryst. Liq. Cryst., 1970, 3, 243–257 CrossRef.
- A. K. Shil, D. Sharma, N. R. Guha and P. Das, Tetrahedron Lett., 2012, 36, 4858–4861 CrossRef.
- S. Sobhani, F. O. Chahkamali and J. M. Sansano, RSC Adv., 2019, 3, 1362–1372 RSC.
- M. Rajabzadeh, H. Eshghi, R. Khalifeh and M. Bakavoli, RSC Adv., 2016, 23, 19331–19340 RSC.
- T. Maejima, Y. Shimoda, K. Nozaki, S. Mori, Y. Sawama, Y. Monguchi and H. Sajiki, Tetrahedron, 2012, 6, 1712–1722 CrossRef.
- J. Li, E. D. Inutan, B. Wang, C. B. Lietz, D. R. Green, C. D. Manly, A. L. Richards, D. D. Marshall, S. Lingenfelter, Y. Ren and S. Trimpin, J. Am. Soc. Mass Spectrom., 2012, 10, 1625–1643 CrossRef PubMed.
- R. Mirbagheri and D. Elhamifar, J. Alloys Compd., 2019, 790, 783–791 CrossRef CAS.
- J. Cai, Y. Zhuang, Y. Chen, L. Xiao, Y. Zhao, X. Jiang, L. Hou and Z. Li, ChemCatChem, 2020, 12(24), 6241–6247 CrossRef CAS.
- J. F. de Souza, G. T. da Silva and A. R. Fajardo, Carbohydr. Polym., 2017, 161, 187–196 CrossRef CAS PubMed.
- N. Salam, B. Banerjeec, A. S. Roy, P. Mondal, A. S. Roy, A. Bhaumik and S. M. Islam, APPL. Catal. A-Gen., 2014, 477, 184–194 CrossRef CAS.
- B. Naik, S. Hazra, V. S. Prasad and N. N. Ghosh, Catal. Commun., 2011, 12, 1104–1108 CrossRef CAS.
- R. K. Rai, A. Mahata, S. Mukhopadhyay, S. Gupta, P. Li, K. T. Nguyen, Y. Zhao, B. Pathak and S. K. Singh, Inorg. Chem., 2014, 53, 2904–2909 CrossRef CAS PubMed.
- M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni, M. Alizadeh and M. Bagherzadeh, J. Colloid Inter. Sci., 2015, 466, 360–368 CrossRef PubMed.
- Z. Duan, G. Ma and W. Zhang, Bull. Korean Chem. Soc., 2012, 33, 4003–4006 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03538j |
| ‡ Currently on leave from CMRDI. |
| This journal is © The Royal Society of Chemistry 2021 |