Open Access Article
Open Access ArticleUntargeted metabolomic and lipid metabolism-related gene expression analyses of the effects and mechanism of aged Liupao tea treatment in HFD-induced obese mice†
Wenliang Wu a,
Yao Hud,
Shuguang Zhanga,
Dongming Liuc,
Qing Lib,
Yong Lin*b and
Zhonghua Liu*b
a,
Yao Hud,
Shuguang Zhanga,
Dongming Liuc,
Qing Lib,
Yong Lin*b and
Zhonghua Liu*b
aTea Research Institute, Hunan Academy of Agricultural Sciences, Changsha, Hunan 410125, PR China
bKey Laboratory of Tea Science of Ministry of Education, Hunan Agricultural University, Changsha 410128, PR China. E-mail: Yong-lin@hunau.edu.cn; zhonghua-liu@hunau.edu.cn
cChangsha University of Science & Technology, Changsha 410114, PR China
dNuclear Agronomy and Aerospace Breeding Research Institute, Hunan Academy of Agricultural Sciences, Changsha, Hunan 410125, PR China
First published on 6th July 2021
Abstract
Liupao tea (LPT) has been demonstrated to have beneficial effects on obesity induced by a high-fat diet (HFD); however, the effects and mechanism of aged Liupao tea (different storage years) treatment on obesity have not yet been reported. In this study, mice were divided into four groups as follows: the control group fed a normal diet; the model group fed an HFD; and the LPT aged 1 year (1Y) and LPT aged 10 years (10Y) groups receiving an HFD and water extractions from LPTs of different ages for 5 weeks. Our results revealed that aged LPT significantly alleviated HFD-induced obesity symptoms, especially in the 10Y group. Additionally, metabolomic analysis identified 11 common differential metabolites that were partly recovered to normal levels after aged LPT treatment, involved mainly in the metabolic pathways of the citrate cycle, purine metabolism, fatty acid metabolism, and amino acid metabolism. Aged LPT treatment also regulated lipid metabolism-related gene expression in the liver, which decreased the mRNA levels of SREBP-1C/HMGR/FAS involved in de novo lipogenesis and increased the mRNA levels of PPARα, LDLR and LCAT. Our study demonstrated that aged LPT may be used as a potential dietary supplement for improving obesity-related diseases caused by an HFD.
1 Introduction
The prevalence of obesity and lipid metabolism disorder has increased sharply in recent years; obesity has become a common problem worldwide, and a high-fat and high-sugar diet is the main reason.1,2 Therefore, the prevention of obesity and improvement of lipid metabolism are urgent matters. Tea, made from the dried buds and leaves of Camellia sinensis L., is one of the most popular beverages consumed in the world. It has been found to prevent and treat many diseases.3 Liupao tea (LPT) comes from Wuzhou City, Guangxi Province, China. LPT is a kind of postfermented Chinese dark tea. Previous studies have demonstrated that LPT could significantly alleviate HFD-induced obesity and hyperlipemia-related symptoms.4–7 However, the alleviative effects of aged LPT (different storage years) on obesity and its potential mechanisms have not been adequately studied. It has been reported that the contents of water extractions, tea polyphenols, free amino acids, water-soluble sugars, and other ingredients in teas change during storage.8 Evidence suggests that aged Oolong tea, aged Hakka stir-fried tea and aged Qingzhuan tea of different storage years have prevented HFD-induced obesity effects in mice to a certain extent.9–11 Hence, it is reasonable to infer that the bioactive strength of aged LPTs should change with storage time.To deeply understand the effects and mechanism of aged LPT on obesity, in this work, we investigated the effects of LPT aged for different years (1 year and 10 years) on HFD-induced obese mice. Biochemical and histological analyses of parameters related to obesity, including in the serum, liver, and epididymal fat tissues, were conducted. Moreover, high-performance liquid chromatography quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS)-based serum metabolomics and related gene expression patterns in the liver are discussed herein.
2 Materials and methods
2.1 Analysis of the major chemical constituents of Liupao tea (LPT)
LPT samples of the same grade were obtained from the Guangxi Wuzhoug Tea Factory (Wuzhou City, Guangxi Province, China) and stored for 1 year and 10 years. The powdered LPT was extracted three times using boiling distilled water (tea/water w/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for 20 min. The extracts were collected and further concentrated at 60 °C and finally freeze dried.
10) for 20 min. The extracts were collected and further concentrated at 60 °C and finally freeze dried.
The contents of tea polyphenols (TP), water extractions, free amino acids (FAAs) and caffeine (CAF) were determined using the Chinese national standards GB/T 8313-2008, GB/T 8302-2002, GB/T 8314-2002 and GB/T 8312-2002, respectively. Water-soluble sugars were measured by anthrone-sulfuric colorimetry.12 Theaflavins (TFs), thearubigins (TRs), theabrownins (TBs) and catechins were measured by using an existing method.13
2.2 Animals and experimental design
Male KM mice were obtained from Hunan SJA Laboratory Animal Co. Ltd, (Changsha City, Hunan Province, China, SCXK (Xiang) 2020-0002). After one week of acclimatization, the body quality of mice determined with deflection was used to eliminate certain mice, and the remaining mice (four weeks of age, average body weight 27 ± 1.2 g) were randomly divided into four groups diet for 5 weeks: the normal control group (NC, n = 10) fed a basic diet, the high-fat model control group (MC, n = 10) fed an HFD, the one-year LPT group (1Y, n = 10) fed an HFD and the water extracts from one-year LPT, and the 10 year LPT group (10Y, n = 10) fed an HFD and the water extracts from 10 year LPT. The HFD contained 63.6% basic feed, 20.0% cane sugar, 15% lard, 1.2% cholesterol, and 0.2% bile salt. The animals were housed at room temperature (22 ± 3 °C) under a 12 h light/dark cycle.Mice in the 1Y and 10Y groups received daily administration of LPT extract (974 and 823 mg kg−1 of body weight, respectively.) by intragastric gavage. The NC and MC group mice were given distilled water at the same time. Each mouse was given a daily dose of LPT extract (tea water extracts frozen into dry powder) (mg kg−1 of body weight) = 166.7 × 20 × tea water extractions rate. The recommended daily dose is that which would be consumed if an adult with a 60 kg body weight drank approximately 10 g tea per day, which corresponds to 166.7 mg kg−1 of body weight for an adult; 20 times this amount is the recommended dose for mice;14 the extraction rates of 1 Y tea and 10 Y tea are 29.21% and 24.69%, respectively. The animal protocols were approved by the Ethical Committee of Hunan Agriculture University (Approval no. HAU-2020-36) and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
2.3 Glucose tolerance test (GTT)
At week 5, oral glucose tolerance test (GTT) was conducted with that the blood glucose level of overnight-fasted mice was measured at 0, 30, 60, and 120 min after glucose (1 g kg−1 BW) oral administration. The tail blood glucose was detected by an OneTouch ultra blood glucose meter (LifeScan, Milpitas, USA).2.4 Histological and biochemical analyses
The formalin-fixed liver and epididymal fat tissues were paraffin-embedded, stained with haematoxylin and eosin (HE), and examined under a camera-equipped light microscope (Olympus, Tokyo, Japan). Sections were analysed in a blinded manner by a trained histopathologist. Serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured by a TBA-40FR automatic analyser (Toshiba, Tokyo, Japan) using commercially available kits (Kehua, Shanghai, China).2.5 RNA preparation and qRT-PCR analysis
To measure the expression of HMGR, FAS, SREBP-1C, LCAT, PPARα and LDLR in liver tissues, total RNA was extracted by an RNA kit (Omega, GA, USA). RNA was used to generate cDNA using a PrimeScript RT reagent kit (Takara, Shiga, Japan). Primers were synthesized by a specialty company (Tsingke, Beijing, China). Quantitative real-time reverse-transcription PCR (qRT-PCR) was performed according to the manufacturer's guidelines using a SYBR green master mix kit (Bio-Rad, Hercules, CA, USA). Experiments were performed in triplicate in a single plate. β-actin was the endogenous normalizer, and the relative expression was calculated using the 2−ΔΔCT method. The primer information was listed in Table 1.| Gene | Gene accession number | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| HMGR | NM_008255.2 | GAATGCCTTGTGATTGGAGTTG | ACCATGTCAGGCGTCCGCCAG |
| FAS | NM_007988.3 | TCGGGTGTGGTGGGTTTGGT | GGCGTGAGATGTGTTGCTGAGG |
| SREBP-1C | BC056922.1 | AACCTCATCCGCCACCTGCT | ATGGTAGACAACAGCCGCATCC |
| LCAT | NM_008490.2 | TGCTGCCCATGAACGAGACAGA | TGGCGACTTAGGAGTGCGGTAG |
| PPARα | XM_030248421.1 | GACCAGCAACAACCCGCCTTT | GCAGCAGTGGAAGAATCGGACC |
| LDLR | NM_001252659.1 | AGGAACTGGCGGCTGAAGAA | ATCGTCCTCCAGGCTGACCATC |
| β-actin | NM_007393.5 | TATGCTCTCCCTCACGCCATCC | CCACGCTCGGTCAGGATCTTCA |
2.6 Sample preparation and HPLC-QTOF-MS conditions
The serum samples were thawed at 4 °C, and a 100 μL serum sample was added to 300 μL methanol/acetonitrile solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), internal standards (stearic acid-1-13C and naringin) and vortexed for 3 min. Then, all samples were stored at 4 °C overnight for protein precipitation. The mixture was centrifuged at 13
1, v/v), internal standards (stearic acid-1-13C and naringin) and vortexed for 3 min. Then, all samples were stored at 4 °C overnight for protein precipitation. The mixture was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, and the supernatant was filtered through a 0.22 μm microporous membrane. A 5 μL filtrate was injected into the instrument for analysis. Quality control (QC) samples were injected after every nine samples to monitor the reproducibility of the metabolomic analysis results.
000 rpm for 10 min at 4 °C, and the supernatant was filtered through a 0.22 μm microporous membrane. A 5 μL filtrate was injected into the instrument for analysis. Quality control (QC) samples were injected after every nine samples to monitor the reproducibility of the metabolomic analysis results.
Serum metabolites were analysed by an HPLC-QTOF-MS system (Agilent, Santa Clara, CA, USA) including a 1290 LC system equipped with a ZORBAX Eclipse XDB-C18 (3.0 × 150 mm, 3.5 μm) and a 6540 Q-TOF-MS system. The mobile phase consisted of acetonitrile (A) and 5 mmol L−1 ammonium acetate containing 0.1% formic acid (B) at a flow rate of 0.4 mL min−1. The gradient was as follows: 0 min, 5% A; 2 min, 30% A; 6 min, 65% A; 7.5 min, 80% A; 12 min, 95% A; and 16 min, 5% A. The analytical column was maintained at 35 °C. Both positive and negative ion modes were operated with the following parameters: the temperature and the flow rate of the drying gas were 250 °C and 10 L min−1, respectively; the temperature and the flow rate of the sheath gas were maintained at 250 °C and 11 L min−1, respectively; the capillary voltage was 3.5 kV; the nebulizer pressure was 40 psi; and the mass scan range was m/z 50–1250.
2.7 Statistical and data analysis
The raw metabolomics data acquired from UHPLC−QTOF/MS were first treated for peak picking using MassHunter Qualitative Analysis software (B.07.00 SP1, Agilent Technologies, Santa Clara, CA, USA) and for peak alignment using Mass Profiler Professional (version 13.0, Agilent Technologies, Santa Clara, CA, USA). The signal-to-noise (S/N) threshold for peak detection was set to 3. The retention time tolerance and mass tolerance for the peak alignment was set to 0.2 min and 0.01 Da, respectively. Data were normalized against the total peak area of each sample with Excel 2010 (Microsoft, USA). The obtained compound ion features with relative standard deviations (RSD) of mass intensities less than 30% in QC samples were used for multivariate and univariate analysis. Compounds were structurally identified based on authentic standards, MS2 spectra, mass accuracy, metabolomics databases (Metlin and HMDB).Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using SIMCA 14.1 software (Umetrics, Umeå, Sweden). To validate the model, permutation analysis (200 times) was carried out. Statistical analyses were performed using SPSS 21 software (IBM, Chicago, IL, USA) via Student's t-test or one-way ANOVA followed by Tukey's multiple comparison. Metabolic pathways were analysed with MetaboAnalyst 4.0 (http://www.metaboanalyst.ca).
3 Results
3.1 Major chemical constituents in Liupao tea (LPT)
As shown in Table 2, the contents of chemical components (water extractions, TP, FAAs, water-soluble sugars and CAF) were significantly lower in the LPT aged for 10 years. However, the TBs content in LPT at 10 years was markedly higher than that at one year (P < 0.01). Our results were basically consistent with those of a previous study,15 which had LPT samples aged one, two, three, five and seven years. In addition, we speculated that TRs and TFs may be converted into TBs during the ageing process.16–20 Moreover, catechins are considered to be the most important polyphenols in teas, compared with LPT at one year, the LPT at 10 years contained lower contents of total catechins and catechins of EGCG, EGC, ECG, EC, GCG, GC and C (P < 0.05 or P < 0.01).| Ingredients/sample | 1 year (1Y) | 10 years (10Y) |
|---|---|---|
| a Student's t-test; ND, not detected; compared to 1Y, *P < 0.05, **P < 0.01. | ||
| Water extractions | 39.20 ± 2.24 | 35.12 ± 2.35* |
| Tea polyphenols (TP) | 12.11 ± 0.87 | 10.12 ± 0.84** |
| Free amino acids (FAAs) | 1.82 ± 0.19 | 1.63 ± 0.18* |
| Water-soluble sugars | 4.25 ± 0.44 | 3.98 ± 0.52** |
| Caffeine (CAF) | 3.63 ± 0.35 | 3.53 ± 0.15* |
| Theaflavins (TFs) | 0.16 ± 0.06 | 0.11 ± 0.06 |
| Thearubigins (TRs) | 2.41 ± 0.24 | 1.84 ± 0.22 |
| Theabrownins (TBs) | 6.93 ± 0.81 | 9.72 ± 0.76** |
| (TFs + TRs)/TBs × 100 | 37.09 | 20.06 |
| Epigallocatechin gallate (EGCG) | 1.86 ± 0.06 | 1.52 ± 0.12* |
| Epigallocatechin (EGC) | 2.2 ± 0.13 | 0.83 ± 0.09** |
| Epicatechin gallate (ECG) | 2.3 ± 0.12 | 1.27 ± 0.08** |
| Epicatechin (EC) | 3.13 ± 0.19 | 2.26 ± 0.16** |
| Gallate (GCG) | 0.76 ± 0.05 | 0.38 ± 0.05** |
| Gallocatechin (GC) | 1.02 ± 0.03 | 0.31 ± 0.08** |
| Catechin gallate (CG) | ND | ND |
| Catechin (C) | 1.76 ± 0.07 | 1.05 ± 0.16** |
| Total catechins | 12.76 ± 0.55 | 7.32 ± 0.69** |
3.2 Body weight, tissue morphology, main biochemical profile and glucose tolerance
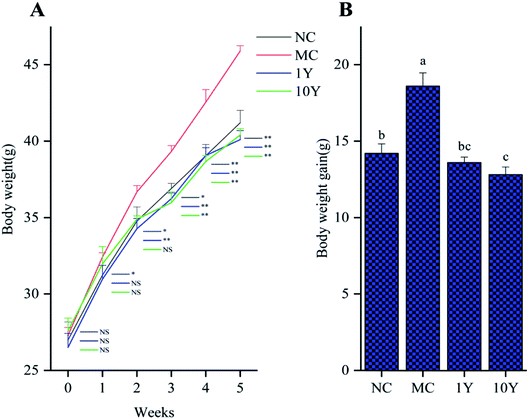
The average body weight variations of the mice in the four groups were shown in Fig. 1. As shown in Fig. 1A, the mouse body weights did not significantly differ in all groups at the start time, but the two LPT-treated groups (1Y and 10Y) had lower body weights than the MC group from the second week. After five weeks of intragastric gavage treatment, the body weight of the LPT-treated groups significantly decreased (P < 0.01) compared with that of the MC group and even reached a level similar to that of the NC group. Furthermore, compared with the MC group, the body weight gain of the 10Y group was lower than that of the 1Y group at the end of the experiment; and body weight gain of 10Y treated mice was even lower than that of NC control (Fig. 1B). Throughout the experiment, aged LTP consumption did not affect food and water consumption compared with the MC group (P > 0.05) (Fig. S1A and B†).The changes in obese mice in the liver and adipose tissues were shown in Fig. 2. As shown in Fig. 2A, the HE-stained liver sections in the MC group showed more circular lipid droplets and macrovesicular steatosis than those in the NC group. At the end of the trial, fatty liver and lipid deposition were markedly alleviated by LPT treatment, especially in the 10Y group. Obesity is associated with increased adipocyte volume and number. In this study, the area of adipocytes in the epididymal fat was significantly increased in the MC group (Fig. 2B). In contrast, the area was definitely restrained in the two LPT-treated groups. Moreover, the fatty cell morphological changes in the 10Y group approximated those in the NC group.
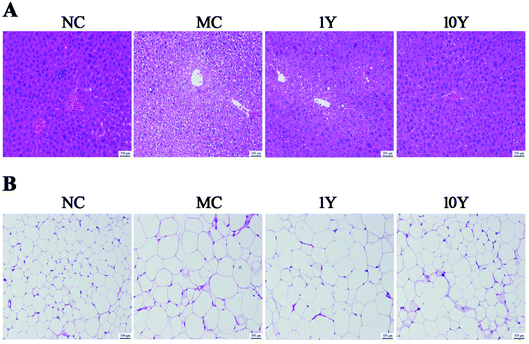 | ||
| Fig. 2 The effect of LPT on mouse liver and epididymal adipose tissues. Representative HE-stained images of (A) the liver and (B) epididymal adipose tissue (scale bar 100 μm). | ||
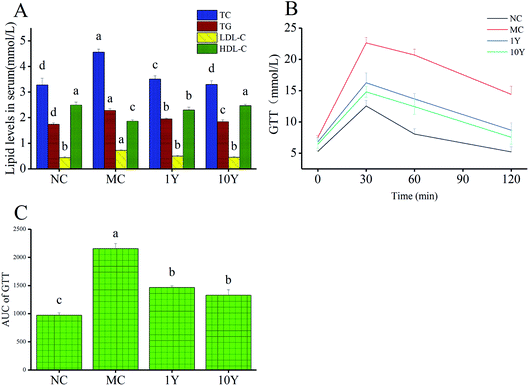
In terms of biochemical profile, compared with the NC group, our data showed that mice in the MC group had significantly higher serum levels of TC, TG and LDL-C, while the level of HDL-C was markedly lower in the MC group (Fig. 3A). Moreover, mice in the two LPT intervention groups (1Y and 10Y) showed better blood and hepatic lipid profiles than mice in the MC group. Obesity may cause glucose-induced hyperglycemia, so the glucose tolerance test was implemented. As shown in Fig. 3B and C, the MC group showed poorer ability of glucose tolerance, while two LPT intervention groups can significantly accelerate the rate of blood glucose reduction after oral administration of glucose. Compared with the blood glucose level at 30 min, the rate of blood glucose reduction in the MC group was 8.65% and 36.47% at 60 min and 120 min, respectively; while the rate of blood glucose reduction in the 1Y and 10Y groups was 15.83% and 15.94% at 60 min, and 46.53% and 49.16% at 120 min, respectively. The level of AUC (area under the blood glucose curve) of the MC group was markedly suppressed by aged LTP intervention (p < 0.05), suggesting that aged LPT can improve glucose tolerance in HFD-induced obese mice. Together, these abovementioned findings suggested that aged LPT could inhibit obesity-associated problems in obese animals.
3.3 Effects of aged LPT on serum metabolic profiles
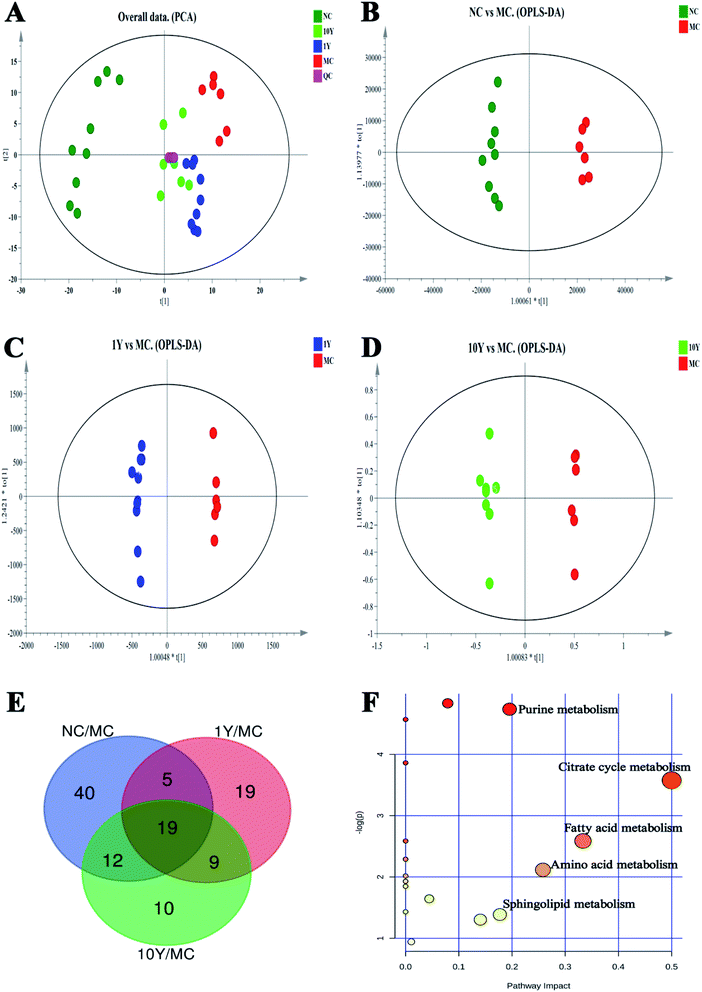
In this study, the compounds in serum samples were detected using metabolomics analysis with HPLC-QTOF-MS. A total of 4218 metabolite ion features were acquired after peak extraction and alignment. Among them, the relative standard deviations (RSDs) of 3166 metabolite ion features were less than 30% in QC samples and were used for further statistical analysis.The serum metabolic profiles of the four groups (NC, MC, 1Y and 10Y) were analysed by PCA (Fig. 4A). PCA showed a clear separation among the four groups. The NC group was completely separated from the MC group, and two aged LPT-intervention groups (1Y and 10Y) were shifted towards the NC group to varying degrees; in particular, the 10Y group was closer to the NC group. The results showed that the aged LPT had a significant positive impact on obese mice.
To search for potential metabolite markers [variable importance in projection (VIP) > 1, P < 0.05], we established OPLS-DA models (NC vs. MC groups, MC vs. 1Y groups, MC vs. 10Y groups, respectively, Fig. 4B–D). Seventy-six differential metabolites were found between the NC and MC groups; likewise, 52 and 50 distinguishing metabolites were obtained in the 1Y and 10Y groups compared to the MC group, respectively. The Venn diagram showed 19 common differential metabolites in all groups (Fig. 4E). Among these metabolites, 11 compounds were ultimately structurally identified based on authentic standards, MS2 spectra, accurate masses, and metabolomics databases (Metlin and HMDB). Table 3 showed the identified biomarkers of obesity syndrome under treatment with aged LPT. Seven of the 11 biomarkers were depressed, and four of them were upregulated in the MC group. After aged LPT intervention, the levels of valine, tryptophan, tyrosine, hypoxanthine, citric acid, succinic acid and lysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were significantly elevated, while the levels of sphingosine, sphinganine, oleamide and palmitic amide were markedly decreased.
1) were significantly elevated, while the levels of sphingosine, sphinganine, oleamide and palmitic amide were markedly decreased.
| Ionization mode | Biomarkers | Formula | Mass (m/z) | KEGG ID | Trendsc | Pathway | ||
|---|---|---|---|---|---|---|---|---|
| MC/NC | 1Y/MC | 10Y/MC | ||||||
| a Metabolites confirmed with authentic standards.b Metabolites putatively annotated by Metlin and HMDB databases.c (↑) and (↓) showed upregulation and downregulation, respectively; 1Y and 10Y groups vs. MC group. *P < 0.05; **P < 0.01. | ||||||||
| Negative | Citric acida | C6H8O7 | 191.0197 | C00158 | ↓** | ↑* | ↑* | Citrate cycle |
| Succinic acida | C4H6O4 | 117.0189 | C00042 | ↓* | ↑* | ↑** | Citrate cycle | |
| Oleamidea | C18H35NO | 280.2655 | C19670 | ↑** | ↓** | ↓* | Fatty acid metabolism | |
| Palmitic amideb | C16H33NO | 254.2472 | — | ↑* | ↓* | ↓* | Fatty acid metabolism | |
| Positive | Hypoxanthinea | C5H4N4O | 137.0453 | C00262 | ↓* | ↑** | ↑* | Purine metabolism |
| L-Valinea | C5H11NO2 | 118.0863 | C00183 | ↓* | ↑** | ↑* | Amino acid metabolism | |
| Tryptophana | C11H12N2O2 | 205.0983 | C00078 | ↓* | ↑* | ↑** | Amino acid metabolism | |
| Tyrosinea | C9H11NO3 | 182.0818 | C00082 | ↓* | ↑* | ↑* | Amino acid metabolism | |
| Sphingosinea | C18H37NO2 | 300.2924 | C00319 | ↑** | ↓* | ↓** | Sphingolipid metabolism | |
| Sphinganineb | C18H39NO2 | 302.3083 | C00836 | ↑* | ↓* | ↓** | Sphingolipid metabolism | |
LysoPCb (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
C26H52NO7P | 522.3553 | C04230 | ↓* | ↑* | ↑** | Lysophosphatidylcholine metabolism | |
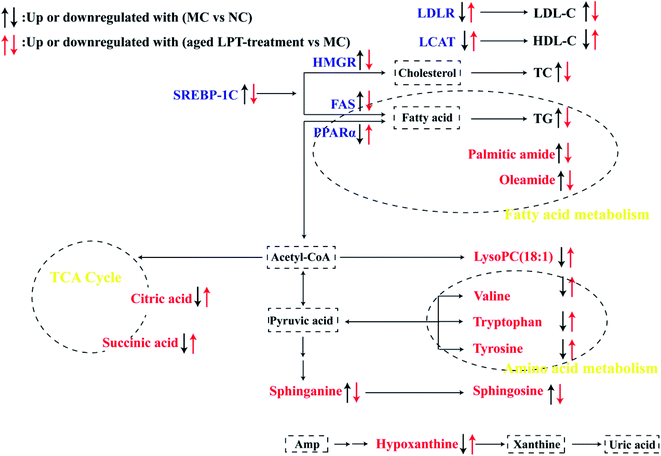
To further understand the metabolic pathways of aged LPT-treated mice, metabolic pathway analysis of the 11 differential metabolites was performed by MetaboAnalyst 4.0 (Fig. 4F). The differential metabolites were mainly involved in the metabolic pathways of the citrate cycle, purine metabolism, fatty acid metabolism, amino acid metabolism, and sphingolipid metabolism.
3.4 Effects of aged LPT on lipid metabolism-related gene expression in the liver
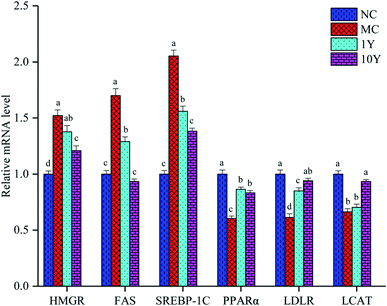
To better understand the molecular mechanism by which aged LPT has beneficial effects on obese mice, we detected some related genes involved in lipid metabolism. As shown in Fig. 5, compared with the NC group, the mRNA expression of HMGR, FAS and SREBP-1C increased significantly (P < 0.05) in the MC group, while the mRNA expression of PPARα, LDLR and LCAT decreased significantly (P < 0.05) in the MC group. In the two aged LPT-treated groups, compared with the MC group, the mRNA expression of HMGR, FAS and SREBP-1C showed a downward trend, although the expression of HMGR in the 1Y group was not significantly different. Moreover, the mRNA expression levels of PPARα, LDLR and LCAT tended to increase, although the expression of LCAT in the 1Y group was not significantly different. The results demonstrated that aged LPT treatment in HFD-induced obese mice can downregulate the expression of HMGR, FAS and SREBP-1C while upregulating the expression of PPARα, LDLR and LCAT.4 Discussion
In the present study, our current results suggested that aged LPT significantly improved obesity symptoms, such as body weight, fatty liver, adiposity, serum biochemical indexes and glucose tolerance in HFD-induced mice. Furthermore, we found that 10Y aged LPT had a better therapeutic effect than 1Y aged LPT to some extent.Metabolomics studies have revealed the physiological state of organisms through the study of changes in small molecule metabolites under different conditions. Metabolomics analysis demonstrated that aged LPT changed the metabolite patterns of the MC group towards those of the NC group to a certain degree (Fig. 4A); 11 differential biomarkers were reversed significantly in aged LPT-treated groups (Table 3). A more detailed network of the 11 differential baseline metabolites was shown in Fig. 6. The TCA cycle is a key pathway in the metabolism of three major nutrients: proteins, lipids, and sugars, and supplies energy for organisms.21 Citric acid and succinic acid are important intermediates in the TCA cycle. Compared with those of the NC group, these parameters were decreased in the MC group, which indicated that the TCA cycle was weakened under obesity and was consistent with the findings of previous studies.21,22 In contrast, the levels of citric acid and succinic acid were higher in the aged LPT-treated groups than in the MC group, which suggested that aged LPT may have a certain mechanism by which it acts against obesity. In purine metabolism, hypoxanthine is converted into xanthine by xanthine oxidase (XOD), eventually changing into uric acid. A number of studies have demonstrated that, compared with the normal diet group, hypoxanthine was significantly decreased in the HFD group,21,23 which was consistent with our results, possibly due to XOD activity was elevated in the HFD group.24,25 In this study, aged LPT-treated groups showed a reverse in this reduction. Palmitic amide and oleamide are amides of palmitic acid and oleic acid (fatty acids), respectively, involved in fatty acid metabolism. In the present study, palmitic amide and oleamide were increased in the MC group compared with the NC group levels, in agreement with the results of a previous study.26 This result suggested that fatty acid oxidation is repressed in obese mice. However, palmitic amide and oleamide levels were significantly reduced in aged LPT-treated groups, which implied that aged LPT could modulate fatty acid metabolic pathways in obese mice. Amino acid metabolism is affected by HFDs, which change the levels of many amino acids. Numerous studies have demonstrated that the levels of valine, tryptophan and tyrosine were decreased in HFD-fed mice.27–29 In this study, the levels in obese mice were markedly increased by aged LPT intervention. Amino acids can enter the TCA cycle through transamination and catabolism, which affect changes in the TCA cycle.30 Sphinganine and sphingosine are involved in sphingolipid metabolism. It has been reported that high accumulation of sphingolipids may decelerate reverse cholesterol transport and increase the risk of hyperlipidaemia-related diseases.31 In this study, we found that the concentrations of sphinganine and sphingosine in HFD-induced mouse serum were elevated, in accordance with the results of previous studies.31,32 Additionally, aged LPT decreased the levels of sphinganine and sphingosine compared with those of the MC group, implying that aged LPT may regulate dysfunctional sphingolipid metabolism. LysoPCs play critical roles in obesity-related diseases derived from phosphatidylcholine hydrolysis.31 In our study, LysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was significantly lower in the model group than in the NC group. This result was also consistent with that of previous studies23,33–35 but disagreed with other conclusions,28,31 likely because of the different experimental animal models of obesity, different sample types (such as serum, liver, urine and faeces) from the animal models, and even the effects on the HFD-induced animals with the length of time. We found that the abnormal LysoPC (18
1) was significantly lower in the model group than in the NC group. This result was also consistent with that of previous studies23,33–35 but disagreed with other conclusions,28,31 likely because of the different experimental animal models of obesity, different sample types (such as serum, liver, urine and faeces) from the animal models, and even the effects on the HFD-induced animals with the length of time. We found that the abnormal LysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) change in obese mice was alleviated by aged LPT treatment.
1) change in obese mice was alleviated by aged LPT treatment.
The liver is the centre of lipid metabolism and secretes a variety of lipid metabolism-related enzymes. Therefore, we selected six important genes (HMGR, FAS, SREBP-1C, PPARα, LDLR and LCAT) involved in lipid synthesis, transformation and oxidation in the liver (Fig. 6) to understand the anti-obesity mechanism of aged LPT. The SREBP pathway plays an important role in the regulation of lipid metabolism, and SREBP may offer therapeutic strategies to attenuate hepatic steatosis and atherosclerosis along with SREBP downstream genes, including HMGCR and FAS.36,37 HMGR is the rate-controlling enzyme in the mevalonate pathway, catalysing the conversion of HMG-CoA to mevalonic acid, which is a necessary step in the production of cholesterol.38 The increased level of HMGR in the HFD mouse liver corresponds to increased cholesterogenesis, as indicated by the higher contents of total cholesterol (TC) and LDL cholesterol (LDL-C) in the plasma.39
FAS is a multienzyme protein catalysing fatty acid synthesis.38 Our results demonstrated that SREBP-1C, HMGCR and FAS, which promote lipid synthesis, were markedly elevated by the HFD; however, aged LPT suppressed these changes. The HFD increased the SREBP-1C, HMGCR and FAS mRNA levels in the liver, identical to the results of previous studies.37,40,41 It has been reported that Pu-erh tea decreased SREBP-1c and FAS mRNA expression in obese mouse livers,41 and Fuzhuan brick tea (FBT) and Kudingcha (KDC) could significantly reduce the expression levels of FAS and SREBP-1C in the HFD group treated with FBT or KDC extracts.42 PPARα is another key regulator of lipid metabolism, regulating the expression of genes encoding proteins related to fatty acid β-oxidation.43 LDLR also plays an important role in lipid metabolism and highly expressed membrane glycoproteins, especially in liver cells. Most LDL-C in plasma is cleared by LDLR on the surface of liver cells when the LDLR gene is upregulated.43 This study showed that the PPARα and LDLR mRNA levels were significantly decreased after HFD feeding; nevertheless, aged LPT effectively increased the PPARα and LDLR levels, which implied that PPARα and LDLR contributed to the therapeutic action of aged LPT in obese mice. This is consistent with a previous report demonstrating that the mRNA expression of PPARα and LDLR was increased in obese mice treated with instant dark tea.44 LCAT is an enzyme that converts cholesterol into cholesteryl esters and may participate in reverse cholesterol transport,43 and enhancing LCAT activity might increase HDL-cholesterol synthesis (HDL-C).39 Our data showed that LCAT gene expression was enhanced in the groups receiving oral administration of aged LPT, suggesting that the effects of aged LPT on improving lipid metabolic disorder were partly due to reverse cholesterol transport.
The 10Y group showed better anti-obesity effects than the 1Y group, indicating that older LPT had a higher effect on improving lipid metabolic disorder than younger LPT during a certain storage time. This was generally consistent with the findings of previous studies.9,11 Oolong tea stored in 2006 showed a better anti-obesity effect than that stored in 2016 and that stored in 1996, and Qingzhuan tea stored for 10 years had a more significant hypolipidaemic effect than that stored for 1 year. However, it is difficult to infer which LPT component was responsible for this difference only considering the data in our study. As shown in Table 2, we determined the contents of the main chemical components of LPT. Most main compounds markedly decreased with storage time; in contrast, the TBs content was the highest in the 10Y group. Theabrownin is known to have significant effects on hyperlipidaemia,45–47 so the lightly better effects of 10Y-LPT treatment on the obesity parameters observed were probably due to the higher concentration of TBs in 10Y group. In fact, which ingredients of aged LPT play a key role in improving lipid metabolic disorder is not easy to answer completely. There may have been combined effects of known constituents of determined and undetermined ingredients, or other unknown substances, that led to these effects.
5 Conclusions
In this work, we demonstrated that aged LPT possessed a good anti-obesity effect. The administration of aged LPT could basically reverse metabolic dysfunction in HFD-induced obese mice by HPLC-QTOF-MS-based metabolomics approaches mainly via possible metabolic pathways associated with the citrate cycle, purine metabolism, fatty acid metabolism, and amino acid metabolism. The mechanism may be related to the downregulation of SREBP-1C/HMGR/FAS signalling pathways and the upregulation of other signalling molecules. Our results suggest that aged LPT may be used as a potential dietary supplement for preventing obesity and related metabolic disorders.Author contributions
Wenliang Wu: conceptualization, methodology, formal analysis, investigation, writing – original draft, writing – review & editing, funding acquisition. Yao Hu: formal analysis. Shuguang Zhang: investigation. Dongming Liu: formal analysis. Qing Li: formal analysis. Yong Lin: conceptualization, Writing – review & editing. Zhonghua Liu: conceptualization, resources, writing – review & editing, project administration, funding acquisition.Conflicts of interest
Authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by the Hunan Provincial Natural Science Foundation of China (2020JJ5277), the Hunan Agricultural Science and Technology Innovation Funds of China (2020CX36) and the Guangxi Innovation Driven Development Special Fund Project of China (AA20302018).References
- P. G. Kopelman, Nature, 2000, 404, 635–643, DOI:10.1038/35007508.
- G. Marrone, C. Guerriero, D. Palazzetti, P. Lido, A. Marolla, F. D. Daniele and A. Noce, Nutrients, 2021, 13, 817, DOI:10.3390/nu13030817.
- D. Heber, Y. Zhang, J. Yang, J. E. Ma, S. M. Henning and Z. Li, J. Nutr., 2014, 144, 1385–1393, DOI:10.3945/jn.114.191007.
- Y. Mao, B. Wei, J. Teng, N. Xia, M. Zhao, L. Huang and Y. Ye, Int. J. Food Sci. Technol., 2018, 53, 599–607, DOI:10.1111/ijfs.13633.
- L. Huang, J. J. Peng, N. Xia, J. W. Teng and B. Y. Wei, Food Sci. Technol., 2013, 38, 123–127, DOI:10.13684/j.cnki.spkj.2013.08.058.
- Q. Ding, B. Zhang, W. Zheng, X. Chen, J. Zhang, R. Yan, T. Zhang, L. Yu, Y. Dong and B. Ma, Biomed. Pharmacother., 2019, 118, 109262, DOI:10.1016/j.biopha.2019.109262.
- Y. Wu, H. Sun, R. Yi, F. Tan and X. Zhao, J. Food Sci., 2021, 86, 215–227, DOI:10.1111/1750-3841.15551.
- K. M. Ku, J. Kim, H. Park, K. Liu and C. H. Lee, J. Agric. Food Chem., 2010, 58, 345–352, DOI:10.1021/jf902818c.
- E. Yuan, X. Duan, L. Xiang, J. Ren, X. Lai, Q. Li, L. Sun and S. Sun, Nutrients, 2018, 10, 187, DOI:10.3390/nu10020187.
- Q. Li, X. Lai, L. Sun, J. Cao, C. Ling, W. Zhang, L. Xiang, R. Chen, D. Li and S. Sun, Food Nutr. Res., 2020, 64 DOI:10.29219/fnr.v64.1681.
- Y. Q. Chen, W. Zhang, D. J. Ni, Q. Cheng, X. H. Liu and D. P. Gan, J. Tea Sci., 2019, 30, 124–128, DOI:10.29219/fnr.v64.1681.
- J. Chung, S. Yoo, Y. Lee, K. Shin, S. Yoo, S. Park, T. Park and S. Shim, Food Funct., 2019, 10, 746–753, 10.1039/c8fo01936c.
- H. Zhu, F. Liu, Y. Ye, L. Chen, J. Liu, A. Gui, J. Zhang and C. Dong, J. Food Eng., 2019, 263, 165–172, DOI:10.1016/j.jfoodeng.2019.06.009.
- Z. Gong, J. Ouyang, X. Wu, F. Zhou, D. Lu, C. Zhao, C. Liu, W. Zhu, J. Zhang and N. Li, Biomed. Pharmacother., 2020, 130, 110514, DOI:10.1016/j.biopha.2020.110514.
- Z. S. Liu, L. X. Wen, M. Z. He, X. R. Li, J. W. Lin, R. Q. Shi, C. M. Xie, F. Zheng and E. F. Ren, Chin. J. Trop. Agric, 2016, 36, 81–86 CAS , http://qikan.cqvip.com/Qikan/Article/Detail?id=671053229.
- F. Lin, X. Wei, H. Liu, H. Li, Y. Xia, D. Wu, P. Zhang, G. R. Gandhi, H.-B. Li and R. Gan, Trends Food Sci. Technol., 2021, 109, 126–138, DOI:10.1016/j.tifs.2021.01.030.
- Y. Jiang, J. Hua, B. Wang, H. Yuan, H. Ma and F. Hernández, J. Food Qual., 2018, 18, 5427302, DOI:10.1155/2018/5427302.
- Y. Xiao, K. Zhong, J. Bai, Y. Wu, J. Zhang and H. Gao, LWT–Food Sci. Technol., 2020, 117, 108629, DOI:10.1016/j.lwt.2019.108629.
- Q. Wang, A. Belščak-Cvitanović, K. Durgo, Y. Chisti, J. Gong, S. Sirisansaneeyakul and D. Komes, LWT–Food Sci. Technol., 2018, 90, 598–605, DOI:10.3390/biom10020204.
- Q. Wang, J. Gong, Y. Chisti and S. Sirisansaneeyakul, J. Food Sci., 2015, 80, M809–M817, DOI:10.1111/1750-3841.12831.
- H. M. Park, K. Park, E. C. Park, S. I. Kim, M. S. Choi, K. Liu and C. H. Lee, Nutrients, 2017, 9, 71, DOI:10.3390/nu9010071.
- Y. Lu, R. Yang, X. Jiang, Y. Yang, F. Peng and H. Yuan, J. Clin. Biochem. Nutr., 2016, 15–72, DOI:10.3164/jcbn.15-72.
- L. S. Lee, J. H. Choi, M. J. Sung, J. Y. Hur, H. J. Hur, J. D. Park, Y. C. Kim, E. J. Gu, B. Min and H. J. Kim, Mol. Nutr. Food Res., 2015, 59, 784–794, DOI:10.1002/mnfr.201400470.
- A. Oberbach, J. Neuhaus, N. Schlichting, J. Kugler, S. Baumann and H. Till, Surg. Obes. Relat. Dis., 2014, 10, 684–690, DOI:10.1016/j.soard.2013.12.010.
- A. Kim, S. Jeon, Y. Jeong, Y. Park, U. Jung and M. Choi, Prev. Nutr. Food Sci., 2011, 16, 95–103, DOI:10.3746/jfn.2011.16.2.095.
- Y. Zhang, Z. Wang, G. Jin, X. Yang and H. Zhou, Int. J. Biol. Macromol., 2017, 101, 107–116, DOI:10.1016/j.ijbiomac.2017.03.084.
- X. C. Ma, Master's thesis, Guangdong Pharmaceutical University, China, 2019, DOI: DOI:10.27690/d.cnki.ggdyk.2019.000122.
- F. Tian, L. Gu, A. Si, Q. Yao, X. Zhang, J. Zhao and D. Hu, Biomed. Chromatogr., 2016, 30, 969–975, DOI:10.1002/bmc.3637.
- J. Sun, X. Liu, L. Cong, H. Li, C. Zhang, J. Chen and C. Wang, Lipids Health Dis., 2017, 16, 1–14, DOI:10.1186/s12944-017-0533-3.
- M. Akram, Cell Biochem. Biophys., 2014, 68, 475–478, DOI:10.1007/s12013-013-9750-1.
- H. Miao, H. Chen, S. Pei, X. Bai, N. D. Vaziri and Y. Zhao, Chem.-Biol. Interact., 2015, 228, 79–87, DOI:10.1016/j.cbi.2015.01.023.
- D. Dai, Y. Tian, H. Guo, P. Zhang, Y. Huang, W. Zhang, F. Xu and Z. Zhang, Metabolomics, 2016, 12, 1–13, DOI:10.1007/s11306-015-0892-6.
- N. Ma, I. Karam, X. Liu, X. Kong, Z. Qin, S. Li, Z. Jiao, P. Dong, Y. Yang and J. Li, Toxicol. Appl. Pharmacol., 2017, 332, 40–51, DOI:10.1016/j.taap.2017.07.013.
- J. Shin, M. H. Nam, H. Lee, J. Lee, H. Kim, M. Chung and J. Seo, Eur. J. Nutr., 2018, 57, 2081–2090, DOI:10.1007/s00394-017-1481-4.
- M. N. Barber, S. Risis, C. Yang, P. J. Meikle, M. Staples, M. A. Febbraio and C. R. Bruce, PLoS One, 2012, 7, e41456, DOI:10.1371/journal.pone.0041456.
- Y. U. Li, S. Xu, M. M. Mihaylova, B. Zheng, X. Hou, B. Jiang, O. Park, Z. Luo, E. Lefai and J. Y. Shyy, Cell Metab., 2011, 13, 376–388, DOI:10.1016/j.cmet.2011.03.009.
- M. S. Brown and J. L. Goldstein, Cell, 1997, 89, 331–340, DOI:10.1016/s0092-8674(00)80213-5.
- R. Tang, Q. Yang, S. Lin, Y. Feng, J. Yang, Q. Lv, G. Wu and J. Hu, Preventive or Curative Administration of Taurine Regulates Lipid Metabolism in the Liver of Rats with Alcoholic Liver Disease, 2019, pp. 119–131 Search PubMed.
- P. B. Naidu, V. S. Uddandrao, R. R. Naik, P. Suresh, B. Meriga, M. S. Begum, R. Pandiyan and G. Saravanan, Mol. Cell. Endocrinol., 2016, 419, 139–147, DOI:10.1016/j.mce.2015.10.007.
- S. Li, W. Yu, X. Guan, K. Huang, J. Liu, D. Liu and R. Duan, J. Funct. Foods, 2019, 59, 49–59, DOI:10.1016/j.jff.2019.05.030.
- Y. Shimamura, M. Yoda, H. Sakakibara, K. Matsunaga and S. Masuda, Biosci., Biotechnol., Biochem., 2013, 77, 1455–1460, DOI:10.1271/bbb.130097.
- G. Chen, M. Xie, Z. Dai, P. Wan, H. Ye, X. Zeng and Y. Sun, Mol. Nutr. Food Res., 2018, 62, 1700485, DOI:10.1002/mnfr.201700485.
- Y. Zhao, L. Peng, W. Lu, Y. Wang, X. Huang, C. Gong, L. He, J. Hong, S. Wu and X. Jin, Exp. Gerontol., 2015, 62, 37–44, DOI:10.1016/j.exger.2014.12.017.
- Y. Sun, Y. Wang, P. Song, H. Wang, N. Xu, Y. Wang, Z. Zhang, P. Yue and X. Gao, Food Funct., 2019, 10, 3502–3513, 10.1039/c9fo00162j.
- Y. Xiao, M. Li, Y. Wu, K. Zhong and H. Gao, Biomolecules, 2020, 10, 204, DOI:10.3390/biom10020204.
- F. Huang, X. Zheng, X. Ma, R. Jiang, W. Zhou, S. Zhou, Y. Zhang, S. Lei, S. Wang and J. Kuang, Nat. Commun., 2019, 10, 1–17, DOI:10.1038/s41467-019-12896-x.
- T. Chen, C. X. Peng, J. S. Gong, Q. P. Wang and H. J. Zhou, J. Chin. Inst. Food Sci. Technol., 2011, 11(01), 20–27, DOI:10.16429/j.1009-7848.2011.01.032.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04438a |
| This journal is © The Royal Society of Chemistry 2021 |