Open Access Article
Open Access ArticleAnaerobic digestion and agronomic applications of microalgae for its sustainable valorization
Doha Elalami
 a,
Abdallah Oukarroum
a and
Abdellatif Barakat
a,
Abdallah Oukarroum
a and
Abdellatif Barakat
 *ab
*ab
aAgroBioSciences, Mohammed VI Polytechnic University (UM6P), Ben Guérir, Morocco. E-mail: abdellatif.barakat@inrae.fr
bIATE, University of Montpellier, INRAE, Agro Institut, Montpellier 34060, France
First published on 3rd August 2021
Abstract
Microalgae are considered potential candidates in biorefinery processes, and due to their biochemical properties, they can be used in the production of biofuels such as biogas, as well as for bioremediation of liquid effluents. The objective of this review is to study the current status of microalgae anaerobic digestion and agricultural uses (as bio-stimulants and biofertilizers), starting from microalgae cultivation. Indeed, the efficiency of these processes necessarily depends on the evaluation of different biotic and abiotic factors that affect the growth of microalgae. However, the adaptation and the optimization of process parameters on a large scale is also limited by energy and economic constraints. Moreover, the integration of biogas production processes with microalgae cultivation allows a nutrients and CO2 virtuous loop, thus promoting the sustainability of the process. Finally, this paper provides a general overview of biogas and biofertilizers production combination, as well as the related challenges and recommended future research perspectives to complement the gap in the literature.
1. Introduction
Microalgae are a promising source for third generation biofuels. The production of microalgae consists of cultivation and harvesting stages. Biofuel production depends also on biochemical properties of microalgae which is highly related to cultivation and harvesting conditions. Light intensity, pH, temperature and nutrients availability as well as mixing conditions are among the main factors influencing the performance of the growth stage.1,2Anaerobic digestion is among the most widely used biological processes converting organic matter to biogas. The efficiency of anaerobic digestion processes can be enhanced when applying a pretreatment to favor hardly degradable matter containing in microalgae.3 However, optimization of pretreatment should be carried out to reduce energy and chemicals consumption. Moreover, pretreatment cannot remedy inhibition problems related to high proteins, heavy metals or polyphenols content in microalgae, that is why co-digestion can be seen as an interesting alternative leading to high methane production.4 In addition, because of the low biomass productivity, monodigestion of microalgae cannot be economically attractive.5
In order to improve the performance of the microalgae production and utilization system, consideration must be given to optimizing processes in such a way that the nutrient and energy loop is closed. Extraction of high-value added matter from microalgae can be carried out first to generate a biostimulant for plants for e.g., and then the solid residues can be subjected to anaerobic digestion. The resulted digestate can be used as biofertilizer or as a source of nutrients for microalgae biomass growth,6 as well as the recycling of CO2 contained in biogas produced from AD can lead to a more cost-effective cultivation stage.
However, more studies need to be oriented towards process life cycle assessment, in order to determine the best possible scenarios for the valorization of microalgae.
Therefore, the aim of this paper is to provide a general overview of the different aspects affecting the production and use of microalgal biomasses. In addition, this paper offers prospects for improving the valorization of microalgae residues by integrating anaerobic digestion and agronomic application of microalgae in an innovative eco-friendly biorefinery in cascade.
2. The potential of microalgae for bioenergy and biofertilization
Microalgae are photosynthetic microorganisms that live in freshwater or seawater. They can be distinguished in two types of microalgae: eukaryotes (green or diatom microalgae) and prokaryotes (blue-green algae). Depending on the growth environment and the adaptability of the species, microalgae can grow by consuming complex organic matter (heterotrophic mode), by consuming organic matter and absorbing light (mixotrophic mode) or by absorbing only light (photoautotrophic mode).The growth conditions as well as the type of microalgae have an impact on the properties of microalgae. Typical species include Chlamydomonas reinhardtii, Dunaliella salina, Chlorella sp., and Botryococcus braunii. Other interesting microalgae species for biotechnology development include Phaeodactylum tricornutum, Thalassiosira pseudonana, Nannochloropsis, and Isochrysis spp.7 In general, microalgae are rich in fatty acids, proteins and sugars as presented in Table 1. In addition, some microalgal biomasses contain calcium (0.1–3%), magnesium (0.3–0.7%), phosphorous (0.7–1.5%), potassium (0.7–2.4%), sodium (0.8–2.7%) and sulfur (0.4–1.4%), as well as heavy metals such as copper (18–100 mg kg−1), iron (1.4–11 g kg−1) and zinc (28–64 mg kg−1), as described by Tibbetts et al. (2015).8 Among the major pigments and polyphenols in microalgae there are chlorophylls (0.5–1%), carotenoids (0.1–0.2%), and phycobiliproteins which are present specifically in cyanobacteria.9
| Carbohydrates (% TS) | Proteins (% TS) | Lipids (% TS) | N (%TS) | P (% TS) | K (% TS) | Ref. | |
|---|---|---|---|---|---|---|---|
| Botryococcus braunii | 4–55 | 1.5 | 25–75 | 8.3 | 1.4 | 0.8 | 11–13 |
| Chlamydomonas reinhardtii | 59.7 | 9.2 | 15–18 | — | — | — | 14 and 15 |
| Chlorella sp. | 12–26 | 53 | 28–32 | 9.7 | 0.91 | 0.91 | 13 and 16 |
| Dunaliella salina | 32 | 57 | 6–25 | 7.0 | 0.15 | 0.43 | 16 and 17 |
| Euglena gracilis | 14–18 | 39–61 | 4–20 | — | — | — | 17 |
| Isochrysis spp. | 4–8 | 5–14 | 7–40 | — | — | — | 18 and 19 |
| Nannochloropsis | 9.3 | 48.3 | 31–68 | 7.0 | 0.7 | 1.5 | 16 and 17 |
| Phaeodactylum tricornutum | 47 | 37 | 18–57 | 8.3 | 1.2 | 2.4 | 20 |
| Neochloris oleoabundans | 37.8 | 30.1 | 29–65 | 6.3 | — | — | 8, 19 and 21 |
Depending on the expected bio-product, the use and choice of microalgae species depends on their composition. The transformation efficiency of microalgae yield for energy, molecules and materials production are highly affected by operating conditions of the growth/harvesting and different process steps.10
3. Microalgae cultivation challenges
3.1. Effect of operating conditions on microalgae growth
According to Jankowska et al. (2017), the most important factors affecting microalgae growth are cultivation system, light intensity, temperature, pH level, CO2 concentration and nutrients availability in addition to biological factors.1Optimization of light intensity is required to avoid photooxidation and photoinhibition. In fact, above the light saturation point, microalgal growth is slowed down and can even be stopped, while light deficiency can lead to photo limitation, since, if present in excess, may cause the generation of harmful oxidative stress and reactive oxygen species (ROS). Liu et al. (2013) found that 150 μmol m−2 s−1 was the optimal light intensity above which microalgae growth rate was decreased.22 Patel et al. (2019) found that the increase of light intensity from 35 to 150 μmol m−2 s−1 increased the biomass yield of Chlorella protothecoides by 124%.2 However, in the presence of an external source of carbon, the growth was slightly improved (+13%) by light intensity increase.2 In the same manner, the growth rate of both Chlorella sorokiniana and Asterarcys quadricellulare doubled after the application of a light intensity of 200 μmol m−2 s−1 compared to 50 μmol m−2 s−1.23 Light intensity acts not only on growth but also on the accumulation of carbohydrates and lipids. Ho et al. (2012) reported that carbohydrates content from Scenedesmus obliquus was improved by 153% when light intensity increased from 60 to 420 μmol m−2 s−1.24 In another study, lipids content using Neochloris oleoabundans was increased by 26% when light intensity varied from 50 to 200 μmol m−2 s−1.25
In the case of high light intensity (greater than the light saturation point), the use of an intermittent light supply mode was recommended. In fact, the ratio of light to darkness periods is also an important parameter impacting the performance of a photo bioreactor (PBR). As explained in Patel et al. (2020), the microalgae absorb light energy under light period and fix CO2 using adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) which have reducing powers favoring the generation of lipids within the mixotrophic cultivation system.26 Compared to continuous illumination at optimal intensity, intermittency is less effective.2 However, from an economic point of view, it reduces energy consumption.
Light absorption is also related to the distance to light source. Liu et al. (2013) reported that at biomass yield was maximal at a distance of 2 cm and decreased above this value.22 Therefore, the position of the energy source must be determined in such a way that the received light intensity is equal to or less than the light saturation intensity. This finding was also reported in Richmond et al. (2003), the length of optical path can highly affect the light absorbance and thus the cultivation of photoautotrophic microorganisms.27 The efficiency of light use is also related to the irradiance conditions, for example, the use of one or more light sources, placed on one side of the bioreactor or on both sides which will increase the fraction of microorganisms exposed to light at any given time. However, as high cell density can negatively affect the light penetration into the culture,27 harvesting needs to be done more frequently.
Also, when increasing temperature from 15 to 23 °C, lipid content was reduced by 33%, while carbohydrate content was enhanced by 43%.10
As seen previously, the temperature tolerance of each species can be different depending on their adaptability and gene regulation sensitivity.30 Photosynthesis is negatively affected by low temperature which reduce the carbon assimilation. However, at high temperatures, photosynthetic proteins can be degraded and cell size can be highly reduced because of the decrease in ribulose-1,5-bisphosphate activity.31
However, pH stress conditions are widely studied in literature to investigate the impact of pH variation on bioprocesses stability. Ho et al. (2014) reported that lipid content and quality in biomass enhanced when increasing pH levels, but as growth decreases, lipid productivity is reduced.30 Carbohydrates content was also found to be affected by the culture pH. Extracellar carbohydrates content in Skeletonema costatum were constant in the range of 6.5–8.0 and increased above this pH level contrarily to growth rate.33
Nitrogen is an important nutrient, since it is essential for proteins synthesis. The three main nitrogen sources used for microalgae growth are nitrate, urea and ammonium. Khanra et al. (2020) reported that the nitrate addition as source of N for Chlorococcum sp. growth, enhanced biomass yield and lipid content by 40% and 117% respectively.36 Similarly, Zarrinmeher et al. (2020) reported that a concentration of 72 mg L−1 of nitrate improved proteins (+112%), lipids productivities (+71%) and growth yield (15-fold high) of I. galbana compared to starvation condition.39 Ammonium was reported to be the easiest nitrogen source to assimilate. However, growth inhibition may occur if ammonium concentration exceeds 100 mg L−1.40 In mixotrophic growth conditions (with 2 g L−1 of glucose added), ammonium addition at 50 mg L−1 enhanced lipids and proteins content, while carbohydrates synthesis was inhibited. However, in autotrophic conditions, nitrogen reduced lipids content in microalgae.41
In the opposite side, insufficiency of nitrogen can highly improve lipid and carbohydrates content in microalgae. Under starvation conditions, microalgae degrade chlorophyll and proteins and convert its Skelton carbon to lipids and carbohydrates.30 However, biomass yield was found to be reduced in nutrient-stressed cultures compared to nutrient-replete cultures. Especially, chlorophyll a, nitrogen, antioxidant activity and phenolic compounds content in biomass (Chlorella, Tetraselmis and Phaeodactylum) was negatively affected by the depletion of nitrogen.42 This finding is in agreement with Ho et al. (2013) in which carbohydrates content doubled under N starvation of Chlorella vulgaris.43
As for nitrogen, phosphorous can highly affect biomass growth and chemical composition. It is essential for microalgae cells and nucleic acids synthesis. The most easily assimilated form of phosphorous is phosphates. Phosphorus addition at 0.04 g L−1 didn't result in a significant change in terms of Podohedriella sp. yield. However, chlorophyll has roughly tripled and proteins content was increased by 82%.44 Phosphorous depletion was found to reduce biomass yield, P and chlorophyll a content of the microalgae. However, vitamins C and E content in biomass was increased.42 Shashirekha et al. (2016) found that a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 0.2
P ratio of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14
0.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8% was the optimal leading to maximized biomass S. obliquus yield in sugar mill effluent.45 The behavior of microalgae in the case of nutrient deficiency or sufficiency differs according to cultivation strategy, nutrients demand and the ability of microalgal species to tolerate environmental stress. Other nutrients such as trace metals are also important for microalgae cultivation as they promote enzymatic activities. Among the trace metals, iron was found to be the most important for its contribution in chlorophyll synthesis, electron transport and nitrogen fixation.46 The presence of iron with phosphorus was found to boost the production of microalgae under 0.05 μM Fe and 50 μM P.47
0.8% was the optimal leading to maximized biomass S. obliquus yield in sugar mill effluent.45 The behavior of microalgae in the case of nutrient deficiency or sufficiency differs according to cultivation strategy, nutrients demand and the ability of microalgal species to tolerate environmental stress. Other nutrients such as trace metals are also important for microalgae cultivation as they promote enzymatic activities. Among the trace metals, iron was found to be the most important for its contribution in chlorophyll synthesis, electron transport and nitrogen fixation.46 The presence of iron with phosphorus was found to boost the production of microalgae under 0.05 μM Fe and 50 μM P.47
Microalgae cultivation can be carried out in open ponds or closed photobioreactors. Photobioreactors are using light source for phototrophic microorganisms' growth. In this system, environmental factors are easy to control. Operating conditions may affect CO2 (then pH), nutrients, heat transfer and light availability in the cultivation system.1 Thus, mixing is among the most important parameters related to reactors functioning. Maximizing mixing condition favors biomass growth, as long as shear stress is taken into account, which is related to the culture medium velocity.1,35 As previously mentioned, light path can also affect microalgae growth. For photobioreactors, construction materials can play an important role in light absorption.48 Light attenuation should also be controlled by modifying the residence time to avoid dark zones within the PBR and then a decrease in biomass productivity.50
Also, an optimized diameter should be considered to accommodate the uniform distribution of light within the reactor.51 The modelling of PBR systems has already been reported in the literature50,52 but has not been addressed in this review.
The raceway pond is the cheapest cultivation system with a cost investment of 0.13 to 0.37 M€ per ha.53 It consists in an oval channel containing a paddle wheel to ensure the continuous water flow. It used sunlight for microalgae growth what makes the strength of this technique.
The most appropriate design of raceway ponds should enhance the productivity and thus, limiting the photoinihibition and photolimitation. These latter are generally avoided by optimizing the water head and reducing pond depth, which favors the contact of microalgae with light. In addition, low water head can be operated by a propeller which consumes less energy compared to paddle wheels.54
The use of existing wastewater lagoons for microalgae cultivation was found to be the most profitable way to produce biomass at large scale.55 In addition, nutrient supply, water and sunlight are provided. Utilization of photobioreactors is generally more productive, but some constraints related to reactor sizing should be considered. Norsker et al. (2020) reported that the maximal recommended length of tubular photobioreactor with diameters between 5–10 cm is around 80–120 m. The dimensions of the photobioreactors depend mainly on dissolved oxygen tolerance and flow velocity. Larger diameters were found to result in lower biomass concentration and density and lower diameters are uneconomical in terms of mixing energy because of shear stress.48 In contrast, open raceway ponds are the most frequently used systems at industrial scale due to their cost effectiveness. It can extend from 1000 to 5000 m2,53 for which the maximal depth is 0.45 m.56 However, contamination issues, harvesting difficulty and environmental conditions changes may be the challenges encountered by this type of systems.
When using open ponds, environmental changes including, temperature and humidity, can affect the productivity of biomass. In addition, loss of water through evaporation can occur.56 The use of sunlight can highly reduce the costs of microalgae production. However, this abundant energy source is not fixed and cannot be controlled. The variability of its intensity can lead to an unreliability of the process and therefore to a non-optimized production of microalgae, especially in cases where the objective is to produce products with high added value.57 For heterotrophic cultivation, the use of organic carbon source such as glucose, can lead to extra costs.
3.2. Biological contamination issues
Biological contaminants affecting the growth of microalgae are classified into 4 categories: zooplankton, microorganisms (bacteria, fungi and protozoa), other microalgae species and viruses.58 Contaminants can also be distinguished according to their contamination mechanisms. Microalgae grazers are found in all-natural aquatic environments. They present the greatest threat on microalgae growth. By ingesting microalgae, they cause reduction of biomass production. Park et al. (2016) reported that Chlorella kessleri can be highly infected by rotifers such as Brachionus calyciflorus. The solution given in the study suggests that addition of chlorine can help preventing pond crash.59 Also, the capacity of Brachionus calyciflorus to consume more than 500 cells of Chlamydomonas reinhardtii per hour and per rotifer was reported.58Grazers prevention can be carried out by sterilizing the medium before introduction in photobioreactors.60 However, the risk of contamination is high in open ponds compared to photobioreactors. In case of open ponds, the use of greenhouses has shown a positive effect on the reduction of the risk of wind infection by cysts and grazers eggs that are threatening microalgae.60 Other chemical treatments such as pesticides were studied, but their cost and impact on environment shows that their application in a larger scale may be inefficient. Moreover, the increase of CO2 concentration was found to be effective in controlling and eliminating grazers.61
In literature, the use of algal–bacterial co-culture based on crashed rotifer culture, had a positive impact on protecting Microchloropsis salina against Brachionus plicatilis. After months of repeating the assays, the algal–bacterial co-culture was no longer effective in protecting microalgae grown in open ponds. This was due to the absence of certain genus in the non-protective community. However, studies need to be carried out to ensure the reliability of this practice.62
4. Biogas production from microalgae
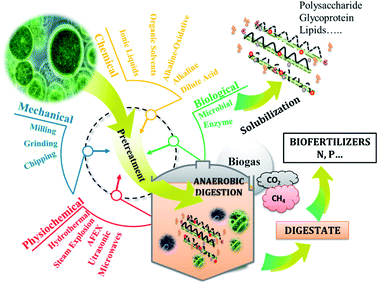
Anaerobic digestion (AD) is the biological process converting organic methane to biogas which is composed mainly of methane and CO2 (Fig. 1). As microalgae is rich in organic matter, its anaerobic digestion had attracted researchers' interest since 1982. Considering that, 1 g of carbohydrates, proteins and lipids produce 415, 496 and 1014 mL of methane. Thus, theoretically, Chlorella sp. can produce around 600 mL of methane per gTS. Mussgnug et al. (2010) found that the methane potentials of Chlamydomonas reinhardtii, Chlorella, Dunaliella salina and Euglena gracilis were 587, 335, 505 and 485 mL g−1 VS respectively.63 Other algal biomasses such as Isochrysis sp. had lower methane potential (135 mL g−1 VS) compared to the others,64 as long as it contained low carbohydrates (4–8%TS) as shown in Table 1.Anaerobic digestion process is the succession of 4 stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. In which the complex organic matter is broken down by the microorganisms into monomers, then volatile fatty acids, then into acetate, hydrogen and CO2, ultimately leading to the production of methane.65 The recalcitrance of microalgal cell walls can be the reason behind the low methane production.66 Thus, pretreatments may be needed to increase solubilization of organic compounds contained in microalgae biomass and therefore, enhance the hydrolysis yield. The latter can be considered as the limiting step of AD process in the case of hardly biodegradable matter.
Many factors can affect the AD process, such as temperature (mesophilic or thermophilic), pH (6.5–7.5), C to N ratio (20–25) and operating conditions (organic loading rate, hydraulic retention time and mixing conditions). In the case of microalgae, Zamalloa et al. (2012) reported that thermophilic AD (54 °C) resulted in lower methane production from Scenedesmus obliquus and Phaeodactylum tricornutum compared to mesophilic temperature (33 °C).67 C to N ratio in Chlorella vulgaris was found to be around 17,68 while Stigonematales sp had lower C to N ratio (around 4.7).69 This suggests that digestion of microalgae with other organic substrates with higher C to N ratio can be carried out to enhance biogas production, which is known as co-digestion. By its simplicity and low energy and chemicals requirements, AD can be an efficient bioprocess for microalgae transformation into bioenergy. When AD of microalgae is applied as downstream process, steps such as dewatering and drying of microalgae are not required. In the case of an existing digester within the wastewater treatment plant, the co-digestion of microalgae with sewage sludge can be feasible and profitable.70
4.1. Pretreatment of microalgae
Pretreatment is a well-known practice in microalgae valorization such as anaerobic digestion (Fig. 1). Hence, the pretreatment of microalgae prior to its valorization is an essential step in order to increase macromolecules accessibility and biodegradability by anaerobic microorganisms. Application of pretreatment steps allows modifying the supramolecular structure of microalgae matrix, thereby changing the natural binding characteristics of microalgae materials and increasing the carbohydrates, proteins, lipids… accessibility for enzymatic or chemical biological action. For the last few years, several physicochemical pretreatments have been developed and applied to microalgae biomass for this purpose, including ultrasonic, mechanical, microwaves, chemical, biological, thermal and combined pretreatments. However, although most of them are known to be effective in hydrolysing the matrix and enhance sugars recovery, they are energy consuming and not always cost effective.71 Microalgae biomass cell is composed of the skelton containing cellulose, mannan and xylans.3 In addition, cell walls are composed of polysaccharide and glycoprotein matrix protecting microalgal cells.72 However, the composition of cell walls varies from species to species. Dunaliella sp. has no cell wall while Chlorella and Scenedesmus sp. can further contain algeanan which is a lignin-like polymer.3,73 The robustness of this wall obviously depends on the growing conditions, such as the attack of the grazers or variable environmental conditions.74 For this reason, the key driver for the successful conversion of microalgae into energy and high values added compounds is the selection of efficient pretreatments that permit to maximize the sugars, lipids and proteins recovery and to minimize their degradation with the consequent formation of toxic derivatives. Thus, the AD of microalgae may require pretreatments of which some recent results are summarized in Table 2.| Biomass | Pretreatment | Conditions | Results | Ref. |
|---|---|---|---|---|
| Consortia (Scenedesmus and Chlorella) | Microwaves | 300 W, 3 min (50 °C) | +280% of VS solubilization | 77 |
| AD: batch test at 35 °C for 45 d | +13% of methane produced | |||
| 900 W, 3 min (98 °C) | +799% of VS solubilization | |||
| AD: batch test at 35 °C for 45 d | +78% of methane produced | |||
| Microalgae-based wastewater treatment system | Enzymatic | 1% Enzyme mix (cellulase, glucohydrolase and xylanase) at 37 °C for 6 h | +243% of soluble VS | 3 |
| AD: batch test at 35 °C for 45 d | +15% of methane produced | |||
| Isochrysis galbana | Chemical | TS (45 g L−1) | +15% of biogas produced | 64 |
| 0.02% H2SO4 at 40 °C for 16 h | ||||
| AD: batch test at 30 °C for 15 d | ||||
| Nannochloropsis Salina | Ultrasounds | TS (35%) | +64% of soluble VS | 79 |
| 200 W, for 45 s | −29% of methane produced | |||
| AD: batch test at 38 °C for 40 d | ||||
| Microwaves | TS (35%) | +130% of soluble VS | ||
| 600 W, 2450 MHz (until boiling) | +40% of methane produced | |||
| AD: batch test at 38 °C for 40 d | ||||
| Thermal | TS (35%) | +116% of soluble VS | ||
| 100 °C for 8 h | +58% of methane produced | |||
| AD: batch test at 38 °C for 40 d | ||||
| Phaeodactylum tricornutum | Ultrasounds | TS (67 g L−1) | +11% of methane | 78 |
| 21 MJ kg−1 TS | ||||
| 36 MJ kg−1 TS | +10% of methane | |||
| 52 MJ kg−1 TS | +11% of methane | |||
| AD: batch test at 33 °C for 29 d | ||||
| Chlorella vulgaris | Thermochemical | TS (16 g L−1) | +600% of released carbohydrates | 75 |
| H2SO4 (4 M, pH 2) at 120 °C for 40 min | +11% of released proteins | |||
| AD: batch test at 35 °C for 30 d | +65% of methane produced | |||
| Thermochemical | TS (16 g L−1) | +400% of released carbohydrates | ||
| NaOH (4 M, pH 10) at 120 °C for 40 min | +94% of released proteins | |||
| AD: batch test at 35 °C for 30 d | +73% of methane produced | |||
| Thermal | TS (16 g L−1) | +340% of released carbohydrates | ||
| 120 °C for 40 min | +17% of released proteins | |||
| AD: batch test at 35 °C for 30 d | +93% of methane produced | |||
| Nannochloropsis salina | Thermal | TS (16–30%) | +150% of biogas produced | 76 |
| 120 °C for 2 h | ||||
| AD: semi-continuous at 38 °C, 1.96 kg per VS per m3 per d for 120 d | ||||
| Botryococcus braunii | Biological | TS (0.5 g L−1) | +67% of methane produced | 80 |
| White-rot fungus (Anthracophyllum | ||||
| 1000 U L−1 for 24 h | ||||
| AD: batch at 30 °C for 55 d | ||||
| Chlamydomonas reinhardtii | Thermal (drying) | 105 °C for 24 h | −18% of biogas produced | 63 |
| AD: natch at 38 °C for 30 d | ||||
| Scenedesmus sp. | Enzymatic | TS (16 g L−1) | +68% of methane produced | 82 |
| Alcalase (0.2 mL g−1) at 75 °C for 30 min. | ||||
| AD: batch at 35 °C for 25 d | ||||
| Chlorella vulgaris | Biological | COD (20 g L−1) | +87% of methane produced | 99 |
| 0.7 g L−1 of cellulase-secreting bacteria was added at 40 °C for 24 h. | ||||
| AD: batch test at 30 °C for 30 d | ||||
| Scenedesmus almeriensis and Chlorella vulgaris | Thermal pretreatment | WAS:microalgae = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
sCOD increase (74-fold) | 98 |
| 60 °C for 24 h | −50% of methane produced | |||
| AD: batch at 37 °C for 50 d | ||||
| Macroalgae | Alkaline | TS (3%) | +16% of methane produced | 70 |
| 37% of HCl at 121 °C, 10 bars for 30 min. | ||||
| AD: batch at 37 °C for 50 d |
Thermal pretreatment of Chlorella vulgaris at 120 °C for 40 min improved organic matter solubilization leading to an enhanced methane production by 93%.75 In the same manner, subjecting Nannochloropsis salina to 120 °C for 2 h resulted in 150% more biogas produced.76 However, according to Mussgnug et al. (2010), thermal pretreatment at 105 °C for 24 h can lead to reduced biogas production by 18%, which may be due to volatilization of organic matter.63 Applying microwaves to a co-culture of Chlorella and Scenedesmus sp. can result in high solubilization of VS with a 13% higher methane production.77 However, Schwede et al. (2013) reported that microwaves can lead to higher methane production (+40%) from Nannochloropsis salina. Microwaves are generally used to substitute conventional heating that consume more energy and take more time. However, the effectiveness of conventional heating can be higher than that of microwaves as reported in Schwede et al. (2013). In this case, it is necessary to consider whether the energy dissipated by the conventional heat pretreatment is compensated by the increase in methane production. Ultrasonic pretreatment is generally used to disrupt flocs and when applied to microalgae, it was found to improve solubilization of VS, but negative or low effect on methane production was reported.78,79 Acid and alkaline pretreatments were also studied and a methane production increase by 15 and 16% were respectively found.64,70 However, by combining thermal and chemical pretreatments, methane production can be enhanced as found in a previous work.75 Moreover, biological pretreatment can achieve high methane production increase. It is the most eco-friendly pretreatment since it is generally carried out at mild conditions. Ciudad et al. (2014) found that fungi pretreatment enhanced methane production of botryococcus braunii by 67%,80 while an increase of 87% of methane production was reported after the biological pretreatment of chlorella vulgaris.81 Methane production from Scenedesmus sp. was also increased by 68% after enzymatic pretreatment.82 The efficiency of pretreatment depends highly on the methane production increase, costs related to energy and chemical consumption as well as the impact of pretreatment on digestate management.
4.2. Codigestion of microalgae
As mentioned previously, codigestion aims to adjusting C to N ratio, humidity and diluting inhibitory compounds such as polyphenols.83 Thus, codigestion of microalgae can improve methane production depending on methane potential of the co-substrate. Codigestion of microalgae with sludge was much studied. Microalgae can be used in WWTPs to treat wastewater effluents (N and P removal), their co-digestion with sludge in already existing digester in WWTPs appears to be an effective and cheaper way to valorize them. Many studies have investigated the effect of different microalgae to sludge ratio on methane production synergism. However, on a real scale, this ratio cannot exceed 50%. It is generally between 5/95 and 20/80 (based on VS).4 In order to achieve the optimal C to N ratio of microalgae and septic sludge mixture, algal proportion should be within the range of 25–75% and a 3-fold improved methane production was observed compared to microalgae monodigestion.84 Similarly, Wang et al. (2013) reported that Chlorella sp. ratios of 4% and 11% resulted in enhanced methane production compared to microalgae alone (73–79%).85 The synergetic effect of sludge and Chlorella sp. codigestion is not always observed, which was the case in.86 Codigestion of sludge with 4% of Chlorella sp. resulted in higher dewaterability of digestate compared to monodigestion of microalgae. In addition, it was richer in ammonium and phosphorus, which suggests that its agronomic quality was improved due to sludge addition.85 In parallel, addition of microalgae to sludge AD can highly affect digestate composition. Solé-Bundo et al. (2019) found that caffeine and triphenyl phosphate were removed from codigestion effluent by 92% and 64%.87 Other organic wastes such as agricultural residues, animal wastes and food waste were studied as co-substrates of microalgae digestion. Agricultural residues are known for having a relatively high C to N ratio.88 Thus, their codigestion with microalgae can improve the methane production. Potato peels wastes codigested with Chlorella vulgaris at a ratio of 25/75 resulted in 32% higher methane production,89 where the C to N ratio increased when increasing potato peels proportion. Wheat straw was also used as cosubstrate for microalgae biomass containing mainly Chlorella sp. at a ratio of 50/50 (VS basis), AD was found to be slightly synergetic with 7% of additional methane production.90 Moreover, codigestion of Microcystis spp. with corn straw was studied to enhance C to N ratio. It was shown that at a C to N ratio of 20/1, methane production was maximal with an increase of 62% compared to microalgae monodigestion.91 Passos et al. (2018) reported that codigestion of Chlorella sp. with coffee husks provided 17% more methane production compared to theoretical production determined based on methane potential of both substrates.92 However, in the literature, many studies are focused on certain species such as Chlorella sp. It is assumed that other microalgal biomasses, especially those rich in proteins such as Dunaliella salina and Euglena gracilis, should be studied in codigestion with carbon-rich organic substrates to adjust their C/N ratio, and avoid inhibition by ammonia.93 Recently, codigestion of Dunaliella salina and olive mill solid waste was studied at a ratio of 5/95 and resulted in 29% high methane production compared to olive mill solid waste alone.94 When mixing Scenedesmus sp. with pig manure at a ratio of 1/1, methane production was enhanced by 50% compared to microalgae alone, however, no synergetic effect was observed.95 Food waste is also a carbon rich material, that is widely used as cosubstrate of sludge. It was already studied in codigestion with a mixture of Chlorella vulgaris and Scenedesmus obliquus. Results showed that at a ratio of 12/88 (microalgae to food waste ratio), methane production was enhanced by 20% compared to monodigestion of food waste.96Anaerobic digestion can be integrated to microalgae valorization processes, such as lipid extraction and biodiesel production. In fact, microalgae residues from biodiesel production can be digested alone or codigested with other organic wastes. Relatively low synergy of methane production was obtained after the codigestion of lipid spend microalgae and glycerol at a ratio of 67/3. This was explained by the low C to N ratio of the mixture and also the potential inhibitory impact of the used solvent for transesterification.97 Lipid extraction can be seen as a pretreatment step, as it can enhance biomass accessibility improving, thus, its methane potential, which was reported in.95 Moreover, codigestion of lipid extracted microalgae with pig manure had the same effect as raw microalgae codigestion with pig manure at similar mixture ratio,95 which raises the question of the effectiveness of combining co-digestion and molecules extraction of microalgae.
4.3. Combining codigestion and pretreatment
Theoretically, the hard digestibility of microalgae and some organic wastes can reduce the benefit of their codigestion. Thus, pretreatments can be carried out to one substrate or to the mixture. In fact, Mahdy et al. (2015) reported that thermal pretreatment at 120 °C for 40 min of Chlorella vulgaris before its mixture with primary sludge, improved methane production by 10%.82 Similarly, pretreated microalgae mixed with sludge and fats, oil and grease resulted in 15% higher methane production compared to untreated microalgae.86 However, the authors do not recommend the pretreatment as it has generally negative energy balance which must be compensated by a greater improvement in methane. Thermal hydrolysis (120 °C for 60 °C) of the mixture of microalgae and coffee husks increased methane production by 15%. However, it is assumed that the impact of pretreatment on individual substrates was more important (18% and 31% for microalgae and coffee husks respectively).92 In some cases, the effect of pre-treatment is reversed. In fact, when low temperature pretreatment (60 °C for 24 h) was applied to microalgae and sludge mixture at a ratio of 1/25, methane production was reduced by 51% compared to untreated mixture.98 However, previous researches assumed that there is no need to pretreat microalgae prior its codigestion. Moreover, additional costs related to chemicals and energy consumption can be generated.5. Microalgae for agriculture
5.1. Impact on soil properties
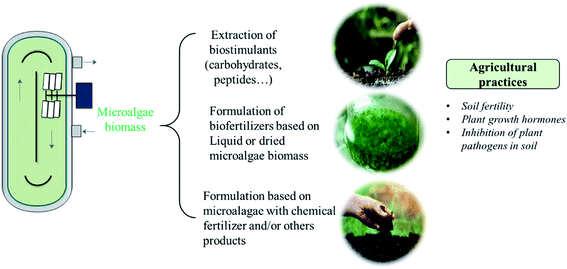
Fig. 2 illustrates the different agronomic applications of microalgae. Soil degradation can be trigged by the extensive agricultural practices in the fields which may affect 30% of the cultivable soils.100 The application of microalgae enhances soil organic matter through production of exopolysaccharides which favors the growth of soil's fauna and flora. In addition, the inoculation of microalgae promotes the solubilization and mineralization of nutrients due to its photosynthetic activity.101 Yilmaz and Sönmez (2017) found that the use of Chlorella vulgaris as biofertilizer added to vermicompost increased the soil aggregates stability and organic carbon by 19%.102 Table 3 presents the effect of some microalgae use as fertilizer on soil nutrients. Chlorella vulgaris and Scenedesmus quadricauda were previously used as biofertilizers. It was found that the enzymatic activity of soil was enhanced by the addition of living microalgae as well as their extracts.103 However, the use of live microalgae allows the in situ growth of these microorganisms at soil level, without the need for a separate cultivation process.104 In addition, this technique reduces the costs related to the extraction of biostimulants or the drying of the algal biomass. Moreover, combining cyanobacteria with microalgae resulted in increased desert soil stability.105 In the same manner, the application of microalgal consortia was found to enrich soil with the essential nutrients (N, P and K).106 However, compared to cyanobacterial consortia, Chlorella application was less effective in enriching soil with nutrients.106 Moreover, Dineshkumar et al. (2018) found that nitrogen, phosphorous and potassium and soil enzymes activities were positively affected by the use of Chlorella and S. platensis as biofertilizers, but the impact of S. platensis was the highest.107 In addition, the use of microalgae and cyanobacteria was found to reduce the required N fertilizer dose.107 Other essential nutrient such as iron, magnesium and zinc were found to be positively affected by microalgae and cyanobacteria addition.108 Moreover, algal biomass can generate siderophores which promote ferric iron chelation and thus make it more accessible to plants and soil’ microbial communities.101| Species | Crop/soil | Conditions | Results | Ref. |
|---|---|---|---|---|
| Microalgal consortia comprising native unicellular strains of sewage (species of Chlorella, Scenedesmus, Chlorococcum, Chroococcus) | Wheat | N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 120 K = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 kg ha−1) 60 kg ha−1) |
+38% of available N in soil at harvest stage | 106 |
| +400% of available P | ||||
| 75% of N and full dose of PK | +20% of available K | |||
| Native filamentous strains isolated from sewage wastewater (species of Phormidium, Anabaena, Westiellopsis, Fischerella, Spirogyra) | +52% of available N in soil at harvest stage | |||
| 50 g of biomass in 6 kg of soil | +480% of available P | |||
| +25% of available K | ||||
| Chlorella vulgaris | Onion | 3 g of dried biomass per kg of soil | N in soil (+14%) | 108 |
| P in soil (+3%) | ||||
| K in soil (+29%) | ||||
| Fe in soil (+21%) | ||||
| Zn in soil (+83%) | ||||
| Mg in soil (+149%) | ||||
| Spirulina platensis | Mixed with 10 g of cow dung manure | N in soil (+17%) | ||
| P in soil (+3%) | ||||
| K in soil (+12%) | ||||
| Fe in soil (+36%) | ||||
| Zn in soil (+114%) | ||||
| Mg in soil (+91%) | ||||
| Calothrix elenkinii | Rice/sandy clay loam (semi-arid climate) | Sterilized soil | Dry weight (+26%) | 122 |
| Available N in soil (−14%) | ||||
| Unsterilized soil | Dry weight (+36%) | |||
| Available N in soil (+13%) | ||||
| Acutodesmus dimorphus | Tomato | Greenhouse conditions at approximately 28 °C, in 85% relative humidity | +180% of branches number | 123 |
| 50 g of dry microalgae before 22 d of transplant | +150% of flower buds numbers | |||
| Total fresh plant weight (10-fold) | ||||
| Chlorella vulgaris | Maize/sandy loam soil | 3 g of dried biomass per kg of soil | Fresh weight (+38%) | 114 |
| Dry weight (+30%) | ||||
| Chlorophyll a (+240%) | ||||
| Chlorophyll b (+225%) | ||||
| 3 g of dried biomass per kg of soil | Fresh weight (+57%) | |||
| Mixed with cow dung manure | Dry weight (+38%) | |||
| Chlorophyll a (+270%) | ||||
| Chlorophyll b (+275%) | ||||
| Spirulina platensis | 3 g of dried biomass per kg of soil | Fresh weight (+41%) | ||
| Dry weight (+35%) | ||||
| Chlorophyll a (+240%) | ||||
| Chlorophyll b (+250%) | ||||
| 3 g of dried biomass per kg of soil | Fresh weight (+87%) | |||
| Mixed with cow dung manure | Dry weight (+49%) | |||
| Chlorophyll a (+270%) | ||||
| Chlorophyll b (+350%) | ||||
| Chlorella vulgaris | Maize | 0.5 L of biomass diluted in 400 L of water | Plant height at 30th day (+60%) | 112 |
| Germination rate (+40%) | ||||
| Wheat | Plant height at 30th day (+50%) | |||
| Germination rate (+17%) | ||||
| Spirulina platensis | Rice/clay loam soil | Soil drench application of microalgae (+75% of recommended nitrogen) | Weight (+29%) | 107 |
| Chlorella vulgaris | Weight (+10%) | |||
| Nannochloropsis oculata | Tomato | Greenhouse conditions. | Dry weight (+42% compared to inorganic fertilizer) | 116 |
| Nutrients: | ||||
| 3600 mg per N per plant | Carotenoids (+50% compared to inorganic fertilizer) | |||
| 4600 mg per K per plant | ||||
| Inorganic fertilizer | Dry weight (+23% compared to organic fertilizer) | |||
| 678 mg per P per plant | ||||
| Organic fertilizer: 1746 mg P per plant | Carotenoids (no significant difference compared to organic fertilizer) | |||
| Microalgae: 7900 mgP per plant | ||||
| S. platensis | Red spinach | 5 g of biomass per pot | Chlorophyll (+34%) | 115 |
| Dry weigh (+156%) | ||||
| 5 g of biomass per pot | Chlorophyll (+54%) | |||
| +Triple Pro 15-15-15, (0.3 g per pot per week) | Dry weigh (+430%) | |||
| Phaeodactylum tricornutum | Bell pepper | NaCl (0 mM) | Germination (+36%) | 113 |
| NaCl (25 mM) | No significant effect on germination | |||
| Dunaliella salina | NaCl (0 mM) | Germination (+36%) | ||
| NaCl (25 mM) | No significant effect on germination | |||
| Chlorella sp. | Wheat/Desert soil | Microalgae grown in wastewater | Plant height (+100%) | 103 |
| Scenedesmus sp. | Plant height (+100%) | |||
| Tetraselmis sp. | Microalgae grown in seawater | Plant height (+77%) | ||
| Nannochloropsis sp. | Plant height (+100%) | |||
| Monoraphidium sp. | Tomato | 170 kg per N per ha | Plant weigh (+32%) | 117 |
| No effect on elemental composition of plants | ||||
| Chlorella vulgaris | Lettuce | Fresh 0.5 g kg−1 of soil | −4% of total pigments | 119 |
| 1 g kg−1 of soil | +30% of total pigments | |||
| 2 g kg−1 of soil | −4% of total pigments | |||
| Dried 0.5 g kg−1 of soil | −9% of total pigments | |||
| 1 g kg−1 of soil | −17% of total pigments | |||
| 2 g kg−1 of soil | −14% of total pigments |
Microalgae can promote the formation of biological soil crusts which help maintaining soil's biota and thus fertility. Yet, the greatest interest in using living microalgae in soils arises in their application to degraded soils such as desert soil. In fact, microalgae can be associated with minerals to form water-stable organomineral formulation which enhance arid soil fertility.109 In addition, microalgae can survive the severe conditions due to their cellular structure especially the presence of cysts. For instance, Protosiphon botryoides, was reported to survive and was still active after 43 years in a dried soil content.110 In the same context, Perera et al. (2018) highlighted the benefit of using the engineered consortia of cyanobacteria/microalgae for rehabilitating desert ecosystems.105 However, the agronomic potential of microalgae may be limited by their growth which may be affected by soil moisture, pH (which need to be slightly acidic to basic) and temperature.100 More studies should be carried out to investigate the mechanisms of soil fertilization by microalgae, especially in arid environments.
5.2. Impact on plant growth and properties
As seen previously, microalgae application can affect soil properties and thus increase availability of nutrients to the plants. In addition, it provides assimilable nitrogen to plant through atmospheric nitrogen fixation.111 As the first step of plant growth is germination, healthy seedlings can be obtained due to microalgae application.103 Some literature results showing the impact of microalgae and cyanobacteria on plant growth, are given in Table 3. Uysal et al. (2015) investigated the impact of Chlorella sp. in wheat and maize germination. It was found that an enhancement of 17% and 40% of germination rate was noticed for wheat and maize respectively.112 Moreover, germination rate of bell pepper was increased by 36% after the application of both Dunaliella salina and Phaeodactylum tricornutum.113Chlorella vulgaris and Spirulina platensis were found to enhance dry weight of maize plant by 30% and 35% which was increased to achieve 38% and 49% when adding a chemical fertilizer.114 Wuang et al. (2016) found that Spirulina platensis enhanced dry weight of red spinach plant and its chlorophyll content and when mixed with chemical fertilizer, the application efficiency was enhanced.115 Similarly, microalgae was reported to have higher impact on plant weight and pigments compared to inorganic fertilizer.116 However, Jimenez et al. (2020) found that microalgae enhanced tomato plant weight and chlorophyll a by 32% and 13% compared to unfertilized soil, while industrial fertilizer had similar effect on tomato plant weight but higher impact on chlorophyll a (+69% with respect to control).117 Also, it was reported that microalgae use had significant impact on nitrogen leaching (7% of loss) compared to industrial fertilizer (50% of loss).117
In fact, during their growth, microalgae uptake macro and micro nutrients and store them in their cells,111 which explains their potential compared to chemical fertilizer. This effect depends strongly on species and their growth conditions,103 dosages118 and crop plant.112 An algal liquid fertilizer was obtained using 500 mg of dried Chorococcum sp. biomass in 200 mL of distilled water. This algal liquid was found to have the best effect on 4 plants growth with a concentration of 50%.118
Moreover, the use of fresh or dried biomass was reported to have different impacts on lettuce growth in which case, fresh microalgae was recommended.119 The enhancement of plant growth after microalgae/cyanobacteria inoculation is owed to their metabolites, which are capable of initiating plant metabolic reactions, such as respiration, photosynthesis, nucleic acid synthesis, chlorophyll production and ion absorption.120 This finding was in agreement with Dineshkumar et al. (2019), in which cyanobacteria and microalgae mixtures with cow dung enhanced pigments and nitrogen, potassium and phosphorus contents in the maize plants.114
However, another study has shown that elemental composition of tomato plants were not significantly affected by microalgae addition as fertilizer, while nitrogen content was slightly increased due to industrial fertilizer incorporation.117 The results of using microalgae in soil fertilisation differ according to the cultivation and agronomic tests conditions. However, the interest in using these species as biofertilisers remains high. More research studies are needed to understand much better the different interactions between these species and the soil/plant and the impact of biofertilisation on the nutritional quality of the fruit of these plants.
Despite the positive impact of applying microalgae biomass and extracts as fertilizer, the optimization of its concentration should be considered in order to maximize its advantage for plant growth. A 60% concentration of microalgae led to higher length, dry weight and chlorophyll content in tomato plant.121
Without any regard to the effectiveness of microalgae application, the use of microalgae as biofertilizer was found to be less beneficial compared to NPK fertilizer, which is mainly caused by microalgae cultivation and harvesting processes.124 In the absence of optimization of these processes, the use of algae in agronomy may be less economically and environmentally attractive. In addition, the abiotic factors related to the growth environment can highly affect both the productivity and the biochemical composition of the microalgae.
To improve the profitability of the conversion of microalgae into valuable products for agronomy, the selection of microalgae strain to be used should be done in the basis of its composition and growth conditions. In addition, depending on extraction methods, several biofertilizers and biostimulants formulations can be realized.
For the commercialization of microalgae products, it is recommended to adopt a quality management system and the standardization of microalgae production processes to control the efficiency of the produced microalgae-based fertilizers.125 Good field practices of these biofertilizers must be established, also the choice of plants and the frequency of treatment play a role in the effectiveness of these products.124
6. Closed-loop valorization of microalgae
6.1. Microalgae digestate as fertilizer
Generally, the composition of digestate depends on substrate composition, inoculum sources, AD conditions and configurations. Table 4 presents some typical properties of digestate. Digestate can be used as an organo-mineral fertilizer substituting the mineral fertilizers, due to its macronutrients elements (nitrogen, phosphorous and potassium).126 In addition, digestate is often rich in metal trace elements that are essential for plant germination and growth. However, an excessive heavy metal concentration or salinity can limit the use of digestate as fertilizer or amender. According to EU regulations, the limit concentrations of biosolids to be applied to soil should not exceed 2, 1000, 800, 10, 200, 1000 and 3000 mg kg−1 TS for Cd, Cu, Pb, Hg, Ni, Cr and Zn respectively. Moreover, digestate is much cheaper than mineral fertilizer, with a cost saving of 390 € per ha.127| Parameters | Typical digestate128,129 | Digestate from microalgae130 |
|---|---|---|
| pH | 7.3–9.0 | 7.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Matter profile | ||
| TS (%) | 1.5–45.7 | 2.9 |
| Organic matter (%TS) | 38–77 | 53 |
| Organic carbon (%TS) | 27–45 | 22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Nutrients profile | ||
| NH4+ (g per N per kg per TS) | 3.3–453.3 | 27.6 |
| C/N | 2–24.8 | 3 |
| TKN (g per N per kg per TS) | 31–140 | 80.6 |
| Ca (g per CaO per kg per TS) | 0.2–66 | 8.9 |
| K (g per K2O per kg per TS) | 19–95 | 5.2 |
| Mg (g per MgO per kg per TS) | 1–47 | 4.2 |
| Na (g per Na2O per kg per TS) | 0.7–25 | 9.4 |
| P (g per P2O5 per kg per TS) | 2–42 | 3.9 |
| Cd (mg kg−1 TS) | — | 2.7 |
| Cu (g kg−1 TS) | 0.01–0.27 | 0.59 |
| Pb (mg kg−1 TS) | — | 49 |
| Hg (mg kg−1 TS) | — | 1.7 |
| Ni (mg kg−1 TS) | — | 127 |
| Cr (mg kg−1 TS) | — | 75 |
| Zn (g kg−1 TS) | 0.07–2.2 | 0.59 |
Microalgae digestate can be used as biofertilizer as it is rich in organic matter, nutrients and heavy metals as presented in Table 4. Microalgae digestates was found to meet the European material requirements for an organic amendment. Available forms of nutrients in liquid and solid fractions of digestate from lipid-extracted C. muelleri were reported in a previous study.131 In fact, 55% of nitrogen was found in solid digestate while 68% of phosphorus was released in liquid fraction. Phosphorus was found to be more available in algal digestates compared to dairy manure.132 Even if the nitrogen was immobilized in the solid fraction, ammonium content in the dissolved organic matter fraction accounted for 40%, which highlights the fertilizing potential of microalgae digestate.131
Moreover, most Oocystis sp. cells were not degraded in digestate. This suggests that: (i) hydrothermal pre-treatment (130 °C for 15 min) improves the degradation of the organic matter contained in the microalgae and thus its methanogenic potential and also, (ii) to preserve organic matter for soil amendment with microalgae digestate, pre-treatments will not be favorable.133 In addition, the presence of living microalgae can promote soil biofertilization. This finding was also reported in another study, where Stigeoclonium sp., diatoms Nitzschia sp. and Amphora sp. were present in digestate. The microalgal cells degradation during anaerobic digestion depend highly on algal stains.134
In addition, Solé-Bundo et al. (2017) study showed that microalgae digestate increased the growth index of cress by 10% which was lower than the growth index after codigestion residue application (75% VS of sludge and 25% VS of microalgae). Despite its lower nutrient content compared to monodigestate, the codigestion residue was found to present less phytotoxicity compared to untreated microalgae digestate, while the digestate of thermally pretreated microalgae had no impact on cress growth,130 which suggest that the impact of codigestion with sludge on digestate agronomic properties was higher compared to the impact of pretreatment.
6.2. Digestate as culture medium
As seen previously, mixotrophic mode was reported to be more advantageous concerning microalgae growth and productivity. As this mode requires nutrients and organic matter supply, wastewater, industrial and agricultural effluents as well as digestates can be used as cheap culture media for microalgae growth.124 The optimized use of effluents as culture media can improve the cost-effectiveness of microalgae cultivation making it more competitive with industrial fertilizers.124 At the same time, the treatment of these effluents can be carried out.In fact, living or non-living algae can be used in heavy metals removal from wastewater. Biosorption using microalgae is more environmentally friendly if compared to surface modified biomass. It is also more cost effective with the possibility of converting the heavy metals to low toxic forms.135 Using Chlorella vulgaris as biosorbent for Cr(VI) removal was found to be effective. At a dose of 1 g L−1 of algae and 50 mg of Cr per L for 4 hours and pH 2, the biosorption efficiency achieved 80%.136 Under the same conditions, Spirulina sp. was found to remove effectively chromium after 30 min.137 Thus, the efficiency of heavy metal biosorption and operating conditions can be different from species to others. Also, pretreatment of microalgae may be needed to enhance its selective removal.138
Carbon, phosphorus and nitrogen from wastewater can also be removed by microalgae. Chlorella vulgaris and Scenedesmus obliquus are among the most studied biomasses for nutrients removal. Galan et al. (2020) reported that phosphorus and ammonium were removed from agricultural runoff with 100% and 93% efficiency respectively.139 However, the use of microalgae for pesticides such as terbutryn, diuron, diazinon and imidacloprid removal was not effective.139 Moreover, the use of microalgae for biosorption of toxic compounds can highly affect its ultimate conversion to biofuels, use for food or agriculture.138 For this reason, the high nutrients and turbidity of the digestate can hinder its use as medium for microalgae culture. As light is the limiting factor for microalgae growth, suspended solids in digestate can absorb light and then increase its attenuation.140 High turbidity can be overcome by pre-treating digestate with centrifugation and filtration for examples which generates additional costs.141 However, the most used method to reduce turbidity is dilution. Rajagopal et al. (2021) reported that above a digestate concentration of 30%, the high N content inhibited microalgae growth.142 Dilution is also used to reduce the ammonia supply when its concentration in digestate exceed 100 mg L−1.141 In the same manner, C to N and N to P ratios in digestates should be maintained into the optimal ranges of 4–8 and 14–16 respectively for an effective microalgae growth.141 Also, if CO2 from anaerobic digestion is used for microalgae growth, attention should be paid to high concentrations of NOx and SOx.143 Many studies have been carried out to assess the effect of digestate on microalgae growth as reported in Table 5. Digestate from different sources: manure, crop residues or municipal wastes showed different impacts depending on microalgae species, the dilution factor and growth conditions. Generally, a dilution factor above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 is used.144 Jimenez et al. (2020) reported that an increase in dilution factor enhanced the specific growth of Monoraphidium sp. as well as ammonium and phosphorus removal efficiencies.117 However, in the case of using digestate from cattle slurry and cheese whey mixture, the final specific growth of three different species of microalgae was found to be reduced when increasing dilution factor. In fact, growth was faster when higher dilution factors were used, but after the 7th day, microalgae growth was slowed down which may be due to low nutrients concentrations in digestate and their fast consumption by microalgae.145 However, the effect of digestate dilution on biomass growth can depend on microalgae strains.146 In the case of Synechocystis sp., biomass productivity was improved by digestate dilution, contrarily to N. salina.146 Digestate source may also affect microalgae growth, which is obviously related to the composition of the substrates.6
10 is used.144 Jimenez et al. (2020) reported that an increase in dilution factor enhanced the specific growth of Monoraphidium sp. as well as ammonium and phosphorus removal efficiencies.117 However, in the case of using digestate from cattle slurry and cheese whey mixture, the final specific growth of three different species of microalgae was found to be reduced when increasing dilution factor. In fact, growth was faster when higher dilution factors were used, but after the 7th day, microalgae growth was slowed down which may be due to low nutrients concentrations in digestate and their fast consumption by microalgae.145 However, the effect of digestate dilution on biomass growth can depend on microalgae strains.146 In the case of Synechocystis sp., biomass productivity was improved by digestate dilution, contrarily to N. salina.146 Digestate source may also affect microalgae growth, which is obviously related to the composition of the substrates.6
| Digestate | Microalgae | Digestate dilution and pretreatment | Growth conditions | Results | Ref. |
|---|---|---|---|---|---|
| Mixture of crop residues and animal manure | Monoraphidium sp. | Separated with screw press, centrifuged and filtered with a 25 μm filter | 33.75 μmol m−2 s−1 | Specific growth = 0.03 d−1 | 117 |
| 21–23 °C | NH4+ removal = 34% | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Continuous | P–PO4−3 removal = 0% | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
Specific growth = 0.05 d−1 | ||||
| NH4+ removal = 66% | |||||
| P–PO4−3 removal = 77% | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Specific growth = 0.13 d−1 | ||||
| NH4+ removal = 100% | |||||
| P–PO4−3 removal = 92% | |||||
| Mixture of cattle slurry and cheese whey | C. vulgaris | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
200 μmol m−2 s−1 | Specific growth = 0.64 d−1 | 145 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Air/CO2 = 97/3 (v/v) | Specific growth = 0.49 d−1 | |||
| S. obliquus | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
25 °C | Specific growth = 0.49 d−1 | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Specific growth = 0.23 d−1 | ||||
| N. oleoabundans | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Continuous | Specific growth = 0.27 d−1 | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Specific growth = 0.26 d−1 | ||||
| Cattle manure | C. sorokiniana (UTEX 1230) | Centrifuged | 160 μmol m−2 s−1 at 25 °C | Biomass productivity (−40% compared to Bold's Basal medium) | 148 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Continuous | P–PO4−3 removal (+318% compared to Bold's Basal medium) | |||
| Agroindustrial wastes | Phaeodactylum tricornutum | Ultrafiltrate digestate | 120 μmol m−2 s−1 at 22 °C | Specific growth = 0.24 d−1 (compared to 0.1 d−1 for f/2) | 147 |
| Pavlova lutheri | Continuous | Specific growth = 0.08 d−1 (compared to 0.05 d−1 for f/2) | |||
| Municipal wastewater and organic waste | Chlorella vulgaris | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
70 μmol m−2 s−1 at 25 °C | Biomass dry mass = 2.1 g L−1 | 6 |
| Sewage sludge | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Light: dark (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
Biomass dry mass = 0.5 g L−1 | ||
| Animal manure and other organic wastes | Neochloris oleoabundans | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
50 μmol m−2 s−1 | Nitrogen content in supernatant = 4633 mg per N per L | 149 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
25 °C | Nitrogen content in supernatant = 4386 mg per N per L | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Light: dark (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
Nitrogen content in supernatant = 4229 mg per N per L | |||
| Tetraselmis sp. | Tetraselmis sp. | 80 mg L−1 | 210 μmol m−2 s−1 | 0.5 × 106 cells per mL (compared to F/2 with 1.8 × 106 cells per mL) | 150 |
| 23–25 °C | |||||
| Continuous | |||||
| Food waste | Chlorella vulgaris | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
8000 lux | Removal of NH4+ (40%) | 151 |
| Autoclaved cooled, and centrifuged | 24–26 °C | P–PO4−3 removal (28%) | |||
| Continuous | |||||
| Agro-industrial wastes | Chlorella vulgaris | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
200 μmol m−2 s−1, 25 °C | 0.161 d−1 | 144 |
| A. obliquus | Centrifuged and filtered | Continuous | 0.145 d−1 | ||
| Municipal wastewater | Synechocystis sp. | 3% | 200 μmol m−2 s−1 | Biomass productivity = 151 mg per L per d | 146 |
| 6% | 25 °C | Biomass productivity = 110 mg per L per d | |||
| N. salina | 3% | Continuous | Biomass productivity = 83 mg per L per d | ||
| 6% | Biomass productivity = 92 mg per L per d |
Compared to synthetic media, digestate was reported to have a higher impact on microalgae growth and productivity.147 However, Kobayashi et al. (2013) found that biomass productivity after digestate addition was 40% lower compared to Bold's Basal medium. This latter is rich in mineral nutrients and vitamins which may be more profitable compared to a diluted digestate.148 Nitrogen removal from digestate was also found to increase when increasing digestate dilution.148,149 The impact of using digestate as nutrient source for microalgae growth was found to be profitable for its agriculture application.117 However, further studies should be carried out to determine to what extent this practice can be beneficial for the soil and the plant.
Rajagopal et al. (2021) studied the energy and nutrient benefit of coupling anaerobic digestion of chicken manure and microalgae production using liquid digestate. It was suggested that the nutrients provided by the digestates were sufficiently beneficial to have microalgae exploitable in the extraction of high value-added molecules.142
7. Challenges and recommendations
The main challenges of microalgae anaerobic digestion are: (i) low yield of biomass; (ii) inhibition occurrence due to high N and salinity content in microalgae is marine; and (iii) need for pretreatment in case of microalgae with thick cell wall; (iv) cultivation and harvesting costs.Anaerobic digestion process requires the use of an available organic matter to feed the digester in case of continuous and semi-continuous reactors. As microalgal biomass yield is generally around 0.5 g L−1,152 monodigestion of microalgae does not appear to be a profitable way to valorize microalgal biomass because of the low methane productivity. Higher biomass recovery efficiency require the use of more expensive harvesting techniques such as centrifugation,153 which is only preferred in the case of microalgae for high value-added products.152
Moreover, salinity can highly affect methanogenesis which may reduce methane production by 50% at a Na+ concentration around 10 g L−1.154 As the optimal sodium content is around 0.23–0.35 g L−1, monodigestion of marine microalgae can lead to operational problems and even to the failure of the anaerobic digestion process.155 Co-digestion can, however, remedy this problem by using a co-substrate with a lower salt content such as sewage sludge or agricultural residues. Codigestion is also beneficial in case of protein-rich microalgae in order to avoid ammonia inhibition related to protein degradation through anaerobic digestion stages.156 In fact, above 1.5 g of ammonium per liter, a reduction of methane production can occur.157 In general, codigestion enables the dilution of potential toxic elements that may hinder anerobic digestion, in addition, it can regulate the C to N ratio leading to more optimal biogas production. Some other problems related to microalgal biomass bioaccessibility can be remedied when applying pretreatments as previously mentioned. The use of an effective pretreatment may require energy and chemicals consumption which generate additional operational costs. Energy and nutrients consumption during cultivation stage is among the most important concerns limiting the application of AD at large scale. Previously, wastewater and digestate use as culture media showed positive impacts on microalgae growth when a dilution is carried out to reduce the toxicity of the elements that may be present in these liquids. In addition, the use of a renewable energy source such as solar or wind power to cover the needs of the upstream processes can greatly reduce the energy inputs.158 So far, microalgae AD is not really economically viable.5 Codigestion of microalgae residues after lipids or proteins extraction can be more interesting, even if the methanogenic potential of these molecules will be deducted from that of the raw microalgal biomass.159 Anaerobic digestion and the use of microalgal residues as fertilizers after extraction of molecules of interest are rather considered as management strategies in the framework of the circular economy.
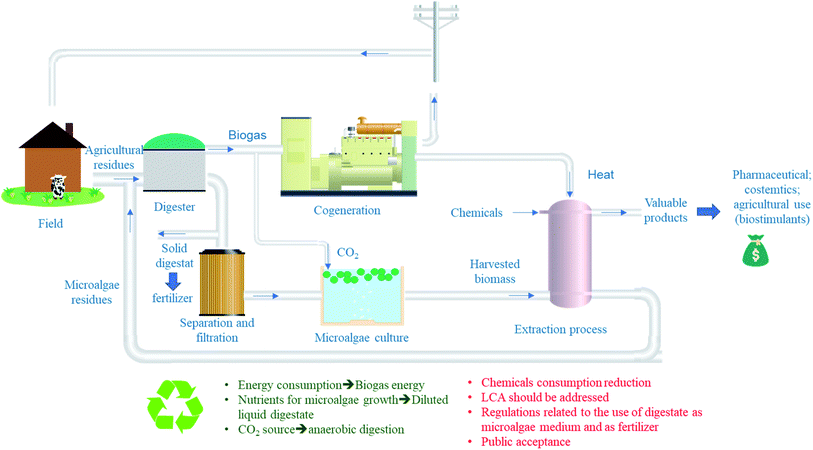
Fig. 3 presents a suggested system for a more sustainable and cost-effective valorization of microalgae. As mentioned previously, the co-digestion of agricultural or municipal solid wastes with microalgae residues after extraction of lipids, proteins or any other value-added molecules. The generated digestate can be then, used as fertilizer (solid fraction) or as nutrient source for microalgae growth (liquid fraction). The main advantage of this system is the recovery of energy, nutrients and CO2 from anaerobic digester which are required for microalgae growth. In addition, to the treatment of microalgae residues which suggest that this process can reach the “Zero waste” objective if well optimized. However, a complete study needs to be carried out regarding technical, economic and energy calculations before establishing technological solutions in large-scale biogas production facilities.17 This study should also take into account potential co-substrates that can boost methane production, their availability in a given geographical area and the costs of transporting these substrates and microalgae. A possible solution to reduce these costs would be the production of microalgae at the WWTP or within the farm in the case of using agricultural residues as cosubstrates. Moreover, sanitary aspect of using digestate for microalgae nutrients should be studied as well as its impact on microalgae properties and its eventual commercial value. As far as regulations are concerned, the use of microalgae for agronomic purposes and their residues should be reviewed and introduced. It is also necessary to study at what level the public will accept this practice, especially as some of the by-products will be returned to the soil.
8. Conclusions
The production of microalgae and their integration into a biorefinery system is among the emerging and much studied strategies. However, their industrial application remains limited, as does their energy and economic efficiency. It is therefore recommended (i) to optimize the conditions of the different stages of the process to favor metabolites accumulation, (ii) to recycle nutrients from anaerobic digestate and CO2 from biogas to reduce microalgae cultivation costs, (iii) to use microalgae residues for biogas production and soil fertilization for its organic and mineral components while meeting the environmental and economic requirements of this practice.Abbreviations
| AD | Anaerobic digestion |
| ATP | Adenosine triphosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PBR | Photobioreactor |
| TS | Total solids |
| VS | Volatile solids |
| WAS | Waste activated sludge |
| WWTP | Wastewater treatment plant |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Special thanks to the University Mohamed VI Polytechnic (UM6P) for providing financial support for this work.References
- E. Jankowska, A. K. Sahu and P. Oleskowicz-Popiel, Renewable Sustainable Energy Rev., 2017, 75, 692–709 CrossRef CAS.
- A. K. Patel, J. M. Joun, M. E. Hong and S. J. Sim, Bioresour. Technol., 2019, 282, 245–253 CrossRef CAS PubMed.
- F. Passos, A. Hom-Diaz, P. Blanquez, T. Vicent and I. Ferrer, Bioresour. Technol., 2016, 199, 347–351 CrossRef CAS.
- M. Solé-Bundó, F. Passos, M. S. Romero-Güiza, I. Ferrer and S. Astals, Renewable Sustainable Energy Rev., 2019, 112, 471–482 CrossRef.
- R. Ganesh Saratale, G. Kumar, R. Banu, A. Xia, S. Periyasamy and G. Dattatraya Saratale, Bioresour. Technol., 2018, 262, 319–332 CrossRef CAS PubMed.
- L. Zuliani, N. Frison, A. Jelic, F. Fatone, D. Bolzonella and M. Ballottari, Int. J. Mol. Sci., 2016, 17, 1692 CrossRef PubMed.
- P. Geada, V. Vasconcelos, A. Vicente and B. Fernandes, in Algal Green Chemistry, Elsevier, 2017, pp. 257–284 Search PubMed.
- S. M. Tibbetts, J. E. Milley and S. P. Lall, J. Appl. Phycol., 2015, 27, 1109–1119 CrossRef CAS.
- Microalgae Biotechnology for Food, Health and High Value Products, ed. M. A. Alam, J.-L. Xu and Z. Wang, Springer Singapore, Singapore, 2020 Search PubMed.
- C. González-Fernández, B. Sialve, N. Bernet and J.-P. Steyer, Biofuels, Bioprod. Biorefin., 2012, 6, 205–218 CrossRef.
- H. Du, J. Ren, Z. Li, H. Zhang, K. Wang, B. Lin, S. Zheng, C. Zhao, C. Meng and Z. Gao, Aquacult. Int., 2020, 28, 1319–1340 CrossRef CAS.
- J. D. Gouveia, J. Ruiz, L. A. M. van den Broek, T. Hesselink, S. Peters, D. M. M. Kleinegris, A. G. Smith, D. van der Veen, M. J. Barbosa and R. H. Wijffels, J. Biotechnol., 2017, 248, 77–86 CrossRef CAS.
- Biofuels Production – Sustainability and Advances in Microbial Bioresources, ed. A. N. Yadav, A. A. Rastegari, N. Yadav and R. Gaur, Springer International Publishing, Cham, 2020, vol. 11 Search PubMed.
- S. P. Choi, M. T. Nguyen and S. J. Sim, Bioresour. Technol., 2010, 101, 5330–5336 CrossRef CAS.
- Y. Li, D. Han, G. Hu, M. Sommerfeld and Q. Hu, Biotechnol. Bioeng., 2010, 107, 258–268 CrossRef CAS.
- A. Kuila and M. Mukhopadhyay, Biorefinery Production Technologies for Chemicals and Energy, Wiley, 2020 Search PubMed.
- H. M. Zabed, S. Akter, J. Yun, G. Zhang, Y. Zhang and X. Qi, Renewable Sustainable Energy Rev., 2020, 117, 109503 CrossRef CAS.
- F. A. Q. Sayegh, N. Radi and D. J. S. Montagnes, Aquaculture, 2007, 273, 665–678 CrossRef.
- Y. Yan, X. Li, G. Wang, X. Gui, G. Li, F. Su, X. Wang and T. Liu, Appl. Energy, 2014, 113, 1614–1631 CrossRef CAS.
- L. German-Báez, M. Valdez-Flores, J. Félix-Medina, C. Norzagaray-Valenzuela, D. Santos-Ballardo, C. Reyes-Moreno, L. Shelton and A. Valdez-Ortiz, Food Sci. Technol. Int., 2017, 23, 681–689 CrossRef PubMed.
- D.-T. Tran, C.-L. Chen and J.-S. Chang, Bioresour. Technol., 2013, 135, 213–221 CrossRef CAS.
- T. Liu, J. Wang, Q. Hu, P. Cheng, B. Ji, J. Liu, Y. Chen, W. Zhang, X. Chen, L. Chen, L. Gao, C. Ji and H. Wang, Bioresour. Technol., 2013, 127, 216–222 CrossRef CAS.
- P. Varshney, J. Beardall, S. Bhattacharya and P. P. Wangikar, Algal Res., 2018, 30, 28–37 CrossRef.
- S.-H. Ho, C.-Y. Chen and J.-S. Chang, Bioresour. Technol., 2012, 113, 244–252 CrossRef CAS PubMed.
- X. Sun, Y. Cao, H. Xu, Y. Liu, J. Sun, D. Qiao and Y. Cao, Bioresour. Technol., 2014, 155, 204–212 CrossRef CAS.
- A. K. Patel, Y. Y. Choi and S. J. Sim, Bioresour. Technol., 2020, 300, 122741 CrossRef CAS PubMed.
- A. Richmond, Z. Cheng-Wu and Y. Zarmi, Biomol. Eng., 2003, 20, 229–236 CrossRef CAS.
- D. P. Kurpan Nogueira, A. F. Silva, O. Q. F. Araújo and R. M. Chaloub, Biomass Bioenergy, 2015, 72, 280–287 CrossRef CAS.
- L. F. Wu, P. C. Chen and C. M. Lee, Int. Biodeterior. Biodegrad., 2013, 85, 506–510 CrossRef CAS.
- S.-H. Ho, X. Ye, T. Hasunuma, J.-S. Chang and A. Kondo, Biotechnol. Adv., 2014, 32, 1448–1459 CrossRef CAS.
- M. I. Khan, J. H. Shin and J. D. Kim, Microb. Cell Fact., 2018, 17, 36 CrossRef.
- K. Ying, K. D. James Gilmour and W. B. Zimmerman, J. Microb. Biochem. Technol., 2014, 6, 167–173 Search PubMed.
- M. Taraldsvik and S. Myklestad, Eur. J. Phycol., 2000, 35, 189–194 CrossRef.
- Z. Lari, N. Moradi-kheibari, H. Ahmadzadeh, P. Abrishamchi, N. R. Moheimani and M. A. Murry, J. Appl. Phycol., 2016, 28, 3235–3250 CrossRef CAS.
- G. Singh and S. K. Patidar, BioEnergy Res., 2020 DOI:10.1007/s12155-020-10195-8.
- A. Khanra, S. Vasistha, P. Kumar and M. P. Rai, 3 Biotech, 2020, 10, 331 CrossRef PubMed.
- C. Y. B. Oliveira, E. B. D'Alessandro, N. R. Antoniosi Filho, R. G. Lopes and R. B. Derner, Sci. Total Environ., 2020, 143476 Search PubMed.
- B. Behera, A. Acharya, I. A. Gargey, N. Aly and B. Paramasivan, Bioresource Technology Reports, 2019, 5, 297–316 CrossRef.
- M. J. Zarrinmehr, O. Farhadian, F. P. Heyrati, J. Keramat, E. Koutra, M. Kornaros and E. Daneshvar, Egypt. J. Aquat. Res., 2020, 46, 153–158 CrossRef.
- G. Markou, Appl. Biochem. Biotechnol., 2014, 172, 2758–2768 CrossRef CAS.
- X. Li, W. Li, J. Zhai, H. Wei and Q. Wang, Bioresour. Technol., 2019, 273, 368–376 CrossRef CAS.
- K. Goiris, W. Van Colen, I. Wilches, F. León-Tamariz, L. De Cooman and K. Muylaert, Algal Res., 2015, 7, 51–57 CrossRef.
- S.-H. Ho, P.-J. Li, C.-C. Liu and J.-S. Chang, Bioresour. Technol., 2013, 145, 142–149 CrossRef CAS.
- A. Ghosh, S. Sarkar, K. Gayen and T. K. Bhowmick, Environ. Prog. Sustainable Energy, 2020, 39, e13378 CAS.
- V. Shashirekha, M. Sivakumar and S. Seshadri, Energy, Ecology and Environment, 2016, 1, 283–295 CrossRef.
- J. Liu, Y. Qiu, L. He, K. Luo and Z. Wang, Arch. Microbiol., 2021, 203, 733–740 CrossRef CAS.
- H.-X. Weng, Y.-C. Qin, X.-W. Sun and J.-F. Chen, Environ. Geol., 2008, 55, 1431–1436 CrossRef CAS.
- N.-H. Norsker, in Handbook of Microalgae-Based Processes and Products, Elsevier, 2020, pp. 861–883 Search PubMed.
- G. Zuccaro, A. Yousuf, A. Pollio and J.-P. Steyer, in Microalgae Cultivation for Biofuels Production, Elsevier, 2020, pp. 11–29 Search PubMed.
- J. Pruvost, F. Le Borgne, A. Artu, J.-F. Cornet and J. Legrand, in Advances in Chemical Engineering, Elsevier, 2016, vol. 48, pp. 257–310 Search PubMed.
- D. S. Wágner, B. Valverde-Pérez and B. Gy. Plósz, Algal Res., 2018, 35, 488–499 CrossRef.
- M. Marsullo, A. Mian, A. V. Ensinas, G. Manente, A. Lazzaretto and F. Marechal, Frontiers in Energy Research, 2015, 3, 41 CrossRef.
- F. G. Acién, E. Molina, A. Reis, G. Torzillo, G. C. Zittelli, C. Sepúlveda and J. Masojídek, in Microalgae-Based Biofuels and Bioproducts, Elsevier, 2017, pp. 1–44 Search PubMed.
- D. Chiaramonti, M. Prussi, D. Casini, M. R. Tredici, L. Rodolfi, N. Bassi, G. C. Zittelli and P. Bondioli, Appl. Energy, 2013, 102, 101–111 CrossRef.
- L. Christenson and R. Sims, Biotechnol. Adv., 2011, 29, 686–702 CrossRef CAS.
- G. S. Murthy, in Biofuels, Elsevier, 2011, pp. 415–437 Search PubMed.
- W. Blanken, M. Cuaresma, R. H. Wijffels and M. Janssen, Algal Res., 2013, 2, 333–340 CrossRef.
- Z. Zhu, J. Jiang and Y. Fa, Molecules, 2020, 25, 5220 CrossRef CAS.
- S. Park, S. W. Van Ginkel, P. Pradeep, T. Igou, C. Yi, T. Snell and Y. Chen, Water Environ. Res., 2016, 88, 70–78 CrossRef CAS.
- J. G. Day, Y. Gong and Q. Hu, Algal Res., 2017, 27, 356–365 CrossRef.
- M. Ma, D. Yuan, Y. He, M. Park, Y. Gong and Q. Hu, Algal Res., 2017, 26, 436–444 CrossRef.
- C. L. Fisher, C. S. Ward, P. D. Lane, J. A. Kimbrel, K. L. Sale, R. K. Stuart, X. Mayali and T. W. Lane, Algal Res., 2019, 40, 101500 CrossRef.
- J. H. Mussgnug, V. Klassen, A. Schlüter and O. Kruse, J. Biotechnol., 2010, 150, 51–56 CrossRef CAS PubMed.
- N. O. Santos, S. M. Oliveira, L. C. Alves and M. C. Cammarota, Bioresour. Technol., 2014, 157, 60–67 CrossRef CAS.
- L. Appels, J. Baeyens, J. Degrève and R. Dewil, Prog. Energy Combust. Sci., 2008, 34, 755–781 CrossRef CAS.
- H. Carrere, G. Antonopoulou, R. Affes, F. Passos, A. Battimelli, G. Lyberatos and I. Ferrer, Bioresour. Technol., 2016, 199, 386–397 CrossRef CAS PubMed.
- C. Zamalloa, N. Boon and W. Verstraete, Appl. Energy, 2012, 92, 733–738 CrossRef CAS.
- M. Anjos, B. D. Fernandes, A. A. Vicente, J. A. Teixeira and G. Dragone, Bioresour. Technol., 2013, 139, 149–154 CrossRef CAS.
- N. Hossain, J. Zaini, T. M. I. Mahlia and A. K. Azad, Renewable Energy, 2019, 131, 617–624 CrossRef CAS.
- P. Bohutskyi, T. A. Keller, D. Phan, M. L. Parris, M. Li, L. Richardson and A. M. Kopachevsky, Frontiers in Energy Research, 2019, 7, 47 CrossRef.
- M. Taherzadeh and K. Karimi, Int. J. Mol. Sci., 2008, 9, 1621–1651 CrossRef CAS PubMed.
- H. G. Gerken, B. Donohoe and E. P. Knoshaug, Planta, 2013, 237, 239–253 CrossRef CAS PubMed.
- M. Audo, M. Paraschiv, C. Queffélec, I. Louvet, J. Hémez, F. Fayon, O. Lépine, J. Legrand, M. Tazerout, E. Chailleux and B. Bujoli, ACS Sustainable Chem. Eng., 2015, 3, 583–590 CrossRef CAS.
- C. Yuan, Y.-L. Zheng, W.-L. Zhang, R. He, Y. Fan, G.-R. Hu and F.-L. Li, J. Appl. Phycol., 2017, 29, 2789–2800 CrossRef CAS.
- L. Mendez, A. Mahdy, R. A. Timmers, M. Ballesteros and C. González-Fernández, Bioresour. Technol., 2013, 149, 136–141 CrossRef CAS.
- S. Schwede, A. Kowalczyk, M. Gerber and R. Span, Influence of different cell disruption techniques on mono digestion of algal, 2011, pp. 41–47 Search PubMed.
- F. Passos, M. Solé, J. García and I. Ferrer, Appl. Energy, 2013, 108, 168–175 CrossRef CAS.
- M. P. Caporgno, M. Olkiewicz, C. Torras, J. Salvadó, E. Clavero and C. Bengoa, J. Environ. Manage., 2016, 177, 240–246 CrossRef CAS.
- S. Schwede, Z.-U. Rehman, M. Gerber, C. Theiss and R. Span, Bioresour. Technol., 2013, 143, 505–511 CrossRef CAS.
- G. Ciudad, O. Rubilar, L. Azócar, C. Toro, M. Cea, Á. Torres, A. Ribera and R. Navia, J. Biosci. Bioeng., 2014, 117, 75–80 CrossRef CAS.
- H. V. Kinnunen, P. E. P. Koskinen and J. Rintala, Bioresour. Technol., 2014, 155, 314–322 CrossRef CAS PubMed.
- A. Mahdy, L. Mendez, M. Ballesteros and C. González-Fernández, Bioresour. Technol., 2015, 184, 236–244 CrossRef CAS.
- D. Elalami, H. Carrere, F. Monlau, K. Abdelouahdi, A. Oukarroum and A. Barakat, Renewable Sustainable Energy Rev., 2019, 114, 109287 CrossRef CAS.
- D. Lu and X. J. Zhang, J. Environ. Eng., 2016, 142, 04016049 CrossRef.
- M. Wang, A. K. Sahu, B. Rusten and C. Park, Bioresour. Technol., 2013, 142, 585–590 CrossRef CAS PubMed.
- M. Solé-Bundó, M. Garfí and I. Ferrer, Bioresour. Technol., 2020, 298, 122563 CrossRef PubMed.
- M. Solé-Bundó, M. Garfí, V. Matamoros and I. Ferrer, Sci. Total Environ., 2019, 660, 974–981 CrossRef.
- R. C. Anyanwu, C. Rodriguez, A. Durrant and A. G. Olabi, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2018, p. B9780128035818093000 Search PubMed.
- Y. Zhang, G. S. Caldwell, A. M. Zealand and P. J. Sallis, Biochem. Eng. J., 2019, 143, 91–100 CrossRef CAS.
- M. Solé-Bundó, C. Eskicioglu, M. Garfí, H. Carrère and I. Ferrer, Bioresour. Technol., 2017, 237, 89–98 CrossRef PubMed.
- W. Zhong, Z. Zhang, Y. Luo, W. Qiao, M. Xiao and M. Zhang, Bioresour. Technol., 2012, 114, 281–286 CrossRef CAS PubMed.
- F. Passos, P. H. M. Cordeiro, B. E. L. Baeta, S. F. de Aquino and S. I. Perez-Elvira, Bioresour. Technol., 2018, 253, 49–54 CrossRef CAS PubMed.
- C. Herrmann, N. Kalita, D. Wall, A. Xia and J. D. Murphy, Bioresour. Technol., 2016, 214, 328–337 CrossRef CAS.
- M. J. Fernández-Rodríguez, D. de la Lama-Calvente, A. Jiménez-Rodríguez, R. Borja and B. Rincón, J. Appl. Phycol., 2021, 33, 419–429 CrossRef.
- S. Astals, R. S. Musenze, X. Bai, S. Tannock, S. Tait, S. Pratt and P. D. Jensen, Bioresour. Technol., 2015, 181, 97–104 CrossRef CAS.
- A. Ajeej, J. V. Thanikal, C. M. Narayanan and R. Senthil Kumar, Renewable Sustainable Energy Rev., 2015, 50, 270–276 CrossRef CAS.
- E. A. Ehimen, S. Connaughton, Z. Sun and G. C. Carrington, GCB Bioenergy, 2009, 1, 371–381 CrossRef CAS.
- R. Avila, E. Carrero, E. Crivillés, M. Mercader, T. Vicent and P. Blánquez, Algal Res., 2020, 50, 101965 CrossRef.
- S. Kavitha, P. Subbulakshmi, J. Rajesh Banu, M. Gobi and I. Tae Yeom, Bioresour. Technol., 2017, 233, 34–43 CrossRef CAS.
- S. Abinandan, S. R. Subashchandrabose, K. Venkateswarlu and M. Megharaj, Crit. Rev. Biotechnol., 2019, 39, 981–998 CrossRef.
- N. Renuka, A. Guldhe, R. Prasanna, P. Singh and F. Bux, Biotechnol. Adv., 2018, 36, 1255–1273 CrossRef CAS.
- E. Yilmaz and M. Sönmez, Soil Tillage Res., 2017, 168, 118–124 CrossRef.
- P. Das, M. A. Quadir, M. I. Thaher, G. S. H. S. Alghasal and H. M. S. J. Aljabri, Int. J. Environ. Sci. Technol., 2019, 16, 3355–3364 CrossRef CAS.
- V. Barone, I. Puglisi, F. Fragalà, P. Stevanato and A. Baglieri, Arch. Agron. Soil Sci., 2019, 65, 712–726 CrossRef CAS.
- I. Perera, S. R. Subashchandrabose, K. Venkateswarlu, R. Naidu and M. Megharaj, Appl. Microbiol. Biotechnol., 2018, 102, 7351–7363 CrossRef CAS.
- N. Renuka, R. Prasanna, A. Sood, A. S. Ahluwalia, R. Bansal, S. Babu, R. Singh, Y. S. Shivay and L. Nain, Environ. Sci. Pollut. Res., 2016, 23, 6608–6620 CrossRef CAS.
- R. Dineshkumar, R. Kumaravel, J. Gopalsamy, M. N. A. Sikder and P. Sampathkumar, Waste Biomass Valorization, 2018, 9, 793–800 CrossRef CAS.
- R. Dineshkumar, J. Subramanian, A. Arumugam, A. Ahamed Rasheeq and P. Sampathkumar, Waste Biomass Valorization, 2020, 11, 77–87 CrossRef CAS.
- S. Geisen, E. A. D. Mitchell, S. Adl, M. Bonkowski, M. Dunthorn, F. Ekelund, L. D. Fernández, A. Jousset, V. Krashevska, D. Singer, F. W. Spiegel, J. Walochnik and E. Lara, FEMS Microbiol. Rev., 2018, 42, 293–323 CrossRef CAS PubMed.
- L. A. Lewis and F. R. Trainor, Phycologia, 2012, 51, 662–665 CrossRef.
- A. L. Gonçalves, Appl. Sci., 2021, 11, 871 CrossRef.
- O. Uysal, F. O. Uysal and K. Ekinci, European Journal of Sustainable Development, 2015, 4(2), 77 CrossRef.
- M. A. Guzmán-Murillo, F. Ascencio and J. A. Larrinaga-Mayoral, Protoplasma, 2013, 250, 33–42 CrossRef.
- R. Dineshkumar, J. Subramanian, J. Gopalsamy, P. Jayasingam, A. Arumugam, S. Kannadasan and P. Sampathkumar, Waste Biomass Valorization, 2019, 10, 1101–1110 CrossRef CAS.
- S. C. Wuang, M. C. Khin, P. Q. D. Chua and Y. D. Luo, Algal Res., 2016, 15, 59–64 CrossRef.
- J. Coppens, O. Grunert, S. Van Den Hende, I. Vanhoutte, N. Boon, G. Haesaert and L. De Gelder, J. Appl. Phycol., 2016, 28, 2367–2377 CrossRef CAS.
- R. Jimenez, G. Markou, S. Tayibi, A. Barakat, C. Chapsal and F. Monlau, Appl. Sci., 2020, 10, 3890 CrossRef CAS.
- P. Deepika and D. MubarakAli, Biocatal. Agric. Biotechnol., 2020, 28, 101701 CrossRef.
- F. A. Faheed and Z. Abd-El Fattah, J. Agri. Soc. Sci., 2008, 4, 165–169 Search PubMed.
- B. Górka, K. Korzeniowska, J. Lipok and P. P. Wieczorek, in Algae Biomass: Characteristics and Applications, ed. K. Chojnacka, P. P. Wieczorek, G. Schroeder and I. Michalak, Springer International Publishing, Cham, 2018, pp. 103–114 Search PubMed.
- K. V. Supraja, B. Behera and B. Paramasivan, Ind. Crops Prod., 2020, 151, 112453 CrossRef CAS.
- K. Ranjan, H. Priya, B. Ramakrishnan, R. Prasanna, S. Venkatachalam, S. Thapa, R. Tiwari, L. Nain, R. Singh and Y. S. Shivay, Applied Soil Ecology, 2016, 108, 195–203 CrossRef.
- J. Garcia-Gonzalez and M. Sommerfeld, J. Appl. Phycol., 2016, 28, 1051–1061 CrossRef PubMed.
- D. Ronga, E. Biazzi, K. Parati, D. Carminati, E. Carminati and A. Tava, Agronomy, 2019, 9, 192 CrossRef CAS.
- G. Colla and Y. Rouphael, Agronomy, 2020, 10, 1240 CrossRef.
- J. A. Alburquerque, C. de la Fuente, A. Ferrer-Costa, L. Carrasco, J. Cegarra, M. Abad and M. P. Bernal, Biomass Bioenergy, 2012, 40, 181–189 CrossRef CAS.
- J. A. Alburquerque, C. de la Fuente, M. Campoy, L. Carrasco, I. Nájera, C. Baixauli, F. Caravaca, A. Roldán, J. Cegarra and M. P. Bernal, Eur. J. Agron., 2012, 43, 119–128 CrossRef.
- F. Monlau, C. Sambusiti, E. Ficara, A. Aboulkas, A. Barakat and H. Carrère, Energy Environ. Sci., 2015, 8, 2600–2621 RSC.
- R. Nkoa, Agron. Sustainable Dev., 2014, 34, 473–492 CrossRef.
- M. Solé-Bundó, M. Cucina, M. Folch, J. Tàpias, G. Gigliotti, M. Garfí and I. Ferrer, Sci. Total Environ., 2017, 586, 1–9 CrossRef PubMed.
- L. M. González-González, E. Eltanahy and P. M. Schenk, Algal Res., 2019, 41, 101534 CrossRef.
- K. Güngör and K. G. Karthikeyan, Bioresour. Technol., 2008, 99, 425–436 CrossRef PubMed.
- F. Passos and I. Ferrer, Water Res., 2015, 68, 364–373 CrossRef CAS PubMed.
- F. Passos and I. Ferrer, Environ. Sci. Technol., 2014, 48, 7171–7178 CrossRef CAS.
- K. G. Pavithra, P. S. Kumar, V. Jaikumar, K. H. Vardhan and P. SundarRajan, Environ. Chem. Lett., 2020, 18, 1905–1923 CrossRef CAS.
- G. Sibi, Green Energy Environ., 2016, 1, 172–177 CrossRef.
- E. Gunasundari and P. Senthil Kumar, IET Nanobiotechnol., 2017, 11, 317–328 CrossRef PubMed.
- S. Maryjoseph and B. Ketheesan, Case Studies in Chemical and Environmental Engineering, 2020, 2, 100046 CrossRef.
- V. García, J. Päkkilä, H. Ojamo, E. Muurinen and R. L. Keiski, Renewable Sustainable Energy Rev., 2011, 15, 964–980 CrossRef.
- L. Wang, Y. Li, P. Chen, M. Min, Y. Chen, J. Zhu and R. R. Ruan, Bioresour. Technol., 2010, 101, 2623–2628 CrossRef CAS PubMed.
- D. Chuka-ogwude, J. Ogbonna and N. R. Moheimani, Algal Res., 2020, 47, 101841 CrossRef.
- R. Rajagopal, S. E. Mousavi, B. Goyette and S. Adhikary, Bioengineering, 2021, 8, 57 CrossRef.
- A. Pires, G. Martinho and N.-B. Chang, J. Environ. Manage., 2011, 92, 1033–1050 CrossRef.
- E. Koutra, C. N. Economou, P. Tsafrakidou and M. Kornaros, Trends Biotechnol., 2018, 36, 819–833 CrossRef CAS.
- M. Franchino, E. Comino, F. Bona and V. A. Riggio, Chemosphere, 2013, 92, 738–744 CrossRef CAS PubMed.
- T. Cai, X. Ge, S. Y. Park and Y. Li, Bioresour. Technol., 2013, 144, 255–260 CrossRef CAS.
- D. Veronesi, A. Ida, G. D. Imporzano and F. Adani, Chemical Engineering Transactions, 2015, 43, 1201–1206 Search PubMed.
- N. Kobayashi, E. A. Noel, A. Barnes, A. Watson, J. N. Rosenberg, G. Erickson and G. A. Oyler, Bioresour. Technol., 2013, 150, 377–386 CrossRef CAS PubMed.
- H. A. Abu Hajar, R. Guy Riefler and B. J. Stuart, Environmental Engineering Research, 2016, 21, 265–275 CrossRef.
- M. Erkelens, A. J. Ward, A. S. Ball and D. M. Lewis, Bioresour. Technol., 2014, 167, 81–86 CrossRef CAS.
- P. Praveen, Y. Guo, H. Kang, C. Lefebvre and K.-C. Loh, Chem. Eng. J., 2018, 354, 905–912 CrossRef CAS.
- K. Muylaert, L. Bastiaens, D. Vandamme and L. Gouveia, in Microalgae-Based Biofuels and Bioproducts, Elsevier, 2017, pp. 113–132 Search PubMed.
- G. Singh and S. K. Patidar, J. Environ. Manage., 2018, 217, 499–508 CrossRef.
- J. Milledge, B. Nielsen, S. Maneein and P. Harvey, Energies, 2019, 12, 1166 CrossRef CAS.
- F. TorresRincón, B. Rincn, J. Bartacek, R. Borja and D. Jeiso, in Biodegradation – Engineering and Technology, ed. R. Chamy, InTech, 2013 Search PubMed.
- V. Klassen, O. Blifernez-Klassen, D. Wibberg, A. Winkler, J. Kalinowski, C. Posten and O. Kruse, Biotechnol. Biofuels, 2017, 10, 186 CrossRef.
- R. Rajagopal, D. I. Massé and G. Singh, Bioresour. Technol., 2013, 143, 632–641 CrossRef CAS.
- L. Lombardi, B. Mendecka and S. Fabrizi, Energies, 2020, 13, 4292 CrossRef CAS.
- J. C. Quinn, A. Hanif, S. Sharvelle and T. H. Bradley, Bioresour. Technol., 2014, 171, 37–43 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |