Open Access Article
Open Access ArticleThe effects of green waste compost on soil N, P, K, and organic matter fractions in forestry soils: elemental analysis evaluation
Xiaojie Fenga,
Xiangyang Sun *a,
Wenjie Zhoua,
Wei Zhangb,
Feiwei Chea and
Suyan Lia
*a,
Wenjie Zhoua,
Wei Zhangb,
Feiwei Chea and
Suyan Lia
aCollege of Forestry, Beijing Forestry University, Beijing 100083, PR China. E-mail: sunxy@bjfu.edu.cn
bBeijing Tongzhou District Gardening and Greening Bureau, Beijing 100013, PR China
First published on 28th September 2021
Abstract
We study the effects of green waste compost on soil fertility to provide a theoretical basis for accurately improving forestry soil quality. This study aims to investigate the effects of green waste compost on soil N, P, K, and soil organic matter (SOM) fractions using elemental and FTIR analyses. Therefore, five fertilization treatments were set up for research, including mineral fertilization (M-fert), green waste compost fertilization (G-fert), standard rate of M-fert plus G-fert (GM-fert), half the standard rate of M-fert plus G-fert (1/2 GM-fert), and a control with no fertilizer addition (N-fert). The results showed that GM-fert treatment significantly increased the content of soil NH4–N, available phosphorus (AP), available potassium (AK), water soluble organic carbon (WSOC), humus (HE), and humic acid (HA), which were 8.53 ± 0.67, 76.1 ± 5.96, 168 ± 3.42, 0.152 ± 0.01, 5.64 ± 0.15, and 4.69 ± 0.21 mg kg−1, respectively. The content of HA (36.7%, F = 7.55, P = 0.01) was positively correlated with the soil N, P, K, and the HA absorption peak. The relative intensities of the alcohol –OH, aliphatic –CH and carbohydrate C–O peaks showed the largest changes, which were 18.6 ± 0.56%, 13.1 ± 0.33%, and 16.3 ± 0.49%. –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (49.8%, F = 12.9, P < 0.01) was also significantly positively correlated with soil N, P, K. In conclusion, green waste compost significantly increased soil N, P, K, and HA in forestry soils, and the –CH/C
C (49.8%, F = 12.9, P < 0.01) was also significantly positively correlated with soil N, P, K. In conclusion, green waste compost significantly increased soil N, P, K, and HA in forestry soils, and the –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of HA was the main factor related to soil nutrients.
C of HA was the main factor related to soil nutrients.
Introduction
Forestry soil is a part of the ground surface, and supplies the living materials of forest plants. Forestry soil is composed of minerals and organic matter, and contains certain amounts of air, moisture, and organisms; it mainly includes natural forest soil, man-made forest soil, nursery soil and urban green space soil. Forestry soil provides the nutrient supply for normal forest growth, and the soil nutrient contents are important indexes to evaluate the forest health status.1,2 Therefore, increasing the N, P, K content of soil provides a better growth environment for forests.Fertilization is an important technical measure to improve soil fertility and forest productivity.3 Soil organic matter (SOM) is the key factor for improving soil fertility; it not only stores mineral elements in the form of organic matter, but also retains part of the available nutrients via ion adsorption.4 Based on differences in acidic and alkali solutions, the SOM fractions were divided into water soluble organic carbon (WSOC), humus (HE), fulvic acid (FA), humic acid (HA) and humin (HM).5,6 M-fert + maize straw or biochar increased HA, and HA had higher carbon storage than HM and FA in agricultural land soils,7,8 which played a major role in stabilizing the organic carbon pool of the soil. The SOM chemical structures were an important component to maintain soil nutrients, and SOM decomposition released nutrients due to organic molecular recombination via microbial activity.9 The development of molecular-scale techniques provided new methods for studying SOM structures.10,11 Many important SOM structures, such as alkyl-C, aromatic-C, and carboxyl-C, were discovered by infrared spectroscopy.12 According to the wave bands and specific structures, four functional groups could be described: alcohol –OH, aliphatic –CH, amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and carbohydrate C–O.13 The characteristics of the functional groups at the molecular scale could reflect the carbon storage and decomposition in forestry soil under M-fert and G-fert treatments. Fertilization had a great influence on the SOM fractions, which were directly related to the release of soil N, P, K. Using infrared spectroscopy technology to explore the structure of the SOM fractions would give a deeper understanding the characteristics of soil organic carbon fixation and the decomposition mechanism in forestry soils.
C and carbohydrate C–O.13 The characteristics of the functional groups at the molecular scale could reflect the carbon storage and decomposition in forestry soil under M-fert and G-fert treatments. Fertilization had a great influence on the SOM fractions, which were directly related to the release of soil N, P, K. Using infrared spectroscopy technology to explore the structure of the SOM fractions would give a deeper understanding the characteristics of soil organic carbon fixation and the decomposition mechanism in forestry soils.
“Green waste compost fertilization” (G-fert) refers to fallen leaves and branch cuttings transformed into potentially safe, stable organic fertilizer using composting technology, which has fast decomposition in the soil.14 The fast turnover of G-fert increased soil N, P, K for tree growth.15 A few studies have investigated the characteristics of the SOM fractions under G-fert treatment, which also formed a circulatory system between green waste resources and forestry soils. As a consequence, the objectives of this study were to determine (1) the effects of green waste compost on soil N, P, K and SOM fractions, and (2) the relationship between the SOM fractions and soil N, P, K. This study provides the mechanisms of the underlying changes in the SOM fractions under different fertilization treatments, which supports soil carbon storage in forestry soils.
Material and methods
Chemical properties of fertilizers
The green waste was composed of tree branches and fallen leaves, the main sources of which were Paulownia, Fraxinus, Maple, which are all common tree species in the Beijing area. The green waste compost used in the study was aerobically composted for two years under natural aerobic conditions with no accelerating agents. The pH was 8.27, with a total organic carbon of 322 g kg−1, total N of 15.5 g kg−1, total P of 2.25 g kg−1, and total K of 7.14 g kg−1. Before incorporation into the soil, the air-dried green waste compost was sieved through a 2 mm sieve. The mineral fertilization (M-fert) treatment consisted of urea (N 46.4%), superphosphate (P2O5 12.0%) and potassium chloride (K2O 60.0%). The M-fert was dissolved into liquid and then mixed evenly with the soil in the pot.Experimental design
Soil was taken from a nursery in which poplar (Populus L.) had been growing for four years. Then soil was air-dried, and coarse rocks and plant residue were removed before sieving the soil through 2 mm nylon mesh. The soil was developed from moisture and river deposits, with a pH of 8.17. The total N was 0.960 g kg−1, the NH4–N was 6.38 mg kg−1, the NO3–N was 0.490 mg kg−1, the AP was 20.6 mg kg−1, and the AK was 97.2 mg kg−1.The pot experiment was carried out from August to November in 2019 at the nursery garden (Sanqinyuan) of Beijing Forestry University. The experiment had five treatments: (1) M-fert; (2) G-fert; (3) standard rate of M-fert plus G-fert (GM-fert); (4) half the standard rate of M-fert plus G-fert (1/2 GM-fert); (5) control with no fertilizer addition (N-fert). The fertilizer types and fertilizer amounts under different fertilization treatments are listed in Table 1. The NPK fertilizers were applied in a single basal application before planting the poplar. Each fertilization treatment had five replicates, and 25 pots were used in total (5 × 5 = 25). The progression of the experiment involved three main steps: (1) 20 kg of air-dried soil was placed in each pot (height 45 cm; volume 20 L). (2) G-fert was mixed uniformity with the air-dried soil under the G-fert, 1/2 GM-fert and GM-fert treatments. (3) After setup, one poplar (Populus L.), which was about 50 cm though cutting, was transplanted into each pot, and then all pots were watered with the M-fert solution. During the pot experiment, according to the dryness or humidity of the soil in the pot, the soil field water holding capacity was kept at about 60% to ensure the normal growth of the poplar trees.
| Treatment | Fertilizer type | |||
|---|---|---|---|---|
| Urea (g) | Superphosphate (g) | KCl (g) | Green waste compost fertilizer (kg) | |
| a M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. | ||||
| M-fert | 22.0 | 12.5 | 7.8 | 0 |
| G-fert | 0 | 0 | 0 | 0.66 |
| GM-fert | 22.0 | 12.5 | 7.8 | 0.66 |
| 1/2 GM-fert | 11.0 | 6.2 | 3.6 | 0.33 |
| N-fert | 0 | 0 | 0 | 0 |
Soil sampling and soil analyses
Soil samples were taken from each pot using an auger 90 days after cutting the poplar (Populus L.) and then air-dried and sieved through a 2 mm and 0.25 mm sieve to measure the soil chemical properties and SOM fractions.The soil chemical properties included the pH value, SOM, total nitrogen (TN), ammonium nitrogen (NH4–N), nitrate nitrogen (NO3–N), available phosphorus (AP) and available potassium (AK).16 Soil pH: 10 g of air-dried soil was weighed out, and 25 mL of carbon dioxide-free water was added. The mixture was stirred vigorously for 1–2 minutes, and then the soil pH was determined after 30 minutes using a pH meter (distinguishability 0.01 pH, error 0.01 pH). SOM: 0.5 g of air-dried soil was weighed out, and 5 mL H2SO4 and 5 mL 0.8 N K2Cr2O7 were added. The mixture was then heated in an oil bath for 5 minutes (180 °C) and titrated using FeSO4. Soil TN: 0.2 g of air-dried soil was weighed out. H2SO4 and mixed catalyst were added, and then the mixture was digested for 2 hours (180–380 °C) and measured using an Automatic Kjeldahl nitrogen analyzer (K1100, China, titration accuracy 1.0 μL per step, repeatability error ≤0.5%). NH4–N: the soil was extracted using 1 mol L−1 KCL and measured using indigo colorimetry with an ultraviolet spectrophotometer (JC-UT2000, China, wavelength precision ±1 nm, photometric accuracy ±0.5% T) at 625 nm. NO3–N: the soil was extracted using 1 mol L−1 KCL and measured using an ultraviolet spectrophotometer (JC-UT2000, China, wavelength precision ±1 nm, photometric accuracy ±0.5% T) at 220 and 275 nm. AP: the soil was extracted using 0.5 mol L−1 NaHCO3 and measured using the molybdenum–antimony colorimetric method using an ultraviolet spectrophotometer (JC-UT2000, China, wavelength precision ±1 nm, photometric accuracy ±0.5% T) at 625 nm. AK: the soil was extracted using 1 mol L−1 CH3COONH4 and determined using a flame spectrophotometer (EP6410, China, stability ≥97%, accuracy ≥97%). For the procedural blank for the soil chemical properties, the operation steps were the same except for the soil samples. The spike recoveries of the soil pH value, SOM, TN, NH4–N, NO3–N, AP and AK were 99.0%, 98.6%, 98.6%, 98.6%, 101.5%, 97.5% and 98.8%.
The SOM was separated into the fractions WSOC, HE, FA, HA and HM, and the extraction steps were as follows: first, 50 mL distilled water was mixed with the soil sample (10 g, <0.25 mm), stirred and shaken in a shock machine. The mixture was then centrifuged, and the supernatant solution was used for the WSOC. Secondly, to the residue was added 40 mL of 0.1 mol L−1 NaOH and 0.1 mol L−1 Na4P2O7 (pH 13) followed by oscillation in a shock machine and centrifugation with a centrifuge. This process was repeated three times, followed by filtration and drying to a constant weight at 55 °C; the residue was the HE. Lastly, 20 mL of 0.5 M H2SO4 (pH 1.0) was used to separate the residue into HA and FA by standing overnight. The soil TOC was measured using wet oxidation with K2Cr2O7 and the semi-micro Kjeldahl methods.16 The concentrations of WSOC, HA, and FA were determined using a TOC analyzer (Shimadzu TOC-V, Japan, precision ≤4%, accuracy ≥98%).6 For the procedural blank for the soil SOM fractions, the operation steps were the same except for the soil samples. The spike recoveries of the soil TOC, WSOC, HA and FA were 98.5%, 99.6%, 99.5% and 99.6%.
HA elemental and FTIR analyses
For HA purification, we first added deionized water to dissolve the ions, then placed the sample in a dialysis bag for dialysis until no Cl− could be detected in the solution, and finally freeze-dried the solution to obtain the purified HA. The purified HA was subjected to elemental and infrared spectroscopy analysis. The purified HA was directly measured in CHNS mode using an elemental analyzer (Elementar VE cube, Germany, precision ≤0.1%, accuracy ≥99.0%) for elemental analysis, and the content of the element O was obtained by the subtraction method. The HA structure was measured using a Fourier Transform Infrared (FT-IR) Spectrometer (Spectrum 100, USA, spectrum precision 0.008 cm−1, spectrum accuracy 0.02 cm−1).17 The specific steps are as follows: firstly, the soil sample was baked in an oven for 8 hours at a temperature of 60 °C. The purpose of this was to remove the moisture in the soil sample and reduce the interference of hydroxyl functional groups in the spectrum. Secondly, the soil sample was mixed with KBr in the desired ratio (soil sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KBr 1
KBr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, w/w) in an agate mortar, and then pressed into tablets until the color of the tablet was light yellow and relatively uniform. Lastly, the tablets were scanned using an infrared spectrometer. The measurement range of the spectrum is 4000–500 cm−1, the resolution is 0.5 cm−1, the spectral precision is 0.008 cm−1 and the spectral accuracy is 0.02 cm−1. For the procedural blanks for HA elemental and infrared spectroscopy analysis, the operation steps were the same except for the HA samples. The spike recoveries of the HA elemental and infrared spectroscopy analyses were 99.0% and 99.6%.
200, w/w) in an agate mortar, and then pressed into tablets until the color of the tablet was light yellow and relatively uniform. Lastly, the tablets were scanned using an infrared spectrometer. The measurement range of the spectrum is 4000–500 cm−1, the resolution is 0.5 cm−1, the spectral precision is 0.008 cm−1 and the spectral accuracy is 0.02 cm−1. For the procedural blanks for HA elemental and infrared spectroscopy analysis, the operation steps were the same except for the HA samples. The spike recoveries of the HA elemental and infrared spectroscopy analyses were 99.0% and 99.6%.
Statistics
Based on the change in the TOC content, the remained carbon (RC), organic carbon decomposition rate (DR) and humification coefficient (HC) of the green waste compost were calculated.| RC = TOCG-fert,GM-fert,1/2GM-fert − (TOCsoilM-fert − TOCsoilN-fert) |
| DR = [1 − (TOCsoilM-fert − TOCsoilN-fert)/TOCG-fert,GM-fert,1/2GM-fert] × 100% |
| HC = (TOCsoilM-fert − TOCsoilN-fert)/TOCG-fert,GM-fert,1/2GM-fert |
Here, TOCG-fert,GM-fert,1/2GM-fert are the TOC values for the G-fert, GM-fert and 1/2 GM-fert treatments, and TOCsoilM-fert and TOCsoilN-fert are the soil TOC for the M-fert and N-fert treatments.
The HE and HM were calculated as shown below:
| HEsoil = HAsoil + FAsoil |
| HMsoil = TOCsoil − (WSOCsoil + HEsoil) |
Here, TOCsoil, WSOCsoil, HAsoil, and FAsoil are the TOC values of soil, WSOC values of soil, HA values of soil and FA values of soil under the M-fert, G-fert, GM-fert, 1/2 GM-fert and N-fert treatments, respectively.
Statistical analyses were conducted using DPS11.0 and Excel 2013. One-way ANOVA and T-tests were applied for the analysis of the variance of the soil properties to determine significant relationships among different fertilization treatments. Transformation of the infrared spectral images from absorbance to transmittance curves was conducted in OMNIC 8.2. Regression analyses were used to evaluate the influence between the soil N, P, K, SOM fractions, and the HA main absorption peak relative intensity under different fertilization treatments. Redundancy analysis (RDA) was used to explain the SOM fractions, and the contribution of the relative intensity of the main HA absorption peak to the variation of the soil N, P, K, and RDA was performed using Canoco 5 and Cano Draw for Windows. For all analyses, P < 0.05 was considered statistically significant. All charts were created in Origin 8.1 and Excel 2013.
Results
The decomposition rate (DR) and humification coefficient (HC) of green waste compost
Table 2 shows the decomposition and residue of green waste compost in forestry soils. The RC was highest under G-fert treatment, and lowest under 1/2 GM-fert. The DR values of the green waste compost were 68.0 ± 1.55%, 65.6 ± 1.32% and 64.2 ± 1.44% under G-fert, GM-fert and 1/2 GM-fert treatment, respectively. The HC values of G-fert were highest under GM-fert and 1/2 GM-fert treatment, at 0.344 ± 0.01 and 0.358 ± 0.02.| Treatment | AC (g) | RC (g) | DR (%) | HC |
|---|---|---|---|---|
| a Table data are mean ± standard deviation. M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. AC = addition of total TOC; RC = residue of total TOC; DR = GF decomposition rate; HC = humification coefficient. Different lowercase letters indicate a significant difference at the P < 0.05 level under different fertilization treatments. | ||||
| M-fert | — | — | — | — |
| G-fert | 209 ± 9.87 a | 142 ± 2.54 a | 68.0 ± 1.55 a | 0.320 ± 0.01 b |
| GM-fert | 209 ± 8.45 a | 137 ± 2.78 b | 65.6 ± 1.32 b | 0.344 ± 0.01 a |
| 1/2 GM-fert | 106 ± 5.22 b | 68.3 ± 1.99 c | 64.2 ± 1.44 b | 0.358 ± 0.02 a |
Soil nutrients under different fertilization treatments
Compared with N-fert treatment, the addition of G-fert increased the OM, TN, and C/N ratio in the soil, which were not influenced under M-fert treatment, and the concentrations of NH4–N, NO3–N, AP, AK increased under the different fertilization treatments (Table 3). The soil pH values under M-fert and GM-fert treatments were significantly decreased by 0.13 and 0.08 units relative to that under N-fert treatment. The soil OM and TN under G-fert, GM-fert, and 1/2 GM-fert treatments were significantly increased by 31.3–59.3% and 17.2–50.5%. The concentrations of NH4–N, AP and AK were highest under the GM-fert treatment, significantly increasing 3.58, 3.50, and 1.77 times compared to those under N-fert treatment, but the concentration of NO3–N was highest under M-fert treatment.| Treatment | pH | OM g kg−1 | TN g kg−1 | NH4–N mg kg−1 | NO3–N mg kg−1 | AP mg kg−1 | AK mg kg−1 |
|---|---|---|---|---|---|---|---|
| a Table data are mean ± standard deviation. M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. Different lowercase letters indicate that soil chemical properties significantly differ at the P < 0.05 level under different fertilization treatments. | |||||||
| M-fert | 8.01 ± 0.01 c | 10.7 ± 0.06 c | 1.04 ± 0.06 bc | 6.66 ± 0.56 b | 5.82 ± 0.15 a | 39.8 ± 2.62 d | 149 ± 2.80 b |
| G-fert | 8.12 ± 0.01 a | 16.3 ± 0.67 a | 1.47 ± 0.04 a | 4.98 ± 0.30 c | 2.48 ± 0.20 c | 51.7 ± 4.83 b | 124 ± 2.89 d |
| GM-fert | 8.06 ± 0.01 b | 16.7 ± 0.36 a | 1.49 ± 0.05 a | 8.53 ± 0.67 a | 5.72 ± 0.28 a | 76.1 ± 5.96 a | 168 ± 3.42 a |
| 1/2 GM-fert | 8.12 ± 0.01 a | 13.8 ± 0.31 b | 1.16 ± 0.09 b | 4.89 ± 0.12 c | 5.06 ± 0.36 b | 46.3 ± 1.86 c | 139 ± 1.39 c |
| N-fert | 8.14 ± 0.01 a | 10.5 ± 0.11 c | 0.99 ± 0.07 c | 2.38 ± 0.18 d | 0.76 ± 0.03 d | 21.8 ± 0.98 e | 95.0 ± 3.85 e |
SOM fractions under different fertilization treatments
The addition of green waste compost significantly increased the TOC, HE, HA, FA and HM in the soil compared to those under N-fert treatment, while M-fert had no effect on the SOM fractions except for the WSOC (Table 4). The concentrations of TOC under G-fert and GM-fert treatments were significantly increased 1.55 times and 1.59 times relative to that under N-fert treatment. The concentrations of WSOC, HE and HA were highest under GM-fert treatment at 0.152 ± 0.01, 5.64 ± 0.15 and 4.69 ± 0.21 mg kg−1, respectively. However, there was no significant difference in the concentrations of WSOC, HE, and HA under M-fert and N-fert treatments, and FA showed no significant difference under M-fert, G-fert, GM-fert and 1/2 GM-fert treatments. The concentration of HM was highest under G-fert and GM-fert treatments at 4.28 ± 0.50 and 3.91 ± 0.26 mg kg−1, respectively.| Treatment | TOC g kg−1 | WSOC mg kg−1 | HE mg kg−1 | HA mg kg−1 | FA mg kg−1 | HM mg kg−1 |
|---|---|---|---|---|---|---|
| a Table data are mean ± standard deviation. M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. Different lowercase letters indicate SOM fractions that significantly differ at the P < 0.05 level under different fertilization treatments. | ||||||
| M-fert | 6.19 ± 0.06 c | 0.0720 ± 0.01 d | 3.65 ± 0.20 d | 2.80 ± 0.15 d | 0.842 ± 0.06 ab | 2.47 ± 0.16 c |
| G-fert | 9.44 ± 0.67 a | 0.101 ± 0.01 c | 5.06 ± 0.20 b | 4.15 ± 0.27 b | 0.911 ± 0.10 a | 4.28 ± 0.50 a |
| GM-fert | 9.69 ± 0.36 a | 0.152 ± 0.01 a | 5.64 ± 0.15 a | 4.69 ± 0.21 a | 0.951 ± 0.12 a | 3.91 ± 0.26 ab |
| 1/2 GM-fert | 7.99 ± 0.31 b | 0.103 ± 0.01 c | 4.42 ± 0.12 c | 3.48 ± 0.15 c | 0.952 ± 0.06 a | 3.47 ± 0.24 b |
| N-fert | 6.09 ± 0.11 c | 0.111 ± 0.01 b | 3.64 ± 0.20 d | 2.87 ± 0.23 d | 0.782 ± 0.03 b | 2.33 ± 0.10 c |
Relationship between soil chemical properties and SOM fractions
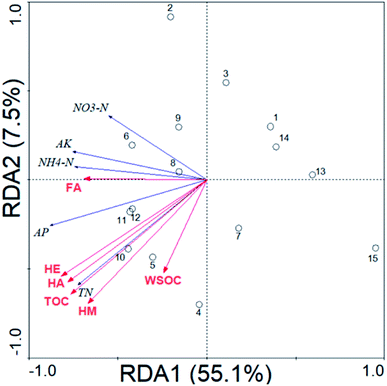
Table 5 shows the Pearson correlation coefficients between the soil chemical properties and SOM fractions. The soil TN was significantly (P < 0.01) and positively correlated with the contents of TOC (r = 0.935), HE (r = 0.916), HA (r = 0.912) and HM (r = 0.892). The soil NH4–N and AK were significantly (P < 0.05) and positively correlated with the content of HA (r = 0.559) and FA (r = 0.521), respectively. The soil AP was significantly (P < 0.05) and positively correlated (r = 0.530–0.904) with the SOM values of each fraction. Though RDA analysis, the SOM fractions could explain the changes (62.6%) of the soil chemical properties (Fig. 1). The soil chemical properties were significantly positively correlated with the content of HE (39.5%, F = 8.49, P = 0.01) and HA (36.7%, F = 7.55, P = 0.01).| pH | OM | TN | NH4–N | NO3–N | AP | AK | |
|---|---|---|---|---|---|---|---|
| a ** means significant correlation at the P < 0.01 level; * means significant correlation at the P < 0.05 level. | |||||||
| TOC | 0.117 | 1.000** | 0.935** | 0.497 | 0.141 | 0.852** | 0.215 |
| WSOC | 0.172 | 0.505 | 0.496 | 0.250 | −0.026 | 0.530* | −0.266 |
| HE | 0.026 | 0.969** | 0.916** | 0.575* | 0.217 | 0.904** | 0.190 |
| HA | 0.039 | 0.962** | 0.912** | 0.559* | 0.183 | 0.889** | 0.137 |
| FA | −0.085 | 0.534* | 0.488 | 0.413 | 0.381 | 0.575* | 0.521* |
| HM | 0.193 | 0.965** | 0.892** | 0.391 | 0.063 | 0.744** | 0.239 |
HA element composition and FTIR analysis
In the different fertilization treatments, the main element composition of HA was C and O, with values of 43.0 ± 0.76 to 60.4 ± 1.45% and 28.0 ± 1.56 to 42.3 ± 1.77% (Table 6). Compared with those in N-fert treatment, the percentages of C and O increased significantly under G-fert treatment, and decreased significantly under M-fert treatment. M-fert treatment decreased C/N, but increased O/C. G-fert treatment increased C/N, but decreased O/C and H/C.| Treatment | C% | N% | O% | H% | C/N | O/C | H/C |
|---|---|---|---|---|---|---|---|
| a Table data are mean ± standard deviation. M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. Different lowercase letters indicate element compositions of HA with significantly differences at the P < 0.05 level under different fertilization treatments. | |||||||
| M-fert | 43.0 ± 0.76 d | 6.13 ± 0.22 a | 42.3 ± 1.77 a | 4.76 ± 0.15 e | 7.04 ± 0.21 d | 0.981 ± 0.02 a | 0.110 ± 0.00 a |
| G-fert | 60.4 ± 1.45 a | 4.01 ± 0.15 e | 28.0 ± 1.56 e | 5.57 ± 0.14 a | 15.1 ± 0.46 a | 0.462 ± 0.01 e | 0.092 ± 0.00 d |
| GM-fert | 55.3 ± 1.55 b | 5.37 ± 0.17 b | 30.1 ± 1.24 d | 5.33 ± 0.11 b | 10.3 ± 0.31 b | 0.543 ± 0.01 d | 0.096 ± 0.00 c |
| 1/2 GM-fert | 47.4 ± 1.22 c | 4.63 ± 0.23 d | 39.8 ± 1.33 c | 5.02 ± 0.17 c | 10.3 ± 0.31 b | 0.837 ± 0.02 c | 0.106 ± 0.00 b |
| N-fert | 46.8 ± 1.03 c | 5.11 ± 0.20 c | 40.2 ± 1.08 b | 4.98 ± 0.12 d | 9.18 ± 0.28 c | 0.857 ± 0.02 b | 0.106 ± 0.00 b |
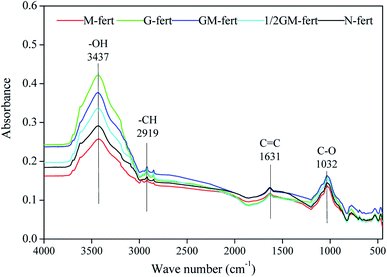
Based on the absorption peaks (Fig. 2 and Table 7), the relative intensities of the main absorption peaks (alcohol –OH, aliphatic –CH, amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, carbohydrate C–O) of HA changed under the different fertilization treatments (Table 8). The relative intensities of the total absorption peaks increased under G-fert, GM-fert and 1/2 GM-fert fertilization treatments and decreased under M-fert treatment relative to those under N-fert. The relative intensity of the –CH/C
C, carbohydrate C–O) of HA changed under the different fertilization treatments (Table 8). The relative intensities of the total absorption peaks increased under G-fert, GM-fert and 1/2 GM-fert fertilization treatments and decreased under M-fert treatment relative to those under N-fert. The relative intensity of the –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C absorption peak showed the largest changes under 1/2 GM-fert treatment, and the relative intensities of the alcohol –OH, aliphatic –CH and carbohydrate C–O peaks showed the largest changes under GM-fert treatment. The relative intensity of the amide group C
C absorption peak showed the largest changes under 1/2 GM-fert treatment, and the relative intensities of the alcohol –OH, aliphatic –CH and carbohydrate C–O peaks showed the largest changes under GM-fert treatment. The relative intensity of the amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C absorption peak decreased 11.3%, 12.0%, 1.50%, and 14.3% under M-fert, G-fert, GM-fert, and 1/2 GM-fert treatments, respectively.
C absorption peak decreased 11.3%, 12.0%, 1.50%, and 14.3% under M-fert, G-fert, GM-fert, and 1/2 GM-fert treatments, respectively.
| Wavenumber/cm−1 | Absorption peak location and assignment |
|---|---|
| 3437 | O–H alcohol stretching vibration |
| 2919 | –CH aliphatic stretching vibration |
| 1631 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration in amide groups C vibration in amide groups |
| 1031 | C–O vibration in carbohydrates |
| Treatment | Total | Alcohol –OH | Aliphatic –CH | Amide groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
Carbohydrates C–O | –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
|---|---|---|---|---|---|---|
| a Table data are mean ± standard deviation. M-fert = mineral fertilizer; G-fert = green waste compost fertilizer; GM-fert = standard rate of M-fert plus G-fert; 1/2 GM-fert = half the standard rate of M-fert plus G-fert; N-fert = control with no fertilizer addition. Different lowercase letters indicate relative absorption peak intensities of HA that are significantly different at the P < 0.05 level under different fertilization treatments. | ||||||
| M-fert | 66.4 ± 0.92 e | 25.7 ± 0.75 e | 15.2 ± 0.46 c | 11.8 ± 0.30 b | 13.7 ± 0.41 d | 1.29 ± 0.01 b |
| G-fert | 88.0 ± 1.55 a | 42.2 ± 1.59 a | 18.0 ± 0.54 a | 11.7 ± 0.29 b | 16.1 ± 0.41 a | 1.53 ± 0.01 d |
| GM-fert | 85.6 ± 2.07 b | 37.7 ± 0.99 b | 18.6 ± 0.56 a | 13.1 ± 0.33 a | 16.3 ± 0.49 a | 1.42 ± 0.01 c |
| 1/2 GM-fert | 78.8 ± 1.76 c | 33.7 ± 0.69 c | 18.3 ± 0.55 a | 11.4 ± 0.29 b | 15.5 ± 0.46 b | 1.60 ± 0.01 a |
| N-fert | 72.9 ± 0.08 d | 29.1 ± 1.32 d | 16.0 ± 0.48 c | 13.3 ± 0.33 a | 14.5 ± 0.44 c | 1.21 ± 0.01 e |
Relationship between soil N, P, K and HA structures
Table 9 shows the Pearson correlation coefficients between the soil chemical properties and HA structures. The correlation coefficients between –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and the TN and AP values were the highest under all fertilization treatments, reaching 0.935 and 0.927. The soil alcohol –OH, aliphatic –CH, amide group C
C and the TN and AP values were the highest under all fertilization treatments, reaching 0.935 and 0.927. The soil alcohol –OH, aliphatic –CH, amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, carbohydrate C–O and –CH/C
C, carbohydrate C–O and –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C were significantly (P < 0.05) correlated (r = −0.669–0.935) with the TN. The soil amide group C
C were significantly (P < 0.05) correlated (r = −0.669–0.935) with the TN. The soil amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was also significantly (P < 0.01) negatively correlated (r = −0.657–(−0.785)) with the NH4–N, AP and AK, and –CH/C
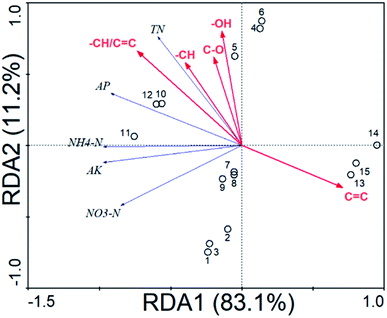
C was also significantly (P < 0.01) negatively correlated (r = −0.657–(−0.785)) with the NH4–N, AP and AK, and –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was significantly (P < 0.05) positively correlated (r = 0.630–0.972) with the NH4–N, AP and AK. Though RDA analysis, the HA structures could explain the changes (94.3%) in the soil N, P, K (Fig. 3 and Table 10). The soil N, P, K was significantly positively correlated with –CH/C
C was significantly (P < 0.05) positively correlated (r = 0.630–0.972) with the NH4–N, AP and AK. Though RDA analysis, the HA structures could explain the changes (94.3%) in the soil N, P, K (Fig. 3 and Table 10). The soil N, P, K was significantly positively correlated with –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (49.8%, F = 12.9, P = 0.004) and significantly negatively correlated with the amide groups C
C (49.8%, F = 12.9, P = 0.004) and significantly negatively correlated with the amide groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (43.1%, F = 9.86, P = 0.004).
C (43.1%, F = 9.86, P = 0.004).
| pH | OM | TN | NH4–N | NO3–N | AP | AK | |
|---|---|---|---|---|---|---|---|
| a ** means significant correlation at the P < 0.01 level; * means significant correlation at the P < 0.05 level. | |||||||
| Alcohol –OH | 0.375 | 0.744* | 0.680** | −0.029 | −0.258 | 0.393 | −0.008 |
| Aliphatic –CH | 0.347 | 0.794** | 0.648** | 0.228 | 0.084 | 0.625* | 0.301 |
Amide groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
0.084 | −0.530* | −0.669** | −0.785** | −0.429 | −0.698** | −0.657** |
| Carbohydrates C–O | −0.371 | 0.701** | 0.551* | 0.011 | −0.109 | 0.433 | 0.086 |
–CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
0.265 | 0.959** | 0.935** | 0.660** | 0.309 | 0.927** | 0.630* |
| F | P | Contribution rate (%) | |
|---|---|---|---|
| a ** means significant correlation at the P < 0.01 level; * means significant correlation at the P < 0.05 level. Contribution rate (%) refers to the percentage contribution of each functional group to the soil N, P, K. | |||
–CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
12.9 | 0.004** | 49.8 |
Amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
9.86 | 0.004** | 43.1 |
| Aliphatic –CH | 2.73 | 0.096 | 17.3 |
| Alcohol –OH | 1.30 | 0.270 | 9.10 |
| Carbohydrate C–O | 1.13 | 0.334 | 8.00 |
Discussion
Our results showed that N-fert treatment resulted in the lowest values of N, P, K in forestry soil, which provided a poor growth environment for plants. Compared with N-fert treatment, M-fert, G-fert, GM-fert and 1/2 GM-fert treatment significantly increased soil N, P, K. The addition of M-fert activated the soil available nutrients, and led to the full release of fertilizer nutrients, but M-fert had no evident influence on the SOM compared to N-fert treatment. The addition of green waste compost significantly increased the SOM and C/N, which indicated that the green waste compost served as a source of nutrients and might enhance the N mineralization rate in the soil. The addition of G-fert in the process of decomposing released effective mineral nutrients, improving the soil fertility for the needs of plants.18–20 However, in forestry regions, the DR of G-fert and the changes in the soil chemical properties and SOM fractions under M-fert and G-fert treatments are less-reported. In this paper, we proved that GM-fert treatment was the optimal treatment in afforestation practices, and that the DR, pH, OM, TN, available nutrients (NH4–N, AP, AK) and HA of G-fert were conducive to fertilization.Firstly, the DR of the green waste compost was 64.2–68.0% under different fertilization treatments, which indicated that the green waste compost had fast turnover rates in forestry soils. The high fast-turnover rates of green waste compost could be applied as a method of organic fertilization in urban regions and field natural forests for reducing the green waste environmental stress.21,22 Secondly, the pH in the soil significantly decreased under M-fert and GM-fert treatment relative to N-fert treatment. A possible reason was that M-fert ameliorated the Ca2+ form dicalcium phosphate in alkaline calcareous soil;23,24 another reason was that green waste compost increased microbial biomass, further leading to CO2 dissolution to lower the pH.25 The soil OM and TN increased with the addition of green waste compost, which was a feature of G-fert, which served as a source of organic fertilizer, and simultaneously enhanced the N mineralization rate in the forestry soil. The soil concentrations of NH4–N, AP, and AK were highest under GM-fert treatment, which showed that M-fert + organic fertilization is more effective than the sole application of either M-fert or organic fertilization.26,27 Thirdly, HA was the most sensitive SOM fraction in forestry after the addition of green waste compost. The soil HA content is related to the soil carbon stabilization pool, which indicates that HA is an important index of soil carbon sequestration in the soil.28 However, our results indicated that the soil FA had no significant difference between the M-fert and N-fert treatments, which showed that M-fert had no effect on the FA to HA process relative to N-fert. Fertilization changed the SOM fractions, and with the changes in the SOM fractions, the available soil nutrients would be released for tree growth.29 Our results showed that the soil chemical properties were mainly affected by HA in forestry soils. The green waste compost increased soil HA, contributing to a higher ratio of HA/FA in the soil, and also could immobilize the nutrients temporarily and prevent them from being lost.6,30
G-fert addition decreased the O% relative to N-fert, which indicated HA formation with aliphatic and aromatic molecules.6,31 The relative intensity of the main absorption peaks of HA indicated the highest amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C under GM-fert and N-fert treatments, showing that HA was more easily stored compared to the M-fert, G-fert and 1/2 GM-fert treatments, suggesting the formation of conjugated groups under N-fert and GM-fert treatments.17,32 HA had lignin-like character with a high molecular weight under GM-fert and N-fert treatments, and increased nutrient retention capacity and chelating micronutrients for plant growth.29,33,34 However, the alcohol –OH bands increased under G-fert treatment, which represented relatively labile carbon accumulated in HA.35 Based on this, we speculated that HA was hard to decompose under N-fert treatment based on the lower alcohol –OH. In our results, we also found that the aliphatic –CH and carbohydrate C–O were highest under G-fert and GM-fert treatments. One reason was that the green waste compost contained abundant cellulose and hemicellulose; after returning to the soil, polysaccharide, protein, cellulose and lignin were the main components in G-fert and could be the main factors to affect the amide group C
C under GM-fert and N-fert treatments, showing that HA was more easily stored compared to the M-fert, G-fert and 1/2 GM-fert treatments, suggesting the formation of conjugated groups under N-fert and GM-fert treatments.17,32 HA had lignin-like character with a high molecular weight under GM-fert and N-fert treatments, and increased nutrient retention capacity and chelating micronutrients for plant growth.29,33,34 However, the alcohol –OH bands increased under G-fert treatment, which represented relatively labile carbon accumulated in HA.35 Based on this, we speculated that HA was hard to decompose under N-fert treatment based on the lower alcohol –OH. In our results, we also found that the aliphatic –CH and carbohydrate C–O were highest under G-fert and GM-fert treatments. One reason was that the green waste compost contained abundant cellulose and hemicellulose; after returning to the soil, polysaccharide, protein, cellulose and lignin were the main components in G-fert and could be the main factors to affect the amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and aliphatic –CH.14,22 The other reason was that G-fert treatment would significantly increase microbial metabolic activity in forestry soil, resulting in increased carbohydrate C–O. Through the coefficient of association and RDA analysis, the –CH/C
C and aliphatic –CH.14,22 The other reason was that G-fert treatment would significantly increase microbial metabolic activity in forestry soil, resulting in increased carbohydrate C–O. Through the coefficient of association and RDA analysis, the –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and amide group C
C and amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of HA were found to have the biggest contributions (49.8% and 43.1%) to soil N, P, K, which showed that green waste compost not only increased the complexity of the structure of HA, but also released soil nutrients for plant growth. As the green waste compost decomposed, the carbohydrate C–O was utilized by microbes,36 and the residual lignin combined with humus to enhance the aromatization degree and stability of the soil humus.
C of HA were found to have the biggest contributions (49.8% and 43.1%) to soil N, P, K, which showed that green waste compost not only increased the complexity of the structure of HA, but also released soil nutrients for plant growth. As the green waste compost decomposed, the carbohydrate C–O was utilized by microbes,36 and the residual lignin combined with humus to enhance the aromatization degree and stability of the soil humus.
In general, the DR of the green waste compost was over 60% after 3 months of fertilization, and provided soil N, P, K for tree growth.15 G-fert could be used as an organic fertilizer in forestry soils, which was beneficial for forest ecological services.14,15 For example, in northern forest system forests, the amount of green waste presents a high risk of wildfires. The fast turnover of green waste compost reduced the accumulation of wastes. In urban regions, poplar (Populus L.) is an afforestation species, and the fast turnover of G-fert instead of human-aided removal was welcomed for sanitation reasons.22 Our findings in this paper indicate that GM-fert treatment was the optimal fertilization treatment for improving soil chemical properties. The increased HA had high nutrient storage capacity, and the decomposition of amide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C released soil N, P, K, which provided a theoretical basis for fertilization in field forestry and urban forestry soils.
C released soil N, P, K, which provided a theoretical basis for fertilization in field forestry and urban forestry soils.
Conclusions
During short-term fertilization, GM-fert treatment significantly increased the content of NH4–N, AP, AK, WSOC, HE, and HA in forestry soil. HA was the main indicator that contributed to soil N, P, K, and the relative intensities of the absorption peaks of alcohol –OH, aliphatic –CH, and carbohydrate C–O showed the largest changes under GM-fert treatment. –CH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was also significantly positively correlated with soil N, P, K. Overall, green waste compost significantly increased soil N, P, K, and SOM fractions, especially the content of HA.
C was also significantly positively correlated with soil N, P, K. Overall, green waste compost significantly increased soil N, P, K, and SOM fractions, especially the content of HA.
Author contributions
Xiaojie Feng: validation, conceptualization, investigation, visualization, writing – original draft, writing – review & editing. Xiangyang Sun: validation, conceptualization, resources. Wenjie Zhou: validation, supervision, investigation. Wei Zhang: validation, supervision. Feiwei Che: validation, investigation. Suyan Li: validation, resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Beijing Natural Science Foundation (Project No. 6202021). We also thank Yi Zheng, Subo Yan and Yinan Li for their assistance with field sampling and laboratory work.References
- S. B. Yang, C. Feng, Y. H. Ma, W. J. Wang, C. Huang, C. J. Qi, S. L. Fu and H. Y. H. Chen, For. Ecol. Manage., 2021, 494, 119298 CrossRef.
- H. T. Xie, Y. Tang, M. Yu and G. G. Wang, Glob. Ecol. Conserv., 2021, 26, e01478 CrossRef.
- A. K. Quaye and T. A. Volk, Biomass Bioenergy, 2013, 57, 113–125 CrossRef CAS.
- M. Schnitzer, Soil Sci., 1991, 151, 41–58 CrossRef.
- C. Hui, B. Liu, R. Wei, H. Jiang, Y. H. Zhao, Y. C. Liang, Q. C. Zhang and L. G. Xu, Environ. Pollut., 2019, 249, 686–695 CrossRef CAS PubMed.
- W. H. Mi, Y. Sun, Q. Gao, M. Y. Liu and L. H. Wu, Soil Tillage Res., 2019, 195, 104421 CrossRef.
- T. A. Doane, O. C. Devêvre and W. R. Horwath, Geoderma, 2003, 114, 319–331 CrossRef CAS.
- J. J. Zhang, Y. X. Wei, J. Z. Liu, J. C. Yuan, Y. Liang, J. Ren and H. G. Cai, Soil Tillage Res., 2019, 190, 1–9 CrossRef.
- S. Fontaine, S. Barot, P. Barre, N. Bdioui, B. Mary and C. Rumpel, Nature, 2007, 450, 277–280 CrossRef CAS PubMed.
- A. Nebbioso and A. Piccolo, Biomacromolecules, 2011, 12, 1187–1199 CrossRef CAS PubMed.
- M. Drosos, A. Nebbioso, P. Mazzei, G. Vinci, R. Spaccini and A. Piccolo, Sci. Total Environ., 2017, 586, 807–816 CrossRef CAS PubMed.
- J. Leifeld, Eur. J. Soil Sci., 2006, 57, 846–857 CrossRef CAS.
- Y. Kavdır, H. Ekinci, O. Yqksel and A. R. Mermut, Geoderma, 2005, 129, 219–229 CrossRef.
- L. Zhang and X. Y. Sun, Bioresour. Technol., 2018, 267, 182–191 CrossRef CAS PubMed.
- P. D. Somerville, P. B. May and S. J. Livesley, J. Environ. Manage., 2018, 227, 365–374 CrossRef PubMed.
- R. K. Lu, Chinese Agriculture, Science and Technology Press, Beijing, 2000 Search PubMed.
- F. Calderón, M. Haddix, R. Conant, K. Magrini-Bair and E. Paul, Soil Sci. Soc. Am. J., 2013, 77, 1591–1600 CrossRef.
- X. Yan, H. Zhou, Q. H. Zhu, X. F. Wang, Y. Z. Zhang, X. C. Yu and X. Peng, Soil Tillage Res., 2013, 130, 42–51 CrossRef.
- Y. T. He, W. J. Zhang, M. G. Xu, X. G. Tong, F. X. Sun, J. Z. Wang, S. M. Huang, P. Zhu and X. H. He, Sci. Total Environ., 2015, 532, 635–644 CrossRef CAS PubMed.
- F. Yang, J. Tian, J. Meersmans, H. J. Fang, H. Yang, Y. L. Lou, Z. F. Li, K. K. Liu, Y. Zhou, E. Blagodatskaya and Y. Kuzyakov, Catena, 2018, 162, 270–277 CrossRef CAS.
- L. Zhang and X. Y. Sun, Bioresour. Technol., 2017, 243, 154–162 CrossRef CAS PubMed.
- L. Zhang and X. Y. Sun, Waste Manag., 2018, 77, 435–446 CrossRef CAS.
- M. A. Naeem, M. Khalid, M. Aon, G. Abbas, M. Amjad, B. Murtaza, W. Khan and N. Ahmad, J. Plant Nutr., 2017, 41, 112–122 CrossRef.
- X. X. Gao, H. T. Liu and J. Zhang, Waste Manag., 2020, 102, 884–899 CrossRef.
- Y. H. Liu, H. D. Zang, T. D. Ge, J. Bai, S. B. Lu, P. Zhou, P. Q. Peng, O. Shibistova, Z. K. Zhu, J. S. Wu and G. Guggenberger, Appl. Soil Ecol., 2018, 127, 51–57 CrossRef.
- K. Uzoma, M. Inoue, H. Andry, H. Fujimaki, A. Zahoor and E. Nishihara, Soil Use Manage., 2011, 27, 205–212 CrossRef.
- Y. E. Cao, Y. M. Gao, Y. B. Qi and J. S. Li, Environ. Sci. Pollut. Res., 2017, 25, 7589–7599 CrossRef PubMed.
- I. A. Navarrete, K. Tsutsuki and R. A. Navarrete, Soil Sci. Plant Nutr., 2010, 56, 289–296 CrossRef CAS.
- H. Shindo, O. Hirahara, M. Yoshida and A. Yamamoto, Biol. Fertil. Soils, 2006, 42, 437–442 CrossRef.
- R. Merckx, J. Diels, B. Vanlauwe, N. Sanginga, K. Denef and K. Oorts, Soil Sci. Soc. Am. J., 2001, 58, 69–89 Search PubMed.
- F. J. Calderón, J. B. Reeves III, H. P. Collins and E. A. Paul, Soil Sci. Soc. Am. J., 2011, 75, 568–579 CrossRef.
- C. P. Assis, I. Jucksch, E. S. Mendonca, J. C. L. Neves, L. H. M. Silva and B. Wendling, Commun. Soil Sci. Plant Anal., 2012, 43, 835–846 CrossRef CAS.
- R. Lal, Land Degrad. Dev., 2006, 17, 197–209 CrossRef.
- H. Khaled and H. A. Fawy, Soil Water Res., 2011, 6, 21–29 CrossRef CAS.
- F. J. Calderón, G. W. McCarty and J. B. Reeves III, J. Anal. Appl. Pyrolysis, 2006, 76, 14–23 CrossRef.
- F. A. Dijkstra, Y. Carrillo, E. Pendall and J. A. Morgan, Front. Microbiol., 2013, 4, 183–190 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |