Open Access Article
Open Access ArticleConsecutive reactions to construct tricarbonyl compounds and synthetic applications thereof†
Diego Madroñero a,
Cesar A. Mujica-Martinez
a,
Cesar A. Mujica-Martinez b and
Alfredo Vázquez
b and
Alfredo Vázquez *a
*a
aDepartamento de Química Orgánica, Facultad de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, Cd. Mx., 04510, Mexico. E-mail: joseavm@unam.mx
bGIFBA, Departamento de Química, Facultad de Ciencias Exactas y Naturales, Centro de Investigación en Materiales CIMA, Universidad de Nariño, San Juan de Pasto, 520002, Colombia
First published on 11th October 2021
Abstract
Lithium anions derived from O-carbonate-protected cyanohydrins undergo conjugate addition to cycloalkenones with the concomitant transfer of the alkoxycarbonyl group to produce tricarbonyl compounds. These products offer numerous possibilities for further elaboration. The synthetic potential of the cascade products was demonstrated by forming bicyclic and tricyclic systems through intramolecular condensation reactions.
Introduction
Since the pioneering work by Stork et al.,1 O-protected cyanohydrins have become recognized as valuable acyl anion synthons.2,3 Over the years, this approach has been widely used4a–k to achieve the chemical synthesis of organic molecules with diverse architectures (Scheme 1).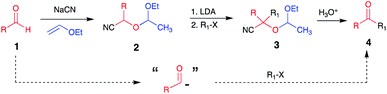 | ||
| Scheme 1 Pioneering work of Stork and Maldonado to use protected cyanohydrins as acyl anion equivalents. | ||
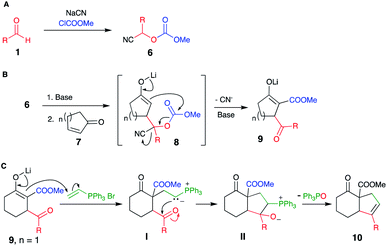
Among the different groups used to protect the hydroxyl group in cyanohydrins, carbonates5a–c display a moderately electrophilic carbon atom. This reactivity can be exploited to perform subsequent transformations to the anions' reaction with electrophiles (i.e., transfer of an acyl group), thus enabling consecutive reactions. Consecutive reactions, also known as cascade or domino reactions,6 are a practical strategy to form multiple bonds sequentially, simplifying the construction of organic molecules.
A recent paper7 demonstrated that anions derived from O-carbonate-protected cyanohydrins undergo conjugate addition to cyclohexenone with concomitant transfer of the alkoxycarbonyl group to produce β-keto-β′-acylcycloalkanecarboxylic acid esters; however, these were isolated as the enol acetate derivatives.
Tricarbonyl compounds have been used for the synthesis of functionalized biphenyls via an oxidative aromatization with iodine8a and for the formal synthesis of (±)-cochlearol A.8b The oxidation of tricarbonyl compounds has been used for the preparation of propellanes, compounds showing a broad spectra of biological and pharmacological activities,8c as well as for the synthesis of heterocycles.8d
Considering the tremendous synthetic utility of the functionalities present in the putative intermediate products namely β-keto ester,9a–f gamma-keto ester,9g and 1,4-dione,10 we attempted to directly obtain these intermediates by modifying Le Lagadec's procedure.7
Herein, we present a procedure to construct synthetically valuable tricarbonyl cyclic compounds featuring consecutive Michael–Claisen reactions of lithium anions derived from O-carbonate-protected cyanohydrins with 5, 6, and 7-membered cycloalkenones. The functionalities introduced into the cycloalkenones was exploited by annulation of a five-membered ring onto the olefin of the original cycloalkenone.
Results and discussion
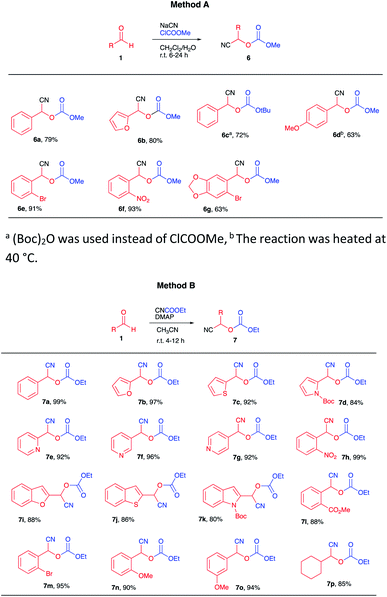
Our strategy is shown in Scheme 2, consisting of the preparation of cyanocarbonates 6 from aldehydes 1 according to literature procedures (Scheme 2A). Deprotonation with a suitable base (2 equiv.), followed by conjugate addition of the corresponding anions onto cyclic enones 7 (Scheme 2B) and subsequent reaction of the tricarbonyl product 9 (as an enolate) with electrophiles would afford highly functionalized products 10 (Scheme 2C).The preparation of some cyanocarbonates 6 was achieved using the two-phase reaction system procedure reported by Kolis et al.11 (Table 1, Method A). In other cases, higher yields were obtained using ethyl carbonocyanidate (CNCOOEt) in the presence of DMAP and CH3CN as the solvent (Table 1, Method B).5c A total of 23 cyanocarbonates were prepared and satisfactorily characterized.
To evaluate the deprotonation ease of the NC-C–H bond, its pKa was determined using a direct approach,12 in which free energies are calculated directly in THF solution at −78 °C (Table 2). Calculations were carried out using the ωB97XD DFT hybrid functional,13 the 6-311++G(d,p) basis set, and the D2 Grimme dispersion correction14 as implemented in the Gaussian 16 suite.15 The solvent was described implicitly using the SMD method.16 This methodology has been used before to determine pKa in several systems.12,17 Fig. 1 shows that the obtained pKa values highly correlate with the Hammett constants18 (σ) of the phenyl substituent for compounds 6a, 6d, 6e, and 6f, for which it was obtained pKa = 37.503–11.527σ and R2 = 0.931. Therefore, the deprotonation ease of these compounds increases with the electron-withdrawing properties of the substituents, which results in the stabilization of the corresponding carbanion. Similar correlations are observed with other molecular parameters and also for compounds 7. The relatively large pKa values indicated the use of a strong base would be required to obtain the corresponding carbanion efficiently.
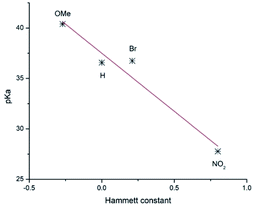 | ||
| Fig. 1 Correlation of pKa with the Hammett constant of the phenyl substituent for compounds 6a, 6d, 6e, 6f. | ||
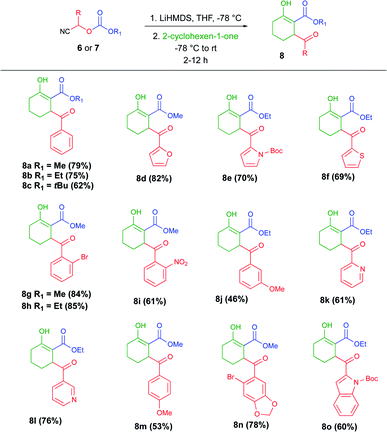
To test our hypothesis and standardize the reaction conditions, we selected cyanocarbonate 6a as the carbanion source and commercially available LiHMDS as the base. The base was added dropwise to a solution of 6a in THF at −78 °C. After 15 min, cyclohex-2-en-1-one was added, and the course of the reaction was monitored by TLC.
To investigate the scope of the cascade process under the optimized conditions described above, the anions of cyanocarbonates 6 and 7 were added to cyclohexanone. The results of those experiments are summarized in Table 2. A total 15 of tricarbonyl compounds (8a–o and 9a–c) were obtained in moderate yields. All the products were successfully characterized.
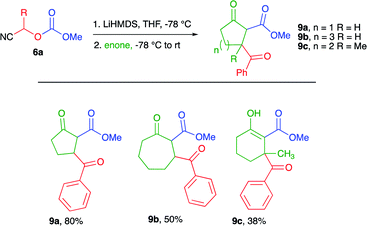
Next, we investigated the use of 2-cyclopenten-1-one, 2-cyclohepten-1-one, and 3-methyl-2-cyclohexen-1-one as the enone component for the cascade process to further explore the scope of the methodology. The results are presented in Table 3. For 2-cylopenten-1-one, the tricarbonyl compound 9a was obtained in 80% yield, whereas for 2-cyclohepten-1-one the desired product 9b was obtained in 50% yield. In the case of 3-methyl-2-cyclohexen-1-one, 9c was obtained in 38% yield. This lower yield was attributed to the steric hindrance caused by the methyl group at C-3 of the cycloalkenone. This assumption is supported by the failure to obtain the cascade product when the more sterically hindered 4,4-dimethyl-2-cyclohexen-1-one was used as the substrate.
Interestingly, 1H NMR spectra for all the cascade products obtained from 2-cyclohexen-1-one and 3-methyl-2-cyclohexen-1-one were isolated as a mixture of keto and enol tautomers. In contrast only a single keto tautomer or the keto diastereomers were observed by 1H NMR for the adducts derived from 2-cyclopenten-1-one and 2-cyclohepten-1-one (i.e., 9a and 9b). Computational results indicate that the keto-tautomer of compounds 9a and 9b is 1.365 and 1.366 kcal mol−1, respectively, more stable than the corresponding enol-tautomer. On the contrary, for compound 8a, the enol-tautomer is only 0.274 kcal mol−1 more stable than the corresponding keto-tautomer. This small energy difference could explain the reason to observe this product as a mixture of tautomers. Further investigation on the tautomeric behavior of these systems is currently underway. Clearly, the cascade process can occur for 5, 6, and 7-membered cycloalkenones, even enones showing moderate steric hindrance.
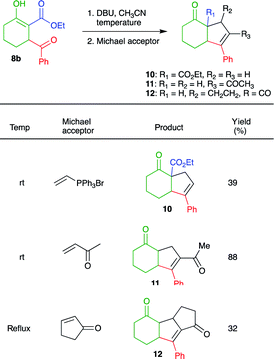
After proving the efficiency of the cascade process, we investigated the possibility of performing annulations via intramolecular condensation reactions. Thus, when 14b was treated with NaH in THF at rt, followed by the addition of triphenylvinylphosphonium bromide (Schweizer's reagent)19 no reaction was observed. If the reaction mixture was heated under reflux, several spots are observed on TLC. However, when DBU was used as the base (in CH3CN), 39% of intramolecular Wittig product 10 was obtained after purification, along with unreacted starting material and traces of two unknown compounds. Treatment of 8b with methylvinyl ketone and cyclopentenone as the Michael acceptors in the presence of DBU (in CH3CN at room temperature), afforded cyclic products 11 and 12 in 88 and 32% yield, respectively (Table 4). It is noteworthy that decarboxylation occurred during the formation of 11 and 12.
The preparation of annulated products 10, 11, and 12 nicely exemplifies the synthetic potential of β-keto-β′-acylcycloalkanecarboxylic acid esters 8 as scaffolds to obtain products with increased structural complexity. With some adjustments, we believe that compounds 8 can be used to obtain diverse molecules such as the core of pacifigorgianes20 13, the sesquiterpenoid cyperolone21 14, indanones22 15, furans23 pyrroles and thiophenes 16, 1,2-azoles 17, pyrimidines 18 and 1,2-diazines 19 (Fig. 2).
Experimental
General information
All experiments involving air and/or moisture-sensitive compounds were conducted in an oven dried round-bottom flask capped with a rubber septum under a positive pressure of nitrogen. Air- and moisture-sensitive liquids were transferred via syringe or stainless-steel cannula. Low temperature baths were ice/water (0 °C), CO2(s)/acetone (−78 °C). Unless otherwise noted, reaction temperatures refer to that of the bath. Concentration refers to removal of volatiles on a rotary evaporator below 35 °C. Analytical thin-layer chromatography (TLC) was performed using glass plates pre-coated with silica gel (0.25 mm, 60 Å pore size, 230–400 mesh, Merck) impregnated with a fluorescent indicator (254 nm). Materials on TLC plates were visualized under an ultraviolet lamp (254 nm) and/or by submersion of the plate in a solution of phosphomolybdic acid (5%) containing a trace of ceric sulfate in aqueous sulfuric acid (5% v/v) followed by charring on a hot plate. Flash column chromatography (FCC) was performed according to Still et al. with silica gel 60 (40–63 μm). All mixed solvent eluents are reported as v/v solutions. Unless otherwise noted, all reported compounds were homogeneous by thin layer chromatography (TLC) and by 1H NMR.Materials and methods
All the reagents were purchased from Sigma-Aldrich and were used as received unless other thing stated. Solvents were distilled prior to use. Anhydrous solvents were distilled under nitrogen atmosphere. THF and diethyl ether were distilled on sodium benzophenone ketyl; MeOH on magnesium activated with 5% iodine. Et3N, CH3CN dichloroethane and DMF were distilled on CaH2.Proton nuclear magnetic resonance (1H NMR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra were recorded on Agilent-Inova-300 and Varian VNMRS-400 NMR spectrometers. Proton chemical shifts are expressed in parts per million (ppm, δ scale) and are referenced to tetramethylsilane (TMS: 0.0). Carbon chemical shifts are expressed in parts per million (ppm, δ scale) and are referenced to tetramethylsilane (TMS: 0.0). For compounds 6e, 7e, 7f, 7h, 7p, 8g, and 8n, CDCl3 was used as a standard. This information is included in the ESI.† Data are represented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, dt = doublet of triplets, td = triplet of doublets, ddd = doublet of doublets of doublets, ddt = doublet of doublets of triplets, dqd = doublet of quartets of doublets, m = multiplet, br = broad, app = apparent), integration, and coupling constant (J) in Hertz (Hz).
IR spectra were recorded on a PerkinElmer Spectrum 400 FT-IR/FIR spectrometer with ATR. Mass spectra were carried out on a JEOL SMX-102a spectrometer.
Method A
A 2 M aqueous solution of NaCN (4.8 mL) was added dropwise to a mixture of the aldehyde (5 mmol), ClCOOMe (0.43 mL, 5.5 mmol) and (nBu)4NBr (160 mg, 0.5 mmol) in CH2Cl2 (6.25 mL) at ambient temperature. The resultant mixture was vigorously stirred overnight, the two phases were separated, and the organic layer was washed with sat. NaCl dried on anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (SiO2).Method B
CNCOOEt (0.108 mL, 1.1 mmol) was slowly added to a mixture of aldehyde (1 mmol) and DMAP (6 mg, 0.05 mmol) in CH3CN (2 mL) under nitrogen atmosphere at ambient temperature. When all the starting material has been consumed (TLC) the reaction mixture is poured on sat NaCl (5 mL) and extracted with EtOAc (3 × 20 mL). The organic extracts were combined and dried on anhydrous Na2SO4, the solvent evaporated in vacuo and the residue purified by column chromatography.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 755 mg (79%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.87 (s, 3H, OCH3), 6.27 (s, 1H, CHCN), 7.47–7.48 (m, 3H, m,p-C6H5), 7.53–7.56 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.0, 66.7, 115.8, 128.0, 129.4, 130.8, 131.3, 154.2 ppm. APCI: m/z calculated for C8H8NO4 [M + H]+ = 192.0661; found: 192.0703.
5) to obtain 755 mg (79%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.87 (s, 3H, OCH3), 6.27 (s, 1H, CHCN), 7.47–7.48 (m, 3H, m,p-C6H5), 7.53–7.56 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.0, 66.7, 115.8, 128.0, 129.4, 130.8, 131.3, 154.2 ppm. APCI: m/z calculated for C8H8NO4 [M + H]+ = 192.0661; found: 192.0703.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 729 mg (80%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.86 (s, 3H, OCH3), 6.34 (s, 1H, CHCN), 6.44–6.45 (dd, 1H, J = 1.8, 3.2 Hz, 3-C4H3O), 6.72–6.73 (d, 1H, J = 3.2 Hz, 4-C4H3O), 7.51–7.52 (d, 1H, J = 1.8 Hz, 5-C4H3O). 13C NMR (400 MHz, CDCl3) δ: 56.1, 59.5, 111.3, 113.3, 113.8, 143.6, 145.4, 154.0 ppm. APCI: m/z calculated for C8H8NO4 [M + H]+ = 182.0453; found: 182.0502.
5) to obtain 729 mg (80%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.86 (s, 3H, OCH3), 6.34 (s, 1H, CHCN), 6.44–6.45 (dd, 1H, J = 1.8, 3.2 Hz, 3-C4H3O), 6.72–6.73 (d, 1H, J = 3.2 Hz, 4-C4H3O), 7.51–7.52 (d, 1H, J = 1.8 Hz, 5-C4H3O). 13C NMR (400 MHz, CDCl3) δ: 56.1, 59.5, 111.3, 113.3, 113.8, 143.6, 145.4, 154.0 ppm. APCI: m/z calculated for C8H8NO4 [M + H]+ = 182.0453; found: 182.0502.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 845 mg (72%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H, Ot-Bu), 6.14 (s, 1H, CHCN), 7.34–7.36 (m, 3H, m,p-C6H5), 7.43–7.45 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 27.7, 65.8, 85.0, 116.2, 128.0, 129.3, 130.6, 131.7, 151.7 ppm. APCI: m/z calculated for C13H16NO3 [M + H]+ = 234.1130; found: 234.1124.
20) to obtain 845 mg (72%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H, Ot-Bu), 6.14 (s, 1H, CHCN), 7.34–7.36 (m, 3H, m,p-C6H5), 7.43–7.45 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 27.7, 65.8, 85.0, 116.2, 128.0, 129.3, 130.6, 131.7, 151.7 ppm. APCI: m/z calculated for C13H16NO3 [M + H]+ = 234.1130; found: 234.1124.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 700 mg (63%) of a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.82 (s, 3H, COOCH3), 3.84 (s, 3H, OCH3), 6.20 (s, 1H, CHCN), 6.95 (m, 2H m-C6H4OMe), 7.47 (m, 2H, o-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 55.5, 55.9, 66.5, 114.7, 116.0, 123.4, 129.9, 154.2, 161.5 ppm. APCI: m/z calculated for C11H12NO4 [M + H]+ = 222.0766; found: 222.0769.
20) to obtain 700 mg (63%) of a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 3.82 (s, 3H, COOCH3), 3.84 (s, 3H, OCH3), 6.20 (s, 1H, CHCN), 6.95 (m, 2H m-C6H4OMe), 7.47 (m, 2H, o-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 55.5, 55.9, 66.5, 114.7, 116.0, 123.4, 129.9, 154.2, 161.5 ppm. APCI: m/z calculated for C11H12NO4 [M + H]+ = 222.0766; found: 222.0769.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 1.23 g (91%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 3.89 (s, 3H, OCH3), 6.57 (s, 1H, CHCN), 7.61–7.64 (m, 1H, m-C6H4Br), 7.73–7.76 (m, 1H, m-C6H4Br), 7.87–7.89 (m, 1H, p-C6H4Br), 8.14–7.16 (m, 1H, o-C6H4Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.4, 63.0, 114.7, 126.0, 126.9, 129.3, 131.6, 134.9, 146.9, 153.6 ppm. ESI+: m/z calculated for C10H9BrNO3 [M + H]+ = 269.9766, found: 269.9775.
10) to obtain 1.23 g (91%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 3.89 (s, 3H, OCH3), 6.57 (s, 1H, CHCN), 7.61–7.64 (m, 1H, m-C6H4Br), 7.73–7.76 (m, 1H, m-C6H4Br), 7.87–7.89 (m, 1H, p-C6H4Br), 8.14–7.16 (m, 1H, o-C6H4Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.4, 63.0, 114.7, 126.0, 126.9, 129.3, 131.6, 134.9, 146.9, 153.6 ppm. ESI+: m/z calculated for C10H9BrNO3 [M + H]+ = 269.9766, found: 269.9775.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 1.16 g (93%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 3.89 (s, 3H, OCH3), 7.01 (s, 1H, CHCN), 7.67–7.71 (m, 1H, m-C6H4NO2), 7.79–7.83 (m, 1H, m-C6H4NO2), 7.94–7.96 (m, 1H, p-C6H4NO2), 8.21–8.24 (m, 1H, o-C6H4NO2) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.4, 63.0, 114.7, 126.0.127.0, 129.3, 131.7, 134.9, 146.9, 153.6 ppm. ESI+: m/z calculated for C10H9N2O5 [M + H]+ = 237.0511, found: 237.0510.
10) to obtain 1.16 g (93%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 3.89 (s, 3H, OCH3), 7.01 (s, 1H, CHCN), 7.67–7.71 (m, 1H, m-C6H4NO2), 7.79–7.83 (m, 1H, m-C6H4NO2), 7.94–7.96 (m, 1H, p-C6H4NO2), 8.21–8.24 (m, 1H, o-C6H4NO2) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.4, 63.0, 114.7, 126.0.127.0, 129.3, 131.7, 134.9, 146.9, 153.6 ppm. ESI+: m/z calculated for C10H9N2O5 [M + H]+ = 237.0511, found: 237.0510.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 1.32 g (84%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 3.88 (s, 3H, OCH3), 6.04 (s, 2H, OCH2O), 6.51 (s, 1H, CHCN), 7.04 (s, 1H, m-C6H2Br), 7.16 (s, 1H, o-C6H2Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.2, 66.2, 102.8, 109.3, 113.3, 114.9, 115.3, 123.8, 148.3, 150.5, 153.9 ppm. ESI+: m/z calculated for C11H9BrNO5 [M + H]+ = 313.9664, found: 313.9658.
20) to obtain 1.32 g (84%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 3.88 (s, 3H, OCH3), 6.04 (s, 2H, OCH2O), 6.51 (s, 1H, CHCN), 7.04 (s, 1H, m-C6H2Br), 7.16 (s, 1H, o-C6H2Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 56.2, 66.2, 102.8, 109.3, 113.3, 114.9, 115.3, 123.8, 148.3, 150.5, 153.9 ppm. ESI+: m/z calculated for C11H9BrNO5 [M + H]+ = 313.9664, found: 313.9658.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 203 mg (99%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.2 Hz, OCH2CH3), 4.21–4.33 (m, 2H, OCH2CH3), 6.26 (s, 1H, CHCN), 7.44–7.45 (m, 3H, m,p-C6H5), 7.52–7.54 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 65.7, 66.5, 115.9, 128.0, 129.4, 130.7, 131.4, 153.5 ppm. APCI: m/z calculated for C11H12NO3 [M + H]+ = 206.0817; found: 206.0849.
20) to obtain 203 mg (99%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.2 Hz, OCH2CH3), 4.21–4.33 (m, 2H, OCH2CH3), 6.26 (s, 1H, CHCN), 7.44–7.45 (m, 3H, m,p-C6H5), 7.52–7.54 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 65.7, 66.5, 115.9, 128.0, 129.4, 130.7, 131.4, 153.5 ppm. APCI: m/z calculated for C11H12NO3 [M + H]+ = 206.0817; found: 206.0849.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 190 mg (97%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.1 Hz, OCH2CH3), 4.22–4.33 (m, 2H, OCH2CH3), 6.32 (s, 1H, CHCN), 6.43 (dd, 1H, J = 1.8, 3.2 Hz, 3-C4H3O), 6.71 (d, 1H, J = 3.2 Hz, 4-C4H3O), 7.5 (d, 1H, J = 1.8 Hz, 5-C4H3O) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 59.3, 65.9, 111.3, 113.1, 113.9, 143.8, 145.4, 153.3 ppm. APCI: m/z calculated for C9H10NO4 [M + H]+ = 196.0610; found: 196.0626.
5) to obtain 190 mg (97%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.1 Hz, OCH2CH3), 4.22–4.33 (m, 2H, OCH2CH3), 6.32 (s, 1H, CHCN), 6.43 (dd, 1H, J = 1.8, 3.2 Hz, 3-C4H3O), 6.71 (d, 1H, J = 3.2 Hz, 4-C4H3O), 7.5 (d, 1H, J = 1.8 Hz, 5-C4H3O) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 59.3, 65.9, 111.3, 113.1, 113.9, 143.8, 145.4, 153.3 ppm. APCI: m/z calculated for C9H10NO4 [M + H]+ = 196.0610; found: 196.0626.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 195 mg (92%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.16 Hz, OCH2CH3), 4.25–4.32 (m, 2H, OCH2CH3), 6.48 (s, 1H, CHCN), 7.04–7.05 (m, 1H, 4-C4H3S), 7.36–7.38 (m, 1H, 3-C4H3S), 7.46–7.47 (m, 1H, 5-C4H3S) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 61.5, 65.8, 115.0, 127.3, 129.4, 130.0, 132.7, 153.2 ppm. APCI: m/z calculated for C9H10NO3S [M + H]+ = 212.0381; found: 212.0395.
20) to obtain 195 mg (92%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.16 Hz, OCH2CH3), 4.25–4.32 (m, 2H, OCH2CH3), 6.48 (s, 1H, CHCN), 7.04–7.05 (m, 1H, 4-C4H3S), 7.36–7.38 (m, 1H, 3-C4H3S), 7.46–7.47 (m, 1H, 5-C4H3S) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 61.5, 65.8, 115.0, 127.3, 129.4, 130.0, 132.7, 153.2 ppm. APCI: m/z calculated for C9H10NO3S [M + H]+ = 212.0381; found: 212.0395.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 248 mg (84%) of a translucent orange oil 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.58 (s, 9H, Ot-Bu), 4.22–4.33 (m, 2H, OCH2CH3), 6.18–6.20 (t, 1H, J = 3.4 Hz, 4-C4H3N), 6.70–6.71 (m, 1H, 3-C4H3N), 6.81 (s, 1H, CHCN), 7.31–7.32 (m, 1H, 5-C4H3N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.3, 28.0, 60.3, 65.5, 85.7, 110.7, 115.4, 117.6, 123.5, 124.7, 148.4, 153.5 ppm. APCI: m/z calculated for C14H19N2O5 [M + H]+ = 295.1294; found: 295.1214.
30) to obtain 248 mg (84%) of a translucent orange oil 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.58 (s, 9H, Ot-Bu), 4.22–4.33 (m, 2H, OCH2CH3), 6.18–6.20 (t, 1H, J = 3.4 Hz, 4-C4H3N), 6.70–6.71 (m, 1H, 3-C4H3N), 6.81 (s, 1H, CHCN), 7.31–7.32 (m, 1H, 5-C4H3N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.3, 28.0, 60.3, 65.5, 85.7, 110.7, 115.4, 117.6, 123.5, 124.7, 148.4, 153.5 ppm. APCI: m/z calculated for C14H19N2O5 [M + H]+ = 295.1294; found: 295.1214.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 190 mg (92%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.35 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.27–4.35 (m, 2H, OCH2CH3), 6.37 (s, 1H, CHCN), 7.37–7.39 (m, 1H, 5-C5H4N), 7.58–7.60 (m, 1H, 3-C5H4N), 7.80–7.83 (m, 1H, 4-C5H4N), 8.66–8.67 (m, 1H, 6-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 66.0, 67.2, 115.3, 121.9, 125.0, 137.8, 150.3, 150.7, 153.4 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0797.
20) to obtain 190 mg (92%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.35 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.27–4.35 (m, 2H, OCH2CH3), 6.37 (s, 1H, CHCN), 7.37–7.39 (m, 1H, 5-C5H4N), 7.58–7.60 (m, 1H, 3-C5H4N), 7.80–7.83 (m, 1H, 4-C5H4N), 8.66–8.67 (m, 1H, 6-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 66.0, 67.2, 115.3, 121.9, 125.0, 137.8, 150.3, 150.7, 153.4 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0797.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 197 mg (96%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.13 Hz, OCH2CH3), 4.23–4.33 (m, 2H, OCH2CH3), 6.30 (s, 1H, CHCN), 7.40–7.42 (m, 1H, 5-C5H4N), 7.89–7.91 (m, 1H, 4-C5H4N), 8.70–8.71 (m, 1H, 6-C5H4N), 8.76–8.77 (m, 1H, 2-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 64.3, 66.1, 115.1, 124.1, 127.6, 135.6, 149.2, 152.0, 153.3 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0797.
10) to obtain 197 mg (96%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.13 Hz, OCH2CH3), 4.23–4.33 (m, 2H, OCH2CH3), 6.30 (s, 1H, CHCN), 7.40–7.42 (m, 1H, 5-C5H4N), 7.89–7.91 (m, 1H, 4-C5H4N), 8.70–8.71 (m, 1H, 6-C5H4N), 8.76–8.77 (m, 1H, 2-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 64.3, 66.1, 115.1, 124.1, 127.6, 135.6, 149.2, 152.0, 153.3 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0797.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 190 mg (92%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.29 (t, 3H, J = 7.14 Hz, OCH2CH3), 4.22–4.29 (m, 2H, OCH2CH3), 6.22 (s, 1H, CHCN), 7.40 (d, 2H, J = 6.19 Hz, 3,5-C5H4N), 8.68 (d, 2H, J = 6.19 Hz, 2,6-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 64.8, 66.2, 114.8, 121.6, 139.8, 150.9, 153.3 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0809.
20) to obtain 190 mg (92%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.29 (t, 3H, J = 7.14 Hz, OCH2CH3), 4.22–4.29 (m, 2H, OCH2CH3), 6.22 (s, 1H, CHCN), 7.40 (d, 2H, J = 6.19 Hz, 3,5-C5H4N), 8.68 (d, 2H, J = 6.19 Hz, 2,6-C5H4N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 64.8, 66.2, 114.8, 121.6, 139.8, 150.9, 153.3 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 207.0770, found: 207.0809.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 245 mg (98%) of colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.35 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.26–4.34 (m, 2H, OCH2CH3), 7.00 (s, 1H, CHCN), 7.67–7.70 (m, 1H, m-C6H4NO2), 7.79–7.83 (m, 1H, m-C6H4NO2), 7.94–7.96 (m, 1H, p-C6H4NO2), 8.21–8.23 (m, 1H, o-C6H4NO2) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 62.8, 66.2, 114.8, 126.0, 127.1, 129.3, 131.6, 134.9, 146.9, 153.0 ppm. APCI: m/z calculated for C11H11N2O5 [M + H]+ = 251.0668; found: 251.0599.
10) to obtain 245 mg (98%) of colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.35 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.26–4.34 (m, 2H, OCH2CH3), 7.00 (s, 1H, CHCN), 7.67–7.70 (m, 1H, m-C6H4NO2), 7.79–7.83 (m, 1H, m-C6H4NO2), 7.94–7.96 (m, 1H, p-C6H4NO2), 8.21–8.23 (m, 1H, o-C6H4NO2) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 62.8, 66.2, 114.8, 126.0, 127.1, 129.3, 131.6, 134.9, 146.9, 153.0 ppm. APCI: m/z calculated for C11H11N2O5 [M + H]+ = 251.0668; found: 251.0599.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 216 mg (88%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.29–4.37 (m, 2H, OCH2CH3), 6.49 (s, 1H, CHCN), 7.10 (s, 1H, 3-C8H5O), 7.28–7.32 (m, 1H, 6-C8H5O), 7.38–7.42 (m, 1H, 5-C8H5O), 7.52–7.54 (m, 1H, 7-C8H5O), 7.62–7.64 (m, 1H, 4-C8H5O) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 59.9, 66.1, 109.5, 112.0, 113.7, 122.2, 123.9, 126.6, 127.0, 145.8, 153.3, 155.9 ppm. APCI: m/z calculated for C13H12NO4 [M + H]+ = 246.0766, found: 246.0770.
20) to obtain 216 mg (88%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.29–4.37 (m, 2H, OCH2CH3), 6.49 (s, 1H, CHCN), 7.10 (s, 1H, 3-C8H5O), 7.28–7.32 (m, 1H, 6-C8H5O), 7.38–7.42 (m, 1H, 5-C8H5O), 7.52–7.54 (m, 1H, 7-C8H5O), 7.62–7.64 (m, 1H, 4-C8H5O) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 59.9, 66.1, 109.5, 112.0, 113.7, 122.2, 123.9, 126.6, 127.0, 145.8, 153.3, 155.9 ppm. APCI: m/z calculated for C13H12NO4 [M + H]+ = 246.0766, found: 246.0770.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 226 mg (86%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.14 Hz, OCH2CH3), 4.28–4.36 (m, 2H, OCH2CH3), 6.58 (s, 1H, CHCN), 7.41–7.44 (m, 2H, 5,6-C8H5S), 7.62–7.63 (m, 1H, 3-C8H5S), 7.81–7.86 (m, 2H, 4,7-C8H5S) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 62.4, 66.0, 114.8, 122.7, 124.8, 125.2, 126.2, 126.8, 133.3, 138.4, 140.9, 153.3 ppm. APCI: m/z calculated for C13H13NO4 [M − OEt]+ = 216.0119, found: 216.0106.
10) to obtain 226 mg (86%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.14 Hz, OCH2CH3), 4.28–4.36 (m, 2H, OCH2CH3), 6.58 (s, 1H, CHCN), 7.41–7.44 (m, 2H, 5,6-C8H5S), 7.62–7.63 (m, 1H, 3-C8H5S), 7.81–7.86 (m, 2H, 4,7-C8H5S) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 62.4, 66.0, 114.8, 122.7, 124.8, 125.2, 126.2, 126.8, 133.3, 138.4, 140.9, 153.3 ppm. APCI: m/z calculated for C13H13NO4 [M − OEt]+ = 216.0119, found: 216.0106.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 275 mg (80%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.10 Hz, OCH2CH3), 1.70 (s, 9H, Ot-Bu), 4.30–4.35 (m, 2H, OCH2CH3), 6.99 (s, 1H, CHCN), 7.08–7.09 (m, 1H, 3-C8H5N), 7.25–7.29 (m, 1H, 5-C8H5N), 7.35–7.39 (m, 1H, 6-C8H5N), 7.57–7.59 (m, 1H, 4-C8H5N), 8.06–8.09 (m, 1H, 7-C8H5N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.3, 28.2, 61.6, 65.7, 86.1, 112.6, 115.3, 116.0, 121.7, 123.6, 126.2, 127.8, 129.3, 137.0, 149.8, 153.4 ppm. APCI: m/z calculated for C18H21N2O5 [M + H]+ = 345.1450, found: 245.1482.
30) to obtain 275 mg (80%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.10 Hz, OCH2CH3), 1.70 (s, 9H, Ot-Bu), 4.30–4.35 (m, 2H, OCH2CH3), 6.99 (s, 1H, CHCN), 7.08–7.09 (m, 1H, 3-C8H5N), 7.25–7.29 (m, 1H, 5-C8H5N), 7.35–7.39 (m, 1H, 6-C8H5N), 7.57–7.59 (m, 1H, 4-C8H5N), 8.06–8.09 (m, 1H, 7-C8H5N) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.3, 28.2, 61.6, 65.7, 86.1, 112.6, 115.3, 116.0, 121.7, 123.6, 126.2, 127.8, 129.3, 137.0, 149.8, 153.4 ppm. APCI: m/z calculated for C18H21N2O5 [M + H]+ = 345.1450, found: 245.1482.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 232 mg (88%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.11 Hz, 3H, OCH2CH3), 3.93 (s, 3H, OMe), 4.25–4.33 (m, 2H, OCH2CH3), 7.28 (s, 1H, CHCN), 7.51–7.55 (m, 1H, m-C6H4CO2Me), 7.63–7.67 (m, 1H m-C6H4CO2Me), 7.83–7.86 (m, 1H, p-C6H4CO2Me), 8.05–8.10 (m, 1H, o-C6H4CO2Me) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 52.7, 63.6, 65.6, 115.9, 128.1, 128.2, 130.2, 131.5, 132.8, 133.4, 153.2, 166.2 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 264.0872, found: 264.0841.
10) to obtain 232 mg (88%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.11 Hz, 3H, OCH2CH3), 3.93 (s, 3H, OMe), 4.25–4.33 (m, 2H, OCH2CH3), 7.28 (s, 1H, CHCN), 7.51–7.55 (m, 1H, m-C6H4CO2Me), 7.63–7.67 (m, 1H m-C6H4CO2Me), 7.83–7.86 (m, 1H, p-C6H4CO2Me), 8.05–8.10 (m, 1H, o-C6H4CO2Me) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 52.7, 63.6, 65.6, 115.9, 128.1, 128.2, 130.2, 131.5, 132.8, 133.4, 153.2, 166.2 ppm. APCI: m/z calculated for C13H14NO5 [M + H]+ = 264.0872, found: 264.0841.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 270 mg (95%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.34 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.27–4.33 (m, 2H, OCH2CH3), 6.57 (s, 1H, CHCN), 7.30–7.34 (m, 1H, m-C6H4Br), 7.40–7.45 (m, 1H, m-C6H4Br), 7.61–7.64 (m, 1H, p-C6H4Br), 7.71–7.74 (m, 1H, o-C6H4Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 65.8 (×2), 115.1, 123.1, 128.3, 129.6, 130.7, 132.0, 133.5, 153.1 ppm. ESI+: m/z calculated for C11H11BrNO3 [M + H]+ = 283.9922, found: 283.9927.
20) to obtain 270 mg (95%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.34 (t, 3H, J = 7.15 Hz, OCH2CH3), 4.27–4.33 (m, 2H, OCH2CH3), 6.57 (s, 1H, CHCN), 7.30–7.34 (m, 1H, m-C6H4Br), 7.40–7.45 (m, 1H, m-C6H4Br), 7.61–7.64 (m, 1H, p-C6H4Br), 7.71–7.74 (m, 1H, o-C6H4Br) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 65.8 (×2), 115.1, 123.1, 128.3, 129.6, 130.7, 132.0, 133.5, 153.1 ppm. ESI+: m/z calculated for C11H11BrNO3 [M + H]+ = 283.9922, found: 283.9927.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 212 mg (90%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.12 Hz, 3H, OCH2CH3), 3.88 (s, 3H, OMe), 4.24–4.32 (m, 2H, OCH2CH3), 6.58 (s, 1H, CHCN), 6.92–6.95 (m, 1H, o-C6H4OMe), 7.00–7.05 (m, 1H, p-C6H4OMe), 7.40–7.44 (m, 1H, m-C6H4OMe), 7.56–7.58 (m, 1H m-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 55.7, 61.7, 65.4, 111.1, 115.9, 119.5, 120.9, 128.9, 132.0, 153.5, 156.7 ppm. APCI: m/z calculated for C12H14NO4 [M + H]+ = 236.0923, found: 236.0930.
10) to obtain 212 mg (90%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.12 Hz, 3H, OCH2CH3), 3.88 (s, 3H, OMe), 4.24–4.32 (m, 2H, OCH2CH3), 6.58 (s, 1H, CHCN), 6.92–6.95 (m, 1H, o-C6H4OMe), 7.00–7.05 (m, 1H, p-C6H4OMe), 7.40–7.44 (m, 1H, m-C6H4OMe), 7.56–7.58 (m, 1H m-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 55.7, 61.7, 65.4, 111.1, 115.9, 119.5, 120.9, 128.9, 132.0, 153.5, 156.7 ppm. APCI: m/z calculated for C12H14NO4 [M + H]+ = 236.0923, found: 236.0930.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 222 mg (94%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.12 Hz, 3H, OCH2CH3), 3.83 (s, 3H, OMe), 4.24–4.32 (m, 2H, OCH2CH3), 6.22 (s, 1H, CHCN), 6.97–7.00 (m, 1H, o-C6H4OMe), 7.04–7.05 (m, 1H, o-C6H4OMe), 7.09–7.11 (m, 1H p-C6H4OMe), 7.33–7.37 (m, 1H, m-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 55.4, 65.6, 66.2, 113.0, 116.4, 116.5, 120.0, 130.4, 132.5, 153.4, 160.1 ppm. APCI: m/z calculated for C12H14NO4 [M + H]+ = 236.0923, found: 236.0955.
10) to obtain 222 mg (94%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.33 (t, J = 7.12 Hz, 3H, OCH2CH3), 3.83 (s, 3H, OMe), 4.24–4.32 (m, 2H, OCH2CH3), 6.22 (s, 1H, CHCN), 6.97–7.00 (m, 1H, o-C6H4OMe), 7.04–7.05 (m, 1H, o-C6H4OMe), 7.09–7.11 (m, 1H p-C6H4OMe), 7.33–7.37 (m, 1H, m-C6H4OMe) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 55.4, 65.6, 66.2, 113.0, 116.4, 116.5, 120.0, 130.4, 132.5, 153.4, 160.1 ppm. APCI: m/z calculated for C12H14NO4 [M + H]+ = 236.0923, found: 236.0955.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 180 mg (85%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.15–1.29 (m, 5H), 1.33 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.68–1.93 (m, 6H), 4.21–4.31 (m, 2H OCH2CH3), 5.03 (d, J = 5.88 Hz, 1H, CHCN) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 25.3, 25.8, 28.1, 40.3, 65.5, 69.4. APCI: m/z calculated for C11H18NO3 [M + H]+ = 212.1287, found: 212.1255.
10) to obtain 180 mg (85%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.15–1.29 (m, 5H), 1.33 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.68–1.93 (m, 6H), 4.21–4.31 (m, 2H OCH2CH3), 5.03 (d, J = 5.88 Hz, 1H, CHCN) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 25.3, 25.8, 28.1, 40.3, 65.5, 69.4. APCI: m/z calculated for C11H18NO3 [M + H]+ = 212.1287, found: 212.1255.General procedure for conjugate additions
1 M LiHMDS (THF) (750 μL, 0.75 mmol) was slowly added to a solution of cyanocarbonate (0.5 mmol) in THF (2.5 mL) at −78 °C under nitrogen atmosphere, and the resultant mixture stirred at this temperature for 15 min. Then, the enone (0.75 mmol) was added dropwise, and after the addition was completed, the mixture was allowed to reach ambient temperature. When the starting material has been consumed (TLC), the reaction was quenched by the slow addition of sat NH4Cl (5 mL). The mixture was diluted with water and extracted with EtOAc (3 × 20 mL); the organics were combined, dried (anh Na2SO4) and concentrated in vacuo. The residue was purified by column chromatography (SiO2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 103 mg (79%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.65–1.95 (m, 4H, CH2CH2CH2CH), 2.17–2.44 (m, 2H, COHCH2CH2), 3.55 (s, 3H, OMe), 4.48–4.50 (m, 1H, CH), 7.44–7.46 (m, 2H, m-C6H5), 7.47–7.48 (m, 1H, p-C6H5), 7.95–7.98 (m, 2H, o-C6H5), 12.34 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 26.1, 28.9, 41.0, 51.5, 97.2, 128.3, 128.7, 132.9, 133.7, 172.0, 174.5, 201.7 ppm. IR (cm−1): 3334, 1738, 1714, 1673, 1333. APCI: m/z calculated for C15H17O4 [M + H]+ = 261.1127, found: 261.1064.
5) to obtain 103 mg (79%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.65–1.95 (m, 4H, CH2CH2CH2CH), 2.17–2.44 (m, 2H, COHCH2CH2), 3.55 (s, 3H, OMe), 4.48–4.50 (m, 1H, CH), 7.44–7.46 (m, 2H, m-C6H5), 7.47–7.48 (m, 1H, p-C6H5), 7.95–7.98 (m, 2H, o-C6H5), 12.34 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 26.1, 28.9, 41.0, 51.5, 97.2, 128.3, 128.7, 132.9, 133.7, 172.0, 174.5, 201.7 ppm. IR (cm−1): 3334, 1738, 1714, 1673, 1333. APCI: m/z calculated for C15H17O4 [M + H]+ = 261.1127, found: 261.1064.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 103 mg (75%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 0.97 (t, 3H, J = 7.12 Hz, OCH2CH3), 1.67–1.94 (m, 4H, CH2CH2CH2CH), 2.32–2.39 (m, 2H, COHCH2CH2), 4.01–4.06 (m, 2H, OCH2CH3), 4.50–4.53 (m, 1H, CH), 7.46–7.49 (m, 2H, m-C6H5), 7.55–7.57 (m, 1H, p-C6H5), 7.96–8.01 (m, 2H, o-C6H5), 12.37 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.9, 18.8, 26.3, 29.0, 41.0, 60.5, 97.6, 128.4, 128.7, 132.9, 136.6, 171.7, 174.5, 202.1 ppm. IR (cm−1): 3448, 1740, 1682, 1651, 1334. APCI: m/z calculated for C16H19O4 [M + H]+ = 275.1283; found: 275.1242.
5) to obtain 103 mg (75%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 0.97 (t, 3H, J = 7.12 Hz, OCH2CH3), 1.67–1.94 (m, 4H, CH2CH2CH2CH), 2.32–2.39 (m, 2H, COHCH2CH2), 4.01–4.06 (m, 2H, OCH2CH3), 4.50–4.53 (m, 1H, CH), 7.46–7.49 (m, 2H, m-C6H5), 7.55–7.57 (m, 1H, p-C6H5), 7.96–8.01 (m, 2H, o-C6H5), 12.37 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.9, 18.8, 26.3, 29.0, 41.0, 60.5, 97.6, 128.4, 128.7, 132.9, 136.6, 171.7, 174.5, 202.1 ppm. IR (cm−1): 3448, 1740, 1682, 1651, 1334. APCI: m/z calculated for C16H19O4 [M + H]+ = 275.1283; found: 275.1242.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 94 mg (62%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.21 (s, 9H, Ot-Bu), 1.63–1.74 (m, 4H, CH2CH2CH2CH), 2.30–2.34 (m, 2H, COHCH2CH2), 4.43–4.47 (m, 1H, CH), 7.43–7.53 (m, 2H, p-C6H5), 7.49–7.55 (m, 1H, m-C6H5), 7.94–8.01 (m, 2H, o-C6H5), 12.55 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 19.0, 26.3, 28.1, 29.1, 41.3, 81.9, 98.8, 128.4, 128.7, 132.9, 133.6, 168.4, 173.8, 202.0 ppm. IR (cm−1): 3061, 1716, 1683, 1648, 1317. APCI: m/z calculated for C14H13O3 [M + H]+ = 303.1596; found: 303.1594.
10) to obtain 94 mg (62%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.21 (s, 9H, Ot-Bu), 1.63–1.74 (m, 4H, CH2CH2CH2CH), 2.30–2.34 (m, 2H, COHCH2CH2), 4.43–4.47 (m, 1H, CH), 7.43–7.53 (m, 2H, p-C6H5), 7.49–7.55 (m, 1H, m-C6H5), 7.94–8.01 (m, 2H, o-C6H5), 12.55 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 19.0, 26.3, 28.1, 29.1, 41.3, 81.9, 98.8, 128.4, 128.7, 132.9, 133.6, 168.4, 173.8, 202.0 ppm. IR (cm−1): 3061, 1716, 1683, 1648, 1317. APCI: m/z calculated for C14H13O3 [M + H]+ = 303.1596; found: 303.1594.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 102 mg (82%) of a light brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.60–1.67 (m, 2H, CH2CH2CH2), 1.83–1.92 (m, 2H, CH2CH2CH), 2.27–2.32 (m, 2H, COHCH2CH2), 3.55 (s, 3H, OMe), 4.22–4.23 (m, 1H, CH), 6.51–6.53 (m, 1H, 4-C4H3O), 7.19–7.20 (m, 1H, 3-C4H3O), 7.57–7.59 (m, 1H, 5-C4H3O), 12.33 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.6, 26.4, 28.9, 41.8, 51.6, 96.5, 112.3, 117.3, 146.4, 147.1, 172.0, 174.9, 190.8 ppm. IR (cm−1): 3321, 1732, 1715, 1666, 1328. APCI: m/z calculated for C13H15O5 [M + H]+ = 251.0919; found: 251.0824.
10) to obtain 102 mg (82%) of a light brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.60–1.67 (m, 2H, CH2CH2CH2), 1.83–1.92 (m, 2H, CH2CH2CH), 2.27–2.32 (m, 2H, COHCH2CH2), 3.55 (s, 3H, OMe), 4.22–4.23 (m, 1H, CH), 6.51–6.53 (m, 1H, 4-C4H3O), 7.19–7.20 (m, 1H, 3-C4H3O), 7.57–7.59 (m, 1H, 5-C4H3O), 12.33 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.6, 26.4, 28.9, 41.8, 51.6, 96.5, 112.3, 117.3, 146.4, 147.1, 172.0, 174.9, 190.8 ppm. IR (cm−1): 3321, 1732, 1715, 1666, 1328. APCI: m/z calculated for C13H15O5 [M + H]+ = 251.0919; found: 251.0824.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 128 mg (70%) of a light brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.09 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.56 (s, 9H, Ot-Bu), 1.80–1.93 (m, 4H, CH2CH2CH2CH), 2.32–2.37 (m, 3H, COHCH2CH2), 3.93–4.15 (m, 3H, OCH2CH3, CH), 6.16–6.20 (m, 1H, 4-C4H3N), 6.98–6.99 (m, 1H, 3-C4H3N), 7.37–7.38 (m, 1H, 5-C4H3N), 12.42 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.0, 18.8, 26.0, 27.7, 29.1, 50.8, 60.6, 84.9, 97.1, 109.7, 121.4, 128.7, 133.3, 149.2, 169.2, 174.7, 192.0 ppm. IR (cm−1): 3476, 1744, 1717, 1673, 1619, 1310. APCI: m/z calculated for C19H25NO6 [M + H]+ = 364.1760; found: 364.1737.
30) to obtain 128 mg (70%) of a light brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.09 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.56 (s, 9H, Ot-Bu), 1.80–1.93 (m, 4H, CH2CH2CH2CH), 2.32–2.37 (m, 3H, COHCH2CH2), 3.93–4.15 (m, 3H, OCH2CH3, CH), 6.16–6.20 (m, 1H, 4-C4H3N), 6.98–6.99 (m, 1H, 3-C4H3N), 7.37–7.38 (m, 1H, 5-C4H3N), 12.42 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.0, 18.8, 26.0, 27.7, 29.1, 50.8, 60.6, 84.9, 97.1, 109.7, 121.4, 128.7, 133.3, 149.2, 169.2, 174.7, 192.0 ppm. IR (cm−1): 3476, 1744, 1717, 1673, 1619, 1310. APCI: m/z calculated for C19H25NO6 [M + H]+ = 364.1760; found: 364.1737.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain 97 mg (69%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, 3H, J = 7.15 Hz, OCH2CH3), 1.84–1.9 (m, 4H, CH2CH2CH2CH), 2.30–2.34 (m, 2H, COHCH2CH2), 3.96–4.02 (m, 2H, OCH2CH3), 4.24–4.27 (m, 1H, CH), 7.10–7.12 (m, 1H, 4-C4H3S), 7.59–7.61 (m, 1H, 3-C4H3S), 7.62–7.77 (m, 1H, 5-C4H3S), 12.36 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.7, 19.0, 26.9, 29.0, 43.0, 60.5, 97.4, 128.1, 131.7, 133.4, 143.3, 171.6, 174.7, 195.0 ppm. IR (cm−1): 3091, 1737, 1715, 1652, 1333, 1216. APCI: m/z calculated for C14H17O4S [M + H]+ = 281.0848; found: 281.0838.
5) to obtain 97 mg (69%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, 3H, J = 7.15 Hz, OCH2CH3), 1.84–1.9 (m, 4H, CH2CH2CH2CH), 2.30–2.34 (m, 2H, COHCH2CH2), 3.96–4.02 (m, 2H, OCH2CH3), 4.24–4.27 (m, 1H, CH), 7.10–7.12 (m, 1H, 4-C4H3S), 7.59–7.61 (m, 1H, 3-C4H3S), 7.62–7.77 (m, 1H, 5-C4H3S), 12.36 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.7, 19.0, 26.9, 29.0, 43.0, 60.5, 97.4, 128.1, 131.7, 133.4, 143.3, 171.6, 174.7, 195.0 ppm. IR (cm−1): 3091, 1737, 1715, 1652, 1333, 1216. APCI: m/z calculated for C14H17O4S [M + H]+ = 281.0848; found: 281.0838.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 143 mg (84%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.68–1.77 (m, 4H, CH2CH2CH2CH), 2.31–2.45 (m, 2H, COHCH2CH2), 3.70 (s, 3H, OMe), 4.22–4.24 (m, 1H, CH), 7.26–7.31 (m, 1H, m-C6H4Br), 7.36–7.40 (m, 1H, m-C6H4Br), 7.52–7.54 (m, 1H, p-C6H4Br), 7.60–7.64 (m, 1H, o-C6H4Br), 12.36 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.4, 29.0, 45.3, 51.7, 96.6, 119.9, 127.3, 128.5, 131.6, 134.1, 141.2, 172.1, 174.9, 203.6 ppm. IR (cm−1): 3440, 1742, 1702, 1655, 1333, 1087. APCI: m/z calculated for C15H16BrO4 [M + H]+ = 339.0232; found: 239.0232.
20) to obtain 143 mg (84%) of a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 1.68–1.77 (m, 4H, CH2CH2CH2CH), 2.31–2.45 (m, 2H, COHCH2CH2), 3.70 (s, 3H, OMe), 4.22–4.24 (m, 1H, CH), 7.26–7.31 (m, 1H, m-C6H4Br), 7.36–7.40 (m, 1H, m-C6H4Br), 7.52–7.54 (m, 1H, p-C6H4Br), 7.60–7.64 (m, 1H, o-C6H4Br), 12.36 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.4, 29.0, 45.3, 51.7, 96.6, 119.9, 127.3, 128.5, 131.6, 134.1, 141.2, 172.1, 174.9, 203.6 ppm. IR (cm−1): 3440, 1742, 1702, 1655, 1333, 1087. APCI: m/z calculated for C15H16BrO4 [M + H]+ = 339.0232; found: 239.0232.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 153 mg (87%) of a colorless oil (mp = 92–94 °C, EtOAc). 1H NMR (400 MHz, CDCl3) δ: 1.20 (t, 3H, J = 7.17 Hz OCH2CH3), 1.63–1.81 (m, 4H, CH2CH2CH2CH) 2.32–2.44 (m, 2H, COHCH2CH2), 4.13–4.19 (m, 2H, 7.38) (m, 1H, m-C6H4Br), 7.54–7.56 (m, 1H, p-C6H4Br), 7.59–7.76 (m, 1H, o-C6H4Br), 12.46 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 18.3, 24.1, 28.9, 45.2, 60.6, 96.5, 119.8, 127.1, 128.3, 131.5, 133.9, 141.1, 169.4, 174.8, 203.4 ppm. IR (cm−1): 3064, 1703, 1651, 1617, 1304, 1086. APCI: m/z calculated for C16H18BrO3 [M + H]+ = 353.0388; found: 353.0352.
10) to obtain 153 mg (87%) of a colorless oil (mp = 92–94 °C, EtOAc). 1H NMR (400 MHz, CDCl3) δ: 1.20 (t, 3H, J = 7.17 Hz OCH2CH3), 1.63–1.81 (m, 4H, CH2CH2CH2CH) 2.32–2.44 (m, 2H, COHCH2CH2), 4.13–4.19 (m, 2H, 7.38) (m, 1H, m-C6H4Br), 7.54–7.56 (m, 1H, p-C6H4Br), 7.59–7.76 (m, 1H, o-C6H4Br), 12.46 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.2, 18.3, 24.1, 28.9, 45.2, 60.6, 96.5, 119.8, 127.1, 128.3, 131.5, 133.9, 141.1, 169.4, 174.8, 203.4 ppm. IR (cm−1): 3064, 1703, 1651, 1617, 1304, 1086. APCI: m/z calculated for C16H18BrO3 [M + H]+ = 353.0388; found: 353.0352.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 132 mg (86%) of a light-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.61–1.71 (m, 4H, CH2CH2CH2CH), 2.26–2.30 (m, 2H, COHCH2CH2), 3.63 (s, 3H, OMe), 4.16–4.17 (m, 1H, CH), 7.20–7.24 (m, 1H, m-C6H4NO2), 7.30–7.33 (m, 1H m-C6H4NO2), 7.46–7.48 (m, 1H, p-C6H4NO2), 7.55–7.57 (m, 1H, o-C6H4NO2), 12.30 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.3, 28.9, 45.3, 51.6, 96.6, 119.8, 127.3, 128.4, 131.6, 134.0, 141.1, 172.0, 174.9, 203.6 ppm. IR (cm−1): 3001, 1742, 1702, 1657, 1440. APCI: m/z calculated for C15H16NO6 [M − CO2Me]+ = 247.0845; found: 247.0862.
10) to obtain 132 mg (86%) of a light-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.61–1.71 (m, 4H, CH2CH2CH2CH), 2.26–2.30 (m, 2H, COHCH2CH2), 3.63 (s, 3H, OMe), 4.16–4.17 (m, 1H, CH), 7.20–7.24 (m, 1H, m-C6H4NO2), 7.30–7.33 (m, 1H m-C6H4NO2), 7.46–7.48 (m, 1H, p-C6H4NO2), 7.55–7.57 (m, 1H, o-C6H4NO2), 12.30 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.3, 28.9, 45.3, 51.6, 96.6, 119.8, 127.3, 128.4, 131.6, 134.0, 141.1, 172.0, 174.9, 203.6 ppm. IR (cm−1): 3001, 1742, 1702, 1657, 1440. APCI: m/z calculated for C15H16NO6 [M − CO2Me]+ = 247.0845; found: 247.0862.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 77 mg (50%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 0.98 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.62–1.79 (m, 4H, CH2CH2CH2CH), 2.31–2.37 (m, 2H, COHCH2CH2), 3.85 (s, 3H, OMe), 4.01–4.02 (m, 2H, OCH2CH3), 4.46–4.48 (m, 1H, CH), 7.08–7.11 (m, 1H, o-C6H4), 7.35–7.39 (m, 1H, o-C6H4), 7.49–7.50 (m, 1H, m-C6H4), 7.56–7.58 (m, 1H, p-C6H4), 12.42 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.8, 18.6, 26.1, 28.8, 41.0, 55.4, 60.4, 97.5, 112.9, 119.0, 120.7, 129.5, 137.8, 159.8, 171.6, 174.3, 201.8 ppm. IR (cm−1): 3074, 1738, 1716, 1682, 1329. APCI: m/z calculated for C17H21O5 [M + H]+ = 305.1389; found: 305.1392.
10) to obtain 77 mg (50%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 0.98 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.62–1.79 (m, 4H, CH2CH2CH2CH), 2.31–2.37 (m, 2H, COHCH2CH2), 3.85 (s, 3H, OMe), 4.01–4.02 (m, 2H, OCH2CH3), 4.46–4.48 (m, 1H, CH), 7.08–7.11 (m, 1H, o-C6H4), 7.35–7.39 (m, 1H, o-C6H4), 7.49–7.50 (m, 1H, m-C6H4), 7.56–7.58 (m, 1H, p-C6H4), 12.42 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.8, 18.6, 26.1, 28.8, 41.0, 55.4, 60.4, 97.5, 112.9, 119.0, 120.7, 129.5, 137.8, 159.8, 171.6, 174.3, 201.8 ppm. IR (cm−1): 3074, 1738, 1716, 1682, 1329. APCI: m/z calculated for C17H21O5 [M + H]+ = 305.1389; found: 305.1392.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 84 mg (61%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 0.88 (t, 3H, J = 7.17 Hz, OCH2CH3), 1.68–1.77 (m, 4H, CH2CH2CH2CH), 2.33–2.36 (m, 2H, COHCH2CH2), 3.96–4.01 (m, 2H, OCH2CH3), 5.12–5.15 (m, 1H, CH), 7.47–7.49 (m, 1H, 5-C5H4N), 7.82–7.84 (m, 1H, 3-C5H4N), 8.05–8.06 (m, 1H, 4-C5H4N), 8.69–8.71 (m, 1H, 6-C5H4N), 12.35 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.6, 19.0, 25.9, 29.0, 39.2, 60.2, 97.7, 122.3, 126.9, 136.9, 148.8, 152.7, 169.2, 174.2, 202.8 ppm. IR (cm−1): 3054.9, 1739, 1695, 1652, 1583, 1347. APCI: m/z calculated for C15H18NO4 [M + H]+ = 276.1236, found: 276.1200.
30) to obtain 84 mg (61%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 0.88 (t, 3H, J = 7.17 Hz, OCH2CH3), 1.68–1.77 (m, 4H, CH2CH2CH2CH), 2.33–2.36 (m, 2H, COHCH2CH2), 3.96–4.01 (m, 2H, OCH2CH3), 5.12–5.15 (m, 1H, CH), 7.47–7.49 (m, 1H, 5-C5H4N), 7.82–7.84 (m, 1H, 3-C5H4N), 8.05–8.06 (m, 1H, 4-C5H4N), 8.69–8.71 (m, 1H, 6-C5H4N), 12.35 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 13.6, 19.0, 25.9, 29.0, 39.2, 60.2, 97.7, 122.3, 126.9, 136.9, 148.8, 152.7, 169.2, 174.2, 202.8 ppm. IR (cm−1): 3054.9, 1739, 1695, 1652, 1583, 1347. APCI: m/z calculated for C15H18NO4 [M + H]+ = 276.1236, found: 276.1200.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 105 mg (76%) of a light-yellow oil. 1H NMR (400 MHz, DMSO) δ: 0.91 (t, 3H, J = 7.12 Hz, OCH2CH3), 1.55–1.72 (m, 4H, CH2CH2CH2CH), 2.25–2.43 (m, 2H, COHCH2CH2), 3.97–4.13 (m, 2H, OCH2CH3), 4.41–4.43 (m, 1H, CH), 7.22–7.39 (m, 1H, 5-C5H4N), 8.19–8.21 (m, 1H, 4-C5H4N), 8.72–8.73 (m, 1H, 6-C5H4N), 9.12–9.16 (m, 1H, 2-C5H4N), 12.29 (bs, 1H, OH) ppm. 13C NMR (400 MHz, DMSO) δ: 14.0, 18.7, 25.9, 28.8, 48.4, 60.7, 97.6, 124.5, 131.5, 136.2, 149.7, 153.9, 171.5, 174.5, 201.7 ppm. IR (cm−1): 3048, 1737, 1716, 1784, 1584, 1331. APCI: m/z calculated for C15H18NO4 [M + H]+ = 276.1236; found: 276.1204.
30) to obtain 105 mg (76%) of a light-yellow oil. 1H NMR (400 MHz, DMSO) δ: 0.91 (t, 3H, J = 7.12 Hz, OCH2CH3), 1.55–1.72 (m, 4H, CH2CH2CH2CH), 2.25–2.43 (m, 2H, COHCH2CH2), 3.97–4.13 (m, 2H, OCH2CH3), 4.41–4.43 (m, 1H, CH), 7.22–7.39 (m, 1H, 5-C5H4N), 8.19–8.21 (m, 1H, 4-C5H4N), 8.72–8.73 (m, 1H, 6-C5H4N), 9.12–9.16 (m, 1H, 2-C5H4N), 12.29 (bs, 1H, OH) ppm. 13C NMR (400 MHz, DMSO) δ: 14.0, 18.7, 25.9, 28.8, 48.4, 60.7, 97.6, 124.5, 131.5, 136.2, 149.7, 153.9, 171.5, 174.5, 201.7 ppm. IR (cm−1): 3048, 1737, 1716, 1784, 1584, 1331. APCI: m/z calculated for C15H18NO4 [M + H]+ = 276.1236; found: 276.1204.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 77 mg (53%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 1.61–1.93 (m, 4H, CH2CH2CH2CH), 2.28–2.40 (m, 2H, COHCH2CH2), 3.57 (s, 3H, CO2Me), 3.88 (s, 3H, OMe), 4.45–4.48 (m, 1H, CH), 6.93–6.98 (m, 2H, m-C6H4OMe), 7.94–8.00 (m, 2H, o-C6H4OMe), 12.35 (bs, 1H, OH) ppm. RMN 13C NMR (400 MHz, CDCl3) δ: 18.7, 26.6, 29.0, 40.7, 48.3, 58.7, 97.5, 113.9, 130.8, 163.5, 170.0, 174.6, 198.3 ppm. IR (cm−1): 3328, 1742, 1714, 1662, 1334. APCI: m/z calculated for C16H19NO5 [M + H]+ = 291.1232; found: 291.1251.
10) to obtain 77 mg (53%) of a white solid. 1H NMR (400 MHz, CDCl3) δ: 1.61–1.93 (m, 4H, CH2CH2CH2CH), 2.28–2.40 (m, 2H, COHCH2CH2), 3.57 (s, 3H, CO2Me), 3.88 (s, 3H, OMe), 4.45–4.48 (m, 1H, CH), 6.93–6.98 (m, 2H, m-C6H4OMe), 7.94–8.00 (m, 2H, o-C6H4OMe), 12.35 (bs, 1H, OH) ppm. RMN 13C NMR (400 MHz, CDCl3) δ: 18.7, 26.6, 29.0, 40.7, 48.3, 58.7, 97.5, 113.9, 130.8, 163.5, 170.0, 174.6, 198.3 ppm. IR (cm−1): 3328, 1742, 1714, 1662, 1334. APCI: m/z calculated for C16H19NO5 [M + H]+ = 291.1232; found: 291.1251.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 150 mg (78%) of a white solid (mp = 92–94 °C AcOEt) 1H NMR (400 MHz, CDCl3) δ: 1.66–1.76 (m, 4H, CH2CH2CH2CH), 2.02–2.35 (m, 2H, COHCH2CH2), 3.69 (s, 3H, OMe), 4.15–4.17 (m, 1H, CH), 6.03 (s, 2H, OCH2O), 7.03 (s, 1H, m-C6H2Br), 7.05 (s, 1H, o-C6H2Br), 12.32 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.6, 29.0, 45.0, 51.7, 96.7, 102.5, 108.5, 112.0, 114.2, 134.0, 147.3, 150.0, 172.0, 174.9, 202.5 ppm. IR (cm−1): 3384, 1716, 1699, 1662, 1328, 1037. APCI: m/z calculated for C16H16BrO6 [M + H]+ = 383.0130; found: 383.0155.
20) to obtain 150 mg (78%) of a white solid (mp = 92–94 °C AcOEt) 1H NMR (400 MHz, CDCl3) δ: 1.66–1.76 (m, 4H, CH2CH2CH2CH), 2.02–2.35 (m, 2H, COHCH2CH2), 3.69 (s, 3H, OMe), 4.15–4.17 (m, 1H, CH), 6.03 (s, 2H, OCH2O), 7.03 (s, 1H, m-C6H2Br), 7.05 (s, 1H, o-C6H2Br), 12.32 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.5, 24.6, 29.0, 45.0, 51.7, 96.7, 102.5, 108.5, 112.0, 114.2, 134.0, 147.3, 150.0, 172.0, 174.9, 202.5 ppm. IR (cm−1): 3384, 1716, 1699, 1662, 1328, 1037. APCI: m/z calculated for C16H16BrO6 [M + H]+ = 383.0130; found: 383.0155.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 123 mg (60%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.05 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.60 (s, 9H, Ot-Bu), 1.89–1.99 (m, 4H, CH2CH2CH2CH), 2.34–2.37 (m, 2H, COHCH2CH2), 3.99–4.14 (m, 2H, OCH2CH3), 4.28–4.30 (m, 1H, CH), 7.23–7.27 (m, 1H, 5-C8H5N), 7.30 (s, 1H, 3-C8H5N) 7.37–7.44 (m, 1H, 6-C8H5N), 7.62–7.65 (m, 1H, 4-C8H5N), 7.98–8.01 (m, 1H, 7-C8H5N), 12.45 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.0, 18.7, 25.8, 27.9, 29.0, 43.4, 60.5, 84.5, 96.7, 114.5, 115.7, 117.0, 122.5, 123.1, 127.3, 137.5, 138.9, 149.6, 169.2, 174.8, 193.5 ppm. IR (cm−1): 3053, 1736, 1679, 1652, 1613, 1321. APCI: m/z calculated for C15H18NO4 [M + H]+ = 414.1917; found: 414.1865.
20) to obtain 123 mg (60%) of a brown oil. 1H NMR (400 MHz, CDCl3) δ: 1.05 (t, 3H, J = 7.13 Hz, OCH2CH3), 1.60 (s, 9H, Ot-Bu), 1.89–1.99 (m, 4H, CH2CH2CH2CH), 2.34–2.37 (m, 2H, COHCH2CH2), 3.99–4.14 (m, 2H, OCH2CH3), 4.28–4.30 (m, 1H, CH), 7.23–7.27 (m, 1H, 5-C8H5N), 7.30 (s, 1H, 3-C8H5N) 7.37–7.44 (m, 1H, 6-C8H5N), 7.62–7.65 (m, 1H, 4-C8H5N), 7.98–8.01 (m, 1H, 7-C8H5N), 12.45 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.0, 18.7, 25.8, 27.9, 29.0, 43.4, 60.5, 84.5, 96.7, 114.5, 115.7, 117.0, 122.5, 123.1, 127.3, 137.5, 138.9, 149.6, 169.2, 174.8, 193.5 ppm. IR (cm−1): 3053, 1736, 1679, 1652, 1613, 1321. APCI: m/z calculated for C15H18NO4 [M + H]+ = 414.1917; found: 414.1865.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 99 mg (80%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.94–1.99 (m, 1H, CH2CH2CH), 2.44–2.58 (m, 3H, COCH2CH2CH), 3.75 (s, 3H, OMe), 3.89–3.92 (d, 1H, J = 9.39 Hz, COCHCO), 4.50–5.57 (m, 1H, CH2CHCO), 7.49–7.60 (m, 2H, m-C6H5), 7.61–7.62 (m, 1H, p-C6H5), 8.02–8.05 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 25.9, 37.9, 47.2, 53.0, 57.1, 128.8, 129.1, 134.0, 135.4, 168.6, 199.0, 209.1 ppm. IR (cm−1): 3053, 1748, 1724, 1678, 1321. APCI: m/z calculated for C13H11O3 [M + H]+ = 247.0970; found: 247.1008.
30) to obtain 99 mg (80%) of a translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.94–1.99 (m, 1H, CH2CH2CH), 2.44–2.58 (m, 3H, COCH2CH2CH), 3.75 (s, 3H, OMe), 3.89–3.92 (d, 1H, J = 9.39 Hz, COCHCO), 4.50–5.57 (m, 1H, CH2CHCO), 7.49–7.60 (m, 2H, m-C6H5), 7.61–7.62 (m, 1H, p-C6H5), 8.02–8.05 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 25.9, 37.9, 47.2, 53.0, 57.1, 128.8, 129.1, 134.0, 135.4, 168.6, 199.0, 209.1 ppm. IR (cm−1): 3053, 1748, 1724, 1678, 1321. APCI: m/z calculated for C13H11O3 [M + H]+ = 247.0970; found: 247.1008.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 68 mg (50%) of a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.49–1.62 (m, 2H, CH2CH2CH2CH), 1.95–2.70 (m, 4H, CH2CH2CH2CH), 2.63–2.73 (m, 2H, COCH2CH2), 3.64 (s, 3H, OMe), 4.01–4.03 (m, 1H, COCHCO), 4.39–4.41 (d, 1H, J = 10.18 Hz, CH2CHCO), 7.45–7.49 (m, 2H, m-C6H5), 7.56–7.57 (m, 1H, p-C6H5), 7.95–7.97 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 24.1, 26.9, 31.7, 43.2, 45.5, 52.6, 59.9, 128.6, 128.9, 133.5, 135.5, 170.0, 201.1, 207.6 ppm. IR (cm−1): 3061, 1742, 1702, 1679, 1322. APCI: m/z calculated for C16H19O4 [M + H]+ = 275.1283; found: 275.1294.
20) to obtain 68 mg (50%) of a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.49–1.62 (m, 2H, CH2CH2CH2CH), 1.95–2.70 (m, 4H, CH2CH2CH2CH), 2.63–2.73 (m, 2H, COCH2CH2), 3.64 (s, 3H, OMe), 4.01–4.03 (m, 1H, COCHCO), 4.39–4.41 (d, 1H, J = 10.18 Hz, CH2CHCO), 7.45–7.49 (m, 2H, m-C6H5), 7.56–7.57 (m, 1H, p-C6H5), 7.95–7.97 (m, 2H, o-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 24.1, 26.9, 31.7, 43.2, 45.5, 52.6, 59.9, 128.6, 128.9, 133.5, 135.5, 170.0, 201.1, 207.6 ppm. IR (cm−1): 3061, 1742, 1702, 1679, 1322. APCI: m/z calculated for C16H19O4 [M + H]+ = 275.1283; found: 275.1294.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 52 mg (38%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.51 (s, 3H, OCH3), 1.70–1.74 (m, 1H, CH2CH2C), 1.90–1.94 (m, 2H, CH2CH2CH2), 2.12–2.18 (m, 1H, CH2CH2C), 2.48–2.54 (m, 2H, COHCH2CH2), 3.39 (s, 3H, OMe), 7.32–7.36 (m, 2H, m-C6H5), 7.40–7.45 (m, 1H, p-C6H5), 7.81–7.83 (m, 2H, o-C6H5), 12.61 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.1, 24.9, 29.6, 34.8, 48.2, 51.1, 104.7, 128.3 (×2), 131.7, 136.7, 172.1, 172.8, 203.9 ppm. 3056, 1717, 1678, 1650, 1308. APCI: m/z calculated for C15H15O3 [M + H]+ = 275.1283; found: 275.1240.
20) to obtain 52 mg (38%) of a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.51 (s, 3H, OCH3), 1.70–1.74 (m, 1H, CH2CH2C), 1.90–1.94 (m, 2H, CH2CH2CH2), 2.12–2.18 (m, 1H, CH2CH2C), 2.48–2.54 (m, 2H, COHCH2CH2), 3.39 (s, 3H, OMe), 7.32–7.36 (m, 2H, m-C6H5), 7.40–7.45 (m, 1H, p-C6H5), 7.81–7.83 (m, 2H, o-C6H5), 12.61 (bs, 1H, OH) ppm. 13C NMR (400 MHz, CDCl3) δ: 18.1, 24.9, 29.6, 34.8, 48.2, 51.1, 104.7, 128.3 (×2), 131.7, 136.7, 172.1, 172.8, 203.9 ppm. 3056, 1717, 1678, 1650, 1308. APCI: m/z calculated for C15H15O3 [M + H]+ = 275.1283; found: 275.1240.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain 40 mg (39%) of translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, 3H, J = 3.17 Hz, OCH2CH3), 1.56–1.78 (m, 4H, CH2CH2CH2CH), 2.11–2.14 (m, 1H, COHCH2CH2), 2.46–2.50 (m, 1H, COHCH2CH2), 2.52–2.54 (m, 1H, CCH2CH), 3.06–3.07 (m, 1H, CCH2CH), 3.984.00 (m, 1H, CH), 4.02–4.22 (m, 2H, OCH2CH3), 5.93–5.95 (m, 1H, CCHCH2), 7.31–7.35 (m, 5H, C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 21.5, 26.3, 38.3, 39.3, 51.5, 61.7, 65.8, 125.0, 126.3, 127.4, 128.4, 135.3, 144.4, 172.1, 208.7. IR (cm−1): 2945, 1709, 1676, 1315. APCI: m/z calculated for C18H21O3 [M + H]+ = 285.1491; found: 285.1467.
30) to obtain 40 mg (39%) of translucent yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, 3H, J = 3.17 Hz, OCH2CH3), 1.56–1.78 (m, 4H, CH2CH2CH2CH), 2.11–2.14 (m, 1H, COHCH2CH2), 2.46–2.50 (m, 1H, COHCH2CH2), 2.52–2.54 (m, 1H, CCH2CH), 3.06–3.07 (m, 1H, CCH2CH), 3.984.00 (m, 1H, CH), 4.02–4.22 (m, 2H, OCH2CH3), 5.93–5.95 (m, 1H, CCHCH2), 7.31–7.35 (m, 5H, C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 14.1, 21.5, 26.3, 38.3, 39.3, 51.5, 61.7, 65.8, 125.0, 126.3, 127.4, 128.4, 135.3, 144.4, 172.1, 208.7. IR (cm−1): 2945, 1709, 1676, 1315. APCI: m/z calculated for C18H21O3 [M + H]+ = 285.1491; found: 285.1467.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 82 mg (88%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.40–1.44 (m, 1H, CH2CH2CH2), 1.62–1.74 (m, 1H, CH2CH2CH2), 1.75–1.77 (m, 2H, CH2CH2CH), 1.81 (s, 3H, COCH3), 2.36–2.39 (m, 2H, COCH2CH2), 2.84–2.85 (m, 1H, CHCH2C), 2.97–2.99 (m, 1H, CHCH2C), 3.12–3.15 (m, 1H, CH), 3.48–3.50 (m, 1H, CH), 7.12–7.15 (m, 2H, m-C6H5), 7.32–7.37 (m, 3H, o,p-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 22.4, 26.2, 29.8, 35.3, 39.4, 48.7, 53.7, 127.8, 128.4, 128.6, 136.2, 138.4, 155.1, 198.8, 212.4 ppm. IR (cm−1): 1348, 1682, 1704, 1720, 2939. APCI: m/z calculated for C17H19O2 [M + H]+ = 255.1385; found: 255.1379.
20) to obtain 82 mg (88%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.40–1.44 (m, 1H, CH2CH2CH2), 1.62–1.74 (m, 1H, CH2CH2CH2), 1.75–1.77 (m, 2H, CH2CH2CH), 1.81 (s, 3H, COCH3), 2.36–2.39 (m, 2H, COCH2CH2), 2.84–2.85 (m, 1H, CHCH2C), 2.97–2.99 (m, 1H, CHCH2C), 3.12–3.15 (m, 1H, CH), 3.48–3.50 (m, 1H, CH), 7.12–7.15 (m, 2H, m-C6H5), 7.32–7.37 (m, 3H, o,p-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 22.4, 26.2, 29.8, 35.3, 39.4, 48.7, 53.7, 127.8, 128.4, 128.6, 136.2, 138.4, 155.1, 198.8, 212.4 ppm. IR (cm−1): 1348, 1682, 1704, 1720, 2939. APCI: m/z calculated for C17H19O2 [M + H]+ = 255.1385; found: 255.1379.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain 31 mg (32%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.51–1.61 (m, 1H), 1.71–1.80 (m, 2H), 2.02–2.10 (m, 2H), 2.24–2.37 (m, 1H), 2.47–2.50 (m, 2H), 2.61–2.65 (m, 2H), 2.84–2.89 (m, 1H), 3.63–3.70 (m, 1H, CH), 3.88–3.94 (m, 1H, CH), 7.39–7.43 (m, 2H, m-C6H5), 7.99–8.02 (m, 3H, o,p-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 24.0, 27.8, 28.9, 39.1, 44.7, 53.3, 54.9, 59.3, 128.5, 129.5, 130.5, 132.1, 137.6, 151.2, 200.5, 212.3 ppm. IR (cm−1): 1335, 1590, 1686, 2951. APCI: m/z calculated for C17H19O2 [M + H]+ = 267.1385; found: 267.1349.
20) to obtain 31 mg (32%) of translucent oil. 1H NMR (400 MHz, CDCl3) δ: 1.51–1.61 (m, 1H), 1.71–1.80 (m, 2H), 2.02–2.10 (m, 2H), 2.24–2.37 (m, 1H), 2.47–2.50 (m, 2H), 2.61–2.65 (m, 2H), 2.84–2.89 (m, 1H), 3.63–3.70 (m, 1H, CH), 3.88–3.94 (m, 1H, CH), 7.39–7.43 (m, 2H, m-C6H5), 7.99–8.02 (m, 3H, o,p-C6H5) ppm. 13C NMR (400 MHz, CDCl3) δ: 24.0, 27.8, 28.9, 39.1, 44.7, 53.3, 54.9, 59.3, 128.5, 129.5, 130.5, 132.1, 137.6, 151.2, 200.5, 212.3 ppm. IR (cm−1): 1335, 1590, 1686, 2951. APCI: m/z calculated for C17H19O2 [M + H]+ = 267.1385; found: 267.1349.Conclusions
We utilized a cascade process to obtain tricarbonyl compounds by adding anions of cyanocarbonates derived from aromatic aldehydes onto 5, 6, and 7-membered cycloalkenones. During the process, we exploited the dual role of cyanocarbonates: as “latent” acylcarbanions and as acylating reagents. The synthetic potential of the cascade products obtained was successfully demonstrated by forming bicyclic and tricyclic systems through intramolecular condensation reactions. These results suggest that tricarbonyl compounds can be used as scaffolds to generate structural diversity. The investigation of further transformations is currently underway and will be published in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Facultad de Química, UNAM for financial support (Grant PAIP 50009062). We thank Professor D. E. Ward for revising the manuscript, and for helpful suggestions. We also thank Rosa Isela del Villar, Nayeli López, Georgina Duarte, and Marisela Gutierrez for recording NMR, MS, and IR spectra. DM thanks CONACYT for Graduate Scholarship.Notes and references
- G. Stork and L. A. Maldonado, J. Am. Chem. Soc., 1971, 93, 5286–5287 CrossRef CAS.
- R. J. H. Gregory, Chem. Rev., 1999, 99, 3649–3682 CrossRef CAS PubMed.
- J. D. Albright, Tetrahedron, 1983, 39, 3207–3233 CrossRef CAS.
- (a) G. Stork and L. Maldonado, J. Am. Chem. Soc., 1974, 96, 5273–5274 Search PubMed; (b) G. Stork, J. C. Depezay and J. DAngelo, Tetrahedron Lett., 1975, 389–392 CrossRef CAS; (c) J. Tsuji, J. Am. Chem. Soc., 1981, 103, 5259–5526 CrossRef; (d) G. Stork and T. Takahashi, J. Am. Chem. Soc., 1977, 99, 1275–1276 CrossRef CAS; (e) G. Stork, T. Takahashi, I. Kawamoto and T. Suzuki, J. Am. Chem. Soc., 1978, 100, 8272–8273 CrossRef CAS; (f) T. Takahashi, K. Kitamura, H. Nemoto, J. Tsuji and I. Miura, Tetrahedron Lett., 1983, 24, 3489–3492 CrossRef CAS; (g) T. Takahashi, H. Iwamoto, K. Nagashima, T. Okabe and T. Doi, Angew. Chem., Int. Ed., 1997, 36, 1319–1321 CrossRef CAS; (h) O. S. Park, H. J. Hwang and W. Y. Lee, Arch. Pharmacal Res., 1993, 16, 205–208 CrossRef CAS; (i) A. Kende, K. Liu, I. Kaldor, G. Dorey and K. Koch, J. Am. Chem. Soc., 1995, 117, 8258–8270 CrossRef CAS; (j) I. Kadota, Y. Hu, G. K. Packard and S. D. Rychnovsky, Proc. Natl. Acad. Sci. U. S. A., 2004, 101(33), 11992–11995 CrossRef CAS PubMed; (k) E. H. Granados-Covarrubias and L. A. Maldonado, J. Org. Chem., 2009, 74, 5097–5099 CrossRef CAS.
- (a) A. T. Au, Synth. Commun., 1984, 14, 743–748 CrossRef CAS , For recent methodologies, see:; (b) H. M. Torres-Domínguez, L. A. Maldonado and R. Le Lagadec, Tetrahedron Lett., 2020, 61, 151414–151417 CrossRef; (c) S. Aoki, S. Kotani, M. Sugiura and M. Nakajima, Tetrahedron Lett., 2010, 51, 3547–3549 CrossRef CAS.
- (a) L. F. Tietze and A. Modi, Med. Res. Rev., 2000, 20(4), 304–322 CrossRef CAS; (b) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS; (c) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993–3009 RSC; (d) J. Poulin, C. M. Grisé-Bard and L. Barriault, Chem. Soc. Rev., 2009, 38, 3092–3101 RSC; (e) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed; (f) H. Pellissier, Chem. Rev., 2013, 113(1), 442–524 CrossRef CAS; (g) S. Zhi, S. Sanjun, X. Ma and W. Zhang, Org. Biomol. Chem., 2019, 17, 7632–7650 RSC.
- H. M. Torres-Domínguez, L. A. Maldonado and R. Le Lagadec, Synth. Commun., 2017, 47, 1250–1255 CrossRef.
- (a) A. Sharma, J. Pandey and R. P. Tripathi, Tetrahedron Lett., 2009, 50, 1812–1816 CrossRef CAS; (b) T. Venkatesh, P. S. Mainkar and S. Chandrasekhar, J. Org. Chem., 2021, 86, 5412–5416 CrossRef CAS; (c) K. Asahi and H. Nishino, Tetrahedron, 2008, 64, 1620–1634 CrossRef CAS; (d) S. R. Lima and F. Coelho, ACS Omega, 2020, 5, 8032–8045 CrossRef CAS , and references therein.
- (a) F. W. Sum and L. Weiler, Tetrahedron, 1981, 37, 303–317 CrossRef; (b) B. Lygo, N. Oconnor and P. R. Wilson, Tetrahedron, 1988, 44, 688–6888 Search PubMed; (c) S. Benetti, R. Romagnoli, C. De Risi, G. Spalluto and V. Zanirato, Chem. Rev., 1995, 95, 1065–1114 CrossRef CAS; (d) C. Simon, C. Thierry and J. Jean Rodriguez, Eur. J. Org. Chem., 2004, 4957–4980 CrossRef CAS; (e) D. Bonne, Y. Coquerel, T. Constantieux and J. Rodriguez, Tetrahedron: Asymmetry, 2010, 21, 1085–1109 CrossRef CAS; (f) T. Govender, P. I. Arvidsson, G. E. M. Maguire, H. G. Kruger and T. Naicker, Chem. Rev., 2016, 116, 9375–9437 CrossRef CAS; (g) Y. S. Rao, Chem. Rev., 1976, 76, 625–694 CrossRef CAS.
- (a) V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, RSC Adv., 2015, 5, 15233–15266 RSC; (b) S. Khaghaninejad and M. M. Heravi, Adv. Heterocycl. Chem., 2014, 111, 95–146 CrossRef CAS; (c) T. J. Donohoe and R. D. C. Pullin, Chem. Commun., 2012, 48, 11924–11938 RSC; (d) C. Schmuck and D. Rupprecht, Synthesis, 2007, 3095–3110 CrossRef CAS.
- S. P. Kolis, M. T. Clayton, J. L. Grutsch and M. M. Faul, Tetrahedron Lett., 2003, 44, 5707–5710 CrossRef CAS.
- B. Thapa and H. Schlegel, J. Phys. Chem. A, 2016, 120, 5726–5735 CrossRef CAS.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- S. J. Grimme, Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS.
- (a) S. Dhers, A. Mondal, D. Aguilà, J. Ramírez, S. Vela, P. Dechambenoit, M. Rouzières, J. R. Nitschke, R. Clérac and J. M. Lehn, J. Am. Chem. Soc., 2018, 140, 8218–8227 CrossRef CAS PubMed; (b) M. A. Rosero-Mafla, J. I. Castro, N. E. Sánchez, C. A. Mujica-Martinez and M. N. Chaur, ChemistrySelect, 2020, 5, 7685–7694 CrossRef CAS.
- (a) C. D. Johnson, The Hammett Equation, Cambridge University Press, Cambridge, 1973 Search PubMed; (b) C. Hansch, A. Leo and R. Tatf, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- A. Kuźnik, R. Mazurkiewicz and B. Fryczkowska, Beilstein J. Org. Chem., 2017, 13, 2710–2738 CrossRef PubMed.
- A. Srikrishn and D. H. Dethe, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2012, 51(2), 345–355 Search PubMed.
- P. Klahn, A. Duschek, C. Liébert and S. F. Kirsch, Org. Lett., 2012, 14, 1250–1253 CrossRef CAS PubMed.
- A. E. Cotman, B. Modec and B. Mohar, Org. Lett., 2018, 20, 2921–2924 CrossRef CAS PubMed.
- C. Zhang, H. Jiang and S. Zhu, Chem. Commun., 2017, 53, 2677–2680 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05187c |
| This journal is © The Royal Society of Chemistry 2021 |