Open Access Article
Open Access ArticleActive and passive adsorption of 47 trace atmospheric volatile organic compounds onto carbon nanotubes
Gwendeline K. S. Wonga and
Richard D. Webster *ab
*ab
aDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Singapore. E-mail: webster@ntu.edu.sg
bNEWRI-ECMG, Nanyang Environment & Water Research Institute, 1 Cleantech Loop, CleanTech One, #06-08, Singapore 637141, Singapore
First published on 7th September 2021
Abstract
Carbon nanotubes (CNTs) of varying sizes and CNTs functionalised with carboxylic acids were examined by thermal desorption gas chromatography mass spectrometry (TD-GCMS) to determine the degree of surface contamination of atmospheric volatile organic compounds (VOCs). The CNTs could be purged of physisorbed VOCs by heating to 380 °C under a stream of purified nitrogen gas. As soon as the cleaned CNTs were exposed to atmospheric air they spontaneously adsorbed trace VOCs. As well as passive adsorption of VOCs, active sampling was carried out by pumping atmospheric air through the CNTs and comparing the results with the standard multisorbent materials Carbopack X and Tenax that are used widely for VOC trapping and analysis. The CNTs were found to trap many VOCs at a comparable level to the standard sorbent materials. Therefore, to maintain the CNTs in a pristine condition, it is recommended that they are first heated under vacuum to remove residual physisorbed VOCs, and then stored under vacuum or in a purified inert gas atmosphere.
Introduction
Carbon nanotubes (CNTs) have been proposed for use in many applications including wastewater treatment,1 hydrogen adsorption,2 and antimicrobial and anti-adhesive functions in medicine.3 These applications all rely on the specific surface properties of the CNTs promoting physisorption processes. Several studies have also shown that CNTs are excellent sorbents for organic species, and have been used in solid phase extraction, chromatographic analysis, and sensor development,4–23 although to the best of our knowledge there are few reports that examine adsorption of volatile organic compounds (VOCs) at their trace atmospheric level (ppb (v/v)).24–26 This leads to concerns whether gaseous VOCs that are naturally present in the atmosphere could spontaneously be introduced into the CNTs during their storage and transportation, thereby affecting their desirable surface features as well as influencing their toxicological properties. Adsorbed VOCs can potentially become involved in chemical reactions, generating unwanted side products, affecting reaction rates, or acquiring false positives or inaccurate data for CNTs when used in other applications. It has already been extensively proven that many of the proposed catalytic effects of CNTs are instead caused by trace metallic impurities,27–29 therefore, trace organic adsorbents could also have deleterious effects on the surface properties of the CNTs.Sensitive microscopy techniques such as SEM and TEM imaging of CNTs will not detect additional adsorbed organic molecules due to their small size and likely monolayer coverages. However, it is important to be able to identify any trapped organic impurities and interferences on CNTs after being subjected to exposure for a known duration of time under ambient conditions. There have been no studies aimed at determining the adsorption effects of naturally present atmospheric organic impurities at trace levels and comparing the results with standard multisorbents used for air quality testing, which have the highest levels of trapping performance and therefore, provide a good means of assessing the likely adulteration by unwanted VOCs. Therefore, in this study, gas chromatography mass spectrometry (GC-MS) coupled with thermal desorption (TD) trapping experiments were performed to determine the extent of contamination of CNTs with 47 trace atmospheric VOCs under passive and active adsorption conditions to determine which atmospheric VOCs are more readily adsorbed, with the results compared with the standard Carbopack X/Tenax multisorbent. The study also investigated how the VOCs can be removed from the CNTs, and the required storage procedures for maintaining the CNTs in pristine condition.
Methods
Materials and chemicals
CNTs were purchased from Nanostructured & Amorphous Materials Inc. (Houston, Texas) and were purified using high temperature conditioning to remove adsorbed VOCs. The dimensions and purities of the CNTs as supplied by the manufacturer are given in Table 1. Thermogravimetric analysis (TGA), Raman spectroscopy and inductively coupled plasma-mass spectrometry (ICP-MS) characterisation of the CNTs have been reported previously.24 VOC standards used for the analysis were obtained from Sigma-Aldrich (St Louis, USA), Merck (Hohenbrunn, Germany), Alfa Aesar (Heysham, Lancaster, UK) and Fluka (Buchs, Switzerland) with purities not less than 97%, except for the following compounds: 1,2,3-trimethylbenzene (93.9%) from Fluka and methacrolein (95%) from Sigma-Aldrich. The neat chemicals were diluted with the appropriate amount of methanol (Schedelco, Malaysia) to prepare (by serial dilutions) VOC standards solution for analysis.| Sorbent | Reported CNT physical dimensions and surface areas | Reported CNT purity/% | Reported wt% COOH | TD tube packed mass/mg | Residual artefacts present after thermal conditioning/ng | ||
|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Hexane | |||||
| a N.A. = not applicable. | |||||||
| sSWCNT | Diameter: 1–2 nm | >95 | N.A. | 75 | 1.07 | 0 | 0.17 |
| Length: 1–3 μm | |||||||
| Surface areas: 300–380 m2 g−1 | |||||||
| SWCNT | Diameter: 1–2 nm | >90 | N.A. | 75 | 1.41 | 0.11 | 0.81 |
| COOH-SWCNT | Length: 5–30 μm | >95 | 2.59–2.87 | 75 | 0.67 | 0 | 0.13 |
| Surface areas: 300–380 m2 g−1 | |||||||
| MWCNT | Outer diameters: 50–80 nm | 95 | N.A. | 100 | 1.71 | 0 | 2.20 |
| Inner diameters: 5–15 nm | |||||||
| COOH-MWCNT | Length: 10–20 mm | 95 | 0.47–0.51 | 100 | 1.78 | 0 | 0.52 |
| Surface areas: 60–80 m2 g−1 | |||||||
Procedures and instrumentation for TD-GCMS experiments
Stainless steel sorbent tubes (89 mm length × 6.4 mm outer diameter) that were pre-packed with 100 mg Carbopack X and 200 mg Tenax TA were obtained from Markes International Limited, Llantrisant, UK. The CNTs for analysis were packed into empty stainless sorbent tubes using a Markes Gauze Loading Rig and Plunger. Due to the higher volumes occupied by the CNTs, the mass of each CNT that could be packed into in the tube was less than for the standard Carbopack X/Tenax multisorbent and was between 75–100 mg (Table 1). The CNTs inside the sorbent tubes were preconditioned to remove existing adsorbed VOCs by heating to 380 °C for up to 20 hours using a Markes tube conditioning dry purge unit (TC-20) under a 70 mL min−1 flow of nitrogen gas. Thermal gravimetric analysis indicated that the CNTs did not undergo any decomposition at this temperature. Atmospheric air samples of the sorbent packed tubes were obtained using a calibrated SKC pocket air pump 210–1002, USA.Thermal desorption experiments were conducted using a Markes UNITY Series 2 heating/trapping unit attached to a Markes Ultra TD-100 autosampler. The autosampler transported the sorbent containing tubes into the UNITY primary desorption compartment that was joined in series to a Peltier cooled Tenax TA trap set at −10 °C, which was used for secondary desorption. Initial desorption involved heating the sorbent containing tubes to 375 °C for CNT sorbents or 280 °C for the Carbopack X/Tenax multisorbent for 10 minutes under a 45 mL min−1 flow of helium (99.999%), causing the desorbed VOCs to be transferred onto the Tenax TA cold trap. Secondary desorption involved heating the pre-concentrated VOCs on the Tenax TA trap to 300 °C for 7 minutes under a reversed flow of 6 mL min−1 helium via 1/6 split mode to transfer the analytes into an Agilent 7890A gas chromatograph.
For separation of the analytes, an Agilent J & W DB-VRX (122–1564, 260 °C, 60 m × 250 μm × 1.4 μm, 1219.45766) column was utilised with helium carrier gas at a flow rate of 1.5 mL min−1. The oven temperature for the GC was maintained at 30 °C for 12 minutes, increased to 60 °C over one minute, then increased at a rate of 40 °C per minute until 124 °C. The oven was isothermal at 124 °C for 2 minutes then raised at 9 °C per minute up to 200 °C and then isothermal at 200 °C for a further 3 minutes. Compounds exiting the column were detected using a 70 eV electron impact ionization Agilent Inert 5975C mass spectrometer with the ion source isothermal at 230 °C and the quadrupole mass analyzer maintained at 150 °C. Scans were performed over a mass range of 35–350 amu utilising scan mode. Origin Pro 8.1 and Microsoft Excel for Microsoft 365 MSO were used for statistical calculations.
Results and discussion
Passive sampling of VOCs
Experiments were first conducted to cleanse the CNTs of pre-adsorbed VOCs using 5 sorbent tubes each containing a nanomaterial: a multi-walled CNT (MWCNT), a carboxylated derivative of the MWCNT (COOH-MWCNT), a single-walled CNT (SWCNT), a functionalized SWCNT (COOH-SWCNT) and a SWCNT with shorter length (sSWCNT). The physical dimensions and residual organic artefacts of the commercially available nanomaterials are summarized in Table 1. During the optimization of the CNT conditioning procedures to achieve acceptable CNT blanks prior to exposure, the total ion current (TIC) chromatograms revealed that multiple VOCs were adsorbed during prolonged storage and required numerous hours of thermal conditioning to be desorbed from the CNT materials.It was found that a temperature of 380 °C under a constant flow of purified nitrogen gas for 20 hours was required to optimally clean the CNTs of residual VOCs. However, benzene and hexane were found in all CNT blank chromatograms and toluene was also detected in the SWCNT sorbents even after optimum conditioning of the sorbent tubes (Table 1). This indicates that these molecules are likely to be always present as artefacts in the CNTs and cannot be removed through the high temperature conditioning processes. The carbon-containing compounds benzene and toluene are employed throughout catalytic CVD processes to produce CNTs consisting of graphene sheets.30 These reagents may remain as residual impurities within the synthesised CNTs or may have been produced during the elevated temperature conditioning process. Carboxylated CNTs were found to contain lesser quantities of hexane in comparison to the non-carboxylated analogues, with the most hexane detected in the MWCNT. This is possibly associated with hexane having a higher isosteric heat of adsorption on non-polar (i.e., non-carboxylated) CNTs, that results in stronger adsorbent–adsorbate interactions,31,32 making it more difficult to desorb hexane from the non-functionalized of CNTs.
The thermally conditioned CNTs inside the sorbent tubes were then placed uncapped on the bench in an analytical laboratory and left exposed to atmospheric air for 72 hours. TD-GCMS analysis was performed for all CNT tubes to qualitatively identify the VOCs that were adsorbed. Mass spectrums of 47 VOC standards were used to determine the relative abundance of qualifier and quantifier ions with respect to the base ion for each compound. The 47 standards were selected based on pre-existing knowledge of background VOCs present in the atmosphere in Singapore.33 Qualitative identification was performed by matching the relative abundance of qualifier ions and retention times (tR) of unknowns to the standards. Table 2 summarizes the presence or absence of VOCs that were adsorbed onto the different CNTs, their tR's and the relative abundance ratios of various ions with respect to the base ion. Representative chromatograms of the desorbed contents of the sorbent tubes after 72 hours of ambient air exposure are shown in Fig. 1.
| Target analytes | Qualifier ionsa | tRb (min) | VOCs detected in CNT sorbents | |||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | MWCNT | COOH-MWCNT | SWCNT | COOH-SWCNT | sSWCNT | ||
| a Identity and () relative abundance of qualifier ions.b Retention times of the analytes. | ||||||||
| Isopropyl alcohol | 43 (17) | 59 (5) | 8.21 | ✗ | ✗ | ✓ | ✗ | ✗ |
| Ethyl ether | 45 (65) | 73 (12) | 8.8 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Isoprene | 68 (69) | 53 (54) | 9.11 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Dichloromethane | 49 (90) | 86 (65) | 10.27 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2-Methylpentane | 43 (100) | 42 (53) | 13.01 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methacrolein | 41 (84) | 39 (73) | 13.25 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3-Methylpentane | 56 (87) | 41 (52) | 13.63 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hexane | 41 (60) | 43 (51) | 14.21 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2-Butanone | 43 (100) | 57 (8) | 14.26 | ✗ | ✗ | ✓ | ✗ | ✓ |
| Trichloromethane | 85 (67) | 47 (17) | 14.7 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ethyl acetate | 61 (19) | 70 (15) | 14.79 | ✗ | ✗ | ✓ | ✓ | ✓ |
| Methylcyclopentane | 69 (48) | 41 (42) | 15.05 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cyclohexane | 56 (95) | 41 (43) | 15.98 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Benzene | 77 (22) | 51 (12) | 16.16 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Heptane | 43 (100) | 57 (64) | 16.89 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Trichloroethylene | 132 (97) | 134 (31) | 16.97 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methyl methacrylate | 41 (85) | 39 (46) | 17.27 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Methyl cyclohexane | 55 (61) | 98 (46) | 17.58 | ✓ | ✓ | ✓ | ✗ | ✓ |
| Methyl isobutyl ketone | 58 (48) | 85 (25) | 17.95 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Pyridine | 52 (47) | 51 (21) | 18.1 | ✗ | ✗ | ✗ | ✗ | ✗ |
| 2-Methylheptane | 43 (78) | 70 (26) | 18.37 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Toluene | 92 (64) | 65 (10) | 18.7 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1-Octene | 41 (77) | 70 (90) | 18.88 | ✗ | ✗ | ✗ | ✓ | ✓ |
| Octane | 85 (71) | 57 (49) | 19.04 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hexanal | 57 (71) | 72 (33) | 19.14 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tetrachloroethylene | 164 (77) | 129 (65) | 19.58 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Furfural | 95 (91) | 39 (33) | 19.95 | ✗ | ✗ | ✓ | ✓ | ✓ |
| Ethylbenzene | 106 (38) | 77 (8) | 20.63 | ✓ | ✓ | ✓ | ✓ | ✓ |
| m,p-Xylene | 106 (56) | 77 (12) | 20.86 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Nonane | 43 (91) | 85 (48) | 21 | ✗ | ✗ | ✓ | ✓ | ✓ |
| Heptanal | 55 (66) | 57 (55) | 21.16 | ✗ | ✗ | ✓ | ✓ | ✓ |
| Styrene | 103 (46) | 78 (37) | 21.29 | ✓ | ✓ | ✓ | ✓ | ✓ |
| o-Xylene | 106 (54) | 105 (21) | 21.39 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Phenol | 66 (24) | 65 (20) | 22.38 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3-Ethyltoluene | 120 (42) | 91 (14) | 22.6 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4-Ethyltoluene | 120 (39) | 91 (12) | 22.68 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Benzaldehyde | 106 (97) | 77 (87) | 22.74 | ✗ | ✗ | ✓ | ✓ | ✓ |
| 1,3,5-Trimethylbenzene | 120 (62) | 91 (11) | 22.85 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Decane | 43 (74) | 71 (45) | 22.9 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2-Ethyltoluene | 120 (42) | 91 (13) | 23.05 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Octanal | 43 (94) | 57 (94) | 23.13 | ✗ | ✗ | ✗ | ✓ | ✗ |
| Benzonitrile | 76 (32) | 50 (10) | 23.18 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1,2,4-Trimethylbenzene | 120 (59) | 91 (11) | 23.41 | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1,2,3-Trimethylbenzene | 120 (51) | 91 (10) | 24.03 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Acetophenone | 77 (66) | 120 (27) | 24.82 | ✓ | ✓ | ✓ | ✗ | ✓ |
| Nonanal | 41 (70) | 70 (40) | 25.03 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Decanal | 41 (81) | 70 (58) | 27.03 | ✗ | ✗ | ✗ | ✗ | ✗ |
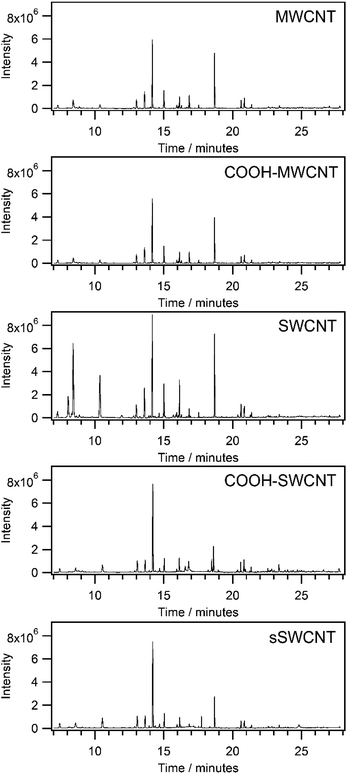 | ||
| Fig. 1 Total ion current chromatograms of atmospheric VOCs adsorbed on different CNT sorbents that had been left inside thermal desorption tubes in ambient air for 72 hours. | ||
Many VOCs in the laboratory air were found to be adsorbed on the CNT materials during the 72 hours of exposure. A total of 33 VOCs were detected in the MWCNTs and between 37 to 40 compounds detected in the SWCNTs. The most visible signals present in all chromatograms belonged to 2-methylpentane, 3-methylpentane, hexane, benzene and toluene. VOCs that were adsorbed on some but not all CNTs were generally alkenes, carbonyl compounds and alcohols except for methyl cyclohexane and nonane.
The preliminary data showed that many organic compounds were retained on the CNTs during exposure to air. As a result, they could potentially participate in chemical reactions. With the garnering interest in utilizing CNTs as a reaction vessel or as a catalyst-support in chemical reactions, it is important to investigate methods of proper containment, transport and purification to simultaneously reduce inorganic and organic contaminants prior to their actual application.34,35
Active sampling of VOCs
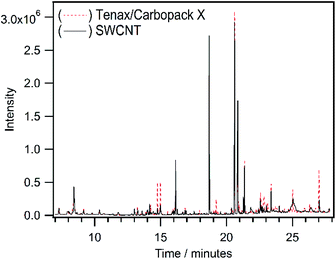
The ability of CNTs to adsorb and desorb gaseous organic compounds during the exposure to ambient air demonstrates the potential of these nanomaterials for trapping these molecules via passive diffusion. To evaluate the possibility of active sampling, air samples were collected using the tube containing the SWCNTs because it showed the highest amounts of VOCs trapped amongst all the nano-sorbents (Fig. 1 and Table 2). A conventional tube containing the widely used commercial Carbopack X/Tenax multisorbent was utilized with which to compare the performance of the SWCNTs for trapping VOCs. Each sorbent tube was connected to a portable air pump and placed on the rooftop of the School of Physical and Mathematical Sciences (SPMS) building in the host University. The air flow was calibrated to 20 mL min−1, with 2.4 L of air acquired after 2 hours with the sorbent tubes and then analysed using TD-GCMS. The overlaid total ion current chromatograms of the 2 sorbent tubes after sampling are shown in Fig. 2. Peak area ratios for each VOC detected were calculated using eqn (1) and are summarized in Table 3 to enable a comparison of the trapping of VOCs by the SWCNTs with respect to the standard commercially available Carbopack X/Tenax. Ratios > 1 for eqn (1) indicate that the SWCNTs are more efficient than the Carbopack X/Tenax multisorbent in trapping the particular VOC.
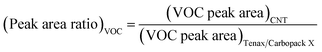 | (1) |
| Functional group | VOC | Normalised peak area ratio |
|---|---|---|
| a 0 represents not detected in the SWCNT while N.A. represents the absence in both SWCNT and Carbopack X/Tenax. | ||
| Alcohol | Isopropyl alcohol | 0 |
| Ether | Ethyl ether | N.A. |
| Alkene | Isoprene | 0 |
| 1-Octene | N.A. | |
| Alkane | 2-Methylpentane | 1.22 |
| 3-Methylpentane | 1.11 | |
| Hexane | 1.32 | |
| Methylcyclopentane | 0.96 | |
| Cyclohexane | 1.08 | |
| Heptane | 0.66 | |
| Methyl cyclohexane | 1.07 | |
| 2-Methylheptane | N.A. | |
| Octane | 0.36 | |
| Nonane | 0.22 | |
| Decane | N.A. | |
| Halogenated alkanes | Dichloromethane | 1.53 |
| Trichloromethane | N.A. | |
| Halogenated alkenes | Trichloroethylene | 1.18 |
| Tetrachloroethylene | 1.13 | |
| Carbonyl compounds | 2-Butanone | 0.2 |
| Methyl isobutyl ketone | 0 | |
| Hexanal | 0 | |
| Heptanal | N.A. | |
| Octanal | 0 | |
| Nonanal | 0 | |
| Decanal | 0.33 | |
| Ethyl acetate | 0.22 | |
| Aromatic compounds | Benzene | 0 |
| Toluene | 1.1 | |
| Ethyl benzene | 0.96 | |
| p,m-Xylene | 0.96 | |
| o-Xylene | 0.92 | |
| 2-Ethyltoluene | 0.83 | |
| 3-Ethyltoluene | 0.8 | |
| 4-Ethyltoluene | 0.77 | |
| 1,3,5-Trimethylbenzene | 1.07 | |
| 1,2,4-Trimethylbenzene | 0.64 | |
| 1,2,3-Trimethylbenzene | 0.73 | |
| Vinyl carbonyls | Methacrolein | 3.17 |
| Methyl methacrylate | N.A. | |
| Aromatic ketones | Acetophenone | 0 |
| Aromatic aldehydes | Benzaldehyde | 1.1 |
| Vinylbenzenes | Styrene | 2.98 |
| Hydroxybenzenes | Phenol | 1.36 |
| Heterocyclic compounds | Pyridine | N.A. |
| Furfural | N.A. | |
| Cyanobenzenes | Benzonitrile | N.A. |
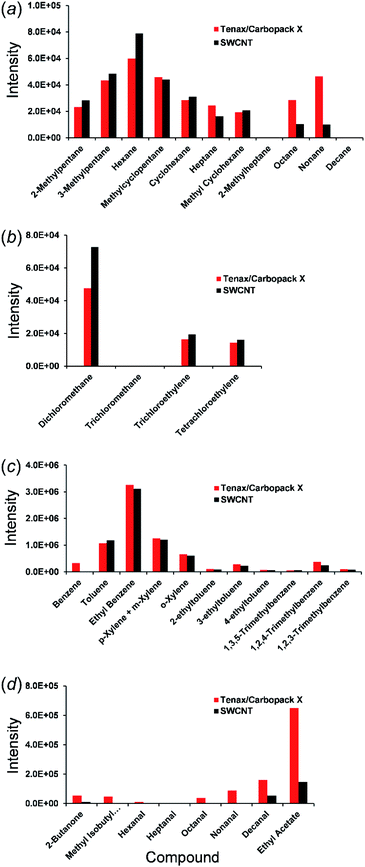
10 of the targeted VOCs were absent from both sorbent tubes during sampling, due to their not being present in the atmosphere at that particular time (or below the detection limits). 37 VOC target analytes were identified in the multisorbent Carbopack X/Tenax tube sample whereas 29 VOC analytes were present in the SWCNT sample tube. Out of the 29 analytes identified in the SWCNT tube, only 7 VOCs (butanone, decanal, ethyl acetate, heptane, octane, nonane and 1,2,4-trimethylbenzene) had peak area ratios <0.7, indicating that most VOCs were favourably retained in comparison to the standard multisorbent material. Fig. 3 shows the quantifier ion peak area of selected target compounds in both sorbent tubes plotted corresponding to their primary functional groups.
VOCs that were found in the Carbopack X/Tenax multisorbent tube but not in the SWCNT sorbent tube are isopropyl alcohol, isoprene, benzene, nonanal, octanal, hexanal, methyl isobutyl ketone and acetophenone. The results from sampling agree with the functional group trends observed previously during the loading of the VOC standards onto the CNT materials,24 except for benzene and 1,2,4-trimethylbenzene. The discrepancies between the benzene ratios in sample tubes and in VOC standards tubes were deemed to be an artifact interference error. Benzene is inherently generated from both sorbent materials during heating and the amount of benzene detected from atmospheric sampling was very low. After background benzene subtraction, zero was obtained on the CNT tube while very low signal intensity was acquired from the Carbopack X/Tenax tube.
Variations in the atmospheric temperature could possibly be the explanation for the lower peak area ratio of 1,2,4-trimethylbenzene during sampling. Although there is no breakthrough of 1,2,4-trimethylbenzene in the SWCNT during the loading of standards,24 it may occur during sampling when temperature in the atmosphere is sufficiently high.
Better recoveries were observed for one third of the VOCs detected in the SWCNTs when the peak areas were compared to the conventional sorbent during sampling (Table 3 and Fig. 3). Methacrolein had the highest ratio, and the signal response was approximately 3.2 times greater than the conventional multi-sorbent material. Styrene was next and had a peak area 3.0 times higher than found from desorption of VOCs from the Carbopack X/Tenax. Dichloromethane had a peak abundance that was 1.5 times higher when using the SWCNT sorbent tube for active sampling. Other compounds having peak area ratios >1.1 include phenol, hexane and 2-methylpentane.
Statistical tests using the Carbopack X/Tenax sorbent demonstrated excellent reproducibility with relative standard deviation (% RSD) values <10%, recoveries between 60% and 120%, tube desorption efficiencies close to 100% and excellent breakthrough values 5% or lower.33 The CNT sorbents have been shown to have % RSD below 25% for most VOCs, which are acceptable values required by the USEPA for environmental sampling.24 Similarly, breakthroughs and recoveries for most of the VOCs were acceptable for the CNTs.
For the 29 VOCs that were detected in both sorbent materials, the paired t-test for comparing individual differences to determine if the data were significantly different (eqn (2)). In eqn (2), d is the is the absolute value of the mean of the difference, sd is the standard deviation of the difference, n is the number of VOCs, and tcal is the calculated comparison value. The tcal value was found to be 1.549, which indicates that the results obtained for the CNTs and Carbopack X/Tenax sorbents are not significantly different at confidence levels of 90% and greater.
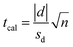 | (2) |
The multi-sorbent Carbopack X/Tenax still remains a superior general sorbent for atmospheric sampling when compared to the CNTs examined in this study since it can retain and desorb a higher number of VOCs. However, it is difficult to exactly quantify the sampling abilities of the CNTs with respect to the Carbopack X/Tenax multisorbent because different amounts of the sorbent materials were used in the tests, due to the CNTs being considerably less dense than the Carbopack X/Tenax. The humidity can also influence the adsorption abilities of the sorbent, but the effects are likely to be relatively small since CNTs are hydrophobic. Nevertheless, it is clear from the peak area ratios obtained from the chromatograms that many of the VOCs are retained and desorbed on the CNTs as efficiently as the multisorbent standard material.
Conclusions
Many studies have focused on the role that metallic impurities have on the catalytic properties of carbonaceous nanomaterials, often concluding that the enhanced properties of the nanomaterials are entirely due to the impurities. This study has shown that CNT sorbents passively exposed to a chemistry laboratory environment for 72 hours spontaneously absorbed a large number of trace atmospheric VOCs. Similarly, the active sampling of outdoor air using SWCNTs in stainless steel sorbent tubes indicated that they retained and desorbed many VOCs at a favorable level to the standard Carbopack X/Tenax multisorbent that is used for sampling VOCs in air quality monitoring studies. Nevertheless, the Carbopack X/Tenax multisorbents can still be considered superior to the CNTs since they are able to adsorb, and then release after heating a larger number of VOCs. Under atmospheric active trace sampling conditions, the CNTs are considerably less efficient than the Carbopack X/Tenax at trapping a range of oxygenated compounds including isopropyl alcohol, isoprene, nonanal, octanal, hexanal, methyl isobutyl ketone and acetophenone, in addition to benzene and isoprene.Since the surface properties of the nanomaterials are considered essential to their applied functions, it is crucial to review the appropriate methods for eliminating organic impurities in purified CNTs prior to their applications and for transporting between apparatus. Although this study has been restricted to CNTs, it is highly likely that other nanoscale carbon-based materials such as graphene and its reduced/oxidized forms also suffer from extensive surface contamination from atmospheric VOCs. CNTs can be cleansed of VOCs by heating to 380 °C under a flow of an inert gas or under vacuum conditions. The cleaned CNTs need to be stored in an inert atmosphere otherwise they will immediately adsorb many atmospheric VOCs at a rate similar to materials utilized specifically as sorbents for atmospheric analysis.
Author contributions
GKSW and RDW conceptualised the project. GKSW conducted the investigation and developed the methodology. Both authors conducted formal analysis of the data. RDW supervised and managed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation, Prime Minister's Office, Singapore under its CREATE program, and a Singapore Government MOE Academic Research Fund Tier 1 Grant (RG3/19).Notes and references
- Z. Yin, C. Cui, H. Chen, Duoni, X. Yu and W. Qian, Small, 2020, 16, 1902301 CrossRef CAS PubMed.
- J. Lyu, V. Kudiiarov and A. Lider, Nanomaterials, 2020, 10, 255 CrossRef CAS PubMed.
- R. Teixeira-Santos, M. Gomes, L. C. Gomes and F. J. Mergulhāo, iScience, 2021, 24, 102001 CrossRef CAS PubMed.
- Y. Q. Cai, G. B. Jiang, J. F. Liu and Q. X. Zhou, Anal. Chem., 2003, 75, 2517–2521 CrossRef CAS PubMed.
- Q. L. Li and D. X. Yuan, J. Chromatogr. A, 2003, 1003, 203–209 CrossRef CAS PubMed.
- Q. L. Li, D. X. Yuan and Q.-M. Lin, J. Chromatogr. A, 2004, 1026, 283–288 CrossRef CAS PubMed.
- C. Saridara and S. Mitra, Anal. Chem., 2005, 77, 7094–7097 CrossRef CAS PubMed.
- Y. Li, Y. Chen, R. Xiang, D. Ciuparu, L. D. Pfefferle, C. Horwath and J. A. Wilkins, Anal. Chem., 2005, 77, 1398–1406 CrossRef CAS PubMed.
- M. Karwa and S. Mitra, Anal. Chem., 2006, 78, 2064–2070 CrossRef CAS PubMed.
- G.-Z. Fang, J.-X. He and S. Wang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2006, 1127, 12–17 CrossRef CAS PubMed.
- E. Menna, F. Della Negra, M. Prato, N. Tagmatarchis, A. Ciogli, F. Gasparrini, D. Misiti and C. Villani, Carbon, 2006, 44, 1609–1613 CrossRef CAS.
- M. Stadermann, A. D. McBrady, B. Dick, V. R. Reid, A. Noy, R. E. Synovec and O. Bakajin, Anal. Chem., 2006, 78, 5639–5644 CrossRef CAS PubMed.
- H. Zhao, L. Wang, Y. Qiu, Z. Zhou, W. Zhong and X. Li, Anal. Chim. Acta, 2007, 586, 399–406 CrossRef CAS PubMed.
- C. M. Hussain, C. Saridara and S. Mitra, J. Chromatogr. A, 2008, 1185, 161–166 CrossRef CAS PubMed.
- E. Díaz, S. Ordóñez and A. Vega, J. Colloid Interface Sci., 2007, 305, 7–16 CrossRef PubMed.
- J. Ma, R. Xiao, J. Li, J. Yu, Y. Zhang and L. Chen, J. Chromatogr. A, 2010, 1217, 5462–5469 CrossRef CAS PubMed.
- J. Ma, L. Jiang, G. Wu, Y. Xia, W. Lu and L. Chen, J. Chromatogr. A, 2016, 1466, 12–20 CrossRef CAS PubMed.
- W. Lu, J. Li, Y. Sheng, X. Zhang, J. You and L. Chen, J. Colloid Interface Sci., 2017, 505, 1134–1146 CrossRef CAS PubMed.
- S. Peng, X. Yang, J. Strong, B. Sarkar, Q. Jiang, F. Peng and D. Liu, J. Hazard. Mater., 2021, 396, 127750 Search PubMed.
- J. N. Hodul, A. K. Murray, N. F. Carneiro, J. R. Meseke, J. Morris, X. He, D. Zemlyanov, G. T.-C. Chiu, J. E. Braun, J. F. Rhoads and B. W. Boudouris, ACS Appl. Nano Mater., 2020, 3, 10389–10398 CrossRef CAS.
- J. Ma, L. Hou, G. Wu, L. Wang, X. Wang and L. Chen, Materials, 2020, 13, 5729 CrossRef CAS PubMed.
- Z. Dai, D. Li, Z. Ao, S. Wang and T. An, J. Hazard. Mater., 2021, 405, 124684 CrossRef CAS PubMed.
- B. Li and C. Mi, Phys. Chem. Chem. Phys., 2021, 23, 2972–2980 RSC.
- G. K. S. Wong, L. Z. Lim, M. J. W. Lim, L. L. Ong, B. Khezri, M. Pumera and R. D. Webster, ChemPlusChem, 2015, 80, 1279–1287 CrossRef CAS PubMed.
- J. Bang, D. W. You, Y. Jang, J.-S. Oh and K. W. Jung, J. Chromatogr. A, 2019, 1605, 460363 CrossRef CAS PubMed.
- Y. Jang and Y.-S. Seon, J. Chromatogr. A, 2020, 1617, 460840 CrossRef CAS PubMed.
- B. Šljukić, C. E. Banks and R. G. Compton, Nano Lett., 2006, 6, 1556–1558 CrossRef PubMed.
- C. E. Banks, A. Crossley, C. Salter, S. J. Wilkins and R. G. Compton, Angew. Chem., Int. Ed., 2006, 45, 2533–2537 CrossRef CAS PubMed.
- A. Ambrosi and M. Pumera, Chem.–Eur. J., 2010, 16, 1786–1792 CrossRef CAS PubMed.
- A. M. Cassell, J. A. Raymakers, J. Kong and H. Dai, J. Phys. Chem. B, 1999, 103, 6484–6492 CrossRef CAS.
- S. Agnihotri, M. J. Rood and M. Rostam-Abadi, Carbon, 2005, 43, 2379–2388 CrossRef CAS.
- C. M. Hussain, C. Saridara and S. Mitra, Analyst, 2009, 134, 1928–1933 RSC.
- G. K. S. Wong, S. J. Ng and R. D. Webster, Anal. Methods, 2013, 5, 219–230 RSC.
- H. B. Zhang, X. L. Pan, X. W. Han, X. M. Liu, X. F. Wang, W. L. Shen and X. H. Bao, Chem. Sci., 2013, 4, 1075–1078 RSC.
- S. L. Deng, Y. Zhang, A. H. Brozena, M. L. Mayes, P. Banerjee, W. A. Chiou, G. W. Rubloff, G. C. Schatz and Y. H. Wang, Nat. Commun., 2011, 2, 382, DOI:10.1038/ncomms1384.
| This journal is © The Royal Society of Chemistry 2021 |