Open Access Article
Open Access Articletert-Butyloxycarbonyl-protected amino acid ionic liquids and their application to dipeptide synthesis†
Ming Chen *a and
Xihan Yub
*a and
Xihan Yub
aSchool of Pharmacy, Yantai University, Qinqquan Road 30, Yantai 264005, China. E-mail: mingchen@mail.ecust.edu.cn
bCollege of Pharmacy, East China University of Science and Technology, Meilong Road 130, Shanghai 200237, China. E-mail: xh_yu@mail.ecust.edu.cn
First published on 13th August 2021
Abstract
Care should be taken when using amino acid ionic liquids (AAILs) for organic synthesis because of their multiple reactive groups. To expand the applicability of AAILs, we prepared a series of room-temperature ionic liquids derived from commercially available tert-butyloxycarbonyl-protected amino acids (Boc-AAILs). The resulting protected AAILs were used as the starting materials in dipeptide synthesis with commonly used coupling reagents. The distinctive coupling reagent N,N′-diethylene-N′′-2-chloroethyl thiophosphoramide was found to enhance amide formation in the Boc-AAILs without addition of base, giving the dipeptides in satisfactory yields in 15 min.
Introduction
Ionic liquids have been broadly used in peptide synthesis as synthetic support,1 cleavage reagent2 and solvents.3–5 In 2005, Ohno and co-workers6,7 reported a series of novel room-temperature ionic liquids (RTILs) called amino acid ionic liquids (AAILs) prepared by neutralization of imidazolium hydroxide with 20 natural amino acids. However, the multiple reactive groups of the amino acid anions can cause unwanted reactions in selective or multistep organic synthesis. Although the AAILs have been used in solution-phase amide formation under thermal heating,8 no controllable strategy for peptide synthesis using AAILs has been reported. Thus, as is the case with using protected amino acids in peptide chemistry, we believe that AAILs could be used as efficient reactants and reaction media in organic synthesis when their reactive side chain and N-terminus are chemically protected. As far as we know, the ionic liquids containing protected amino acids have not been studied before. We therefore commenced our study with development of novel RTILs consisting of the 1-ethyl-3-methylimidazolium cation ([emim]) and the anions of 20 commercially available tert-butyloxycarbonyl (Boc)-protected amino acids.Results and discussion
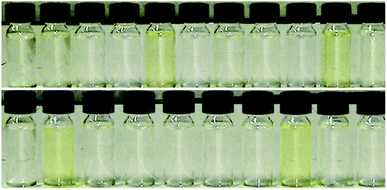
Because the halide–anion exchange method is considered to be unsuitable for AAIL preparation, we synthesized the Boc-protected amino acid ionic liquids (Boc-AAILs) by simple neutralization of [emim][OH] with commercially available Boc-protected amino acids.6 The neat Boc-AAILs are clear and nearly colorless to pale yellow liquids at room temperature (Fig. 1). NMR, elemental analysis and IR spectroscopic analysis of the Boc-AAILs were consistent with their proposed structures (see ESI†). All of the Boc-AAILs are miscible in acetonitrile, methanol, dimethylformamide (DMF) and dimethyl sulfoxide (DMSO), partially miscible in water, but immiscible in diethyl ether, ethyl acetate and hexane at room temperature.The thermophysical properties of the Boc-AAILs were investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) (Table 1). From the measured values, decomposition of the Boc-AAILs began at temperatures of 73–105 °C (entries 1–10 and 12–21), except for [emim][Boc-Asn] which began to decompose at 42 °C (entry 11). The DSC data showed that all of the Boc-AAILs do not have a melting point, but the glass transition temperatures range from−42 to 6 °C. [emim][Boc-Gly] and the group of Boc-AAILs containing small aliphatic or rigid side groups have the lowest Tg values (entries 1–5, 16, and 21). The other Boc-AAILs containing aromatic rings (side chains or protecting groups) have higher Tg values, possibly because of aromatic stacking interactions (entries 6, 8–10, 13, 14, and 17–20). The Tg values of [emim][Boc-Asn], [emim][Boc-Gln], and [emim][Boc-Trp(For)] are close to or above 0 °C, indicating involvement of strong intramolecular hydrogen bonds (entries 7, 11, and 15). In addition to the thermal properties, the viscosities of the Boc-AAILs were measured with a capillary viscometer at 25 °C (Table 1). The high η values reveal that the pure Boc-AAILs are not suitable for use as a single solvent in solid-phase synthesis, while the fluidity (η′−1) is significantly improved by diluting the Boc-AAILs with DMF.
| Entry | Anion of AAIL | Tga (°C) | Tdb (°C) | ηc (cP) | η′d (cP) |
|---|---|---|---|---|---|
| a Glass transition temperature.b Thermal decomposition temperature, at which 5% mass loss occurs.c η is the viscosity of the neat Boc-AAIL at 25 °C.d η′ is the viscosity of 80 wt% Boc-AAIL in DMF at 25 °C. | |||||
| 1 | [Boc-Gly] | −42 | 105 | 52.5 | 11.5 |
| 2 | [Boc-Ala] | −39 | 103 | 53.9 | 11.6 |
| 3 | [Boc-Val] | −37 | 102 | 54.6 | 11.8 |
| 4 | [Boc-Leu] | −37 | 103 | 57.3 | 12.1 |
| 5 | [Boc-Ile] | −39 | 100 | 60.3 | 12.5 |
| 6 | [Boc-Phe] | −25 | 105 | 71.2 | 14.7 |
| 7 | [Boc-Trp(For)] | 6 | 79 | 501.7 | 35.2 |
| 8 | [Boc-Tyr(Bn)] | −11 | 102 | 146.0 | 20.1 |
| 9 | [Boc-Asp(Bn)] | −13 | 82 | 187.8 | 24.5 |
| 10 | [Boc-His(Ts)] | −17 | 80 | 183.1 | 24.2 |
| 11 | [Boc-Asn] | −6 | 42 | 243.5 | 28.6 |
| 12 | [Boc-Asn(Trt)] | −17 | 80 | 146.6 | 23.7 |
| 13 | [Boc-Glu(Bn)] | −14 | 92 | 148.3 | 23.8 |
| 14 | [Boc-Lys(Z)] | −21 | 96 | 132.9 | 23.5 |
| 15 | [Boc-Gln] | −2 | 101 | 267.5 | 28.7 |
| 16 | [Boc-Met] | −37 | 75 | 54.4 | 11.7 |
| 17 | [Boc-Arg(Ts)] | −12 | 78 | 187.3 | 22.5 |
| 18 | [Boc-Ser(Bn)] | −24 | 95 | 75.8 | 14.2 |
| 19 | [Boc-Thr(Bn)] | −28 | 96 | 74.0 | 14.5 |
| 20 | [Boc-Cys(Meb)] | −14 | 73 | 96.1 | 16.9 |
| 21 | [Boc-Pro] | −41 | 100 | 71.7 | 14.7 |
To investigate the efficiencies of the Boc-AAILs in SPPS, we first performed model coupling reactions of [emim][Boc-Ala] (2.0 mL, 80 wt% in DMF) with H-L-phenylalanine-HMPB-ChemMatrix resin (100 mg, 0.58 mmol g−1 loading) as the solid support, which possesses excellent swelling properties in both DMF and ionic liquid (Table 2, entries 9–11).9 The L-Ala–L-Phe dipeptide was obtained as the desired product after cleavage and purification. For comparison, the coupling reactions were also performed under the standard conditions of Boc-SPPS using DMF (entries 1–3) and [bmim][PF6] (1-butyl-3-methylimidazolium hexafluorophosphate) (entries 5–7) as the solvent. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) with the HOBt (hydroxybenzotriazole) additive, 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), and benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) were chosen as alternative coupling reagents and N,N-diisopropylethylamine (DIEA) was used as the base for all of the model reactions. In the case of coupling in [bmim][PF6] (entries 5–7), HATU and PyBOP were both less effective than the reactions achieved by the standard Boc strategy using DMF (entries 1–3), and no coupling occurred using EDC. In contrast, the HATU- and PyBOP-mediated reactions with AAIL rapidly proceeded in the beginning (entries 10 and 11), although the reactions did not give satisfactory yields after 1 h. The screening studies showed that in presence of an imidazolium-based ionic liquid, the efficiencies of the coupling reagents decrease in the order HATU > PyBOP > EDC. Because of the disappointing results, we attempted to find a novel coupling reagent that is appropriate for peptide synthesis with Boc-AAILs.
| Entry | Amino acid | Reagent, additive, solvent | Yield¼a (%) | Yieldb (%) |
|---|---|---|---|---|
| a Isolated yield in 15 min.b Isolated yield in 1 h.c 4.0 equiv. Boc-Ala-OH, 8.0 equiv. EDC, 4.0 equiv. HOBt, 8.0 equiv. DIEA, 2.0 mL DMF.d 4.0 equiv. Boc-Ala-OH, 4.0 equiv. HATU, 4.0 equiv. HOBt, 8.0 equiv. DIEA.e 4.0 equiv. Boc-Ala-OH, 4.0 equiv. PyBOP, 8.0 equiv. DIEA, 2.0 mL DMF.f 4.0 equiv. Boc-Ala-OH, 4.0 equiv. CTPA, 8.0 equiv. DIEA, 2.0 mL DMF.g 4.0 equiv. Boc-Ala-OH, 8.0 equiv. EDC, 4.0 equiv. HOBt, 8.0 equiv. DIEA, 2.0 mL [bmim][PF6].h 4.0 equiv. Boc-Ala-OH, 4.0 equiv. HATU, 4.0 equiv. HOBt, 8.0 equiv. DIEA, 2.0 mL [bmim][PF6].i 4.0 equiv. Boc-Ala-OH, 4.0 equiv. PyBOP, 8.0 equiv. DIEA, 2.0 mL [bmim][PF6].j 4.0 equiv. Boc-Ala-OH, 4.0 equiv. CTPA, 8.0 equiv. DIEA, 2.0 mL [bmim][PF6].k 4.0 equiv. Boc-Ala-OH, 4.0 equiv. CTPA, 2.0 mL [bmim][PF6].l 2.0 mL [emim][Boc-Ala] (80% wt% in DMF), 8.0 equiv. EDC, 4.0 equiv. HOBt, 8.0 equiv. DIEA.m 2.0 mL [emim][Boc-Ala] (80 wt% in DMF), 4.0 equiv. HATU, 4.0 equiv. HOBt, 8.0 equiv. DIEA.n 2.0 mL [emim][Boc-Ala] (80 wt% in DMF, 2.0 mL), 4.0 equiv. PyBOP, 8.0 equiv. DIEA.o 2.0 mL [emim][Boc-Ala] (20 wt% in DMF), 4.0 equiv. CTPA.p 2.0 mL [emim][Boc-Ala] (40 wt% in DMF), 4.0 equiv. CTPA.q 2.0 mL [emim][Boc-Ala] (80 wt% in DMF), 4.0 equiv. CTPA. | ||||
| 1c | Boc-Ala | EDC, HOBt, DIEA, DMF | 38 | 92 |
| 2d | Boc-Ala | HATU, HOBt, DIEA, DMF | 39 | 96 |
| 3e | Boc-Ala | PyBOP, DIEA, DMF | 33 | 95 |
| 4f | Boc-Ala | CTPA, DIEA, DMF | 0 | 0 |
| 5g | Boc-Ala | EDC, HOBt, [bmim][PF6] | 0 | 0 |
| 6h | Boc-Ala | HATU, DIEA, [bmim][PF6] | 18 | 68 |
| 7i | Boc-Ala | PyBOP, DIEA, [bmim][PF6] | 16 | 43 |
| 8j | Boc-Ala | CTPA, DIEA, [bmim][PF6] | 31 | 78 |
| 9k | Boc-Ala | CTPA, [bmim][PF6] | 23 | 35 |
| Entry | Anion of AAIL | Reagent, additive | Yield¼a (%) | Yieldb (%) |
|---|---|---|---|---|
| 10l | [Boc-Ala] | EDC, HOBt, DIEA | 0 | 0 |
| 11m | [Boc-Ala] | HATU, HOBt, DIEA | 70 | 71 |
| 12n | [Boc-Ala] | PyBOP, DIEA | 35 | 35 |
| 13o | [Boc-Ala] | CTPA | 26 | 72 |
| 14p | [Boc-Ala] | CTPA | 64 | 77 |
| 15q | [Boc-Ala] | CTPA | 95 | 95 |
| 16q | [Boc-Gly] | CTPA | 93 | — |
| 17q | [Boc-Val] | CTPA | 95 | — |
| 18q | [Boc-Leu] | CTPA | 94 | — |
| 19q | [Boc-Ile] | CTPA | 96 | — |
| 20q | [Boc-Phe] | CTPA | 92 | — |
| 21q | [Boc-Trp(For)] | CTPA | 92 | — |
| 22q | [Boc-Tyr(Bn)] | CTPA | 93 | — |
| 23q | [Boc-Asp(Bn)] | CTPA | 89 | — |
| 24q | [Boc-His(Ts)] | CTPA | 90 | — |
| 25q | [Boc-Asn] | CTPA | 10 | — |
| 26q | [Boc-Asn(Trt)] | CTPA | 86 | — |
| 27q | [Boc-Glu(Bn)] | CTPA | 93 | — |
| 28q | [Boc-Lys(Z)] | CTPA | 91 | — |
| 29q | [Boc-Gln] | CTPA | 90 | — |
| 30q | [Boc-Met] | CTPA | 93 | — |
| 31q | [Boc-Arg(Ts)] | CTPA | 89 | — |
| 32q | [Boc-Ser(Bn)] | CTPA | 92 | — |
| 33q | [Boc-Thr(Bn)] | CTPA | 89 | — |
| 34q | [Boc-Cys(Meb)] | CTPA | 91 | — |
| 35q | [Boc-Pro] | CTPA | 89 | — |
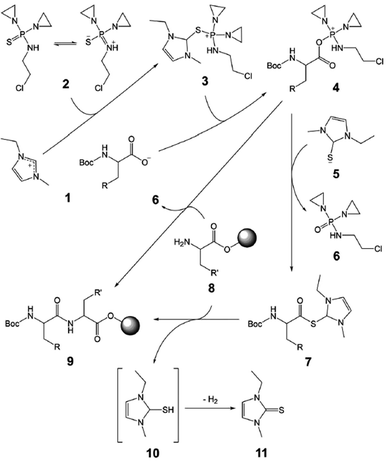
Next, N,N′-diethylene-N′′-2-chloroethyl thiophosphoramide (CTPA, Scheme 1, 2), which is a degradation product of chemotherapy drug Thiotepa, was used as a potential coupling reagent in the model reactions. CTPA-mediated peptide synthesis in [bmim][PF6] was achieved in 78% final yield with serious epimerization (18% D-Ala–L-Phe) in the presence of DIEA (Table 2, entry 8), but the yield was only 35% in the absence of base (entry 9). The results showed that DIEA facilitates the reaction but causes epimerization. In contrast, there was no amide formation for the same synthesis in DMF (entry 4). Pleasingly, coupling in 80 wt% [emim][Boc-Ala] smoothly completed with 99% high-performance liquid chromatography (HPLC) purity and 95% yield in only 15 min and required no base and additive (entry 15). As the concentration of [emim][Boc-Ala] in DMF was increased, the reaction efficiency increased significantly (entries 13–15). From the above findings, we deduced that, at least for CTPA-mediated method, the Boc-AAIL could not be replaced with a mixture of the Boc-amino acid and imidazolium-based RTIL. Encouraged by this success, the reactivity tests of CTPA were then extended to include reactions with other types of Boc-AAILs, in which almost all the Boc-AAILs performed outstandingly well with little epimerization (entries 15–24 and 27–35), except for [emim][Boc-Asn] (entry 25). The use of CTPA led to succinimide formation from [emim][Boc-Asn]. However, this problem can be overcome by introducing a sidechain protecting trityl group (entry 26). Notably, [emim][Boc-Gln] was scarcely deamidated under treatment with CTPA (entry 29), despite it having a similar carboxamide side chain to [emim][Boc-Asn].
Besides the applicability, another important issue is the recyclability of the Boc-AAILs. After coupling, the liquid phase composed of the Boc-AAIL, DMF, and the by-products (N,N′- diethylene-N′′-2-chloroethyl phosphoramide (Scheme 1, 6) and imidazole-2-thione (11)), was separated and evaporated. The resultant Boc-AAIL was then subjected to extraction with diethyl ether followed by saturated aqueous NaHCO3 solution. A recovery test indicated that the [emim][Boc-Ala] can be recycled at least four times in the model reaction without significant loss of activity (Table 3).
Based on the characterization results (see the ESI†) and partial structural similarity of CTPA with phosphonium reagents,10,11 the proposed mechanism of CTPA-mediated peptide synthesis is shown in Scheme 1. The proposed mechanism includes the following steps: (1) attack of the 2-position of imidazolium ionic liquid 1 by CTPA to give phosphonium intermediate 3, (2) formation of acyloxyphosphonium 4 from the protected amino acid anion of 1 and generation of hydrosulphide 5, (3) an alternative pathway where the salt 4 directly reacts with resin-bound amino acid 8 to give peptide product 9, and (4) another possible pathway where 4 first transfers to active thioester 7, which then couples with 8 to form product 9. By-product 11 is generated instead of unstable dihydroimidazole-2-thiol 10.12 According to the proposed mechanism, it is of interest to note that the Boc-AAIL can play multiple roles as the reactant, reaction medium, coupling additive, and CTPA pre-activator. This mechanism can also help to understand why CTPA is completely inefficient in DMF in absence of the imidazolium cation.
Conclusions
In conclusion, we have prepared RTILs derived from Boc-protected amino acids. The structural characteristics and chemophysical properties of the resulting Boc-AAILs were investigated. These protected AAILs are selectively reactive and show high potential for application in the field of organic synthesis. In this application, examples of using Boc-AAILs as multifunctional reactants in dipeptide synthesis have been provided. Further, we found that the special coupling reagent CTPA is particularly suitable for rapid SPPS using Boc-AAILs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Shanghai Pujiang Program for funding.Notes and references
- W. Miao and T.-H. Chan, J. Org. Chem., 2005, 70, 3251–3255 CrossRef CAS PubMed.
- S. Majumdar, J. De, A. Chakraborty, D. Roy and D. K. Maiti, RSC Adv., 2015, 5, 3200–3205 RSC.
- H. Vallette, L. Ferron, G. Coquerel, A.-C. Gaumont and J.-C. Plaquevent, Tetrahedron Lett., 2004, 45, 1617–1619 CrossRef CAS.
- M. L. Di Gioia, A. Barattucci, P. Bonaccorsi, A. Leggio, L. Minuti, E. Romio, A. Temperini and C. Siciliano, RSC Adv., 2014, 4, 2678–2686 RSC.
- S. S. Bhawal, R. A. Patil and D. W. Armstrong, RSC Adv., 2015, 5, 95854–95856 RSC.
- K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef CAS PubMed.
- For selected reviews on AAIL: (a) H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS PubMed; (b) S. Kirchhecker and D. Esposito, Curr. Opin. Green Sustain. Chem., 2016, 2, 28–33 CrossRef; (c) C. Verma, E. Ebenso and M. Quraishi, Clin. Med. Biochem., 2018, 4, 1–5 Search PubMed.
- S. Furukawa, T. Fukuyama, A. Matsui, M. Kuratsu, R. Nakaya, T. Ineyama, H. Ueda and I. Ryu, Chem.–Eur. J., 2015, 21, 11980–11983 CrossRef CAS PubMed.
- S. Lawrenson, M. North, F. Peigneguy and A. Routledge, Green Chem., 2017, 19, 952–962 RSC.
- E. Frérot, J. Coste, A. Pantaloni, M.-N. Dufour and P. Jouin, Tetrahedron, 1991, 47, 259–270 CrossRef.
- For selected reviews on the mechanism of phosphonium coupling: (a) F. Albeiicio, R. Chinchilla, D. J. Dodsworth and C. Nájera, Org. Prep. Proced. Int., 2001, 33, 203–303 CrossRef; (b) S.-Y. Han and Y.-A. Kim, Tetrahedron, 2004, 60, 2447–2467 CrossRef CAS; (c) C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852 CrossRef CAS; (d) A. El-Faham and F. Albericio, Chem. Rev., 2011, 111, 6557–6602 CrossRef CAS PubMed; (e) T. I. Al-Warhi, H. M. A. Al-Hazimi and A. El-Faham, J. Saudi Chem. Soc., 2012, 16, 97–116 CrossRef CAS.
- (a) O. Hollóczki, D. Gerhard, K. Massone, L. Szarvas, B. Németh, T. Veszprémi and L. Nyulászi, New J. Chem., 2010, 34, 3004 RSC; (b) L. H. Finger, F. Wohde, E. I. Grigoryev, A.-K. Hansmann, R. Berger, B. Roling and J. Sundermeyer, Chem. Commun., 2015, 51, 16169–16172 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05597f |
| This journal is © The Royal Society of Chemistry 2021 |