Open Access Article
Open Access ArticleQualitative and quantitative adsorption mechanisms of zinc ions from aqueous solutions onto dead carp derived biochar†
Hong-tao Qiao a,
Yong-sheng Qiaoa,
Xiao-hang Luoa,
Bao-wei Zhao
a,
Yong-sheng Qiaoa,
Xiao-hang Luoa,
Bao-wei Zhao *b and
Qiu-ying Caia
*b and
Qiu-ying Caia
aInstitute of Applied Chemistry, Department of Chemistry, Xinzhou Teachers University, Xinzhou 034000, China. E-mail: 469494248@qq.com
bSchool of Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou 730070, China. E-mail: zhbw2001@sina.com
First published on 29th November 2021
Abstract
The objective of this study is to investigate the qualitative mechanisms of Zn2+ adsorption on carp biochars (CMBx) produced from dead carp at different temperatures (450–650 °C) and their quantitative contribution. The pseudo second order kinetic model and the Langmuir model could fit the kinetic and isothermal adsorption data well, respectively. The intra-particle diffusion was the main rate-limiting step but not the only rate-limiting step. The maximum adsorption capacity obtained from the Langmuir model for CMB650 was 87.7 mg g−1 which was greater than those of other biochars. Precipitation with minerals, ion exchange, and complexation with functional groups (OFGs) were the main adsorption mechanisms. Quantum chemistry calculations confirmed that the functional groups (e.g., hydroxyl, carboxyl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) tended to bind with Zn2+ more strongly than with Ca2+ and Mg2+, because the structure of the complex formed by the former was more stable. The contribution of different adsorption mechanisms varied with the pyrolysis temperature to prepare biochar. With increasing pyrolysis temperature, the contribution of the interaction between Zn2+ and the minerals increased from 46.4% to 84.7%, while that of complexation with OFGs decreased from 41.7% to 4.7%. Overall, the mechanism of Zn2+ adsorption on CMB450 was dominated by complexation with OFGs and exchange with cations (accounting for 73.2%), while the mechanisms on CMB650 were dominated by the interaction with minerals. In view of the total adsorption capacity, 650 °C was the optimized pyrolysis temperature for CMBx preparation and adsorption treatment of Zn-contaminated water. These results are useful for screening effective biochars as engineered sorbents to treat Zn-containing wastewater.
C) tended to bind with Zn2+ more strongly than with Ca2+ and Mg2+, because the structure of the complex formed by the former was more stable. The contribution of different adsorption mechanisms varied with the pyrolysis temperature to prepare biochar. With increasing pyrolysis temperature, the contribution of the interaction between Zn2+ and the minerals increased from 46.4% to 84.7%, while that of complexation with OFGs decreased from 41.7% to 4.7%. Overall, the mechanism of Zn2+ adsorption on CMB450 was dominated by complexation with OFGs and exchange with cations (accounting for 73.2%), while the mechanisms on CMB650 were dominated by the interaction with minerals. In view of the total adsorption capacity, 650 °C was the optimized pyrolysis temperature for CMBx preparation and adsorption treatment of Zn-contaminated water. These results are useful for screening effective biochars as engineered sorbents to treat Zn-containing wastewater.
1. Introduction
With the rapid development of industrialization and urbanization, massive amounts of zinc ion-containing wastewater (e.g., industrial effluents, galvanized waste liquids, and leachates from landfills) are discharged into the environment. Although zinc ions are originally beneficial to the growth of human beings, animals and plants, they act as a heavy metal pollutant because of their excessive amounts.1–4 Large amounts of Zn2+ could lead to strong toxicity for human beings and lead to serious environmental pollution and ecological damage (e.g., Zn2+ with concentration > 5 mg L−1 in water).5–7 In addition, compared to other heavy metal ions, Zn2+ ions are not readily removed from water environment due to their higher electronegativity.7 Among the many treatment technologies for heavy metal polluted water, the adsorption by biochar is considered to have broad application prospects.8 Biochar is a kind of carbon-containing, stable and highly aromatized carbonaceous solid material prepared by pyrolysis of biomass (e.g., plant residues, livestock and poultry manure, and other organic solid wastes) under oxygen-limited conditions and the temperature below 700 °C.8–10 In recent years, China supplies about 60.5% of aquatic products worldwide.11,12 The large amount of wastes (e.g., dead or sick aquatic carcasses, residual baits and feces) that produced during the intensive farming process are burned or buried, which will cause not only waste of biomass energy and environmental pollution, but also risks of zoonotic diseases.11,12 Alternatively, if these wastes were converted into biochar and employed for the removal of heavy metal ions (e.g. Zn2+) from wastewater, it would be quite beneficial to resource utilization of waste and remediation of heavy metal polluted wastewater.According to recently published research results, the mechanisms responsible for heavy metal adsorption on biochar mainly included physical adsorption, chemical precipitation, surface complexation with oxygen-containing functional groups (OFGs), cation exchange, electrostatic attraction, and cation–π interaction.13–18 However, the relative contribution of these mechanisms for heavy metal adsorption on biochar largely varies with the type of biomass and pyrolysis temperature. Specifically speaking, Zhao et al. indicated that for the biochars derived from rice straw, chicken manure, and sewage sludge at 350 and 550 °C, adsorption capacities and mechanisms for Pb2+ and Zn2+ were quite different.18 Deng et al. found that for the rice straw biochars prepared at 400 and 700 °C, the contribution of complexation with surface acid groups was negligible and the dominant adsorption mechanisms for heavy metals on the low temperature biochar and high temperature biochar were cation exchange and precipitation with minerals respectively.15 Additionally, Lei et al. reported that animal derived biochars (ADBs) were more effective in immobilizing Pb2+, Cd2+ and Cu2+ than plant or manure derived biochars; and Pb2+ was effectively immobilized via precipitation, whereas ion exchange was the dominant mechanism in combining Cd2+ and Cu2+ with ADBs.16,17 The reason is that the ADBs had large amount of hydroxyapatite (HAP) and relatively low carbon contents, and HAP had a significant effect on the adsorption of heavy metal ions on ADBs.16,17,19,20 These results suggest that the quantitative information of different adsorption mechanisms is of great significance for the application of biochar in the remediation of heavy metal pollution. However, the qualitative and quantitative adsorption mechanism of Zn removal by aquatic animal biochar is still unknown, which makes it lack the necessary theoretical support for its application in the remediation of heavy metal pollution.
In order to fill this gap, dead carp was selected as the research object of aquatic animals, and the objectives of this work are to: (i) investigate the adsorption performance of Zn2+ on biochars prepared from dead carp at different pyrolysis temperature; (ii) understand the contributions of each mechanism to total adsorption by the biochars on a qualitative and quantitative basis; (iii) assess the removal potential of Zn2+ by biochars and discuss the relationship between the mechanisms and the specific properties of biochars. These results will have guiding significance for screening effective biochar as engineering adsorbent to treat Zn-containing wastewater.
2. Experimental
2.1 Biochar preparation and characterization
Dead carps were obtained from a fish farm in Xinzhou, Shanxi Province, China. Carp was cut into pieces, washed with distilled water, air-dried for 2 D, dried in an oven at 70 °C for 24 h to make carp meat and bone meal, and then crushed and passed through a 0.84 mm sieve (20 mesh). Carp biomass was tightly placed in a ceramic pot, and then pyrolyzed in the muffle furnace under limited air with a heating rate of 15 °C min−1 and held at the peak temperature (450, 550, and 650 °C) for 6 h before cooling to room temperature. All biochar samples were passed through a 0.17 mm sieve (80 mesh), and stored in airtight containers and labelled as CMB450, CMB550, and CMBC650 in accordance with the pyrolysis temperature, respectively. Demineralized biochars were obtained according to the method by Wang et al.21 and identified as DCMB450, DCMB550, and DCMB650, respectively (ESI†).The physicochemical (yield, zeta potential, pH, specific surface area, elemental composition and thermogravimetric curves) and spectral (SEM-EDS, XRD, XPS and FTIR) properties of biochars were measured. More detailed analytical methods were described in the ESI.†
2.2 Adsorption experiments
Batch adsorption experiments were carried out to elucidate the characteristics of Zn2+ adsorption on biochars. A certain amount of biochars (0.02 g) was added into a 50 mL conical flask, and mixed with 20 mL solutions containing initial Zn2+ concentration. The initial pH value for Zn2+ solutions was adjusted to 5.0 ± 0.05 by adding 0.1 M HNO3 or NaOH solutions and the tested Zn2+ solutions for the adsorption experiments contained 0.01 M NaNO3 (analytical reagent, Sinopharm, China) as background electrolyte to keep a constant ionic strength. The mixture was shaken in the air bath thermostat oscillator (THZ-82A, Putian Instrument Manufacturing Co., Ltd., China) at 150 rpm and 25 °C for a certain time. The mixture was filtered with a 0.45 μm filter membrane after shaking, and the filtrate was used to measure of Zn2+ concentration by inductively coupled plasma emission spectrometer (ICP-OES) (Optima 8000, PerkinElmer, USA). For adsorption kinetics experiments, the initial Zn2+ concentration was 100 mg L−1 and the oscillating adsorption time was 2–420 min. Adsorption isotherm experiments were carried out as mentioned above with initial Zn2+ concentrations in the range of 60–500 mg L−1 for 7 h. Kinetic and isothermal adsorption models were used to illustrate the adsorption process, and the fitting analysis was described in the ESI.†In order to illustrate the contribution of each mechanism to the overall adsorption of Zn2+ on biochars, the CMBx (‘x’ represents the pyrolysis temperature of biochar) and DCMBx were placed into Zn2+ solution (100 mg L−1), and distilled water as a contrast under the same conditions above, respectively. Then, the pH values and the concentrations of K+, Ca2+, Na+, Mg2+ and Zn2+ in solutions were measured and analyzed by the pH meter and ICP-OES after filtering through 0.45 μm filters. The biochars loaded with Zn2+ were individually collected for FTIR, XRD, XPS and SEM-EDS analyses.
2.3 Quantitative contributions of different mechanisms to Zn2+ adsorption on biochars
The contributions of Zn2+ adsorption mechanisms on CMBx were determined using the method reported by Cui et al.,13 Wang et al.,22 Deng et al.15 and further modified by Gao et al.8 The specific calculation methods about the contributions of precipitation with minerals (Qpm), exchange with cation (Qec), complexation with OFGs (Qcf) and other potential mechanisms (Qop) (including physical adsorption and Zn2+–π interaction) were described as following:(i) The contribution of the interaction with minerals (Qim) was calculated as the difference between adsorption amount of Zn2+ on CMBx and DCMBx.23,24 In addition, (Fig. 1) the yield of DCMBx from CMBx was considered to offset the concentration effect. The calculation formula is as following eqn (1):
| Qim = Qo − Qd × Y | (1) |
(ii) The adsorption capacity Qec resulting from exchange with cation (K+, Na+, Ca2+, and Mg2+) was determined by calculating the net release of these cations before and after adsorption in solution (as shown by eqn (2)).
| Qec = QK + QNa + QCa + QMg | (2) |
(iii) The effects of interaction with minerals on Zn2+ adsorption includes precipitation with minerals and exchange with cations. Therefore, the adsorption capacity of Zn2+ due to precipitation with minerals could be calculated according to the difference between the Qim and the Qec values (as shown in eqn (3)).
| Qpm = Qim − Qec | (3) |
(iv) A decrease in pH before and after Zn2+ adsorption on DCMBx was due to the interaction of Zn2+ with the surface acid functional groups (as shown in eqn (4)). The amount of H+ released could be calculated by the decreased pH value, and then the amount of Zn2+ adsorbed by the surface acid functional groups complexation (Qcfd) could be calculated accordingly. The yield of DCMBx from CMBx (Y) was calculated to offset the concentration effect (as shown in eqn (5)).
| –COOH/–OH + Zn2+ + H2O → –COOZn+/–OZn+ + H3O+ | (4) |
| Qcf = Qcfd × Y | (5) |
(v) The adsorption capacity of other potential mechanisms (Qop), including physical adsorption and Zn2+–π interaction, could be calculated by subtracting the interaction with minerals (Qim) and complexation with acid functional groups (Qcf) from the total adsorption capacity (as shown in eqn (6)).
| Qop = Qo − Qim − Qcf | (6) |
In addition, the proportions of the contributions of different mechanisms to the Zn2+ adsorption were defined by following the ratios Qpm/Qo, Qec/Qo, Qcf/Qo and Qop/Qo.
2.4 Statistical analysis
The batch experiments of Zn2+ adsorption were performed in triplicate. Since the characterization of microstructures and other properties was only measured once, no statistical analysis was carried. The means and standard deviations were calculated by Microsoft 2007 Excel software, and the adsorption kinetics and isotherms were fitted using Origin 8.5. Correlations were analyzed with the Pearson test at P = 0.01 or 0.05 by SPSS 18.0.3. Results and discussion
3.1 Biochar characterization
The major physicochemical properties of CMBx are shown in Table 1. With increasing pyrolytic temperature from 450 to 650 °C, lower mass yield of CMBx was obtained and the BET surface area increased from 63.46 to 76.76 m2 g−1, mainly due to the slow decomposition of organic matter and accumulation of minerals in the process of biomass pyrolysis, such as bone, muscle, fat, and connective tissue in the carp meat and bone meal.17,25 This was consistent with the SEM images observation showing more pores but less white impurities on the surface of CMB650 compared to CMB 450 and CMB550 (Fig. S1†). In addition, it can be seen from the SEM images (Fig. S1†) that the cracks on the surface of biochars gradually increase and expand with the increasing pyrolysis temperature, resulting in the increase of pore volume. CMBx were alkaline and higher pyrolysis temperature resulted in much higher pH values, which could be attributed to the decomposition of acidic functional groups of biochar at higher pyrolysis temperature and the presence of abundant hydroxyapatite (HAP) on biochars because of high ingredient of HAP in bone (60–70%).16,17 There was a significant correlation between the zeta potential of biochar at the initial pH and the pyrolysis temperature (r = −0.999, P = 0.008 and less than 0.01), indicating that the amount of negative charges on the surface of the biochars increased as the pyrolysis temperature increases, and the effect of electrostatic attraction on the adsorption of metal cations increased. According to the results of zeta potential, CMB650 had the highest stability in aqueous solution. The change of C, H, N and O contents in CMBx resulted in a decrease in the H/C ratios, which showed a significant negative correlation with the pyrolysis temperature (Fig. S2†), suggesting that CMB650 had been highly carbonized. The thermogravimetric (TG) and derivative thermogravimetry (DTG) curves of CMBx are shown in Fig. S3.† As the temperature ranging from 200 to 500 °C and 500 to 700 °C, the mass loss of CMB450, CMB550, and CMB650 were about 3.29, 5.50, 6.17% and 15.32, 8.90, 5.12%, respectively. The evaporation of moisture and structural bound water from the biochars, and the burning of organic matters including fat and proteins occurred at the temperature ranging from 200 to 500 °C, and decomposition of calcite and degradation of organics with strong thermal stability occurred at the temperature ranging from 500 to 700 °C.25,26 Relative to CMB450 and CMB550, CMB650 had a high thermal stability. In short, pyrolysis temperature had a significant influence on the physicochemical properties of CMBx, which might have a profound influence on their adsorption mechanisms for Zn2+.| Sample | Yield (%) | pH | SBETa (m2 g−1) | Elemental content (%) | Atomic ratio | PVa (m3 g−1) | APa (nm) | ZPa (mV) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | O/C | H/C | (O + N)/C | |||||||
| a SBET, PV, AP, and ZP are the BET surface area, pore volume, average pore size and zeta potential, respectively.b The different lowercase letters (a, b, and c) behind the data on the same column indicate significant difference, which is analyzed by the Duncan's multiple range test using SPSS 18.0. | |||||||||||||
| CMB450 | 34.37 ± 0.46ab | 9.44 ± 0.02c | 63.46 | 42.05 | 3.36 | 6.88 | 11.87 | 0.21 | 0.96 | 0.35 | 0.07 | 4.72 | −50.8 ± 0.89c |
| CMB550 | 25.92 ± 0.18b | 9.63 ± 0.02b | 63.62 | 31.12 | 2.09 | 4.91 | 12.30 | 0.30 | 0.81 | 0.43 | 0.14 | 8.90 | −55.1 ± 0.70b |
| CMB650 | 19.48 ± 0.66c | 9.87 ± 0.03a | 76.76 | 30.81 | 1.21 | 5.16 | 13.58 | 0.33 | 0.43 | 0.47 | 0.16 | 8.52 | −59.6 ± 0.75a |
3.2 Adsorption properties
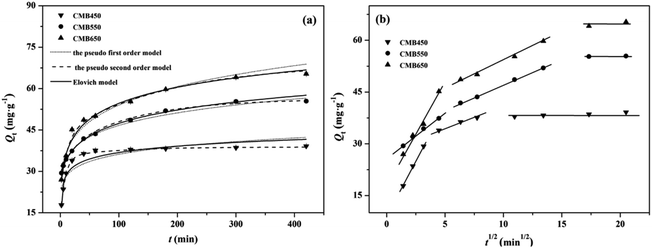
The kinetic model has been widely used to evaluate the adsorption process of heavy metals on biochars.8,15,21 The adsorption kinetics of Zn2+ on CMB450, CMB550 and CMB650 are presented in Fig. 1a, and the parameters fitted by the kinetic models are listed in Table 2. All the correlation coefficients R2 obtained using the pseudo second order kinetic model were greater than 0.997, which were much larger than those using the pseudo first order kinetic model. In addition, the calculated adsorption capacities (Qcal) based on the pseudo second order kinetic model were more accordant with the experimental value (Qexp) than on the pseudo first order kinetic model, indicating that the adsorption processes of Zn2+ on CMB450, CMB550 and CMB650 conformed to the pseudo second order kinetic model, and the adsorption processes were dominated by chemisorption.15,27 Elovich model reflects the heterogeneous diffusion process controlled by reaction rate and diffusion factor, which could be used to better evaluate the importance of chemical adsorption than the pseudo second order kinetic model.17,20 In this study, the correlation coefficients (R2) of Elovich model for CMB650 were greater than 0.99 and significantly greater than those for CMB450 (0.86) and CMB550 (0.97). This shows that the dominance of chemisorption in the process of Zn2+ adsorption on CMB6500 was obviously stronger than those on CMB450 and CMB550. In short, with the increase of pyrolysis temperature, the ability of CMBx to adsorb Zn2+ became stronger and the dominant role of chemisorption was more obvious in the adsorption process.| Sample | Qexp | Pseudo first order model | Pseudo second order model | Elovich model | Intra-particle diffusion model | |
|---|---|---|---|---|---|---|
| a Qexp (mg g−1) and Qcal (mg g−1) are the amount of Zn2+ adsorption at equilibrium and the adsorption capacity calculated by the pseudo first order and pseudo second order models at equilibrium, respectively. k1 (min−1), k2 (g mg−1 min−1) and kdi (mg g−1 min−1/2) are the rate constant of the pseudo first order equation, the rate constant of the pseudo second order equation and the intra-particle diffusion equation, respectively. α (mg g−1 min−1) and β (g mg−1) are the initial adsorption rate constant and a parameter of the Elovich equation, respectively. ci is representing the thickness of the liquid film, mg g−1. | ||||||
| CMB450 | 39.17 | Qcal = 8.62 | Qcal = 39.22 | α = 19.05 | kd1 = 6.56 | kd2 = 1.14 |
| k1 = 0.006 | k2 = 0.007 | β = 3.82 | c1 = 8.64 | c2 = 28.93 | ||
| R2 = 0.6708 | R2 = 0.9999 | R2 = 0.8668 | R2 = 0.9989 | R2 = 0.9853 | ||
| CMB550 | 55.44 | Qcal = 22.54 | Qcal = 56.50 | α = 23.32 | kd1 = 2.58 | kd2 = 1.42 |
| k1 = 0.007 | k2 = 0.002 | β = 5.30 | c1 = 26.01 | c2 = 32.56 | ||
| R2 = 0.9903 | R2 = 0.9982 | R2 = 0.9785 | R2 = 0.9939 | R2 = 0.9988 | ||
| CMB650 | 65.34 | Qcal = 28.91 | Qcal = 66.23 | α = 20.90 | kd1 = 5.78 | kd2 = 1.59 |
| k1 = 0.009 | k2 = 0.001 | β = 7.40 | c1 = 18.79 | c2 = 38.20 | ||
| R2 = 0.9609 | R2 = 0.9976 | R2 = 0.9911 | R2 = 0.9847 | R2 = 0.9939 | ||
The adsorption of Zn2+ on CMB450, CMB550 and CMB650 reached equilibrium within 420 min. The adsorptive amounts increased rapidly during the initial 40 min, which accounted for 74.5–92.9% of total amount of adsorption, and showed only a small increment over the subsequent period until adsorption reached equilibrium. This was a typical three-stage adsorption process, including an initial rapid increase, a period of weakening growth and the final equilibrium stage. The intra-particle diffusion model is helpful to determine processes and mechanisms of adsorption and evaluate rate controlling stages.15,27 The adsorption of Zn2+ on CMBx showed the three stages including the first fast stage by external diffusion, slower stage by intra-particle diffusion, and the third stage by adsorption equilibrium (Fig. 1b). kd1 values corresponding to the first fast stage were higher than kd2 values, and the line fitted at the second stage did not pass through the coordinate origin, implying that the intra-particle diffusion was the main rate-limiting step but not the only rate-limiting step mechanisms. Values of the intercept ci (Table 2), which could reflect the thickness of the adsorbent boundary layer, were greater than 0 for all biochars, and the values of c2 were higher than c1. These findings indicate that the transport process of Zn2+ from solution to biochar particle through the boundary layer might influence the associated adsorption kinetics.13,15
The adsorption isotherms of Zn2+ on CMBx are shown in Fig. 2 and the parameters fitted by Langmuir, Freundlich and Temkin models are listed in Table 3. The Langmuir model fitted the isothermal adsorption data well with higher correlation coefficient R2 (0.995–0.999) than Freundlich model (0.880–0.716), implying that the adsorption mechanisms of Zn2+ on CMBx were mainly dominated by chemical adsorption, and the monolayer adsorption occurred on the homogeneous surfaces of CMBx.20,21 In the fitting of Langmuir model, the R2 value of CMB650 (0.9996) was obviously higher than those of CMB450 (0.9952) and CMB550 (0.9951), indicating that the predominant role of chemisorption of high temperature biochars was more significant for CMB650, which was consistent with the kinetic results. The parameter KL was related to the affinity of the binding sites and represents the affinity between biochars and heavy metal ions. On the basis of KL values, CMB650 had a higher affinity for Zn2+ than CMB450 and CMB550 (Table 3).13 Additionally, the calculated maximum monolayer adsorption capacities based on Langmuir model for CMBx were 80.65–87.72 mg g−1. The observed maximum monolayer adsorption capacities of CMBx for Zn2+ were higher than those of rice straw biochars (39.7 and 40.2 mg g−1),18,28 chicken manure biochars (11.2 mg g−1),18 sewage sludge biochars (4.3 mg g−1),18 wheat straw biochars (41.8 mg g−1),5 S. hermaphrodita biochars (48.1 mg g−1),5 hardwood biochars (4.3 mg g−1)27 and corn straw biochars (4.3 mg g−1),27 suggesting that carp biochar was a promising one for Zn2+ adsorption (Table S1†). The RL values (ESI†) for CMB450, CMB550 and CMB650 were obtained in the range of 0.091–0.455, 0.063–0.357 and 0.032–0.217, respectively, indicating that the adsorption of Zn2+ on CMBx was the favorable adsorption (0 < RL < 1).13,29 Moreover, the Temkin equation also fitted the adsorption data well, suggesting that Zn2+ adsorption process might be partly affected by the adsorbate/adsorbent interactions.13
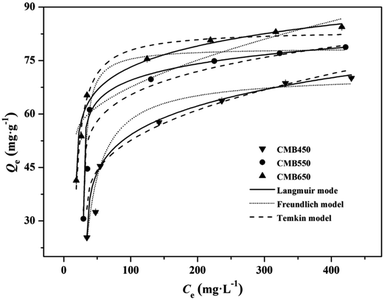 | ||
| Fig. 2 Adsorption isotherms of Zn2+ on biochars with Langmuir model, Freundlich model and Temkin model fitting curves, respectively. | ||
| Sample | Langmuir model | Freundlich model | Temkin model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL | Qm | R2 | KF | n | R2 | KT | A | R2 | |
| a KL (L mg−1), KF [mg(1−1/n) L1/n g−1], and KT (L mg−1) are the adsorption coefficient of the Langmuir model, Freundlich model and Temkin model. Qm is the maximum adsorption capacity calculated by Langmuir model, mg g−1. The dimensionless constant n is experimentally derived exponent of Freundlich model. A (kJ mol−1) is a coefficient related to the heat of adsorption in the Temkin model. | |||||||||
| CMB450 | 0.02 | 80.65 | 0.9952 | 8.32 | 0.37 | 0.8801 | 5.91 | 17.10 | 0.9427 |
| CMB550 | 0.03 | 84.75 | 0.9951 | 17.22 | 0.27 | 0.7163 | 1.50 | 14.62 | 0.8056 |
| CMB650 | 0.06 | 87.72 | 0.9996 | 27.92 | 0.19 | 0.8478 | 2.96 | 12.31 | 0.9061 |
3.3 Qualitative characterization of adsorption mechanisms
According to previous research results,30–32 there are four main mechanisms for biochars to adsorb Zn2+, including cation exchange between protons or alkaline metals and Zn2+, complexation with functional groups, precipitation with minerals, and electrostatic interaction between biochar and Zn2+.Large amounts of metal cations (such as K+, Na+, Ca2+ and Mg2+) were retained on the biochar by electrostatic attraction, forming complexes with functional groups (e.g., –COO−, –R–O− and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) or precipitation.15,17,22 These metals could be exchanged by Zn2+ during adsorption process. To confirm the above mechanism, the net release of metals ions was investigated, which was calculated from the difference between the release amount of metal ions from biochars in salt solution (0.01 M NaNO3) (as the background electrolyte) and Zn2+ solution (100 mg L−1) with 0.01 M NaNO3 (Fig. 3). As shown in Fig. 3, significant amounts of K+, Na+, Ca2+ and Mg2+ were released into solution during the Zn2+ adsorption on CMB450, CMB550 and CMB650, and the total amounts of metal ions released were 0.44, 0.97 and 1.25 meq g−1, respectively, where the release of divalent cations (Ca2+ and Mg2+) was significantly higher than that of monovalent cations (K+ and Na+), and Ca2+ played a dominant role in these released cations because its release amount accounted for 79%, 83% and 81% of the total release, respectively. This was mainly due to two reasons. The first was that the monovalent cation could be retained on biochars by electrostatic attraction,14,15 and could not be complexed with the functional groups or formed precipitates on the surface of the biochars, while the divalent cations could be retained on biochars by precipitation with CO32− and PO43− or complexation with functional groups (e.g., –COOCa+/Mg+, –R–O–Ca+/Mg+ and Ca+/Mg+–π).13–15 Secondly, there were a lot of hydroxyapatite (HAP, CaI4CaII6(PO4)6(OH)2) in animal derived biochars,16,17 and CaI and CaII were considered to be stable and exchangeable, respectively, indicating that the large number of exchangeable crystal lattice metal cations in HAP crystal structures could exchange with Zn2+. The characterization results of XRD (Fig. S4†) and SEM-EDS (Fig. S5–S7†) could provide direct evidence. Both the distribution of Ca–P–O in CMBx surface and Ca–P–O–Zn in CMBx + Zn surface exhibited a notable correlation before and after Zn2+ adsorption in the elemental dot maps of EDS spectra, and such correlations increased as the pyrolysis temperature of biochar increased, which indicated that the pyrolysis temperature had a significant effect on the adsorption mechanism of Zn2+.
C) or precipitation.15,17,22 These metals could be exchanged by Zn2+ during adsorption process. To confirm the above mechanism, the net release of metals ions was investigated, which was calculated from the difference between the release amount of metal ions from biochars in salt solution (0.01 M NaNO3) (as the background electrolyte) and Zn2+ solution (100 mg L−1) with 0.01 M NaNO3 (Fig. 3). As shown in Fig. 3, significant amounts of K+, Na+, Ca2+ and Mg2+ were released into solution during the Zn2+ adsorption on CMB450, CMB550 and CMB650, and the total amounts of metal ions released were 0.44, 0.97 and 1.25 meq g−1, respectively, where the release of divalent cations (Ca2+ and Mg2+) was significantly higher than that of monovalent cations (K+ and Na+), and Ca2+ played a dominant role in these released cations because its release amount accounted for 79%, 83% and 81% of the total release, respectively. This was mainly due to two reasons. The first was that the monovalent cation could be retained on biochars by electrostatic attraction,14,15 and could not be complexed with the functional groups or formed precipitates on the surface of the biochars, while the divalent cations could be retained on biochars by precipitation with CO32− and PO43− or complexation with functional groups (e.g., –COOCa+/Mg+, –R–O–Ca+/Mg+ and Ca+/Mg+–π).13–15 Secondly, there were a lot of hydroxyapatite (HAP, CaI4CaII6(PO4)6(OH)2) in animal derived biochars,16,17 and CaI and CaII were considered to be stable and exchangeable, respectively, indicating that the large number of exchangeable crystal lattice metal cations in HAP crystal structures could exchange with Zn2+. The characterization results of XRD (Fig. S4†) and SEM-EDS (Fig. S5–S7†) could provide direct evidence. Both the distribution of Ca–P–O in CMBx surface and Ca–P–O–Zn in CMBx + Zn surface exhibited a notable correlation before and after Zn2+ adsorption in the elemental dot maps of EDS spectra, and such correlations increased as the pyrolysis temperature of biochar increased, which indicated that the pyrolysis temperature had a significant effect on the adsorption mechanism of Zn2+.
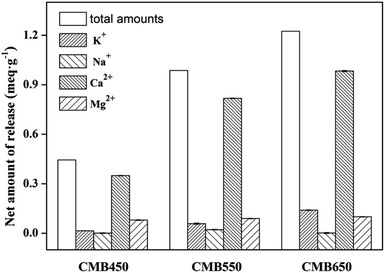 | ||
| Fig. 3 The net amount of released cations (K+, Na+, Ca2+ and Mg2+) from the mixed solution of CMBx with and without Zn2+ after equilibration. | ||
The research by Xue et al. showed that precipitation occurred between Zn2+ and PO43− released from animal (cattle) derived biochars.20 Compared with the CMBx, a larger number of white granular crystals were dispersedly scattered on the surfaces of CMBx + Zn according to the results of SEM images (Fig. S1†). Analysis of the FTIR results of Zn2+ loaded biochars showed that, after Zn2+ adsorption, the peaks at 2013, 1029, 563 and 468 cm−1 (assigned to the v1, v3, v4 and v2 modes of PO43−) had a significant displacement and stronger,16,17,20 and the new peak at 603 cm−1 was attributed to the formation of Zn–O (Fig. S8†).33 In addition, the stability of metal ions forming precipitates in solution was closely related to the solubility product constant (Ksp). For example, for Ca2+, Mg2+ and Zn2+, Ksp values of Ca3(PO4)2 and Mg3(PO4)2 are 2.07 × 10−29 and 1.04 × 10−24 respectively, which are significantly larger than Ksp values of precipitates Zn3(PO4)2 (9.0 × 10−33). That is to say, Zn2+ could form precipitates to be adsorbed on biochars through ion exchange with metal ions. XRD analysis proved that the precipitate formed on the surface of CMBx was zinc phosphate (Fig. S4†), which was similar to the results of Xue et al.20
Complexation of Zn2+ with functional groups through ion exchange has been suggested as an important mechanism for Zn2+ adsorption on biochars, including unsaturated carbon (e.g. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and ionized oxygen-containing functional groups (e.g. hydroxyl and carboxyl groups).18,20,34 Compared with the original biochar, the FTIR results of Zn2+ loaded biochars showed that, after Zn2+ adsorption, the peaks at 3498, 3034 and 1649 cm−1 (assigned to the –OH, –CO–OH and C
C) and ionized oxygen-containing functional groups (e.g. hydroxyl and carboxyl groups).18,20,34 Compared with the original biochar, the FTIR results of Zn2+ loaded biochars showed that, after Zn2+ adsorption, the peaks at 3498, 3034 and 1649 cm−1 (assigned to the –OH, –CO–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) had a significant displacement and were broadened, indicating that the hydroxyl, carboxyl and C
C) had a significant displacement and were broadened, indicating that the hydroxyl, carboxyl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C were involved in the adsorption process of Zn2+ on CMBx. Analysis of the XPS wide scan spectra show that, after Zn2+ adsorption on CMB550, the photoelectron peaks of Zn 2p1/2 and Zn 2p3/2 appeared in the binding energy of 1022.13 eV and 1045.31 eV (Fig. 4f), respectively, indicating that Zn2+ were adsorbed on CMB550 successfully;7,18 the binding energy of C
C were involved in the adsorption process of Zn2+ on CMBx. Analysis of the XPS wide scan spectra show that, after Zn2+ adsorption on CMB550, the photoelectron peaks of Zn 2p1/2 and Zn 2p3/2 appeared in the binding energy of 1022.13 eV and 1045.31 eV (Fig. 4f), respectively, indicating that Zn2+ were adsorbed on CMB550 successfully;7,18 the binding energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/OFGs in C 1s/O 1s XPS spectra had a minor shift due to the interaction between Zn2+ and π electrons/OFGs.18 In order to further explain the mechanism of functional groups in the adsorption reaction, simpler biochar molecules containing C
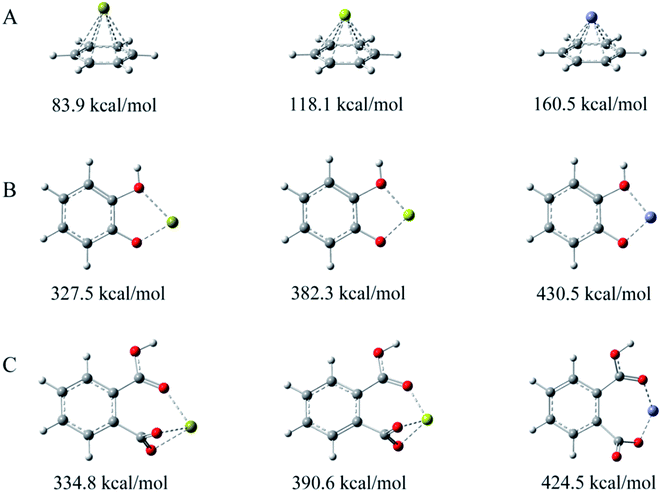
C/OFGs in C 1s/O 1s XPS spectra had a minor shift due to the interaction between Zn2+ and π electrons/OFGs.18 In order to further explain the mechanism of functional groups in the adsorption reaction, simpler biochar molecules containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, hydroxyl and carboxyl groups (e.g., benzene rings, catechol or phthalic acid) were used to model the interactions between biochar and Zn2+. The binding energy between the heavy metal ions (Ca2+, Mg2+ or Zn2+) and the benzene, catechol or phthalic acid were calculated using the G09 package. M2+–C6H6, M+–C6H5O2 or M+–C6H5O4 complexes were calculated at the B3LYP/6-311+G** level (M = Ca, Mg or Zn). The binding energy between the heavy metal ions (Ca2+, Mg2+ and Zn2+) and the benzene, catechol or phthalic was calculated (ESI†), and the results of the optimized stable structure of the M2+–C6H6, M+–C6H5O2 or M+–C6H5O4 complexes and its corresponding binding energy are shown in Fig. 5. For benzene ring, the binding energy for Ca2+ (83.9 kcal mol−1) and Mg2+ (118.1 kcal mol−1) with benzene ring via the combination mode of surface complexation was smaller than that for Zn2+ (160.5 kcal mol−1); therefore, it was easier for Zn2+ complexes with benzene ring through ion exchange with Ca2+ and Mg2+. Similar results were obtained when catechol or phthalic acid combined with Ca2+, Mg2+ and Zn2+. The binding energy difference between benzene ring and Zn2+ (160.5 kcal mol−1) was smaller compared to that of catechol (430.5 kcal mol−1) and phthalic acid (424.5 kcal mol−1), suggesting that Zn2+ adsorption occurred more easily on carboxyl and hydroxyl groups than on C
C, hydroxyl and carboxyl groups (e.g., benzene rings, catechol or phthalic acid) were used to model the interactions between biochar and Zn2+. The binding energy between the heavy metal ions (Ca2+, Mg2+ or Zn2+) and the benzene, catechol or phthalic acid were calculated using the G09 package. M2+–C6H6, M+–C6H5O2 or M+–C6H5O4 complexes were calculated at the B3LYP/6-311+G** level (M = Ca, Mg or Zn). The binding energy between the heavy metal ions (Ca2+, Mg2+ and Zn2+) and the benzene, catechol or phthalic was calculated (ESI†), and the results of the optimized stable structure of the M2+–C6H6, M+–C6H5O2 or M+–C6H5O4 complexes and its corresponding binding energy are shown in Fig. 5. For benzene ring, the binding energy for Ca2+ (83.9 kcal mol−1) and Mg2+ (118.1 kcal mol−1) with benzene ring via the combination mode of surface complexation was smaller than that for Zn2+ (160.5 kcal mol−1); therefore, it was easier for Zn2+ complexes with benzene ring through ion exchange with Ca2+ and Mg2+. Similar results were obtained when catechol or phthalic acid combined with Ca2+, Mg2+ and Zn2+. The binding energy difference between benzene ring and Zn2+ (160.5 kcal mol−1) was smaller compared to that of catechol (430.5 kcal mol−1) and phthalic acid (424.5 kcal mol−1), suggesting that Zn2+ adsorption occurred more easily on carboxyl and hydroxyl groups than on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. Zn2+–π interaction had a certain contribution to Zn2+ adsorption on CMBx but its effect was far less than that of OFGs. There was a fascinating phenomenon: for phthalic acid, since the radius of Zn2+ ion was smaller than that of Ca2+ or Mg2+, the stable structure formed by complexing Zn2+ with phthalic acid was a quasi-planar seven-membered ring, while the larger radius of Ca2+ and Mg2+ were not conducive to the formation of seven-membered ring and eventually coordinated with the three oxygen to form a stable structure.
C. Zn2+–π interaction had a certain contribution to Zn2+ adsorption on CMBx but its effect was far less than that of OFGs. There was a fascinating phenomenon: for phthalic acid, since the radius of Zn2+ ion was smaller than that of Ca2+ or Mg2+, the stable structure formed by complexing Zn2+ with phthalic acid was a quasi-planar seven-membered ring, while the larger radius of Ca2+ and Mg2+ were not conducive to the formation of seven-membered ring and eventually coordinated with the three oxygen to form a stable structure.
 | ||
| Fig. 4 XPS spectra of CMB550 and CMB550 + Zn (a), high resolution spectra of C 1s (b and c), O 1s (d and e) and Zn 2p (f). | ||
The occurrence of complexation of Zn2+ with OFGs (e.g., –COOH, –OH) was usually accompanied by the release of H+ into solution, causing the pH value of solution to decrease.13,21,35 Demineralized biochars (DCMBx) were used to explore the release of H+ due to the occurrence of complexation of Zn2+ with –OH or –COOH in the Zn2+ adsorption process. The results showed that there was a decreasing trend of pH value after Zn2+ adsorption and the decrease of pH values was more significant for DCMB450 than for DCMB650 (Fig. S9†), indicating that the occurrence of complexation of Zn2+ with OFGs might contribute more for the low temperature biochars than high temperature biochars. In summary, the pyrolysis temperature had a significant effect on the adsorption mechanism.
3.4 Quantitative characterisation of adsorption mechanisms
According to the adsorption of Zn2+ on the original biochars or demineralized biochars and related control experiments, the contribution of different mechanisms to the Zn2+ adsorption were calculated. The contributions of precipitation with minerals (Qpm), exchange with cation (Qec), complexation with OFGs (Qcf) and other potential mechanisms (Qop) to the total Zn2+ adsorption (Qo) are shown in Fig. 6. As the pyrolysis temperature increased from 450 to 650 °C, the contribution of both Qec and Qec/Qo increased, while those of Qcf and Qcf/Qo showed the reverse trend. Specifically, the values of Qec and Qec/Qo for CMB450 were 12.4 mg (Zn) g−1 and 31.5%, respectively, while those for CMB650 were 40.7 mg (Zn) g−1 and 61.2% respectively (Fig. 6). On the contrary, the values of Qcf and Qcf/Qo for CMB450 were 16.3 mg (Zn) g−1 and 41.7%, respectively, while those for CMB650 were 3.15 mg (Zn) g−1 and 4.7% respectively. This phenomenon indicated that the contribution of complexation with OFGs to adsorption in high-temperature biochar was reduced, which was supported by the correlation between the pyrolysis temperature and the carbonization degree of biochars (Fig. S2†). In addition, the BET specific surface area and pore volume of biochars increased as the pyrolysis temperature increased (Table 1), which would cause more ion exchange between Ca2+ (from HAP crystal structures) and Zn2+. More HAP crystal structures would be exposed with the higher temperature carbonization of fish bones. Therefore, the exchange with cation dominated the process of adsorption of Zn2+ by high-temperature biochars, while the complexation with OFGs dominated the process by low-temperature biochar. The values of Qpm and Qpm/Qo for CMB650 were higher than those for CMB450 and CMB550, indicating that the precipitation mechanism was more obvious for CMB650, because CMB650 had the highest pH value. However, the values of Qop and Qop/Qo for CMB450 were lower than those for CMB550 and CMB650, indicating that the other potential mechanisms, including physical adsorption, Zn2+–π interaction and electrostatic attraction, were not obvious for CMB450 compared to CMB550 and CMB650, because CMB450 had the lowest BET surface area, aromaticity and zeta potential (Table 1).Overall, the pyrolysis temperature had an important influence on the adsorption performance of Zn2+ on CMBx and the contribution of different mechanisms. With the pyrolysis temperature increased, the effect of the minerals on Zn2+ adsorption (Qec/Qo + Qpm/Qo) increased significantly, while the contribution of OFGs decreased. The value of Qim (Qec + Qpm) for CMBx accounted for 46.4–84.8% of their total adsorption, indicating that the mechanisms of Zn2+ adsorption on CMB650 (84.8%) were dominated by the interaction between Zn2+ and the minerals. On the contrary, the mechanisms of Zn2+ on CMB450 was dominated by complexation with OFGs and exchange with cation due to the value of Qcf/Qo + Qec/Qo for CMB450 accounted for 73.2%. Considering the total adsorption capacity, 650 °C was the optimized pyrolysis temperature for the preparation of biochar from dead carp for treating Zn-contaminated wastewater.
4. Conclusions
The adsorption of Zn2+ on CMBx was dependent on the effect of pyrolysis temperature on properties of biochar. CMB650 had a larger adsorption capacity for Zn2+ than other biochars. The kinetic and isothermal adsorption data could be best fitted by the pseudo second order model and the Langmuir model, respectively. The adsorption mechanism of Zn2+ on biochar mainly included precipitation with minerals, metal ion exchange and complexation with functional groups (e.g. hydroxy, carboxyl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The interaction with minerals including precipitation with the minerals and metal ion exchange dominated the Zn2+ adsorption on CMB550 and CMB650, while the contribution of complexation with OFGs and metal ion exchange were the dominant mechanism in combining Zn2+ with CMB450. Therefore, CMB650 has great potential as an engineering adsorbent to treat Zn-contaminated wastewater.
C). The interaction with minerals including precipitation with the minerals and metal ion exchange dominated the Zn2+ adsorption on CMB550 and CMB650, while the contribution of complexation with OFGs and metal ion exchange were the dominant mechanism in combining Zn2+ with CMB450. Therefore, CMB650 has great potential as an engineering adsorbent to treat Zn-contaminated wastewater.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51766008), Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi (2019L0836 and 2021L460), the research fund for the Xinzhou Teachers University (2018KY10).References
- E. Gozzard, W. M. Mayes, H. A. B. Potter and A. P. Jarvis, Environ. Pollut., 2011, 159, 3113–3122 CrossRef CAS PubMed.
- M. Mekapogu, J. Nadimikeri, P. K. Madri and S. Devi, Int. J. Sediment Res., 2018, 33, 510–517 CrossRef.
- M. Chen, D. Wang, S. Ding, X. Fan, Z. Jin, Y. Wu, Y. Wang and C. Zhang, Sci. Total Environ., 2019, 670, 361–368 CrossRef CAS PubMed.
- J. Deng, D. Fu, W. Hu, X. Lu and H. Bryan, Bioresour. Technol., 2020, 303, 122963, DOI:10.1016/j.biortech.2020.122963.
- A. Bogusz, P. Oleszczuk and R. Dobrowolski, Bioresour. Technol., 2015, 196, 540–549 CrossRef CAS PubMed.
- S. M. Sorouraddin and S. Nouri, Anal. Methods, 2016, 8(6), 1396–1404 RSC.
- J. Song, S. Zhang, G. Li, Q. Du and F. Yang, J. Hazard. Mater., 2020, 391, 121692, DOI:10.1016/j.jhazmat.2019.121692.
- L. Gao, J. Deng, G. Huang, K. Li, K. Cai, Y. Liu and F. Huang, Bioresour. Technol., 2019, 272, 114–122 CrossRef CAS PubMed.
- F. M. Pellera, A. Giannis, D. Kalderis, K. Anastasiadou, R. Stegmann, J. Y. Wang and E. Gidarakos, J. Environ. Manage., 2012, 96, 35–42 CrossRef CAS PubMed.
- W. Yan and R. Liu, Sci. Total Environ., 2018, 628, 1139–1148 Search PubMed.
- H. Zheng, H. Mu and X. Zhao, Mar. Policy, 2018, 96, 152–162 CrossRef.
- W. Qiao, Y. Zhang, X. Xu and L. Zhu, Ind. Water Treat., 2019, 39(10), 26–31 Search PubMed.
- X. Cui, S. Fang, Y. Yao, T. Li, Q. Ni, X. Yang and Z. He, Sci. Total Environ., 2016, 562, 517–525 CrossRef CAS PubMed.
- Z. Mahdi, Q. J. Yu and A. E. Hanandeh, J. Environ. Chem. Eng., 2018, 6, 1171–1181 CrossRef CAS.
- Y. Deng, S. Huang, D. A. Larid, X. Wang and Z. Meng, Chemosphere, 2019, 218, 308–318 CrossRef CAS PubMed.
- S. Lei, Y. Shi, Y. Qiu, L. Che and C. Xue, Sci. Total Environ., 2019, 646, 1281–1289 CrossRef CAS PubMed.
- S. Lei, L. Zhu, C. Xue, C. Hong, J. Wang, L. Che, Y. Hu and Y. Qiu, Environ. Pollut., 2019, 258, 113675, DOI:10.1016/j.envpol.2019.113675.
- M. Zhao, Y. Dai, M. Zhang, C. Feng and R. Qiu, Sci. Total Environ., 2020, 717, 136894, DOI:10.1016/j.scitotenv.2020.136894.
- A. R. Betts, N. Chen, J. G. Hamilton and D. Peak, Environ. Sci. Technol., 2013, 47, 14350–14357 CrossRef CAS PubMed.
- C. Xue, L. Zhu, S. Lei, M. Liu and Y. Qiu, Sci. Total Environ., 2020, 713, 136395, DOI:10.1016/j.scitotenv.2019.136395.
- Z. Wang, G. Liu, H. Zheng, F. Li, H. H. Ngo, W. Guo, C. Liu, L. Chen and B. Xing, Bioresour. Technol., 2015, 177, 308–317 CrossRef CAS PubMed.
- R. Wang, D. Huang, Y. Liu, C. Zhang, C. Lai, G. Zeng, M. Cheng, X. Gong, J. Wan and H. Luo, Bioresour. Technol., 2018, 261, 265–271 CrossRef CAS PubMed.
- Y. Qiu, H. Cheng, C. Xu and G. D. Sheng, Water Res., 2008, 42, 567–574 CrossRef CAS PubMed.
- D. I. Mendoza-Castillo, A. Bonilla-Petriciolet and J. Jáuregui-Rincón, Water Treat., 2015, 54, 1651–1662 CAS.
- X. Cao and W. Harris, Bioresour. Technol., 2010, 101, 5222–5228 CrossRef CAS PubMed.
- Y. Xu and B. Chen, Bioresour. Technol., 2013, 146, 485–493 CrossRef CAS PubMed.
- X. Chen, G. Chen, L. Chen, Y. Chen, J. Lehmann, M. B. McBride and A. G. Hay, Bioresour. Technol., 2011, 102, 8877–8884 CrossRef CAS PubMed.
- J. H. Park, J. J. Wang, S. H. Kim, J. S. Cho, S. W. Kang, R. D. Delaune, K. J. Han and D. C. Seo, Colloids Surf., A, 2017, 533, 330–337 CrossRef CAS.
- L. Wang, Y. M. Xu, X. F. Liang, Y. B. Sun and R. Y. Dong, China Environ. Sci., 2014, 11, 2851–2858 Search PubMed.
- H. Lu, W. Zhang, Y. Yang, X. Huang, S. Wang and R. Qiu, Water Res., 2012, 46, 854–862 CrossRef CAS PubMed.
- M. I. Inyang, B. Gao, Y. Yao, Y. Xue, A. Zimmerman, A. Mosa, P. Pullammanappallil, Y. S. Ok and X. Cao, Crit. Rev. Environ. Sci. Technol., 2016, 46, 406–433 CrossRef CAS.
- H. Li, X. Dong, E. B. D. Silva, L. M. de Oliveira, Y. Chen and L. Q. Ma, Chemosphere, 2017, 178, 466–478 CrossRef CAS PubMed.
- R. Fernández-González, M. A. Martín-Lara, J. A. Moreno, G. Blazquez and M. Calero, J. Cleaner Prod., 2019, 227, 634–663 CrossRef.
- Z. L. Chen, J. Q. Zhang, L. Huang, Z. H. Yuan, Z. J. Li and M. C. Liu, J. Integr. Agric., 2019, 18(1), 201–210 CrossRef CAS.
- X. Cao, L. Ma, B. Gao and W. Harris, Environ. Sci. Technol., 2009, 43, 3285–3291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05636k |
| This journal is © The Royal Society of Chemistry 2021 |