Open Access Article
Open Access ArticleFerrocenium complex aided O-glycosylation of glycosyl halides†
Deva Saroja Talasila and
Eike B. Bauer *
*
University of Missouri – St. Louis, Department of Chemistry and Biochemistry, One University Boulevard, St. Louis, MO 63121, USA. E-mail: bauere@umsl.edu; Fax: +1 314 516 5342; Tel: +1 314 516 5311
First published on 17th November 2021
Abstract
A new strategy for the activation of glycosyl halide donors to be utilized in glycosylation reactions is presented, utilizing the ferrocenium (Fc) complexes [FcB(OH)2]SbF6 and FcBF4 as promoters. The scope of the new system has been investigated using glycosyl chloride and glycosyl fluoride donors in combination with common glycosyl acceptors, such as protected glucose. The corresponding glycosylation products were formed in 95 to 10% isolated yields with α/β ratios ranging from 1/1 to β only (2 to 14 h reaction time at room temperature, 40 to 100% ferrocenium promoter load).
Introduction
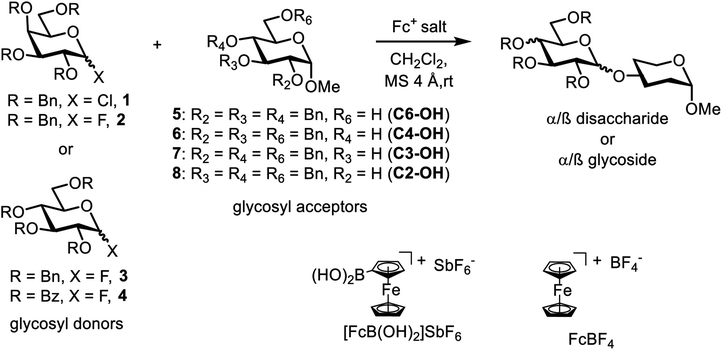
Carbohydrates are a large class of diverse biopolymers with complex structures that are involved in many key biological processes such as cellular communication,1 the regulation of tumor proliferation,2 and immune response.3 Extraction of pure carbohydrates from natural sources is difficult.4 Chemical synthesis through glycosylation provides the most efficient access to carbohydrates and has become the main approach for the investigation and synthesis of carbohydrate-based vaccines and pharmaceuticals.5 Glycosylation is the construction of glycosidic linkages between electrophilic glycosyl donors (with a leaving group at the anomeric position) and the free hydroxyl unit of a glycosyl acceptor; it is a key step in the synthesis of carbohydrates (Scheme 1).6 Two epimers α and β can form, differing only in the configuration at the anomeric carbon, and a high excess of one over the other one is highly desirable.7 The glycosyl donors are typically activated through their leaving group.8 Organic as well as Brønsted acids are employed for that purpose in stoichiometric or catalytic quantities.9 Metal-based activators have been employed as well, in catalytic or stoichiometric quantities.10 Transition-metal catalyzed or promoted glycosylation reactions are less common but known in the literature.10,11 Transition metals in the form of metal complexes can be tuned through the attached ligands, allowing for improvement of performance through catalyst modifications. As such, their employment can advance the selective formation of glycosidic bonds and the synthesis of valuable carbohydrates through glycosylation.As part of our long-standing interest in the catalytic activation of propargylic alcohols to obtain propargylic ethers with alcohol nucleophiles,12 we found that ferrocenium cations can be employed for that purpose.12a,e Ferrocenium complexes (Scheme 1, Fc = ferrocenium) are a large compound class, that have been employed as catalysts or reagents in organic transformations.13 They can be tuned through the substituents on the cyclopentadienyl ring systems coordinated to the iron. Given some similarities between propargylic etherification and glycosylation reactions (both may proceed through a carbocation intermediate and form a carbon–oxygen bond), we were hypothesizing that ferrocenium cations may also promote glycosylation reactions. Iron is an abundant, inexpensive, non-toxic, and environmentally friendly metal, and its metal complexes have been intensely investigated as catalysts to substitute catalyst systems based on more expensive or more harmful metals.14 Iron salts of the general formula FeX3 (with X = Cl,15 CF3SO3,16 NO3 (ref. 17) or SO4)18 have successfully been employed as catalysts or reagents in O-glycosylation reactions or Ferrier rearrangements. However, we are not aware of the application of iron-based complexes beyond these simple, Lewis acidic iron salts. Simple iron salts are not very well soluble in organic solvents. Furthermore, it has been argued that iron salts like Fe(CF3SO3)2 may create small amounts of protons in solution, and that the corresponding glycosylation reactions are actually Brønsted acid catalyzed.19 The application of metal complexes may circumvent that problem. We introduce here for the first time ferrocenium complexes as a new, tunable class of iron(III) complexes promoting glycosylation reactions. Ferrocenium salts are well soluble in CH2Cl2, the solvent of choice for many glycosylation reactions.
Results and discussion
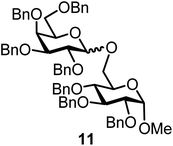
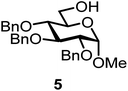
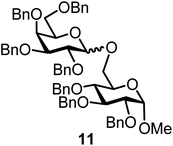
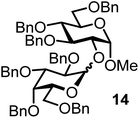
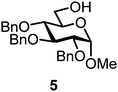
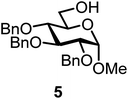
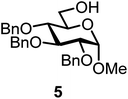
We decided to employ the known galactosyl halides 1 and 2 and the glucosyl halides 3 and 4 as donors (Scheme 1); they are readily available and iron has an affinity to halides, which may promote activation. Furthermore, we selected a set of four known, benzyl-protected acceptors 5 to 8, differing in the position of the free OH group to be engaged in the glycosylation reaction. As promoters, we selected the known iron(III) ferrocenium salts [FcB(OH)2]SbF6 and FcBF4 (Scheme 1). Details of the synthesis of the donors and acceptors as well as the glycosylation reactions are given in the ESI.†In test reactions, we investigated these donors and acceptors employing the two promoters, and optimized the reaction conditions. When the freshly synthesized donors, the glucosyl acceptors and 40–70 mol% of the ferrocenium promoter were combined in CH2Cl2 along with 4 Å molecular sieves under argon at room temperature, the corresponding products 11 to 27 were obtained in 95 to 10% yield after chromatographic workup. No further additives were required. The results of these optimization efforts are compiled in Table 1.
| Entry | Donor | Acceptor | Product | Promoter | Time yielde α/β |
|---|---|---|---|---|---|
| a The galactosyl chloride donor (0.035 mmol), glycosyl acceptor (0.015 mmol), 4 Å molecular sieves and the ferrocenium salt in CH2Cl2 at room temperature.b The glycosyl fluoride donor (0.05 mmol), glycosyl acceptor (0.06 mmol), 4 Å molecular sieves and the promoter in CH2Cl2 at room temperature.c The glycosyl fluoride donor (0.05 mmol), glycosyl acceptor (0.04 mmol), 4 Å molecular sieves and the promoter in CH2Cl2 at room temperature.d The glycosyl fluoride donor (0.02 mmol), glycosyl acceptor (0.02 mmol), 4 Å molecular sieves and the promoter in CH2Cl2 at room temperature.e Isolated yield after chromatographic workup.f In the presence of 100 mol% promoter, only 42% isolated yield were obtained, and hydrolysis was observed. The reaction virtually shut down in the presence of 0.25 to 1 equivalents 2,6-di-tert-butyl-pyridine.g In the presence of 70 mol% promoter, only 10% isolated yields were obtained. | |||||
| 1a |  |
 |
 |
[FcB(OH)2]SbF6, 60 mol% | 4 h 86%, 1/1.1 |
| 2a | 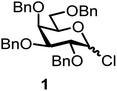 |
 |
 |
[FcB(OH)2]SbF6, 100 mol% | 2 h 95%, 1/1.1 |
| 3a | 1 | 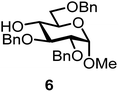 |
 |
[FcB(OH)2]SbF6, 60 mol% | 6 h, 64%, 1.2/1, |
| 4a | 1 | 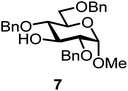 |
 |
[FcB(OH)2]SbF6 60 mol% | 6 h, 63%, 1.6/1 |
| 5a | 1 | 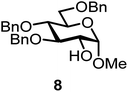 |
 |
[FcB(OH)2]SbF6 60 mol% | 6 h, 45%, 1.2/1 |
| 6b,f | 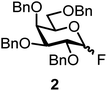 |
 |
 |
FcBF4, 70 mol% | 2 h, 50%, 1/1.3 |
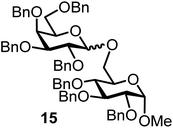
| 7b | 2 | 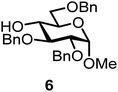 |
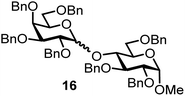 |
FcBF4, 70 mol% | 6 h, 31%, 1/1 |
| 8b | 2 | 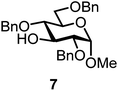 |
 |
FcBF4, 70 mol% | 6 h, 34%, 2.3/1 |
| 9b | 2 | 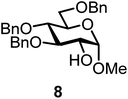 |
 |
FcBF4, 70 mol% | 6 h, 32%, 1.6/1 |
| 10b,g | 2 | 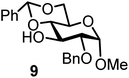 |
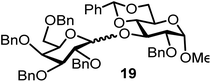 |
FcBF4, 100 mol% | 12 h, 42%, 1.8/1 |
| 11b | 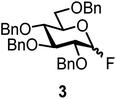 |
 |
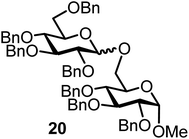 |
FcBF4, 70 mol% | 4 h, 45%, 1/1 |
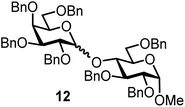
| 12b | 3 | 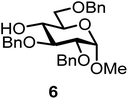 |
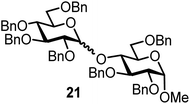 |
FcBF4, 70 mol% | 8 h, 52%, 1.5/1 |
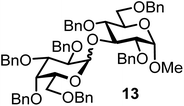
| 13b | 3 | 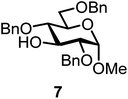 |
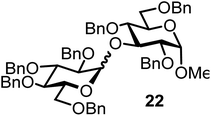 |
FcBF4, 70 mol% | 8 h, 48%, 1/1 |
| 14b | 3 | 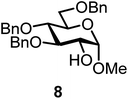 |
 |
FcBF4, 70 mol% | 8 h, 46%, 1/1 |
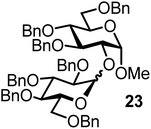
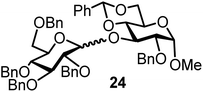
| 15b | 3 | 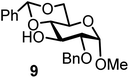 |
 |
FcBF4, 70 mol% | 16 h, 12%, 1/1 |
| 16c | 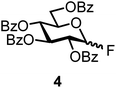 |
 |
 |
FcBF4, 40 mol% | 8 h, 24%, β-only |
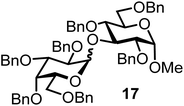
| 17d | 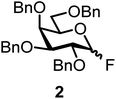 |
 |
 |
FcBF4, 40 mol% | 8 h, 87%, 1/5 |
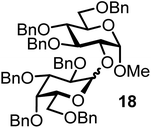
| 18d | 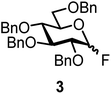 |
10 | 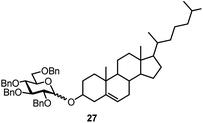 |
FcBF4, 40 mol% | 14 h, 55%, 1/1 |
It turned out that galactosyl donor 1 gave in combination with the most reactive glycosyl acceptor 5 (the free OH group is located on a primary carbon atom) the highest yield of the corresponding disaccharide (11, 86%, entry 1). When employing 100 mol% of the ferrocenium promoter, the yield improved to 95% (entry 2). However, we decided to keep employing 60 mol% of the promoter for practical reasons. The yields dropped to 64 to 45% when the secondary glycosyl acceptors 6 to 8 were employed (entries 3 to 5). Given the steric differences between 5 and 6 to 8, the somewhat lower yields are not surprising. The ferrocenium complex [FcB(OH)2]SbF6 was the promoter of choice for the galactosyl chloride donor 1. Interestingly, the corresponding glucosyl chloride donor did not give any glycosylation, we only observed hydrolysis.
Glycosylation between galactosyl fluoride donor 2 and glycosyl acceptors 5 to 9 gave the corresponding disaccharides 15 to 19 in somewhat lower yields of 50 to 10%, when the promoter FcBF4 was employed (entries 6 to 10). To investigate the impact of an increased amount of promoter in the yield, we performed the reaction in entry 6 with 100 mol% of the promoter. The yield decreased from 50% to 42% and we observed hydrolysis. The benzylidene acetal protected acceptor 9 (entry 10) resulted in an isolate yield of only 10% with a promoter loading of 70 mol%; however, when the promoter was increased to 100 mol%, a 42% yield was obtained. In order to investigate whether an acid scavenger has an impact on the yield, we performed the reaction in entry 6 in the presence of various amounts of 2,6-di-tert-butyl-pyridine. Surprisingly, the yield decreased drastically in the presence of the scavenger. It has been described that metal salts can form small amounts of Brønsted acids in solution, which may be the actual catalysts.19 However, there seems a promoter-dependency on the reaction, which makes mere Brønsted acid catalysis unlikely. In general, the interrelation between promoter load and yield seems not to be straightforward.
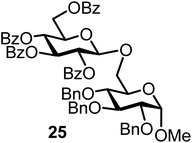
The fluoride glycosyl donor 3 together with the glycosyl acceptors 5 to 9 gave the corresponding disaccharides 20 to 24 in 52 to 12% isolated yields (entries 11 to 15). For these reactions, FcBF4 was the promoter of choice, as [FcB(OH)2]SbF6 did not lead to any conversion. The benzylidene acetal protected acceptor 9 gave the poorest yield of 12% (entry 15). As exemplified in entry 16, the benzoyl-protected fluoride glucosyl donor 4 gave also a poor yield of 24% of the corresponding disaccharide 25, when the acceptor 5 was employed.
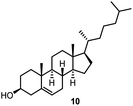
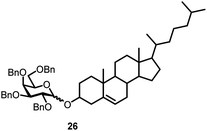
Finally, we investigated whether other secondary alcohols can be employed in the title reaction and selected cholesterol (10) as an acceptor (entries 17 and 18). Certain sterol glycosides can exhibit pharmaceutical activity.20 Utilizing FcBF4 as the promoter, the glycosylation products 26 and 27 were obtained in 87 and 55% isolated yields. All α/β ratios in Table 1 ranged from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to β only with no clear trend obvious. However, only the β epimer was obtained when the OBz protected donor 4 was employed (entry 16), which may be attributed to the directing effect the OBz group can exert.21
1 to β only with no clear trend obvious. However, only the β epimer was obtained when the OBz protected donor 4 was employed (entry 16), which may be attributed to the directing effect the OBz group can exert.21
The results in Table 1 reveal a number of important trends, which will guide the development of refined versions of the ferrocenium complexes as glycosylation promoters or catalysts. First, the nature of the leaving group determines which promoter is the most suitable. For the chloride donor 1, [FcB(OH)2]SbF6 is the promoter of choice, whereas for fluoride donors, FcBF4 is better suitable, as determined during our optimization efforts. To what extent the B(OH)2 group plays a role in the glycosylation mechanism requires further research. It is known, though, that boron has some affinity to OH groups,22 and the B(OH)2 unit may exert a directing effect on the glycosylation reactions. When we reduced the load of the promoter, lower yields were obtained and with increased promoter load, the yields either increased somewhat (Table 1, entries 2 and 10) or they decreased somewhat due to hydrolysis (Table 1, entry 6). These findings point toward an involvement of the promoter in the key step of the glycosylation.
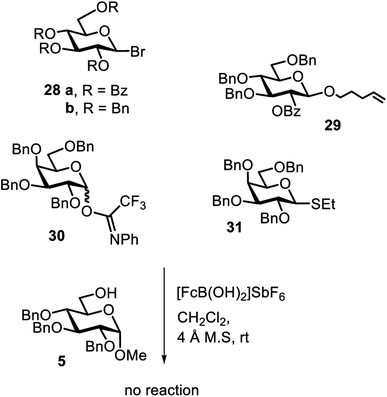
To obtain an understanding about the mode of activation, we also investigated other leaving groups (Fig. 1). The donors 28 with the bromide leaving group and Bn and Bz protecting groups did not give any appreciable amount of product, and so did not donor 29 with an n-pentenyl leaving group, donor 30 with the trichloroacetimidate leaving group or donor 31 with a sulfide leaving group.19 These findings may give some hint about potential activation modes. The leaving groups in Fig. 1 are generally activated by Brønsted or Lewis acids.19 As such, the ferrocenium promoters do not merely play the role of a Lewis acid, because donor 30 with a nitrogen atom as well as donor 31 with a sulfur atom should interact well with the iron center. The trichloroacetimidate leaving group was successfully activated by FeCl3,15b which may be more Lewis acidic compared to the ferrocenium complexes employed herein. On the other hand, an n-pentenyl leaving group was previously proven to also not be activated by Fe(CF3SO3)3,23 which may be due to the low affinity of Fe(III) to isolated double bonds, through which the leaving group may be activated. The mode of activation is currently under investigation. However, the interplay between the leaving group, the protecting groups, the promoter and the promoter load seems to be multifaceted, opening avenues for directed, selective activation of corresponding donors by systematic promoter tuning.
The challenge of iron-promoted or iron-catalyzed glycosylation reactions is the general affinity of iron to oxygen. Both the donors and acceptors feature a number of oxygen atoms. The oxygen atoms of the protected hydroxyl groups in the sugars may be complexed by the iron promoter. When complexed to atoms other than the leaving group, the ferrocenium promoter will not be able to activate the donor, which explains the relatively high promoter loadings of 40 to 70 mol% required for the reaction to proceed efficiently. Along these lines, the benzoyl-protected donor 4 gave a relatively low yield of only 24% (entry 16), a trend we also observed during our optimization efforts utilizing other acceptors. The carbonyl units in the protection groups of donor 4 provide an extra number of oxygen atoms, offering additional opportunities for the ferrocenium promoter to coordinate. In turn, the cholesterol acceptor 10 (featuring only one oxygen atom from the –OH group) gave relatively high yields from 87 to 55% (entries 17 and 18), however, no glycosylation was observed with the benzoyl-protected donor 4. There is clearly a correlation between the number of oxygen atoms present in the substrates and the efficiency of the ferrocenium promoter.
A large number of ferrocenium complexes are known in the literature.13 The advantages of the ferrocenium systems described herein is their tunability through substituent to be placed on the cyclopentadienyl ligand. Furthermore, as outlined above, they exhibit good solubility in organic solvents such as CH2Cl2. Future investigations of iron-promoted or catalyzed glycosylation reactions may need to find a balance between the affinity of the ferrocenium complexes to the leaving group and the oxygen atoms present in the donor and acceptor molecules, and such studies are currently underway.
In summary, we introduced for the first time ferrocenium complexes as a tunable platform to promote glycosylation reactions. The combination of the appropriate ferrocenium complexes with the donors seems to be crucial and will require further research. Given the large structural diversity of ferrocenium salts available, the research described herein offers new avenues for the glycosylation of a variety of donors and acceptors. As opposed to simple metal salts, ferrocenium cations can be tuned through their substituents on the cyclopentadienyl rings to improve reactivity and selectivity and they tend to be well soluble in organic solvents, which may lead to more efficient and selective glycosylation reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the University of Missouri (Tier 2 – emphasis in grand challenge projects) for financial support. We would like to thank Professor Alexei V. Demchenko and his research group for fruitful discussions and advice and Jessica Raj Doshi for technical assistance with data collection.References
- R. S. Haltiwanger and J. B. Lowe, Annu. Rev. Biochem., 2004, 73, 491–537 CrossRef CAS PubMed
.
- M. M. Fuster and J. D. Esko, Nat. Rev. Cancer, 2005, 5, 526–542 CrossRef CAS PubMed
.
- C. M. Park and Y. S. Song, Nutr. Res. Pract., 2013, 7, 423–429 CrossRef CAS PubMed
.
- Z. Wang, Z. S. Chinoy, S. G. Ambre, W. Peng, R. McBride, R. P. de Vries, J. Glushka, J. C. Paulson and G.-J. Boons, Science, 2013, 341, 379–383 CrossRef CAS PubMed
.
-
(a) C. F. Dixon, A. N. Nottingham, A. F. Lozano, J. A. Sizemore, L. A. Russell, C. Valiton, K. L. Newell, D. Babin, W. T. Bridges, M. R. Parris, D. V. Shchirov, N. L. Snyder and J. V. Ruppel, RSC Adv., 2021, 11, 7037–7042 RSC
; (b) S. Kapil, C. Petit, V. N. Drago, D. R. Ronning and S. J. Sucheck, ChemBioChem, 2019, 20, 260–269 CrossRef CAS PubMed
; (c) K. Liu, X. Jiang and P. Hunziker, Nanoscale, 2016, 8, 16091–16156 RSC
; (d) M. Kilcoyne and L. Joshi, Carbohydrates in Therapeutics, 2007, 5, 186–197 CAS
.
-
(a) H. Liao, J. Ma, H. Yao and X.-W. Liu, Org. Biomol. Chem., 2018, 16, 1791–1806 RSC
; (b) J. T. Smoot and A. V. Demchenko, Adv. Carbohydr. Chem. Biochem., 2009, 62, 161–250 CrossRef CAS PubMed
.
- B. Yang, W. Yang, S. Ramadan and X. Huang, Eur. J. Org. Chem., 2018, 1075–1096 CrossRef CAS PubMed
.
- K. Toshima and K. Tatsuta, Chem. Rev., 1993, 93, 1503–1531 CrossRef CAS
.
-
(a) S. A. Blaszczyk, T. C. Homan and W. Tang, Carbohydr. Res., 2019, 471, 64–77 CrossRef CAS PubMed
; (b) R. Williams and M. C. Galan, Eur. J. Org. Chem., 2017, 6247–6264 CrossRef CAS
; (c) S. Zhu, G. Samala, E. T. Sletten, J. L. Stockdill and H. M. Nguyen, Chem. Sci., 2019, 10, 10475–10480 RSC
.
-
(a) E. B. Bauer, Org. Biomol. Chem., 2020, 18, 9160–9180 RSC
; (b) M. M. Nielsen and C. M. Pedersen, Chem. Rev., 2018, 118, 8285–8358 CrossRef CAS PubMed
; (c) X. Li and J. Zhu, Eur. J. Org. Chem., 2016, 4724–4767 CrossRef CAS
; (d) M. J. McKay and H. M. Nguyen, ACS Catal., 2012, 2, 1563–1595 CrossRef CAS PubMed
; (e) X. Li and J. Zhu, J. Carbohydr. Chem., 2012, 31, 284–324 CrossRef
.
- Representative examples of metal-catalyzed glycosylation reactions:
(a) P. Peng and R. R. Schmidt, J. Am. Chem. Soc., 2015, 137, 12653–12659 CrossRef CAS PubMed
; (b) B. P. Schuff, G. J. Mercer and H. M. Nguyen, Org. Lett., 2007, 9, 3173–3176 CrossRef CAS PubMed
; (c) S. Hotha and S. Kashyap, J. Am. Chem. Soc., 2006, 128, 9620–9621 CrossRef CAS PubMed
.
-
(a) D. S. Talasila, M. J. Queensen, M. Barnes-Flaspoler, K. Jurkowski, E. Stephenson, J. M. Rabus and E. B. Bauer, Eur. J. Org. Chem., 2019, 7348–7358 CrossRef CAS
; (b) N. Jourabchian, K. Jurkowski and E. B. Bauer, Catal. Commun., 2018, 106, 92–95 CrossRef CAS
; (c) M. J. Stark, M. J. Shaw, A. Fadamin, M. P. Rath and E. B. Bauer, J. Organomet. Chem., 2017, 847, 41–53 CrossRef CAS
; (d) M. J. Stark, M. J. Shaw, N. P. Rath and E. B. Bauer, Eur. J. Inorg. Chem., 2016, 1093–1102 CrossRef CAS
; (e) M. Q. Queensen, J. M. Rabus and E. B. Bauer, J. Mol. Catal. A: Chem., 2015, 407, 221–229 CrossRef CAS
; (f) D. F. Alkhaleeli, K. J. Baum, J. M. Rabus and E. B. Bauer, Catal. Commun., 2014, 47, 45–48 CrossRef CAS
; (g) A. K. Widaman, N. P. Rath and E. B. Bauer, New J. Chem., 2011, 35, 2427–2434 RSC
; (h) S. Costin, N. P. Rath and E. B. Bauer, Adv. Synth. Catal., 2008, 350, 2414–2424 CrossRef CAS
.
- Š. Toma and R. Šebesta, Synthesis, 2015, 47, 1683–1695 CrossRef
.
-
(a) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed
; (b) E. B. Bauer, Curr. Org. Chem., 2008, 12, 1341–1369 CrossRef CAS
.
-
(a) M. M. Mukherjee and R. Ghosh, J. Org. Chem., 2017, 82, 5751–5760 CrossRef CAS PubMed
; (b) M. M. Mukherjee, N. Basu and R. Ghosh, RSC Adv., 2016, 6, 105589–105606 RSC
; (c) S. A. Geringer and A. V. Demchenko, Org. Biomol. Chem., 2018, 16, 9133–9137 RSC
; (d) G. S. Y. Wu, A. Liu, S. Qiu, W. Zhang, Z. Wang and J. Zhang, Synlett, 2018, 29, 668–672 CrossRef
; (e) S. Narayanaperumal, R. C. da Silva, J. L. Monteiro, A. G. Corrêa and M. W. Paixão, J. Braz. Chem. Soc., 2012, 23, 1982–1988 CrossRef CAS
; (f) O. Monasson, G. Sizun-Thomé, N. Lubin-Germain, J. Uziel and J. Augé, Carbohydr. Res., 2012, 352, 202–205 CrossRef CAS PubMed
; (g) S. B. Salunke, N. S. Babuz and C.-T. Chen, Chem. Commun., 2011, 47, 10440–10442 RSC
; (h) H. Tachallait, M. S. Filho, H. Marzag, K. Bougrin, L. Demange, A. R. Martin and R. Benhida, New J. Chem., 2019, 43, 5551–5558 RSC
; (i) H. Guo, W. Si, J. Li, G. Yang, T. Tang, Z. Wang, J. Tang and J. Zhang, Synthesis, 2019, 51, 2984–3000 CrossRef CAS
.
-
(a) L. Yang, C. H. Hammelev and C. M. Pedersen, ChemSusChem, 2020, 13, 3166–3171 CrossRef CAS PubMed
; (b) K. Kowalska and C. M. Pedersen, Org. Biomol. Chem., 2020, 18, 1918–1925 RSC
; (c) A. Xolin, A. Stévenin, M. Pucheault, S. Norsikian, F.-D. Boyer and J.-M. Beau, Org. Chem. Front., 2014, 1, 992–1000 RSC
; (d) A. Xolin, S. Norsikian, F.-D. Boyer and J.-M. Beau, Eur. J. Org. Chem., 2016, 3408–3418 CrossRef CAS
.
- P. U. Naik, S. J. Nara, J. R. Harjani and M. M. Salunkhe, J. Mol. Catal. A: Chem., 2005, 234, 35–43 CrossRef CAS
.
- G. Zhang, Q. Liu, L. Shi and J. Wang, Tetrahedron, 2008, 64, 339–344 CrossRef CAS
.
- E. T. Sletten, Y.-J. Tu, H. B. Schlegel and H. M. Nguyen, ACS Catal., 2019, 9, 2110–2123 CrossRef CAS PubMed
.
- A. Verma and B. Ahmed, Med. Chem. Res., 2012, 21, 2449–2453 CrossRef CAS
.
- Reactivity Tuning in Oligosaccharide Assembly, B. Fraser-Reid and J. Cristóbal López, Springer, New York, 2011 Search PubMed
.
- A. Harada, T. Takagi, S. Kataoka, T. Yamamoto and A. Endo, Adsorption, 2011, 17, 171–178 CrossRef CAS
.
- Y. Zu, C. Cai, J. Sheng, L. Cheng, Y. Feng, S. Zhang, Q. Zhang and Y. Chai, Org. Lett., 2019, 21, 8270–8274 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05788j |
| This journal is © The Royal Society of Chemistry 2021 |