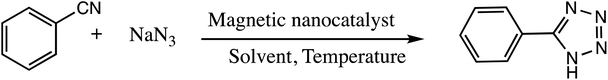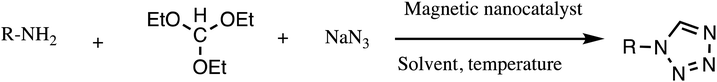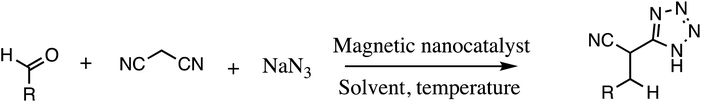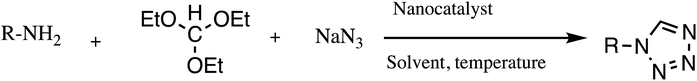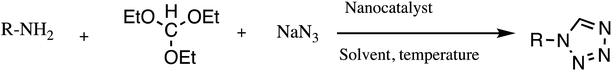Open Access Article
Open Access ArticleNanomaterial catalyzed green synthesis of tetrazoles and its derivatives: a review on recent advancements
Suman Swami
 a,
Satya Narayan Sahu
a,
Satya Narayan Sahu
 b and
Rahul Shrivastava
b and
Rahul Shrivastava
 *a
*a
aDepartment of Chemistry, Manipal University Jaipur, VPO-Dehmi-Kalan, Off Jaipur-Ajmer Express Way, Jaipur, Rajasthan, India-303007. E-mail: chem.rahul@gmail.com
bSchool of Chemistry, Sambalpur University, Jyoti Vihar, Burla, Sambalpur, Orissa, India-768019
First published on 7th December 2021
Abstract
Tetrazoles are indispensable nitrogen containing heterocyclic scaffolds that offer a broad spectrum of applications in various domains such as medicinal chemistry, high energy material science, biochemistry, pharmacology etc. Owing to their useful applications, a wide range of catalysts have been explored for green synthesis of tetrazole derivatives. In recent times, nanomaterials have been emerged as extremely efficient catalysts for different organic transformations because of their high surface area-to-volume ratio, easy surface modification, simple fabrications, easy recovery and reusability. In this article, we have presented an overview of utilization of various nano-catalysts, nanocomposites and other solid-supported nanomaterials as an efficient environmental benign catalytic system for green synthesis of tetrazoles and derivatives. This review will provide an exclusive emphasis on boehmite, magnetic, copper, carbon, MCM-41, and composite based nanomaterials that have been developed since the year 2010 for the synthesis of tetrazole derivatives. In addition, we have briefly discussed the fabrication, functionalization and characterization of some novel nanomaterials and their advantages in the synthesis of tetrazole and its derivatives along with the reaction mechanism that involves synthesis of tetrazole derivatives via nanomaterials catalysed reactions.
1. Introduction
Chemicals are necessary for production of various essential products, pharmaceutically relevant moieties, food additives, pesticides, fertilizers and many more. The production of these necessary products involves well established chemical routes that have not been upgraded or modified over the years by chemical industries. Many of these chemical processes accomplished with high temperature, pressure, toxic solvent, catalyst, chemicals and generate enormous amount of toxic chemical waste which impose hazardous impacts on environment and health.1,2 In recent years, environmental policies have become more stringent because of increased awareness about harmful impacts of generated by-products and reagents on environment and living species. Further, owing to global health crisis generated by industrial toxic chemical wastes, various regulatory bodies demand to adopt eco-compatible greener synthetic processes against the conventional synthetic procedures in chemical industries. Among the various aspects for advancement in chemical process, use of green and efficient catalyst played a key role with aim of reducing temperature, pressure, reagent-based waste with minimum unwanted toxic by-products. In this context, designing and development of sustainable environmental benign catalysts are one of the urgently indispensable challenge facing by chemists. The idea of green chemistry has become an integral part of sustainability which makes catalysis science even more innovative. From the viewpoint of green chemistry, sustainable catalyst must possess a series of distinct advantages like high activity, selectivity and efficiency, reasonable recovery, high stability and excellent recyclability.Catalysts can be generally divided into two types based on reaction phase: (i) homogeneous (ii) heterogeneous catalyst. Homogenous catalysts have several advantages such as high selectivity, better yield, and high turnover numbers due to its solubility in reaction medium which makes catalytic sites of homogeneous catalyst more accessible. Despite its significant advantages, separation of homogenous catalyst from reaction medium is time-consuming and laborious task and needs specific techniques. Also, homogenous catalyst derived chemical process are not compatible with green chemistry principles in modern-day catalysis science, especially from environmental and economic standpoint. Therefore, in recent years, chemists have focused their effort to transform homogenous catalytic process by heterogeneous catalysts so that they can avoids indorsed limitation. During the last two decades, chemists all over the world are fascinated toward nanomaterials that can be used as an excellent alternative of conventional catalytic system thereby can reduce gap between homogenous and heterogeneous catalysis. The nanomaterials could provide incredible solid support for immobilization of homogeneous catalysts to produce combine benefits of homogeneous and heterogeneous catalysis. Nano catalysts or nanomaterials support can assist chemists in development of sustainable catalyst with outstanding activity, selectivity and stability by fine-tuning their morphologies, shapes and sizes. These nanomaterials based catalytic systems are useful for numerous reasons such as:
• The interaction between nano catalyst and reactant are significantly higher due to the large surface area of nano catalyst which facilitates excellent rate of reaction in comparison to its homogeneous counterpart.
• Due to nano meter size, their surface area-to-volume ratio are very high.
• The sizes, shapes, and morphology of nano catalysts can be easily tuned as per the necessity of catalytic applications which would generally be tricky in their homogeneous counterparts.
• The recovery of magnetic nano catalysts from reaction mixtures with help of an external magnet is easy and convenient.
• Nanomaterials based catalysts are recyclable and can be reused many times which could bring them for more industrial applications, especially from economic standpoint.
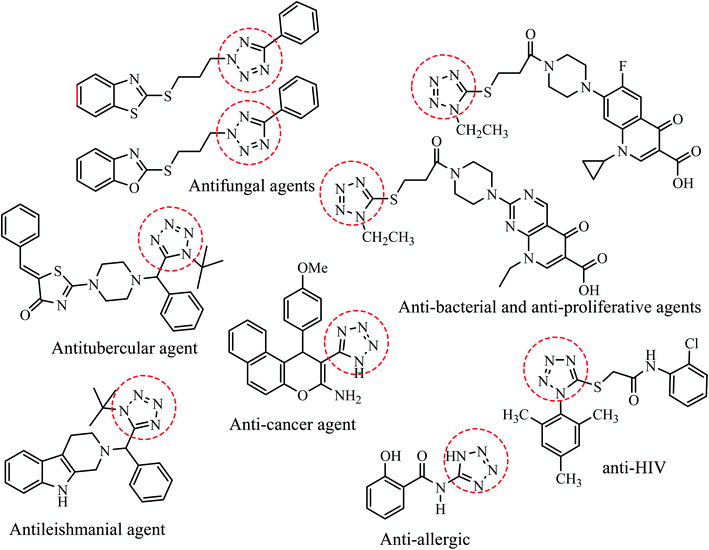
To develop innovative pharmaceutical entities, number of novel heterocyclic scaffolds have been explored based on their intrinsic toxicity towards numerous pathogens. Amongst the various nitrogen-containing heterocycles, tetrazole based molecules have attracted considerable attention due to their potential biological applications towards antifungal,3–7 anti-HIV,8 antitubercular,9 antibacterial,10 antiulcer,11,12 antiallergic,13 antihypertensive,14 antileishmanial,15 anticancer,16–19 antibacterial and antiproliferative20 activities (Fig. 1). Further, tetrazole derivatives are leading bio-isosteres of carboxylic acids/cis-amide which play important roles in enhancement of lipophilicity, bioavailability, pharmacokinetic properties and reduce side effects of the drug molecules.16 Besides medicinal utility, tetrazoles are also widely employed in various fields of research such as high energy material science, photography, bioimaging agents, explosives and organometallic chemistry etc.21,22
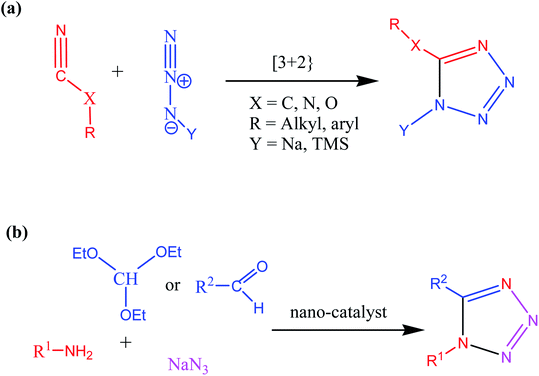
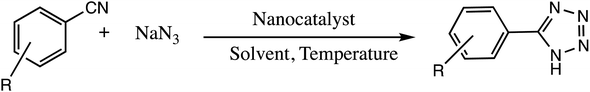
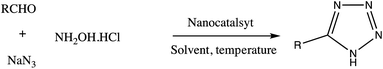
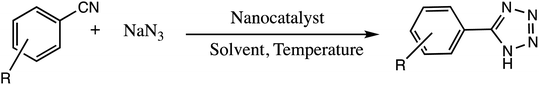
Consequently, owing to the potent applications of tetrazole scaffolds in diverse areas, different methodology for synthesis of tetrazole entities have been attempted. In general, tetrazoles are directly prepared either by: (a) [3+2] cycloaddition reaction between isocyanides and hydrazoic acid/trimethyl azide23–25 or (b) one-pot multicomponent reaction (MCR) between amine/organic nitrile, triethyl orthoformate/aldehyde and sodium azide21 under influence of an appropriate catalyst and solvent conditions as shown in Fig. 2.
It was observed that despite several advantages of conventional synthetic route [3+2] cycloaddition reaction of isocyanides and trimethyl azide/hydrazoic acids, this methodology also suffers some limitations such as uses of hazardous acids, toxic solvents, high temperatures and low to moderate yields and longer reaction times. Therefore, various research group offered multicomponent reaction (MCR) approaches with suitable catalysts, solvents, and reaction conditions to overcome limitations associated with conventional methodology. In this context, number of acidic homogenous and heterogenous catalysts were investigated for synthesis of tetrazole derivatives via multicomponent reaction. It was found that despite high selectivity and activity of homogenous catalyst, it suffers from few drawbacks such as tedious workup and purification procedure, difficulties in separation of catalysts and high temperature whereas using traditional heterogeneous catalyst, low yield and long reaction time are main disadvantages for synthesis of tetrazoles derivatives. Therefore, considering limitations associate with both homogenous and heterogeneous catalyst systems, researcher devoted massive efforts to achieve green synthetic methodology for synthesis of tetrazole via introducing nanostructured catalyst systems.
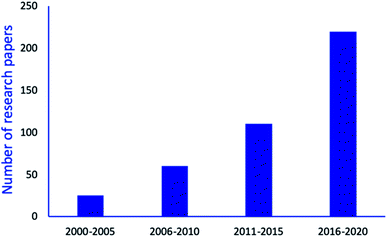
As a result, several nano-catalysts or nanomaterials supported catalysts have been explored to avoid drawbacks associated with the conventional catalytic system for synthesize of tetrazole derivatives. It is observed that interest in synthesis of tetrazole derivatives via environmental benign process by using various nanomaterials have continuously risen as depicted by Fig. 3. As shown in figure, ample magnetic and non-magnetic nanomaterials-based research articles are published since 2010 for tetrazoles synthesis comprising the green chemical processes.
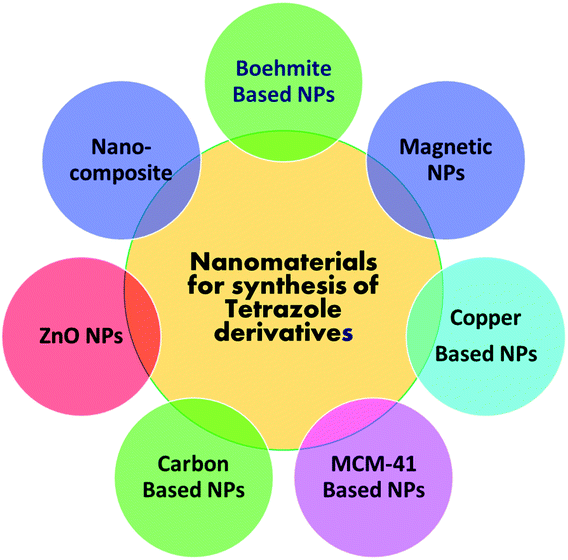
The detail literature survey reflects that no systematic and comprehensive overview approaching nanomaterials catalyzed tetrazoles synthesis have been reported so far. The published reviews on nanoparticles catalyzed heterocyclic compounds are majorly discussed on synthesis of numerous diverse heterocyclic ring system rather than emphasis only on tetrazole and derivatives. Thus, we believe that an insightful overview of the established nano-catalysts employed for synthesis of tetrazole derivatives, would be a driving step to the scientific community working in this domain. In the present review, we have emphasized mainly on exploring applications of different nanoparticle-based catalysts used in tetrazole synthesis through multicomponent reaction so far. Examples described in the present review are grouped under different sections (Fig. 4) based on boehmite, magnetic, non-magnetic, copper, ZnO, MCM-41 and carbon nanoparticles catalyzed synthesis of tetrazole derivatives with comprehensive analysis on synthesis and advantages thereof.
2. Boehmite based nano-catalyzed synthesis of 5-substitute tetrazoles
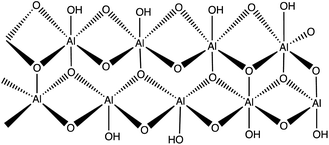
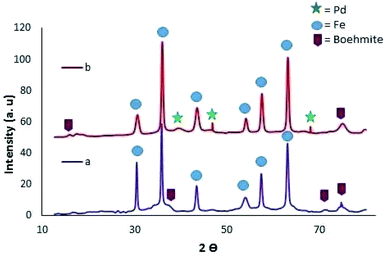
Boehmite is an aluminum oxide hydroxide (γ-AlOOH) having large number of hydroxyl groups on its surface (Fig. 5).26–28 These terminal hydroxyl groups provide high reactivity and hydrophilic environment to boehmite which enhances catalytic activities. In view of high reactivity and hydrophilic nature of boehmite, it was envisaged that transforming boehmite from molecular scale level to nanoscale level makes them even more attractive and efficient catalyst for various organic transformations. It was found that at nanoscale, boehmite devises several appealing features such as high thermal, mechanical, and non-toxicity, high dispersion of the active phases, chemical stability, high specific surface area, readily and easily available and favorable biocompatibility.26,29–35The high specific surface area (more than 120 m2 g−1), hydroxyl groups on surface, and ease of surface modification of boehmite29,36 make them suitable green nano-catalyst for several organic transformation. The overwhelming performance of boehmite@nanostructure as catalyst in tetrazole synthesis motivates researchers to explore it at large extends. In this connection, a boehmite supported Pd-SMTU@boehmite nano-catalyst was prepared and examined their catalytic utility in 5-substituted-1H-tetrazole synthesis (Table 1, entry 1).37 Optimization of reaction conditions involved a reaction between nitrile (1 mmol) and sodium azide (1.4 mmol) in the presence of Pd-SMTU@boehmite at 120 °C in poly(ethylene glycol)-400 as green solvent. The optimized condition reveals that 0.3 mol% catalyst loading (Pd-SMTU@boehmite) are sufficient to achieve maximum yield. Similarly, boehmite@SiO2@Tris-Cu(I) nano-catalyst was prepared for synthesis of 5-substituted-1H-tetrazole derivatives (Table 1, entry 2).38 The boehmite@SiO2@Tris-Cu(I) synthesis comprises immobilization of a Cu(I) complex on modified-surface of boehmite nanoparticles and characterized through FT-IR, XRD, EDS, ICP, X-ray mapping, TGA and SEM techniques. Further, nickel-anchored curcumin-functionalized boehmite nanoparticle (BNPs@Cur-Ni) was prepared via the sequential process as depicted in the Scheme 1 (Table 1, entry 3). The synthesized nano-catalyst was successfully employed in 5-substituted-1H-tetrazole synthesis.39 The BNPs@Cur-Ni NPs exhibited excellent catalytic activity for 5-substituted-1H-tetrazole synthesis with magnificent turnover number (TON) and turnover frequency (TOF) results.
| Entry | Boehmite@nano-structures | Catalyst loading (mg or mol%) | Temperature (°C) | Solvent | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Pd-SMTU@boehmite | 0.30 mol% | 120 | PEG-400 | 2.5 | 95 | 37 |
| 2 | Boehmite@SiO2@Tris-Cu(I) | 0.20 mol% | 120 | PEG-400 | 2 | 97 | 38 |
| 3 | Boehmite Nps@Cur-Ni | 40 mg | 120 | PEG-400 | 1.25 | 97 | 39 |
| 4 | Ni-SMTU@boehmite | 25 mg | 120 | PEG-400 | 1.33 | 94 | 32 |
| 5 | Pd-isatin@boehmite | 35 mg | 120 | PEG-400 | 7 | 96 | 36 |
| 6 | Pd-Arg@boehmite | 25 mg | 120 | PEG-400 | 7 | 97 | 40 |
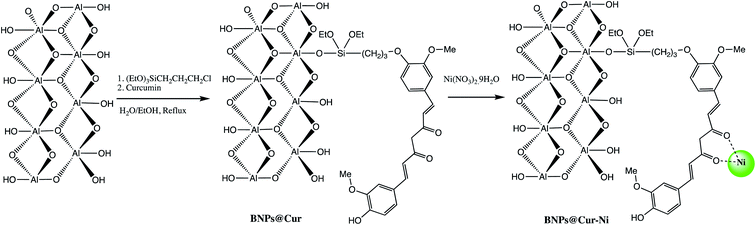 | ||
| Scheme 1 Synthesis of BNPs@Cur-Ni nano-catalyst.39 | ||
In another attempt, boehmite based nano catalyst was synthesized through three-step process as shown in Scheme 2. The synthesized Ni-SMTU@boehmite was effectively used for synthesis of tetrazole derivatives with good yield (Table 1, entry 4).32 However organic moiety functionalized nanostructure always demanding in organic transformation, thus a Schiff base functionalized boehmite nanostructure was synthesized and utilized for the same (Table 1, entry 5).36
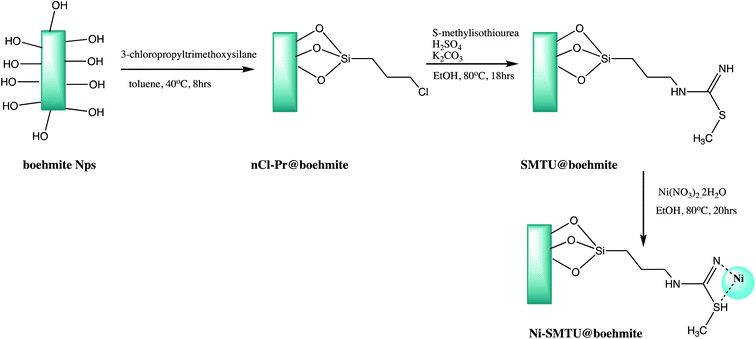 | ||
| Scheme 2 Synthesis of Ni-SMTU@boehmite nanoparticles.32 | ||
A palladium–arginine complex immobilized on boehmite nanoparticles (Pd-Arg@boehmite) was synthesized to serve as a green catalyst in tetrazole synthesis (Table 1, entry 6).40 The reported boehmite supported catalytic systems offers several advantages like high substrate competency, use of environmental-friendly and commercially accessible starting materials, operational simplicity, shorter reaction time, ease of catalyst separation and reusability over several times without leaching of the braced metal ions (Cu, Pd, Ni) and high yield.
3. Magnetic nano-catalyzed synthesis of tetrazoles
Magnetic nano particles have received tremendous attention in the last few decades as an excellent catalyst or supporting materials for the organic transformation. Magnetic nanomaterials have number of remarkable features like easy preparation, reusability, high catalytic efficiency, and handy separation from reaction mixtures through an external magnet which makes them ideal and efficient nano-catalyst.37–39 For example, Zarghania and Akhlaghinia have explored magnetite-chitin (Fe3O4@chitin) as an eco-friendly magnetic nano-catalyst for the synthesis of 5-substituted-1H-tetrazoles under solvent-free conditions (Table 2, entry 1).41 The magnetite-chitin was prepared via a hydrothermal synthesis and characterized with XRD, TEM, SEM, FT-IR, TGA and VSM analytical techniques. It was observed that Fe3O4@chitin catalyst deals with the synthesis of a different tetrazoles from reaction of 1-butyl-3-methylimidazolium azide ([bmim][N3]) with nitriles under solvent-free condition. The excellent yield, shorter reaction times (15–120 minutes), easy preparation of the catalyst, abating chemical waste and good reusability without any significant loss of catalytic efficiency are some of the advantages of the present protocol.| Entry | Magnetic@nanocatalyst | Solvent | Catalyst loading mg or mol% | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4@chitin | — | 0.03 g | 110 | 0.33 | 95 | 41 |
| 2 | Fe3O4@tryptophan–La | H2O | 8 mg | 80 | 0.1 | 96 | 42 |
| 3 | Fe3O4@Nd | H2O | 4 mg | 80 | 0.16 | 95 | 43 |
| 4 | Fe3O4-adenine-Zn | PEG-400 | 50 mg | 120 | 1.33 | 96 | 44 |
| 5 | Fe3O4@MCM-41@Cu-P2C | PEG | 20 mg | 120 | 3 | 96 | 45 |
| 6 | Fe3O4@L-aspartic-Gd | PEG | 70 mg | 100 | 2.08 | 93 | 46 |
| 7 | Fe3O4@tryptophan@Ni | PEG-400 | 0.08 g | 120 | 0.33 | 97 | 47 |
| 8 | Fe3O4@SiO2-APTES-TFA | Ethanol | 0.1 g | 80 | 4 | 97 | 48 |
| 9 | Fe3O4@L-lysine-Pd(0) | H2O | 0.30% | 100 | 1 | 99 | 49 |
| 10 | Fe3O4/HT-GLYMO-TA(IV) | H2O | 0.005 g | 70 | 0.33 | 95 | 50 |
| 11 | Methionine@Fe3O4 | DMSO | 0.05 g | 120 | 0.166 | 100 | 51 |
| 12 | CoFe2O4@glycine-Yb | PEG | 0.07 g | 120 | 2.33 | 93 | 52 |
| 13 | Chitosan supported magnetic ionic liquid nanoparticles (CSMIL) | — | 2.5 mol% | 70 | 6 | 92 | 54 |
In another interesting report, magnetic Fe3O4@tryptophan–La catalyst was prepared via anchoring lanthanum on the surface of Fe3O4 nanoparticles and characterized through FT-IR, SEM, TGA, XRD, EDX, and ICP techniques. The catalytic behavior of Fe3O4@tryptophan–La was investigated as recyclable catalytic system for synthesis of 5-substituted tetrazoles using various substituted aromatic nitrile and NaN3 in neat reaction condition (Table 2, entry 2). Along with substrate generality, La leaching was studied by La loading amount before and after recycling of catalyst by ICP-OEIS technique which shows a very negligible leaching of La metal from catalyst surface. This catalyst offers excellent product yield, high reusability cycles, negligible leaching of La and easy separation of catalyst via an external magnetic field.42 A similar catalyst (Fe3O4@Nd) was synthesized by T. Tamoradi et al. for purpose of synthesis of 5-substituted-1H-tetrazoles (Table 2, entry 3). The catalyst was synthesized through surface functionalization of Fe3O4 nanoparticles with tryptophan ligand followed by Nd immobilization. The prepared Fe3O4@Nd nano-catalyst exhibits remarkable catalytic activity toward the synthesis of aromatic tetrazoles with excellent yield.43
Further, in another report, Fe3O4 functionalized with zinc(II)-adenine complex was successfully synthesized and employed for synthesis of 5-substituted tetrazoles (Table 2, entry 4). The catalytic activity of Fe3O4-adenine-Zn was investigated with model reactants 4-chlorobenzonitrile and sodium azide in PEG at 120 °C. It was observed that Fe3O4-adenine-Zn nano-catalyst offers 96% yield within 80 minutes. Additionally, nano-catalyst Fe3O4-adenine-Zn gave excellent product yield with variety of aromatic and aliphatic nitriles.44
A magnetic nano-catalyst, Fe3O4@MCM-41@Cu-P2C was developed by Nikoorazm and Erfani and examined it catalyst efficiency for synthesis of 5-substituted-1H-tetrazoles derivatives using wide variety of nitriles (Table 2, entry 5). The Fe3O4@MCM-41@Cu-P2C nano-catalyst offers many advantages like simple handling, excellent yield, easy recovery by an external magnet and reusability up to five times without any significant loss of its catalytic activity.45 Similarly, Fe3O4@L-aspartic-Gd nano-catalyst was prepared and characterized. The Fe3O4@L-aspartic-Gd nano-catalyst was utilized for synthesis of 5-substituted-1H-tetrazoles (Table 2, entry 6). The reaction conditions were optimized with model reaction of sodium azide (1.2 mmol) and 4-chlorobenzonitrile (1 mmol). The solvent and temperature influence studies revealed that 100 °C with PEG solvent provided maximum yield of product. Further, Gd leaching studies was also examined by ICP-OEIS technique which indicated that traces of Gd was leached from the initial load to the next run. The beneficial features associated with the protocol were easy separation of catalyst, high catalytic activity, chemical and physical stability, easy workups, short reaction-time and good yields.46
An efficient Fe3O4@tryptophan@Ni (Table 2, entry 7) nano-catalyst was prepared by immobilization of Ni metal on tryptophane coated Fe3O4 nanoparticles as depicted in Scheme 3. The prepared Fe3O4@tryptophan@Ni was characterized using various spectroscopic techniques. The synthesized Fe3O4@tryptophan@Ni nano-catalyst was successfully furnished desired tetrazole derivatives in excellent yield and shorter reaction time (20 minutes). The reusability and metal leaching studies revealed that catalyst can be used up to seven cycles without significant leaching of Ni metal. The easy recovery, reusability and thermal stability are features advantages of Fe3O4@tryptophan@Ni nano-catalyzed synthesis of tetrazole derivatives.47
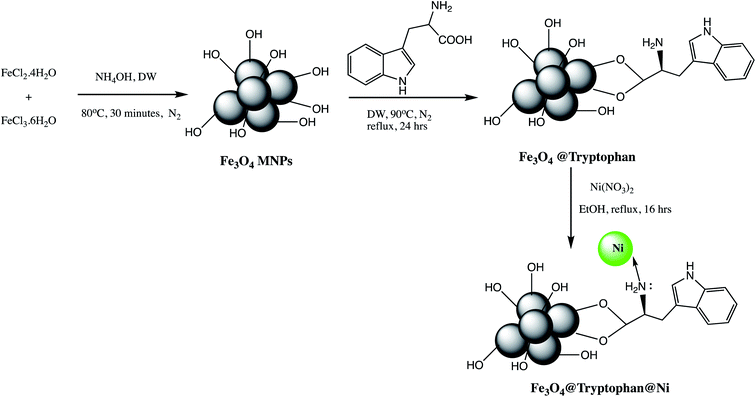 | ||
| Scheme 3 Preparation of Fe3O4@tryptophan@Ni nanoparticles.47 | ||
In another attempt, Fe3O4@SiO2-APTES-TFA nano-catalyst was developed by Fatahi et al. for synthesis of 5-substituted-1H-tetrazoles from various nitriles and sodium azide in EtOH at 80 °C (Table 2, entry 8). The synthesized nano-catalyst produced target tetrazoles in excellent yields in short reaction time of 4 h. Along with this, nano catalyst was easily recovered from external magnet and reused several times without significant loss of catalytic activity.48 Similarly, structurally diverse wide library of 5-substituted-1H-tetrazoles drug-like molecules was synthesized by Ashraf et al. by introducing new magnetic nano-catalyst Fe3O4@L-lysine-Pd(0) catalyzed protocol (Table 2, entry 9). The Fe3O4@L-lysine-Pd(0) catalyzed green sustainable protocol involved [3+2] cycloaddition reactions of various aryl nitriles (1.0 mmol) possessing either electron-withdrawing or electron-donating substituents with sodium azide (1.3 mmol) in water. It was found from optimization study that 0.30 mol% amount of catalyst is sufficient to yield maximum yield of desired tetrazole derivatives. Furthermore, it was noticed that nano catalyst could be reused up to eight cycles without losing appreciable activity and leaching of Pd in solution.49 In another study, Ghasemzadeh and Akhlaghinia was prepared Fe3O4/HT-GLYMO-TA(IV) as magnetic nano-catalyst for synthesis of 1- and 5-substituted tetrazole synthesis (Tables 2 and 3, entries 10 & 8). The catalytic activity of Fe3O4/HT-GLYMO-TA(IV) was studied for both 1-substituted-tetrazole and 5-substituted-tetrazoles synthesis under similar reaction condition. It was observed that 1-substituted-1H-tetrazoles were synthesized using triethyl orthoformate, amines, and [bmim]N3 whereas 5-substituted-1H-tetrazoles, using various nitriles and [bmim][N3]. Both electron-donating and electron-withdrawing groups containing aromatic nitriles were successfully gave target product in high to excellent yields within 20 minutes. However, main advantages reported for nano catalyst are high yield, easy work-up procedure and recyclable up to eight cycle without losing significant activity.50
| Entry | Catalyst | Solvent | Catalyst loading | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4@silica sulfonic acid | — | 0.02 g | 100 | 50 | 97 | 53 |
| 2 | Chitosan supported magnetic ionic liquid nanoparticles (CSMIL) | — | 2.5 mol% | 70 | 1 | 92 | 54 |
| 3 | Fe3O4@Quinol@Cu | — | 0.01 g | 100 | 40 | 92 | 55 |
| 4 | Fe3O4@Phen@Mn | — | 50 mg | 100 | 40 | 92 | 56 |
| 5 | Fe3O4@WO3-EAE-SO3H(III) | H2O | 0.01 g | 60 | 30 | 95 | 57 |
| 6 | Fe3O4/HT-NH2-CuII | H2O | 0.005 g | 90 | 1 | 94 | 58 |
| 7 | Fe3O4@HT@AEPH2-CoII | H2O | 0.005 g | 90 | 60 | 95 | 59 |
| 8 | Fe3O4/HT-GLYMO-TA | H2O | 0.02 g | 90 | 45 | 95 | 50 |
Methionine-coated Fe3O4 nanoparticles was successfully employed in 5-substituted-1H-tetrazoles synthesis by Karimian et al. (Table 2, entry 11). Moreover, they found that synthesized catalyst was easily separate from reaction mixture and re-claimed in many cycles without loss of activity. This nano catalyst also offers several other benefits such as cost-effective, quick reaction, low loading of catalyst, clean process, high yields, and simple operation.51 Similarly, CoFe2O4@glycine-M (Pr, Tb and Yb) (Table 2, entry 12) have been prepared and utilized in the 5-substituted tetrazole synthesis.52
It has been found that synthesis of various 5-substituted tetrazole derivatives have been widely documents, their 1-substituted analogs also have been subject of investigation in view their useful medical and biological applications. Most reported synthetic protocols for 1-substituted analogs involve cyclization among triethyl orthoformate, primary amines and sodium azide by using suitable catalyst. In this context and to follow advancement in catalyst, Naeimi and Mohamadabadi carried out functionalization at Fe3O4 as core structure to form sulfonic acid-functionalized silica-coated magnetic nanoparticles. The catalyst was characterized by FTIR, SEM, TGA, XRD, and VSM. The catalytic activity of Fe3O4@silica sulfonic acid was investigated in model reaction of triethyl orthoformate, sodium azide and 4-chloroaniline at 100 °C (Table 3, entry 1). The Fe3O4@silica sulfonic acid offers excellent yield in a shorter time (50 minutes) and efficiently apply for a range of substrates. It was observed that anilines containing electron-withdrawing and electron-donating groups gave complete reaction in lesser reaction times in comparison to unsubstituted amines. The para-substituted anilines produce better results compared to ortho-substituted aniline due to steric hindrance at ortho position.53 In an alternative method, chitosan biopolymer supported magnetic ionic liquid nanoparticles (CSMIL) were developed for both 1- and 5-substituted tetrazole synthesis as novel heterogeneous catalyst (Tables 2 and 3, entries 13 & 2). Authors explored this new environmental-friendly nano-catalyst in tetrazole synthesis under solvent-free condition at 70 °C. Furthermore, they compared catalytic activity of this catalyst with other reported catalyst like In(OTf)3, SSA, [HBIm]BF4, and natrolite zeolite. In the comparison studies, they found that 2.5 mol% of this nano-catalyst is sufficient to give maximum yield in shorter time compared to other reported catalyst. Moreover, the reported synthetic protocol offered other several advantages such as operation easiness, high competence, low cost, easily recyclable and competency for large-scale synthesis.54
To carry out nano-catalyst development for 1-substituted-tetrazole synthesis, Habibi et al. have prepared Fe3O4@5,10-dihydropyrido[2,3-b]quinoxaline-7,8-diol copper complex as a heterogeneous magnetic nano-catalyst (Table 3, entry 3). The catalyst successfully furnished tetrazole formation (84–92%) in one-pot reaction from various substituted anilines with triethyl orthoformate and electron-donating or electron-withdrawing groups with sodium azide. Further, ICP analysis revealed that amount of Cu leaching was about 0.25 after first run and 2.5% after five consecutive runs while no leaching was observed for iron from nanocatalyst.55 Similarly, Habibi et al. also developed Fe3O4@1,10-phenanthroline-5,6-diol@Mn nano-catalyst for 1-substituted-tetrazole synthesis (Table 3, entry 4).56
A functionalized magnetic Fe3O4@WO3-EAE-SO3H nano-catalyst was prepared by Ghasemzadeh and Akhlaghinia as depicted in the Scheme 4. This nano-catalyst serves as a significant replacement of Brønsted acids and used in rapid preparation of 1-substituted-1H-tetrazoles via cyclization reaction between 1-butyl-3-methylimidazolium azide ([bmim][N3]), triethyl orthoformate and primary amines (Table 3, entry 5). This protocol offered characteristics advantages including operational simplicity, excellent yield, high purity, gracefully separation of the catalyst via an external magnet and its potential reusability.57
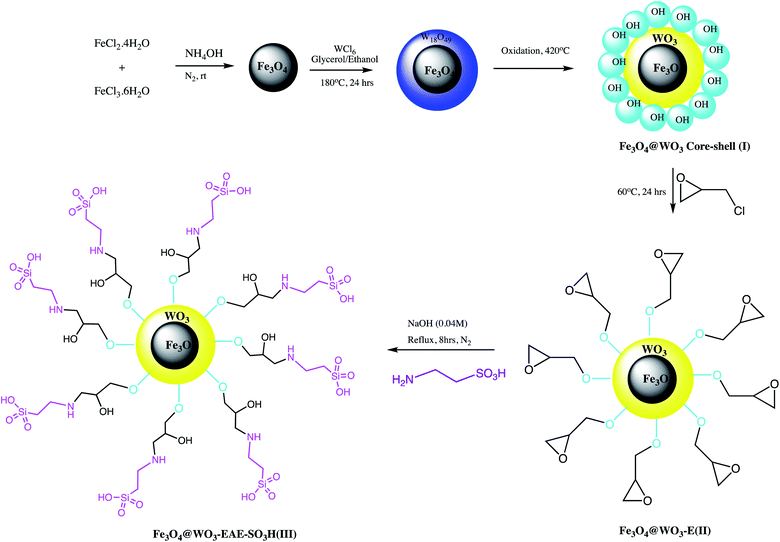 | ||
| Scheme 4 Synthesis of Fe3O4@WO3-EAE-SO3H(III) NPs.57 | ||
A bifunctional magnetite nano-catalyst (Fe3O4/HT-NH2-CuII) was prepared and characterized by Salimi and Zamanpour research group and examined its catalytic efficiency for synthesis of 1-substituted-1H-tetrazoles derivatives (Table 3, entry 6). The catalyst was prepared via five consecutive steps as depicted in the Scheme 5. The catalytic activity of Fe3O4/HT-NH2-CuII was monitored through reaction of different aromatic amine, (1 mmol) triethyl orthoformate, (1.4 mmol) and sodium azide (1.1 mmol) 90 °C in H2O solvent. Authors also extend range of diverse substrates like arylamines with electron-releasing or electron-withdrawing group that gracefully succeeded 1-substituted-1H-tetrazoles.58 Similarly, Salimi's research group was prepared Fe3O4@HT@AEPH2-CoII nano-catalyst for 1-substituted-tetrazole synthesis (Table 3, entry 7). This catalyst also exhibits catalytic competence as previous one in preparation of 1-substituted-1H-tetrazoles from triethyl orthoformate (1.2 mmol), sodium azide, (1 mmol) and amine (1.0 mmol).59
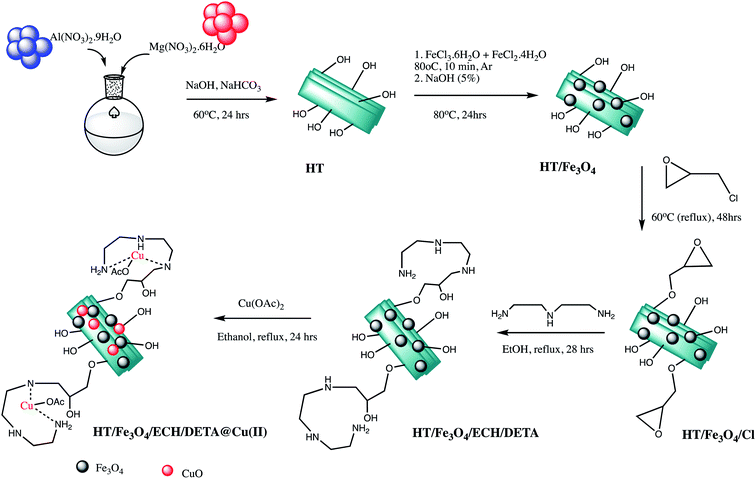 | ||
| Scheme 5 Synthesis of the catalyst Fe3O4/HT-NH2-CuII NPs.58 | ||
To utilize non-toxic and cost-effective starting reactants for synthesis of 5-substituted tetrazoles, one-pot multicomponent reaction approach using easily available, lower toxic and diverse aldehydes with active methylene compound and azides were explored in presence of suitable catalyst systems. For example, fibroin based magnetic nano-catalyst (Fe3O4@fibroin) was reported by Parouch's research group for synthesis of tetrazole derivatives (Table 4, entry 1). The Fe3O4@fibroin-SO3H was synthesized by coating of Fe3O4 NPs with fibroin followed by sulfonation. The catalytic activity of Fe3O4@fibroin-SO3H was investigated in a reaction between malononitrile (1.00 mmol) sodium azide (2.00 mmol) and aryl aldehyde (1.00 mmol) to furnish tetrazoles derivatives. It was observed that 10 mol% amount of catalyst is sufficient to yield 86% of tetrazole product.60 Further, Khodamorady et al. have prepared magnetic boehmite based nano-catalyst immobilized with palladium chitosan complex (Fe3O4@BNPs-CPTMS-chitosan-Pd(0)) (Table 4, entry 2) for development of green synthetic protocol for synthesis of 5-substituted tetrazole derivatives by reaction between from aldehyde, malononitrile and sodium azide. However, literature reports stated that crystallinity of Fe3O4 NPs was altered during functionalization with various coating/immobilization.61–64 In the current instance, crystallinity of Fe3O4@BNPs was not altered with binding of CPTMS linker and chitosan–palladium complex on the surface of catalyst which was confirmed by SEM and XRD results as shown in Fig. 6 and 7 respectively. The Fe3O4@BNPs-CPTMS-chitosan-Pd(0) nano-catalyst was initially catalyzed for chemo-selective oxidation of alcohol in presence of H2O2 in ethanol to furnish corresponding aldehydes. Then resulting aldehydes were utilized in the homo-selective synthesis of tetrazoles. The reaction condition was optimized from mixture of malononitrile (1 mmol), benzaldehyde (1 mmol), sodium azide (1.5 mmol) using different amounts of nano-catalyst in solvent-free conditions at 70 °C which showed the maximum yield (96%) of tetrazole derivatives at 2.8 mol% of catalyst. The Fe3O4@BNPs-CPTMS-chitosan-Pd(0) offered numerous advantages such as green ligand (Chitosan), thermal and mechanical stability, high-grade magnetic strength, high surface area, reusability (5 cycles), an easy and fast separation.65
 | ||
| Fig. 6 (a) FESEM of the Fe3O4@BNPs and (b) FESEM of the Fe3O4@BNPs-CPTMS-chitosan-Pd(0). Adapted with consent from ref. 65. Copyright John Wiley and Sons. | ||
 | ||
| Fig. 7 (a) XRD of Fe3O4@BNPs and (b) XRD of Fe3O4@BNPs-CPTMS-chitosan-Pd(0). Adapted with consent from ref. 65. Copyright John Wiley and Sons. | ||
Akbarzadeh et al. embedded chlorosulfonic acid functionalized carbon nanotubes on Fe3O4 nanoparticles to produce Fe3O4-CNT-SO3H nano catalyst as depicted in Scheme 6. The Fe3O4-CNT-SO3H nano catalyst was employed for 2-(1-H-tetrazole-5-yl) acrylonitrile synthesis via multicomponent domino Knoevenagel condensation/1,3-dipolar cycloaddition reaction among aromatic aldehydes, (1.0 mmol) malononitrile, (1.0 mmol) and sodium azide (1.5 mmol) under solvent-free conditions (Table 4, entry 3). Further authors compared catalytic performance of Fe3O4-CNT-SO3H with previously reported heterogeneous catalysts including, nano ZrP2O7, OPNSA, Nano NiO, Fe3O4. They revealed that this catalytic system produced high product yield within a shorter reaction time and eliminates toxic and volatile solvents. Further tempting to green catalyst, recyclability of Fe3O4-CNT-SO3H is much easier compared to other stated.66
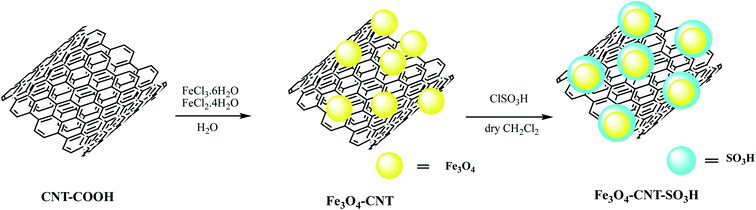 | ||
| Scheme 6 Preparation of the Fe3O4-CNT-SO3H nano-catalyst.66 | ||
Similarly, it was found that, unmodified nano Fe3O4 exhibited remarkable catalytic activity for 5-substituted tetrazole synthesis from malononitrile (1 mmol), aromatic aldehyde (1 mmol), sodium azide (1 mmol) as tabulated in Table 4, entry 4. They observed that nano Fe3O4 gave best result at 20 mol% amount. Prominent features of the presented protocol comprise of reactions under microwave condition, short reaction time, exclusion of toxic solvents, reasonable and reclaimable catalyst, mild reaction condition and easy workup.67
4. Copper-based nanomaterial catalyzed synthesis of tetrazoles
Development of copper-based nanomaterials received huge attention in recent years specially in domain of nano-catalysis organic transformations due to its extensive range of oxidation states which can enables reactivity through both one- and two-electron pathways.66 Also, copper-based nano-materials found applications in various other areas like nanotechnology, electrocatalysis, and photocatalysis.68–70 In this context, Cu-immobilized heterogenous nano-catalysts have been extensively utilized in synthesis of tetrazoles derivatives (Tables 5–8). For example, Cu(II) immobilized magnetic Cu(II)/Fe3O4@APTMS-DFX nano-catalyst was developed and examined for [3+2] cycloaddition reactions of sodium azide and various organic nitriles to provided biologically active 5-substituted-1H-tetrazoles (Table 5, entry 1). It was found that 2.5 mol% of nano-catalyst produced a series of 5-substituted-1H-tetrazoles in DMSO at 120 °C. This nano-catalyzed protocol offered advantage of recyclability up to five runs with insignificant leaching of copper from catalyst.71| Entry | Catalyst | Solvent | Catalyst loading | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Cu(II)/Fe3O4@APTMS-DFXn | DMSO | 2.5 mol% | 120 | 1 | 98 | 71 |
| 2 | Cu-MOFs-1 (Cu-TMU-17-NH2) | PEG | 0.05 g | 110 | 5 | 94 | 72 |
| 3 | Cu-MOFs-2 (Cu-TMU-17-NH2) | PEG | 0.05 g | 110 | 5 | 95 | 72 |
| 4 | CuO/aluminosilicate | DMF | 35 mg | 110 | 2–13 | 80–93 | 73 |
| 5 | Cu-TBA@biochar | PEG | 0.78 mol% | 130 | 7 | 98 | 74 |
| 6 | SBA-15@glycine-Ni | PEG | 0.04 g | 100 | 110 | 98 | 78 |
| 7 | SBA-15@glycine-Cu | PEG | 0.04 g | 100 | 110 | 98 | 78 |
| 8 | SBA-15/thioamide-Cu(I) | H2O | 1 mol% | 80 | 240 | 97 | 79 |
| 9 | Cu(II)-DCC-CMK-3 | PEG | 20 mg | 120 | 90 | 97 | 80 |
| 10 | Cu(II) immobilized on Fe3O4@SiO2@L-arginine | PEG | 1.0 mol% | 120 | 3 | 95 | 81 |
| 11 | Fe3O4@SiO2-DAQ-Cu(II) | DMF | 0.9 mol% | 110 | 2.5 | 95 | 82 |
| 12 | Fe3O4@SiO2/ligand/Cu(II) | DMF | 0.4 mol% | 110 | 4 | 92 | 83 |
| 13 | Fe3O4@SiO2/salen Cu(II) | DMF | 0.4 mol% | 120 | 6 | 92 | 84 |
| 14 | Fe3O4-AMPD-Cu | 85 | |||||
| 15 | Cu(II) immobilized on aminated epichlorohydrin activated silica (CAES) | DMSO | 1.0 mol% | 130 | 1 | 95 | 86 |
| 16 | Cu(II) immobilized on Fe3O4@SiO2@L-histidine | PEG | 0.05 g | 120 | 60 | 95 | 87 |
Two analogous of Cu-TMU-17-NH2's framework entitled Cu-MOF-1 and Cu-MOF-2 were prepared by Salahshournia et al. as efficient and recoverable heterogeneous nano-catalyst for the 5-substituted-1-H-tetrazoles synthesis (Table 5, entries 2–3). The Cu-MOF-1 and Cu-MOF-2 were obtained via post-synthetic metalation of a Zn-MOF (TMU-17-NH2) with Cu(OAc)2 and CuCl2·2H2O respectively as copper precursors. The synthesized catalysts offer excellent yield of 5-substituted-1H-tetrazoles (more than 94%) in PEG as a green solvent probably due to the porous nature of nano Cu-MOFs. Further, it also provide several advantages such as feasible synthesis, smooth recovery, and reusability (five cycles) without dropping its catalytic activity.72 In this line, CuO/aluminosilicate, as an effective heterogeneous nano-catalyst was developed by Movaheditabar et al. for preparation of 5-substituted-1-H-tetrazoles (Table 5, entry 4). The nano-catalyst affords good to excellent yield of tetrazoles from a series of aliphatic and aromatic nitriles.73
In the progress for sustainable nano-catalyst development, Moradi et al. have explored the use of biochar as a solid support material for preparation of functionalized biochar-based nano-catalyst (Table 5, entry 5). Biochar is a carbon-rich solid having carbonyl, carboxylic acid, and hydroxyl functional groups on its surfaces. In the process of preparation of nano catalyst, initially biochar nanoparticles were synthesized via pyrolysis of chicken manure and then functionalized with 3-choloropropyltrimthoxy silane to obtain CPTMS@biochar. Subsequently, 2-(thiophene-2-yl)-1H-benzo[d]imidazole was embedded on CPTMS@biocar to form TBA@biochar for Cu immobilization. Finally, copper was immobilized on TBA@biochar. The prepared Cu-TBA@biochar characterized by TGA, XRD, SEM, EDS, N2 adsorption–desorption isotherms, AAS and FT-IR techniques which indicated that particle formed in less than 100 nm range (Fig. 8). It was observed that 0.78 mol% of Cu-TBA@biochar was sufficient to give excellent yield (98%) of tetrazoles derivatives with reusability of several runs without loss of copper leaching and catalytic activity.74
 | ||
| Fig. 8 SEM image of Cu-TBA@biochar nanoparticles. Adapted with consent from ref. 74. Copyright Elsevier. | ||
In view of stringent demand of green nano-catalyst for heterocyclic transformation, different solid supports like zeolites, silica, carbon nanotubes, organic polymers, alumina and other metal oxides have been used for development of nano-catalyst.75–77 Among different solid supports, mesoporous silica nanoparticles especially SBA-15 have extensively used in different catalytic systems due to their hexagonally packed arrays and hierarchical structure.23 Tamoradi et al. utilized SBA-15 as catalyst support for preparation of two heterogeneous catalysts SBA-15@glycine-Ni and SBA-15@glycine-Cu (Table 5, entries 6–7) by immobilization of Ni and Cu metal on ordered SBA-15 coated with glycine. The structure of SBA-15@glycine-Ni and SBA-15@glycine-Cu was analyzed by FT-IR, TGA, BET, ICP-OES, XRD and EDX analysis and successfully employed for synthesis of 5-substituted tetrazoles derivatives. The high yield (83–98%) of desired tetrazole derivatives revealed high catalytic activity of prepared catalysts. It was observed that improved Lewis's acid characteristic of SBA-15@glycine-Cu is mainly responsible for its high catalytic performance than SBA-15@glycine-Ni. Further, the present catalysts offers some exclusive advantages such as high catalytic nature, use of a green solvent (ethanol and PEG) and metals (Ni & Cu) and ligand (glycine).78
Similarly, an SBA-15 modified thioamide-Cu(I) (Table 5, entry 8) catalyst was developed by Pourhassan and Eshghi as illustrated in Scheme 7. In synthesis of SBA-15/thioamide-Cu(I), SBA-15 channels was modified with tris(2-aminoethyl)amine (TAEA) groups and then reacted with S8 and phenylacetylene to form thioamide groups. This synthesized porous material was found to be an active host for immobilization of economical Cu(II) ions. The 1.0 mol% amount of catalyst gave 95% yield of tetrazoles derivative at room temperature. Further, practical application of catalyst was examined by recovery and reusability of catalyst, and it was observed that catalyst can be reused consecutively up to eleven runs in a model reaction without losing its catalytic efficiency. The copper ion leaching was monitored by ICP-OES analysis. It was reported that after eleven successful runs of tetrazole synthesis only 0.6 wt% of Cu species leached in solution.79
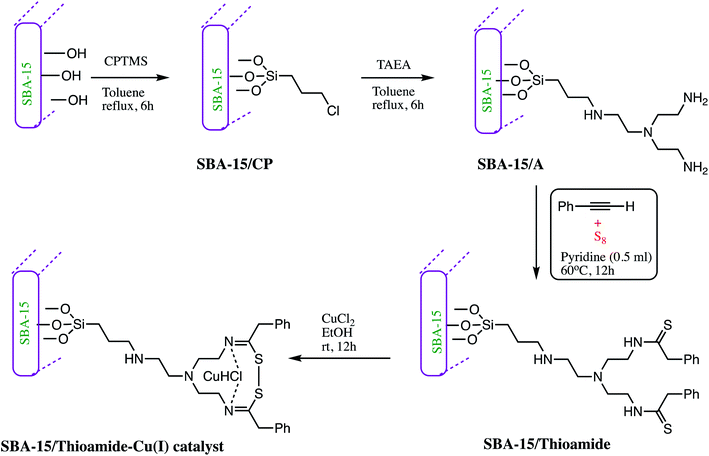 | ||
| Scheme 7 Preparation of the SBA-15/thioamide-Cu(I) NPs.79 | ||
Another nano-catalyst Cu(II)-DCC-CMK-3 was synthesized using SBA-15 as a solid template. The synthesis of Cu(II)-DCC-CMK-3 was achieved via multi-step process as depicted in Scheme 8. In the first step mesoporous carbon CMK-3 was synthesized from SBA-15 silica which was used for preparation of Cu(II)-DCC-CMK-3 catalyst. The catalyst was analyzed by FT-IR, TGA, BET, XRD, SEM, ICP-OES and EDX techniques. The SEM image of the nano-catalyst indicated that CMK-3 carbon particles maintain rod-like structures, whereas, TEM image of Cu(II)-DCC-CMK-3 reveals well-ordered mesostructured with hexagonal symmetry. The synthesized catalyst was successfully employed for synthesis of tetrazole derivatives (Table 5, entry 9).80
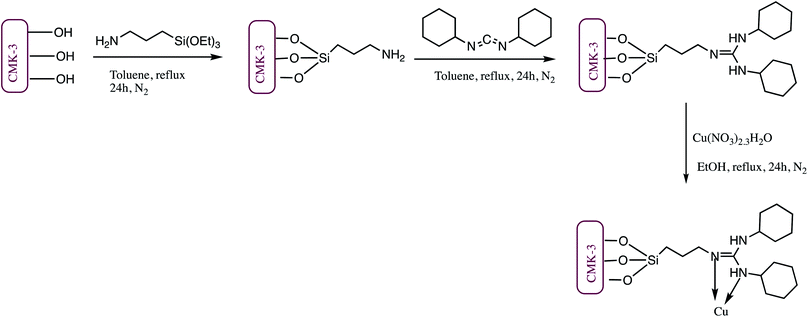 | ||
| Scheme 8 Preparation of Cu(II)-DCC-CMK-3 nano-catalyst.80 | ||
A non-corrosive Cu(II) immobilized Fe3O4@SiO2@L-arginine, nano catalyst was developed for synthesis of 5-substituted-1H-tetrazoles via cycloaddition reaction of various organic nitriles with sodium azide (Table 5, entry 10). The optimization results with aryl nitrile (1 mmol), NaN3 (1 mmol) in the presence of Cu(II) immobilized Fe3O4@SiO2@L-arginine catalyst revealed that 1.020 mol% of catalyst gave 95% yield at 120 °C in PEG solvent. Furthermore, high competence, ease of operation, easy recovery, low cost and reusability are some significant beneficial features of this catalytic system.81 In an another report, 1,4-dihydroxyanthraquinone–copper(II) was immobilized on superparamagnetic Fe3O4@SiO2 NPs to developed Fe3O4@SiO2-DAQ-Cu(II) nano-catalyst for synthesis of 1- and 5-substituted-1H-tetrazoles as illustrated in Scheme 9 (Tables 5 and 6, entry 11 & 4). It was reported that 0.9 mol% of this catalyst was sufficient for 5-substituted-1-H-tetrazole and 0.8 mol% for 1-substituted-1-H-tetrazoles synthesis. It was observed that different aromatic nitriles containing electron-donating and electron-withdrawing groups were successfully employed for tetrazole synthesis with excellent product yields and the substituents on benzonitriles play significant impact on the reaction time. For example, model reaction with electron-poor aromatic and heteroaromatic nitriles completed within a few hours whereas nitriles having electron-donating substituents took longer reaction time. The Fe3O4@SiO2-DAQ-Cu(II) catalyzed synthetic protocol serves many advantages like operational simplicity, reusability up to six runs and negligible copper leaching and excellent product yield.82
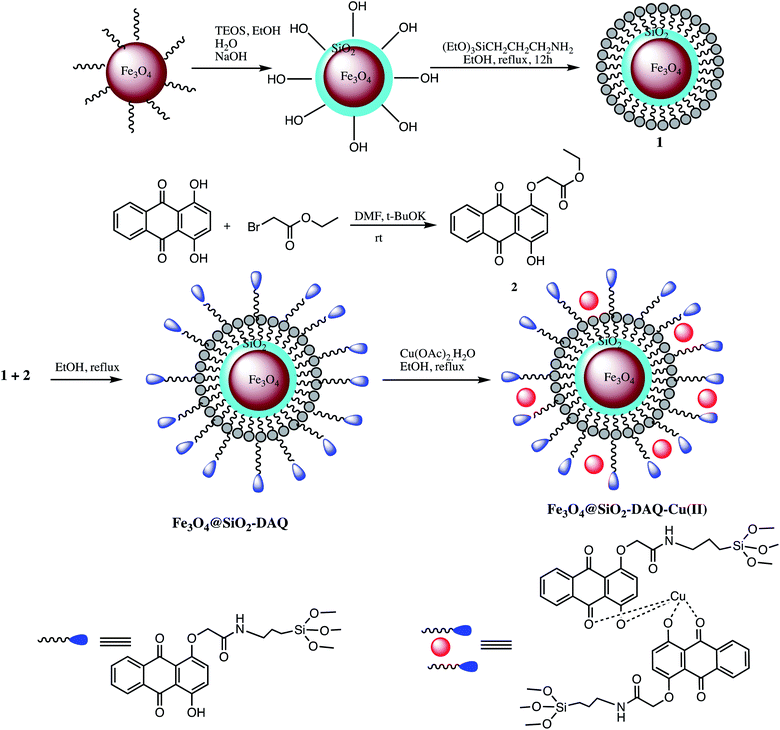 | ||
| Scheme 9 Preparation of the Fe3O4@SiO2-DAQ-Cu(II) nano-catalyst.82 | ||
| Entry | Catalyst | Solvent | Catalyst loading | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4/SiO2/CPTMS/MT/Cu | — | 50 mg | 40 | 2 | 94 | 88 |
| 2 | Fe3O4@SiO2/salen Cu(II) | — | 0.4 mol% | 100 | 75 | 92 | 84 |
| 3 | Fe3O4@SiO2/ligand/Cu(II) | — | 0.4 mol% | 100 | 0.75 | 93 | 83 |
| 4 | Fe3O4@SiO2-DAQ-Cu(II) | — | 0.8 mol% | 100 | 1 | 96 | 82 |
| 5 | Fe3O4/SiO2/CPTMS/MT/Cu | H2O | 20 mg | 40 | 2 | 94 | 89 |
| 6 | Fe3O4/SiO2/CPTMS/AT/Cu | Ethanol | 20 mg | 100 | 1 | 97 | 90 |
Similarly, Esmaeilpour et al. prepared Fe3O4@SiO2/ligand/Cu(II) magnetic nano-catalyst for 1- and 5-substituted-1-H-tetrazoles synthesis such as illustrated in Scheme 10 (Tables 5 and 6, entry 12 & 3). It was observed that 0.4 mol% of the nano catalyst are sufficient to yield more than 90% of the targeted product. Furthermore, super magnetic nature of the catalyst eliminates catalyst filtration after completion of reaction which represents its main advantage in the expression of energy-consuming and environmental-friendly.83
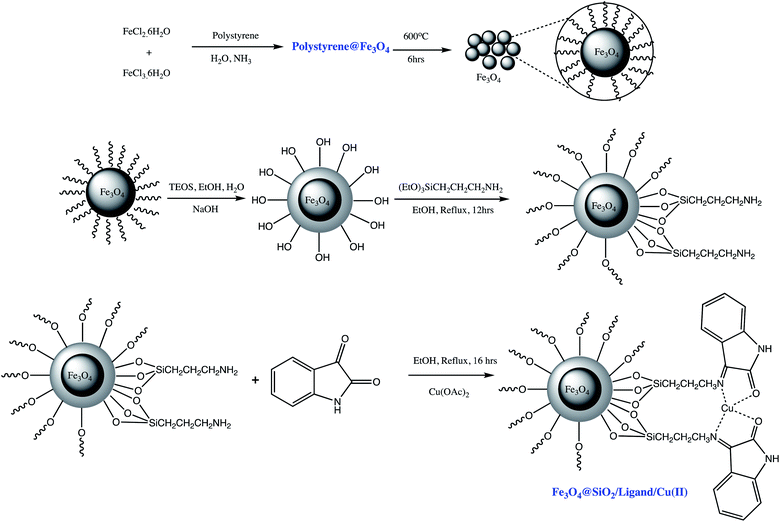 | ||
| Scheme 10 Preparation of the Fe3O4@SiO2/ligand/Cu(II) nano-catalyst.83 | ||
In another method from Dehghani et al., 1- and 5-substituted-1H-tetrazoles were synthesized from nitriles and amines using, Fe3O4@SiO2/salen of Cu(II) based magnetite nanoparticles was developed by (Tables 5 and 6, entry 13 & 2). The structural and magnetic properties of Fe3O4@SiO2/Salen of Cu(II) was elucidated by TEM, SEM, FTIR, and VSM analysis. The reported catalyst successfully furnished synthesis of various 1- and 5-substituted tetrazole derivative with wide substrate scope in good to excellent. Furthermore, it was observed that 0.4 mol% of catalyst is best for both 1- and 5-substituted tetrazole derivatives. Authors also compared catalytic competence of reported catalyst with other previously reported catalysts such as nano natrolite zeolite, ZnHAP, ZnO/Co3O4 and Zn/Al-HT etc. which showed that Fe3O4@SiO2/Salen of Cu(II) gave better product yield in shorter reaction time compare to other ones.84
Another copper immobilized Fe3O4-AMPD-Cu catalyst was prepared and employed for 5-substituted tetrazole synthesis (Table 5, entry 14). The nano-catalyst offers several advantages including environmental-benign condition, inexpensive, chemically inert reagents, shorter reaction time (28 minutes), easy separation via an external magnet, and reusability.85 Analogously, Cu(II) immobilized on aminated epichlorohydrin activated silica (CAES) as efficient heterogeneous nano-catalyst was synthesized via five-step synthetic method for synthesis of 5-substituted-1H-tetrazoles as depicted in Scheme 11 (Table 5, entry 15). The synthesized CAES catalyst in 0.1 mol% gave excellent yield of tetrazoles via [3+2] cycloaddition reaction of sodium azide and nitriles with wide substrate scope.86 Further, Azadi et al. reported synthesis of copper(II) immobilization on Fe3O4@SiO2@L-histidine as a reusable nano-catalyst for the synthesis of 5-substituted tetrazoles (entry 16, Table 5). This synthetic protocol provide excellent yield in a shorter time with efficient reusability.87
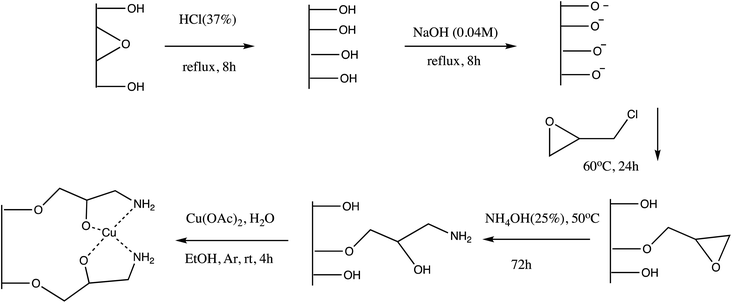 | ||
| Scheme 11 Preparation of Cu(II) immobilized on aminated epichlorohydrin activated silica (CAES)86 | ||
A Cu(II) immobilized Fe3O4/SiO2/CPTMS/MT/Cu magnetic catalyst was prepared and characterized by distinctive methods includes TEM, SEM, XRD, ICP, EDX, VSM and TG-DTA analysis etc. The reported catalyst successfully utilized for synthesis of tetrazole derivatives by reaction of aromatic amines with sodium azide in presence of triethyl orthoformate in a solvent-free condition at 100 °C (Table 6, entry 1). The catalyst exhibits excellent competence in the expression of 94% yield within 2 h88
In some report's amino acids was utilized for synthesis of tetrazole derivatives via one-pot MCR pathway using triethyl orthoformate, and azide. For example, Cu(II) immobilized nano catalyst (Fe3O4/SiO2/CPTMS/MT/Cu) was prepared via five-step synthetic protocol. The prepared magnetic nano-catalyst was effectively utilized for synthesis of tetrazoles using several amino acids in aqueous medium at 40 °C (entry 5, Table 6).89 Similarly, in another alternative method, Fe3O4/SiO2/CPTMS/AT/Cu nano catalyst was developed for tetrazole synthesis from triethyl orthoformate, sodium azide and amino acids in ethanol at reflux condition (Table 6, entry 6).90
A copper-based magnetic heterogeneous catalyst was prepared via functionalization surface of nano Fe3O4@SiO2 with polyvinyl alcohol and the resultant Fe3O4@SiO2-PVA was then immobilized with copper(II) complex. The nano-catalyst Fe3O4@SiO2-PVA/Cu(II) was well-characterized and employed for green synthesis of 5-substituted-1H-tetrazoles using hydroxylamine hydrochloride (1.5 mmol), aldehydes (1.0 mmol) and sodium azide (1.5 mmol) in aqueous medium (Table 7, entry 1). It was observed that 0.5 mol% of Fe3O4@SiO2-PVA/Cu(II) nano-catalyst was found to be enough for the maximum yield. Further, easy handling, easy separation of catalyst, reusability (seven successive runs), and yielding high to excellent yields of the anticipated products were few beneficial features of present protocol.91
| Entry | Catalyst | Solvent | Catalyst loading | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2-PVA/Cu(II) | H2O | 0.5 mol% | 100 | 4 | 92 | 91 |
5. Zinc oxide derived nanomaterials catalyzed synthesis of tetrazoles
Some inorganic metal oxide nanoparticles are well known to display both Lewis acid–base and redox properties on their surfaces. In this context, zinc oxide nanoparticles exhibits Lewis acidic sites at their surfaces and outstanding physical and chemical properties like wide bandgaps energy,92,93 large surface area, high pores volume, reusability and environmental sustainability94 which was widely employed as heterogeneous acid catalyst. These interesting physical properties of ZnO nanoparticles prompts to deployed in various fields such as photodegradation of microorganisms, electrical engineering, catalysis, optical and optoelectronic devices and solar cells.95–98 Further, ZnO nanoparticles offers excellent catalytic activity for acid catalyzed organic transformations.99 A nanocrystalline ZnO as a heterogenous green catalyst was employed for preparation of 5-substituted-1H-tetrazoles through [3+2] cycloaddition of sodium azide and nitriles (Table 8, entry 1). Nanocrystalline ZnO effectively yielded 5-substituted-1H-tetrazoles from various nitriles at 120–130 °C with product yields of 69–82%. Furthermore, catalyst graciously recovered and reused in the consecutive run.100 It was established in literature that role of zinc oxide in tetrazole synthesis is to activate –CN triple bond of nitriles.101–103 The acidity of zinc oxide (ZnO) could be enhanced by mixing it with other metal oxides like TiO2, CeO2 and Co3O4. A mixed metal oxide (ZnO/Co3O4) was employed as pioneering catalyst for 5-substituted-1H-tetrazoles (Table 8, entry 2). The mixed nano metal oxide exhibits excellent catalytic activity compared to solitary ZnO or Co3O4. This protocol offers significant advantages comprises easy applicability, catalyst recyclability, simple work-up, and high yield.104 Further, in an another report, silver doped ZnO nanorods catalyzed photo-triggered synthesis of 1,5-disubstituted tetrazoles was reported. This synthetic approach involved one-pot MCR of aldehyde, chromenocyl bromide, sodium azide in presence of triethylamine using ZnO nanorods and Ag-doped ZnO nanocomposites (NCs) as photocatalysts. Optimized reaction conditions revealed that at room temperature in dark, 10 mol% of silver doped ZnO nanorods gave 97% yield of 1,5-disubstituted tetrazole derivatives.105 Similarly, in another attempt, green protocol was developed for tetrazole synthesis using ZnO nano particles as nano catalyst under ultrasound irradiation through reaction of triethyl orthoformate, sodium azide, ethyl esters and a series of α-amino acid (Table 9, entry 1). It was observed that under irritation at 200 W, reaction time shortened to 30 min with excellent yield (88–96%). The major advantages of this protocol are use of mild reaction conditions that eliminated formation of HN3, no side products formation, high yields, and easy work-up procedure. The recyclability and reusability of nano-catalysts were adequate which showed cost proficiency and green aspect of the methodology.106| Entry | Catalyst | Solvent | Catalyst loading | Temperature (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | ZnO | CH3CN/H2O | 1 wt% | 200 W | 1 | 90 | 106 |
6. Carbon-based nanomaterials catalyzed synthesis of tetrazoles
Carbon nanostructures like fullerene, graphene, graphene oxide, carbon nanotubes, carbon nanowires, carbon nanoparticles (CNPs) and nano-diamonds widely used by researcher in form of compatible catalyst support for various organic transformations due to their inevitable exclusive physical and chemical properties like high thermal, mechanical and chemical stability, large surface area, inertness, low cost, easy functionalization and tunable carbon framework.107,108 In line of this, a multi-walled carbon nanotube supported AMWCNTs-O-Cu(II)-PhTPY nano-catalyst was reported for facile preparation of 1-substituted and 5-substituted-1H-tetrazole derivatives. The AMWCNTs-O-Cu(II)-PhTPY preparation involved immobilization of copper(II) complex of 4′-phenyl-2,2′:6′,2′′-terpyridine on activated multi-walled carbon nanotubes. The optimization of the 5-substituted-1H-tetrazole synthesis was carried out by taking model reactants such as nitrile, azide, ammonium acetate and DMF as solvent. Optimization studies revealed that 4.0 mol% of catalyst loading is sufficient to achieve maximum product yield at 70 °C s. The AMWCNTs-O-Cu(II)-PhTPY nano-catalyst also examined for wide substrates scope with hetero-aryl nitriles substituted with the electron-withdrawing as well as electron donating groups. It was noticed that electron-deficient nitriles furnished the target products in shorter reaction time than the electron-rich counterparts with comparatively higher yields. The lesser reaction time, easy recovery of catalyst, wide substrate scope, catalyst reusability up to five cycles without showing copper leaching are some advantages of the reported protocol.104 Similarly, a carbon nanotube supported heterogeneous Fe3O4-CNT-TEA-Cu(II) nano-catalyst was prepared and characterized (FTIR, XRD, FESM, TGA, and VSM) for the 5-substituted-1H-tetrazoles synthesis. The Fe3O4-CNT-TEA-Cu(II) nano-catalyst was prepared by growing metallic copper(II) nanoparticles on the surface of magnetic carbon nanotube and supported with triethanolamine (TEA) that acts as a non-toxic ligand to capture the copper nanoparticles. The prepared Fe3O4-CNT-TEA-Cu(II) successfully employed in synthesis of 5-substituted-1H-tetrazoles through one-pot MCR of aldehyde, hydroxylamine, and sodium azide. The authors revealed that protocol furnish product in high yield with low catalyst loading without leaching copper ions in the solution. Moreover, it was found that catalyst performed the recyclability up to eight cycles with same extent without losing noticeable catalytic activity. The reusability, separation with a magnet, substrate scope, high yield and lesser reaction time are some of aids with this protocol.109Among different carbon nanostructures, graphene oxide has potentials to behave as excellent catalyst support for fabrication of an ideal nano-catalyst for organic transformations because of their distinctive properties such as high thermal and chemical stability, nano-size dimension with high surface area, sheet structure, and possibility of functionalization with different organic entities etc. In this context, a graphene oxide (GO) supported GO/Fe3O4@PAA-Cu-complex, nano-catalyst was prepared for 1- and 5-substituted tetrazoles synthesis by one-pot multicomponent reaction. The GO/Fe3O4@PAA-Cu-complex nanostructure synthesis comprises multi-step protocol as depicted in Scheme 12. Graphene oxide synthesis commenced through modified Hummer's method and GO-Fe3O4 hybrid by chemical co-precipitation. The final step involved immobilization of the poly(α-amino acid)-Cu(II) complex on GO-Fe3O4 hybrid. The prepared GO/Fe3O4@PAA-Cu-complex were characterized through XRD, EDS, FESEM, TEM, TGA, VSM and DLS analysis and utilized in tetrazole synthesis. The present method offered diversity in reaction, recyclability of catalyst over six successive turns with insignificant metal leaching.110
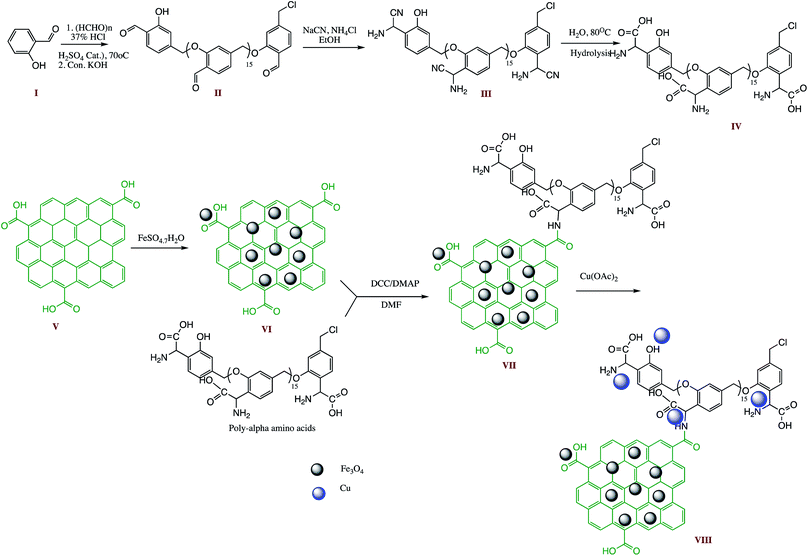 | ||
| Scheme 12 Schematic synthesis route for GO/Fe3O4@PAA-Cu-complex preparation.110 | ||
In another report, GO supported Cu/AC/r-GO nanohybrid nano-catalyst (Fig. 9) was prepared for 5-substituted-1H-tetrazoles synthesis bearing bioactive N-heterocyclic cores. Different types of nitriles with diverse N-heterocyclic cores reacted with sodium azide in water/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) system under catalytic effect of Cu/AC/r-GO nanohybrid at reflux condition gave corresponding 5-substituted-1H-tetrazoles. The nanohybrid exhibited chemical and thermal stability, inexpensive, and environmentally benign nano-catalyst that significantly endorsed cycloaddition of the azide and nitrile. Further, the advantage of recyclability and reused for many consecutive runs without significant loss of its activity makes this system more effective.111
50, v/v) system under catalytic effect of Cu/AC/r-GO nanohybrid at reflux condition gave corresponding 5-substituted-1H-tetrazoles. The nanohybrid exhibited chemical and thermal stability, inexpensive, and environmentally benign nano-catalyst that significantly endorsed cycloaddition of the azide and nitrile. Further, the advantage of recyclability and reused for many consecutive runs without significant loss of its activity makes this system more effective.111
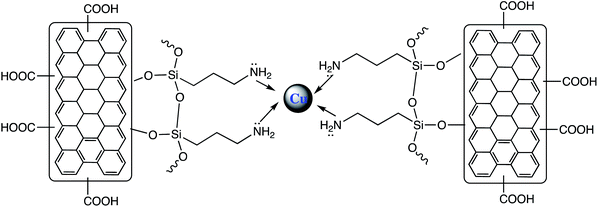 | ||
| Fig. 9 Cu/AC/r-GO nanohybrid.111 | ||
7. Composite nanomaterial catalyzed synthesis of tetrazoles
Composites are superintended or naturally transpiring solid materials in which two or more different constituent materials are combined to produce modified materials with superior physical and chemical properties.112 In recent years, to overcome limitations associated with various engineering materials, nanocomposites has emerged as beneficial alternatives. Therefore, numerous nanocomposites were explored in different organic transformation as heterogeneous catalyst. For instance, a biosynthesized Pd/MnO2 nanocomposite catalyst explored for the 5-aryl-1H-tetrazoles synthesis from various aryl halides. It was observed that this strategy suit to a range of aryl halides having electron withdrawing groups on benzene and heterocyclic ring. Further authors also clarified that MnO2 play important role in stabilizing Pd NPs, by preventing agglomeration of Pd nanoparticles and leaching during reactions. The biocatalyst (Pd/MnO2) offers several advantages like high yields, easygoing preparation, use of K4[Fe(CN)6] as a non-toxic cyanide source, simple work-up procedure, escaping formation of destructive hydrazoic acid, and accessibility of MnO2 as support.113A magnetic Fe3O4@MCM-41-SB-Cu nanocomposite as a catalyst was prepared for 5-substituted-1H-tetrazoles synthesis. The Fe3O4@MCM-41-SB-Cu nanocomposite synthesis involved the several steps shown in Scheme 13. In the first step magnetic mesoporous silica nanocomposite was prepared and functionalized with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane. Thereafter Schiff base grafted NPs were prepared by condensation reaction between 5,5′-methylene bis (salicylaldehyde) and N-(2-aminoethyl)-3-aminopropyltrimethoxysilane which was then reacted with benzhydrazide followed by (CH3COOH)2. H2O to give Fe3O4@MCM-41-SB-Cu. The new organic–inorganic hybrid nanocomposite characterized by various spectroscopic analytical techniques. The catalytic performance of Fe3O4@MCM-41-SB-Cu was assessed in one-pot MCR of using aldehydes, hydroxylamine hydrochloride and sodium azide for the synthesis of 5-substituted-1H-tetrazoles (Scheme 14.) The Fe3O4@MCM-41-SB-Cu nano catalyst offers easy separation due to magnetic nature and reused several times without dropping catalytic stability and activity.114 Similarly, a magnetic nanocomposite (Fe3O4@SiO2/aza-crown ether-Cu(II)) was prepared for the synthesis of 1,2,3-triazoles, 1-substituted-1H-tetrazoles and 5-substituted-1H-tetrazoles synthesis. The fabrication of the nanocomposite catalyst could be achieved via covalent grafting of an aza-crown ether Cu(II) complex on a silica-coated iron oxide support. The resulted nanocomposite characterized by various techniques like TEM, SEM, XRD, TGA, FT-IR and ICP. The nanocomposite catalyzed the 1,2,3-triazoles, 5-phenyl-1H-tetrazoles and 1-phenyl-1-H-tetrazoles synthesis with several advantages including thermal stability, easygoing preparation, convenient separation, short reaction time, reusability (five runs) and admirable yields.115
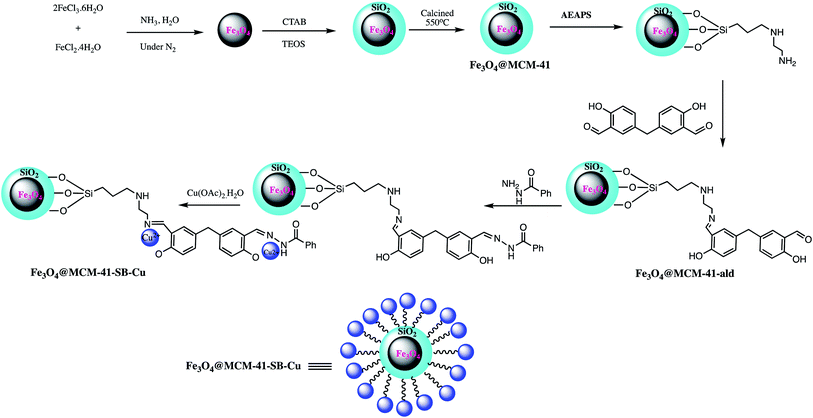 | ||
| Scheme 13 Preparation of the Fe3O4@MCM-41-SB-Cu nano-catalyst.114 | ||
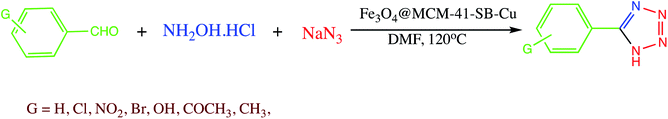 | ||
| Scheme 14 Synthesis of 5-substituted-1H-tetrazoles catalyzed by Fe3O4@MCM-41-SB-Cu nanocomposite.114 | ||
Further, Cu NPs@Fe3O4-chitosan nano-catalyst was prepared and characterized for different tetrazoles synthesis using sodium azide and various secondary or tertiary cyanamides. The Cu NPs@Fe3O4-chitosan nano-catalyst preparation involved immobilization of copper nanoparticles on the magnetic chitosan using Euphorbia falcata leaf extract as reducing and stabilizing agent such as depicted in the Scheme 15. The tetrazole formation was optimized using 2,4-dimethylphenylcyanamide and NaN3 as model reactants. It was observed that Cu NPs@Fe3O4-chitosan display high catalytic activity for reaction of various N-arylcyanamides possessing diverse functionalities with NaN3 in aqueous medium with high yields. Further, numerous advantages also recognized with this protocol such as easy work-up procedure, minimum use of toxic hydrazoic acid, use of green solvent (H2O), catalyst stability, reusability, and high yields.116
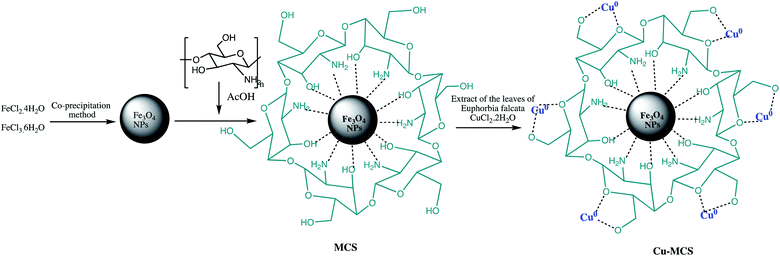 | ||
| Scheme 15 Preparation of the Cu NPs@Fe3O4-chitosan nano-catalyst.116 | ||
A biosynthesized Ag/sodium borosilicate nanocomposite was explored by Nasrollahzadeh et al. for 1-substituted-1H-tetrazoles synthesis. The Ag/sodium borosilicate nanocomposite (ASBN) catalyst was prepared using Aleurites moluccana leaf extract as stabilizing and reducing agent. The formation of the catalyst was characterized via various spectroscopical analysis such as FESEM, TEM, EDS, FT-IR, XRD. This synthetic protocol offered high yields, easy work-up, short reaction time, green synthesis without using harmful reducing agents. Further, reported catalyst was recycled and reused multiple times without any significant loss of activity.117
Another nanocomposite RuO2/MMT was synthesized and employed in one-pot three-component (3-CR) synthesis for tetrazole using different sodium azide, amine and triethyl orthoformate under solvent-free condition. The RuO2/MMT catalyzed synthesis of tetrazole derivatives offered excellent yield (84–97%), high reusability (five cycles), short reaction time and simple work up procedure. The enhanced catalytic activity of reported bifunctional nanocomposite is attributed by the uniform dispersion of RuO2 NPs on surface of montmorillonite (MMT). In this protocol RuO2 sites are accountable for the coordination of isocyanide intermediate while strong acidic character of MMT induces condensation and cyclization steps in a synergic mode.118
8. MCM-41 based nanomaterials catalyzed synthesis of tetrazoles
MCM-41 offered as auspicious catalyst support in liquid phase reactions due to its well-defined mesoporous structure with a high specific surface area (>1000 m2 g−1) and thermal stability.119 MCM-41 was first time reported in 1992 by scientists of Mobil Company during their project for finding new highly porous materials. Since then, mesoporous MCM-41 fascinated cumulative research attention due to their chemical versatility, large specific surface area with huge silanol groups which can functionalized with other suitable functional groups. The 1.3 ml g−1 pore volume of MCM-41 allows anchoring of diverse molecules such as metal complexes and organic ligands into highly ordered hexagonal channels.96,120,121 In recent years MCM-41 widely utilized as catalyst support in organic transformation like SO3H functionalized MCM-41 nonordered heterogenous catalyst was prepared for 1- and 5-substituted-1H-tetrazole synthesis (Table 10, entry 1).122| Entry | MCM-41@nanostructure | Catalyst loading (mg or mol%) | Temperature (°C) | Solvent | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | SO3H@MCM-41 | 50 mg | 80 | DMF | 1.6 | 90 | 122 |
| 2 | Cu-cytosine@MCM-41 | 3.1% | 120 | PEG-400 | 0.5 | 96 | 123 |
| 3 | Ni-cytosine@MCM-41 | 0.1% | 120 | PEG-400 | 1 | 92 | 123 |
| 4 | L-Cysteine@MCM-41 | 2.9% | 100 | PEG-400 | 2.5 | 97 | 124 |
| 5 | Pd-SBT@MCM-41 | 35 mg | 120 | PEG-400 | 6.75 | 97 | 125 |
Cu and Ni immobilized on cytosine@MCM-41 nano catalysts (Cu-cytosine@MCM-41 & Ni-cytosine@MCM-41) were synthesized and monitored for 5-substituted-1H-tetrazoles synthesis (Table 10, entries 2–3) as depicted in the Scheme 16. The synthesized organic–inorganic hybrid nano-catalysts characterized by TGA, BET, XRD, TEM, EDS, SEM, WDX, AAS and FT-IR techniques. The SEM images of synthesized nano catalyst exhibited that nano catalysts are nearly spherical in shape whereas TEM image exemplified ordered hexagonal mesoporous structure less than 5 nm and uniform quasi-spherical morphology (Fig. 10 and 11). The reaction condition was optimized by varying catalyst loading, solvents, sodium azide concentration and temperature in the [3+2] cycloaddition of benzonitrile and sodium azide. It was observed that optimum results were obtained with 3.1 mol% Cu-cytosine@MCM-41 and 0.1 mol% of Ni-cytosine@MCM-41 catalyst loading in PEG-400 at 120 °C using 1.3 mmol of sodium azide. Further, to establish the role of Cu-cytosine@MCM-41 and Ni-cytosine@MCM-41 in the tetrazoles synthesis, authors also demonstrated comparative catalytic study of nickel nitrate (Ni(NO3)2), copper nitrate (Cu(NO3)2), MCM-41, and cytosine as a catalyst. It was noticed that compared to Cu-cytosine@MCM-41 and Ni-cytosine@MCM-41, model reaction was not completed in presence of either Ni(NO3)2, Cu(NO3)2, MCM-41, cytosine even when reaction time was prolonged. However, reported organic–inorganic hybrid catalyst system offers several beneficial features such as high catalytic activity due to porous large surface area, reusability, low metal leaching in the reaction solution and high yield.123
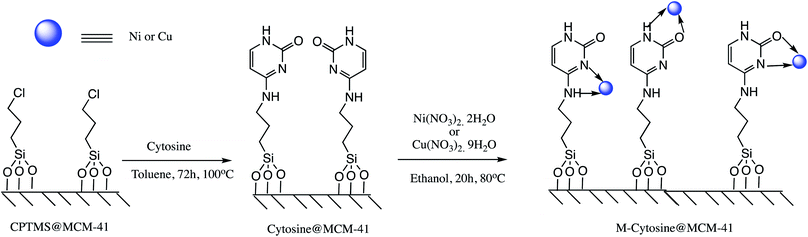 | ||
| Scheme 16 Synthesis of the Ni-cytosine@MCM-41 and Cu-cytosine@MCM-41 nano-catalyst.123 | ||
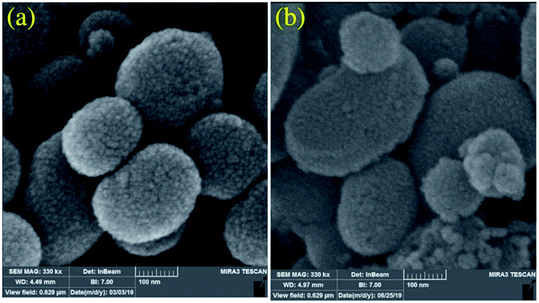 | ||
| Fig. 10 SEM images of the (a) Cu-cytosine@MCM-41 and (b) Ni-cytosine@MCM-41 (Adapted with consent from ref. 123. Copyright John Wiley and Sons). | ||
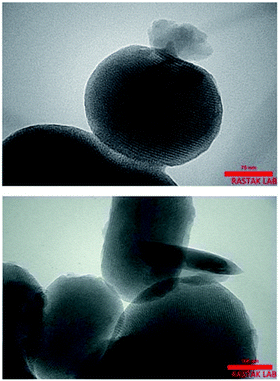 | ||
| Fig. 11 TEM images of Cu-cytosine@MCM-41. Adapted with consent from ref. 123. Copyright John Wiley and Sons. | ||
In another research, L-cysteine-Pd@MCM-41 organic–inorganic hybrid nano-catalyst was synthesized by the functionalization of L-cysteine on mesoporous MCM-41 channel followed by addition of palladium particles on surface of L-cysteine@MCM-41. The L-cysteine-Pd@MCM-41 organic–inorganic hybrid nano-catalyst was used for synthesis of 5-substituted-1H-tetrazoles (Table 10, entry 4). The catalyst was characterized by various spectroscopic techniques such as FTIR, XRD, TGA, EDS, WDX, SEM ICP, and N2 adsorption–desorption measurement isotherms. The collected data suggest that palladium complex immobilized onto MCM-41 pores. Additionally, protocol offered several advantages such as simple methodology, easy separation of catalyst, shorter reaction times, high product yields and catalyst retrieval up to six times without losing significant activity and selectivity.124
Similarly, Pd-SBT@MCM-41 nano-catalyst was prepared and examined as catalyst for 5-substituted-1H-tetrazoles synthesis (Table 10, entry 5). The Pd-SBT@MCM-41 nano-catalyst was prepared via sequential process depicted in the Scheme 17. The characterization of reported nanomaterial was done through XRD, SEM, TGA, ICP-OES and N2 absorption–desorption isotherms techniques. The SEM analysis revealed that Pd-SBTU@MCM-41 are spherical with a mean diameter of 80 ± 20 nm. Further, TEM image exhibited that catalyst had well-ordered hexagonal type of array of regular hexagonal arrangement before and after coordination of palladium. The TEM image also reveals most of Pd particles supported on MCM-41 with an average diameter of about 4 ± 1 nm. The synthesized Pd-SBT@MCM-41 was successfully employed in 5-substituted-1H-tetrazole synthesis via reaction of various nitriles with sodium azide. This protocol offered several advantages like high stability, easy handling, simple application procedure and reusability of the catalyst.125
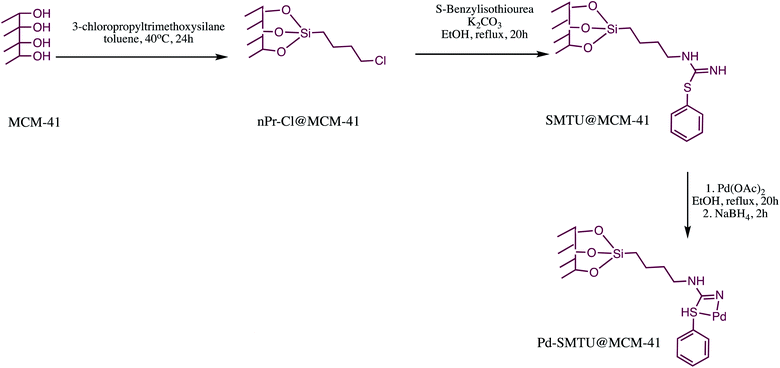 | ||
| Scheme 17 Preparation of the Pd-SMTU@MCM-41 nano-catalyst.125 | ||
9. Miscellaneous nano-catalyst for the synthesis of tetrazole
Besides these broad sorts of nanostructured catalyst some miscellaneous nano-catalysts were also explored by various research groups. Such as Movaheditabar and Javaherian explored nano-silica melamine trisulfonic acid as heterogeneous catalyst for synthesis of 5-substituted-1H-tetrazoles. The reaction was optimized via model reaction of benzonitrile with sodium azide. It was observed that nitrile activity is critical toward azide ion in [3+2] cycloaddition reactions. Thus, in shorter time better yield was obtain when aliphatic nitriles were reacted with sodium azide contrary to aromatic nitriles. The lower activity of aromatic nitriles may be due to their significant resonance between the aromatic ring and cyano group, which decreases its electrophilicity. Additionally, authors reported that if electron-donating and withdrawing substituents were located at para- or meta-positions in aromatic compounds, no major difference reaction times or in product yields were observed. It has also observed that synthesized catalyst efficiently recovered after reaction completion and could be applicable at the industrial scale.126 Similarly, a nickel zirconium phosphate (NiZrP) based heterogeneous catalyst was prepared and characterized for synthesis of 5-substituted-1H-tetrazoles via the [3+2] cycloaddition reaction of sodium azide with nitriles. They found that 5 mol% of nickel zirconium phosphate is optimum to achieve maximum yield of the tetrazoles. Additionally, short reaction time with excellent product formation are attractive advantages of this method.127 The β-Ni(OH)2 nanoparticles was reported as a green heterogeneous catalyst for synthesis of versatile substituted tetrazoles. Authors found that 4.32 mol% of β-Ni(OH)2 catalyst loading in water is sufficient for tetrazoles synthesis via simple, one-pot reaction. Owing to nanocrystalline nature, good thermal stability, large surface area and small particle size of β-Ni(OH)2 offer excellent yield (98%) of the 5-substituted-1H-tetrazoles. Another heterogeneous nano catalyst TiCl4·SiO2 was developed by Zamani et al. for 5-substituted-1H-tetrazoles synthesis. They noticed that 0.1 mol% catalyst loading is sufficient for the maximum yield of the product.128 In this line, magnetic Fe3O4@SiO2@CPTMS@AMTDA@Co heterogeneous catalyst was developed via sequential process for synthesis of diverse tetrazoles as depicted in Scheme 18. The Fe3O4@SiO2@CPTMS@AMTDA@Co activity optimized in a model reaction of aniline with sodium azide and triethyl orthoformate (TEOF) to form 1-phenyl-1H-tetrazole. Moreover, the reusability of catalyst also performed up to five runs without losing noticeable catalytic activity.129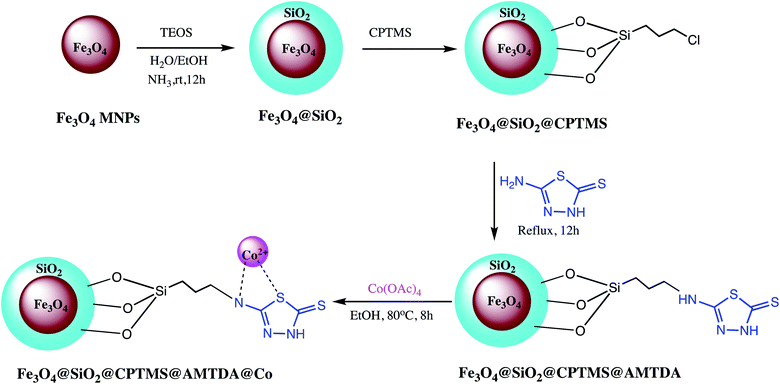 | ||
| Scheme 18 Preparation of Fe3O4@SiO2@CPTMS@AMTDA@Co nano-catalyst.129 | ||
10. Generalized reaction mechanism of the nano-catalyzed tetrazole synthesis
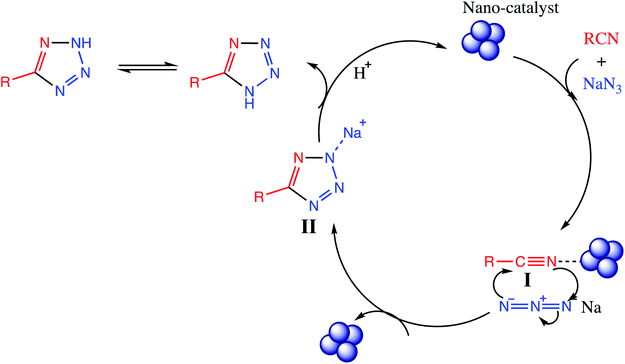
In this section, general mechanism involves in synthesis of tetrazoles in both [3+2] cycloaddition reaction and one pot multicomponent reaction are described in detail using suitable reactants.11. Mechanism for tetrazoles synthesis via [3+2] cycloaddition
The [3+2] cycloaddition nano-catalyzed tetrazole synthesis was initiated with interaction of nitrile group with nano catalyst and forms an intermediate which accelerates [3+2] cycloaddition step. Certainly, nano-catalyst activates nitrile groups via coordination to nitrogen and/or triple bond which enhances electrophilic character of cyanide group (intermediate I). Thereafter, sodium azide reacts with this complex and produces second intermediate (II). Finally, 1,3-H-shift produce tetrazole product with acidic work up (Fig. 12).12. One-pot multicomponent reaction of amine/amino acids, triethyl orthoformate and sodium azide
One pot multicomponent reaction of amine/amino acids with triethyl orthoformate and azide is another alternative route for synthesis of tetrazole derivatives. The reaction mechanism of nanomaterials catalyzed synthesis of tetrazole derivatives via multicomponent reaction approach is well described in literature in which nanoparticle attack to oxygen atom of ethoxy group of triethyl orthoformate thereby facilitate nucleophilic attack of amino group on intermediate A. Successive removal of two molecule of ethanol followed by nucleophilic attack of azide to give intermediate B which than cyclize to produce final tetrazole (Fig. 13).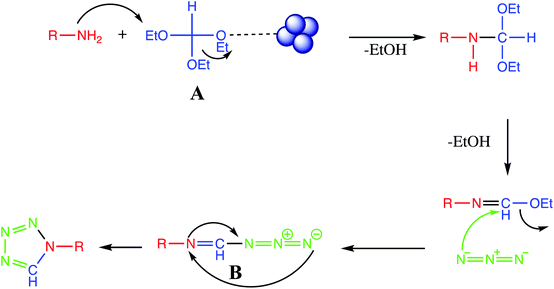 | ||
| Fig. 13 Reaction mechanism for tetrazole via one-pot MCR of amine/amino acids, triethyl orthoformate and sodium azide. | ||
Similarly, the reaction mechanism of nanomaterial catalyzed multicomponent reaction between aldehyde, malononitrile and azide is depicted in Fig. 14. Initially, carbonyl oxygen atom of aldehyde was activated by nanoparticle to produce intermediate A. The activated carbonyl compound (A) reacts with malononitrile to formed arylidene malononitrile (B) via Knoevenagel condensation. The nitrogen atom of arylidene malononitrile (B) activated by nanoparticles form complex (C) which subsequently activates towards the attack of the azide ion. The [3+2] cycloaddition reaction between arylidene malononitrile and azide ion gave intermediate (D) Finally addition of acid produce target tetrazole.
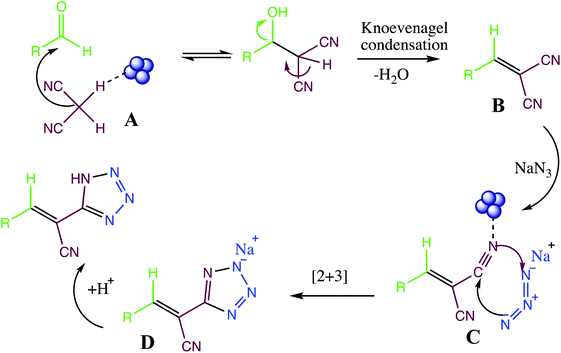 | ||
| Fig. 14 Reaction mechanism for tetrazole via aldehyde, active methylene compound (malononitrile) and sodium azide. | ||
13. Conclusions
Tetrazoles and its derivatives are vital heterocyclic scaffolds having wide applications in various fields such as biochemistry, medicinal chemistry, high energy material science and many more. The synthesis of tetrazole and its derivatives using conventional homogeneous and heterogeneous catalytic system are associated several constraints such as long reaction time, moderate yield, tedious workup, and purification etc. Therefore, the prime focus of chemists working in this domain are to develop efficient environmental benign catalytic system for synthesis of tetrazoles and its derivatives with the aim of high yield, minimum by-products, low temperature condition, catalyst reusability, easy workup procedure and economic and eco-friendly methodology. In this context, different nanomaterial derived catalytic systems have undoubtedly proven as an efficient and eco-friendly catalyst for tetrazoles synthesis. This review article demonstrated synthesis, characterization and benefits of various class nanomaterials derived catalysts for example boehmite, magnetic and nonmagnetic nanoparticles, nanomaterials supported copper, zinc, carbon derived nanomaterials, MCM-41, and composite nanostructures under different reaction condition in tetrazoles synthesis. Along with this, we have also focused on the fabrication, functionalization, and characterization of some novel nanomaterials. We hope that this review would enrich the process chemists to design and develop eco-friendly and novel synthetic methodology for tetrazole and their derivatives considering environmental and chemical industrial apprehensions and area of nanomaterials derived synthesis of tetrazole will continue to flourish.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S.S. and R.S. also would like to acknowledge Manipal University Jaipur for the laboratory facility.References
- A. Corma and H. Garcia, Adv. Synth. Catal., 2006, 348, 1391–1412 CrossRef CAS.
- M. B. Gawande, Y. Monga, R. Zboril and R. Sharma, Coord. Chem. Rev., 2015, 288, 118–143 CrossRef CAS.
- R. S. Upadhayaya, S. Jain, N. Sinha, N. Kishore, R. Chandra and S. K. Arora, Eur. J. Med. Chem., 2004, 39, 579–592 CrossRef CAS PubMed.
- E. Łukowska-Chojnacka, J. Mierzejewska, M. Milner-Krawczyk, M. Bondaryk and M. Staniszewska, Bioorg. Med. Chem., 2016, 24, 6058–6065 CrossRef PubMed.
- M. Asif, Pharm. Methods, 2014, 5, 39–46 Search PubMed.
- A. Qian, Y. Zheng, R. Wang, J. Wei, Y. Cui, X. Cao and Y. Yang, Bioorg. Med. Chem. Lett., 2018, 28, 344–350 CrossRef CAS PubMed.
- S. Vembu, P. Parasuraman and M. Gopalakrishnan, Pharma Chem., 2014, 6, 35–44 Search PubMed.
- P. Zhan, Z. Li, X. Liu and E. De Clercq, Mini-Rev. Med. Chem., 2009, 9, 1014–1023 CrossRef PubMed.
- K. Chauhan, M. Sharma, P. Trivedi, V. Chaturvedi and P. M. Chauhan, Bioorg. Med. Chem. Lett., 2014, 24, 4166–4170 CrossRef CAS PubMed.
- R. M. DeMarinis, J. R. Hoover, G. L. Dunn, P. Actor, J. V. Uri and J. A. Weisbach, J. Antibiot., 1975, 28, 463–470 CrossRef CAS PubMed.
- M. Uchida, M. Komatsu, S. Morita, T. Kanbe and K. Nakagawa, Chem. Pharm. Bull., 1989, 37, 322–326 CrossRef CAS PubMed.
- M. Uchida, M. Komatsu, S. Morita, T. Kanbe, K. Yamasaki and K. Nakagawa, Chem. Pharm. Bull., 1989, 37, 958–961 CrossRef CAS PubMed.
- R. E. Ford, P. Knowles, E. Lunt, S. M. Marshall, A. J. Penrose, C. A. Ramsden, A. J. Summers, J. L. Walker and D. E. Wright, J. Med. Chem., 1986, 29, 538–549 CrossRef CAS PubMed.
- S. Hayao, H. J. Havera, W. G. Strycker, T. Leipzig and R. Rodriguez, J. Med. Chem., 1967, 10, 400–402 CrossRef CAS PubMed.
- P. Purohit, A. K. Pandey, D. Singh, P. S. Chouhan, K. Ramalingam, M. Shukla, N. Goyal, J. Lal and P. M. Chauhan, MedChemComm, 2017, 8, 1824–1834 RSC.
- C. G. Neochoritis, T. Zhao and A. Dömling, Chem. Rev., 2019, 119, 1970–2042 CrossRef CAS PubMed.
- F. Gao, J. Xiao and G. Huang, Eur. J. Med. Chem., 2019, 184, 111744 CrossRef CAS PubMed.
- V. Bhaskar and P. Mohite, J. Optoelectron. Biomed. Mater., 2010, 2, 249–259 Search PubMed.
- L. Myznikov, A. Hrabalek and G. Koldobskii, Chem. Heterocycl. Compd., 2007, 43, 1–9 CrossRef CAS.
- K. Dileep, S. Polepalli, N. Jain, S. K. Buddana, R. Prakasham and M. Murty, Mol. Diversity, 2018, 22, 83–93 CrossRef CAS PubMed.
- A. K. Gupta, C. H. Song and C. H. Oh, Tetrahedron Lett., 2004, 45, 4113–4116 CrossRef CAS.
- D. R. Patil, Y. B. Wagh, P. G. Ingole, K. Singh and D. S. Dalal, New J. Chem., 2013, 37, 3261–3266 RSC.
- J. Dhainaut, J.-P. Dacquin, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 296–303 RSC.
- F. G. Fallon and R. M. Herbst, J. Org. Chem., 1957, 22, 933–936 CrossRef CAS.
- T. Jin, S. Kamijo and Y. Yamamoto, Tetrahedron Lett., 2004, 45, 9435–9437 CrossRef CAS.
- K. Bahrami, M. M. Khodaei and M. Roostaei, New J. Chem., 2014, 38, 5515–5520 RSC.
- E. Carbonell, E. Delgado-Pinar, J. Pitarch-Jarque, J. Alarcón and E. García-España, J. Phys. Chem. C, 2013, 117, 14325–14331 CrossRef CAS.
- A. Keivanloo, M. Bakherad, E. Imanifar and M. Mirzaee, Appl. Catal., A, 2013, 467, 291–300 CrossRef CAS.
- M. Mirzaee, B. Bahramian and A. Amoli, Appl. Organomet. Chem., 2015, 29, 593–600 CrossRef CAS.
- Z. Wu, Y. Mao, M. Song, X. Yin and M. Zhang, Catal. Commun., 2013, 32, 52–57 CrossRef CAS.
- S.-M. Kim, Y.-J. Lee, K.-W. Jun, J.-Y. Park and H. Potdar, Mater. Chem. Phys., 2007, 104, 56–61 CrossRef CAS.
- A. Ghorbani-Choghamarani, P. Moradi and B. Tahmasbi, RSC Adv., 2016, 6, 56638–56646 RSC.
- A. Ghorbani-Choghamarani, P. Moradi and B. Tahmasbi, RSC Adv., 2016, 6, 56458–56466 RSC.
- V. Vatanpour, S. S. Madaeni, L. Rajabi, S. Zinadini and A. A. Derakhshan, J. Membr. Sci., 2012, 401, 132–143 CrossRef.
- B. Tahmasbi, A. Ghorbani-Choghamarani and P. Moradi, New J. Chem., 2020, 44, 3717–3727 RSC.
- A. Jabbari, B. Tahmasbi, M. Nikoorazm and A. Ghorbani-Choghamarani, Appl. Organomet. Chem., 2018, 32, e4295 CrossRef.
- P. Moradi and A. Ghorbani-Choghamarani, Appl. Organomet. Chem., 2017, 31, e3602 CrossRef.
- A. Ghorbani-Choghamarani, H. Aghavandi and M. Mohammadi, Appl. Organomet. Chem., 2020, 34, e5804 CrossRef CAS.
- M. A. Jani and K. Bahrami, Appl. Organomet. Chem., 2020, 34, e6014 CrossRef CAS.
- B. Tahmasbi and A. Ghorbani-Choghamarani, Appl. Organomet. Chem., 2017, 31, e3644 CrossRef.
- M. Zarghani and B. Akhlaghinia, RSC Adv., 2016, 6, 31850–31860 RSC.
- T. Tamoradi, A. Irandoust and M. Ghadermazi, J. Iran. Chem. Soc., 2019, 16, 1723–1733 CrossRef CAS.
- T. Tamoradi, S. Taherabadi and M. Ghadermazi, Polyhedron, 2019, 171, 305–311 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, New J. Chem., 2017, 41, 11714–11721 RSC.
- M. Nikoorazm and Z. Erfani, Chem. Phys. Lett., 2019, 737, 136784 CrossRef CAS.
- M. Nemati, T. Tamoradi and H. Veisi, Polyhedron, 2019, 167, 75–84 CrossRef CAS.
- N. Moeini, T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Appl. Organomet. Chem., 2018, 32, e4445 CrossRef.
- H. Fatahi, M. Jafarzadeh and Z. Pourmanouchehri, J. Heterocycl. Chem., 2019, 56, 2090–2098 CrossRef CAS.
- M. A. Ashraf, Z. Liu, C. Li and D. Zhang, Appl. Organomet. Chem., 2020, e6133 Search PubMed.
- M. S. Ghasemzadeh and B. Akhlaghinia, ChemistrySelect, 2020, 5, 6440–6452 CrossRef CAS.
- A. Karimian, M. Namvar-Mhaboub and R. Abbasi, Russ. J. Org. Chem., 2020, 56, 1646–1653 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, Solid State Sci., 2019, 88, 81–94 CrossRef CAS.
- H. Naeimi and S. Mohamadabadi, Dalton Trans., 2014, 43, 12967–12973 RSC.
- A. Khalafi-Nezhad and S. Mohammadi, RSC Adv., 2013, 3, 4362–4371 RSC.
- D. Habibi, N. Pakravan, A. Arabi and Z. Kaboudvand, Appl. Organomet. Chem., 2018, 32, e3988 CrossRef.
- D. Habibi, S. Heydari, A. Gil, M. Afsharfarnia, A. Faraji, R. Karamian and M. Asadbegy, Appl. Organomet. Chem., 2018, 32, e4005 CrossRef.
- M. S. Ghasemzadeh and B. Akhlaghinia, Bull. Chem. Soc. Jpn., 2017, 90, 1119–1128 CrossRef CAS.
- M. Salimi and A. Zamanpour, Appl. Organomet. Chem., 2020, 34, e5682 CAS.
- M. Salimi, F. Esmaeli-nasrabadi and R. Sandaroos, Inorg. Chem. Commun., 2020, 122, 108287 CrossRef CAS.
- A. N. Parouch, N. Koukabi and E. Abdous, Res. Chem. Intermed., 2020, 46, 3295–3310 CrossRef.
- R. Gao, Z. Yang, L. Zheng, L. Gu, L. Liu, Y. Lee, Z. Hu and X. Liu, ACS Catal., 2018, 8, 1955–1963 CrossRef CAS.
- N. Zhou, Q. An, Z. Xiao, S. Zhai and Z. Shi, ACS Sustainable Chem. Eng., 2017, 5, 5394–5407 CrossRef CAS.
- W. Xie, Z. Guo, F. Gao, Q. Gao, D. Wang, B.-s. Liaw, Q. Cai, X. Sun, X. Wang and L. Zhao, Theranostics, 2018, 8, 3284 CrossRef CAS PubMed.
- X. Sun, M. Sui, G. Cui, L. Li, X. Li, X. Lv, F. Wu and G. Gu, RSC Adv., 2018, 8, 17489–17496 RSC.
- M. Khodamorady and K. Bahrami, ChemistrySelect, 2019, 4, 8183–8194 CrossRef CAS.
- P. Akbarzadeh, N. Koukabi and M. M. Hosseini, J. Heterocycl. Chem., 2020, 57, 2455–2465 CrossRef CAS.
- P. Akbarzadeh, N. Koukabi and E. Kolvari, Res. Chem. Intermed., 2019, 45, 1009–1024 CrossRef CAS.
- B. C. Ranu, R. Dey, T. Chatterjee and S. Ahammed, ChemSusChem, 2012, 5, 22–44 CrossRef CAS PubMed.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- K. Pan, H. Ming, H. Yu, Y. Liu, Z. Kang, H. Zhang and S. T. Lee, Cryst. Res. Technol., 2011, 46, 1167–1174 CrossRef CAS.
- F. Taghavi, M. Gholizadeh, A. S. Saljooghi and M. Ramezani, MedChemComm, 2017, 8, 1953–1964 RSC.
- B. Salahshournia, H. Hamadi and V. Nobakht, Appl. Organomet. Chem., 2018, 32, e4416 CrossRef.
- P. Movaheditabar, M. Javaherian and V. Nobakht, React. Kinet., Mech. Catal., 2017, 122, 217–228 CrossRef CAS.
- P. Moradi, M. Hajjami and B. Tahmasbi, Polyhedron, 2020, 175, 114169 CrossRef.
- J. Y. Kim, K. Park, S. Y. Bae, G. C. Kim, S. Lee and H. C. Choi, J. Mater. Chem., 2011, 21, 5999–6005 RSC.
- T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, New J. Chem., 2018, 42, 5479–5488 RSC.
- L. Wei, S. Yan, H. Wang and H. Yang, NPG Asia Mater., 2018, 10, 899–911 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani, M. Ghadermazi and H. Veisi, Solid State Sci., 2019, 91, 96–107 CrossRef CAS.
- F. Pourhassan and H. Eshghi, Catal. Lett., 2020, 150, 1287–1300 CrossRef CAS.
- R. Ghafouri-Nejad and M. Hajjami, React. Kinet., Mech. Catal., 2020, 129, 371–389 CrossRef CAS.
- A. Ghorbani-Choghamarani, L. Shiri and G. Azadi, RSC Adv., 2016, 6, 32653–32660 RSC.
- M. Esmaeilpour, J. Javidi and S. Zahmatkesh, Appl. Organomet. Chem., 2016, 30, 897–904 CrossRef CAS.
- M. Esmaeilpour, J. Javidi, F. N. Dodeji and M. M. Abarghoui, J. Mol. Catal. A: Chem., 2014, 393, 18–29 CrossRef CAS.
- F. Dehghani, A. R. Sardarian and M. Esmaeilpour, J. Organomet. Chem., 2013, 743, 87–96 CrossRef CAS.
- M. Darabi, T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Transition Met. Chem., 2017, 42, 703–710 CrossRef CAS.
- N. Razavi and B. Akhlaghinia, RSC Adv., 2015, 5, 12372–12381 RSC.
- G. Azadi, A. Ghorbani-Choghamarani and L. Shiri, Transition Met. Chem., 2017, 42, 131–136 CrossRef CAS.
- V. Khorramabadi, D. Habibi and S. Heydari, Green Chem. Lett. Rev., 2020, 13, 50–59 CrossRef CAS.
- V. Khorramabadi, D. Habibi and S. Heydari, Org. Prep. Proced. Int., 2020, 52, 139–146 CrossRef CAS.
- M. Ariannezhad, D. Habibi and S. Heydari, Polyhedron, 2019, 160, 170–179 CrossRef CAS.
- A. R. Sardarian, H. Eslahi and M. Esmaeilpour, ChemistrySelect, 2018, 3, 1499–1511 CrossRef CAS.
- T. Sun, J. Qiu and C. Liang, J. Phys. Chem. C, 2008, 112, 715–721 CrossRef CAS.
- E. S. Jang, J. H. Won, S. J. Hwang and J. H. Choy, Adv. Mater., 2006, 18, 3309–3312 CrossRef CAS.
- Y. A. Attia, Mater. Express, 2016, 6, 211–219 CrossRef CAS.
- T.-J. Hsueh, C.-L. Hsu, S.-J. Chang, P.-W. Guo, J.-H. Hsieh and I.-C. Chen, Scr. Mater., 2007, 57, 53–56 CrossRef CAS.
- C. Huo, J. Ouyang and H. Yang, Sci. Rep., 2014, 4, 1–9 Search PubMed.
- R. S. Mane, W. J. Lee, H. M. Pathan and S.-H. Han, J. Phys. Chem. B, 2005, 109, 24254–24259 CrossRef CAS PubMed.
- O. Seven, B. Dindar, S. Aydemir, D. Metin, M. Ozinel and S. Icli, J. Photochem. Photobiol., A, 2004, 165, 103–107 CrossRef CAS.
- P. Chhattise, S. Saleh, V. Pandit, S. Arbuj and V. Chabukswar, Mater. Adv., 2020, 1, 2339–2345 RSC.
- M. Lakshmi Kantam, K. S. Kumar and C. Sridhar, Adv. Synth. Catal., 2005, 347, 1212–1214 CrossRef.
- F. Himo, Z. P. Demko and L. Noodleman, J. Org. Chem., 2003, 68, 9076–9080 CrossRef CAS PubMed.
- F. Himo, Z. P. Demko, L. Noodleman and K. B. Sharpless, J. Am. Chem. Soc., 2002, 124, 12210–12216 CrossRef CAS PubMed.
- F. Himo, Z. P. Demko, L. Noodleman and K. B. Sharpless, J. Am. Chem. Soc., 2003, 125, 9983–9987 CrossRef CAS PubMed.
- S. M. Agawane and J. M. Nagarkar, Catal. Sci. Technol., 2012, 2, 1324–1327 RSC.
- Y. A. Attia, Y. M. Mohamed, M. M. Awad and S. Alexeree, J. Organomet. Chem., 2020, 919, 121320 CrossRef CAS.
- Y. M. A. Mohamed and Y. A. Attia, Appl. Organomet. Chem., 2020, 34, e5758 CrossRef CAS.
- C. Testa, A. Zammataro, A. Pappalardo and G. T. Sfrazzetto, RSC Adv., 2019, 9, 27659–27664 RSC.
- A. V. Vakhrushev, V. I. Kodolov, A. Haghi and S. C. Ameta, Carbon Nanotubes and Nanoparticles: Current and Potential Applications, CRC Press, 2019 Search PubMed.
- P. Akbarzadeh, N. Koukabi and E. Kolvari, Mol. Diversity, 2019, 1–15 Search PubMed.
- M. Kazemnejadi, B. Mahmoudi, Z. Sharafi, M. A. Nasseri, A. Allahresani and M. Esmaeilpour, Appl. Organomet. Chem., 2020, 34, e5273 CAS.
- M. N. Soltani Rad, S. Behrouz, V. Sadeghi Dehchenari and S. J. Hoseini, J. Heterocycl. Chem., 2017, 54, 355–365 CrossRef CAS.
- M. Sen, in Nanotechnology and the Environment, IntechOpen, 2020 Search PubMed.
- M. Nasrollahzadeh, F. Ghorbannezhad and S. M. Sajadi, Appl. Organomet. Chem., 2019, 33, e4698 CrossRef.
- A. Ahmadi, T. Sedaghat, H. Motamedi and R. Azadi, Appl. Organomet. Chem., 2020, 34, e5572 CrossRef CAS.
- F. Rezaei, M. A. Amrollahi and R. Khalifeh, Inorg. Chim. Acta, 2019, 489, 8–18 CrossRef CAS.
- N. Motahharifar, M. Nasrollahzadeh, A. Taheri-Kafrani, R. S. Varma and M. Shokouhimehr, Carbohydr. Polym., 2020, 232, 115819 CrossRef CAS PubMed.
- M. Nasrollahzadeh, M. Sajjadi, M. R. Tahsili, M. Shokouhimehr and R. S. Varma, ACS Omega, 2019, 4, 8985–9000 CrossRef CAS PubMed.
- H. R. Pawar and R. C. Chikate, J. Mol. Struct., 2021, 1225, 128985 CrossRef CAS.
- J. Choi, D. Kim, S. Chang and W. Ahn, Appl. Catal., A, 2003, 254, 225–237 CrossRef CAS.
- A. S. Diez, M. Alvarez and M. A. Volpe, J. Braz. Chem. Soc., 2015, 26, 1542–1550 CAS.
- S. Alahmad, Orient. J. Chem., 2012, 28, 1 CrossRef.
- F. Matloubi Moghaddam, M. Eslami and N. Ghadirian, Sci. Iran., 2019, 26, 1463–1473 Search PubMed.
- M. Nikoorazm, B. Tahmasbi, S. Gholami and P. Moradi, Appl. Organomet. Chem., 2020, 34, e5919 CAS.
- M. Nikoorazm, P. Moradi and N. Noori, J. Porous Mater., 2020, 27, 1159–1169 CrossRef CAS.
- M. Nikoorazm, A. Ghorbani-Choghamarani, M. Ghobadi and S. Massahi, Appl. Organomet. Chem., 2017, 31, e3848 CrossRef.
- P. Movaheditabar and M. Javaherian, J. Org. Chem. Res., 2019, 5, 174–189 Search PubMed.
- F. Abrishami, M. Ebrahimikia and F. Rafiee, Iran. J. Catal., 2016, 6, 245–251 CAS.
- M. Halder, M. M. Islam, P. Singh, A. Singha Roy, S. M. Islam and K. Sen, ACS Omega, 2018, 3, 8169–8180 CrossRef CAS PubMed.
- F. Sarrafioun, S. Jamehbozorgi and M. Ramezani, Russ. J. Org. Chem., 2019, 55, 1777–1784 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |