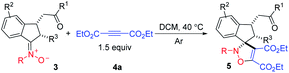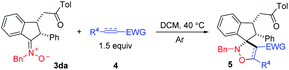Open Access Article
Open Access ArticleSynthesis of spirocyclic Δ4-isoxazolines via [3 + 2] cycloaddition of indanone-derived ketonitrones with alkynes†
Yilin Liu *ab,
Jiaxue Liua,
Yan-Yun Liu
*ab,
Jiaxue Liua,
Yan-Yun Liu *a,
Boxiao Tanga,
Hongwei Lina,
Yuanxiang Lia and
Lin Zhanga
*a,
Boxiao Tanga,
Hongwei Lina,
Yuanxiang Lia and
Lin Zhanga
aHunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material, Institute of Organic Synthesis, Huaihua University, Huaihua 418000, China. E-mail: liuyilinhn@126.com; liuyanyun314@sina.com
bCAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 13th September 2021
Abstract
A [3 + 2] cycloaddition of indanone-derived nitrones and alkynes under mild conditions is developed, allowing facile synthesis of spirocyclicindenyl isoxazolines with structural diversity. The sequential protocol of generated in situ ketonitrone from unsaturated ketones and N-alkylhydroxylamines is also achieved successfully, affording the desired products in considerable yield with moderate to good diastereoselectivity. Moreover, the spirocyclic product can be conveniently transformed into indenyl-based allylic alcohol and enamide.
Introduction
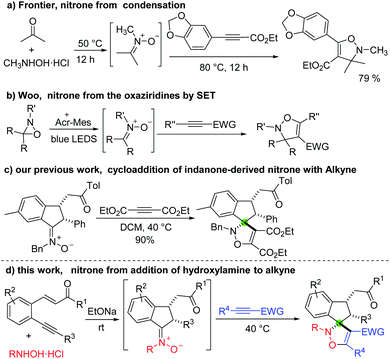
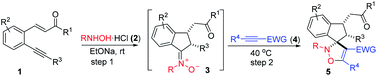
Isoxazolines are part of an important class of N,O-containing heterocycles, since they are well known for biological properties1 and could be used as versatile intermediates for the synthesis of many complex compounds.2 Among the many methods, the cycloaddition of nitrones has been widely used in the synthesis of this skeleton.3 Recently, one-pot cycloaddition reactions of nitrones generated in situ have attracted much attention due to their high efficiency and avoidance of complicated operation and the separation of unstable nitrones. Despite great progress being made in the cycloaddition of aldonitrones with cyclopropanes,4 olefins,5 and alkynes,6 however, the cycloaddition of ketonitrones generated in situ for preparation of 4-isoxazoline remains scarce. In 2009, Frontier and coworkers reported that the [3 + 2] dipolar cycloaddition of an electron-deficient alkyne and a ketonitrone generated in situ from condensation of acetone and N-methylhydroxylamine gives an isolable isoxazoline in 79% yield (Scheme 1a).7 Recently, Woo developed an efficient visible-light photoredox-catalyzed [3 + 2] cycloaddition of oxaziridines with alkynes to give 4-isoxazoline in high yield, this novel strategy involves in situ generation of ketonitrones from oxaziridines through a SET way (Scheme 1b).8 However, most of these powerful approaches suffer from the use of transition metal catalysts and unstable and expensive reagents,4,5c,6c or multistep manipulations as well as uneconomical atomic transformations.5f,6a Consequently, the development of an environmentally friendly and atom-economical cycloaddition of novel nitrone generated in situ for the synthesis of highly functionalized isoxazoline is still of great interest.Spiroisoxazolines have been received intensive attention because the incorporation of a rigid spiro-ring can reduce the conformational entropy penalty upon binding with a protein target in modern drug discovery.9 However, the application of cycloaddition reaction of nitrone to construct spiroisoxazolines is largely undeveloped, because methods for the synthesis of cyclic ketonitrone are still not rich and mostly limited to specific substrates scope such as cyclic ketone derived nitrones,10 isatin ketonitrones,11 sugar ketonitrones,12 fluorenone nitrones.13 Therefore, only a few examples using these compounds as substrates to synthesize spirocyclic compounds have been reported. Oxyallyl cations,14 cyclopropanes,11f aza-oxyallyl cations,15 olefins11a,b,d,12,16 gave the expected cycloadducts in high yields, while reactions with acetylenes seldom gave the corresponding spiroisoxazolines. Instead, non spiro products arising from transformations of the initially formed spiroisoxazoline are produced in these cases.13c,17 For example, in 2012, Anderson and co-workers reported that the reaction of alkynes with N-vinyl fluorenone nitrones provides the fluorene-tethered isoxazoles at high temperature via a cyclization and elimination process.13c In contrast, Prathapan and co-workers discovered that the cycloadduct resulting from the reaction of N-phenyl fluorenone nitrones and electron deficient acetylenes was formed predominantly initially and then could undergo rearrangement easily to give 3(2H)-furanone at room temperature.17b Therefore, the continuous development of novel cyclic ketonitrones for the discovery of spirocyclic drug candidates is highly desirable.
Recently, we developed a carbonyl-directed addition of N-alkylhydroxylamines to unactivated alkynes with high stereoselectivity. This strategy enables the facile synthesis of indanone-derived nitrones, which was subjected successfully to [3 + 2] cycloadditions with diethyl acetylenedicarboxylate (DEAD) in dichloromethane (DCM) to give spiro-isoxazoline in 90% yield (Scheme 1c).20 This privilege skeleton prompted us to further expand the substrate scope. Herein, we wish to report our efforts on the [3 + 2] cycloaddition of indanone ketonitrones with alkynes under mild conditions (Scheme 1d).
Results and discussion
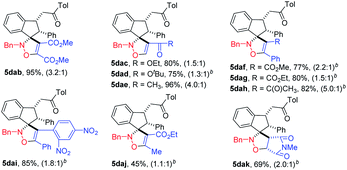
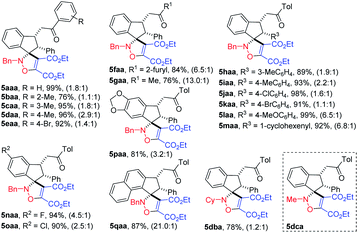
It was found that the amount of DEAD 4a in cycloadditions could be decreased to 1.5 equivalent, and the same reactivity was observed. The relative configuration of product spiroindenyl isoxazoline 5 was assigned by its analogue X-ray diffraction analysis reported by us.20 As shown in Table 1, a variety of N-benzyl indanone-derived nitrones underwent cycloadditions with DEAD 4a in DCM at 40 °C smoothly, affording the corresponding spiroindenyl isoxazolines in good to excellent yields, albeit with low dr values (5aaa–qaa). It was interesting to find that when the R1 group was methyl substituent, the nitrone 3ga produced the desired products in higher dr value, compared to nitrones with aryl group at position R1 (5aaa–faa vs. 5gaa). Spiroindenyl isoxazolines could be obtained in excellent yields when indanone-derived nitrones 3ha–ma were used as substrates. The reaction tolerated different groups at position R2 in nitrones 3, affording the desired products in good to excellent yields (5naa–qaa). It is worth noting that naphthalenyl isoxazoline 5qaa was furnished in 87% yield with a high 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, which may attribute to the steric hindrance effect. To our pleasure, N-cyclohexenyl indanone-derived nitrone 3db was also an effective substrate for this reaction to furnish the desired product 5dba in good yield. However, N-methyl isoxazoline 5dca failed to be furnished, because nitrone 3dc is too unstable to be separated.
1 dr, which may attribute to the steric hindrance effect. To our pleasure, N-cyclohexenyl indanone-derived nitrone 3db was also an effective substrate for this reaction to furnish the desired product 5dba in good yield. However, N-methyl isoxazoline 5dca failed to be furnished, because nitrone 3dc is too unstable to be separated.
| a All reactions were carried out with 3 (0.20 mmol), 4a (1.5 equiv.), and DCM (3.0 mL), 17–21 h unless otherwise stated; isolated yield based on 3; the dr ratio is given in brackets and determined by 1H NMR analysis (see ESI for details). |
|---|
 |
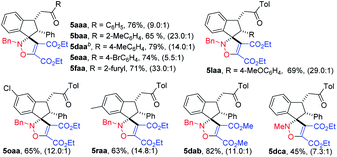
Next, to further probe the generality of this cycloaddition reaction, a variety of alkynes 4b–j were treated with indanone-derived nitrone 3da. The results are shown in Table 2 (5dab–daj). Although dimethyl acetylenedicarboxylate, ethyl propionate, and 3-butyn-2-one gave the corresponding spiroindenyl isoxazoline in high yields in dichloromethane, the reaction of tert-butyl propionate, methyl phenylpropiolate, ethyl phenylpropiolate, 4-phenyl-3-butyn-2-one, and ethyl 2-butynoate needed to be carried out in chloroform at higher temperature to obtain satisfactory yields (5dab, 5dac, 5dae vs. 5dad, 5daf–dah, 5daj). To our delight, cycloaddition reaction of nitrone 3da with diphenylethyne also went smoothly to give diphenyl-4-isoxazoline (5dai), which may be a potential inhibitor of cyclooxygenase-2 with analgesic and antiinflammatory activity according to the study reported by Knaus.1a It was found that electron deficient olefin was also a good partner in cycloaddition reaction with indanone-derived nitrone, as N-methylmaleimide could afford the spiroindenyl isoxazolidine in moderate yield (5dak).
DCM was used as the solvent in both the preparation of indanone-derived nitrone and the cycloaddition reaction of nitrone with electron deficient alkyne, therefore, we envisioned that cycloaddition of nitrones generated in situ from unsaturated ketone 1, and N-alkylhydroxylamine 2, with alkyne 4 for synthesis of spiroindenyl isoxazoline was possible. Indeed, the cycloaddition of nitrones generated in situ went smoothly to afford the spiroindenyl isoxazoline in good yield, and the results are summarized in Table 3. Of note is that 5dca can be afforded successfully in a yield of 45%. Surprisingly, this cycloaddition gave higher dr value than cycloaddition of pre-prepared nitrone in Table 1. The mechanism is still not clear currently, according to the previous literature17–19 and experimental results, the reaction process may be determined by the attack of nucleophilic oxygen anion in nitrone moiety on the carbon–carbon triple bond in alkynes,19 and the reason is probably that this cycloaddition, at least in part, follows a two-step mechanism, while cycloaddition of pre-prepared nitrone in Table 1 proceeds in a concerted manner.
| a All reactions were carried out with 1 (0.50 mmol), 2 (0.50 mmol), EtONa (1.3 equiv.), and DCM (5.0 mL), 12–24 h for step one, then 4 (1.5 equiv.) was added, 17–21 h for step two unless otherwise stated; isolated yield based on 1; the dr ratio is given in brackets and determined by 1H NMR analysis (see ESI for details).b The reactions were carried out with 4 mmol scale of 1d. |
|---|
 |
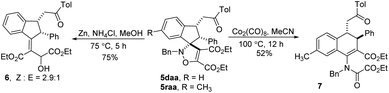
With the novel spiroisoxazolines in hand, subsequently, transformations of isoxazoline were investigated (Scheme 2). 4-Isoxazoline 5daa underwent reductive cleavage of the N–O bond successfully in the present of zinc powder and NH4Cl at 75 °C, affording allylic alcohol 6 in a yield of 75%.8,21 Besides, it was found that Co2(CO)8 catalyzed rearrangement of 4-isoxazoline 5raa could occur in MeCN, giving enamide 7 in 52% yield, instead of ring contraction product acylaziridines.22 While the mechanism for Co2(CO)8 catalyzed rearrangement is not clear at present, further examination of the rearrangement reaction conditions and mechanism will be carried out in due course.
Conclusions
In summary, we have reported a novel [3 + 2] cycloaddition between indanone-derived nitrones and electron deficient alkynes to give a series of spiroindenyl isoxazolines under mild conditions in moderate to good yields. To the best of our knowledge, this is the first example of [3 + 2] cycloaddition of indanone-derived nitrones generated in situ, giving the corresponding spiroindenyl isoxazolines in high diastereoselectivity. Application of these spiroindenyl isoxazolines and expansion of the scope of dipolarophiles for synthesis of other novel spiroindenyl compounds are currently under investigation in our laboratory.Experimental
General information
All 1H, 13C, and 19F NMR spectra were recorded at ambient temperatures on a Bruker 400 MHz or 500 MHz advance spectrometer with tetramethylsilane as internal standard. High-resolution mass spectra (HRMS) were recorded on an Agilent 1290 or GCT Premier Mass Spectrometer using ESI-TOF or EI (electrospray ionization time of flight). All reactions were monitored by thin-layer chromatography. Column chromatography (petroleum ether/ethyl acetate) was performed on silica gel (200–300 mesh). 2,4-Dinitro-1-(phenylethynyl)benzene 4i23 was prepared according to literature procedure, and other reagents were purchased from commercial suppliers and used without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the desired product 5aaa as a light yellow solid (0.1191 g, 99% yield, 1.8
1, v/v) to afford the desired product 5aaa as a light yellow solid (0.1191 g, 99% yield, 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).Diethyl 2′-benzyl-3-(2-oxo-2-phenylethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5aaa), 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 119.1 mg, 99% yield; light yellow solid, mp 120–122 °C, IR (film) 1739, 1709, 1474, 1303, 1231, 1188 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 10.0 Hz, 0.7H), 7.79 (d, J = 10.0 Hz, 1.3H), 7.67 (t, J = 5.0 Hz, 1.3H), 7.57–7.33 (m, 5.7H), 7.32–7.11 (m, 10H), 4.50 (t, J = 10.0 Hz, 0.3H), 4.25–4.07 (m, 5.3H), 4.02 (d, J = 15.0 Hz, 0.7H), 3.87 (dd, J = 15.0, 5.0 Hz, 0.7H), 3.57–3.43 (m, 2.3H), 3.36 (dd, J = 20.0, 10.0 Hz, 0.4H), 3.22 (dd, J = 15.0, 5.0 Hz, 0.3H), 1.29–1.20 (m, 4.1H), 1.13 (d, J = 10.0 Hz, 1.9H); 13C NMR (125 MHz, CDCl3) δ 199.5, 199.1, 162.7, 162.4, 159.2, 155.4, 154.8, 148.0, 147.0, 139.8, 137.8, 137.5, 137.4, 137.4, 137.2, 137.0, 135.5, 133.6, 133.4, 133.1, 131.6, 130.5, 130.0, 129.7, 129.1, 129.0, 128.8, 128.7, 128.6, 128.6, 128.4, 128.3, 128.2, 128.1, 128.0, 127.6, 127.6, 127.4, 127.2, 127.2, 127.1, 126.6, 126.3, 126.0, 124.7, 113.1, 108.7, 100.2, 85.3, 84.0, 62.6, 62.6, 61.4, 60.9, 60.8, 60.6, 60.2, 58.4, 43.9, 42.6, 42.3, 40.4, 14.2,14.1, 14.0, 14.0; HRMS(ESI) calcd for C38H36NO6 [M + H]+ 602.2537, found 602.2543.
1 petroleum ether/ethyl acetate): 119.1 mg, 99% yield; light yellow solid, mp 120–122 °C, IR (film) 1739, 1709, 1474, 1303, 1231, 1188 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 10.0 Hz, 0.7H), 7.79 (d, J = 10.0 Hz, 1.3H), 7.67 (t, J = 5.0 Hz, 1.3H), 7.57–7.33 (m, 5.7H), 7.32–7.11 (m, 10H), 4.50 (t, J = 10.0 Hz, 0.3H), 4.25–4.07 (m, 5.3H), 4.02 (d, J = 15.0 Hz, 0.7H), 3.87 (dd, J = 15.0, 5.0 Hz, 0.7H), 3.57–3.43 (m, 2.3H), 3.36 (dd, J = 20.0, 10.0 Hz, 0.4H), 3.22 (dd, J = 15.0, 5.0 Hz, 0.3H), 1.29–1.20 (m, 4.1H), 1.13 (d, J = 10.0 Hz, 1.9H); 13C NMR (125 MHz, CDCl3) δ 199.5, 199.1, 162.7, 162.4, 159.2, 155.4, 154.8, 148.0, 147.0, 139.8, 137.8, 137.5, 137.4, 137.4, 137.2, 137.0, 135.5, 133.6, 133.4, 133.1, 131.6, 130.5, 130.0, 129.7, 129.1, 129.0, 128.8, 128.7, 128.6, 128.6, 128.4, 128.3, 128.2, 128.1, 128.0, 127.6, 127.6, 127.4, 127.2, 127.2, 127.1, 126.6, 126.3, 126.0, 124.7, 113.1, 108.7, 100.2, 85.3, 84.0, 62.6, 62.6, 61.4, 60.9, 60.8, 60.6, 60.2, 58.4, 43.9, 42.6, 42.3, 40.4, 14.2,14.1, 14.0, 14.0; HRMS(ESI) calcd for C38H36NO6 [M + H]+ 602.2537, found 602.2543.
Diethyl 2′-benzyl-3-(2-oxo-2-(o-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5baa), 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 93.6 mg, 76% yield; light yellow solid, mp 87–89 °C, IR (film) 1747, 1712, 1456, 1371, 1299, 1187 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.64–7.62 (m, 1H), 7.58 (d, J = 8.0 Hz, 0.5H), 7.45–7.43 (m, 2H), 7.39–7.36 (m, 1.5H), 7.34–7.26 (m, 4H), 7.25–7.10 (m, 9H), 4.49–4.43 (m, 0.6H), 4.21–4.12 (m, 4.8H), 4.00 (d, J = 12.0 Hz, 0.5H), 3.86 (d, J = 16.0 Hz, 0.5H), 3.82 (d, J = 12.0 Hz, 0.5H), 3.52 (d, J = 12.0 Hz, 0.5H), 3.50–3.42 (m, 1.3H), 3.32–3.25 (m, 0.6H), 3.16 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.48 (s, 1.6H), 2.36 (s, 1.4H), 1.29–1.18 (m, 4.6H), 1.13 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 203.3, 202.8, 162.7, 162.4, 159.3, 159.2, 155.5, 154.8, 148.2, 147.0, 138.7, 138.4, 138.3, 137.8, 137.6, 137.5, 137.3, 137.2, 137.0, 135.5, 132.3, 132.1, 131.7, 131.7, 131.4, 130.5, 130.0, 129.7, 129.1, 129.0, 128.7, 128.6, 128.5, 128.3, 128.2, 128.0, 127.6, 127.4, 127.2, 127.2, 127.1, 126.5, 126.4, 126.1, 125.9, 125.7, 124.5, 108.7, 127.2, 85.3, 84.1, 62.6, 61.5, 60.9, 60.8, 60.6, 60.2, 58.4, 45.6, 45.0, 43.9, 40.7, 29.9, 21.7, 21.4, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2690.
1 petroleum ether/ethyl acetate): 93.6 mg, 76% yield; light yellow solid, mp 87–89 °C, IR (film) 1747, 1712, 1456, 1371, 1299, 1187 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.64–7.62 (m, 1H), 7.58 (d, J = 8.0 Hz, 0.5H), 7.45–7.43 (m, 2H), 7.39–7.36 (m, 1.5H), 7.34–7.26 (m, 4H), 7.25–7.10 (m, 9H), 4.49–4.43 (m, 0.6H), 4.21–4.12 (m, 4.8H), 4.00 (d, J = 12.0 Hz, 0.5H), 3.86 (d, J = 16.0 Hz, 0.5H), 3.82 (d, J = 12.0 Hz, 0.5H), 3.52 (d, J = 12.0 Hz, 0.5H), 3.50–3.42 (m, 1.3H), 3.32–3.25 (m, 0.6H), 3.16 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.48 (s, 1.6H), 2.36 (s, 1.4H), 1.29–1.18 (m, 4.6H), 1.13 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 203.3, 202.8, 162.7, 162.4, 159.3, 159.2, 155.5, 154.8, 148.2, 147.0, 138.7, 138.4, 138.3, 137.8, 137.6, 137.5, 137.3, 137.2, 137.0, 135.5, 132.3, 132.1, 131.7, 131.7, 131.4, 130.5, 130.0, 129.7, 129.1, 129.0, 128.7, 128.6, 128.5, 128.3, 128.2, 128.0, 127.6, 127.4, 127.2, 127.2, 127.1, 126.5, 126.4, 126.1, 125.9, 125.7, 124.5, 108.7, 127.2, 85.3, 84.1, 62.6, 61.5, 60.9, 60.8, 60.6, 60.2, 58.4, 45.6, 45.0, 43.9, 40.7, 29.9, 21.7, 21.4, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2690.
Diethyl 2′-benzyl-3-(2-oxo-2-(m-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5caa), 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 117.0 mg, 95% yield; light yellow oil, IR (film) 1745, 1701, 1496, 1370, 1181, 1108 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 10.0 Hz, 2H), 7.59 (s, 0.7H), 7.46–7.40 (m, 2H), 7.36–7.26 (m, 7H), 7.24–7.12 (m, 5H), 6.69 (t, J = 10.0 Hz, 0.5H), 6.79 (d, J = 5.0 Hz, 1H), 4.48 (t, J = 10.0 Hz, 0.6H), 4.26–4.10 (m, 4.5H), 4.02 (d, J = 15.0 Hz, 0.4H), 3.88–3.85 (m, 1.3H), 3.53–3.43 (m, 1.7H), 3.34 (dd, J = 20.0, 10.0 Hz, 0.7H), 3.20 (d, J = 15.0 Hz, 0.6H), 2.38 (s, 1.9H), 2.31 (s, 1.1H), 1.28–1.20 (m, 5H), 1.12 (t, J = 10.0 Hz, 1.3H); 13C NMR (125 MHz, CDCl3) δ 200.0, 199.6, 162.9, 162.4, 159.3, 156.1, 155.6, 154.9, 148.0, 147.0, 138.6, 138.5, 137.5, 137.4, 137.2, 137.1, 137.0, 135.6, 134.2, 133.9, 131.6, 130.5, 130.0, 129.8, 129.7, 128.9, 128.7, 128.6, 128.6, 128.2, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 127.2, 127.1, 126.6, 126.3, 126.0, 125.6, 125.4, 124.7, 120.6, 115.5, 107.3, 85.3, 84.0, 62.6, 61.4, 60.9, 60.7, 60.2, 58.4, 44.0, 42.6, 42.3, 40.5, 29.9, 21.5, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2696.
1 petroleum ether/ethyl acetate): 117.0 mg, 95% yield; light yellow oil, IR (film) 1745, 1701, 1496, 1370, 1181, 1108 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 10.0 Hz, 2H), 7.59 (s, 0.7H), 7.46–7.40 (m, 2H), 7.36–7.26 (m, 7H), 7.24–7.12 (m, 5H), 6.69 (t, J = 10.0 Hz, 0.5H), 6.79 (d, J = 5.0 Hz, 1H), 4.48 (t, J = 10.0 Hz, 0.6H), 4.26–4.10 (m, 4.5H), 4.02 (d, J = 15.0 Hz, 0.4H), 3.88–3.85 (m, 1.3H), 3.53–3.43 (m, 1.7H), 3.34 (dd, J = 20.0, 10.0 Hz, 0.7H), 3.20 (d, J = 15.0 Hz, 0.6H), 2.38 (s, 1.9H), 2.31 (s, 1.1H), 1.28–1.20 (m, 5H), 1.12 (t, J = 10.0 Hz, 1.3H); 13C NMR (125 MHz, CDCl3) δ 200.0, 199.6, 162.9, 162.4, 159.3, 156.1, 155.6, 154.9, 148.0, 147.0, 138.6, 138.5, 137.5, 137.4, 137.2, 137.1, 137.0, 135.6, 134.2, 133.9, 131.6, 130.5, 130.0, 129.8, 129.7, 128.9, 128.7, 128.6, 128.6, 128.2, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 127.2, 127.1, 126.6, 126.3, 126.0, 125.6, 125.4, 124.7, 120.6, 115.5, 107.3, 85.3, 84.0, 62.6, 61.4, 60.9, 60.7, 60.2, 58.4, 44.0, 42.6, 42.3, 40.5, 29.9, 21.5, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2696.
Diethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5daa), 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 118.2 mg, 96% yield; light yellow solid, mp 127–129 °C, IR (film) 1743, 1709, 1474, 1303, 1231, 1181 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 10.0 Hz, 1.5H), 7.70–7.66 (m, 1H), 7.47–7.12 (m, 15.5H), 4.50 (t, J = 10.0 Hz, 0.7H), 4.26–4.10 (m, 4.5H), 4.02 (d, J = 15.0 Hz, 0.3H), 3.87 (t, J = 10.0 Hz, 1.5H), 3.57–3.40 (m, 1.4H), 3.33 (dd, J = 20.0, 10.0 Hz, 0.8H), 3.18 (d, J = 20.0 Hz, 0.7H), 2.38 (s, 2.2H), 2.36 (s, 0.8H), 1.28–1.20 (m, 5.2H), 1.12 (t, J = 10.0 Hz, 0.8H); 13C NMR (125 MHz, CDCl3) δ 199.1, 198.8, 162.8, 162.5, 159.2, 155.4, 154.8, 148.0, 147.0, 144.2, 143.8, 137.4, 137.0, 135.6, 135.0, 134.6, 131.6, 130.5, 130.0, 129.6, 129.4, 129.3, 129.0, 128.6, 128.6, 128.5, 128.3, 128.2, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 127.1, 127.0, 126.6, 126.3, 125.9, 124.7, 108.8, 107.3, 85.3, 84.0, 62.5, 61.4, 60.8, 60.6, 60.2, 58.4, 43.9, 42.4, 42.1, 40.5, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2697.
1 petroleum ether/ethyl acetate): 118.2 mg, 96% yield; light yellow solid, mp 127–129 °C, IR (film) 1743, 1709, 1474, 1303, 1231, 1181 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 10.0 Hz, 1.5H), 7.70–7.66 (m, 1H), 7.47–7.12 (m, 15.5H), 4.50 (t, J = 10.0 Hz, 0.7H), 4.26–4.10 (m, 4.5H), 4.02 (d, J = 15.0 Hz, 0.3H), 3.87 (t, J = 10.0 Hz, 1.5H), 3.57–3.40 (m, 1.4H), 3.33 (dd, J = 20.0, 10.0 Hz, 0.8H), 3.18 (d, J = 20.0 Hz, 0.7H), 2.38 (s, 2.2H), 2.36 (s, 0.8H), 1.28–1.20 (m, 5.2H), 1.12 (t, J = 10.0 Hz, 0.8H); 13C NMR (125 MHz, CDCl3) δ 199.1, 198.8, 162.8, 162.5, 159.2, 155.4, 154.8, 148.0, 147.0, 144.2, 143.8, 137.4, 137.0, 135.6, 135.0, 134.6, 131.6, 130.5, 130.0, 129.6, 129.4, 129.3, 129.0, 128.6, 128.6, 128.5, 128.3, 128.2, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 127.1, 127.0, 126.6, 126.3, 125.9, 124.7, 108.8, 107.3, 85.3, 84.0, 62.5, 61.4, 60.8, 60.6, 60.2, 58.4, 43.9, 42.4, 42.1, 40.5, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H38NO6 [M + H]+ 616.2694, found 616.2697.
Diethyl 2′-benzyl-3-(2-(4-bromophenyl)-2-oxoethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5eaa), 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 125.2 mg, 92% yield; white solid, mp 96–98 °C, IR (film) 1735, 1701, 1499, 1373, 1223, 1167 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 5.0 Hz, 1H), 7.65–7.60 (m, 2H), 7.56 (d, J = 10.0 Hz, 1H), 7.49–7.38 (m, 4H), 7.33–7.09 (m, 10H), 4.44 (t, J = 10.0 Hz, 0.4H), 4.24–4.10 (m, 5.3H), 4.03 (d, J = 15.0 Hz, 0.6H), 3.86 (q, J = 10.0 Hz, 0.8H), 3.53–3.45 (m, 1.6H), 3.37–3.28 (m, 1H), 3.17 (d, J = 15.0 Hz, 0.4H), 1.28–1.20 (m, 4.3H), 1.13 (t, J = 10.0 Hz, 1.7H); 13C NMR (125 MHz, CDCl3) δ 198.6, 198.1, 162.7, 162.4, 159.2, 159.2, 155.6, 154.8, 147.8, 146.7, 137.9, 137.5, 137.4, 137.1, 136.9, 136.2, 135.7, 135.4, 132.1, 132.0, 131.5, 130.5, 130.0, 129.9, 129.7, 129.7, 128.9, 128.6, 128.6, 128.3, 128.2, 128.0, 127.7, 127.6, 127.4, 127.3, 127.2, 127.2, 126.4, 126.4, 126.1, 124.5, 108.7, 107.2, 85.3, 84.0, 62.6, 62.6, 61.4, 60.9, 60.8, 60.6, 60.2, 58.3, 43.9, 42.4, 42.2, 40.6, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C38H35BrNO6 [M + H]+ 680.1642, found 680.1648.
1 petroleum ether/ethyl acetate): 125.2 mg, 92% yield; white solid, mp 96–98 °C, IR (film) 1735, 1701, 1499, 1373, 1223, 1167 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 5.0 Hz, 1H), 7.65–7.60 (m, 2H), 7.56 (d, J = 10.0 Hz, 1H), 7.49–7.38 (m, 4H), 7.33–7.09 (m, 10H), 4.44 (t, J = 10.0 Hz, 0.4H), 4.24–4.10 (m, 5.3H), 4.03 (d, J = 15.0 Hz, 0.6H), 3.86 (q, J = 10.0 Hz, 0.8H), 3.53–3.45 (m, 1.6H), 3.37–3.28 (m, 1H), 3.17 (d, J = 15.0 Hz, 0.4H), 1.28–1.20 (m, 4.3H), 1.13 (t, J = 10.0 Hz, 1.7H); 13C NMR (125 MHz, CDCl3) δ 198.6, 198.1, 162.7, 162.4, 159.2, 159.2, 155.6, 154.8, 147.8, 146.7, 137.9, 137.5, 137.4, 137.1, 136.9, 136.2, 135.7, 135.4, 132.1, 132.0, 131.5, 130.5, 130.0, 129.9, 129.7, 129.7, 128.9, 128.6, 128.6, 128.3, 128.2, 128.0, 127.7, 127.6, 127.4, 127.3, 127.2, 127.2, 126.4, 126.4, 126.1, 124.5, 108.7, 107.2, 85.3, 84.0, 62.6, 62.6, 61.4, 60.9, 60.8, 60.6, 60.2, 58.3, 43.9, 42.4, 42.2, 40.6, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C38H35BrNO6 [M + H]+ 680.1642, found 680.1648.
Diethyl 2′-benzyl-3-(2-(furan-2-yl)-2-oxoethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5faa), 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 99.4 mg, 84% yield; light yellow solid, mp 65–67 °C, IR (film) 1740, 1710, 1467, 1300, 1253, 1189 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.53 (s, 1H), 7.43 (d, J = 10.0 Hz, 2H), 7.38–7.07 (m, 14H), 6.48 (t, J = 5.0 Hz, 1H), 6.42 (s, 0.1H), 4.41 (d, J = 5.0 Hz, 1H), 4.24–4.17 (m, 4H), 3.88–3.82 (m, 2H), 3.46 (d, J = 15.0 Hz, 1H), 3.20 (dd, J = 20.0, 10.0 Hz, 1H), 3.09 (dd, J = 15.0, 5.0 Hz, 1H), 1.28–1.19 (m, 5.7H), 1.12 (t, J = 5.0 Hz, 0.4H); 13C NMR (125 MHz, CDCl3) δ 188.5, 162.7, 159.2, 155.5, 152.8, 146.7, 146.6, 137.4, 136.9, 135.4, 130.4, 130.0, 129.0, 128.6, 128.2, 127.9, 127.5, 127.2, 127.1, 126.0, 124.6, 117.4, 112.4, 107.2, 84.0, 62.6, 61.5, 60.8, 60.2, 41.9, 40.4, 29.9, 14.2, 14.0; HRMS(ESI) calcd for C36H34NO7 [M + H]+ 592.2330, found 592.2333.
1 petroleum ether/ethyl acetate): 99.4 mg, 84% yield; light yellow solid, mp 65–67 °C, IR (film) 1740, 1710, 1467, 1300, 1253, 1189 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.53 (s, 1H), 7.43 (d, J = 10.0 Hz, 2H), 7.38–7.07 (m, 14H), 6.48 (t, J = 5.0 Hz, 1H), 6.42 (s, 0.1H), 4.41 (d, J = 5.0 Hz, 1H), 4.24–4.17 (m, 4H), 3.88–3.82 (m, 2H), 3.46 (d, J = 15.0 Hz, 1H), 3.20 (dd, J = 20.0, 10.0 Hz, 1H), 3.09 (dd, J = 15.0, 5.0 Hz, 1H), 1.28–1.19 (m, 5.7H), 1.12 (t, J = 5.0 Hz, 0.4H); 13C NMR (125 MHz, CDCl3) δ 188.5, 162.7, 159.2, 155.5, 152.8, 146.7, 146.6, 137.4, 136.9, 135.4, 130.4, 130.0, 129.0, 128.6, 128.2, 127.9, 127.5, 127.2, 127.1, 126.0, 124.6, 117.4, 112.4, 107.2, 84.0, 62.6, 61.5, 60.8, 60.2, 41.9, 40.4, 29.9, 14.2, 14.0; HRMS(ESI) calcd for C36H34NO7 [M + H]+ 592.2330, found 592.2333.
Diethyl 2′-benzyl-3-(2-oxopropyl)-2-phenyl-2,3-dihydro-2′H-spiro-[indene-1,3′-is-oxazole]-4′,5′-dicarboxylate (5gaa), 13.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (30
1 dr. Purified by silica gel column chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 82.0 mg, 76% yield; Light yellow oil, IR (film) 1712, 1685, 1448, 1370, 1223, 1108 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 4.0 Hz, 2H), 7.45–7.21 (m, 12H), 4.20–4.05 (m, 5H), 4.01–3.84 (m, 2H), 3.52–3.42 (m, 1H), 3.03–2.98 (m, 1H), 2.88–2.81 (m, 1H), 2.75 (d, J = 4.0 Hz, 0.1H), 2.15 (s, 0.2H), 2.02 (s, 2.8H), 1.29–1.19 (m, 3.2H), 1.12 (d, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 207.7, 162.4, 159.2, 154.7, 147.9, 137.6, 137.2, 137.1, 131.7, 129.7, 129.0, 128.6, 128.1, 127.6, 127.4, 127.2, 126.4, 126.2, 108.7, 85.2, 62.6, 60.9, 60.5, 58.0, 47.4, 43.4, 31.0, 14.1, 13.9. HRMS (ESI) calcd for C33H33NO6Na [M + Na]+ 562.2200, found 562.2199.
1 petroleum ether/ethyl acetate): 82.0 mg, 76% yield; Light yellow oil, IR (film) 1712, 1685, 1448, 1370, 1223, 1108 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 4.0 Hz, 2H), 7.45–7.21 (m, 12H), 4.20–4.05 (m, 5H), 4.01–3.84 (m, 2H), 3.52–3.42 (m, 1H), 3.03–2.98 (m, 1H), 2.88–2.81 (m, 1H), 2.75 (d, J = 4.0 Hz, 0.1H), 2.15 (s, 0.2H), 2.02 (s, 2.8H), 1.29–1.19 (m, 3.2H), 1.12 (d, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 207.7, 162.4, 159.2, 154.7, 147.9, 137.6, 137.2, 137.1, 131.7, 129.7, 129.0, 128.6, 128.1, 127.6, 127.4, 127.2, 126.4, 126.2, 108.7, 85.2, 62.6, 60.9, 60.5, 58.0, 47.4, 43.4, 31.0, 14.1, 13.9. HRMS (ESI) calcd for C33H33NO6Na [M + Na]+ 562.2200, found 562.2199.
Diethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-(m-tolyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5haa), 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 112.1 mg, 89% yield; white yellow solid, mp 50–52 °C, IR (film) 1741, 1712, 1496, 1370, 1300, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.7H), 7.67 (d, J = 8.0 Hz, 1.3H), 7.55 (d, J = 8.0 Hz, 0.7H), 7.45–7.27 (m, 7.6H), 7.24–7.11 (m, 5.9H), 7.06 (d, J = 8.0 Hz, 0.4H), 7.01 (d, J = 8.0 Hz, 0.7H), 4.48 (t, J = 8.0 Hz, 0.3H), 4.26–4.07 (m, 5.1H), 4.02 (d, J = 16.0 Hz, 0.8H), 3.85 (dd, J = 16.0, 8.0 Hz, 0.7H), 3.55–3.45 (m, 1.6H), 3.34 (dd, J = 20.0, 8.0 Hz, 1H), 3.20 (dd, J = 20.0, 4.0 Hz, 0.4H), 2.39 (s, 0.9H), 2.36 (s, 1.9H), 2.32 (s, 1.1H), 2.21 (s, 2H), 1.30–1.19 (m, 4.1H), 1.12 (t, J = 8.0 Hz, 1.9H); 13C NMR (100 MHz, CDCl3) δ 199.3, 198.8, 162.8, 162.4, 159.4, 159.3, 155.6, 154.8, 148.0, 147.0, 144.2, 143.8, 138.0, 137.6, 137.5, 137.4, 137.2, 137.1, 135.5, 135.0, 134.6, 132.6, 131.3, 129.9, 129.6, 129.4, 129.3, 128.8, 128.5, 128.5, 128.3, 128.2, 128.1, 127.9, 127.9, 127.5, 127.2, 127.1, 127.0, 126.4, 126.2, 125.9, 124.8, 108.8, 107.2, 85.2, 83.9, 62.6, 61.1, 60.8, 60.7, 60.6, 60.2, 58.2, 44.0, 42.4, 42.3, 40.4, 29.9, 21.8, 21.8, 21.7, 21.6, 14.2, 14.1, 14.1, 14.0; HRMS (ESI) calcd for C40H39NO6Na [M + Na]+ 652.2670, found 652.2669.
1 petroleum ether/ethyl acetate): 112.1 mg, 89% yield; white yellow solid, mp 50–52 °C, IR (film) 1741, 1712, 1496, 1370, 1300, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.7H), 7.67 (d, J = 8.0 Hz, 1.3H), 7.55 (d, J = 8.0 Hz, 0.7H), 7.45–7.27 (m, 7.6H), 7.24–7.11 (m, 5.9H), 7.06 (d, J = 8.0 Hz, 0.4H), 7.01 (d, J = 8.0 Hz, 0.7H), 4.48 (t, J = 8.0 Hz, 0.3H), 4.26–4.07 (m, 5.1H), 4.02 (d, J = 16.0 Hz, 0.8H), 3.85 (dd, J = 16.0, 8.0 Hz, 0.7H), 3.55–3.45 (m, 1.6H), 3.34 (dd, J = 20.0, 8.0 Hz, 1H), 3.20 (dd, J = 20.0, 4.0 Hz, 0.4H), 2.39 (s, 0.9H), 2.36 (s, 1.9H), 2.32 (s, 1.1H), 2.21 (s, 2H), 1.30–1.19 (m, 4.1H), 1.12 (t, J = 8.0 Hz, 1.9H); 13C NMR (100 MHz, CDCl3) δ 199.3, 198.8, 162.8, 162.4, 159.4, 159.3, 155.6, 154.8, 148.0, 147.0, 144.2, 143.8, 138.0, 137.6, 137.5, 137.4, 137.2, 137.1, 135.5, 135.0, 134.6, 132.6, 131.3, 129.9, 129.6, 129.4, 129.3, 128.8, 128.5, 128.5, 128.3, 128.2, 128.1, 127.9, 127.9, 127.5, 127.2, 127.1, 127.0, 126.4, 126.2, 125.9, 124.8, 108.8, 107.2, 85.2, 83.9, 62.6, 61.1, 60.8, 60.7, 60.6, 60.2, 58.2, 44.0, 42.4, 42.3, 40.4, 29.9, 21.8, 21.8, 21.7, 21.6, 14.2, 14.1, 14.1, 14.0; HRMS (ESI) calcd for C40H39NO6Na [M + Na]+ 652.2670, found 652.2669.
Diethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-(p-tolyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5iaa), 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 117.1 mg, 93% yield; light yellow solid, mp 55–57 °C, IR (film) 1739, 1709, 1497, 1371, 1302, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1.5H), 7.69 (d, J = 8.0 Hz, 0.5H), 7.55 (d, J = 8.0 Hz, 0.6H), 7.44–7.39 (m, 0.7H), 7.37–7.32 (m, 3.3H), 7.29–7.09 (m, 10H), 7.05 (d, J = 8.0 Hz, 0.6H), 4.47 (t, J = 8.0 Hz, 0.8H), 4.25–4.07 (m, 4.5H), 4.01 (d, J = 12.0 Hz, 0.3H), 3.84 (dd, J = 20.0, 12.0 Hz, 1.5H), 3.53–3.38 (m, 1.5H), 3.31 (dd, J = 16.0, 8.0 Hz, 0.8H), 3.20 (dd, J = 16.0, 4.0 Hz, 0.8H), 2.39 (s, 2.1H), 2.36 (s, 0.9H), 2.30 (s, 2.1H), 2.27 (s, 0.9H), 1.29–1.19 (m, 5.1H), 1.12 (t, J = 8.0 Hz, 0.9H); 13C NMR (100 MHz, CDCl3) δ 199.3, 198.9, 162.8, 162.4, 159.3, 155.3, 154.7, 148.1, 147.2, 144.2, 143.8, 137.8, 137.5, 137.1, 137.0, 136.7, 135.1, 134.6, 134.1, 132.5, 131.5, 130.4, 129.9, 129.6, 129.4, 129.3, 129.0, 128.9, 128.8, 128.6, 128.6, 128.5, 128.3, 128.3, 127.6, 127.2, 127.1, 127.0, 126.6, 126.3, 126.0, 124.7, 108.9, 107.5, 85.3, 84.0, 62.5, 61.1, 60.8, 60.6, 60.3, 58.1, 44.0, 42.5, 42.1, 40.5, 29.9, 21.8, 21.4, 21.3, 21.2, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C40H40NO6 [M + H]+ 630.2850, found 630.2852.
1 petroleum ether/ethyl acetate): 117.1 mg, 93% yield; light yellow solid, mp 55–57 °C, IR (film) 1739, 1709, 1497, 1371, 1302, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1.5H), 7.69 (d, J = 8.0 Hz, 0.5H), 7.55 (d, J = 8.0 Hz, 0.6H), 7.44–7.39 (m, 0.7H), 7.37–7.32 (m, 3.3H), 7.29–7.09 (m, 10H), 7.05 (d, J = 8.0 Hz, 0.6H), 4.47 (t, J = 8.0 Hz, 0.8H), 4.25–4.07 (m, 4.5H), 4.01 (d, J = 12.0 Hz, 0.3H), 3.84 (dd, J = 20.0, 12.0 Hz, 1.5H), 3.53–3.38 (m, 1.5H), 3.31 (dd, J = 16.0, 8.0 Hz, 0.8H), 3.20 (dd, J = 16.0, 4.0 Hz, 0.8H), 2.39 (s, 2.1H), 2.36 (s, 0.9H), 2.30 (s, 2.1H), 2.27 (s, 0.9H), 1.29–1.19 (m, 5.1H), 1.12 (t, J = 8.0 Hz, 0.9H); 13C NMR (100 MHz, CDCl3) δ 199.3, 198.9, 162.8, 162.4, 159.3, 155.3, 154.7, 148.1, 147.2, 144.2, 143.8, 137.8, 137.5, 137.1, 137.0, 136.7, 135.1, 134.6, 134.1, 132.5, 131.5, 130.4, 129.9, 129.6, 129.4, 129.3, 129.0, 128.9, 128.8, 128.6, 128.6, 128.5, 128.3, 128.3, 127.6, 127.2, 127.1, 127.0, 126.6, 126.3, 126.0, 124.7, 108.9, 107.5, 85.3, 84.0, 62.5, 61.1, 60.8, 60.6, 60.3, 58.1, 44.0, 42.5, 42.1, 40.5, 29.9, 21.8, 21.4, 21.3, 21.2, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C40H40NO6 [M + H]+ 630.2850, found 630.2852.
Diethyl 2′-benzyl-2-(4-chlorophenyl)-3-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5jaa), 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 127.4 mg, 98% yield; light yellow solid, mp 95–97 °C, IR (film) 1741, 1701, 1476, 1353, 1226, 1156 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.7H), 7.70 (d, J = 8.0 Hz, 1.2H), 7.60 (d, J = 8.0 Hz, 1.3H), 7.44–7.37 (m, 2.6H), 7.34–7.28 (m, 5H), 7.25–7.11 (m, 6.4H), 4.44 (t, J = 8.0 Hz, 0.4H), 4.25–4.08 (m, 5.2H), 4.01 (d, J = 12.0 Hz, 0.7H), 3.88 (d, J = 16.0 Hz, 0.4H), 3.79 (d, J = 12.0 Hz, 0.4H), 3.52–3.45 (m, 1.5H), 3.42–3.31 (m, 1.2H), 3.13 (dd, J = 16.0, 4.0 Hz, 0.4H), 2.40 (s, 1.2H), 2.38 (s, 1.8H), 1.30–1.20 (m, 4.2H), 1.13 (t, J = 8.0 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ 199.0, 198.5, 162.7, 162.3, 159.1, 155.4, 154.8, 147.9, 146.8, 144.3, 144.0, 137.3, 137.2, 137.1, 136.8, 135.7, 134.9, 134.4, 134.1, 133.3, 133.3, 132.9, 131.8, 130.1, 129.8, 129.5, 129.4, 129.0, 128.6, 128.4, 128.3, 128.3, 128.2, 128.1, 127.7, 127.4, 127.3, 127.2, 126.6, 126.3, 126.0, 124.7, 108.5, 107.2, 100.1, 85.2, 84.0, 62.8, 61.0, 60.6, 60.2, 57.6, 43.8, 42.4, 41.9, 40.7, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS (ESI) calcd for C39H36NO6ClNa [M + Na]+ 672.2123, found 672.2128.
1 petroleum ether/ethyl acetate): 127.4 mg, 98% yield; light yellow solid, mp 95–97 °C, IR (film) 1741, 1701, 1476, 1353, 1226, 1156 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.7H), 7.70 (d, J = 8.0 Hz, 1.2H), 7.60 (d, J = 8.0 Hz, 1.3H), 7.44–7.37 (m, 2.6H), 7.34–7.28 (m, 5H), 7.25–7.11 (m, 6.4H), 4.44 (t, J = 8.0 Hz, 0.4H), 4.25–4.08 (m, 5.2H), 4.01 (d, J = 12.0 Hz, 0.7H), 3.88 (d, J = 16.0 Hz, 0.4H), 3.79 (d, J = 12.0 Hz, 0.4H), 3.52–3.45 (m, 1.5H), 3.42–3.31 (m, 1.2H), 3.13 (dd, J = 16.0, 4.0 Hz, 0.4H), 2.40 (s, 1.2H), 2.38 (s, 1.8H), 1.30–1.20 (m, 4.2H), 1.13 (t, J = 8.0 Hz, 1.8H); 13C NMR (100 MHz, CDCl3) δ 199.0, 198.5, 162.7, 162.3, 159.1, 155.4, 154.8, 147.9, 146.8, 144.3, 144.0, 137.3, 137.2, 137.1, 136.8, 135.7, 134.9, 134.4, 134.1, 133.3, 133.3, 132.9, 131.8, 130.1, 129.8, 129.5, 129.4, 129.0, 128.6, 128.4, 128.3, 128.3, 128.2, 128.1, 127.7, 127.4, 127.3, 127.2, 126.6, 126.3, 126.0, 124.7, 108.5, 107.2, 100.1, 85.2, 84.0, 62.8, 61.0, 60.6, 60.2, 57.6, 43.8, 42.4, 41.9, 40.7, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS (ESI) calcd for C39H36NO6ClNa [M + Na]+ 672.2123, found 672.2128.
Diethyl 2′-benzyl-2-(4-bromophenyl)-3-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5kaa), 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 126.4 mg, 91% yield; white solid, mp 82–84 °C, IR (film) 1749, 1712, 1488, 1371, 1299, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.44–7.27 (m, 8H), 7.25–7.10 (m, 6H), 4.43 (t, J = 8.0 Hz, 0.5H), 4.25–4.15 (m, 3H), 4.13–4.07 (m, 1.8H), 4.00 (d, J = 16.0 Hz, 0.5H), 3.88 (d, J = 12.0 Hz, 0.6H), 3.78 (d, J = 12.0 Hz, 0.6H), 3.52–3.30 (m, 2.5H), 3.12 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.40 (s, 1.5H), 2.38 (s, 1.5H), 1.30–1.19 (m, 4.4H), 1.13 (t, J = 8.0 Hz, 1.6H); 13C NMR (100 MHz, CDCl3) δ 199.0, 198.5, 162.7, 162.3, 159.1, 155.4, 154.8, 147.9, 146.8, 144.3, 144.0, 137.2, 137.1, 136.8, 136.2, 135.0, 134.7, 134.5, 133.3, 132.2, 131.3, 131.1, 130.1, 129.8, 129.5, 129.4, 129.0, 128.7, 128.6, 128.5, 128.3, 128.3, 127.7, 127.4, 127.3, 127.2, 126.6, 126.3, 126.0, 124.7, 121.6, 121.6, 108.5, 107.2, 85.3, 84.0, 62.8, 62.7, 61.1, 61.0, 60.6, 60.3, 57.7, 43.8, 42.4, 41.9, 40.7, 29.9, 21.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H37O6BrN [M + H] + 694.1799; found: 694.1799.
1 petroleum ether/ethyl acetate): 126.4 mg, 91% yield; white solid, mp 82–84 °C, IR (film) 1749, 1712, 1488, 1371, 1299, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.44–7.27 (m, 8H), 7.25–7.10 (m, 6H), 4.43 (t, J = 8.0 Hz, 0.5H), 4.25–4.15 (m, 3H), 4.13–4.07 (m, 1.8H), 4.00 (d, J = 16.0 Hz, 0.5H), 3.88 (d, J = 12.0 Hz, 0.6H), 3.78 (d, J = 12.0 Hz, 0.6H), 3.52–3.30 (m, 2.5H), 3.12 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.40 (s, 1.5H), 2.38 (s, 1.5H), 1.30–1.19 (m, 4.4H), 1.13 (t, J = 8.0 Hz, 1.6H); 13C NMR (100 MHz, CDCl3) δ 199.0, 198.5, 162.7, 162.3, 159.1, 155.4, 154.8, 147.9, 146.8, 144.3, 144.0, 137.2, 137.1, 136.8, 136.2, 135.0, 134.7, 134.5, 133.3, 132.2, 131.3, 131.1, 130.1, 129.8, 129.5, 129.4, 129.0, 128.7, 128.6, 128.5, 128.3, 128.3, 127.7, 127.4, 127.3, 127.2, 126.6, 126.3, 126.0, 124.7, 121.6, 121.6, 108.5, 107.2, 85.3, 84.0, 62.8, 62.7, 61.1, 61.0, 60.6, 60.3, 57.7, 43.8, 42.4, 41.9, 40.7, 29.9, 21.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H37O6BrN [M + H] + 694.1799; found: 694.1799.
Diethyl 2′-benzyl-2-(4-methoxyphenyl)-3-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5laa), 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 127.9 mg, 99% yield; light yellow solid, mp 78–80 °C, IR (film) 1739, 1711, 1491, 1370, 1300, 1107 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.2H), 7.71 (d, J = 8.0 Hz, 1.8H), 7.59 (d, J = 8.0 Hz, 2H), 7.44–7.37 (m, 2H), 7.34–7.21 (m, 7H), 7.17 (t, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 0.2H), 6.78 (d, J = 8.0 Hz, 1.8H), 4.22–4.05 (m, 5.9H), 4.01 (d, J = 12.0 Hz, 0.9H), 3.77 (s, 0.4H), 3.75 (s, 2.6H), 3.52–3.38 (m, 2.8H), 2.40 (s, 0.4H), 2.37 (s, 2.6H), 1.30–1.19 (m, 3.4H), 1.13 (t, J = 8.0 Hz, 2.6H); 13C NMR (100 MHz, CDCl3) δ 198.9, 162.4, 159.2, 158.8, 154.6, 148.1, 143.9, 137.7, 137.4, 135.0, 132.7, 131.5, 129.6, 129.3, 129.2, 129.0, 128.6, 128.6, 128.5, 128.3, 127.6, 127.1, 126.6, 126.3, 113.5, 108.9, 85.3, 62.6, 60.9, 60.6, 57.7, 55.3, 44.0, 42.5, 40.8, 21.8, 14.1, 14.0; HRMS (ESI) calcd for C40H39NO7Na [M + Na]+ 668.2619, found 668.2615.
1 petroleum ether/ethyl acetate): 127.9 mg, 99% yield; light yellow solid, mp 78–80 °C, IR (film) 1739, 1711, 1491, 1370, 1300, 1107 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.2H), 7.71 (d, J = 8.0 Hz, 1.8H), 7.59 (d, J = 8.0 Hz, 2H), 7.44–7.37 (m, 2H), 7.34–7.21 (m, 7H), 7.17 (t, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 0.2H), 6.78 (d, J = 8.0 Hz, 1.8H), 4.22–4.05 (m, 5.9H), 4.01 (d, J = 12.0 Hz, 0.9H), 3.77 (s, 0.4H), 3.75 (s, 2.6H), 3.52–3.38 (m, 2.8H), 2.40 (s, 0.4H), 2.37 (s, 2.6H), 1.30–1.19 (m, 3.4H), 1.13 (t, J = 8.0 Hz, 2.6H); 13C NMR (100 MHz, CDCl3) δ 198.9, 162.4, 159.2, 158.8, 154.6, 148.1, 143.9, 137.7, 137.4, 135.0, 132.7, 131.5, 129.6, 129.3, 129.2, 129.0, 128.6, 128.6, 128.5, 128.3, 127.6, 127.1, 126.6, 126.3, 113.5, 108.9, 85.3, 62.6, 60.9, 60.6, 57.7, 55.3, 44.0, 42.5, 40.8, 21.8, 14.1, 14.0; HRMS (ESI) calcd for C40H39NO7Na [M + Na]+ 668.2619, found 668.2615.
Diethyl 2′-benzyl-2-(cyclohex-1-en-1-yl)-3-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5maa), 6.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (40
1 dr. Purified by silica gel column chromatography (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 114.0 mg, 92% yield; light yellow solid, mp 85–87 °C, IR (film) 1733, 1710, 1372, 1298, 1200, 1175 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 10.0 Hz, 0.2H), 7.81 (d, J = 10.0 Hz, 1.7H), 7.39 (d, J = 5.0 Hz, 1H), 7.34–7.23 (m, 8.2H), 7.18 (d, J = 10.0 Hz, 1.8H), 6.22 (s, 0.8H), 4.34–4.28 (m, 2H), 4.16–4.06 (m, 2.8H), 3.99 (d, J = 15.0 Hz, 0.8H), 3.81–3.76 (m, 0.9H), 3.41 (dd, J = 15.0, 10.0 Hz, 0.9H), 3.32–3.20 (m, 1.1H), 3.01 (dd, J = 15.0, 5.0 Hz, 0.8H), 2.43 (s, 0.4H), 2.39 (s, 2.6H), 2.26–2.13 (m, 0.9H), 2.04–1.99 (m, 0.3H), 1.90–1.82 (m, 1.6H), 1.62–1.56 (m, 2.8H), 1.49–1.40 (m, 2.7H), 1.36–1.33 (m, 3H), 1.17 (d, J = 10.0 Hz, 0.4H), 1.13 (d, J = 10.0 Hz, 2.6H); 13C NMR (100 MHz, CDCl3) δ 199.4, 198.6, 162.7, 162.5, 160.0, 159.8, 155.3, 148.1, 147.1, 144.2, 143.7, 137.7, 137.6, 137.4, 135.1, 134.8, 133.2, 132.6, 129.8, 129.5, 129.3, 128.8, 128.6, 128.6, 128.5, 128.4, 128.3, 127.5, 127.3, 126.9, 126.8, 126.1, 126.0, 125.8, 124.6, 108.1, 85.0, 84.4, 62.7, 60.8, 60.6, 59.9, 58.2, 42.6, 42.3, 42.0, 38.4, 31.5, 29.9, 28.5, 27.8, 27.1, 25.9, 25.8, 23.5, 23.4, 22.6, 22.2, 21.9, 21.8, 14.2, 14.1; HRMS (ESI) calcd for C39H41NO6Na [M + Na]+ 642.2826, found 642.2823.
1 petroleum ether/ethyl acetate): 114.0 mg, 92% yield; light yellow solid, mp 85–87 °C, IR (film) 1733, 1710, 1372, 1298, 1200, 1175 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 10.0 Hz, 0.2H), 7.81 (d, J = 10.0 Hz, 1.7H), 7.39 (d, J = 5.0 Hz, 1H), 7.34–7.23 (m, 8.2H), 7.18 (d, J = 10.0 Hz, 1.8H), 6.22 (s, 0.8H), 4.34–4.28 (m, 2H), 4.16–4.06 (m, 2.8H), 3.99 (d, J = 15.0 Hz, 0.8H), 3.81–3.76 (m, 0.9H), 3.41 (dd, J = 15.0, 10.0 Hz, 0.9H), 3.32–3.20 (m, 1.1H), 3.01 (dd, J = 15.0, 5.0 Hz, 0.8H), 2.43 (s, 0.4H), 2.39 (s, 2.6H), 2.26–2.13 (m, 0.9H), 2.04–1.99 (m, 0.3H), 1.90–1.82 (m, 1.6H), 1.62–1.56 (m, 2.8H), 1.49–1.40 (m, 2.7H), 1.36–1.33 (m, 3H), 1.17 (d, J = 10.0 Hz, 0.4H), 1.13 (d, J = 10.0 Hz, 2.6H); 13C NMR (100 MHz, CDCl3) δ 199.4, 198.6, 162.7, 162.5, 160.0, 159.8, 155.3, 148.1, 147.1, 144.2, 143.7, 137.7, 137.6, 137.4, 135.1, 134.8, 133.2, 132.6, 129.8, 129.5, 129.3, 128.8, 128.6, 128.6, 128.5, 128.4, 128.3, 127.5, 127.3, 126.9, 126.8, 126.1, 126.0, 125.8, 124.6, 108.1, 85.0, 84.4, 62.7, 60.8, 60.6, 59.9, 58.2, 42.6, 42.3, 42.0, 38.4, 31.5, 29.9, 28.5, 27.8, 27.1, 25.9, 25.8, 23.5, 23.4, 22.6, 22.2, 21.9, 21.8, 14.2, 14.1; HRMS (ESI) calcd for C39H41NO6Na [M + Na]+ 642.2826, found 642.2823.
Diethyl 2′-benzyl-5-fluoro-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5naa), 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 119.1 mg, 94% yield; yellowish white solid, mp 116–118 °C, IR (film) 1743, 1709, 1474, 1303, 1231, 1181 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.4H), 7.71 (d, J = 8.0 Hz, 1.6H), 7.65 (d, J = 8.0 Hz, 1.6H), 7.42–7.36 (m, 1.4H), 7.35–7.14 (m, 11H), 7.02–6.97 (m, 1H), 6.82 (d, J = 8.0 Hz, 0.2H), 4.45 (t, J = 8.0 Hz, 0.2H), 4.30–4.07 (m, 5.6H), 3.99 (d, J = 12.0 Hz, 0.8H), 3.87 (d, J = 12.0 Hz, 0.4H), 3.53–3.40 (m, 2.6H), 3.33–3.14 (m, 0.4H), 2.40 (s, 0.5H), 2.38 (s, 2.5H), 1.29–1.22 (m, 3.5H), 1.15 (t, J = 8.0 Hz, 2.5H); 13C NMR (100 MHz, CDCl3) δ 198.8, 198.4, 168.4, 163.7 (d, J = 246.8 Hz), 162.7, 162.3, 159.2, 159.1, 155.7, 155.0, 150.6, 150.5, 149.4, 144.4, 144.1, 141.1, 137.2, 136.8, 136.7, 135.2, 134.8, 134.3, 133.5 (d, J = 2.6 Hz), 131.6, 130.4, 130.2, 129.5, 129.4, 129.0, 128.6, 128.6, 128.5, 128.3, 128.3, 128.1, 127.7, 127.6, 127.5, 127.5, 127.3, 120.5, 114.6 (d, J = 22.9 Hz), 114.0 (d, J = 22.4 Hz), 112.4, 112.2, 108.2, 106.8, 92.9, 84.6, 84.0, 83.3, 62.7, 61.6, 61.0, 60.5, 60.1, 58.8, 43.7, 42.1, 41.9, 40.4, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; 19F NMR (282 MHz, CDCl3): δ −111.5 (s, 1F); HRMS(ESI) calcd for C39H37FNO6 [M + H]+ 634.2599, found 634.2603.
1 petroleum ether/ethyl acetate): 119.1 mg, 94% yield; yellowish white solid, mp 116–118 °C, IR (film) 1743, 1709, 1474, 1303, 1231, 1181 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.4H), 7.71 (d, J = 8.0 Hz, 1.6H), 7.65 (d, J = 8.0 Hz, 1.6H), 7.42–7.36 (m, 1.4H), 7.35–7.14 (m, 11H), 7.02–6.97 (m, 1H), 6.82 (d, J = 8.0 Hz, 0.2H), 4.45 (t, J = 8.0 Hz, 0.2H), 4.30–4.07 (m, 5.6H), 3.99 (d, J = 12.0 Hz, 0.8H), 3.87 (d, J = 12.0 Hz, 0.4H), 3.53–3.40 (m, 2.6H), 3.33–3.14 (m, 0.4H), 2.40 (s, 0.5H), 2.38 (s, 2.5H), 1.29–1.22 (m, 3.5H), 1.15 (t, J = 8.0 Hz, 2.5H); 13C NMR (100 MHz, CDCl3) δ 198.8, 198.4, 168.4, 163.7 (d, J = 246.8 Hz), 162.7, 162.3, 159.2, 159.1, 155.7, 155.0, 150.6, 150.5, 149.4, 144.4, 144.1, 141.1, 137.2, 136.8, 136.7, 135.2, 134.8, 134.3, 133.5 (d, J = 2.6 Hz), 131.6, 130.4, 130.2, 129.5, 129.4, 129.0, 128.6, 128.6, 128.5, 128.3, 128.3, 128.1, 127.7, 127.6, 127.5, 127.5, 127.3, 120.5, 114.6 (d, J = 22.9 Hz), 114.0 (d, J = 22.4 Hz), 112.4, 112.2, 108.2, 106.8, 92.9, 84.6, 84.0, 83.3, 62.7, 61.6, 61.0, 60.5, 60.1, 58.8, 43.7, 42.1, 41.9, 40.4, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; 19F NMR (282 MHz, CDCl3): δ −111.5 (s, 1F); HRMS(ESI) calcd for C39H37FNO6 [M + H]+ 634.2599, found 634.2603.
Diethyl 2′-benzyl-5-chloro-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5oaa), 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 117.0 mg, 90% yield; light yellow solid, mp 112–114 °C, IR (film) 1740, 1709, 1466, 1304, 1261, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1.4H), 7.71 (d, J = 12.0 Hz, 0.5H), 7.64 (d, J = 4.0 Hz, 0.5H), 7.47 (d, J = 1.6 Hz, 0.3H), 7.42 (d, J = 8.0 Hz, 1.5H), 7.39–7.10 (m, 13H), 4.45 (t, J = 8.0 Hz, 0.7H), 4.26–4.09 (m, 4.4H), 3.99 (d, J = 12.0 Hz, 0.3H), 3.89–3.84 (m, 1.5H), 3.52–3.42 (m, 1.5H), 3.33–3.18 (m, 1.6H), 2.40 (s, 2H), 2.38 (s, 1H), 1.29–1.21 (m, 5.1H), 1.16 (t, J = 8.0 Hz, 0.9H); 13C NMR (100 MHz, CDCl3) δ 198.7, 198.3, 162.6, 162.3, 159.1, 155.8, 149.9, 149.0, 144.4, 144.1, 137.1, 136.7, 136.6, 136.5, 136.1, 135.9, 135.7, 135.1, 134.8, 134.3, 131.5, 130.4, 129.5, 129.4, 129.0, 128.6, 128.6, 128.5, 128.3, 128.3, 128.3, 128.1, 127.7, 127.6, 127.5, 127.4, 127.3, 127.0, 125.3, 108.1, 106.6, 84.7, 83.4, 62.7, 61.3, 60.9, 60.6, 60.2, 58.5, 43.6, 42.0, 41.9, 40.3, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H37ClNO6 [M + H]+ 650.2304, found 650.2308.
1 petroleum ether/ethyl acetate): 117.0 mg, 90% yield; light yellow solid, mp 112–114 °C, IR (film) 1740, 1709, 1466, 1304, 1261, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1.4H), 7.71 (d, J = 12.0 Hz, 0.5H), 7.64 (d, J = 4.0 Hz, 0.5H), 7.47 (d, J = 1.6 Hz, 0.3H), 7.42 (d, J = 8.0 Hz, 1.5H), 7.39–7.10 (m, 13H), 4.45 (t, J = 8.0 Hz, 0.7H), 4.26–4.09 (m, 4.4H), 3.99 (d, J = 12.0 Hz, 0.3H), 3.89–3.84 (m, 1.5H), 3.52–3.42 (m, 1.5H), 3.33–3.18 (m, 1.6H), 2.40 (s, 2H), 2.38 (s, 1H), 1.29–1.21 (m, 5.1H), 1.16 (t, J = 8.0 Hz, 0.9H); 13C NMR (100 MHz, CDCl3) δ 198.7, 198.3, 162.6, 162.3, 159.1, 155.8, 149.9, 149.0, 144.4, 144.1, 137.1, 136.7, 136.6, 136.5, 136.1, 135.9, 135.7, 135.1, 134.8, 134.3, 131.5, 130.4, 129.5, 129.4, 129.0, 128.6, 128.6, 128.5, 128.3, 128.3, 128.3, 128.1, 127.7, 127.6, 127.5, 127.4, 127.3, 127.0, 125.3, 108.1, 106.6, 84.7, 83.4, 62.7, 61.3, 60.9, 60.6, 60.2, 58.5, 43.6, 42.0, 41.9, 40.3, 29.9, 21.8, 14.2, 14.1, 14.0, 14.0; HRMS(ESI) calcd for C39H37ClNO6 [M + H]+ 650.2304, found 650.2308.
Diethyl 2′-benzyl-7-(2-oxo-2-(p-tolyl)ethyl)-6-phenyl-6,7-dihydro-2′H-spiro[indeno-[5,6-d][1,3]dioxole-5,3′-isoxazole]-4′,5′-dicarboxylate (5paa), 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 106.9 mg, 81% yield; yellowish white solid, mp 149–151 °C, IR (film) 1734, 1705, 1497, 1370, 1258, 1143 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 12.0 Hz, 1.6H), 7.71–7.65 (m, 1H), 7.42 (d, J = 8.0 Hz, 1.6H), 7.35 (t, J = 4.0 Hz, 1.3H), 7.31–7.15 (m, 9H), 6.86 (d, J = 8.0 Hz, 0.5H), 6.80 (s, 0.7H), 6.60 (s, 0.7H), 5.97–5.93 (m, 1.9H), 4.40–4.35 (m, 0.9H), 4.27–4.12 (m, 4.2H), 4.03–3.95 (m, 0.5H), 3.91–3.83 (m, 1.4H), 3.57 (dd, J = 20.0, 12.0 Hz, 1H), 3.41 (d, J = 8.0 Hz, 0.4H), 3.27 (dd, J = 16.0, 8.0 Hz, 0.8H), 3.16 (dd, J = 16.0, 4.0 Hz, 0.7H), 2.39 (s, 2.3H), 2.37 (s, 0.7H), 1.29–1.21 (m, 5.3H), 1.18 (t, J = 8.0 Hz, 0.7H); 13C NMR (100 MHz, CDCl3) δ 199.2, 198.9, 162.8, 162.4, 159.3, 155.5, 154.8, 149.4, 149.1, 147.3, 147.2, 144.3, 143.9, 142.0, 140.9, 137.5, 137.2, 137.0, 136.1, 135.7, 135.0, 134.5, 131.6, 130.5, 130.3, 129.5, 129.4, 129.0, 128.9, 128.6, 128.5, 128.5, 128.3, 128.3, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 107.0, 106.9, 106.0, 105.9, 105.5, 101.6, 85.2, 83.9, 62.6, 61.5, 60.9, 60.9, 60.6, 60.0, 58.9, 43.7, 42.6, 42.4, 40.2, 21.8, 14.3, 14.2, 14.0, 14.0; HRMS(ESI) calcd for C40H37NO8Na [M + Na]+ 682.2411, found 682.2406.
1 petroleum ether/ethyl acetate): 106.9 mg, 81% yield; yellowish white solid, mp 149–151 °C, IR (film) 1734, 1705, 1497, 1370, 1258, 1143 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 12.0 Hz, 1.6H), 7.71–7.65 (m, 1H), 7.42 (d, J = 8.0 Hz, 1.6H), 7.35 (t, J = 4.0 Hz, 1.3H), 7.31–7.15 (m, 9H), 6.86 (d, J = 8.0 Hz, 0.5H), 6.80 (s, 0.7H), 6.60 (s, 0.7H), 5.97–5.93 (m, 1.9H), 4.40–4.35 (m, 0.9H), 4.27–4.12 (m, 4.2H), 4.03–3.95 (m, 0.5H), 3.91–3.83 (m, 1.4H), 3.57 (dd, J = 20.0, 12.0 Hz, 1H), 3.41 (d, J = 8.0 Hz, 0.4H), 3.27 (dd, J = 16.0, 8.0 Hz, 0.8H), 3.16 (dd, J = 16.0, 4.0 Hz, 0.7H), 2.39 (s, 2.3H), 2.37 (s, 0.7H), 1.29–1.21 (m, 5.3H), 1.18 (t, J = 8.0 Hz, 0.7H); 13C NMR (100 MHz, CDCl3) δ 199.2, 198.9, 162.8, 162.4, 159.3, 155.5, 154.8, 149.4, 149.1, 147.3, 147.2, 144.3, 143.9, 142.0, 140.9, 137.5, 137.2, 137.0, 136.1, 135.7, 135.0, 134.5, 131.6, 130.5, 130.3, 129.5, 129.4, 129.0, 128.9, 128.6, 128.5, 128.5, 128.3, 128.3, 128.2, 128.0, 127.6, 127.5, 127.3, 127.2, 107.0, 106.9, 106.0, 105.9, 105.5, 101.6, 85.2, 83.9, 62.6, 61.5, 60.9, 60.9, 60.6, 60.0, 58.9, 43.7, 42.6, 42.4, 40.2, 21.8, 14.3, 14.2, 14.0, 14.0; HRMS(ESI) calcd for C40H37NO8Na [M + Na]+ 682.2411, found 682.2406.
Diethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[cyclopenta[a]naphthalene-1,3′-isoxazole]-4′,5′-dicarboxylate (5qaa), 21.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (40
1 dr. Purified by silica gel column chromatography (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 115.8 mg, 87% yield; yellowish white solid, mp 158–160 °C, IR (film) 1747, 1711, 1370, 1300, 1181, 1094 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.0 Hz, 4H), 7.55 (t, J = 8.0 Hz, 2H), 7.45 (t, J = 8.0 Hz, 1H),7.32–7.20 (m, 8H), 7.15 (d, J = 8.0 Hz, 2H), 4.30–4.24 (m, 3H), 4.20 (d, J = 8.0 Hz, 2H), 4.05–3.90 (m, 2H), 3.75–3.55 (m, 3H), 2.37 (s, 3H), 1.23 (t, J = 8.0 Hz, 3H), 0.98 (d, J = 8.0 Hz, 0.1H), 0.85 (d, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 198.9, 162.4, 159.4, 154.9, 148.1, 143.9, 137.7, 137.2, 135.0, 133.6, 132.0, 132.0, 130.8, 129.4, 129.3, 128.8, 128.6, 128.3, 128.1, 127.5, 127.4, 127.1, 125.4, 124.4, 124.1, 108.7, 85.8, 62.7, 60.6, 60.6, 59.1, 44.0, 43.1, 21.8, 14.0, 13.9; HRMS(ESI) calcd for C43H40NO6 [M + H]+ 666.2850, found 666.2855.
1 petroleum ether/ethyl acetate): 115.8 mg, 87% yield; yellowish white solid, mp 158–160 °C, IR (film) 1747, 1711, 1370, 1300, 1181, 1094 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.0 Hz, 4H), 7.55 (t, J = 8.0 Hz, 2H), 7.45 (t, J = 8.0 Hz, 1H),7.32–7.20 (m, 8H), 7.15 (d, J = 8.0 Hz, 2H), 4.30–4.24 (m, 3H), 4.20 (d, J = 8.0 Hz, 2H), 4.05–3.90 (m, 2H), 3.75–3.55 (m, 3H), 2.37 (s, 3H), 1.23 (t, J = 8.0 Hz, 3H), 0.98 (d, J = 8.0 Hz, 0.1H), 0.85 (d, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 198.9, 162.4, 159.4, 154.9, 148.1, 143.9, 137.7, 137.2, 135.0, 133.6, 132.0, 132.0, 130.8, 129.4, 129.3, 128.8, 128.6, 128.3, 128.1, 127.5, 127.4, 127.1, 125.4, 124.4, 124.1, 108.7, 85.8, 62.7, 60.6, 60.6, 59.1, 44.0, 43.1, 21.8, 14.0, 13.9; HRMS(ESI) calcd for C43H40NO6 [M + H]+ 666.2850, found 666.2855.
Diethyl 2′-cyclohexyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5dba), 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 94.8 mg, 78% yield; light yellow solid, mp 142–144 °C, IR (film) 1746, 1703, 1455, 1353, 1278, 1171 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H),7.68–7.65 (m, 1H), 7.42–7.11 (m, 10H), 4.47 (d, J = 8.0 Hz, 0.4H), 4.26–3.99 (m, 4H), 3.94 (d, J = 8.0 Hz, 0.5H), 3.57 (d, J = 12.0 Hz, 0.4H), 3.52–3.39 (m, 0.9H), 3.29 (dd, J = 16.0, 8.0 Hz, 0.4H), 3.12 (dd, J = 16.0, 4.0 Hz, 0.4H), 2.40 (s, 1.6H), 2.38 (s, 1.4H), 2.30–2.25 (m, 0.5H), 2.13 (d, J = 12.0 Hz, 0.5H), 1.97 (d, J = 12.0 Hz, 0.5H), 1.82–1.69 (m, 1.7H), 1.65–1.51 (m, 3H), 1.31–1.18 (m, 6H), 1.11 (t, J = 8.0 Hz, 2H), 1.02–0.86 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.4, 162.7, 162.4, 159.3, 159.2, 156.3, 155.7, 148.5, 147.9, 144.2, 143.8, 137.6, 137.5, 137.4, 135.7, 135.3, 134.6, 132.0, 130.7, 129.6, 129.4, 129.4, 129.4, 128.5, 128.2, 127.9, 127.6, 127.4, 127.3, 126.9, 126.7, 126.5, 125.7, 125.7, 124.5, 110.3, 108.8, 85.0, 83.7, 63.8, 63.7, 63.2, 62.4, 62.4, 60.7, 59.3, 44.0, 42.1, 41.8, 40.0, 33.8, 32.7, 29.9, 27.7, 27.6, 26.2, 25.9, 25.7, 25.2, 25.1, 21.8, 21.8, 14.2, 14.2, 14.1, 14.1, 14.0; HRMS (ESI) calcd for C38H42NO6 [M + H]+ 608.3007, found 608.3012.
1 petroleum ether/ethyl acetate): 94.8 mg, 78% yield; light yellow solid, mp 142–144 °C, IR (film) 1746, 1703, 1455, 1353, 1278, 1171 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H),7.68–7.65 (m, 1H), 7.42–7.11 (m, 10H), 4.47 (d, J = 8.0 Hz, 0.4H), 4.26–3.99 (m, 4H), 3.94 (d, J = 8.0 Hz, 0.5H), 3.57 (d, J = 12.0 Hz, 0.4H), 3.52–3.39 (m, 0.9H), 3.29 (dd, J = 16.0, 8.0 Hz, 0.4H), 3.12 (dd, J = 16.0, 4.0 Hz, 0.4H), 2.40 (s, 1.6H), 2.38 (s, 1.4H), 2.30–2.25 (m, 0.5H), 2.13 (d, J = 12.0 Hz, 0.5H), 1.97 (d, J = 12.0 Hz, 0.5H), 1.82–1.69 (m, 1.7H), 1.65–1.51 (m, 3H), 1.31–1.18 (m, 6H), 1.11 (t, J = 8.0 Hz, 2H), 1.02–0.86 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.4, 162.7, 162.4, 159.3, 159.2, 156.3, 155.7, 148.5, 147.9, 144.2, 143.8, 137.6, 137.5, 137.4, 135.7, 135.3, 134.6, 132.0, 130.7, 129.6, 129.4, 129.4, 129.4, 128.5, 128.2, 127.9, 127.6, 127.4, 127.3, 126.9, 126.7, 126.5, 125.7, 125.7, 124.5, 110.3, 108.8, 85.0, 83.7, 63.8, 63.7, 63.2, 62.4, 62.4, 60.7, 59.3, 44.0, 42.1, 41.8, 40.0, 33.8, 32.7, 29.9, 27.7, 27.6, 26.2, 25.9, 25.7, 25.2, 25.1, 21.8, 21.8, 14.2, 14.2, 14.1, 14.1, 14.0; HRMS (ESI) calcd for C38H42NO6 [M + H]+ 608.3007, found 608.3012.
Dimethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5dab), 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 111.7 mg, 95% yield; light yellow solid, mp 123–125 °C, IR (film) 1763, 1754, 1443, 1352, 1296, 1142 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.5H), 7.70–7.67 (m, 3H), 7.47–7.40 (m, 2.5H), 7.36–7.15 (m, 10H), 7.13 (d, J = 4.0 Hz, 2H), 4.50 (t, J = 8.0 Hz, 0.2H), 4.19–4.10 (m, 1.8H), 4.00 (d, J = 16.0 Hz, 0.9H), 3.91–3.81 (m, 0.7H), 3.75 (d, J = 4.0 Hz, 1.2H), 3.73 (s, 2.2H), 3.66 (s, 2.2H), 3.54–3.32 (m, 2.7H), 3.18 (dd, J = 4.0, 16.0 Hz, 0.2H), 2.39 (s, 0.7H), 2.36 (s, 2.2H); 13C NMR (100 MHz, CDCl3) δ 199.1, 198.8, 163.3, 162.9, 159.5, 154.9, 154.4, 148.0, 147.0, 144.2, 143.9, 137.6, 137.3, 137.1, 136.9, 135.5, 135.4, 135.0, 134.6, 131.6, 130.5, 130.1, 129.7, 129.4, 129.3, 128.9, 128.6, 128.5, 128.3, 128.2, 128.0, 127.6, 127.4, 127.3, 127.2, 126.7, 126.1, 125.8, 124.8, 109.2, 107.9, 100.2, 85.4, 84.1, 61.3, 60.7, 60.2, 58.4, 53.2, 52.1, 44.0, 42.4, 42.1, 40.5, 29.9, 21.8; HRMS (ESI) calcd for C37H34NO6 [M + H]+ 588.2381, found 588.2379.
1 petroleum ether/ethyl acetate): 111.7 mg, 95% yield; light yellow solid, mp 123–125 °C, IR (film) 1763, 1754, 1443, 1352, 1296, 1142 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 0.5H), 7.70–7.67 (m, 3H), 7.47–7.40 (m, 2.5H), 7.36–7.15 (m, 10H), 7.13 (d, J = 4.0 Hz, 2H), 4.50 (t, J = 8.0 Hz, 0.2H), 4.19–4.10 (m, 1.8H), 4.00 (d, J = 16.0 Hz, 0.9H), 3.91–3.81 (m, 0.7H), 3.75 (d, J = 4.0 Hz, 1.2H), 3.73 (s, 2.2H), 3.66 (s, 2.2H), 3.54–3.32 (m, 2.7H), 3.18 (dd, J = 4.0, 16.0 Hz, 0.2H), 2.39 (s, 0.7H), 2.36 (s, 2.2H); 13C NMR (100 MHz, CDCl3) δ 199.1, 198.8, 163.3, 162.9, 159.5, 154.9, 154.4, 148.0, 147.0, 144.2, 143.9, 137.6, 137.3, 137.1, 136.9, 135.5, 135.4, 135.0, 134.6, 131.6, 130.5, 130.1, 129.7, 129.4, 129.3, 128.9, 128.6, 128.5, 128.3, 128.2, 128.0, 127.6, 127.4, 127.3, 127.2, 126.7, 126.1, 125.8, 124.8, 109.2, 107.9, 100.2, 85.4, 84.1, 61.3, 60.7, 60.2, 58.4, 53.2, 52.1, 44.0, 42.4, 42.1, 40.5, 29.9, 21.8; HRMS (ESI) calcd for C37H34NO6 [M + H]+ 588.2381, found 588.2379.
Ethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′-carboxylate (5dac), 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 87.0 mg, 80% yield; light yellow oil, IR (film) 1704, 1682, 1454, 1372, 1278, 1106 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1H),7.73–7.71 (m, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.46–7.39 (m, 2H), 7.37 (d, J = 4.0 Hz, 2H), 7.34–7.27 (m, 4H), 7.26–7.21 (m, 4H), 7.19–7.12 (m, 3H), 4.45 (t, J = 8.0 Hz, 0.6H), 4.23–4.16 (m, 1.5H), 4.14–4.07 (m, 1.3H), 3.95–3.88 (m, 1H), 3.73 (d, J = 16.0 Hz, 0.6H), 3.50–3.33 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.39 (s, 1.8H), 2.35 (s, 1.2H), 1.26–1.23 (m, 1.8H), 1.15 (d, J = 8.0 Hz, 1.2H), 13C NMR (100 MHz, CDCl3) δ 199.3, 198.8, 163.8, 163.3, 156.4, 156.2, 147.8, 146.6, 144.2, 143.8, 138.3, 138.3, 138.0, 137.9, 137.5, 136.3, 135.1, 134.6, 131.6, 130.7, 129.7, 129.4, 129.4, 129.3, 128.7, 128.5, 128.4, 128.3, 128.3, 128.2, 127.9, 127.6, 127.4, 127.3, 127.0, 126.6, 126.0, 125.7, 124.7, 108.3, 127.7, 83.3, 82.1, 61.1, 60.6, 60.6, 60.1, 57.9, 43.9, 42.4, 42.3, 40.8, 29.9, 21.8, 21.8, 14.5, 14.4; HRMS (ESI) calcd for C36H34NO4 [M + H]+ 544.2482, found 544.2479.
1 petroleum ether/ethyl acetate): 87.0 mg, 80% yield; light yellow oil, IR (film) 1704, 1682, 1454, 1372, 1278, 1106 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1H),7.73–7.71 (m, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.46–7.39 (m, 2H), 7.37 (d, J = 4.0 Hz, 2H), 7.34–7.27 (m, 4H), 7.26–7.21 (m, 4H), 7.19–7.12 (m, 3H), 4.45 (t, J = 8.0 Hz, 0.6H), 4.23–4.16 (m, 1.5H), 4.14–4.07 (m, 1.3H), 3.95–3.88 (m, 1H), 3.73 (d, J = 16.0 Hz, 0.6H), 3.50–3.33 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.39 (s, 1.8H), 2.35 (s, 1.2H), 1.26–1.23 (m, 1.8H), 1.15 (d, J = 8.0 Hz, 1.2H), 13C NMR (100 MHz, CDCl3) δ 199.3, 198.8, 163.8, 163.3, 156.4, 156.2, 147.8, 146.6, 144.2, 143.8, 138.3, 138.3, 138.0, 137.9, 137.5, 136.3, 135.1, 134.6, 131.6, 130.7, 129.7, 129.4, 129.4, 129.3, 128.7, 128.5, 128.4, 128.3, 128.3, 128.2, 127.9, 127.6, 127.4, 127.3, 127.0, 126.6, 126.0, 125.7, 124.7, 108.3, 127.7, 83.3, 82.1, 61.1, 60.6, 60.6, 60.1, 57.9, 43.9, 42.4, 42.3, 40.8, 29.9, 21.8, 21.8, 14.5, 14.4; HRMS (ESI) calcd for C36H34NO4 [M + H]+ 544.2482, found 544.2479.
tert-Butyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′-carboxylate (5dad), 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 85.8 mg, 75% yield; reddish brown solid, mp 85–87 °C, IR (film) 1699, 1624, 1455, 1364, 1228, 1134 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 5.0 Hz, 1H), 7.12 (d, J = 5.0 Hz, 1H), 7.68 (d, J = 10.0 Hz, 1H), 7.47–7.36 (m, 4H), 7.34–7.21 (m, 8H), 7.18–7.11 (m, 3H), 4.43 (t, J = 10.0 Hz, 0.5H), 4.13 (d, J = 5.0 Hz, 0.4H), 4.10–4.05 (m, 0.5H), 3.93–3.85 (m, 0.9H), 3.75 (d, J = 20.0 Hz, 0.5H), 3.51–3.37 (m, 1.8H), 3.31 (dd, J = 20.0, 10.0 Hz, 0.6H), 3.17 (dd, J = 15.0, 5.0 Hz, 0.5H), 2.40 (s, 1.7H), 2.36 (s, 1.3H), 1.40 (s, 5H), 1.30 (s, 4H); 13C NMR (125 MHz, CDCl3) δ 199.3, 198.9, 163.3, 162.8, 156.0, 155.9, 147.9, 146.6, 144.2, 143.8, 138.6, 138.6, 138.1, 138.0, 137.7, 136.4, 135.1, 134.6, 131.7, 130.7, 129.6, 129.4, 129.4, 129.3, 129.2, 128.6, 128.5, 128.4, 128.3, 128.2, 128.2, 127.9, 127.5, 127.4, 127.2, 127.2, 126.9, 126.9, 126.5, 126.2, 125.8, 124.6, 109.9, 109.1, 83.3, 82.1, 80.6, 80.5, 61.1, 60.9, 60.7, 58.0, 43.9, 42.5, 42.4, 40.7, 28.4, 28.2, 21.8, 21.8; HRMS (ESI) calcd for C38H37NO6Na [M + Na]+ 594.2615, found 594.2615.
1 petroleum ether/ethyl acetate): 85.8 mg, 75% yield; reddish brown solid, mp 85–87 °C, IR (film) 1699, 1624, 1455, 1364, 1228, 1134 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 5.0 Hz, 1H), 7.12 (d, J = 5.0 Hz, 1H), 7.68 (d, J = 10.0 Hz, 1H), 7.47–7.36 (m, 4H), 7.34–7.21 (m, 8H), 7.18–7.11 (m, 3H), 4.43 (t, J = 10.0 Hz, 0.5H), 4.13 (d, J = 5.0 Hz, 0.4H), 4.10–4.05 (m, 0.5H), 3.93–3.85 (m, 0.9H), 3.75 (d, J = 20.0 Hz, 0.5H), 3.51–3.37 (m, 1.8H), 3.31 (dd, J = 20.0, 10.0 Hz, 0.6H), 3.17 (dd, J = 15.0, 5.0 Hz, 0.5H), 2.40 (s, 1.7H), 2.36 (s, 1.3H), 1.40 (s, 5H), 1.30 (s, 4H); 13C NMR (125 MHz, CDCl3) δ 199.3, 198.9, 163.3, 162.8, 156.0, 155.9, 147.9, 146.6, 144.2, 143.8, 138.6, 138.6, 138.1, 138.0, 137.7, 136.4, 135.1, 134.6, 131.7, 130.7, 129.6, 129.4, 129.4, 129.3, 129.2, 128.6, 128.5, 128.4, 128.3, 128.2, 128.2, 127.9, 127.5, 127.4, 127.2, 127.2, 126.9, 126.9, 126.5, 126.2, 125.8, 124.6, 109.9, 109.1, 83.3, 82.1, 80.6, 80.5, 61.1, 60.9, 60.7, 58.0, 43.9, 42.5, 42.4, 40.7, 28.4, 28.2, 21.8, 21.8; HRMS (ESI) calcd for C38H37NO6Na [M + Na]+ 594.2615, found 594.2615.
2-(4′-Acetyl-2′-benzyl-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazol]-3-yl)-1-(p-tolyl)ethan-1-one (5dae), 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (30
1 dr. Purified by silica gel column chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 98.6 mg, 96% yield; white solid, mp 71–73 °C, IR (film) 1679, 1653, 1457, 1374, 1228, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1.5H),7.70–7.65 (m, 0.6H), 7.45–7.34 (m, 3H), 7.31–7.17 (m, 12H), 7.13 (d, J = 4.0 Hz, 1H), 4.44 (d, J = 8.0 Hz, 0.8H), 4.20 (d, J = 8.0 Hz, 0.2H), 4.13–4.07 (m, 0.2H), 4.03 (d, J = 12.0 Hz, 0.8H), 3.86 (d, J = 16.0 Hz, 0.2H), 3.70 (d, J = 16.0 Hz, 0.8H), 3.48–3.36 (m, 2H), 3.13 (dd, J = 16.0, 4.0 Hz, 0.8H), 2.38 (s, 2.4H), 2.35 (s, 0.6H), 2.32 (s, 2.3H), 2.22 (s, 0.5H); 13C NMR (100 MHz, CDCl3) δ 199.4, 198.8, 191.7, 191.3, 157.7, 157.6, 147.6, 146.5, 144.1, 143.8, 138.2, 137.9, 137.9, 137.8, 137.4, 136.5, 135.0, 134.6, 131.6, 130.7, 129.6, 129.4, 129.3, 128.7, 128.5, 128.3, 128.2, 128.2, 127.9, 127.6, 127.3, 127.3, 127.2, 127.0, 126.7, 125.9, 125.4, 124.8, 118.1, 117.9, 83.6, 82.3, 61.2, 60.7, 59.8, 57.2, 43.9, 42.4, 42.2, 40.8, 29.9, 28.1, 28.0, 21.8, 21.8; HRMS (ESI) calcd for C35H32NO3 [M + H]+ 514.2377, found 514.2380.
1 petroleum ether/ethyl acetate): 98.6 mg, 96% yield; white solid, mp 71–73 °C, IR (film) 1679, 1653, 1457, 1374, 1228, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.82 (d, J = 8.0 Hz, 1.5H),7.70–7.65 (m, 0.6H), 7.45–7.34 (m, 3H), 7.31–7.17 (m, 12H), 7.13 (d, J = 4.0 Hz, 1H), 4.44 (d, J = 8.0 Hz, 0.8H), 4.20 (d, J = 8.0 Hz, 0.2H), 4.13–4.07 (m, 0.2H), 4.03 (d, J = 12.0 Hz, 0.8H), 3.86 (d, J = 16.0 Hz, 0.2H), 3.70 (d, J = 16.0 Hz, 0.8H), 3.48–3.36 (m, 2H), 3.13 (dd, J = 16.0, 4.0 Hz, 0.8H), 2.38 (s, 2.4H), 2.35 (s, 0.6H), 2.32 (s, 2.3H), 2.22 (s, 0.5H); 13C NMR (100 MHz, CDCl3) δ 199.4, 198.8, 191.7, 191.3, 157.7, 157.6, 147.6, 146.5, 144.1, 143.8, 138.2, 137.9, 137.9, 137.8, 137.4, 136.5, 135.0, 134.6, 131.6, 130.7, 129.6, 129.4, 129.3, 128.7, 128.5, 128.3, 128.2, 128.2, 127.9, 127.6, 127.3, 127.3, 127.2, 127.0, 126.7, 125.9, 125.4, 124.8, 118.1, 117.9, 83.6, 82.3, 61.2, 60.7, 59.8, 57.2, 43.9, 42.4, 42.2, 40.8, 29.9, 28.1, 28.0, 21.8, 21.8; HRMS (ESI) calcd for C35H32NO3 [M + H]+ 514.2377, found 514.2380.
Methyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2,5′-diphenyl-2,3-dihydro-2′H-spiro-[indene-1,3′-isoxazole]-4′-carboxylate (5daf), 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 93.2 mg, 77% yield; yellow white solid, mp 73–75 °C, IR (film) 1738, 1697, 1495, 1350, 1241, 1092 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.5H), 7.74 (t, J = 8.0 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1.5H), 7.43–7.13 (m, 19H), 4.59 (t, J = 8.0 Hz, 0.7H), 4.25 (d, J = 8.0 Hz, 0.3H), 4.17–4.12 (m, 0.3H), 4.05 (d, J = 16.0 Hz, 0.3H), 3.93–3.85 (m, 1.3H), 3.59 (s, 2.7H), 3.55–3.45 (m, 1.5H), 3.41–3.34 (m, 0.8H), 3.22–3.17 (m, 0.8H), 2.39 (s, 2.1H), 2.37 (s, 0.9H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.2, 166.7, 164.9, 148.1, 147.1, 144.1, 143.8, 138.6, 138.1, 138.0, 137.8, 136.4, 134.7, 131.9, 130.6, 130.5, 129.6, 129.4, 129.4, 129.3, 129.2, 128.6, 128.6, 128.5, 128.4, 128.3, 128.3, 128.2, 128.0, 127.9, 127.9, 127.8, 127.4, 127.3, 127.0, 127.0, 126.8, 126.7, 126.2, 125.8, 124.7, 101.7, 86.1, 84.8, 62.2, 60.5, 60.2, 58.8, 51.2, 3.9, 42.9, 42.2, 40.5, 29.9, 21.8, 21.8; HRMS (ESI) calcd for C41H36NO4 [M + H]+ 606.2639, found 606.2646.
1 petroleum ether/ethyl acetate): 93.2 mg, 77% yield; yellow white solid, mp 73–75 °C, IR (film) 1738, 1697, 1495, 1350, 1241, 1092 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.5H), 7.74 (t, J = 8.0 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1.5H), 7.43–7.13 (m, 19H), 4.59 (t, J = 8.0 Hz, 0.7H), 4.25 (d, J = 8.0 Hz, 0.3H), 4.17–4.12 (m, 0.3H), 4.05 (d, J = 16.0 Hz, 0.3H), 3.93–3.85 (m, 1.3H), 3.59 (s, 2.7H), 3.55–3.45 (m, 1.5H), 3.41–3.34 (m, 0.8H), 3.22–3.17 (m, 0.8H), 2.39 (s, 2.1H), 2.37 (s, 0.9H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.2, 166.7, 164.9, 148.1, 147.1, 144.1, 143.8, 138.6, 138.1, 138.0, 137.8, 136.4, 134.7, 131.9, 130.6, 130.5, 129.6, 129.4, 129.4, 129.3, 129.2, 128.6, 128.6, 128.5, 128.4, 128.3, 128.3, 128.2, 128.0, 127.9, 127.9, 127.8, 127.4, 127.3, 127.0, 127.0, 126.8, 126.7, 126.2, 125.8, 124.7, 101.7, 86.1, 84.8, 62.2, 60.5, 60.2, 58.8, 51.2, 3.9, 42.9, 42.2, 40.5, 29.9, 21.8, 21.8; HRMS (ESI) calcd for C41H36NO4 [M + H]+ 606.2639, found 606.2646.
Ethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2,5′-diphenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′-carboxylate (5dag), 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 99.2 mg, 80% yield; yellow white solid, mp 81–83 °C, IR (film) 1695, 1646, 1454, 1372, 1336, 1091 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.3H), 7.74 (t, J = 8.0 Hz, 1.4H), 7.53 (d, J = 8.0 Hz, 1.4H), 7.44–7.13 (m, 19H), 4.58 (t, J = 8.0 Hz, 0.6H), 4.26 (d, J = 8.0 Hz, 0.4H), 4.17–3.96 (m, 2.3H), 3.94–3.86 (m, 1.5H), 3.66–3.59 (m, 1.1H), 3.53 (d, J = 12.0 Hz, 0.7H), 3.35 (dd, J = 20.0, 8.0 Hz, 0.7H), 3.20 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.40 (s, 1.8H), 2.37 (s, 1.2H), 0.99 (d, J = 8.0 Hz, 1.8H), 0.90 (d, J = 8.0 Hz, 1.2H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.2, 166.5, 166.4, 166.0, 148.2, 147.1, 144.1, 143.8, 141.3, 138.9, 138.8, 138.2, 138.0, 137.9, 136.5, 135.2, 134.7, 131.9, 130.6, 130.6, 129.5, 129.4, 129.4, 129.3, 129.2, 128.6, 128.6, 128.5, 128.4, 128.3, 128.3, 128.2, 128.0, 127.9, 127.8, 127.8, 127.4, 127.2, 127.0, 126.9, 126.8, 126.6, 126.3, 125.9, 124.6, 102.7, 102.0, 86.0, 84.8, 62.2, 60.5, 60.2, 60.0, 59.9, 58.8, 43.9, 42.9, 42.2, 40.6, 32.1, 29.9, 21.8, 21.8, 14.0, 13.9; HRMS (ESI) calcd for C42H37NO4Na [M + Na]+ 642.2615, found 642.2610.
1 petroleum ether/ethyl acetate): 99.2 mg, 80% yield; yellow white solid, mp 81–83 °C, IR (film) 1695, 1646, 1454, 1372, 1336, 1091 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.3H), 7.74 (t, J = 8.0 Hz, 1.4H), 7.53 (d, J = 8.0 Hz, 1.4H), 7.44–7.13 (m, 19H), 4.58 (t, J = 8.0 Hz, 0.6H), 4.26 (d, J = 8.0 Hz, 0.4H), 4.17–3.96 (m, 2.3H), 3.94–3.86 (m, 1.5H), 3.66–3.59 (m, 1.1H), 3.53 (d, J = 12.0 Hz, 0.7H), 3.35 (dd, J = 20.0, 8.0 Hz, 0.7H), 3.20 (dd, J = 16.0, 4.0 Hz, 0.6H), 2.40 (s, 1.8H), 2.37 (s, 1.2H), 0.99 (d, J = 8.0 Hz, 1.8H), 0.90 (d, J = 8.0 Hz, 1.2H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.2, 166.5, 166.4, 166.0, 148.2, 147.1, 144.1, 143.8, 141.3, 138.9, 138.8, 138.2, 138.0, 137.9, 136.5, 135.2, 134.7, 131.9, 130.6, 130.6, 129.5, 129.4, 129.4, 129.3, 129.2, 128.6, 128.6, 128.5, 128.4, 128.3, 128.3, 128.2, 128.0, 127.9, 127.8, 127.8, 127.4, 127.2, 127.0, 126.9, 126.8, 126.6, 126.3, 125.9, 124.6, 102.7, 102.0, 86.0, 84.8, 62.2, 60.5, 60.2, 60.0, 59.9, 58.8, 43.9, 42.9, 42.2, 40.6, 32.1, 29.9, 21.8, 21.8, 14.0, 13.9; HRMS (ESI) calcd for C42H37NO4Na [M + Na]+ 642.2615, found 642.2610.
2-(4′-Acetyl-2′-benzyl-2,5′-diphenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazol]-3-yl)-1-(p-tolyl)ethan-1-one (5dah), 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 96.7 mg, 82% yield; light yellow solid, mp 70–72 °C, IR (film) 1736, 1680, 1454, 1371, 1241, 1118 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.5H), 7.75–7.72 (m, 0.6H), 7.53 (d, J = 8.0 Hz, 1.5H), 7.42–7.13 (m, 19H), 6.97 (d, J = 8.0 Hz, 0.6H), 4.59 (t, J = 8.0 Hz, 0.8H), 4.32–4.26 (m, 0.2H), 4.17–4.09 (m, 0.2H), 4.05 (d, J = 12.0 Hz, 0.1H), 3.95–3.88 (m, 1.7H), 3.64 (t, J = 8.0 Hz, 0.5H), 3.52–3.39 (m, 1.8H), 3.16 (dd, J = 16.0, 4.0 Hz,1H), 2.39 (s, 2.4H), 2.37 (s, 0.6H), 1.97 (s, 2.5H), 1.83 (s, 0.5H); 13C NMR (100 MHz, CDCl3) δ 199.5, 199.2, 194.0, 167.9, 148.2, 147.1, 144.1, 143.7, 138.3, 138.1, 137.8, 136.6, 135.3, 134.7, 132.0, 131.0, 130.9, 130.7, 129.5, 129.4, 129.4, 129.3, 129.2, 128.9, 128.8, 128.7, 128.6, 128.5, 128.3, 128.0, 127.8, 127.5, 127.3, 127.1, 126.9, 126.8, 126.2, 125.7, 124.8, 113.4, 85.5, 61.7, 60.3, 58.5, 43.8, 43.0, 42.1, 40.7, 30.2, 30.0, 29.9, 21.8; HRMS (ESI) calcd for C41H36NO3 [M + H]+ 590.2690, found 590.2692.
1 petroleum ether/ethyl acetate): 96.7 mg, 82% yield; light yellow solid, mp 70–72 °C, IR (film) 1736, 1680, 1454, 1371, 1241, 1118 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1.5H), 7.75–7.72 (m, 0.6H), 7.53 (d, J = 8.0 Hz, 1.5H), 7.42–7.13 (m, 19H), 6.97 (d, J = 8.0 Hz, 0.6H), 4.59 (t, J = 8.0 Hz, 0.8H), 4.32–4.26 (m, 0.2H), 4.17–4.09 (m, 0.2H), 4.05 (d, J = 12.0 Hz, 0.1H), 3.95–3.88 (m, 1.7H), 3.64 (t, J = 8.0 Hz, 0.5H), 3.52–3.39 (m, 1.8H), 3.16 (dd, J = 16.0, 4.0 Hz,1H), 2.39 (s, 2.4H), 2.37 (s, 0.6H), 1.97 (s, 2.5H), 1.83 (s, 0.5H); 13C NMR (100 MHz, CDCl3) δ 199.5, 199.2, 194.0, 167.9, 148.2, 147.1, 144.1, 143.7, 138.3, 138.1, 137.8, 136.6, 135.3, 134.7, 132.0, 131.0, 130.9, 130.7, 129.5, 129.4, 129.4, 129.3, 129.2, 128.9, 128.8, 128.7, 128.6, 128.5, 128.3, 128.0, 127.8, 127.5, 127.3, 127.1, 126.9, 126.8, 126.2, 125.7, 124.8, 113.4, 85.5, 61.7, 60.3, 58.5, 43.8, 43.0, 42.1, 40.7, 30.2, 30.0, 29.9, 21.8; HRMS (ESI) calcd for C41H36NO3 [M + H]+ 590.2690, found 590.2692.
2-(2′-Benzyl-4'-(2,4-dinitrophenyl)-2,5′-diphenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazol]-3-yl)-1-(p-tolyl)ethan-1-one (5dai), 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 121.3 mg, 85% yield; brownish red solid, mp 135–137 °C, IR (film) 1721, 1680, 1536, 1453, 1345, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 2H), 7.53–7.14 (m, 20H), 6.97 (d, J = 8.0 Hz, 2H), 4.70 (s, 0.7H), 4.25–4.03 (m, 1.6H), 3.90 (d, J = 8.0 Hz, 1H), 3.63 (d, J = 12.0 Hz, 1H), 3.41 (d, J = 4.0 Hz, 1.7H), 2.42 (s, 1.9H), 2.37 (s, 1.1H), 3.71 (d, J = 16.0 Hz, 0.6H), 3.49–3.28 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.39 (s, 1.6H), 2.35 (s, 1.4H), 2.06 (s, 1.5H), 2.00 (s, 1.4H), 1.14 (t, J = 8.0 Hz, 1.6H), 1.05 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 198.7, 150.5, 145.5, 144.4, 144.0, 138.1, 137.8, 135.7, 135.6, 135.4, 134.7, 131.1, 131.0, 130.1, 129.5, 129.3, 129.0, 128.8, 128.7, 128.6, 128.5, 128.4, 128.1, 128.0, 127.8, 127.6, 127.4, 127.2, 126.8, 126.6, 124.6, 121.0, 117.9, 88.8, 86.9, 59.6, 41.3, 34.6, 29.8, 21.9; HRMS (ESI) calcd for C45H35N3O6Na [M + Na]+ 736.2418, found 736.2425.
1 petroleum ether/ethyl acetate): 121.3 mg, 85% yield; brownish red solid, mp 135–137 °C, IR (film) 1721, 1680, 1536, 1453, 1345, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 2H), 7.53–7.14 (m, 20H), 6.97 (d, J = 8.0 Hz, 2H), 4.70 (s, 0.7H), 4.25–4.03 (m, 1.6H), 3.90 (d, J = 8.0 Hz, 1H), 3.63 (d, J = 12.0 Hz, 1H), 3.41 (d, J = 4.0 Hz, 1.7H), 2.42 (s, 1.9H), 2.37 (s, 1.1H), 3.71 (d, J = 16.0 Hz, 0.6H), 3.49–3.28 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.39 (s, 1.6H), 2.35 (s, 1.4H), 2.06 (s, 1.5H), 2.00 (s, 1.4H), 1.14 (t, J = 8.0 Hz, 1.6H), 1.05 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 198.7, 150.5, 145.5, 144.4, 144.0, 138.1, 137.8, 135.7, 135.6, 135.4, 134.7, 131.1, 131.0, 130.1, 129.5, 129.3, 129.0, 128.8, 128.7, 128.6, 128.5, 128.4, 128.1, 128.0, 127.8, 127.6, 127.4, 127.2, 126.8, 126.6, 124.6, 121.0, 117.9, 88.8, 86.9, 59.6, 41.3, 34.6, 29.8, 21.9; HRMS (ESI) calcd for C45H35N3O6Na [M + Na]+ 736.2418, found 736.2425.
Ethyl 2′-benzyl-5′-methyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′-carboxylate (5daj), 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (20
1 dr. Purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 50.2 mg, 45% yield; brown solid, mp 39–41 °C, IR (film) 1734, 1696, 1473, 1374, 1258, 1107 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 2H), 7.41–7.33 (m, 4H), 7.31–7.09 (m, 11H), 4.48 (t, J = 8.0 Hz, 0.5H), 4.23–3.98 (m, 3H), 3.87–3.78 (m, 1H), 3.71 (d, J = 16.0 Hz, 0.6H), 3.49–3.28 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.39 (s, 1.6H), 2.35 (s, 1.4H), 2.06 (s, 1.5H), 2.00 (s, 1.4H), 1.14 (t, J = 8.0 Hz, 1.6H), 1.05 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.1, 168.5, 168.3, 164.9, 164.6, 147.7, 146.7, 144.1, 143.7, 139.4, 139.1, 138.3, 137.9, 136.6, 135.1, 134.7, 131.6, 130.5, 129.4, 129.3, 129.3, 129.0, 128.6, 128.5, 128.5, 128.3, 128.2, 128.1, 127.7, 127.4, 127.3, 127.0, 126.7, 126.7, 126.4, 126.2, 125.9, 124.5, 102.1, 101.6, 84.7, 83.5, 61.4, 60.8, 60.3, 59.7, 59.6, 58.1, 43.9, 42.6, 42.3, 40.3, 29.9, 21.8, 21.8, 14.4, 14.3, 13.2, 13.1; HRMS (ESI) calcd for C37H35NO4Na [M + Na]+ 580.2458, found 580.2460.
1 petroleum ether/ethyl acetate): 50.2 mg, 45% yield; brown solid, mp 39–41 °C, IR (film) 1734, 1696, 1473, 1374, 1258, 1107 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 2H), 7.41–7.33 (m, 4H), 7.31–7.09 (m, 11H), 4.48 (t, J = 8.0 Hz, 0.5H), 4.23–3.98 (m, 3H), 3.87–3.78 (m, 1H), 3.71 (d, J = 16.0 Hz, 0.6H), 3.49–3.28 (m, 2.4H), 3.17 (dd, J = 16.0, 4.0 Hz, 0.5H), 2.39 (s, 1.6H), 2.35 (s, 1.4H), 2.06 (s, 1.5H), 2.00 (s, 1.4H), 1.14 (t, J = 8.0 Hz, 1.6H), 1.05 (d, J = 8.0 Hz, 1.4H); 13C NMR (100 MHz, CDCl3) δ 199.4, 199.1, 168.5, 168.3, 164.9, 164.6, 147.7, 146.7, 144.1, 143.7, 139.4, 139.1, 138.3, 137.9, 136.6, 135.1, 134.7, 131.6, 130.5, 129.4, 129.3, 129.3, 129.0, 128.6, 128.5, 128.5, 128.3, 128.2, 128.1, 127.7, 127.4, 127.3, 127.0, 126.7, 126.7, 126.4, 126.2, 125.9, 124.5, 102.1, 101.6, 84.7, 83.5, 61.4, 60.8, 60.3, 59.7, 59.6, 58.1, 43.9, 42.6, 42.3, 40.3, 29.9, 21.8, 21.8, 14.4, 14.3, 13.2, 13.1; HRMS (ESI) calcd for C37H35NO4Na [M + Na]+ 580.2458, found 580.2460.
2′-Benzyl-5′-methyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3,3a′,6a′-tetrahydro-2′H,4′H-spiro[indene-1,3′-pyrrolo[3,4-d]isoxazole]-4′,6′(5′H)-dione (5dak), 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (10
1 dr. Purified by silica gel column chromatography (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/ethyl acetate): 76.8 mg, 69% yield; yellowish white solid, mp 110–112 °C, IR (film) 1709, 1677, 1453, 1377, 1281, 1181 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.0 Hz, 0.7H), 7.54 (d, J = 8.0 Hz, 1.2H), 7.41–7.17 (m, 15H), 7.10 (d, J = 4.0 Hz, 0.8H), 7.00 (d, J = 8.0 Hz, 0.5H), 4.94 (d, J = 8.0 Hz, 0.6H), 4.66 (d, J = 8.0 Hz, 0.3H), 4.56–4.51 (m, 0.1H), 4.37–4.29 (m, 0.5H), 4.11–3.95 (m, 2.1H), 3.90–3.78 (m, 1H), 3.73–3.67 (m, 0.4H), 3.51–3.44 (m, 0.4H), 3.36–3.29 (m, 1.2H), 3.21–3.15 (m, 1.3H), 3.00 (s, 1H), 2.50 (s, 2H), 2.39 (s, 1H), 2.38 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 198.8, 175.2, 173.9, 172.7, 146.5, 145.0, 144.3, 141.3, 137.4, 137.2, 136.8, 134.9, 134.7, 134.4, 134.4, 132.1, 130.7, 129.8, 129.6, 129.5, 128.8, 128.5, 128.4, 128.4, 128.3, 128.2, 128.2, 128.1, 128.0, 127.7, 127.6, 127.2, 127.1, 125.3, 124.9, 124.7, 124.7, 79.8, 78.8, 75.4, 60.3, 58.7, 58.1, 56.8, 56.4, 56.0, 46.5, 43.4, 43.0, 42.6, 29.9, 24.9, 24.4, 21.8. HRMS (ESI) calcd for C36H32N2O4Na [M + Na]+ 579.2254, found 579.2259.
1 petroleum ether/ethyl acetate): 76.8 mg, 69% yield; yellowish white solid, mp 110–112 °C, IR (film) 1709, 1677, 1453, 1377, 1281, 1181 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.0 Hz, 0.7H), 7.54 (d, J = 8.0 Hz, 1.2H), 7.41–7.17 (m, 15H), 7.10 (d, J = 4.0 Hz, 0.8H), 7.00 (d, J = 8.0 Hz, 0.5H), 4.94 (d, J = 8.0 Hz, 0.6H), 4.66 (d, J = 8.0 Hz, 0.3H), 4.56–4.51 (m, 0.1H), 4.37–4.29 (m, 0.5H), 4.11–3.95 (m, 2.1H), 3.90–3.78 (m, 1H), 3.73–3.67 (m, 0.4H), 3.51–3.44 (m, 0.4H), 3.36–3.29 (m, 1.2H), 3.21–3.15 (m, 1.3H), 3.00 (s, 1H), 2.50 (s, 2H), 2.39 (s, 1H), 2.38 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 198.8, 175.2, 173.9, 172.7, 146.5, 145.0, 144.3, 141.3, 137.4, 137.2, 136.8, 134.9, 134.7, 134.4, 134.4, 132.1, 130.7, 129.8, 129.6, 129.5, 128.8, 128.5, 128.4, 128.4, 128.3, 128.2, 128.2, 128.1, 128.0, 127.7, 127.6, 127.2, 127.1, 125.3, 124.9, 124.7, 124.7, 79.8, 78.8, 75.4, 60.3, 58.7, 58.1, 56.8, 56.4, 56.0, 46.5, 43.4, 43.0, 42.6, 29.9, 24.9, 24.4, 21.8. HRMS (ESI) calcd for C36H32N2O4Na [M + Na]+ 579.2254, found 579.2259.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired product 5aaa as a light yellow solid (0.2286 g, 76% yield, 9
1) to afford the desired product 5aaa as a light yellow solid (0.2286 g, 76% yield, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).Diethyl 2′-benzyl-3-(2-oxo-2-phenylethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5aaa), 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 228.6 mg, 76% yield; light yellow solid, mp 127–129 °C, IR (film) 1740, 1706, 1454, 1393, 1239, 1106 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 2H), 7.52–7.42 (m, 3H), 7.39–7.20 (m, 12H), 4.21–4.08 (m, 6H), 4.02 (d, J = 16.0 Hz, 1H), 3.56–3.42 (m, 3H), 1.31–1.19 (m, 3.3H), 1.13 (t, J = 8.0 Hz, 2.7H); 13C NMR (100 MHz, CDCl3) δ 199.2, 162.4, 159.2, 154.8, 148.0, 137.8, 137.5, 137.4, 137.2, 133.1, 131.6, 129.7, 129.0, 128.7, 128.6, 128.2, 128.1, 127.6, 127.3, 127.2, 126.6, 126.3, 108.7, 85.3, 62.6, 60.9, 60.6, 58.4, 43.9, 42.6, 14.1, 14.0.
1): 228.6 mg, 76% yield; light yellow solid, mp 127–129 °C, IR (film) 1740, 1706, 1454, 1393, 1239, 1106 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 2H), 7.52–7.42 (m, 3H), 7.39–7.20 (m, 12H), 4.21–4.08 (m, 6H), 4.02 (d, J = 16.0 Hz, 1H), 3.56–3.42 (m, 3H), 1.31–1.19 (m, 3.3H), 1.13 (t, J = 8.0 Hz, 2.7H); 13C NMR (100 MHz, CDCl3) δ 199.2, 162.4, 159.2, 154.8, 148.0, 137.8, 137.5, 137.4, 137.2, 133.1, 131.6, 129.7, 129.0, 128.7, 128.6, 128.2, 128.1, 127.6, 127.3, 127.2, 126.6, 126.3, 108.7, 85.3, 62.6, 60.9, 60.6, 58.4, 43.9, 42.6, 14.1, 14.0.
Diethyl 2′-benzyl-3-(2-oxo-2-(o-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5baa), 23.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 200.1 mg, 65% yield; light yellow solid, mp 96–97 °C, IR (film) 1743, 1719, 1495, 1393, 1300, 1176 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.64–7.62 (m, 2H), 7.46–7.43 (m, 2H), 7.39 (d, J = 8.0 Hz, 1H), 7.34–7.31 (m, 3H), 7.29–7.18 (m, 9H), 7.16–7.12 (m, 1H), 4.19–4.08 (m, 6H), 3.99 (d, J = 12.0 Hz, 1H), 3.52 (d, J = 12.0 Hz, 1H), 3.48–3.41 (m, 2H), 2.49 (s, 0.1H), 2.36 (s, 3H), 1.22 (t, J = 8.0 Hz, 3H), 1.13 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 202.8, 162.4, 159.2, 154.8, 148.2, 138.4, 138.3, 137.8, 137.3, 137.2, 132.1, 131.7, 131.4, 129.7, 129.0, 128.7, 128.5, 128.2, 127.6, 127.4, 127.2, 126.4, 126.3, 125.7, 108.7, 85.3, 62.6, 60.9, 60.6, 58.4, 45.6, 43.9, 21.4, 14.1, 14.0.
1): 200.1 mg, 65% yield; light yellow solid, mp 96–97 °C, IR (film) 1743, 1719, 1495, 1393, 1300, 1176 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.64–7.62 (m, 2H), 7.46–7.43 (m, 2H), 7.39 (d, J = 8.0 Hz, 1H), 7.34–7.31 (m, 3H), 7.29–7.18 (m, 9H), 7.16–7.12 (m, 1H), 4.19–4.08 (m, 6H), 3.99 (d, J = 12.0 Hz, 1H), 3.52 (d, J = 12.0 Hz, 1H), 3.48–3.41 (m, 2H), 2.49 (s, 0.1H), 2.36 (s, 3H), 1.22 (t, J = 8.0 Hz, 3H), 1.13 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 202.8, 162.4, 159.2, 154.8, 148.2, 138.4, 138.3, 137.8, 137.3, 137.2, 132.1, 131.7, 131.4, 129.7, 129.0, 128.7, 128.5, 128.2, 127.6, 127.4, 127.2, 126.4, 126.3, 125.7, 108.7, 85.3, 62.6, 60.9, 60.6, 58.4, 45.6, 43.9, 21.4, 14.1, 14.0.
Diethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5daa), 14.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 243.2 mg, 79% yield; 112.1 mg, 89% yield; light yellow solid, mp 140–142 °C, IR (film) 1740, 1713, 1474, 1370, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 8.0 Hz, 4H), 7.43–7.39 (m, 2H), 7.32–7.15 (m, 12H), 4.21–4.10 (m, 6H), 4.02 (d, J = 12.0 Hz, 1H), 3.54–3.39 (m, 3H), 2.40 (s, 0.2H), 2.37 (s, 2.8H), 1.23 (t, J = 8.0 Hz, 3.2H), 1.13 (t, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 198.8, 162.4, 159.2, 154.8, 148.1, 143.9, 137.8, 137.5, 137.2, 135.1, 131.6, 129.7, 129.4, 129.0, 128.6, 128.3, 128.2, 127.6, 127.3, 127.1, 126.6, 126.3, 108.8, 85.3, 62.6, 60.9, 60.6, 58.4, 44.0, 42.5, 21.8, 14.1, 14.0.
1): 243.2 mg, 79% yield; 112.1 mg, 89% yield; light yellow solid, mp 140–142 °C, IR (film) 1740, 1713, 1474, 1370, 1241, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 8.0 Hz, 4H), 7.43–7.39 (m, 2H), 7.32–7.15 (m, 12H), 4.21–4.10 (m, 6H), 4.02 (d, J = 12.0 Hz, 1H), 3.54–3.39 (m, 3H), 2.40 (s, 0.2H), 2.37 (s, 2.8H), 1.23 (t, J = 8.0 Hz, 3.2H), 1.13 (t, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 198.8, 162.4, 159.2, 154.8, 148.1, 143.9, 137.8, 137.5, 137.2, 135.1, 131.6, 129.7, 129.4, 129.0, 128.6, 128.3, 128.2, 127.6, 127.3, 127.1, 126.6, 126.3, 108.8, 85.3, 62.6, 60.9, 60.6, 58.4, 44.0, 42.5, 21.8, 14.1, 14.0.
Diethyl 2′-benzyl-3-(2-(4-bromophenyl)-2-oxoethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5eaa), 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 251.8 mg, 74% yield; light yellow solid, mp115–117 °C, IR (film) 1735, 1707, 1499, 1370, 1242, 1169 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 0.4H), 7.66–7.56 (m, 3.5H), 7.50–7.37 (m, 4H), 7.35–7.14 (m, 10H), 4.22–4.08 (m, 5.2H), 4.03 (d, J = 12.0 Hz, 0.9H), 3.89–3.76 (m, 0.4H), 3.54–3.48 (m, 1.8H), 3.38–3.28 (m, 1H), 3.20–3.14 (m, 0.2H), 1.29–1.20 (m, 3.6H), 1.13 (t, J = 8.0 Hz, 2.5H); 13C NMR (100 MHz, CDCl3) δ 198.1, 162.4, 159.2, 154.8, 147.7, 137.8, 137.4, 137.1, 136.1, 132.1, 131.9, 131.5, 130.5, 129.9, 129.7, 129.7, 128.9, 128.6, 128.6, 128.3, 128.2, 128.0, 127.7, 127.4, 127.3, 126.4, 126.4, 108.6, 85.3, 62.7, 60.9, 60.6, 58.3, 43.9, 42.4, 14.1, 14.0.
1): 251.8 mg, 74% yield; light yellow solid, mp115–117 °C, IR (film) 1735, 1707, 1499, 1370, 1242, 1169 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 0.4H), 7.66–7.56 (m, 3.5H), 7.50–7.37 (m, 4H), 7.35–7.14 (m, 10H), 4.22–4.08 (m, 5.2H), 4.03 (d, J = 12.0 Hz, 0.9H), 3.89–3.76 (m, 0.4H), 3.54–3.48 (m, 1.8H), 3.38–3.28 (m, 1H), 3.20–3.14 (m, 0.2H), 1.29–1.20 (m, 3.6H), 1.13 (t, J = 8.0 Hz, 2.5H); 13C NMR (100 MHz, CDCl3) δ 198.1, 162.4, 159.2, 154.8, 147.7, 137.8, 137.4, 137.1, 136.1, 132.1, 131.9, 131.5, 130.5, 129.9, 129.7, 129.7, 128.9, 128.6, 128.6, 128.3, 128.2, 128.0, 127.7, 127.4, 127.3, 126.4, 126.4, 108.6, 85.3, 62.7, 60.9, 60.6, 58.3, 43.9, 42.4, 14.1, 14.0.
Diethyl 2′-benzyl-3-(2-(furan-2-yl)-2-oxoethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5faa), 33.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 210.1 mg, 71% yield; light yellow solid, mp 141–143 °C, IR (film) 1739, 1710, 1465, 1371, 1189, 1140 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 10.0 Hz, 2H), 7.45–7.42 (m, 2H), 7.40 (d, J = 5.0 Hz, 2H), 7.36–7.33 (m, 3H), 7.30–7.20 (m, 6H), 7.00 (d, J = 5.0 Hz, 1H), 6.50–6.49 (m, 0.03H), 6.44–6.43 (m, 1H), 4.20–4.15 (m, 3H), 4.14–4.06 (m, 3H), 4.01 (d, J = 15.0 Hz, 1H), 3.54 (d, J = 15.0 Hz, 1H), 3.47 (dd, J = 20.0, 10.0 Hz, 1H), 3.21 (dd, J = 20.0, 5.0 Hz, 1H), 1.23 (t, J = 10.0 Hz, 3H), 1.12 (t, J = 10.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 188.7, 162.4, 159.2, 154.9, 153.3, 147.5, 146.2, 137.8, 137.5, 137.0, 131.6, 129.6, 129.1, 128.6, 128.1, 127.6, 127.4, 127.2, 126.4, 126.3, 116.8, 112.5, 108.5, 85.2, 62.7, 60.9, 60.7, 58.3, 44.0, 42.3, 14.1, 14.0.
1): 210.1 mg, 71% yield; light yellow solid, mp 141–143 °C, IR (film) 1739, 1710, 1465, 1371, 1189, 1140 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 10.0 Hz, 2H), 7.45–7.42 (m, 2H), 7.40 (d, J = 5.0 Hz, 2H), 7.36–7.33 (m, 3H), 7.30–7.20 (m, 6H), 7.00 (d, J = 5.0 Hz, 1H), 6.50–6.49 (m, 0.03H), 6.44–6.43 (m, 1H), 4.20–4.15 (m, 3H), 4.14–4.06 (m, 3H), 4.01 (d, J = 15.0 Hz, 1H), 3.54 (d, J = 15.0 Hz, 1H), 3.47 (dd, J = 20.0, 10.0 Hz, 1H), 3.21 (dd, J = 20.0, 5.0 Hz, 1H), 1.23 (t, J = 10.0 Hz, 3H), 1.12 (t, J = 10.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 188.7, 162.4, 159.2, 154.9, 153.3, 147.5, 146.2, 137.8, 137.5, 137.0, 131.6, 129.6, 129.1, 128.6, 128.1, 127.6, 127.4, 127.2, 126.4, 126.3, 116.8, 112.5, 108.5, 85.2, 62.7, 60.9, 60.7, 58.3, 44.0, 42.3, 14.1, 14.0.
Diethyl 2′-benzyl-2-(4-methoxyphenyl)-3-(2-oxo-2-(p-tolyl)ethyl)-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5laa), 29.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 222.8 mg, 69% yield; light yellow solid, mp 85–86 °C, IR (film) 1738, 1712, 1496, 1393, 1305, 1140 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 5.0 Hz, 2H), 7.59 (d, J = 10.0 Hz, 2H), 7.44–7.39 (m, 2H), 7.36–7.32 (m, 4H), 7.30–7.27 (m, 3H), 7.17 (d, J = 10.0 Hz, 2H), 6.78 (d, J = 10.0 Hz, 2H), 4.21–4.16 (m, 2H), 4.15–4.05 (m, 4H), 4.01 (d, J = 15.0 Hz, 1H), 3.75 (s, 3H), 3.52–3.48 (m, 2H), 3.44–3.38 (m, 1H), 2.37 (s, 2.9H), 1.25 (t, J = 10.0 Hz, 3.1H), 1.13 (t, J = 10.0 Hz, 2.9H); 13C NMR (125 MHz, CDCl3) δ 198.9, 162.5, 159.2, 158.9, 154.6, 148.1, 143.9, 137.7, 137.5, 135.1, 132.7, 129.6, 129.3, 129.2, 129.0, 128.6, 128.3, 127.6, 127.1, 126.6, 126.3, 113.6, 108.9, 85.3, 62.6, 60.9, 60.6, 57.7, 55.3, 44.1, 42.5, 21.8, 14.1, 14.0.
1): 222.8 mg, 69% yield; light yellow solid, mp 85–86 °C, IR (film) 1738, 1712, 1496, 1393, 1305, 1140 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 5.0 Hz, 2H), 7.59 (d, J = 10.0 Hz, 2H), 7.44–7.39 (m, 2H), 7.36–7.32 (m, 4H), 7.30–7.27 (m, 3H), 7.17 (d, J = 10.0 Hz, 2H), 6.78 (d, J = 10.0 Hz, 2H), 4.21–4.16 (m, 2H), 4.15–4.05 (m, 4H), 4.01 (d, J = 15.0 Hz, 1H), 3.75 (s, 3H), 3.52–3.48 (m, 2H), 3.44–3.38 (m, 1H), 2.37 (s, 2.9H), 1.25 (t, J = 10.0 Hz, 3.1H), 1.13 (t, J = 10.0 Hz, 2.9H); 13C NMR (125 MHz, CDCl3) δ 198.9, 162.5, 159.2, 158.9, 154.6, 148.1, 143.9, 137.7, 137.5, 135.1, 132.7, 129.6, 129.3, 129.2, 129.0, 128.6, 128.3, 127.6, 127.1, 126.6, 126.3, 113.6, 108.9, 85.3, 62.6, 60.9, 60.6, 57.7, 55.3, 44.1, 42.5, 21.8, 14.1, 14.0.
Diethyl 2′-benzyl-5-chloro-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5oaa), 12.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 211.3 mg, 65% yield; light yellow solid, mp 118–120 °C, IR (film) 1740, 1710, 1454, 1394, 1304, 1143 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.2H), 7.71 (d, J = 8.0 Hz, 2H), 7.65–7.62 (m, 2H), 7.48 (d, J = 4.0 Hz, 1H), 7.38–7.16 (m, 12H), 4.21–4.09 (m, 6H), 3.99 (d, J = 12.0 Hz, 1H), 3.52–3.38 (m, 3H), 2.40 (s, 0.2H), 2.38 (s, 2.7H), 1.23 (t, J = 8.0 Hz, 3H), 1.16 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 198.3, 162.3, 159.1, 155.1, 149.9, 144.1, 137.1, 136.7, 136.5, 135.6, 134.8, 131.5, 129.4, 129.0, 128.6, 128.3, 128.2, 127.7, 127.6, 127.5, 127.3, 127.1, 108.1, 84.7, 62.7, 61.0, 60.6, 58.5, 43.6, 42.0, 21.8, 14.1, 14.0.
1): 211.3 mg, 65% yield; light yellow solid, mp 118–120 °C, IR (film) 1740, 1710, 1454, 1394, 1304, 1143 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.0 Hz, 0.2H), 7.71 (d, J = 8.0 Hz, 2H), 7.65–7.62 (m, 2H), 7.48 (d, J = 4.0 Hz, 1H), 7.38–7.16 (m, 12H), 4.21–4.09 (m, 6H), 3.99 (d, J = 12.0 Hz, 1H), 3.52–3.38 (m, 3H), 2.40 (s, 0.2H), 2.38 (s, 2.7H), 1.23 (t, J = 8.0 Hz, 3H), 1.16 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 198.3, 162.3, 159.1, 155.1, 149.9, 144.1, 137.1, 136.7, 136.5, 135.6, 134.8, 131.5, 129.4, 129.0, 128.6, 128.3, 128.2, 127.7, 127.6, 127.5, 127.3, 127.1, 108.1, 84.7, 62.7, 61.0, 60.6, 58.5, 43.6, 42.0, 21.8, 14.1, 14.0.
Diethyl 2′-benzyl-6-methyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydrospiro[indene-1,3′-isoxazolidine]-4′,5′-dicarboxylate (5raa), 14.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 198.4 mg, 63% yield; yellowish white solid, mp 145–147 °C, IR (film) 1749, 1712, 1453, 1305, 1200, 1178 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70–7.65 (m, 4H), 7.34 (t, J = 4.0 Hz, 4H), 7.30–7.20 (m, 6H), 7.16 (d, J = 8.0 Hz, 2H), 7.19 (d, J = 8.0 Hz, 1H), 4.21–4.05 (m, 6H), 4.00 (d, J = 16.0 Hz, 1H), 3.53 (d, J = 12.0 Hz, 1H), 3.49–3.37 (m, 2H), 2.37 (s, 3H), 2.36 (s, 3H), 1.25 (t, J = 8.0 Hz, 3.2H), 1.13 (t, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 199.0, 162.5, 159.2, 154.5, 145.0, 143.8, 137.8, 137.5, 137.3, 136.8, 135.1, 131.6, 130.6, 129.3, 129.1, 128.6, 128.3, 128.2, 127.6, 127.3, 126.6, 126.2, 123.0, 109.0, 100.1, 93.3, 85.3, 62.6, 60.8, 60.6, 58.6, 43.7, 42.6, 21.8, 21.6, 14.1, 14.0.
1): 198.4 mg, 63% yield; yellowish white solid, mp 145–147 °C, IR (film) 1749, 1712, 1453, 1305, 1200, 1178 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70–7.65 (m, 4H), 7.34 (t, J = 4.0 Hz, 4H), 7.30–7.20 (m, 6H), 7.16 (d, J = 8.0 Hz, 2H), 7.19 (d, J = 8.0 Hz, 1H), 4.21–4.05 (m, 6H), 4.00 (d, J = 16.0 Hz, 1H), 3.53 (d, J = 12.0 Hz, 1H), 3.49–3.37 (m, 2H), 2.37 (s, 3H), 2.36 (s, 3H), 1.25 (t, J = 8.0 Hz, 3.2H), 1.13 (t, J = 8.0 Hz, 2.8H); 13C NMR (100 MHz, CDCl3) δ 199.0, 162.5, 159.2, 154.5, 145.0, 143.8, 137.8, 137.5, 137.3, 136.8, 135.1, 131.6, 130.6, 129.3, 129.1, 128.6, 128.3, 128.2, 127.6, 127.3, 126.6, 126.2, 123.0, 109.0, 100.1, 93.3, 85.3, 62.6, 60.8, 60.6, 58.6, 43.7, 42.6, 21.8, 21.6, 14.1, 14.0.
Dimethyl 2′-benzyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-2′H-spiro[indene-1,3′-isoxazole]-4′,5′-dicarboxylate (5dab), 11.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 241.0 mg, 82% yield; light yellow solid, mp 132–134 °C, IR (film) 1765, 1750, 1448, 1352, 1307, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.68 (t, J = 8.0 Hz, 4H), 7.47–7.41 (m, 2H), 7.36–7.20 (m, 10H), 7.16 (d, J = 8.0 Hz, 2H), 4.19–4.10 (m, 2H), 4.00 (d, J = 16.0 Hz, 1H), 3.73 (s, 3H), 3.66 (s, 3H), 3.52–3.39 (m, 3H), 2.39 (s, 0.3H), 2.37 (s, 2.7H); 13C NMR (100 MHz, CDCl3) δ 198.8, 162.9, 159.5, 154.4, 148.0, 143.9, 137.6, 137.3, 137.1, 135.0, 131.6, 129.7, 129.3, 128.9, 128.6, 128.3, 128.2, 127.6, 127.4, 127.3, 126.7, 126.1, 109.2, 85.3, 60.7, 58.4, 53.3, 52.1, 44.0, 42.4, 21.8.
1): 241.0 mg, 82% yield; light yellow solid, mp 132–134 °C, IR (film) 1765, 1750, 1448, 1352, 1307, 1140 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.68 (t, J = 8.0 Hz, 4H), 7.47–7.41 (m, 2H), 7.36–7.20 (m, 10H), 7.16 (d, J = 8.0 Hz, 2H), 4.19–4.10 (m, 2H), 4.00 (d, J = 16.0 Hz, 1H), 3.73 (s, 3H), 3.66 (s, 3H), 3.52–3.39 (m, 3H), 2.39 (s, 0.3H), 2.37 (s, 2.7H); 13C NMR (100 MHz, CDCl3) δ 198.8, 162.9, 159.5, 154.4, 148.0, 143.9, 137.6, 137.3, 137.1, 135.0, 131.6, 129.7, 129.3, 128.9, 128.6, 128.3, 128.2, 127.6, 127.4, 127.3, 126.7, 126.1, 109.2, 85.3, 60.7, 58.4, 53.3, 52.1, 44.0, 42.4, 21.8.
Diethyl 2′-methyl-3-(2-oxo-2-(p-tolyl)ethyl)-2-phenyl-2,3-dihydro-spiro[indene-1,3′-isoxazolidine]-4′,5′-dicarboxylate (5dca), 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50
1 dr. Purified by silica gel column chromatography (petroleum ether/ethyl acetate from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 121.4 mg, 45% yield; brown solid, mp 74–76 °C, IR (film) 1737, 1702, 1460, 1370, 1291, 1186 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 10.0 Hz, 0.3H), 7.72 (d, J = 10.0 Hz, 1.7H), 7.68 (d, J = 5.0 Hz, 1.7H), 7.44 (d, J = 5.0 Hz, 0.3H), 7.37 (d, J = 10.0 Hz, 1H), 7.32 (t, J = 5.0 Hz, 1H), 7.29–7.19 (m, 7H), 7.14 (d, J = 10.0 Hz, 0.2H), 4.26–4.14 (m, 2.3H), 4.13–4.04 (m, 3.5H), 3.43 (dd, J = 15.0, 5.0 Hz, 0.9H), 3.32 (dd, J = 15.0, 10.0 Hz, 1H), 2.62 (s, 2.6H), 2.45 (s, 0.4H), 2.40 (s, 0.4H), 2.38 (s, 2.6H), 1.31–1.19 (m, 3.4H), 1.11 (t, J = 10.0 Hz, 2.6H); 13C NMR (125 MHz, CDCl3) δ 199.0, 162.6, 159.1, 153.8, 148.0, 143.8, 137.3, 137.2, 135.1, 131.8, 130.4, 129.9, 129.5, 129.4, 129.4, 128.5, 128.3, 128.0, 127.9, 127.4, 126.9, 126.5, 126.5, 108.9, 85.6, 62.6, 60.9, 58.1, 43.9, 43.7, 42.5, 21.8, 14.1, 14.0. HRMS (ESI) calcd for C33H34NO6 [M + H]+ 540.2381, found 540.2386.
1): 121.4 mg, 45% yield; brown solid, mp 74–76 °C, IR (film) 1737, 1702, 1460, 1370, 1291, 1186 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 10.0 Hz, 0.3H), 7.72 (d, J = 10.0 Hz, 1.7H), 7.68 (d, J = 5.0 Hz, 1.7H), 7.44 (d, J = 5.0 Hz, 0.3H), 7.37 (d, J = 10.0 Hz, 1H), 7.32 (t, J = 5.0 Hz, 1H), 7.29–7.19 (m, 7H), 7.14 (d, J = 10.0 Hz, 0.2H), 4.26–4.14 (m, 2.3H), 4.13–4.04 (m, 3.5H), 3.43 (dd, J = 15.0, 5.0 Hz, 0.9H), 3.32 (dd, J = 15.0, 10.0 Hz, 1H), 2.62 (s, 2.6H), 2.45 (s, 0.4H), 2.40 (s, 0.4H), 2.38 (s, 2.6H), 1.31–1.19 (m, 3.4H), 1.11 (t, J = 10.0 Hz, 2.6H); 13C NMR (125 MHz, CDCl3) δ 199.0, 162.6, 159.1, 153.8, 148.0, 143.8, 137.3, 137.2, 135.1, 131.8, 130.4, 129.9, 129.5, 129.4, 129.4, 128.5, 128.3, 128.0, 127.9, 127.4, 126.9, 126.5, 126.5, 108.9, 85.6, 62.6, 60.9, 58.1, 43.9, 43.7, 42.5, 21.8, 14.1, 14.0. HRMS (ESI) calcd for C33H34NO6 [M + H]+ 540.2381, found 540.2386.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the product 6 as a white solid (0.1153 g, 75% yield), mp. 50–52 °C, IR (film) 3486, 1730, 1680, 1606, 1453, 1366, 1224, 1079 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 10.0 Hz, 0.1H), 7.88–7.82 (m, 0.3H), 7.75 (d, J = 10.0 Hz, 0.1H), 7.67 (d, J = 5.0 Hz, 0.7H), 7.57–7.51 (m, 1.9H), 7.42 (d, J = 10.0 Hz, 0.2H), 7.40–7.35 (m, 1H), 7.32–7.22 (m, 2.6H), 7.19 (d, J = 5.0 Hz, 0.3H), 7.17–7.13 (m, 2H), 7.04–6.96 (m, 2.8H), 6.85 (d, J = 5.0 Hz, 0.2H), 6.74 (t, J = 10.0 Hz, 1.7H), 5.79 (d, J = 10.0 Hz, 0.2H), 5.47 (d, J = 10.0 Hz, 0.2H), 5.05–5.02 (m, 0.8H), 4.87 (d, J = 10.0 Hz, 0.8H), 3.40 (t, J = 10.0 Hz, 0.9H), 4.31–4.24 (m, 2.1H), 4.10–3.90 (m, 0.5H), 3.89–3.75 (m, 1.8H), 3.43 (d, J = 5.0 Hz, 0.8H), 3.19–3.01 (m, 1H), 2.84–2.74 (m, 0.9H), 2.41 (s, 0.2H), 2.37 (s, 0.5H), 2.36 (s, 2.2H), 1.30–1.21 (m, 3.5H), 1.11 (t, J = 5.0 Hz, 0.5H), 0.82 (t, J = 10.0 Hz, 2.1H); 13C NMR (125 MHz, CDCl3) δ 198.9, 173.4, 173.3, 167.5, 156.5, 152.7, 150.0, 143.8, 140.6, 140.3, 139.2, 134.7, 131.0, 130.5, 129.5, 129.3, 129.2, 128.9, 128.5, 128.5, 128.2, 128.1, 128.0, 127.5, 127.0, 127.0, 126.9, 126.7, 126.4, 125.7, 124.9, 124.6, 124.0, 70.3, 68.6, 62.3, 62.0, 61.2, 60.9, 55.4, 54.8, 43.4, 43.1, 39.7, 39.0, 21.8, 14.3, 14.2, 14.0, 13.8; HRMS (ESI) calcd for C32H33O6[M + H]+ 513.2272, found 513.2277.
1, v/v) to afford the product 6 as a white solid (0.1153 g, 75% yield), mp. 50–52 °C, IR (film) 3486, 1730, 1680, 1606, 1453, 1366, 1224, 1079 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 10.0 Hz, 0.1H), 7.88–7.82 (m, 0.3H), 7.75 (d, J = 10.0 Hz, 0.1H), 7.67 (d, J = 5.0 Hz, 0.7H), 7.57–7.51 (m, 1.9H), 7.42 (d, J = 10.0 Hz, 0.2H), 7.40–7.35 (m, 1H), 7.32–7.22 (m, 2.6H), 7.19 (d, J = 5.0 Hz, 0.3H), 7.17–7.13 (m, 2H), 7.04–6.96 (m, 2.8H), 6.85 (d, J = 5.0 Hz, 0.2H), 6.74 (t, J = 10.0 Hz, 1.7H), 5.79 (d, J = 10.0 Hz, 0.2H), 5.47 (d, J = 10.0 Hz, 0.2H), 5.05–5.02 (m, 0.8H), 4.87 (d, J = 10.0 Hz, 0.8H), 3.40 (t, J = 10.0 Hz, 0.9H), 4.31–4.24 (m, 2.1H), 4.10–3.90 (m, 0.5H), 3.89–3.75 (m, 1.8H), 3.43 (d, J = 5.0 Hz, 0.8H), 3.19–3.01 (m, 1H), 2.84–2.74 (m, 0.9H), 2.41 (s, 0.2H), 2.37 (s, 0.5H), 2.36 (s, 2.2H), 1.30–1.21 (m, 3.5H), 1.11 (t, J = 5.0 Hz, 0.5H), 0.82 (t, J = 10.0 Hz, 2.1H); 13C NMR (125 MHz, CDCl3) δ 198.9, 173.4, 173.3, 167.5, 156.5, 152.7, 150.0, 143.8, 140.6, 140.3, 139.2, 134.7, 131.0, 130.5, 129.5, 129.3, 129.2, 128.9, 128.5, 128.5, 128.2, 128.1, 128.0, 127.5, 127.0, 127.0, 126.9, 126.7, 126.4, 125.7, 124.9, 124.6, 124.0, 70.3, 68.6, 62.3, 62.0, 61.2, 60.9, 55.4, 54.8, 43.4, 43.1, 39.7, 39.0, 21.8, 14.3, 14.2, 14.0, 13.8; HRMS (ESI) calcd for C32H33O6[M + H]+ 513.2272, found 513.2277.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the product 7 as a light yellow solid (0.0655 g, 52% yield), mp 45–47 °C, IR (film) 1733, 1710, 1679, 1494, 1284, 1215, 1093 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.0 Hz, 0.3H), 7.77 (d, J = 8.0 Hz, 1.7H), 7.39–7.33 (m, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.15–7.09 (m, 4H), 7.07–6.98 (m, 3H), 5.48 (d, J = 16.0 Hz, 0.8H), 5.20 (d, J = 16.0 Hz, 0.2H), 4.69 (d, J = 16.0 Hz, 0.2H), 4.19 (d, J = 16.0 Hz, 0.9H), 4.08–4.02 (m, 1.3H), 3.98–3.92 (m, 1.7H), 3.83–3.73 (m, 2.9H), 3.62–3.56 (m, 0.2H), 3.32–3.26 (m, 0.9H), 3.15–3.03 (m, 0.3H), 2.89 (dd, J = 16.0, 4.0 Hz, 0.9H), 2.44 (s, 2.5H), 2.38 (s, 0.5H), 2.36 (s, 2.5H), 2.08 (s, 0.5H), 1.07 (d, J = 8.0 Hz, 0.6H), 1.03–0.97 (m, 5.4H); 13C NMR (100 MHz, CDCl3) δ 197.9, 165.8, 162.0, 161.7, 144.3, 141.5, 141.4, 137.3, 136.8, 135.7, 134.8, 131.9, 131.8, 130.9, 130.6, 129.5, 129.4, 129.3, 129.2, 128.6, 128.5, 128.5, 128.5, 128.4, 128.4, 128.3, 127.8, 127.7, 127.3, 127.0, 62.3, 61.7, 61.4, 51.4, 46.0, 45.9, 45.4, 44.9, 44.0, 40.2, 21.9, 21.6, 13.8, 13.7; HRMS (ESI) calcd for C40H40NO6 [M + H]+ 630.2850, found 630.2857.
1, v/v) to afford the product 7 as a light yellow solid (0.0655 g, 52% yield), mp 45–47 °C, IR (film) 1733, 1710, 1679, 1494, 1284, 1215, 1093 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.0 Hz, 0.3H), 7.77 (d, J = 8.0 Hz, 1.7H), 7.39–7.33 (m, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.15–7.09 (m, 4H), 7.07–6.98 (m, 3H), 5.48 (d, J = 16.0 Hz, 0.8H), 5.20 (d, J = 16.0 Hz, 0.2H), 4.69 (d, J = 16.0 Hz, 0.2H), 4.19 (d, J = 16.0 Hz, 0.9H), 4.08–4.02 (m, 1.3H), 3.98–3.92 (m, 1.7H), 3.83–3.73 (m, 2.9H), 3.62–3.56 (m, 0.2H), 3.32–3.26 (m, 0.9H), 3.15–3.03 (m, 0.3H), 2.89 (dd, J = 16.0, 4.0 Hz, 0.9H), 2.44 (s, 2.5H), 2.38 (s, 0.5H), 2.36 (s, 2.5H), 2.08 (s, 0.5H), 1.07 (d, J = 8.0 Hz, 0.6H), 1.03–0.97 (m, 5.4H); 13C NMR (100 MHz, CDCl3) δ 197.9, 165.8, 162.0, 161.7, 144.3, 141.5, 141.4, 137.3, 136.8, 135.7, 134.8, 131.9, 131.8, 130.9, 130.6, 129.5, 129.4, 129.3, 129.2, 128.6, 128.5, 128.5, 128.5, 128.4, 128.4, 128.3, 127.8, 127.7, 127.3, 127.0, 62.3, 61.7, 61.4, 51.4, 46.0, 45.9, 45.4, 44.9, 44.0, 40.2, 21.9, 21.6, 13.8, 13.7; HRMS (ESI) calcd for C40H40NO6 [M + H]+ 630.2850, found 630.2857.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21502065, 21702072), Scientific Research Fund of Hunan Provincial Education Department (No. 19A389), Opening Fund of CAS Key Laboratory of Molecular Recognition and Function (Chinese Academy of Sciences) (No. 2017LRMF009) for financial support.Notes and references
- (a) A. G. Habeeb, P. N. Praveen Rao and E. E. Knaus, J. Med. Chem., 2001, 44, 2921–2927 CrossRef CAS PubMed; (b) R. D. Cramer, R. J. Jilek, S. Guessregen, S. J. Clark, B. Wendt and R. D. Clark, J. Med. Chem., 2004, 47, 6777–6791 CrossRef CAS PubMed; (c) A. I. Hubich, T. A. Zheldakova, T. V. Chernikhova, E. V. Koroleva, F. A. Lakhvich and M. V. Sholukh, Biochem. Biophys. Res. Commun., 2006, 341, 357–362 CrossRef CAS PubMed.
- For selected examples: (a) A. Brandi, F. Cardona, S. Cicchi, F. M. Cordero and A. Goti, Chem.–Eur. J., 2009, 15, 7808–7821 CrossRef CAS PubMed; (b) H. Wei, C. Qiao, G. Liu, Z. Yang and C. Li, Angew. Chem., Int. Ed., 2013, 52, 620–624 CrossRef CAS PubMed ;and references therein..
- Selected reviews: (a) J. P. Freeman, Chem. Rev., 1983, 83, 241–261 CrossRef CAS; (b) T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2010, 3363–3376 CrossRef CAS; (c) N. V. Chukanov and V. A. Reznikov, Russ. Chem. Bull., Int. Ed., 2011, 60, 379–399 CrossRef CAS.
- (a) I. S. Young and M. A. Kerr, Org. Lett., 2004, 6, 139–141 CrossRef CAS PubMed; (b) P.-W. Xu, C. Chen, J.-K. Liu, Y.-T. Song, F. Zhou, J. Yan and J. Zhou, J. Org. Chem., 2018, 83, 12763–12774 CrossRef CAS PubMed.
- (a) S. A. Hussain, A. H. Sharma and M. J. Perkin, J. Chem. Soc. D, 1979, 289–291 RSC; (b) K. M. Partridge, M. E. Anzovino and T. P. Yoon, J. Am. Chem. Soc., 2008, 130, 2920–2921 CrossRef CAS PubMed; (c) A. R. Reddy, Z. Guo, F.-M. Siu, C.-N. Lok, F. Liu, K.-C. Yeung, C.-Y. Zhou and C.-M. Che, Org. Biomol. Chem., 2012, 10, 9165–9174 RSC; (d) H. Hou, S. Zhu, F. Pan and M. Rueping, Org. Lett., 2014, 16, 2872–2875 CrossRef CAS PubMed; (e) Y. Liu, J. Ao, S. Paladhi, C. E. Song and H. Yan, J. Am. Chem. Soc., 2016, 138, 16486–16492 CrossRef CAS PubMed; (f) C. Gioia, F. Fini, A. Mazzanti, L. Bernardi and A. Ricci, J. Am. Chem. Soc., 2009, 131, 9614–9615 CrossRef CAS PubMed; (g) Z.-J. Xu, D. Zhu, X. Zeng, F. Wang, B. Tan, Y. Hou, Y. Lv and G. Zhong, Chem. Commun., 2010, 46, 2504–2506 RSC; (h) N. Morita, R. Kono, K. Fukui, A. Miyazawa, H. Masu, I. Azumaya, S. Ban, Y. Hashimoto, I. Okamoto and O. Tamura, J. Org. Chem., 2015, 80, 4797–4802 CrossRef CAS PubMed; (i) X. Li, T. Feng, D. Li, H. Chang, W. Gao and W. Wei, J. Org. Chem., 2019, 84, 4402–4412 CrossRef CAS PubMed.
- (a) X. Guinchard, Y. Vallée and J.-N. Denis, Org. Lett., 2005, 7, 5147–5150 CrossRef CAS PubMed; (b) A. Gini, M. Segler, D. Kellner and O. G. Mancheño, Chem.–Eur. J., 2015, 21, 12053–12060 CrossRef CAS PubMed; (c) A. R. Reddy, C.-Y. Zhou and C.-M. Che, Org. Lett., 2014, 16, 1048–1051 CrossRef CAS PubMed; (d) K. Wu, C.-Y. Zhou and C.-M. Che, Org. Lett., 2019, 21, 85–89 CrossRef CAS PubMed; (e) X. Li, T. Feng, D. Li, H. Chang, W. Gao and W. Wei, J. Org. Chem., 2020, 85, 9538–9547 CrossRef CAS PubMed.
- D. P. Canterbury, Il. R. Herrick, J. Um, K. N. Houk and A. J. Frontier, Tetrahedron, 2009, 65, 3165–3179 CrossRef CAS PubMed.
- G. S. Jang, J. Lee, J. Seo and S. K. Woo, Org. Lett., 2017, 19, 6448–6451 CrossRef CAS PubMed.
- Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed.
- (a) J. Y. Pfeiffer and A. M. Beauchemin, J. Org. Chem., 2009, 74, 8381–8383 CrossRef CAS PubMed; (b) S. Franco, F. L. Merchán, P. Merino and T. Tejero, Synth. Commun., 1995, 25, 2275–2284 CrossRef CAS.
- (a) C.-K. Pei, Yu. Jiang and M. Shi, Eur. J. Org. Chem., 2012, 4206–4216 CrossRef CAS; (b) M. Mehrdad, L. Faraji and K. Jadidi, Monatsh. Chem., 2011, 142, 917–921 CrossRef CAS; (c) J. Feng, P.-J. Ma, Y.-M. Zeng, Y.-J. Xu and C.-D. Lu, Chem. Commun., 2018, 54, 2882–2885 RSC; (d) L. Maiuolo, P. Merino, V. Algieri, M. Nardi, M. L. D. Gioia, B. Russo, I. Delso, M. A. Tallarida and A. D. Nino, RSC Adv., 2017, 7, 48980–48988 RSC; (e) C.-H. Chen, Q.-Q. Liu, X.-P. Ma, Y. Feng, C. Liang, C.-X. Pan, G.-F. Su and D.-L. Mo, J. Org. Chem., 2017, 82, 6417–6425 CrossRef CAS PubMed; (f) H.-B. Yang and M. Shi, Org. Biomol. Chem., 2012, 10, 8236–8243 RSC.
- For selected examples, see: (a) J. Rong, P. Roselt, J. Plavec and J. Chattopadhyaya, Tetrahedron, 1994, 50, 4921–4936 CrossRef CAS; (b) S. Torrente, B. Noya, M. D. Paredes and A. Alonso, J. Org. Chem., 1997, 62, 6710–6711 CrossRef CAS; (c) T. S. orrente, B. Noya, V. Branchadell and R. Alonso, J. Org. Chem., 2003, 68, 4772–4783 CrossRef PubMed.
- (a) A. W. Johnson, J. Org. Chem., 1963, 28, 252–254 CrossRef; (b) M. A. Abou-Gharbia and M. M. Joullie, J. Org. Chem., 1979, 44, 2961–2966 CrossRef CAS; (c) D.-L. Mo, D. A. Wink and L. L. Anderson, Org. Lett., 2012, 14, 5180–5183 CrossRef CAS PubMed; (d) W. H. Pecak, J. Son, A. J. Burnstine and L. L. Anderson, Org. Lett., 2014, 16, 3440–3443 CrossRef CAS PubMed.
- M. Cordier and A. Archambeau, Org. Lett., 2018, 20, 2265–2268 CrossRef CAS PubMed.
- Y. Luo, C.-H. Chen, J.-Q. Zhang, C. Liang and D.-L. Mo, Synthesis, 2020, 52, 424–432 CrossRef CAS.
- (a) B. B. Snider and H. Lin, J. Am. Chem. Soc., 1999, 121, 7778–7786 CrossRef CAS; (b) B. B. Snider and H. Lin, Org. Lett., 2000, 2, 643–646 CrossRef CAS PubMed; (c) K. Shuji and T. Takashi, Chem. Lett., 1995, 24, 49–50 CrossRef.
- (a) R. Natarajan, P. A. Unnikrishnan, S. Radhamani, J. P. Rappai and S. Prathapan, Tetrahedron Lett., 2016, 57(27–28), 2981–2984 CrossRef CAS; (b) S. Radhamani, R. Natarajan, P. A. Unnikrishnan, S. Prathapan and J. P. Rappai, New J. Chem., 2015, 39, 5580–5588 RSC.
- For selected examples about detailed calculation on the mechanism of [3 + 2] cycloaddition reaction, please see: (a) R. Jasiński, M. Ziółkowska, O. M. Demchuk and A. Maziarka, Cent. Eur. J. Chem., 2014, 12, 586–593 Search PubMed; (b) R. Jasiński, RSC Adv., 2015, 5, 101045–101048 RSC; (c) R. Jasiński, Tetrahedron Lett., 2015, 56, 532–535 CrossRef; (d) R. Jasiński, Comput. Theor. Chem., 2018, 1125, 77–85 CrossRef; (e) R. Jasiński, J. Mol. Graphics Modell., 2020, 94, 107461–107465 CrossRef PubMed; (f) R. Jasiński and E. Dresler, Organics, 2020, 1, 49–69 CrossRef.
- R. Jasiński, K. Mróz and A. Kącka, J. Heterocycl. Chem., 2016, 53, 1424–1429 CrossRef.
- Y. Liu, X. Feng, Y. Liu, H. Lin, Y. Li, Y. Gong, L. Cao and L. Chen, Org. Lett., 2019, 21, 382–386 CrossRef CAS PubMed.
- (a) F. A. Khan, J. Dash, C. Sudheer and R. K. Gupta, Tetrahedron Lett., 2003, 44, 7783–7787 CrossRef CAS; (b) S. M. Kelly and B. H. Lipshutz, Org. Lett., 2014, 16, 98–101 CrossRef CAS PubMed.
- T. Ishikawa, T. Kudoh, J. Yoshida, A. Yasuhara, S. Manabe and S. Saito, Org. Lett., 2002, 4, 1907–1910 CrossRef CAS PubMed.
- B. Prüger, G. E. Hofmeister, C. B. Jacobsen, D. G. Alberg, M. Nielsen and K. A. Jørgensen, Chem.–Eur. J., 2010, 16, 3783–3790 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, X-ray crystal structures of compounds 6 and 7. CCDC 2068718 and 2068719. Copies of NMR spectra. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra06063e |
| This journal is © The Royal Society of Chemistry 2021 |