 Open Access Article
Open Access ArticleAn overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae)†
Hong Thien Van *a,
Tran Dinh Thang
*a,
Tran Dinh Thang a,
Thao Nguyen Luu
a,
Thao Nguyen Luu a and
Van Dat Doan
a and
Van Dat Doan b
b
aInstitute of Biotechnology and Food Technology, Industrial University of Ho Chi Minh City, No. 12 Nguyen Van Bao Street, Ward 4, Go Vap District, Ho Chi Minh City, Vietnam. E-mail: vanhongthien@iuh.edu.vn
bFaculty of Chemical Engineering, Industrial University of Ho Chi Minh City, No. 12 Nguyen Van Bao, Ward 4, Go Vap District, Ho Chi Minh City, Vietnam
First published on 23rd November 2021
Abstract
Alpinia Roxb. is the largest genus of the Zingiberaceae family. A large number of Alpinia species has been used as food and traditional medicines. Alpinia essential oils have been studied for their chemical profiles, in which 1,8-cineole, β-pinene, α-pinene, β-myrcene, camphor, γ-terpinene, p-cymene, geraniol, α-fenchyl acetate, ocimene, methyl cinnamate, and β-caryophyllene have been found to be the major compounds. Essential oils isolated from Alpinia plants have been reported to have antimicrobial, cytotoxic, antioxidant, anti-inflammatory, anti-asthmatic, tyrosinase inhibitory, insecticidal, and larvicidal activities and slimming aromatherapy. In this review, the comprehensive information regarding the volatile components of various Alpinia plants, the bioactivities of Alpinia essential oils and their major compounds are provided.
1. Introduction
Alpinia Roxb. is a large genus belonging to the Zingiberaceae family with around 230 species. This genus is widely distributed in tropical and subtropical regions, including Andamans, Australia, Burma, the Carolines, China, Fiji, India, Indochina, Indonesia, Japan, New Guinea, New Hebrides, New Caledonia, Malaysia, Philippines, Sri Lanka, Solomons, Samoa and Thailand.1,2 The Alpinia species are herbal plants, usually 2–4 meters, but sometimes up to 12 meters in height.1 Many Alpinia species are considered ethnomedicinal and spice plants in several countries such as China, India, Japan and Vietnam.3,4 For instance, the plant parts of Alpinia species are commonly used to cure digestion, gastralgia, vomiting, and enterozoa.5,6 The classes of chemical components generally found in Alpinia plants are terpenes, phenylpropanoids, diarylheptanoids, flavonoids, volatile oils, and lignins.4 In addition, this genus has been reported to possess various bioactivities such as antitumor,7,8 antiulcer,9 antimicrobial,10,11 hypoglycemic,12 antiemetic,13,14 cardioprotection,15 neuroprotection,16,17 and antianxiety activities.18Alpinia plants, which are also known as aromatic herbs, have many parts that contain essential oils such as fruits, seeds, leaves, rhizomes, roots, shoots, stems, pseudostems, inflorescences, flowers, petals and seeds. Alpinia essential oils have been reported to contain oxygenated monoterpenes, monoterpene hydrocarbons and oxygenated sesquiterpenes as the major compounds.19,20 They also exhibit a wide variety of medicinal properties, such as antimicrobial, antioxidant, cytotoxic, anti-inflammatory, larvicidal, anti-asthmatic, tyrosinase inhibitory activity and slimming aromatherapy.21,22 In addition, the compounds in Alpinia essential oils have a wide variety of pharmacological resources.23–25 However, to date, there is no overall review on the chemical profiles and bioactivities of Alpinia essential oils. Therefore, this review aims to provide comprehensive information on the chemical composition and biological activities of the essential oils and their major components isolated from various parts of Alpinia plants.
2. Volatile compounds of Alpinia spp.
In many studies, the gas chromatography-mass spectrometry method has been routinely used for the identification of the volatile compounds of Alpinia spp. Table S1† presents the chemical compositions of the essential oil isolated from different parts of Alpinia plants, including rhizomes, leaves, pseudostems, stems, roots, seeds, fruits, flowers, inflorescences and petals.25,262.1. Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. Synonym: A. speciosa (J.C.Wendl.) K.Schum., A. cristata Griff., A. fimbriata Gagnep., A. fluvitialis Hayata, A. schumanniana Valeton, A. nutans var. longiramosa Gagnep.
A. zerumbet, commonly known as “shell ginger” and the synonym “A. speciosa (J.C.Wendl.) K.Schum.”, is an endemic species to India and also found in the subtropical and tropical areas of South America, Oceania and Asia.27 A. zerumbet is one of the large species in the Zingiberaceae whose height can reach up to 2 or 3 meters.28 This species is known for its traditional medicine to treat inflammation, hypertension, colds, and cardiovascular disorders and as an antispasmodic agent.29,30 Also, several plant parts of A. zerumbet are used as food. Notably, the leaves and rhizomes of this species are commonly used as herbal tea and spices, respectively.31The major components of A. zerumbet essential oils are mainly composed of oxygenated monoterpenes, followed by monoterpene hydrocarbons and oxygenated sesquiterpenes. The leaf is the most common part used in the studies of the essential oil of A. zerumbet. Accordingly, the major components of the leaf essential oils of A. zerumbet collected from Okinawa, Japan at different times of the year included p-cymene, 1,8-cineole, terpinen-4-ol, α-pinene, limonene, sabinene and camphor.32,33 Meanwhile, the leaf oils from Nishieue, Japan had 3,4-dimethyl-3-cyclohexen-1-carboxaldehyde and α-humulene as their main constituents.34 The leaf oil samples from Maja and Toyohara, Japan contained 3,4-dimethyl-3-cyclohexen-1-carboxaldehyde and camphor as their major compounds.34 The leaf oils from Futami, Japan,34 Fiji,35 Rio de Janeiro, Brazil,36,37 Ceara, Brazil,38 and São Cristóvão, Brazil26 were identified as a mixture of terpinen-4-ol and 1,8-cineole, whereas that from Kushi, Japan possessed caryophyllene oxide and 3,4-dimethyl-3-cyclohexen-1-carboxaldehyde as their major constituents.34 Moreover, linalool has been reported as the most constituent in the leaf oils collected from Higashieue and Nago, Japan,34 while that from Rio de Janeiro, Brazil had terpinen-7-al, sabinene hydrate and p-mentha-1,3,8-triene as the major compounds.37
The essential oils of A. zerumbet flowers collected from three different regions of France, including São Cristóvão, Morne Rouge and François have been reported to contain 1,8-cineole and terpinen-4-ol as their major components.39 These compounds were also the main constituents in the rhizome oils from Fiji.35 Meanwhile, terpinen-4-ol and α-terpineol were the major constituents in the petal and rhizome oils collected from São Cristóvão, Brazil.26 Furthermore, the leaf essential oil from A. zerumbet var. variegata from Rio de Janeiro, Brazil was reported to contain 1,8-cineole, β-pinene, and β-caryophyllene as its main constituents.40
Alpinia speciosa (J.C.Wendl.) K.Schum., also known as Alpinia zerumbet, has been also reported for its phytochemical composition of essential oils. Accordingly, the leaf and seed oils of A. speciosa from Chu-Tung, Taiwan were mainly composed of camphor and sabinene, followed by ocimene and 1,8-cineole,41 while terpene-4-ol and 1,8-cineole were the major constituents in the rhizome and leaf oils of this species from Amazonas, Brazil42 and Dehradun, India.43 The major constituents of the essential oil of the A. speciosa leaves from Martinique, France were terpinen-4-ol, 1,8-cineole and p-cymene,24 while β-pinene and 1,8-cineole were the major compounds in the leaf oils from Mexico44 and Egypt.45 Finally, the leaf essential oil of A. speciosa from Japan was found to be rich in (E)-methyl cinnamate, camphor and camphene.46
2.2. Alpinia galanga (L.) Willd. Synonym: Alpinia alba (Retz.) Roscoe, A. bifida Warb., A. carnea Griff., A. pyramidata Blume, A. rheedei Wight, A. viridiflora Griff., A. galanga var. galanga, A. galanga var. pyramidata (Blume) K.Schum.
Alpinia galanga is commonly known as “galangal” in English and “Riêng nếp” in Vietnamese. It is widely cultivated in subtropical and tropical regions, including Sri Lanka, India, Malaysia, Philippines, Indonesia, Thailand, India, China and Vietnam.3,47 Several parts of the galangal plant are broadly used as a condiment for foods and as traditional medicine in some Asian countries.3,48 Notably, in traditional Vietnamese medicine, the dried fruits, rhizomes and seeds of A. galanga are used for treating abdominal aches, dysentery, diarrhea, flatulence, vomiting, coughs, intoxication and sore throat. Rhizomes soaked in salt have been used as anti-thirst agent and remedy for tiredness. People in Thai Nguyen Province, Vietnam use rhizome of this plant soaked in alcohol to treat ringworm.3 In addition, this plant has been reported to possess many pharmacological effects such as antioxidant, antiplatelet, antidiabetic, hypolipidemic, antiprotozoal, antibacterial, antifungal and antiviral activities.49,50Furthermore, the chemical profiles of the essential oils isolated from various parts of the galangal plant have been reported in previous studies. Accordingly, the main constituents of A. galanga essential oils are composed of relatively equal amounts of monoterpene hydrocarbons and oxygenated monoterpenes, followed by sesquiterpene hydrocarbons. The major compounds are 1,8-cineole, α-fenchyl acetate, β-myrcene, β-ocimene, camphor, and limonene. Some galangal rhizome essential oils from several locations of India contained 1,8-cineole as the most abundant compound, followed by α-terpineol, germacrene D,51 α-fenchyl acetate, camphor,52 chavibetol acetate53 (Kerala, India), geranyl acetate, β-caryophyllene54 (New Delhi, India), β-sesquiphellandrene, chavicol, (E)-β-farnesene and eugenol acetate55 (Imphal, India). Furthermore, the leaf and stem essential oils of galangal from Kerala, India mainly contained 1,8-cineole, camphor, β-pinene and (E)-methyl cinnamate, while its root oil was found to be rich in α-fenchyl acetate and 1,8-cineole.52 The essential oil isolated from the galangal oil of the whole plant from Tamil Nadu, India contained 1,8-cineole, β-farnesene, and β-sesquiphellandrene as the main compounds.52
The rhizome and leaf essential oils of A. galanga from Alabama, United States were characterized by the predominance of β-myrcene, β-ocimene and β-pinene.56 The rhizome oil of galangal from Thailand contained a mixture of 1,8-cineole, chavicol, α-bisabolene, 5-t-butyl-hexa-3 and DL-limonene.57,58 Moreover, 1,8-cineole, 4-allylphenyl acetate and α-farnesene were the main constituents of the rhizome oils of galangal from Indonesia,59 while sample from China was found to be rich in 1,8-cineole, β-pinene and α-pinene.60 The chemical compositions of the rhizome and seed essential oils of A. galanga from Malaysia were reported in a study by Jantan et al.61 in which 1,8-cineole, β-bisabolene, (Z,E)-farnesol (leaf), β-bisabolene, (E)-β-farnesene, and (E,E)-farnesyl acetate (seed) were present as the major constituents.61 The rhizome essential oil of galangal from Nawinna, Sri Lanka mainly contained zerumbone, p-cymene and camphene,62 whereas the samples from Indonesia were characterized by the predominance of β-bisabolene and trans-caryophyllene.63 In addition, the whole plant oil of galangal from Phu Tho, Vietnam mainly contained limonene, borneol and geranyllinelol as the major constituents,64 while the rhizome oil from Ha Noi, Vietnam was found to be rich in 1,8-cineole, neral and geranial.65
2.3. Alpinia malaccensis (Burm.f.) Roscoe. Synonym: Alpinia malaccensis var. malaccensis, A. nutans var. sericea Baker
A. malaccensis is commonly known as “Kha Pa” in Thai and “Riêng Malacca” in Vietnamese. It is a medicinal plant native to both Indonesia and Malaysia. In addition, it is widely distributed in other tropical and subtropical such as Bangladesh, Bhutan, India, Myanmar, Thailand and Vietnam.3,66,67 Several plant parts of A. malaccensis were used to treat sores and wounds as well as make the voice clear.66,67 In Vietnam, the rhizome extract of A. malaccensis is used to cure intestinal diseases and scabies.3The main constituents of A. malaccensis essential oils belong to monoterpene hydrocarbons and non-terpenoid, followed by oxygenated monoterpenes. The major constituents in the essential oil of A. malaccensis rhizomes from Thailand mainly contained 1,8-cineole, linalool and fenchyl acetate,58 while the rhizome oils from Kerala, South India possessed α-phellandrene, p-cymene and β-pinene as their major compounds.53 In addition, α-phellandrene was found as the most abundant constituent in the leaf oil of A. malaccensis from Chittagong, Bangladesh and Orissa, India, followed by notable amounts of 1,8-cineole, O-cymene, β-cymene and β-pinene.67,68 Other essential oils extracted from different plant parts of A. malaccensis grown in Vietnam such as leaves, stems and fruits had β-pinene as the most abundant constituent, followed by α-pinene, δ-3-carene and β-phellandrene, whereas (E)-methyl cinnamate, β-pinene and β-phellandrene were the main constituents in the root oil.69 Furthermore, A. malaccensis var. nobilis, a variant of A. malaccensis endemic to Peninsular Malaysia70 has been reported for its essential oil compositions. Accordingly, the essential oils extracted from the fruits, leaves and rhizomes of this variant contained (E)-methyl cinnamate as the highest percentage, followed by notable amounts of 1,8-cineole, p-cymene, β-pinene and β-phellandrene.70 Furthermore, the leaf oil of A. malaccensis var. nobilis from Janda Baik, Malaysia contained methyl cinnamate, α-terpineol and 1,8-cineole as their major compounds.22
2.4. Alpinia officinarum Hance. Synonym: Languas officinarum (Hance) Farw., L. officinarum (Hance) P.H. Hộ
A. officinarum is also known as “Gaoliangjiang” in Chinese and “Riêng” in Vietnamese. It is a rhizomatous perennial herb widely distributed in India, southeast Asia and China.3,71,72 In Chinese pharmacopoeia, dried rhizomes of A. officinarum are used to treat several diseases such as colds, stomachaches, and vomiting, invigorate the circulatory system, and alleviate swelling.73 Juice from boiled A. officinarum rhizomes is used to cure intestinal diseases and treat scabies in Vietnamese traditional medicine.3 Notably, various important bioactive compounds belonging to flavonoids, diarylheptanoids, phenylpropanoids and glycosides extracted from the rhizome essential oils of A. officinarum have been recorded in several previous studies.74,75The major constituents of A. officinarum essential oils are mainly composed of oxygenated monoterpenes, followed by sesquiterpene hydrocarbons. For instance, dozens of chemical components have been identified in the essential oils of A. officinarum rhizomes collected from several regions of China, including Sanming, Guilin, Yulin, Guigang, Qiandongnan, Panzhihua, Xishuangbanna and Bozhou. Notably, the most abundant constituent was 1,8-cineole, followed by notable amounts of γ-cadinene, α-farnesene, α-terpineol, α-bergamotene and globulol.73 In addition, the rhizome oils of A. officinarum collected from Gaozhou, China were found to contain trans-β-farnesene, α-bergamotene and linalool as dominant constituents, while α-farnesene, γ-cadinene and δ-cadinene were the major compounds in the sample from Xuwen, China.73 Moreover, the main compounds of A. officinarum rhizome essential oil from Hainan island were found to be 1,8-cineole, trans-carveol and piperitol,76 while the major constituents of the rhizome oil of this species collected from Imphal, India were 1,8-cineole, α-fenchyl acetate, carotol and β-pinene.55,77 Finally, the essential oil of A. officinarum rhizomes from Thailand possessed α-bisabolene, α-trans-bergamotene and β-sesquiphellandrene as its major constituents,58 whereas 1,8-cineole, exo-2-hydroxy-1,8-cineole acetate and β-caryophyllene were the main constituents in the sample from Vietnam.78
2.5. Alpinia calcarata (Haw.) Roscoe. Synonym: Alpinia alata A.Dietr., A. bracteata Roscoe, A. calcarata var. compacta Gagnep., A. cernua Sims, A. erecta Lodd. ex Steud., A. roscoeana Steud., A. simsii Gasp., A. spicata Roxb.
A. calcarata is known as an economic and medicinal plant. It is a slender and perennial rhizomatous herb, reaching a height of 60–120 cm. This species is widely distributed in tropical and subtropical regions such as India, Myanmar, Indonesia, Thailand and Sri Lanka.79 In traditional medicine, this species is used to cure rheumatism, diabetes, and fever and stomach aches.79 Notably, its rhizomes have been widely used to treat several diseases, such as bronchitis, throat inflammation, colds and asthma.80 Furthermore, this species was also reported to have some pharmacological properties such as anti-emetic, antibacterial, antispasmodic, antifungal, antiulcer, antioxidant and anti-inflammatory activities.81The major constituents of A. calcarata are mainly composed of oxygenated monoterpenes, followed by monoterpene hydrocarbons, oxygenated sesquiterpenes and non-terpenoids. Accordingly, the rhizome and leaf oils of A. calcarata collected from Sri Lanka contained 1,8-cineole and α-terpineol as their main compounds,23 while 1,8-cineole and β-fenchyl acetate were found as the major constituents in the rhizome oils from Kerala, India.51 The leaf oil of A. calcarata from Tamil Nadu, India was mainly composed of 1,8-cineole, camphor and α-myrcene,82 while the whole plant oil of this species grown in Karnataka, India was composed of α-fenchyl acetate, 1,8-cineole and (E)-methyl cinnamate.82 Rout et al.83 reported the chemical constituents of the essential oils of A. calcarata collected from Bhubaneswar and Bangalore, India. Accordingly, the root oils from these two locations contained α-fenchyl acetate, 1,8-cineole and camphene as their major constituents, while β-pinene, 1,8-cineole, camphor and camphene were the major compounds in the leaf oils. The rhizome oil from Bhubaneswar region was found to be rich in α-fenchyl acetate, 1,8-cineole, and camphene, whereas the sample from Bangalore mainly contained geraniol, 1,8-cineole and α-fenchyl acetate.83
The leaf and rhizome essential oils of A. calcarata grown in Pantnagar, India had 1,8-cineole as their most abundant constituent, followed by camphor, β-pinene (leaf), α-fenchyl acetate, and camphene (rhizome).84 The rhizome and root essential oils of this plant from south India were dominated by α-fenchyl acetate and 1,8-cineole, followed by camphene (root) and α-terpineol (rhizome), while 1,8-cineole, α-fenchyl acetate and camphor were the major compounds in the shoot oil of the same species.81 In addition, the major components of the rhizome, leaf and root essential oils of A. calcarata from Veyangoda, Sri Lanka were 1,8-cineole, α-fenchyl acetate, β-pinene, camphene and camphor.85
2.6. Alpinia mutica Roxb. Synonym: Alpinia korthalsii K.Schum., A. laxiflora Gagnep., Catimbium muticum (Roxb.) Holttum, Languas korthalsii (K.Schum.) Merr. L. laxiflora (Gagnep.) Merr., L. mutica (Roxb.) Merr., Renealmia mutica (Roxb.) Salisb.
A. mutica is a herbaceous perennial species, which is endemic to the southern regions of Malaysia and also found in Borneo, Singapore, India and Vietnam.3,86 Several plant parts of this species are used to treat flatulence and diarrhea by local people in southern India.87 In Malaysia, A. mutica is cultivated as ornamental trees, while its rhizomes are used as a stomachic.88 Vietnamese people use crushed rhizomes mixed with water to cure stomachaches and abdominalaches.3 Furthermore, A. mutica has been reported to possess some biological activities. For examples, its crude extract had a cytotoxic effect against human cancer cells, including KB, MCF7 and CaSki cells89 and CEMss (human T4 lymphoblastoid) cancer cells.90 The hexane extract of A. mutica showed antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa,91 while its dichloromethane extract presented an antibacterial effect against Bacillus subtilis and Staphylococcus aureus.90 Moreover, the ethyl acetate fractions isolated from A. mutica rhizome have been reported to exhibit antioxidant activity.91,92The chemical composition of the essential oils from A. mutica is characterized by the predominance of monoterpene hydrocarbons and oxygenated monoterpenes, followed by sesquiterpene hydrocarbons, and oxygenated sesquiterpenes. The major volatile components of A. mutica unripe and ripe fruits from Peninsular, Malaysia included camphor, camphene and β-pinene.93 Furthermore, the main components of the essential oils of the young and mature fruits of A. mutica from Johor, Malaysia were (E,E)-farnesol and α-farnesene, followed by α-humulene and 1,8-cineole,94 while the rhizome oil from the same location possessed camphor, 1,8-cineole and borneol as the major compounds.95 The rhizome and fruit essential oils of this plant from Kerala, India were characterized by large quantities of β-pinene, camphor and 1,8-cineole.96 In addition, the diversity of the chemical composition in the essential oils from different plant parts of A. mutica collected from Phong Nha-Ke Ban National Park, Vietnam has been reported.86 Accordingly, the quantitatively significant constituents of the leaf and root essential oils were β-pinene, 1,8-cineole and α-pinene, while the other plant parts were found to be rich in 1,8-cineole, β-pinene, and α-pinene (pseudostem) and β-caryophyllene, β-cadinol, and camphor (fruit).86
2.7. Alpinia nigra (Gaertn.) Burtt. Synonym: Alpinia allughas (Retz.) Roscoe, Amomum bifidum Stokes, A. nigrum (Gaertn.) Raeusch., A. taraca Horan., Hellenia allughas (Retz.) Willd., Heritiera allughas Retz., Languas allughas (Retz.) Burkill, L. aquatica J. König, Zingiber nigrum Gaertn.
A. nigra is a perennial and aromatic plant widely distributed in Bhutan, China, India, Sri Lanka and Thailand. This species is used as a spice and medicinal agent. In Thai folk remedies, A. nigra was used to cure stomachicum, gastric diseases, bronchitis, jaundice, dyspepsia, gastric ulcers and insect bites and as antibacterial and antifungal agents.97 In addition, the aerial parts of A. nigra were used in curry as a flavour and anthelmintics by the native tribes of Tripura, northeast India.98 Studies showed that the leaf extract of A. nigra has analgesic, antibacterial and cytotoxic activities,99,100 while its rhizome extract possesses anti-inflammatory and analgesic effects.101The chemical profiles of the essential oils isolated from A. nigra have been reported in previous studies. For instance, the essential oils obtained from four plant parts such as the leaves, rhizomes, flowers and seeds of A. nigra grown in Guwahati, India contained β-caryophyllene, β-pinene and α-humulene as their major compounds.102 The rhizome and leaf essential oils from Kalimpong, India were characterized by the presence of β-pinene as the most abundant compound, followed by myrtenol, α-humulene and α-farnesene,103 whereas β-pinene, α-caryophyllene and α-farnesene were the major constituents in the leaf oil from West Bengal, India.104 In addition, β-pinene was the most abundant component in the leaf and rhizome essential oils of A. allughas (a synonym of Alpinia nigra) from Terai, India, followed by α-pinene, 7-epi-α-eudesmol (rhizome), 1,8-cineole, α-humulene (leaf).105
2.8. Alpinia hainanensis K.Schum. Synonym: Alpinia katsumadai Hayata, A. henryi K.Schum., A. henryi var. densihispida H.Dong & G.J.Xu, A. kainantensis Masam., Languas hainanensis (K.Schum.) Merr., L. henryi (K.Schum.) Merr., L. katsumadai (Hayata) Merr.
Alpinia hainanensis K.Schum. is commonly known as “Riêng Hải Nam” in Vietnamese and the synonym “Alpinia katsumadai Hayata”. This species is distributed in several locations in China (Guangdong, Guangxi, Hainan) and northern Vietnam.3 A. hainanensis is extensively used in traditional Chinese medicine to treat emesis and gastric disorders.25 Also, Vietnamese people use A. hainanensis to cure abdominal aches and bloating diseases by drinking juice extract from its fruits.3 The extracts of A. katsumadai and its fractions have been reported to possess in vitro antiviral effects against influenza virus type A, especially human A/PR/8/34 (H1N1) and avian A/Chicken/Korea/MS96/96 (H9N2).106 Moreover, the antioxidant107 and antibacterial108 activities of A. katsumadai extracts have been also reported.107The chemical composition of the essential oils isolated from A. hainanensis were reported in previous studies. For example, the leaf and flower essential oils of A. hainanensis collected from Hainan island, China were found to be rich in ocimene and β-pinene, followed by octadecenoic acid and terpinene.109 Furthermore, the components of the oils from A. katsumadai, a synonym of A. hainanensis, have been recorded. Accordingly, the leaf oil of A. katsumadai from Hainan island, China contained p-menth-1-en-ol, terpinen and 4-carene as its major compounds, while p-menth-1-en-ol, 1,8-cineole and terpinen were the prominent constituents in its flower oil.109 The seed oil of A. katsumadai from Guangxi, China contained methyl cinnamate, cis-4-decen-1-ol and octahydro-cis-2H-inden-2-one as its major constituents.25 Furthermore, the essential oil of A. katsumadai from Vietnam was mainly composed of geraniol, fenchone and 1,8-cineole. The major constituents of the stem essential oil were fenchone, geraniol and 1,8-cineole, while the seed oil was dominated by geraniol, linalool and decanol.110
2.9. Alpinia conchigera Griff. Synonym: Alpinia laosensis Gagnep., A. humilis Teijsm. & Binn., A. sumatrana (Miq.) K.Schum., Languas conchigera (Griff.) Burkill, L. sumatrana (Miq.) Merr., Strobidia conchigera (Griff.) Kuntze, S. oligosperma Kuntze, S. sumatrana Miq.
A. conchigera is commonly known as “Lengkuas ranting” in Malay, “Riêng rừng” in Vietnamese and the synonym “A. laosensis Gagnep.”3 This species is a native plant in Malaysia and also found in Bangladesh and Vietnam.67,111 Several plant parts of this species have been used as a food flavour and Malaysian traditional remedies for rheumatism, arthritis, stimulation, diaphoretic and regulatory in uterine hemorrhage.112 In Vietnam, boiled juice from its leaf and rhizome is traditionally used as a folk remedy to cure spleen and abdominal pain.3 In addition, A. conchigera has been reported to possess anti-inflammatory,113 antifungal and antibacterial67 activities. Previous studies showed that A. conchigera extracts contained various bioactive compounds such as β-sitosterol, 1′-acetoxychavicol acetate, 1′-acetoxyeugenol acetate,114 chavicol, chavicol acetate, 1-hydroxychavicol acetate, 4-acetoxycinnamyl alcohol and 4-acetoxycinnamyl acetate.114There have been several studies investigating the chemical composition of the essential oils isolated from A. conchigera. For example, the rhizome oil of A. conchigera from Penang, Malaysia had β-bisabolene, 1,8-cineole and β-caryophyllene as its main compounds,114 while the rhizome oil collected from Pagoh, Malaysia was characterized by the predominance of β-sesquiphellandrene, β-bisabolene and 1,8-cineole.115 The leaf essential oil of A. conchigera from Chittagong, Bangladesh was obviously dominated by 1,8-cineole, chavicol and β-pinene.67 In addition, the phytochemical profiles of the essential oil of Alpinia laosensis Gagnep., a synonym of A. conchigera, have been reported. Accordingly, the essential oil isolated from A. laosensis rhizomes grown in northern Vietnam was mainly composed of 1,8-cineole, caryophyllene oxide and methyl eugenol.111
2.10. Alpinia latilabris Ridl. Synonym: Alpinia hookeriana Valeton, A. sericea Ridl., Catimbium latilabre (Ridl.) Holttum, Languas hookeriana (Valeton) Merr., L. sericea (Ridl.) Merr.
A. latilabris is commonly known as “Ry” in Vietnamese and the synonym “Catimbium latilabre (Ridl.) Holttum”. It is a herbaceous plant, reaching a height of 3 meters and usually found in lowland forests in Borneo, Malaya, Myanmar and Vietnam.3,116,117 In Vietnam, the rhizome extract of A. latilabris is traditionally used as a folk remedy to cure gastrointestinal diseases.3The components of the essential oils of three plant parts of A. latilabris such as rhizomes, unripe and ripe fruits collected from Peninsular, Malaysia have been identified. Accordingly, 1,8-cineole, β-pinene and α-pinene were the dominant compounds in the unripe fruit and ripe fruit oils,93 while the oil obtained from its rhizome was dominated by camphor, 1,8-cineole and β-pinene.114 The leaf essential oil of this plant from Janda Baik, Malaysia possessed phytol, carvone and β-sesquiphellandrene as its major compounds.22 In addition, the leaf, stem and root essential oils of A. latilabris grown in Pu Mat National Park, Vietnam were characterized by the predominance of α-cadinol, γ-terpinen and β-pinen.118 Catimbium latilabre (Ridl.) Holttum, a synonym of A. latilabris, collected from Hue, Vietnam has been also investigated for the components of its essential oils. The rhizome oil of C. latilabre was mainly composed of 1,8-cineole, linalool and carotol, while its root oil contained citronellol, 1,8-cineole and camphene as is major compounds.119 The major constituents in C. latilabre seed oil were β-caryophyllene, camphor and caryophyllene oxide, whereas β-pinene, 1,8-cineole and α-pinene were the main constituents in the fruit skin essential oil.120
2.11. Alpinia pinnanensis T.L.Wu & S.J.Chen
A. pinnanensis is also known as “Riêng pina” in Vietnamese. It is native to both China and northern Vietnam. This plant can reach up 1.5 meters tall and usually grows along streams, wet slopes and under forest canopies.1,3 In traditional Vietnamese medicine, the rhizome and tuber extracts of A. pinnanensis are used to treat coughs, fever, abdominal aches, flatulence and stomachaches.3 Furthermore, the extracts of this plant exhibited antimicrobial and cytotoxic effects.121 Also, some bioactive compounds, including β-sitosterol, alpinetin, chalcones, stigmasterol, flavanones, diarylheptanoids, alpinnanins A–C (1–3), (3S,5S)-trans-3,5-dihydroxy-1,7-diphenyl-1-heptene, 2′,4′-dihydroxy-6′-methoxychalcone, naringenin 5-O-methyl ether, 4′,6′-dimethylchalconaringenin and β-sitosterol 3-O-β-D-glucopyranoside were isolated from A. pinnanensis rhizomes.121The chemical compositions of the essential oils extracted from A. pinnanensis grown in Vu Quang National Park, Vietnam have been reported.122 Accordingly, the leaf oil contained 1,8-cineole, 4-phenyl-2-butanol and α-phellandrene as its major components. The rhizome oil was mainly characterized by the presence of 1,8-cineole, β-elemene and α-gurjunene. Its fruit essential oil was comprised mainly of α-cadinol, β-caryophyllene and (E,E)-farnesol, whereas (E,E)-farnesol, α-gurjunene and camphene were found to be the main compounds in its root bark oil.122
2.12. Alpinia roxburghii Sweet. Synonym: Alpinia blepharocalyx K.Schum., A. blepharocalyx var. glabrior (Hand.-Mazz.) T.L.Wu, A. bracteata Roxb., Languas blepharocalyx (K.Schum.) Hand.-Mazz., Languas blepharocalyx var. glabrior Hand.-Mazz.
Alpinia roxburghii is commonly known as “Alpinia blepharocalyx K.Schum.” This species usually grows on wet slopes and is native to both China and Vietnam.3 A. blepharocalyx is a pseudostem, reaching a height of up to 3 meters and used as a flavor and fragrance.123 In Chinese traditional medicine, A. blepharocalyx rhizomes are used to treat abdominal pain and abdominal distension.123 The juice obtained from the fruits, rhizomes and seeds of this plant has been used as Vietnamese traditional remedies to cure stomachache digestive disorders and abdominal aches due to the cold.3 Moreover, studies demonstrated that this plant contains diarylheptanoids and phenolic compounds.124,125The chemical profiles of the essential oils isolated from A. blepharocalyx have been investigated. Accordingly, the rhizome essential oils of A. blepharocalyx collected from Xishuangbanna, China were mainly characterized by the presence of camphor, sabinene and α-pinene,123 while the rhizome oil of this plant grown in Pu Hoat Natural Reserve, Vietnam contained δ-cadinene, τ-muurolol and α-cadinol as its major components.20 Also, the principal compounds in the pseudostem oil from Pu Hoat Natural Reserve were (E,E)-α-farnesene, β-pinene, τ-muurolol and α-cadinol, whereas δ-cadinene, β-pinene and γ-cadinene constituted the bulk of the leaf oil.20
2.13. Alpinia purpurata (Vieill.) K.Schum. Synonym: Alpinia grandis K.Schum., A. purpurata var. albobracteata K.Schum., A. purpurata var. anomala Gagnep., A. purpurata var. grandis (K.Schum.) K.Schum.
A. purpurata is commonly known as an ornamental plant. This species is native to the Pacific islands.37,126 There is little information about the medicinal use of A. purpurata, only that this species was used in Venezuelan traditional medicine to cure coughs.127 The major constituents of A. purpurata essential oils were mainly characterized by the predominance of monoterpene hydrocarbons, followed by oxygenated monoterpenes and sesquiterpene hydrocarbons. The inflorescence essential oils of the red variant and pink variant of A. purpurata collected from Paulista, Brazil contained β-caryophyllene and β-pinene, respectively as the most dominant constituents, followed by notable amounts of linalool, α-pinene, bornyl acetate and 7-epi-selinene.128 Similarly, β-pinene was found as the major constituent in the essential oil from the leaf, rhizome and flower oils of the red variant and pink variant of A. purpurata grown in Fiji.35 Meanwhile, the leaf oil of A. purpurata from Rio de Janeiro, Brazil had β-pinene, α-pinene and trans-β-guaiene as its major compounds.372.14. Alpinia breviligulata (Gagnep.) Gagnep. Synonym: Alpinia calcarata var. breviligulata Gagnep., Catimbium breviligulatum (Gagnep.) P.H.Hô
A. breviligulata is commonly known as “Riêng lưỡi ngăn” in Vietnamese. This species usually grows along the streams in secondary forests. A. breviligulata is native to both China and Vietnam.3 In traditional Vietnamese medicine, the rhizome of A. breviligulata is used to treat abdominal aches by drinking water with it or topically on the abdomen.3 Several studies also reported the compositions of the essential oils of A. breviligulata collected from Hue, Vietnam. Accordingly, the seed oil of A. breviligulata contained (E,E)-farnesol, geranyl acetate and α-humulene as its major compounds.19 The fruit peel oil was mainly characterized by the presence of β-pinene, α-terpineol and caryophyllene oxide.19 The flower oil was made of β-pinene, β-caryophyllene and α-pinene,129 while caryophyllene oxide, α-pinene and α-copaene were found to be the main compounds in its leaf oil.1302.15. Other Alpinia species
Other Alpinia species have been reported much less due to their limited distribution and commercial interest. The rhizome oil of A. aquatica (Retz.) Roscoe from Kuching, Malaysia had β-pinene, α-humulene and aromadendrene as its dominant compounds, while its leaf oil contained notable amounts of germacrene D, β-pinene and sabinene. Also, α-humulene, germacrene D and β-caryophyllene were the main constituents in the pseudostem oil of this species.131 The leaf oil of A. murdochii Ridl. from Pahang, Malaysia was composed of β-pinene, sabinene and terpinene-4-ol, while the rhizome oil of this plant possessed γ-selinene, (E,E)-farnesyl acetate and terpinen 4-ol as its main components.132 The fruit and rhizome essential oils obtained from A. rafflesiana Wall. ex Baker grown in Selangor Darul Ehsan, Malaysia contained tetracosane as the most abundant constituent, followed by τ-cadinol, α-terpineol and (2E,6E)-farnesol. The leaf oil of this species was found to be rich in β-caryophyllene, caryophyllene oxide and (2E,6E)-farnesol, while the pseudostem oil was made of 1,8-cineole, β-myrcene and α-terpineol.133 In addition, the major volatile components of A. scabra (Blume) Náves leaves collected from Pahang, Malaysia contained β-pinene, α-pinene and borneol as their major constituents, whereas the rhizome oil was obviously dominated by γ-selinene, α-selinene and α-terpineol.132A. polyantha D. Fang, an Alpinia species collected from Nghe An, Vietnam, has been reported to possess a diverse chemical composition in the essential oils from its different plant parts. The leaf oil contained camphor, α-pinene and β-agarofuran as its main constituents. The stem oil was found to be rich in α-pinene, β-cubebene and β-agarofuran. The major compounds of the root oil were found to be β-cubebene, fenchyl acetate and β-maaliene, whereas the fruit oil was characterized by the predominance of δ-cadinene, β-caryophyllene and β-pinene.134 The chemical constituents of A. macroura K.Schum. essential oils, another Alpinia species from Nghe An, Vietnam, was also investigated. Accordingly, γ-terpinene, β-pinene and 1,8-cineole were found in its root and stem oils as their major compounds. The leaf oil of this species was mainly composed of 1,8-cineole, γ-terpinene and β-pinene. Meanwhile, β-caryophyllene was the most abundant constituent in the fruit and flower oils of A. macroura, followed by β-pinene, 1,8-cineole and sabinene.135 In addition, the essential oil obtained from the fruits, leaves, stems and roots of A. menghaiensis S.Q.Tong & Y.M.Xia grown in Nghe An, Vietnam was dominated by β-pinene and α-pinene,136,137 whereas γ-terpinene, 1,8-cineole and α-terpinene were the major compounds in the leaf, stem and root essential oils of A. nantoensis F.Y.Lu & Y.W.Kuo from Pu Mat National Park, Vietnam.138 Meanwhile, the leaf and rhizome oils of A. nantoensis collected from Nantou County, Taiwan were dominated by camphor, camphene and β-pinene.139
The leaf, pseudostem and stem essential oils of A. strobiliformis T.L.Wu & S.J.Chen collected from Pu Hoat Natural Reserve, Vietnam consisted mainly of 1,8-cineole, γ-terpinene and β-pinene.20 The leaf and stem oils of A. maclurei Merr. from Bach Ma National Park, Vietnam were dominated by β-pinene and α-pinene, whereas its root oil contained β-pinene, β-phellandrene and fenchyl acetate.137 The diversity of the chemical compositions in the essential oils from different plant parts of A. chinensis (Retz.) Roscoe collected from Hue, Vietnam has been reported. Accordingly, the leaf oils of the species mainly comprised β-bisabolene, (E,E)-farnesylacetate and β-pinene. The root oil was found to be rich in caryophyllene oxide, γ-selinene and α-humulene, while (E,E)-α-farnesene, α-humulene and β-bisabolene constituted the bulk of the flower oil.140 In addition, β-pinene was the most abundant compound in the leaf essential oils of A. tonkinensis Gagnep. and A. globosa (Lour.) Horan. collected from Ben En National Park, Vietnam, followed by (E)-β-ocimene and γ-terpinene (A. tonkinensis), α-gurjunene and (Z)-13-docosenamide (A. globosa).141 Finally, the rhizome oil of A. henryi K.Schum. from Vinh Phuc, Vietnam contained 1,8-cineole, α-terpineol and β-pinene as its major compounds.142
The major volatile components of A. kwangsiensis T.L.Wu & S.J.Chen rhizome from Xishuangbanna, China included camphor, 1,8-cineole and β-pinene as their major constituents.143 The leaf oil of A. vittata W.Bull from Rio de Janeiro, Brazil was mainly composed of β-pinene, epi-cubebol and α-pinene.36 The essential oil from the aerial parts of A. nutans (L.) Roscoe from Uttarakhand, India possessed sabinene, 1,8-cineole and terpinen-4-ol as its major compounds, while the flower oil was mainly composed of terpinen-4-ol, γ-terpinene and sabinene.144 Moreover, β-caryophyllene was found to be the most abundant constituent in the leaf and rhizome oils of A. smithiae M.Sabu & Mangaly from Kerala, India, followed by sabinene, β-myrcene and β-pinene.145 Finally, the leaf oil of A. carinata Valeton collected from Gorakhpur, India consisted mainly of β-pinene, terpinen-4-ol and p-cymene.146
According to Table S1† with the major chemical patterns of Alpinia essential oils, the oils can be classified into the following groups.
(1) Oils dominated by monoterpenes and their oxygenated derivatives;
(2) Oil mainly with monoterpenes and sesquiterpenes; and
(3) Oils with an ester of carboxylic acid as the main compound.
It can be seen that, the oils dominated by monoterpenes and their oxygenated derivatives are from A. allughas, A. aquatica, A. breviligulata, A. calcarata, A. chinensis, A. conchigera, A. galanga, A. hainanensis, A. katsumadai, A. laosensis, A. malaccensis, A. macroura, A. maclurei, A. menghaiensis, A. murdochii, A. mutica, A. nantoensis, A. nutans, A. pinnanensis, A. polyantha, A. purpurata, A. rafflesiana, A. scabra, A. speciosa, A. tonkinensis, A. strobiliformis, A. vittata and A. zerumbet. The oils rich in both monoterpenes and sesquiterpenes are from A. aquatica, A. blepharocalyx, A. globosa, A. nigra, A. officinarum, A. smithiae and A. zerumbet var. variegata. The oils consisting of carboxylic acid as the main compound are from A. malaccensis var. nobilis. The chemical patterns of the Alpinia oils including their chemical composition and the number of major compounds vary significantly depending on the part of the plant and its habitat (Fig. 1).
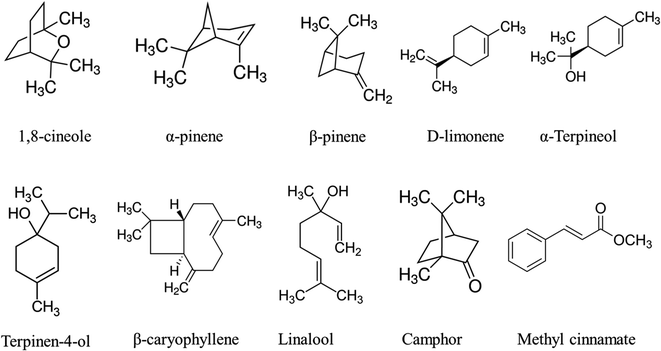 | ||
| Fig. 1 Major chemical compounds isolated from the essential oils of Alpinia spp. exhibiting biological activities. | ||
3. Biological activities of Alpinia oils
Table S2† presents a summary of the biological activities of the various Alpinia essential oils. Generally, the Alpinia oils possess some important bioactivities such as antifungal, antibacterial, cytotoxic, anti-inflammatory, antioxidant, insecticidal, and larvicidal activities and slimming aromatherapy. This information gives evidence for the future applications of the species from the genus Alpinia in medicine and other relevant fields.3.1. Antimicrobial activity
The essential oils isolated from A. galanga rhizomes grown in Samut Sakhon, Thailand could inhibit the growth of Escherichia coli, Staphylococcus aureus and Salmonella typhimurium with MIC values of 0.78, 1.56 and 0.78 μL mL−1, respectively.57 The rhizome oil of A. galanga from Central Java, Indonesia exhibited an antibacterial effect against Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Salmonella typhimurium and Vibrio cholera with MIC values ranging from 62.5 to 1000 μg mL−1.59 The essential oils extracted from A. galanga grown in Phu Tho, Vietnam had an inhibitory effect on Salmonella typhimurium, Bacillus cereus, Staphylococcus aureus and Escherichia coli.64 In addition, the essential oil from the leaf of A. malaccensis was reported to have antimicrobial activity against oral bacteria and fungi, including Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger with MIC values of 1.95, 7.81, 5.5, 6.7 μL mL−1, respectively.68 The rhizome essential oil of A. galanga from Vietnam showed antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Streptococcus faecalis, Escherichia coli, Proteus vulgaris, Salmonella enteritidis and Saccharomyces cerevisiae with MIC values ranging from 2.5 to 20 μL mL−1.65The essential oils from the unripe fruit of A. mutica showed strong antimicrobial activities against Staphylococcus aureus, Escherichia coli, Trichophyton mentagrophytes and Trichophyton rubrum, followed by Bacillus subtilis, Pseudomonas aeruginosa, Candida glabrata and Microsporum canis.93 Meanwhile, the ripe fruit oil of A. mutica had a potent antimicrobial effect against S. aureus, Escherichia coli, M. canis, T. mentagrophytes and T. rubrum, followed by B. subtilis, P. aeruginosa and Candida glabrata.93 Furthermore, the rhizome and fruit essential oils of A. mutica from southern India also exhibited moderate antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermis, Staphylococcus simulans, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Vibrio cholerae, Klebsiella pneumonia and Salmonella typhi.96 Similarly, the unripe and ripe fruit oils extracted from another Alpinia species grown in Peninsular Malaysia, A. latilabris, showed strong antimicrobial effects against Staphylococcus aureus, Trichophyton mentagrophytes and Candida glabrata, followed by Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Microsporum canis, Trichophyton mentagrophytes and Trichophyton rubrum.93 In addition, the leaf oil of A. latilabris from Pahang, Malaysia possessed potent antibacterial activity against Klebsiella pneumonia and Staphylococcus aureus, followed by Bacillus subtilis, Acinetobacter baumannii, Pseudomonas aeruginosa and Salmonella typhii.22
Additionally, the essential oil from the flower of A. zerumbet from Martinique island, France showed potent antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus, Escherichia coli, Listeria innocua and Salmonella arizonae. It also showed strong antifungal effects against Aspergillus niger and Candida albicans.39 Meanwhile, the leaf oil of A. zerumbet grown in Rio de Janeiro, Brazil presented interesting antimicrobial activity against Escherichia coli, Staphylococcus aureus, S. epidermidis, Candida albicans and Cryptococcus neoformans.147 In addition, the leaf oil of A. zerumbet collected from Ceara, Brazil was active against many bacterial strains, including Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Aeromonas caviae, Klebsiella pneumonia, Shigella flexneri, Vibrio cholerae and Listeria monocytogenes with MIC values of 32, 512, 128, 1024, 256, 256, 512, 1024 and 256 μL mL−1.38
The essential oil from the rhizomes of A. officinarum collected from ten different habitats in China, including Sanming, Guilin, Yulin, Guigang, Qiandongnan, Panzhihua, Xishuangbanna, Gaozhou, Xuwen and Bozhou possessed antimicrobial activity. Accordingly, all ten samples showed potent antibacterial activity against two Gram positive bacteria, Staphylococcus aureus and Bacillus subtilis, while the oils from Yulin and Xishuangbanna could inhibit Escherichia coli, and only the oil sample from Yulin was found to be effective against Pseudomonas aeruginosa. Furthermore, four oil samples also showed strong antifungal effects against Candida albicans.73 The inflorescence oil of A. purpurata also showed strong antibacterial activity against Gram-positive and Gram-negative bacteria such as Staphylococcus aureus and S. epidermis with MIC values of 10 μg mL−1, while this sample presented weak inhibition against Pseudomonas aeruginosa, Escherichia coli, Salmonella sp., Shigella sp., Klebsiella sp. and Proteus sp. with MIC values of 1000 μg mL−1.128 The leaf, pseudostem, rhizome and fruit oils of A. rafflesiana grown in Selangor, Malaysia have been reported to possess antimicrobial and antifungal activities by using the MIC test.133 Accordingly, the leaf oil showed strong antimicrobial activity against Staphylococcus aureus and Escherichia coli with MIC values of 7.81 and 15.63 μg mL−1, respectively, and weak effects against Pseudomonas putida and C. albicans with MIC values of 125 μg mL−1. The pseudostem oil possessed potent antimicrobial activities against S. aureus and Bacillus subtilis (MIC = 31.25 μg mL−1), moderate effects against E. coli (MIC = 62.5 μg mL−1) and low activities against Pseudomonas aeruginosa, P. putida, Candida albicans and Aspergillus niger (MICs = 125 μg mL−1). The fruit oil was moderately active against S. aureus (MIC = 31.25 μg mL−1), E. coli, B. subtilis, P. aeruginosa, P. putida, C. albicans and A. niger (MICs = 125 μg mL−1). Finally, the rhizome oil showed weak antifungal activity against A. niger (MIC = 125 μg mL−1), while it was inactive against all the tested bacteria and C. albicans.133
The seed and leaf oils from A. speciosa grown in Taiwan exhibited strong broad-spectrum antimicrobial activity against Malassezia pachydermatis, Candida albicans, Staphylococcus aureus and Escherichia coli.41 The rhizome oil of A. speciosa collected from Dehradun, India exhibited strong antibacterial activity against Bacillus subtilis and Salmonella typhimurium, followed by Micrococcus luteus, Streptococcus mutans, Staphylococcus aureus and Pseudomonas aeruginosa.43 The leaf and rhizome essential oils of A. scabra were also demonstrated to exhibit potent antimicrobial effects against Staphylococcus aureus ATCC 29213, S. aureus ATCC 33591, S. aureus ATCC 700699, S. aureus VISA24, S. aureus VRSA156, Candida albicans, C. glabrata, Microsporum canis and Trichophyton rubrum.132 Moreover, the leaf oil of A. speciosa grown in Martinique, France showed potent antibacterial effects against Staphylococcus aureus, Escherichia coli and Mycobacterium smegmatis and moderate effects against Streptococcus faecalis and Pseudomonas aeruginosa, whereas this sample presented strong antifungal activity against Candida albicans, Aspergillus niger, Cylindrocarpon mali, SotIyfis cinerea, Stereum purpureum and Sclerotinia sclerotiorum.24
The oils from the leaf and rhizome of A. murdochii showed antifungal activity against Candida albicans, C. glabrata, Microsporum canis and Trichophyton rubrum with MIC values of 2.5 mg mL−1, while MIC values of 2.5 and 0.63 mg mL−1, 2.5 and 2.5 mg mL−1, 2.5 and 0.63 mg mL−1, 1.25 and 0.08 mg mL−1, 0.31 and 0.04 mg mL−1 were recorded for the leaf and rhizome oils towards five S. aureus strains (ATCC 29213, ATCC 33591, ATCC 700699, VISA24, VRSA156), respectively.132 Furthermore, the rhizome, leaf, seed and flower oils of A. nigra from India showed antibacterial activity against 3 Gram positive bacteria (Staphylococcus aureus, Bacillus cereus and Listeria monocytogenes) and 4 Gram negative bacteria (Escherichia coli, Salmonella paratyphi, E. coli enterotoxic and Yersinia enterocolitica) with MIC and MBC values ranging from 3.12 to 6.25 μL mL−1.102 The rhizome oil of A. nigra from India also possessed potent antimicrobial effects against Pseudomonas aeruginosa, followed by Escherichia coli, Staphylococcus aureus, Candida albicans and Aspergillus niger.104
The aerial part and flower essential oils of A. nutans from India showed strong antibacterial activities against Staphylococcus aureus, Pasteurella multocida, Salmonella enterica enterica, Shigella flexneri and Escherichia coli.144 Moreover, the leaf oil of A. malaccensis var. nobilis from Malaysia showed strong inhibition of the growth of Cryptococcus neoformans and Candida tropicalis with IC50 values of 1.97 and 1.75 mg mL−1, respectively. Meanwhile, this sample possessed moderate antibacterial effects against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and Salmonella typhii.22 Finally, the leaf essential oils of two Alpinia species from Vietnam, including A. globosa and A. tonkinensis, had an inhibitory effect on Escherichia coli, Staphylococcus aureus subsp. aureus, Fusarium oxysporum and Saccharomyces cerevisiae.141
The antimicrobial activities of the essential oils obtained from different parts of Alpinia plants are established by the following major compounds, including α-pinene, 1,8-cineole, β-pinene, terpinen-4-ol, β-caryophyllene, linalool, D-limonene, β-myrcene, p-cymene and camphor. Among them, α-pinene, 1,8-cineole and linalool possess strong antimicrobial activities. It has been reported that α-pinene isolated from the leaf oil of A. speciosa grown in Martinique, France showed a strong antimicrobial effect against Escherichia coli (MIC = 2 mg mL−1), Candida albicans, and Sclerotinia sclerotiorum (MICs = 0.25-0.5 mg mL−1), followed by Mycobacterium smegmatis, Cylindrocarpon mali, Staphylococcus aureus, Streptococcus faecalis, Pseudomonas aeruginosa, Aspergillus niger and Stereum purpureum with MIC values of more than 4 mg mL−1.24 Furthermore, α-pinene showed antibacterial activities against Staphylococcus aureus (MIC = 20 μL mL−1), S. epidermidis (MIC = 5 μL mL−1), Streptococcus pyogenes (MIC = 10 μL mL−1) and S. pneumonia (MIC = 5 μL mL−1).148 Additionally, α-pinene had antifungal activities against Candida albicans, Cryptococcus neoformans and Rhizopus oryzae with MIC values of 3.13, 117 and 390 μg mL−1, respectively.149 1,8-Cineole isolated from the leaf oil of A. speciosa grown in Martinique, France showed potent antimicrobial activity against Mycobacterium smegmatis (MIC = 2–4 mg mL−1) and Cylindrocarpon mali (MIC = 0.5–1 mg mL−1), followed by Staphylococcus aureus, Escherichia coli, Streptococcus faecalis, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger, SotIyfis cinerea, Stereum purpureum and Sclerotinia sclerotiorum with MIC values of more than 4 mg mL−1.24 Furthermore, 1,8-cineole had antimicrobial activity against microorganisms grown in suspension and biofilm. Accordingly, 1,8-cineole showed the potent antimicrobial properties against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans grown in suspension and biofilm with MIC values of 16 and 512 mg L−1, 256 and 512 mg L−1, 64 and 128 mg L−1, and 8 and 4 mg L−1, respectively whereas MBC values of 256 and 512 mg L−1, 256 and 512 mg L−1, 64 and 256 mg L−1, 64 and 8 mg L−1 respectively were shown by 1,8-cineole towards the same microorganisms grown in suspension and biofilm.150 Linalool has been reported to possess strong antibacterial and antifungal activities. This compound can inhibit the growth of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans.151 Also, linalool displayed activity against many periodontopathogens, including Porphyromonas gingivalis, Prevotella intermedia, P. nigrescens, Fusobacterium nucleatum subsp. nucleatum, F. nucleatum subsp. polymorphum, F. nucleatum subsp. vincentii, F. nucleatum subsp. fusiforme, F. nucleatum subsp. animalis, Streptococcus mutans, S. sobrinus and Aggregatibacter actinomycetemcomitans.152 In addition, linalool showed strong activity against Pasteurella multocida153 and Listeria monocytogenes.154
In addition, β-pinene, terpinen-4-ol, β-caryophyllene, D-limonene, β-myrcene, p-cymene and camphor also contribute to the antimicrobial properties of Alpinia essential oils. Namely, β-pinene has been shown to exhibit antibacterial activity against Gram-positive bacteria that cause the potential infectious endocarditis, including Staphylococcus aureus, S. epidermidis, Streptococcus pyogenes and S. pneumonia with MIC values of 20 μL mL−1.148 Also, β-pinene exhibited antifungal activity against Candida albicans, Cryptococcus neoformans and Rhizopus oryzae with MIC values of 187, 234 and 780 μg mL−1, respectively.149 Terpinen-4-ol has been shown to be an antibacterial and antibiofilm agent against Staphylococcus aureus. It showed potent antibacterial activity against ten S. aureus strains, including ATCC-25923, ATCC-13150, LM-02, LM-40, LM-45, LM-116, LM-222, LM-232, LM-297 and LM-314 with MIC and MBC values of 0.25% and 0.5% (v/v), respectively.155 β-caryophyllene has been reported to possess antimicrobial activities against Bacillus cereus, B. subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Aspergillus niger, Penicillium citrinum, Rhizopus oryzae and Trichoderma reesei with MIC values ranging from 4 to 14 μM.156 D-Limonene, a bioactive component, has been shown to be biologically active against a wide range of microorganisms. This compound was active against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Saccharomyces cerevisiae with MIC values of 12.5, 3.2, 12.5 and 6.3 μg mL−1, respectively.157 β-myrcene has been reported to possess antibacterial activities against Enterococcus faecalis, Streptococcus salivarius and S. sanguinis with MIC values of 2.0, 0.4 and 1.5 mg mL−1, respectively.158 Finally, p-cymene and camphor isolated from the leaf oil of Alpinia speciosa grown in Martinique, France showed antimicrobial activity against many bacterial and fungal strains, including Escherichia coli, Mycobacterium smegmatis, and Cylindrocarpon mali followed by Staphylococcus aureus, Streptococcus faecalis, Pseudomonas aeruginosa, Candida albicans, Sclerotinia sclerotiorum, Aspergillus niger, SotIyfis cinerea and Stereum purpureum.24
The antimicrobial mechanisms of the above-mentioned compounds can be attributed to their lipophilicity and/or hydrophobicity and the presence of a hydroxyl group (–OH), playing a key role in the sequential inhibition of common biochemical pathways, inhibition of protective enzymes and the use of cell wall active agents to enhance the uptake of other antimicrobials.159,160 Although several components isolated from Alpinia essential oil were found to be effective against various microorganisms, the mechanism of the antimicrobial activity using the initial Alpinia essential oil is still unclear.
3.2. Antioxidant effect
The antioxidant activity of the essential oil of A. galanga collected from Phu Tho, Vietnam was determined using 1,1-diphenyl-2-picrylhydrazol (DPPH) with a percentage inhibition of 47.15%.64 The essential oils obtained from the leaves of A. malaccensis from Odisha, India showed strong radical-scavenging activities, as evaluated using the DPPH and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays, with IC50 values of 18.26 μg mL−1 and 20 μg mL−1, respectively.68 Meanwhile, the antioxidant effect of the essential oil from the leaf of A. malaccensis var. nobilis from Malaysia was investigated using the DPPH radical scavenging test (IC50 = 32.67 mg mL−1), ABTS (GAE = 26.59 mg GAE per g) and ferric reducing ability of plasma (FRAP) (TE = 24.56 M TE per g) analysis.22 In addition, four plant parts of A. nigra collected from Guwahati, India showed strong radical-scavenging activities, as evaluated by the DPPH assay. Accordingly, the rhizome oil was found to be the best antioxidant with an IC50 value of 36.83 μg mL−1, followed by the seed, leaf and flower oils with IC50 values of 40.11, 42.13 and 43.41 μg mL−1, respectively.102 The antioxidant effect determined using DPPH and ABTS assays was investigated for the leaf essential oil of A. nigra from West Bengal, India with IC50 values of 4.05 and 15.55 μg mL−1, respectively.104 Moreover, the seed and leaf essential oil of A. speciose grown in Chu-Tung, Taiwan showed potent DPPH radical-scavenging activities. At doses of 0.25%, 0.5% and 1%, the seed oil was found effective against the DPPH radical scavenger with the percentage inhibition of 58.0%, 59.9% and 63.1%, whereas that of the leaf oil was 50%, 56.4% and 67%, respectively.41Kuraya et al.34 showed the relationship among the antioxidant activity, chemical composition and yield of the essential oil isolated from A. zerumbet leaves collected from various locations in Japan. Their report demonstrated that the antioxidant activity and yield of the oils varied significantly between individuals and collecting seasons, and the yields of the essential oils and their antioxidant activity had an inverse correlation. Furthermore, the antioxidant effects of the A. zerumbet oils were dependent on highly polar compounds, while the chemical compounds in each plant was not affected by the season or its growth habitat. Thus, this suggests that the essential oils from different individuals of A. zerumbet possess different antioxidants. This can be the useful information for the selection of lineages of A. zerumbet to optimize the production of compounds with particular medicinal value.34 In addition, the fruit rind and rhizome essential oils from India showed antioxidant activities, as evaluated using the DPPH radical scavenging assay. Accordingly, the fruit rind oils possessed a stronger antioxidant effect with 56.5% inhibition at a dose of 20 μg mL−1 (IC50 value of 15.17 μg mL−1), while 8.9% inhibition was shown by the rhizome oil at the same oil concentration.96 Finally, the leaf essential oil of A. latilabris from Malaysia was investigated using three different methods. Accordingly, the IC50 using the DPPH free radical scavenging assay was 54.33 mg mL−1, whereas measurements by the ABTS and FRAP assays provided a GAE value of 14.47 mg GAE per g and TE value of 17.51 M TE per g, respectively.22 The antioxidant efficacy of Alpinia essential oil is due to the presence of β-caryophyllene, as reported in a recent study.156 Accordingly, β-caryophyllene showed antioxidant activities with IC50 values of 1.25 and 3.23 μM for the DPPH and FRAP assays, respectively. However, the antioxidant reaction mechanisms of β-caryophyllene has not been thoroughly studied to date and there is almost no literature on the transformation of this compound after it reacts with free radicals under diverse conditions.
3.3. Larvicidal activity
The flower oil of A. zerumbet from Le Morne Rouge, France presented repellent and irritant activities against Aedes aegypti at a dose of 0.1% and interesting toxic activity at 1%.39 Similarly, Cavalcanti et al.161 demonstrated that the leaf and branch of A. zerumbet collected from Brazil possessed larvicidal activity against Ae. aegypti with an LC50 value of 313 ppm.161 Moreover, the leaf essential oil of A. speciosa grown in Brazil had larvicidal activity against Ae. Aegypti. Accordingly, the oil exhibited a larvae mortality of 100% after 5 min of its application at doses of 2.0 and 2.5 μL mL−1, and the lethal concentration values for 50% (LC50) and 90% (LC90) of 0.94 and 1.2 μL mL−1, respectively.161 The leaf and seed oils of A. speciosa from Chu-Tung, Taiwan have been reported to exhibit mosquito larvicidal activity. The leaf oil showed larvicidal activity against Ae. Aegypti with LC50 values of 64 and 32 ppm after 2 h and 24 h, while the LC50 values were 125 and 87 ppm, respectively, for the seed oil towards the same mosquito.41 The essential oils of pink and red variants of A. purpurata from Paulista, Brazil showed potent larvicidal activities against Ae. aegypti with LC50 values of 71.5 and 80.7 ppm, respectively.128 The oils isolated from different plant parts (leaf, rhizome, flower and seed) of A. nigra exhibited mosquito larvicidal activity. The leaf, rhizome and seed oils of A. nigra exhibited 100% mortality at the dose of 125 ppm. Furthermore, the oil from four plant parts also presented biting deterrent activity against female Ae. Aegypti. The proportion not biting for the flower, rhizome, leaf and seed oils was 49%, 52%, 58% and 62% at dose of 10 μg cm−2.102The larvicidal activity of Alpinia essential oil is ascribed to α-pinene, β-pinene, and β-caryophyllene. It has been found that α-pinene obtained from the essential oil of Alpinia purpurata exhibited larvicidal effects against fourth stage larvae of Aedes aegypti. The compound could inhibit against this mosquito with 12%, 27%, 33%, 45 and 67% mortality at doses of 150, 200, 250, 300 and 400 ppm, respectively.128 The β-pinene from Alpinia purpurata oil has been shown to possess larvicidal activity against Ae. aegypti. At doses of 150, 200, 250, 300 and 400 ppm, β-pinene was found to be effective against this mosquito with 35%, 40%, 65%, 78% and 90% mortality, respectively.128 β-Caryophyllene from Alpinia purpurata oil has been shown to possess weak larvicidal activity against Ae. aegypti. At doses of 150, 200, 250, 300 and 400 ppm, the compound presented larvicidal effects against this mosquito with 3.3%, 3.3%, 3.3%, 5.0% and 3.3% mortality, respectively.128 However, to date, the mechanism of larvicidal activity of essential oils and of Alpinia essential oil and their constituents in particular has not been fully reported. Many researchers have supposed that the mechanism of larvicidal activity can be due to the inhibition of the acetylcholinesterase enzyme, a similar neurotoxic effect produced by organophosphorus and carbamate insecticides.162–164
3.4. Insecticidal activity
The essential oils derived from seeds of A. katsumadai grown in Guangxi, China showed insecticidal effects against Tribolium castaneum, Liposcelis bostrychophila and Lasioderma serricorne with LD50 values of 52.6, 35.6 and 17.4 μg cm−2, respectively. Similarly, the essential oil of A. kwangsiensis rhizomes from Xishuangbanna, China was found to possess strong contact toxicity against L. serricorne with an LC50 value of 24.59 μg per adult, while the fumigant toxicity had an LC50 value of 9.91 μg mL−1 air.143 Also, the rhizome oil of A. galanga from Yunnan, China showed high fumigant toxicity against L. serricorne adults with an LC50 value of 3.5 mg L−1, whereas the LD50 value of 12.2 μg per adult was shown by the contact toxicity towards the same beetle.60 Insecticidal activity against Callosobruchus maculatus has been reported in the oil of A. calcarata rhizome with LC50 values of 0.685 and 0.141 g L−1 for the fumigant toxicity and contact toxicity, respectively.21.Souza et al.36 showed the insecticidal activity against Rhodnius nasutus (a vector of chagas disease) of the leaf oils of two Alpinia species grown in Rio de Janeiro, Brazil, including A. zerumbet and A. vittata. In the first 10 min of application, the A. vittata oil at 125 g mL−1 showed 73.3% of mortality, while 83.3% mortality was recorded in A. zerumbet oil.36 In addition, the rhizome essential oil of A. blepharocalyx from China showed strong insecticidal effects against Lasioderma serricorne with an LD50 value of 15.02 μg per adult (contact toxicity) and LC50 value of 3.83 mg L−1 (fumigant toxicity).123 Maize weevil (Sitophilus zeamais) is one of the most cosmopolitan pests of stored grains, including rice (Oryza sativa), wheat (Triticum spp.), triticale (Triticum aestivum L. and Secale cereale) and barley (Hordeum vulgare).165 De Lira et al.166 investigated the fumigant toxicity of the inflorescence essential oil obtained from Alpinia purpurata against Sitophilus zeamais adults with an LC50 value of 41.4 mL per L in air.166
The insecticidal property is mainly related to β-pinene, α-pinene, methyl cinnamate, α-terpineol and camphor as the major compounds presented in Alpinia essential oils. Accordingly, β-pinene isolated from Alpinia kwangsiensis oils displayed insecticidal effects against Lasioderma serricorne with an LC50 value of 35.69 μg mL−1 air for fumigant toxicity and LD50 value of 65.87 μg per adult for contact toxicity.143 Similarly, β-pinene as the major compound isolated from Alpinia galanga oil showed insecticidal effects against L. serricorne with an LD50 value of 65.6 μg per adult for contact toxicity and LC50 value of 29.0 mg L−1 air for fumigant toxicity.60 Souza et al.36 showed that the essential oil from Alpinia vittata contained β-pinene as the most abundant compound, which was topically applied on Rhodnius nasutus fifth-instar nymphs with 100% mortality within the first 10 min of application at a dose of 44 μg mL−1.36 Similarly, α-pinene has been recorded as a major compound of Alpinia galanga oil. The compound had insecticidal effects against L. serricorne with an LD50 value of 76.8 μg per adult for contact toxicity and LC50 value of 38.1 mg L−1 air for fumigant toxicity.60 Methyl cinnamate, the most abundant compound of Alpinia katsumadai oil, has been reported to possess potential insecticidal activities against three stored product insects, including Tribolium castaneum, Liposcelis bostrychophila and Lasioderma serricorne, with LD50 values of 5.0 μg per adult, 2.2 μg per adult and 23.5 μg cm−2, respectively.25 α-Terpineol as a major compound of Alpinia galanga oil exhibited insecticidal activities against Lasioderma serricorne with an LD50 value of 13.3 μg per adult for contact toxicity and LC50 value of 2.8 mg L−1 air for fumigant toxicity.60 Camphor isolated from the essential oil of Alpinia kwangsiensis has been reported to possess strong insecticidal activity against Lasioderma serricorne adults. This compound could inhibit by two potential contacts with an LC50 value of 2.91 mg L−1 air for contact toxicity while an LD50 value of 11.30 μg per adult was recorded for fumigant toxicity.143 Several studies indicated that the above-mentioned compounds can affect the cuticle of insects, thereby favoring the insecticidal action of some synthetic compounds. In addition, they also affect the components of the epicuticular waxes of insects, which could be a mechanism of pesticidal activity.167
3.5. Cytotoxic activity
The essential oil isolated from the whole plant of A. calcarata collected from Sri Lanka has been reported to exhibit cytotoxic activities against several cell lines, including RAW264.7, HaCaT, HepG2 and IEC-6 cells. At a dose of 100 μg mL−1, the oil caused a reduction in the viability of the above-mentioned cell lines.23 In another study, Zhang et al.73 showed the cytotoxic activities of A. officinarum essential oils collected from ten locations in China. Accordingly, the rhizome oils from different habitats such as Sanming, Guilin, Yulin, Guigang, Qiandongnan, Panzhihua, Xishuangbanna, Gaozhou, Xuwen and Bozhou showed cytotoxic activities against BV2 cells with IC50 values of 211.07, 269.22, 769.06, 258.08, 231.79, 552.49, 305.77, 239.95, 233.85 and 302.37 μg mL−1, respectively.73 Moreover, the rhizome and fruit rind essential oils of A. mutica from India showed cytotoxic activities against Dalton's lymphoma ascites (DHA). Accordingly, the fruit rind oil possessed a strong effect against DHA cells with a CD50 (curative dose) value of 0.06 μg mL−1, while the rhizome oil showed weaker activity with a CD50 value of 13 μg mL−1.96 Also, at dose of 20 μg mL−1, the rhizome oil of A. nigra from India was highly cytotoxic to MCF-7 and HeLa cells with 70.9% and 79% inhibition, while 50% and 60% inhibition, respectively, were shown by the leaf oil towards the same cells.103The cytotoxic activity of Alpinia essential oil can be due to the presence of terpinen-4-ol, linalool, β-caryophyllene, and D-limonene. Terpinen-4-ol has been shown to exhibit moderate cytotoxicity against colorectal, pancreatic, prostate and gastric cancer cells. At a dose of 10%, this chemical component showed 90% growth inhibition towards cancer cells.168 Besides, the cytotoxicity of linalool against human prostate cancer cells (DU145) has also been reported. At doses of 20, 40 and 80 μM, the compound induced sub-G1 cell cycle arrest, and therefore DNA damage.169 Furthermore, linalool has been demonstrated to possess potential anti-cancer properties against some cancer cell lines, including HepG2 (IC50 = 290 μM), A549 (IC50 = 438 μM), SW620 (IC50 = 222 μM), T-47D (IC50 = 224 μM),170 RPMI 7932 (IC50 = 5.60 μM),171 HeLa (IC50 = 2.59 μM), U937 (IC50 = 11.02 μM).172 β-Caryophyllene possessed enhanced activities based on the anticancer effects of other compounds such as α-humulene, isocaryophyllene and paclitaxel against MCF-7, DLD-1 and L-929 human tumour cells.173 β-Caryophyllene also had cytotoxicity against some human cancer cell lines, including HCT 116, PANC-1, HT-29, ME-180, PC3, K562, MCF-7, CCD-18Co, NIH/3T3-L1 and RGC5 with IC50 values of 19, 27, 63, 95, 104, 105, 285, 612, 530 and 156 μM, respectively.156 Finally, D-limonene has been proven to have bioactivity against breast cancer. After limonene intervention, cyclin D1 expression was reduced by 22% in tumor tissue, while its effect was hardly found in Ki67 tissue, cleaved caspase-3 expression, serum leptin, adiponectin, TGF-b1, insulin-like growth factor binding protein-3 (IGFBP-3) and interleukin-6 (IL-6) levels.174 The main mechanism for the cytotoxic effects of the reported constituents includes induction of cell death by apoptosis and/or necrosis, cell cycle arrest, and loss of key organelle function.175
3.6. Anti-inflammatory activity
The essential oil extracted from the leaves of A. calcarata collected from Tamil Nadu, India exhibited a good anti-inflammatory effect in a paw edema model in albino Wistar rats. At doses of 200 mg kg−1 and 300 mg kg−1, the oil could inhibit in vivo anti-inflammatory capacity with the percentage inhibition of around 75.78% and 78.15%, respectively.82 Furthermore, the essential oils from the leaves and rhizomes of A. calcarata grown in Sri Lanka showed anti-inflammatory activity on COX enzymes (COX-1 and COX-2). At doses of 0.5, 5.0 and 50 μg mL−1, the rhizome oil possessed the anti-inflammatory effects on COX-1 enzyme with a percentage inhibition of 19.45%, 28.5% and 76.47%, while that of 10.4%, 27.14% and 65.61%, respectively, was shown by the leaf oil towards the same enzyme. Meanwhile, the COX-2 enzyme was inhibited by the rhizome oil by about 21.4%, 36.3% and 85.7% at doses of 0.5, 5.0 and 50 μg mL−1, while the leaf oil had an anti-inflammatory effect with the percentage inhibition of 5.84%, 27.92% and 70.12%, respectively.23 The anti-inflammatory properties of the essential oils of A. officinarum rhizome collected from different habitats in China, including Sanming, Guilin, Yulin, Guigang, Qiandongnan, Panzhihua, Xishuangbanna, Gaozhou, Xuwen and Bozhou were determined using a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema assay. At a dose of 100 mg kg−1, the A. officinarum oils from these ten habitats showed anti-inflammatory activity on the TPA-induced mouse ear edema with the percentage inhibition of 72.16%, 76.20%, 91.62%, 81.14%, 64.42%, 70.96%, 82.34%, 63.17%, 79.34% and 34.43%, respectively.73The anti-inflammatory properties of Alpinia essential oils correspond to β-caryophyllene, linalool, and γ-terpinene. It has been reported that β-caryophyllene possesses anti-inflammatory activity, which was effective in reducing platelet activating factor-, bradykinin- and ovalbumin-induced mouse paw oedema.176 Linalool exhibited anti-inflammatory activities in LPS-stimulated RAW 264.6 cells by blocking the activation of the NF-κB pathway and the mitogen-activated protein kinase (MAPK) pathway.177 Moreover, LPS-induced inflammation in BV2 microglia cells was suggested to be inhibited by linalool through both the NF-κB pathway and the activation of the erythroid 2-related factor/heme oxygenase-1 (Nrf2/HO-1) signaling pathway.178 Linalool exhibited hypocholesterolemic effects in high-fat fed C57BL/6J mice and HepG2 cells. At a dose of 0.57 mg per mouse per day, linalool was sufficient to reduce plasma cholesterol levels in mice.179 Ramalho et al.180 demonstrated that γ-terpinene possessed the anti-inflammatory effects. At a dose of 25 mg kg−1 or 50 mg kg−1, γ-terpinene could decrease the paw edema in mice within one hour by 67.3% or 53.3%, respectively.180
3.7. Slimming aromatherapy
Aromatherapy, a holistic healing treatment involving the use of natural products from plants, has been recently explored as a slimming alternative medicine. Notably, the essential oils isolated from plants have high potential as slimming aromatherapy.181,182 The essential oil of A. galanga grown in Indonesia and its two fractions, n-hexane and ethyl acetate, have been reported to exhibit a slimming aromatherapy effect through in vivo observation in adult male Sprague Dawley rats.63 Accordingly, the average body weights of a male rat at pretreatment and post treatment with A. galanga oil and the n-hexane and ethyl acetate fractions were 283.2 g and 244.8 g, 287.7 g and 266.8 g, 289.0 g and 258.9 g, respectively. Additionally, the total plasma cholesterol and triglyceride concentration in Sprague Dawley rats after 5 weeks of treatment with A. galanga oil and n-hexane and ethyl acetate fractions were recorded. Consequently, the rats treated with oils had a total plasma cholesterol and triglyceride concentration of 106.6 and 47.97 mg dL−1, whereas those treated with the n-hexane and ethyl acetate fractions had values of 79.9 and 37.83 mg dL−1, 83.5 and 43.51 mg dL−1, respectively.633.8. Other biological activities
Patil et al.183 demonstrated that the essential oil of A. galanga collected from Belgaum, India showed anti-asthmatic activity in mice. Accordingly, A. galanga oil had a beneficial effect not only on histamine-induced bronchospasm in Guinea pigs but also on ovalbumin-induced allergic airway inflammation in a mouse model.183 Cândido et al.184 reported that the association of kinesiotherapy with A. zerumbet essential oils could improve the muscle recruitment of people with spasticity who needed to perform motor rehabilitation. This result showed a significant decrease in the spasticity of pathological legs during the best muscle contraction after application of essential oil at doses of 0.5 mL/2 kg and 0.05 mL/4 kg.184 Additionally, the inhibition of mushroom tyrosinase by the oils isolated from the seeds and leaves of A. speciosa was evaluated. At a dose of 1000 ppm, the seed oils showed 74% inhibition of mushroom tyrosinase, while the leaf oil revealed an 81% inhibitory effect.41 The essential oil isolated from the pseudostems, leaves and rhizomes of A. aquatica exhibited weak tyrosinase inhibitory activity with the percentage inhibition of 1.4%, 6.6% and 9.5%, repectively.131 In a previous study, Cavalcanti and collaborators160 also reported that the essential oil of Alpinia zerumbet exerted scavenging effects against DPPH radicals. Moreover, cotreatment with essential oil could increase the intracellular GSH content and decrease lipid peroxidation, oxidation of purine bases and intracellular ROS after H2O2 challenge.1854. Conclusion and future perspectives
This overview provided a summary of the available information on the chemical compositions and biological activities of Alpinia essential oils. The essential oils and their major compounds isolated from different parts of the Alpinia plant have been found to produce dynamic biological activities, including antimicrobial, cytotoxic, antioxidant, anti-inflammatory, anti-asthmatic, insecticidal, and larvicidal activities and slimming aromatherapy.Currently, the primary focus of research is the examination of the chemical composition and biological properties of essential oils from different parts of newly found species. The chemical compositions are mainly identified using the LC-MS and GC-MS techniques. However, in many works, the n-alkane standard for calculating the retention indices has not been run, the retention indices have not been analyzed and compared, and a running reference standard is missing to enable a more accurate conclusion about the essential oil composition. In addition, there are not many studies related to the activities of the individual components of the Alpinia essential oil and their mechanisms of action. Therefore, future research should consider the correction of the composition and percentage of compounds, especially those with a low content, in the essential oils of newly discovered Alpinia species. Importantly, the mechanisms of action of the Alpinia essential oils and their individual components should be elucidated to design appropriate isolation methods for individual natural components to improve their biological properties.
Conflicts of interest
There are no conflicts to declare.References
- R. M. Smith, Edinb. J. Bot., 1990, 47, 1–75 CrossRef.
- X. N. Ma, C. L. Xie, Z. Miao, Q. Yang and X. W. Yang, RSC Adv., 2017, 7, 14114–14144 RSC.
- H. N. Phuong, B. N. Quoc and B. S. Adhikar, Endanger. Species., 2014, vol. 2, pp. 2332–2343 Search PubMed.
- W. J. Zhang, J. G. Luo and L. Y. Kong, World J. Tradit. Chin. Med., 2016, 2, 26–41 CrossRef.
- H. Itokawa, K. Watanabe, H. Morita, S. Mihashi and Y. Iitaka, Chem. Pharm. Bull., 1985, 33, 2023–2027 CrossRef CAS.
- B. Roy and A. Swargiary, J. Parasit. Dis., 2009, 33, 48–53 CrossRef PubMed.
- K. S. Chun, Y. Sohn, H. S. Kim, O. H. Kim, K. K. Park and J. M. Lee, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1999, 428, 49–57 CrossRef CAS.
- S. Samarghandian, M. A. R. Hadjzadeh, J. T. Afshari and M. Hosseini, BMC Complementary Altern. Med., 2014, 14, 192 CrossRef PubMed.
- M. A. Al-Yahya, S. Rafatullah, J. S. Mossa, A. M. Ageel, M. S. Al-Said and M. Tariq, Phytother. Res., 1990, 4, 112–114 CrossRef.
- K. Rao, B. Ch, L. M. Narasu and A. Giri, Appl. Biochem. Biotechnol., 2010, 162, 871–884 CrossRef CAS PubMed.
- P. Niyomkam, S. Kaewbumrung, S. Kaewnpparat and P. Panichayupakaranant, Pharm. Biol., 2010, 48, 375–380 CrossRef CAS PubMed.
- R. Rajasekar, K. Manokaran, N. Rajasekaran, G. Duraisamy and D. Kanakasabapathi, J. Diabetes Metab. Disord., 2014, 13, 33 CrossRef PubMed.
- D. Shin, K. Kinoshita, K. Koyama and K. Takahashi, J. Nat. Prod., 2002, 65, 1315–1318 CrossRef CAS PubMed.
- Y. Yang, K. Kinoshita, K. Koyama, K. Takahashi, S. Kondo and K. Watanabe, Phytomedicine, 2002, 9, 146–152 CrossRef CAS PubMed.
- Y. M. Chang, C. T. Te, C. C. R. Wang, Y. S. Chen, Y. M. Lin and C. H. Kou, Biosci., Biotechnol., Biochem., 2013, 77, 229–234 CrossRef CAS PubMed.
- S. Shi, X. Zhao, A. Liu, B. Liu, H. Li and B. Wu, Physiol. Behav., 2015, 139, 13–20 CrossRef CAS PubMed.
- X. Z. Li, S. N. Zhang, S. M. Liu and F. Lu, Fitoterapia, 2013, 84, 273–285 CrossRef CAS PubMed.
- D. P. Sousa, S. H. P. Almeida, L. N. Andrade and R. Andreatini, Molecules, 2015, 20, 18620–18660 CrossRef PubMed.
- N. X. Dung, T. D. Chinh, D. D. Rang and P. A. Leclercq, J. Essent. Oil Res., 1994, 6, 295–297 CrossRef.
- N. D. Hung, L. T. Huong, D. N. Dai, T. M. Hoi and I. A. Ogunwande, J. Essent. Oil-Bear. Plants, 2018, 21, 1585–1593 CrossRef CAS.
- K. Abeywickrama, A. A. C. K. Adhikari, P. Paranagama and C. S. P. Gamage, Can. J. Plant Sci., 2006, 86, 821–827 CrossRef CAS.
- J. Vejayan, N. E. Selladuri, H. Ibrahim, A. S. Shuib and M. M. Yusoff, J. Essent. Oil-Bear. Plants, 2017, 20, 959–971 CrossRef CAS.
- M. Chandrakanthan, S. M. Handunnetti, G. S. A. Premakumara and S. Kathirgamanathar, J. Evidence-Based Complementary Altern. Med., 2020, 2020, 2035671 Search PubMed.
- D. Prudent, F. Perineau, J. M. Bessiere, G. Michel and R. Bravo, J. Essent. Oil Res., 1993, 5, 255–264 CrossRef CAS.
- Z. Chen, X. Pang, S. Guo, W. Zhang, Z. Geng and Z. Zhang, J. Essent. Oil-Bear. Plants, 2019, 22, 504–515 CrossRef CAS.
- C. N. Jezler, R. S. Batista, P. B. Aves, D. C. Silva and L. C. B. Costa, Cienc. Rural, 2013, 43, 1811–1816 CrossRef CAS.
- L. Tao, H. S. Hu and X. C. Shen, Phytomedicine, 2013, 20, 387–393 CrossRef CAS PubMed.
- E. W. C. Chan, S. K. Wong and H. T. Chan, J. Chin. Pharm. Sci., 2017, 26, 775–788 CAS.
- A. Elzaawely, T. Xuan, H. Koyama and S. Tawata, Food Chem., 2007, 104, 1648–1653 CrossRef CAS.
- A. Upadhyay, J. Chompoo, N. Taira, M. Fukuta and S. Tawata, Biosci., Biotechnol., Biochem., 2013, 77, 217–223 CrossRef CAS PubMed.
- E. W. C. Chan, Y. Y. Lim, L. F. Wong, F. S. Lianto, S. K. Wong and K. K. Lim, Food Chem., 2008, 109, 477–483 CrossRef CAS.
- S. Murakami, W. Li, M. Matsuura, T. Satou, S. Hayashi and K. Koike, J. Nat. Med., 2009, 63, 204–208 CrossRef CAS PubMed.
- H. Kawai, E. Kuraya, A. Touyama, O. Higac, K. Hokamoto and K. Tokeshi, Food Bioprod. Process., 2021, 125, 134–140 CrossRef CAS.
- E. Kuraya, R. Yamashiro, A. Touyama, S. Nakada, K. Watanabe and A. Iguchi, Nat. Prod. Commun., 2017, 12, 1321–1325 CrossRef.
- S. Ali, S. Sotheeswaran, M. Tuiwawa and R. M. Smith, J. Essent. Oil Res., 2002, 14, 409–411 CrossRef CAS.
- T. A. Souza, M. B. P. Lopes, A. S. Ramos, J. L. P. Ferreira, J. R. A. Silva and M. M. C. Queiroz, Sci. World J., 2018, 2018, 2393858 Search PubMed.
- C. Victorio, S. Leitao and C. Lage, J. Essent. Oil Res., 2010, 22, 2252–2254 CrossRef.
- F. R. S. Mendes, F. G. E. Silva, E. O. Sousa, F. F. G. Rodrigues, J. G. M. Costa and F. J. Q. Monte, J. Essent. Oil Res., 2015, 27, 259–263 CrossRef CAS.
- A. Kerdudo, E. N. Ellong, P. Burger, V. Gonnot, L. Boyer and F. Chandre, Chem. Biodiversity, 2017, 14, 27935668 CrossRef PubMed.
- A. D. S. Ramos, T. D. A. Souza, Y. M. Dias, T. A. L. Oliveira, J. L. P. Ferreira and M. A. M. Silva, Comparative study on essential oils of Alpinia zerumbet varieties, 8th Brazilian Symposium on Essential Oils-International Symposium on Essential Oils, Brazil, 2015 Search PubMed.
- J. C. Ho, J. Chin. Chem. Soc., 2010, 57, 758–763 CrossRef CAS.
- A. Luz, M. Zoghbi, J. Maia and M. Silva, J. Nat. Prod., 1984, 47, 907–908 CrossRef CAS PubMed.
- A. K. Indrayan, P. K. Tyagi and N. K. Agrawal, J. Essent. Oil Res., 2010, 22, 179–182 CrossRef CAS.
- G. Silva, N. Siqueira, L. Bauer and B. Sant'Ana, Rev. CENIC, Cienc. Biol., 1977, 5, 51–54 Search PubMed.
- M. Haggag and A. El-Shamy, Egypt. J. Pharm. Sci., 1977, 18, 465–476 CAS.
- H. Fujita and M. Yamashita, J. Pharm. Soc. Jpn., 1973, 93, 1635–1638 CrossRef CAS PubMed.
- A. Chouni and S. Paul, Pharmacogn. J., 2018, 10, 9–15 CrossRef CAS.
- X. Yang and R. G. Eilerman, J. Agric. Food Chem., 1999, 47, 1657–1662 CrossRef CAS PubMed.
- H. L. D. Pooter, M. N. Omar, B. A. Coolsaet and N. M. Schamp, Phytochemistry, 1985, 24, 93–96 CrossRef.
- F. Kiuchi, K. Matsuo, Y. Itano, M. Ito, G. Honda and T. K. Qui, Nat. Med., 2002, 56, 64–68 CAS.
- S. V. Nampoothiri, A. N. Menon, T. Esakkidurai and K. Pitchumani, J. Essent. Oil-Bear. Plants, 2016, 19, 82–87 CrossRef CAS.
- L. Jirovetz, G. Buchbauer, M. P. Shafi and N. K. Leela, Acta Pharm., 2003, 53(2), 73–78 CAS.
- G. Raj, D. P. Pradeep, C. Yusufali, M. Dan and S. Baby, J. Essent. Oil Res., 2013, 25, 97–102 CrossRef CAS.
- P. Akhtar, M. Ali, S. R. Mir and M. P. Sharma, J. Essent. Oil-Bear. Plants, 2004, 7, 243–246 CrossRef CAS.
- A. P. Raina, S. K. Verma and Z. Abraham, J. Essent. Oil Res., 2014, 26, 24–28 CrossRef CAS.
- D. J. Charles, J. E. Simon and N. K. Singh, J. Essent. Oil Res., 1992, 4, 81–82 CrossRef CAS.
- W. Sripor and N. Jinda, Effect of Alpinia galanga essential oil on bacteria spoilage, 26th annual meeting of the Thai Society for Biotechnology and International Conference, Thailand, 2014 Search PubMed.
- P. Pripdeevech, N. Nuntawong and S. Wongpornchai, Chem. Nat. Compd., 2009, 45, 562–564 CrossRef CAS.
- A. Hamad, A. Alifah, A. Permadi and D. Hartanti, Int. Food Res. J., 2016, 23, 837–841 CAS.
- Y. Wu, Y. Wang, Z. H. Li, C. F. Wang, J. Y. Wei, X. L. Li, P. J. Wang, Z. F. Zhou, S. S. Du, D. Y. Huang and Z. W. Deng, Bull. Insectology, 2014, 67, 247–254 Search PubMed.
- B. Jantan, F. B. Ahmad and A. S. Ahmad, J. Essent. Oil Res., 2004, 16, 174–176 CrossRef.
- L. S. R. Arambewela, M. Arawwawala, N. L. Owen and B. Jarvis, J. Essent. Oil Res., 2007, 19, 455–456 CrossRef CAS.
- R. Damayanti, I. Batubara and I. H. Suparto, Int. J. Pharma Bio Sci., 2015, 6, 283–289 CAS.
- V. L. Nguyen and Q. U. Nguyen, Afr. J. Biotechnol., 2016, 15, 2739–2742 CrossRef.
- T. T. Q. Vuong and W. D. Reinhard, J. Essent. Oil-Bear. Plants, 2004, 7, 165–170 CrossRef.
- W. J. Kress, A. Z. Liu, M. Newman and Q. J. Li, Am. J. Bot., 2005, 92, 167–178 CrossRef CAS PubMed.
- M. N. I. Bhuiyan, J. U. Chowdhury, J. Begum and N. C. Nandi, Afr. J. Plant Sci., 2010, 4, 197–201 CAS.
- S. Sahoo, S. Singh and S. Nayak, Int. J. Pharm. Pharm. Sci., 2014, 7, 183–188 Search PubMed.
- L. T. Huong, T. D. Thang and I. O. Ogunwade, Eur. J. Med. Plants, 2015, 7, 118–124 CrossRef.
- M. Azah, Y. Sam, J. Mailina and L. S. L. Chua, J. Trop. For. Sci., 2005, 17, 631–633 Search PubMed.
- V. K. Raina, S. K. Srivastava and K. V. Syamasunder, Flavour Fragrance J., 2002, 17, 358–360 CrossRef CAS.
- L. Y. Lin, C. C. Peng, X. Y. Yeh, B. Y. Huang, H. E. Wang and K. C. Chen, Food Funct., 2015, 6, 1600–1610 RSC.
- L. Zhang, C. Pan, Z. Ou, X. Liang, Y. Shi and L. Chi, Ind. Crops Prod., 2020, 153, 112583 CrossRef CAS.
- G. Eumkeb, S. Sakdarat and S. Siriwong, Phytomedicine, 2010, 18, 40–45 CrossRef CAS PubMed.
- M. Gazal, M. R. Valente, B. A. Acosta, F. N. Kaufmann, E. Braganhol and C. L. Lencina, Eur. J. Pharmacol., 2014, 724, 132–139 CrossRef CAS PubMed.
- L. Wu, L. W. Lei, W. L. Mei, H. F. Dai and M. Peng, Chem. Nat. Compd., 2012, 48, 325–326 CrossRef.
- V. S. Rana, M. Verdeguer and M. A. Blazquez, J. Essent. Oil Res., 2010, 22, 521–524 CrossRef CAS.
- T. L. Ngoc, R. Yamauchi and K. Kato, Food Sci. Technol. Res., 2001, 7, 303–306 CrossRef.
- L. D. A. M. Arawwawala, N. Athauda and L. Arambewela, J. Ayurveda Integr. Med., 2010, 1, 199–202 CrossRef PubMed.
- C. P. Khare, Indian medicinal plant-An illustrated dictionary, Springer, US, 2007, pp. 37–42 Search PubMed.
- A. P. Raina and Z. Abraham, J. Essent. Oil Res., 2015, 27, 238–243 CrossRef CAS.
- K. Poonkodi, S. K. V. Baranika, P. Udayakumar, R. Jeevitha and G. Priyanka, Int. J. Pharm. Sci. Rev. Res., 2017, 42, 207–210 CAS.
- P. K. Rout, S. Sahoo, S. P. Rath and Y. R. Rao, J. Essent. Oil Res., 2005, 17, 398–400 CrossRef CAS.
- A. Tewari, A. Pant, C. Mathela, E. Kohl and H. Bestmann, J. Essent. Oil Res., 1999, 11, 739–741 CrossRef CAS.
- L. S. R. Arambewela, A. Kumaratunge, M. Arawwawela, N. L. Owen and L. Du, J. Essent. Oil Res., 2005, 17, 124–125 CrossRef.
- L. T. Huong, D. N. Dai, T. D. Thang, T. T. Bach and I. A. Ogunwande, J. Essent. Oil-Bear. Plants, 2016, 19, 2049–2055 CrossRef CAS.
- R. Smith, Edinburgh. J. Bot., 1990, 47, 19–21 Search PubMed.
- I. H. Burkill, A dictionary of the economic products of the Malay Peninsula, Ministry of Agriculture and Co-operatives, Kuala Lumpur, Malaysia, 1966, pp. 11–32 Search PubMed.
- S. N. A. Malek, C. W. Phang, H. Ibrahim, W. N. Abdul and K. S. Sim, Molecules, 2011, 16, 583–589 CrossRef PubMed.
- N. A. Mustahil, M. A. Sukari, A. B. Abdul, N. A. Ali and G. E. C. Lian, Pak. J. Pharm. Sci., 2013, 26, 391–395 CAS.
- M. Habsah, M. Amran, M. Mackeen, N. H. Lajis, H. Kikuzaki and N. Nakatani, J. Ethnopharmacol., 2000, 72, 403–410 CrossRef CAS PubMed.
- P. C. Weng, S. Nurestri, A. Malek, H. Ibrahim and N. A. Wahab, Afr. J. Pharm. Pharmacol., 2010, 5, 842–852 Search PubMed.
- H. Ibrahim, Y. Sivasothy, D. R. Syamsir, N. H. Nagoor, N. Jamil and K. Awang, Sci. World J., 2014, 2014, 430831 Search PubMed.
- H. M. Sirat, N. F. M. Khalid, N. A. Jani and N. Basar, J. Essent. Oil Res., 2009, 21, 457–458 CrossRef CAS.
- H. M. Sirat and A. A. Rahman, J. Essent. Oil Res., 1998, 10, 83–84 CrossRef CAS.
- M. Salim, R. Rajendran, N. S. Ajikumaran, M. Dan and S. Baby, J. Essent. Oil Res., 2016, 28, 428–435 CrossRef CAS.
- C. Qiao, Q. Han, J. Song, Z. Wang, L. Xu and H. Xu, Asian J. Tradit. Med., 2007, 2, 85–91 CAS.
- B. Roy, A. Swargiary and G. B. Ranjan, Adv. Life Sci., 2012, 2, 39–51 CrossRef.
- S. Ghosh, K. Indukuri, S. Bondalapati, A. K. Saikia and L. Rangan, Eur. J. Med. Chem., 2013, 66, 1–5 CrossRef PubMed.
- A. A. M. Ahmed, F. Sharmen, A. Mannan and M. A. Rahman, Afr. J. Tradit., Complementary Altern. Med., 2015, 5, 1–5 CrossRef PubMed.
- B. N. Das and N. Qais, Pharm. Globale, 2012, 3, 1–3 Search PubMed.
- S. Ghosh, T. Ozek, N. Tabanca, A. Ali, J. Rehman and I. A. Khan, Ind. Crops Prod., 2014, 53, 111–119 CrossRef CAS.
- S. Sahoo, B. Kar, S. Dash, M. Ray, K. G. Acharya and S. Singh, J. Essent. Oil-Bear. Plants, 2018, 21, 869–875 CrossRef CAS.
- S. Sahoo, B. Kar, A. Sahoo and S. Nayak, J. Essent. Oil-Bear. Plants, 2018, 20, 1461–1471 CrossRef.
- O. Prakash, S. Joshi, A. K. Pant, C. S. Chanotiya and C. S. Mathela, J. Essent. Oil Res., 2007, 19, 407–409 CrossRef CAS.
- H. J. Kwon, H. H. Kim, S. Y. Yoon, Y. B. Ryu, J. S. Chang and K. O. Cho, Virol. J., 2010, 7, 307 CrossRef PubMed.
- S. E. Lee, H. T. Shin, H. J. Hwang and J. H. Kim, Pharmacol. Res., 2003, 17, 1041–1047 Search PubMed.
- J. Kovac, N. Gavaric, F. Bucar and S. Mozina, Food Technol. Biotechnol., 2014, 52, 248–254 Search PubMed.
- P. Nan, Y. Hu, J. Zhao, Y. Feng and Y. Zhong, Z. Naturforsch. C. Biosci., 2004, 59, 157–160 CrossRef PubMed.
- N. X. Dung, D. L. Phuong and P. A. Leclercq, J. Essent. Oil Res., 1990, 2, 259–261 CrossRef.
- N. X. Dung, H. Q. Trung, V. N. Huong, N. X. Phuong and P. A. Leclercq, J. Essent. Oil Res., 2000, 12, 213–215 CrossRef CAS.
- L. Perry, Medicinal plants of east and Southeast Asia: Attributed properties and uses, MIT Press, Cambridge, MA, 1980 Search PubMed.
- J. H. Lee, H. S. Jung, P. M. Giang, X. Jin, S. Lee and P. T. Son, J. Pharmacol. Exp. Ther., 2006, 316, 271–278 CrossRef CAS PubMed.
- K. C. Wong, B. C. Lee, N. F. Lam and P. Ibrahim, Flavour Fragrance J., 2005, 20, 431–433 CrossRef CAS.
- H. M. Sirat and A. B. Nordin, J. Essent. Oil Res., 1995, 7, 195–197 CrossRef CAS.
- R. Holttum, The Zingiberaceae of the Malay Peninsula. Gardens' Bulletin, Singapore, 1950 Search PubMed.
- H. H. Pham, Araceae, in Pham-Hoang, Cây Cỏ Việt Nam: An Illustrated Flora of Vietnam, Youth Publishing House, Ho Chi Minh City, Vietnam, 2000 Search PubMed.
- T. H. Le, T. B. Tran and Q. B. Nguyen, Utilization pattern of genera Alpinia and Amomum (Zingiberaceae) in north central Vietnam, Proceedings of the 6th National Conference on Ecology and Biological Resources, Vietnam, 2015, pp. 1150–1154 Search PubMed.
- N. X. Dung, T. D. Chinh, D. D. Rang and P. A. Leclercq, J. Essent. Oil Res., 1994, 6, 327–329 CrossRef.
- P. A. Leclercq, N. X. Dung, T. D. Chinh and D. D. Rang, J. Essent. Oil Res., 1994, 6, 541–543 CrossRef CAS.
- P. M. Giang, P. T. Son, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2005, 53, 1335–1337 CrossRef CAS PubMed.
- L. T. Huong, D. N. Dai, T. D. Thang, T. T. Bach and I. A. Ogunwande, J. Essent. Oil-Bear. Plants, 2017, 20, 264–271 CrossRef CAS.
- Y. Wang, C. You, K. Yang, R. Chen, E. Juan and Y. Wu, J. Serb. Chem. Soc., 2015, 80, 171–178 CrossRef CAS.
- H. Dong, S. X. Chen, H. X. Xu, S. Kadota and T. Namba, J. Nat. Prod., 1998, 61, 142–144 CrossRef CAS PubMed.
- S. Kadota, Y. Tezuka, J. Prasain, A. M. Shawkat and A. Banskota, Curr. Top. Med. Chem., 2003, 3, 203–225 CrossRef CAS PubMed.
- M. D. G. B. Zoghbi, E. H. A. Andrade and J. G. S. Maia, Flavour Fragrance J., 1999, 14, 411–414 CrossRef CAS.
- A. Bermúdez and D. Velázquez, Rev. Cient. Fac. Cienc. Quim. Farm., 2002, 44, 2–6 Search PubMed.
- G. K. N. Santos, K. A. Dutra, R. A. Barros, C. A. G. Camara, D. D. Lira and N. B. Gusmao, Ind. Crops Prod., 2012, 40, 254–260 CrossRef CAS.
- N. X. Dung, T. D. Chinh, D. D. Rang and P. A. Leclercq, J. Essent. Oil Res., 1993, 5, 575–576 CrossRef CAS.
- N. X. Dung, T. D. Chinh, D. D. Rang and P. A. Leclercq, J. Essent. Oil Res., 1994, 6, 181–182 CrossRef CAS.
- N. B. Romes, N. Basar, H. M. Sirat, S. E. Hashim and Z. Asim, Nat. Prod. Commun., 2018, 13, 787–789 CrossRef.
- D. R. Syamsir, N. Tohar, K. Awang, N. A. M. Ali, M. Mokhtar and Y. Sivasothy Y, Sains Malays., 2020, 49, 43–48 CrossRef CAS.
- S. Jusoh, H. M. Sirat and F. Ahmad, Nat. Prod. Commun., 2013, 8, 317–320 CrossRef.
- L. T. Huong, T. D. Thang and I. A. Ogunwande, Nat. Prod. Commun., 2015, 10, 367–368 CrossRef.
- L. T. Huong, D. N. Dai, V. C. Mai, M. D. Doan and I. A. Ogunwande, Bol. Latinoam. Caribe Plant. Med. Aromat., 2017, 16, 26–33 CAS.
- D. N. Dai, L. T. Huong, T. D. Thang, T. O. Olayiwola, A. A. Ogunmoye and I. A. Ogunwande, Br. Biotechnol. J., 2016, 11, 1–7 CrossRef.
- D. N. Dai, L. T. Huong, T. D. Thang and I. A. Ogunwande, Chem. Nat. Compd., 2017, 53, 570–573 CrossRef CAS.
- L. T. Huong, D. N. Dai, L. T. M. Chau and I. A. Ogunwande, Chem. Nat. Compd., 2018, 54, 992–994 CrossRef CAS.
- S. K. J. Kumar, V. M. Gokila, P. C. Wu, H. J. Lee, Y. H. Tseng and S. Y. Wang, Plants, 2020, 9, 1–17 Search PubMed.
- P. A. Leclercq, N. X. Dung, T. D. Chinh and D. D. Rang, J. Essent. Oil Res., 1994, 6, 401–402 CrossRef CAS.
- D. N. Dai, L. T. Huong, N. H. Hung, V. H. Chinh and I. A. Ogunwande, J. Essent. Oil-Bear. Plants, 2020, 23, 322–330 CrossRef CAS.
- G. M. Phan, S. T. Phan and W. A. Konig, J. Essent. Oil Res., 2007, 19, 507–508 CrossRef CAS.
- Y. Wu, W. J. Zhang, D. Y. Huang, Y. Wang, J. Y. Wei and Z. H. Li, Molecules, 2015, 20, 21939–21945 CrossRef CAS PubMed.
- S. Joshi, O. Prakash, A. K. Pant and C. S. Mathela, J. Essent. Oil Res., 2010, 22, 85–90 CrossRef CAS.
- R. Joseph, T. Joseph and J. Joseph, Rev. Biol. Trop., 2001, 49, 509–512 CAS.
- G. Singh, S. K. Panday and P. A. Leclercq, J. Essent. Oil Res., 1999, 11, 335–336 CrossRef CAS.
- C. P. Victório, D. S. Alviano, C. S. Alviano and C. L. S. Lage, Rev. Bras. Farmacogn., 2009, 19, 697–701 CrossRef.
- A. M. Leite, E. O. Lima, E. L. Souza, M. F. F. M. Diniz, V. N. Trajano and I. A. Medeiros, Braz. J. Pharm. Sci., 2007, 43, 122–126 Search PubMed.
- A. C. S. Rivas, P. M. Lopes, M. M. A. Barros, D. C. C. Machado, C. S. Alviano and D. S. Alviano, Molecules, 2012, 17, 6305–6316 CrossRef PubMed.
- E. R. Hendry, T. Worthington, B. R. Conway and P. A. Lambert, J. Antimicrob. Chemother., 2009, 64, 1219–1225 CrossRef CAS PubMed.
- A. Herman, K. Tambor and A. Herman, Curr. Microbiol., 2016, 72, 165–172 CrossRef CAS PubMed.
- S. N. Park, Y. K. Lim, M. O. Freire, E. Cho, D. Jin and J. K. Kook, Anaerobe, 2012, 18, 369–372 CrossRef CAS PubMed.
- Q. Wu, L. Yu, J. Qiu, B. Shen, D. Wang and L. W. Soromou, Int. Immunopharmacol., 2014, 21, 456–463 CrossRef CAS PubMed.
- Z. Gao, V. J. D. Nostrand, J. Zhou, W. Zhong, K. Chen and J. Guo, Anti-listeria activities of linalool and its mechanism revealed by comparative transcriptome analysis, Front. Microbiol., 2019, 10, 2947 CrossRef PubMed.
- L. Cordeiro, P. Figueiredo, H. Souza, P. Tirosh and N. Arber, Int. J. Mol. Sci., 2020, 21, 1–14 Search PubMed.
- S. S. Dahham, Y. M. Tabana, M. A. Iqbal, O. B. K. Ahamed, M. O. Ezzat and A. S. A. Majid, Molecules, 2015, 20, 11808–11829 CrossRef CAS PubMed.
- M. R. Zahi, H. Liang and Q. Yuan, Food Control, 2015, 50, 554–559 CrossRef CAS.
- A. Koziol, A. Stryjewska, T. Librowski, K. Sałat, M. Gaweł and A. Moniczewski, Mini-Rev. Med. Chem., 2014, 14, 1156–1168 CrossRef CAS PubMed.
- I. H. N. Bassolé and H. R. Juliani, Molecules, 2012, 17, 3989–4006 CrossRef PubMed.
- H. Zengin and A. Baysal, Molecules, 2014, 19, 17773–177798 CrossRef PubMed.
- E. S. B. Cavalcanti, S. M. Morais, M. A. A. Lima and E. W. P. Santana, Mem. Inst. Oswaldo Cruz, 2004, 99, 541–544 CrossRef CAS PubMed.
- M. Isman, Crop Prot., 2000, 19, 603–608 CrossRef CAS.
- P. Houghton, Y. Ren and M. Howes, Nat. Prod. Rep., 2006, 23, 181–199 RSC.
- S. Andrade-Ochoa, J. Correa-Basurto, L. M. Rodríguez-Valdez, L. E. Sánchez-Torres, B. Nogueda-Torres and G. V. Nevárez-Moorillón, Chem. Cent. J., 2018, 12, 53 CrossRef CAS PubMed.
- S. D. Sharma, R. B. Thapa, K. C. Bahadur, G. Bhandari and S. Tiwari, J. Maize Res. Dev., 2016, 2, 58–65 CrossRef.
- C. S. de Lira, E. V. Pontual, L. P. Albuquerque, L. M. de Paiva, P. M. G. Paiva, J. V. de Oliveira, T. H. Napoleao and D. M. d. A. F. Navarro, Crop Prot., 2015, 71, 95–100 CrossRef CAS.
- J. S. Dambolena, M. P. Zunino, J. M. Herrera, R. P. Pizzolitto, V. A. Areco and J. A. Zygadlo, Psyche, 2016, 2016, 4595823 Search PubMed.
- S. Shapira, S. Pleban, D. Kazanov, P. Tirosh and N. Arber, PLoS One, 2016, 11, 0156540 Search PubMed.
- X. Sun, S. Wang, T. Li and Y. Yang, Trop. J. Pharm. Res., 2015, 14, 619–625 CrossRef CAS.
- M. Y. Chang and Y. L. Shen, Molecules, 2014, 19, 6694–6706 CrossRef PubMed.
- T. Cerchiara, S. V. Straface, E. Brunelli, S. Tripepi, M. C. Gallucci and G. Chidichimo, Nat. Prod. Commun., 2015, 10, 547–549 CrossRef PubMed.
- M. Y. Chang, D. E. Shieh, C. C. Chen, C. S. Yeh and H. P. Dong, Int. J. Mol. Sci., 2015, 16, 28169–28179 CrossRef CAS PubMed.
- J. Legault and A. Pichette, J. Pharm. Pharmacol., 2010, 59, 1643–1647 CrossRef PubMed.
- J. A. Miller, J. E. Lang, M. Ley, Y. Jia, K. H. Kim and S. J. Lee, Cancer Prev. Res., 2013, 6, 577–584 CrossRef CAS PubMed.
- R. Russo, M. T. Corasaniti, G. Bagetta and L. A. Morrone, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 397821 Search PubMed.
- E. S. Fernandes, G. F. Passos, R. Medeiros, F. M. Cunha, J. Ferreira and M. M. Campos, Eur. J. Pharmacol., 2007, 569, 228–236 CrossRef CAS PubMed.
- M. Huo, X. Cui, J. Xue, G. Chi, R. Gao and X. Deng, J. Surg. Res., 2013, 180, 47–54 CrossRef PubMed.
- Y. Li, O. Lv, F. Zhou, Q. Li, Z. Wu and Y. Zheng, Neurochem. Res., 2015, 40, 1520–1525 CrossRef CAS PubMed.
- S. Y. Cho, H. Jun, J. H. Lee, Y. Jia, K. H. Kim and S. J. Lee, FEBS Lett., 2011, 585, 3289–3296 CrossRef CAS PubMed.
- T. R. D. O. Ramalho, M. T. P. Oliveira, A. L. D. A. Lima, C. R. B. Santos and M. R. Piuvezam, Planta Med., 2015, 81, 1248–1254 CrossRef CAS PubMed.
- S. Aggarwal, S. Agarwal and S. Jalhan, Int. J. Pharm. Biol. Sci., 2013, 4, 857–868 CAS.
- A. Kumar, Int. J. Pharma Bio Sci., 2014, 5, 225–231 CAS.
- J. L. Patil, S. Us, M. Mk and P. Shetti, J. Pharm. BioSci., 2018, 6, 27–33 CrossRef CAS.
- J. F. Cândido, R. M. Lopes, X. F. Lauro and E. A. F. Cândidoostst, Int. J. Drug Dev. Res., 2017, 7, 15837–15843 Search PubMed.
- B. C. Cavalcanti, J. R. O. Ferreira, I. O. Cabral, H. I. F. Magalhaes, C. C. Oliveira and F. A. R. Rodrigues, Food Chem. Toxicol., 2012, 50, 4051–4061 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07370b |
| This journal is © The Royal Society of Chemistry 2021 |
