Open Access Article
Open Access ArticleZein as a versatile biopolymer: different shapes for different biomedical applications
Silvia Tortorella ab,
Mirko Maturi
ab,
Mirko Maturi a,
Veronica Vetri Buratti
a,
Veronica Vetri Buratti a,
Giulia Vozzolo
a,
Giulia Vozzolo a,
Erica Locatelli
a,
Erica Locatelli a,
Letizia Sambri
a,
Letizia Sambri *a and
Mauro Comes Franchini
*a and
Mauro Comes Franchini *a
*a
aDepartment of Industrial Chemistry “Toso Montanari”, Alma Mater Studiorum – University of Bologna, Viale Risorgimento 4, 40136 Bologna, Italy. E-mail: mauro.comesfranchini@unibo.it
bIstituto per l’Endocrinologia e l’Oncologia Sperimentale “G. Salvatore” (IEOS), Consiglio Nazionale delle Ricerche (CNR), Via S. Pansini 5, 80131 Naples, Italy
First published on 6th December 2021
Abstract
In recent years, the interest regarding the use of proteins as renewable resources has deeply intensified. The strongest impact of these biomaterials is clear in the field of smart medicines and biomedical engineering. Zein, a vegetal protein extracted from corn, is a suitable biomaterial for all the above-mentioned purposes due to its biodegradability and biocompatibility. The controlled drug delivery of small molecules, fabrication of bioactive membranes, and 3D assembly of scaffold for tissue regeneration are just some of the topics now being extensively investigated and reported in the literature. Herein, we review the recent literature on zein as a biopolymer and its applications in the biomedical world, focusing on the different shapes and sizes through which it can be manipulated.
Introduction
Amongst natural and synthetic materials, the former is more favorable considering the enormous availability of raw materials and their advantages in terms of manufacturing costs and low environmental impact. The search for biopolymers from natural and sustainable sources is one of the most important goals for the scientific community working on the boundary of chemistry and biology. The purpose is the fabrication of novel systems, which could be exploited for diverse applications in the biomedical field, such as targeted drug delivery, bioimaging, or tissue engineering. In the last decades, several natural and synthetic polymers have been investigated in order to develop biocompatible smart materials for the above-mentioned purposes.1,2 Proteins extracted from plants, in particular, have the benefit of being extensively available on earth and can be easily worked into different shapes, as fibers, films, micro-, and nanoparticles, or even hydrogels for medical applications.3,4 They are surely favored compared to carbohydrates because proteins carry many functional groups (such as amino and carboxyl groups) that can be exploited, for example, for labeling targeting molecules or in order to allow fast and stable crosslinking in 3D scaffolds' fabrication. Moreover, the amphipathic nature of proteins gives them a versatile structure for easily and dynamically interacting with carbohydrates and lipids, just as at the interface between air and water.5Many plant proteins, such as wheat gluten and soy proteins, have been used for fabricating biomaterials and have demonstrated biocompatibility in vitro but further issues arise due to their very poor solubility (either in water or organic solvents) and their inferior mechanical properties compared to the more well-known animal proteins, collagen and silk.6
Zein is a class of alcohol-soluble proteins rich in prolamin, approved as a Generally Recognized As Safe (GRAS) excipient in 1985 by the United States Food and Drug Administration (US-FDA) for the film coating of pharmaceuticals.7,8 In 2019, the global corn fiber market size was valued at USD 718.9 million and is expected to grow at a compound annual growth rate (CAGR) of 6.3% from 2020 to 2027.9 The rising usage of corn in the pharmaceutical field is expected to drive the market growth over the forecast period, supported by the simultaneously increasing consumption of food and beverage products.
According to solubility and sequence homology, we can classify zein in 4 groups: α-zein (19 and 22 kDa), which includes 70–85% of the total fraction of zein mass, β-zein (14 kDa), γ-zein (16 and 27 kDa), the second most abundant fraction, and δ-zein (10 kDa).10 All zein classes have several hydrophobic and neutral amino acids (as leucine, proline, and alanine) and also contain some polar amino acid residues, such as glutamine. Zein stands out from other proteins because it almost completely lacks lysine and tryptophan, including a few arginine and histidine residues. This amino acid composition is the reason for its unique solubility, mainly restricted to acetone, acetic acid, aqueous alcohols, and aqueous alkaline solutions.11
Novel biomedical applications of zein such as the controlled and targeted delivery of bioactive molecules and tissue engineering are the current research interests of the scientific community.
Zein-based formulations are focused on the properties possessed by this interesting natural material: highly resistant to heat, water, abrasion, and humidity; zein protein from corn is able to enhance the possibility for a longer shelf-life of biomolecules. A variety of biomedical fields take advantage of zein and research is nowadays extremely prolific; therefore, conscious of all the respectable works already published on zein, we acted rationally, in order to make our review relevant and innovative. We decided to reserve our attention to the very recent literature and strictly to those examples where zein has been used as the major component of innovative materials or applications, limiting those studies where zein protein has been used only as a minor additive. In this way, we reported a selected and high-impact summary of the whole literature concerning this material, focusing on the very recent applications of zein as an attractive promising biopolymer exclusively in the biomedical field and biomedical applications. Moreover, we rationalized the literature based on zein forms in terms of shape and size. We emphasized on chapter arrangement in order to help the reader find the desired application in a wide panning shot, from the 1D to the final 3D perspective. Furthermore, we think that this review can provide a multidisciplinary scenario of the emerging area of nanomedicine, which is increasingly becoming the glue between different scientific fields.
As touched upon, due to the huge number of cases that must be reported, we have divided the current survey into 3 chapters, following a logical thread based on the dimension of the zein-based system: in the first chapter zein nanoparticles and nanocomplexes are discussed; the second chapter is dedicated to fibers, films, and membranes; eventually, 3D objects as microbeads, gel, and scaffolds are examined in the third chapter.
Zein particles
The hydrophobicity of zein is well known and is attributed to the high percentage content of non-polar amino acids such as alanine, proline, and leucine.14 Zein solubility characteristics have been exploited to generate nano or microspheres that are employed for drug, nutraceutical, or essential oil encapsulation. Colloidal particles are generated exploiting zein solubility in binary solvent mixtures, with procedures such as anti-solvent precipitation, liquid–liquid dispersion, and phase separation or coacervation. A typical workflow of this method, reported by Zhong et al.15 involves the dissolution of zein together with the active compound in 55–90% of ethanol-deionized water binary solvent mixture. A key step for obtaining nanoparticles with appropriate features is the selection of a suitable solvent and antisolvent couple, considering their miscibility in the concentration range in which they will be used. The addition of a non-solvent to the previously prepared ethanolic solution induces supersaturation, leading to the precipitation of the solute and the formation of nanoparticles; after freeze-drying, a powder consisting of zein nanoparticles is finally formed. Recently, the importance of implementation of the freeze-drying process after the production of resveratrol-loaded zein NPs by means of the nanoprecipitation technique has resulted in more extensive drug release compared with fresh NPs, as demonstrated by Nunes and coworkers.16 The properties, drug loading capacity, and finally, the drug release profile of the resulting nanoparticles are closely related to some experimental parameters, as reported in Fig. 1. Numerous studies have been carried out in order to understand how the variation of all these factors affects the controlled release curve of bioactive species.17
 | ||
| Fig. 1 Schematic representation of the antisolvent precipitation method for generating zein colloidal particles and factors affecting the particle properties, drug loading, and release profile. This figure has been adapted/reproduced from ref. 17 with permission from Elsevier, 2021. | ||
This methodology does not require complex equipment and has straightforward preparation conditions, providing low-cost and satisfactory encapsulation efficiencies for bioactive compounds. Due to its simplicity, the anti-solvent precipitation method still represents the most commonly used technique to afford zein NPs (diameters ranging from less than 100 nm to a few micrometers) but it also presents some drawbacks.18 Accurate characterization has highlighted an excessive variability in size and morphology depending on the choice of experimental parameters, which can result in poor reproducibility of the procedure. Moreover, while these techniques are suitable for the optimization of formulations using small volumes of samples, the same results are not ensured at larger scales. Sometimes, this synthetic process has been slightly improved in the attempt of obtaining specific carrier morphologies and properties. Xu et al., for instance, reported a procedure to fabricate hollow zein nanoparticles by a modified antisolvent precipitation method.19
This strategy, as described in Fig. 2, involves the precipitation of zein in solution in the presence of sodium carbonate as a sacrificial template; after zein precipitation on the inorganic compound, sodium carbonate was dissolved by adding water, giving rise to hollow nanoparticles of zein, which are suitable for controlled release and direct cell delivery of the encapsulated drug molecules.
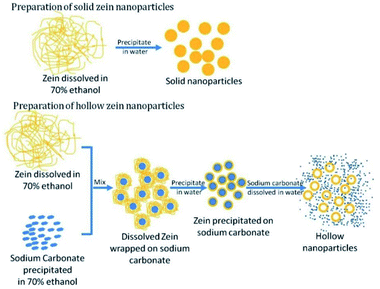 | ||
| Fig. 2 Preparation method for solid and hollow zein nanoparticles. This figure has been adapted/reproduced from ref. 19 with permission from the Royal Society of Chemistry, 2021. | ||
The possible synthetic alternatives are represented by supercritical antisolvent precipitation techniques (SAS) and spray-drying, which are strictly related techniques that differ for the process of solvent removal, respectively carried out using an aerosol solvent extraction process mediated by supercritical CO2 and a warm air stream. Closely related to the spray-drying technique is the electrohydrodynamic atomization, well-known as electrospraying, which is based on the separation of a liquid into charged droplets under the influence of an electric field. Taking advantage of this technique, it is possible to prepare monodisperse nanoparticles in several morphologies and sizes, depending on the different parameters that control the process.20 Kang et al. showed how new generation drug–polymer@lipid hybrid nanoparticles, achieved via modified coaxial electrospraying, perfectly release Tamoxifen citrate with a typical Fickian diffusion mechanism.21 Despite the scalability of this single-step manufacturing process favoring a variety of applications within the pharmaceutical, biotechnology, and medical industries, its applicability remains limited due to the high implementation cost.
The aforementioned supercritical antisolvent technique (SAS) shows the possibility to overcome some practical problems associated with the processes described before. Among these, the most common include the possibility of scaling up the process without particular complications, the thermal degradation of the bioactive species, the usage of multistage processing for organic solvents removal, the broad size distribution, and the wide variability due to the setting if the experimental parameters.22 The supercritical antisolvent process and its relative modifications consist of a feed continuously sprayed into supercritical CO2 (scCO2) that acts as an anti-solvent for a majority of polymers. Recently, Rosa et al. studied, for the first time, the incidence of the operating conditions on the encapsulation of the vitamin complex containing riboflavin, δ-tocopherol, and β-carotene through the SAS technique.23 According to the experimental results, microcapsules with the mean particle size varying from 8 to 18 μm and spherical morphology were formed, depending on the vitamin-encapsulated nature.
Despite the many advantages, this technique cannot completely replace the conventional methodologies because of the elevated pressure required, high maintenance cost, and requirement of accessories/auxiliary equipment. Furthermore, its incompatibility with some active species limits the use of supercritical fluid techniques for a large class of pharmaceuticals.24
More recent generation single step and reproducible techniques are represented by built-in ultrasonic dialysis process (BDUP) and antisolvent-dialysis method in order to get zein micro and nanoparticles by means of self-assembly induced by the concentration gradient and polarity change of the solvents. In both cases, the nucleation process is triggered when the concentration of the biopolymer reaches the critical limit of saturation and the interface between the biopolymer and the antisolvent is broken.25
Features such as the high thermal resistance and the great oxygen barrier properties make the zein protein an attractive matrix for the encapsulation of compounds sensitive to temperature and/or oxidation. Among the major benefits of this practise, it is possible to obtain an improvement in the solubility of poorly soluble molecules, the ability to deliver the molecule in a sustained or controlled way, the protection from degradation, and the possibility of targeting different cells and/or tissues.26 When orally administered, zein nanoparticles move slowly along the gastrointestinal tract, interacting with the protective mucus layer lining the small intestine epithelium. These nanocarriers are not capable of entering into the circulation; consequently, they display a long residence time within the gut, about 24–48 h, before being degraded and/or eliminated. This is in line with the fact that zein is relatively resistant to digestive enzymes. Thorough research of the literature has revealed numerous examples that show how zein is the ideal candidate to fill the role of a nanocarrier; therefore, the most recent works are listed below, subdividing them according to the class to which the bioactive species belong. Among all the bioactive compounds, polyphenols have often been chosen as a model for hydrophobic drugs to be encapsulated into the zein nanocarriers. Polyphenols are known to interact well with prolamin residues via hydrophobic interactions and hydrogen bonding. Moreover, they have excellent solubility in lower alcohols, a reason why they can be encapsulated in zein colloidal particles using the process of anti-solvent precipitation.
Curcumin is probably the most investigated natural polyphenol due to its clinical potential. However, curcumin exhibits low oral bioavailability because of its limited solubility in water, physicochemical and biological instability, and extensive pre-systemic metabolism. Consequently, the delivery of curcumin in zein NPs has been widely studied. The encapsulation of curcumin in the polymeric matrix of zein colloidal particles was reported to give the expected results, improving the photostability and stability of curcumin at all physiologically relevant pH. The colloidal formulation was found to be stable under gastrointestinal conditions, overcoming the first-pass metabolism; in addition, the particles showed mucoadhesive properties, which were also studied by Patel et al. in vitro on Caco-2-cells.27 Liu et al. studied a novel built-in ultrasonic dialysis process to generate zein microspheres loaded with curcumin, developing a semi-empirical model for predicting the particle size.28
Apart from curcumin, other polyphenols have been encapsulated in zein NPs. Quercetin is another well-known polyphenol that acts as a good lipid peroxidation inhibitor and shows strong anti-inflammatory activity, attributed to its ability to inhibit the proinflammatory cytokine TNF-α (tumor necrosis factor alpha).29 However low aqueous solubility and degradation in physiological conditions limit its oral bioavailability.30 In their work, Penalva et al. encapsulated quercetin into zein NPs with the assistance of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), chosen for its capability to both increase the encapsulation of lipophilic molecules and to promote the oral bioavailability of specific compounds such as quercetin. The administration of zein NPs loaded with quercetin showed sustained release in time up to 72 h, while quercetin oral availability was calculated to be close to 60% in rats.
Procyanidins are oligomers and polymers of flavan-3-ols, which are known to have potent antioxidant activity and may reduce the risk of chronic diseases.31,32 However, similar to other polyphenols, their oral bioavailability is extremely low. Zou et al. fabricated procyanidin-loaded zein nanoparticles (CPs-zein NPs) with particle size lower than 450 nm, using a modified liquid–liquid dispersion method. In addition, their cytotoxic activity using human promyelocytic leukaemia HL-60 cells was evaluated, and they found that, even if the bioavailability of procyanidins was increased by the encapsulation, the encapsulated CPs had decreased cytotoxicity compared to the CPs in solution.33,34
Similarly, de Rosa et al. evaluated the antioxidant and antimicrobial activity of phenolic monoterpene-loaded zein nanoparticles, whose efficiency depends on preservation and bioavailability. SEM, TEM, and DLS analyses revealed the characteristic tracts of thymol and carvacrol-loaded nanoparticles and demonstrated the storage stability during the 90 day monitoring between +6 and 20 °C. The strong interaction of the encapsulated compounds with the wall material led to a controlled drug release over time (50% in 72 h) without burst effects, ensuring antimicrobial activity against Gram-positive bacteria.35
Driven by the enormous efforts that the research community is spending in designing innovative anticancer treatments, several chemotherapeutic agents have been encapsulated into zein nanocarriers with consequent evaluation studies about their efficiency in targeting the specific sites and accumulation, thanks to the EPR effect.
In particular, 5-fluorouracil (5-FU)-loaded zein NPs using a phase separation procedure were prepared by Lai et al.36 5-FU is a chemotherapeutic agent that suffers from low oral bioavailability and toxicity to the bone marrow and gastrointestinal tract; thus, it may benefit from NPs encapsulation and delivery. Indeed, in vitro 5-FU-loaded zein NPs revealed a sustained release profile of the drug. In vivo experiments on mice revealed a nanocarrier distribution mostly located in the liver after intravenous administration, displaying blood retention for 24 h. This study demonstrated that these drug-loaded zein NPs could be efficiently targeted at the liver by intravenous delivery.
Doxorubicin-loaded zein NPs formation and in vitro release studies on HeLa cells cancer lines were reported in 2016 by Dong et al.37 Doxorubicin (DOX), a water-soluble anti-cancer drug, has limited clinical use because it is harmful to healthy tissues and has significant side effects, such as cardiotoxicity and myelosuppression. The site-specific supply can be achieved using a drug delivery nanosystem, designed to localize drugs at the target sites. These studies indicated that DOX-zein-NPs exhibited a sustained pH-responsible release and that at the same concentration of DOX, DOX-zein-NPs killed more HeLa cells than free DOX. Furthermore, DOX-zein-NPs entered the cytoplasm of HeLa cells and the DOX release was delayed and controlled by the zein encapsulation structure.
An interesting study was carried out by Thapa et al. in 2017 on a potential prostate cancer therapy by means of Vorinostat (Vor) and Bortezomib (Bor), a histone deacetylase inhibitor and a proteasomal, respectively.38 Their combination chemotherapy could be an attractive strategy for treating prostate cancer because Vor enhances protein unfolding and Bor prevents unfolded protein degradation, thereby enhancing ER stress-mediated apoptosis. After preparing Vor and Bor zein NPs (ZNP/VB), the ZNP/VB treatment was evaluated both in vitro in various prostate cancer cells lines (PC3, DU145, and LNCaP) and in vivo. In vivo studies have been performed in a PC3 tumor xenograft model using BALB/c nude mice (7 week-old) by subcutaneously injecting 2 × 107 PC3 cells per mice into the right thigh flank: the results highlighted a significant and encouraging reduction in the tumor volume. Compared to control and free drug-treated groups, the antitumor effect was enhanced by the combination drug treatment and was further increased significantly by the ZNPs.
Zein nanoparticles and microparticles were evaluated mainly as drug delivery systems of hydrophobic pharmaceutics but recently, Lau et al. were able to also report the encapsulation of hydrophilic drugs.39,40 In particular, their studies concerning the production of zein microparticles by coacervation and their investigation as carriers for prednisolone and hydrocortisone, two corticosteroids, and Mesalazine. Zein NPs were also promising for peptides and protein delivery; it has been reported as an effective release of insulin after oral administration, with a loading of about 17% of insulin in zein microspheres.41 Lee et al. developed a zein-based drug delivery system for the encapsulation of catalase and superoxide dismutase (SOD), two enzymes that have therapeutic potential, acting as scavengers of reactive oxygen species (ROS).42 These therapeutic proteins are easily degraded in gastrointestinal conditions and their encapsulation into zein nanoparticles strongly increased their bioavailability.
In addition to the encapsulation of bioactive molecules, peptides, and proteins, zein NPs are useful tools for the controlled and targeted delivery of genetic material to intracellular compartments. Gene delivery, the introduction of exogenous DNA into cells with subsequent expression, applies to the fields of gene therapy, DNA vaccination, functional genomics and diagnostics, and tissue engineering. The use of plasmid DNA (pDNA), which has lower immunogenicity, more flexibility in transgene capacity, and with the potential for industrial production, is a more appealing solution for gene transfer than viral gene delivery.43 Zein NPs encapsulating plasmid DNA designed for oral delivery have been reported by Regier et al.44 Zein NPs were easily fabricated using a simple coacervation technique, which is employed without the use of unfavorable harsh solvents or high temperatures. DNA encapsulation efficiencies were maximized and the zein nanospheres exhibited a sustained release of the plasmid for 7 days in vitro. Concerning their encouraging results, the authors suggested specific applications including oral gene delivery, intramuscular delivery, and the fabrication of tissue engineering scaffolds.
In order to give a panning shot on this last chapter part, a table has been generated, containing all the encapsulated bioactive molecules described (Table 1).
| Drug or therapeutic agent | NPs preparation method | Delivery target/disease | In vitro/in vivo studies | Ref. |
|---|---|---|---|---|
| Resveratrol | Nanoprecipitation | Antioxidant, anti-inflammatory, cardio- and neuroprotective,anticancer | Cytotoxicity and permeability studies on Caco-2 and HT29-MTX cell lines | 16 |
| Vitamin complex | Supercritical anti-solvent technique | Vitamin deficiency | — | 23 |
| Polyphenols | Antisolvent precipitation method | Antioxidant, anti-inflammatory, antiproliferative, antiangiogenic | In vitro mucoadhesion study on Caco-2 cells | 27 |
| Curcumin | Built-in ultrasonic dialysis process | — | 28 | |
| Quercetin | Desolvation procedure of an hydroalcoholic solution | Antioxidant, anti-inflammatory | In vitro release in simulated gastric fluid, in vivo pharmacokinetic after i.v. administration, anti-inflammatory efficacy after intraperitoneal injection | 30 |
| Procyanidine | Liquid–liquid dispersion method | Antioxidant, cardioprotective | Apoptotic and cytotoxic properties on HL-60 cancer cells | 32 |
| Phenolic monoterpenes | Nanoprecipitation | Antimicrobial, antioxidant, anti-inflammatory | Antibacterial activity against gram + and gram − bacteria | 35 |
| Thymol | ||||
| Carvacrol | ||||
| 5-FU | Phase separation process | Chemotherapeutic agent | In vitro drug release in buffer, biodistribution and target efficiency in vivo in mice after i.v. administration | 36 |
| Doxorubicin | Phase separation method | Chemotherapeutic agent | Anti-proliferative effect on HeLa cells | 37 |
| Vorinostat/bortezomib, Vor/Bor | Phase separation method | Histone deacetylase inhibitors for cancer therapy | In vitro cellular uptake by prostate cancer cells and cytotoxicity study, in vivo antitumor efficacy on tumor xenograft model in BALB/c nude mice | 38 |
| Prednisolone | Phase separation method | Inflammatory bowel disease | In vitro release in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) | 39 |
| Hydrocortisone | Coacervation from 70% (v/v) ethanol | Inflammatory bowel disease | In vitro digestibility assay | 40 |
| Mesalazine | ||||
| SOD | Phase separation method | Rheumatoid arthritis | In vitro determination of enzyme activities by SOD assay kit, delivery of catalase or SOD in zein nanoparticles to activated RAW-264.7 macrophages | 42 |
| Genetic material | Coacervation | Gene therapy and DNA vaccination | pDNA release in PBS buffer, in vitro biocompatibility, cellular association and internalization in Caco-2 and HEK 293T cell lines | 43, 44 |
Zein-based multicomposite nanoparticles for improving the stability
Although zein NPs are biocompatible and can be easily prepared, they tend to form aggregates during freeze-drying (lyophilization) and cannot be redispersed into water. The low colloidal stability after centrifugation and lyophilization limits the applications of zein-based colloidal carrier systems. At the physiological pH, close to the zein isoelectric point (pI = 6.2), or at high ionic strength, an enhancement of hydrophilic interactions occurs, while electrostatic repulsions decrease, inducing the aggregation of the biopolymer. The pH adjustment during and after centrifugation is usually not sufficient or not applicable; adding other ingredients into the formulation can lead to the formation of core–shell particles, resulting in improved colloidal stability and a better-controlled release profile of bioactive compounds.17 Podaralla and co-workers, for instance, demonstrate how sometimes the effect of blending or chemical modification is not limited to stabilization but also has other beneficial implications for the final product. Zein-based micelles prepared via conjugation with methoxy poly(ethylene glycol) (mPEG) are found to be nonimmunogenic and do not elicit any antibodies when injected subcutaneously in mice. The chemical modification of bioactive compounds with polyethylene glycol chain, so-called PEGylation, usually leads to improved pharmacokinetics and biological function. In addition, it is possible to prevent opsonization and provide steric hindrance for the enzymatic degradation of the protein. Curcumin-loaded micelles can be efficiently taken up by endocytosis, leading to a cellular uptake that is 3-fold higher than free curcumin against drug-resistant ovarian human cancer cells.45Although blending is often preferred to the use of surfactants/emulsifiers in order to maintain the characteristics of biocompatibility and biodegradability, in very recent studies, it has been shown how those two concepts can sometimes go at the same pace, with the introduction of compatible surfactants counting Tween 80 (ref. 46), Tween 20 (ref. 47), Brij®,48 sodium deoxycholate,49 lecithin, and Pluronic F127®. In particular, Chuacharoen et al. displayed the use of those latter in the synthesis of lutein-loaded nanoparticles, taking advantage of a solvent-free liquid–liquid dispersion method. In addition to increased size, improved polydispersity index, and decreased zeta potential, nanoparticles stabilized with a combination of phospholipid soybean lecithin and tri-block copolymer Pluronic F127® showed a controlled release of lutein, preventing its degradation under different temperatures and in the presence of UV light.50
In the last years, the use of stabilizers has been widely investigated and includes lecithin/poloxamer-188,38,45,51 sodium caseinate,27,52,53 β-lactoglobulin,54 pectin,53,55 and lysine.56,57
In this framework, Lucio et al. recently evaluated the capability of zein nanoparticles with lysine as an emulsifier for acting as oral carriers of glibenclamide (GB).57 Glibenclamide, a sulfonylurea commonly used for the treatment of type II diabetes mellitus (T2DM), presents low solubility and its dissolution in a physiological medium is considered to be the rate-limiting step for its adsorption. The so-formed zein NPs with lysine as the stabilizing agent presented a mean size of 190 nm. The nanoencapsulated GB showed a hypolipidemic activity in C. elegans worms and may be of interest to develop a new oral formulation of this antidiabetic drug.
More recent studies have focused on the development of multilayer nanoparticles to increase the stability of the system under several environmental stresses, such as pH, salt, thermal processing, chilling, freezing, dehydration, and mechanical agitation. For this purpose, McClements summarized in a very detailed review all the potential advantages and drawbacks of multilayer emulsions over conventional emulsions for industrial and medical applications and how it is possible to tune the composition and properties of the nanolaminated coatings, leading to the increased stability of the colloidal particles toward aggregation.58 The article is not related to zein but we believe it is very relevant in the field because it reviews the latest developments in the creation of structured emulsions, including multiple emulsions, multilayer emulsions, colloidosomes, microclusters, filled hydrogel microspheres, and hybrid systems, discussing the structure, fabrication, properties, and potential applications of each type of structured emulsion. Moreover, since emulsion droplets are the basic building blocks of structured emulsions, recent advances in their fabrication with specific properties (size, charge, interfacial properties, and physical state) have also been revised by the author.
In order to prepare multilayer nanoparticles, additional ingredients for coating and processing steps are required. Several biopolymers, either proteins (sodium caseinate and gelatin,59,60), polysaccharides (chitosan or modified chitosan, pectin, alginate, hyaluronic acid53,60–63), and glycolipid (rhamnolipid64), have been investigated as coating materials for the outer surface of zein nanoparticles, to enhance their stability by electrostatic and steric effects.
Sodium caseinate, for example, is a mixture of several caseins (αs1, αs2, β, and κ) widely used in the food industry as an emulsifier/stabilizer; it has been reported to act for a combination of electrostatic and steric stabilization.27,52,53 For this purpose in 2018, Zhang et al. investigated how some experimental conditions such as the initial concentration of biopolymers, the drug dosage, and ethanol volume fractions significantly affected the antioxidant activity in an aqueous medium of novel rutin-loaded zein-sodium caseinate nanoparticles prepared via antisolvent nanoprecipitation technique.65
More in-depth studies evaluated the different physicochemical properties of rutin-integrated biopolymeric nanoparticles. Zein nanosystems showed a higher ability to retain the drug if compared with the PLGA (poly(lactic-co-glycolic acid) analogue; the protein matrix and the use of sodium deoxycholate monohydrate as a stabilizer promoted great entrapment efficiency in addition to the prolonged release of the bioactive compound. The protective effect exerted by the active compound is increased, even though further studies will be performed in order to investigate the mechanisms involved in the pharmacological synergistic outcomes of zein and rutin nanoencapsulation.66 Similarly, as reported by Heep and co-workers, the use of zein-casein-lysine-based NPs was tested for the encapsulation of ferulic acid (FA) to improve the FA biological effects by the oral route. The in vitro release assay indicated that zein-casein-lysine NPs are promising carriers for sustained FA release. Furthermore, the cytotoxicity assay showed that NPs did not present cytotoxicity over Caco-2 and Caco-2/HT29-MTX cells for 4 h-incubation. Overall, zein-casein-lysine NPs were presented as a potential oral drug delivery system in the treatment of several diseases where oxidative stress is presented.
However, protein-coated zein NPs are still highly sensitive to aggregation at pH values near the isoelectric point of the absorbed protein layer; under these conditions, electrostatic repulsions are not sufficient anymore to overcome the attractive interactions (van der Waals and hydrophobic) and the steric stabilization could be unsatisfactory. In addition, casein-zein NPs have been reported to have low aggregation stability under gastrointestinal conditions.67 A way to overcome this problem was proposed by Davidov-Pardo, McClements et al. who developed caseinate–dextran conjugates as the coating of zein nanoparticles.68 The chemical conjugation of amino groups of caseinate with the carbonyl groups of dextran is achieved via the Maillard reaction to give Schiff bases; the thermolabile glycosylamine subsequently undergoes Amadori rearrangement, affording a 1-amino-1-deoxy-2-ketose (Fig. 3). The conjugates were then used as a coating material for zein nanoparticles. As expected, zein NPs coated with conjugates showed improved stability, being stable at pH changes (from 2 to 9), in the presence of CaCl2 100 mM, and at a temperature between 30 °C and 90 °C for 30 minutes.68
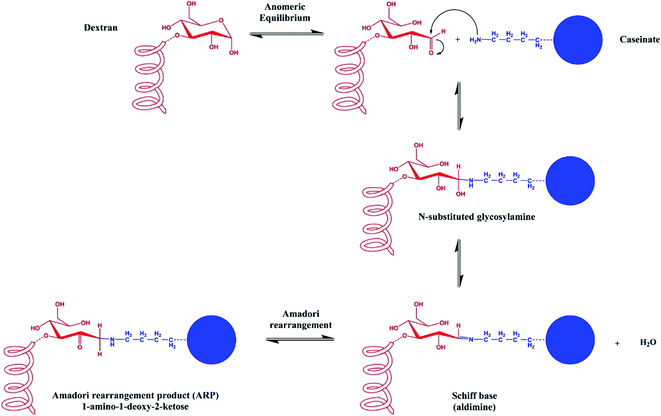 | ||
| Fig. 3 Schematic representation of the Maillard conjugation reaction between the reducing end of a sugar molecule and a free amine group of a protein, affording a Schiff base and then the Amadori rearrangement product (ARP). This figure has been adapted/reproduced from ref. 68 with permission from the American Chemical Society, 2021. | ||
As already mentioned, polysaccharide coated-zein NPs have been developed for obtaining more stability and gastrointestinal resistance. Zein-chitosan particles have been used to encapsulate curcumin,61 α-tocopherol,69 vitamin D3,70 indole-3-carbinol, and 3,3′-diindolylmethane,71 thymol,62 and resveratrol.72 In addition, several composites NPs have been developed, including zein/casein/pectin,53 zein/casein/chitosan,62 zein/gelatin/alginate,60 zein/hyaluronan,63 and other blends.
Zhang et al. investigated the potentiality of water-soluble chitosan hydrochloride (CHC) and sodium caseinate (SC) coating on thymol-loaded zein NPs to improve their physicochemical properties and antimicrobial activity.62 Due to the presence of sodium caseinate, the stabilized zein NPs showed a shift of the isoelectric point from 6.18 to 5.05 and a desirable redispersibility in water at neutral pH after lyophilization. The CHC coating onto the SC stabilized zein NPs resulted in increased particle size, reversal of zeta potential value from negative to positive, and improved encapsulation efficiency. Furthermore, such encapsulated thymol was reported to have stronger antimicrobial activity against S. aureus, suggesting that these colloidal NPs could potentially be used as all-natural delivery systems for antimicrobial agents and benefit in food, pharmaceutical, and agricultural formulations. More recently, chitosan-coated zein NPs for the encapsulation of resveratrol, a natural antioxidant stilbenoid, were developed and characterized by Pauluk et al.72 In addition to the enhanced resveratrol encapsulation stability in simulated gastrointestinal fluids (SGIF), thanks to the chitosan coating, in vitro mucoadhesion assay showed that chitosan played an important role in the improvement of mucin adsorption on particles surface, suggesting the possibility to exploit chitosan coating not only for an improvement of the zein NPs physicochemical stability but also for the development of mucoadhesive properties.
Thanks to the recent studies of Chang et al., it has been confirmed that pectin coating is able to stabilize nanoparticles under gastrointestinal conditions. The authors prepared sodium caseinate-zein NPs coated with pectin via a pH and heating-induced electrostatic adsorption process.53 They found that only about 40% of curcumin was released in the simulated gastric fluid (SGF), followed by sustained release in the simulated intestinal fluid (SIF), leading to a general improvement of the sustained release of this hydrophobic compound in both gastric and intestinal physiological conditions.
Another formulation that led to an improvement of curcumin sustained release in SGIF was developed by Chen et al., who prepared zein-hyaluronan NPs. Circular dichroism and fluorescence results revealed that the zein secondary structure changed after its combination with hyaluronan.63
Recent efforts to improve the encapsulation efficiency, thermal and photo-stability, and in vitro release profiles of curcumin were also made by Dai et al., who fabricated zein–rhamnolipid complex NPs.64 Rhamnolipid is a glycolipid that generally consists of one or two polar moieties together with a non-polar fatty acid chain; its nonpolar alkanoic acid derivative unit provides the anionic behavior at an appropriate pH range. In this study, it was demonstrated that the presence of rhamnolipid highly increased the encapsulation efficiency of curcumin in zein NPs from 17.64% to 98.05%, as well as its thermal and UV irradiation stability.
Cold fish gelatine is a natural biopolymer that can be used as a gelling, foaming, or emulsifying agent in foods. Studies have shown that gelatine can interact with anionic polysaccharides (such as gum arabic, k-carrageenan, and pectin) through electrostatic interactions, which results in the formation of films or gels. Oppositely charged proteins and polysaccharides can assemble into electrostatic complexes (coacervates), forming a thick layer around the hydrophobic particles, such as lipid droplets, thus improving their stability by generating a strong steric repulsion. Consequently, Yao et al. examined the possibility of using electrostatic complexes consisting of gelatine and alginate to coat the zein NPs.60
Zein nanocomplexes for imaging, targeting, and photothermal cancer therapy
We already extensively discussed how zein NPs are widely exploited in drug delivery; however, they also find applications in imaging and theranostics.Indeed, zein NPs have been exploited for the development of fluorescent nanoagents.73,74 In their work, Aswathy et al. developed zein-QD NPs for the nanoscale delivery of 5-FU. Quantum dots (QDs) are nanometer-sized inorganic semiconductor fluorophores, which absorb light in a particular wavelength and re-emit in another one. Although QDs find interesting applications in the medical field as excellent probes for bioimaging, their toxicity limits their applications. Hence, QDs have been coated with a variety of natural and synthetic biopolymers to make them biocompatible. In this work, zein NPS were made fluorescent by the addition of ZnS:Mn QDs;73 the biocompatibility of zein and zein-QD was demonstrated and the fluorescence imparted by the QD was exploited for cellular imaging. Furthermore, the cytotoxicity of 5-FU-loaded zein-QD NPS was proved in vitro on human breast cancer cell line MCF-7 and mouse fibroblast cell line L929.
As previously described, chemically modified zein NPs are obtained via Schiff base formation, by conjugation with QD630 exploiting 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) as the coupling agent.74 The fluorescent images indicated different uptake kinetics between QD630 and QD630/Zein by the NIH3T3 cells. The in vivo experiment of skin penetrating ability using nude mice was performed, revealing that QD630 particles penetrated through the stratum corneum barrier and localized within the epidermal and dermal layers by 8 h, and no QDs was found after 24 h, while for QD630/Zein, no significant penetration of the NPs was observed until 24 h. In conclusion, these studies proved that zein may alter QD630 skin penetration characteristics, suggesting a potential solution for reducing the QD630 toxicity.
One of the greatest challenges in anti-tumoral drugs development is to avoid their severe side effects against the normal tissues, generally arising from their lack of tumor selectivity. To minimize or avoid their toxic side effects, nanodrug delivery systems must circulate with an appropriate long half-life to reach the target tumor and must bind and enter the tumor cells. Without the aid of exogenous targeting ligands, NPs have been observed to target tumor passively through the EPR, or specific tissues, such as the lymphatic system, through molecular sieving.75 However, the chemical modification of the nanocomplex with cell-specific ligands can promote cell adhesion and its internalization via multiple endocytosis mechanisms, obtaining highly tumor-specific probes. In their work, Wang et al. developed novel self-assembled gold nanoparticles (AuNPs)-zein conjugated with folate.75 High expression of folate receptors in many tumor cells would allow their preferential internalization via receptor-mediated endocytosis. The conjugation with folate was achieved by a versatile surface modification method based on dopamine polymerization. Once polydopamine (PDA) shells are formed, folate was conjugated. The so-formed nanodrug delivery system was used to target tumor cells for the delivery of hydroxycamptothecin, an anticancer drug. In vitro and in vivo studies confirmed that PDA-coated nanocomplexes with the conjugation of folic acid were stable in the bloodstream, with minimal drug release in extracellular conditions, leading to prolonged blood circulation and high accumulation in tumor tissues, thus emerging as promising tools for the development of multiple-modal treatments in the future.
Besides anticancer drugs, great results have been achieved using alternative therapies, such as hyperthermia. Plasmonic nanomaterials, especially those that can convert near-infrared (NIR) light into heat, proved to be excellent as photothermal agents for localized hyperthermia cancer therapy. A series of coated gold plasmonic NPs have been fabricated to kill carcinogenic cells without damaging, or with minimum damage, to normal cells; among these systems gold nanorods (GNRs), nanocages or nanoshells, assembled gold NPs, and many other multifunctional nanocomposites are being explored for NIR light-driven photothermal therapy. In this framework, Chauhan et al. developed a facile, green, and scalable synthesis of gold deposited zein nanoshells (AuZNS) for the imaging-guided photothermal therapy of cancer.76 Water-soluble glycol chitosan was used as a stabilizer as well as for the cationic functionalization of zein NPs; multiple hydroxyl substitutions and free amino groups impart excellent water solubility at neutral and acidic pH. It helps in decreasing the toxicity induced due to acidic conditions and shows better in vivo stability. After glycol chitosan-functionalized zein NPs (GCHT-ZNPs) preparation, gold deposition was performed via an ex situ method at ambient conditions. The AuZNS size was reported to be about 100 nm and their size, polydispersity, and photothermal efficacy were reported to be comparable to the already developed polymer and liposome-based nanoshells. In vitro tests showed that AuZNS increased the temperature from 37 °C to 43 °C within 1 minute of the two different cancer cell lines, MCF-7 (breast cancer) and C33A (cervical cancer). The authors concluded that these novel AuZNS could provide a wide range of possibilities for the further development of nanomaterials, following the principle of green chemistry and facile synthesis, for cancer theranostic applications.
To conclude this chapter, it is appropriate to mention a novel zein drug delivery system that appears promising in the photothermal therapy of cancers and other diseases, and which exhibits controllable pH-dependent degradability and intracellular drug delivery activity.77 In this work, metal-tannic acid-coated zein NPs (zein-TA/metal NPs) were developed for anticancer drug delivery. The supramolecular shell was generated onto the zein NPs surface by mixing TA and metal within the as-prepared zein NPs solution. TA acted as an organic ligand while metal ions were used as the inorganic cross-linkers. DOX was encapsulated and, to test the antitumor activity from the self-assembled NPs, the cytotoxicity of free DOX NPs against HepG2 cells was investigated. In addition, biocompatible AuNPs were generated using zein-TA/metal NPs as reducing and stabilizing agents. Overall, the intense surface plasmon-enhanced absorption of AuNPs made Au@zeinTA/metal NPs greatly promising in the photothermal therapy of cancers and other diseases.
Zein films can be easily produced by spin casting, extrusion, and solvent casting, the last being the most popular approach to obtain zein-based films.78,79 Protein films can also be produced via scalable processes relying on compression molding or high-temperature extrusion, which generally results in increased plasticization effectiveness.80 Moreover, a less common strategy exploits the use of a spin coater to achieve ultrathin biodegradable films with high thickness control efficiency.81
A polymeric film for coating applications generally requires both mechanical properties, such as elongation or tensile strength, and barrier behavior to water and gas permeability.82,83 In particular, the mechanical and barrier properties of the film can be tailored by modulating the starting zein concentration, film casting solvent, and drying conditions.78 Tomoyuki et al. demonstrated that films obtained by high-concentration zein solutions display a lower transition temperature and elongation than films obtained by low-concentrated zein.84 More complicated effects can be observed starting from equal zein concentration solutions, tuning solvents, and drying conditions. Increasing the relative humidity, for instance, water acts as a strong plasticizer that alters both the internal and superficial films' microstructure, resulting in different surface morphologies and contact angles.85
The use of pure zein films is commonly limited owing to their brittleness and poor ability to act as a water barrier. In order to overcome this issue, it is strategically feasible to add plasticizers or crosslinkers, introduce chemical modifications, or even incorporate other biopolymers to get hybrid films. Plasticizers lead to a drop in the intermolecular/intramolecular interactions, which lower the glass transition temperature (Tg).86 At the same time, it improves the flexibility and chain mobility without altering the film barrier properties.87 Moreover, plasticizers can avoid shrinking during handling and storage and to slow down the aging process. The way plasticizers affect the properties of zein films is related not only to the intrinsic plasticization efficiency, depending on the number and positions of hydroxyl groups, but also to the extent of interactions between the zein and plasticizer molecules.88 The introduction of a plasticizer into a polymeric film can be achieved by directly adding it during the fabrication process or later, by absorption, and/or permeation. Besides water, which remains the most powerful natural plasticizer for hydrocolloid-based films, other plasticizers can be classified as amphiphilic and polar ones. The former generally include fatty acids and citrate esters, such as tributyl citrate,89 palmitic, stearic, oleic, and linoleic acids, whereas polar plasticizers consist of polyols such as mannitol, glycerol, and polyethylene glycerol.90,91
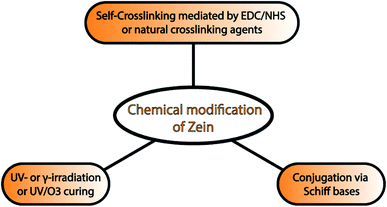
Currently, chemical modification can also be used to improve the toughness, mechanical strength, and water resistance of zein films. This approach includes self-crosslinking of zein molecules,92 conjugation,93 UV- and γ-irradiation, and UV/ozone curing of zein films. Kim et al. reported for the first time that the self-crosslinking of zein molecules mediated by EDC and N-hydroxysuccinimide (NHS) was able to suppress the aggregation phenomenon in solution and improve both the film-forming properties and the tensile strength. An alternative and interesting approach is the exploitation of natural crosslinking agents. As demonstrated by Khalil et al., PEG400/zein cross-linked with natural crosslinking agents, such as succinic acid, citric acid or succinic anhydride, and eugenol, exhibit enhanced mechanical properties and anti-pathogenic activities.94
Similarly, Soliman and coworkers showed how the chemical modification of zein with phenolic aldehydes via Schiff bases formation led to improved mechanical properties and antibacterial activity.93 Zein films with increased hydrophilicity can be obtained after UV/ozone treatment by converting some of the surface methyl groups into carbonyl groups. This chemical modification is an effective strategy to modulate zein film properties, which would further widen the application of zein as a barrier material and in drug delivery systems.81 A summary of chemical modification strategies is provided in Fig. 4.
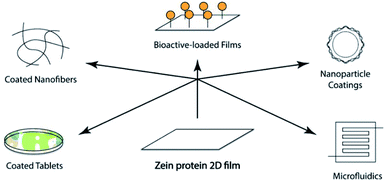
The last strategy evaluates the possibility of creating blended films embedding another natural or semisynthetic polymer. Once a proper solvent is selected, the blends can be obtained through chemical crosslinking reactions or by lamination reprocessing of a zein film and another polymeric film. Xia et al. fabricated tea polyphenol-doped multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity.95 Although the usage of zein for tablet coating has a long history, nowadays, it is also considered a promising film coating material for novel biomedical applications. These last, as summarized in Fig. 5, ranging from bioactive loaded films, are useful for the controlled release of pharmaceuticals in drug delivery, to tissue engineering96 and coatings of nanofiber, nanoparticle, and stent.78 Zein protein-based films, once degraded by proteolytic enzymes, lead to the production of amino acids, not toxic and easily re-absorbable by the body. In addition to full biocompatibility, they appear tough, glossy, hydrophobic, and perfect for any route of administration (oral, buccal, and transdermal).
Tablets are composed of a complex mixture of bioactive pharmaceutical ingredients and excipients. A polymeric coating is needed to hide unpleasant odors or tastes or for protecting ingredients from environmental risks such as moisture, oxygen, and light.97 Furthermore, this trick allows to ensure a site-specific controlled release of the drug and to increase the gastric retention time for drugs with narrow therapeutic windows.98
Recently, a pH-sensitive layer obtained by the combination of zein and Kollicoat® MAE 100P has been used to control the in vitro release of prednisolone for colon-targeted oral delivery in the treatment of inflammatory bowel diseases, such as Crohn's disease.99 Similarly, high colon-specific targeting of paracetamol can be achieved using amylose maize starch-zein coatings.
Coated tablets showed limited drug release under upper gastrointestinal tract conditions, indicating that zein effectively suppressed the swelling of starch, which remains untouched by pepsin and pancreatic enzymes.100 There are also plenty of examples showing the use of zein protein in NPs and implants coating. For example, Luo et al. focused their research on selenite-loaded chitosan nanoparticles.101 Their investigations validate how the encapsulation efficiency and release profile in simulated gastrointestinal conditions are improved after zein coating. In 2013, Han and coworkers published a detailed study on the histocompatibility of zein-coated vascular implants testing human umbilical vein-derived endothelial cells adhesion and ability to resist flow-shear.102 These studies are performed under simulated flow shear conditions, as well as to evaluate adhesion and cell spreading inside an environment that mimics the human body.
Several studies revealed that the zein film itself could act as a controlled release matrix for the delivery of bioactive compounds.13 The mechanism of drug release is not yet fully understood but film hydration and consequently swelling led to the formation of aqueous channels, which seems to be recognized as the dominant diffusional pathways. Being one of its original uses, multiple works are focused on the development of antimicrobial zein coatings. Arcan et al. obtained free radical scavenger zein films by incorporating lysozymes and catechins.103 The introduction of fatty acids into zein films resulted in lower drug release rates, depending on fatty acid chain length and double bond number, due to the increased film hydrophobicity and altered film morphology. Similarly, nisin and thymol-loaded zein films plasticized with glycerol were tested for their antimicrobial activity against Gram-positive bacteria.104,105
Nowadays, zein-based films find several applications in the study of alternative therapeutic pathways to ensure the controlled release and high-efficiency drug absorption. Innovative approaches that have recently received growing attention rely upon stimuli-responsive polymeric systems for controlled drug release. They included stimuli-responsive zein films containing magnetite NPs in order to provide a magnetically tunable device for the release of acetaminophen, used as a model drug, keeping the NPs features unmodified after film formation.106
Zein films can also be interestingly formed from drug-loaded nanoparticles/microparticles.107 Antioxidant nanocomposite films have been formulated using curcumin encapsulated into zein/sodium caseinate NPs, implementing an innovative self-assembling “pH-driven” method.108 This provides an alternative eco-friendly method to produce functional nanocomposites avoiding alcohol or other organic solvents usage. One of the most promising novel points is also represented by the film-forming nanogel. Ngo et al. reported the fabrication of a curcumin-loaded film-forming nanogel in accordance with the high attention to transdermal administration as a favorable route for drug delivery.109 The hydrogel quickly transforms into a versatile film with a uniform drug's dispersion, available for immediate treatment onto the site of application. This kind of administration allows prolonged release of the drug over a long period of time for an improved treatment and sustained tissue permeation.
Electrospinning is certainly the most popular method because of its reliability and versatility to produce fibers with diameters ranging from 10 nm to 10 μm.113 By applying coaxial electrospinning, it is possible to create fibers with a core-sheath structure, where zein constitutes the core and an un-spinnable solvent containing no polymer forms the sheath. Huang et al. described the preparation of ibuprofen-loaded corn zein nanofibers (NFs), where N,N-dimethylformamide was used as sheath fluid.114 In vitro dissolution tests showed that all the fibers could provide sustained drug release over a time period of 10 h. Zein NFs are characterized by poor mechanical strength and morphological instability, especially in wet conditions. Numerous efforts are adopted to improve their strength, either by blending with other polymers or using chemical crosslinking agents. For this purpose, Jiang et al. exploited citric acid as a non-toxic crosslinker agent to improve the tensile strength and water stability of zein-based electrospun fibers, resulting in the preservation of the fibrous structure even after immersion at 37 °C in PBS for up to 15 days.115 Furthermore, they showed enhanced attachment, spreading, and growth of mouse fibroblast cells if compared with those displayed by polylactic acid (PLA) electrospun fibers and not crosslinked electrospun zein fibers. The crosslinking process also contributes to modulating the zein surface hydrophobicity, which is appropriate for drug delivery vehicle ability (resistance to hydrolysis); but, on the other, can inhibit cell adhesion. Lu et al. realized water-stable zein/ethyl cellulose composite NFs, investigating its ability to release poorly water soluble indomethacine.116 In vitro dissolution tests revealed that nanofibrous membrane with blend ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had a sustained drug release profile, which can be further exploited as wound dressing material and tissue engineering scaffold. In a similar way, novel antibacterial structures have been obtained by co-electrospinning of zein/collagen blends with different weight ratio in aqueous acetic acid solution by means of berberine as drug model.117 Modifying the blending ratio, the electrospun membrane shows tunable fiber diameter, surface wettability, mechanical, and in vitro degradable properties as well as cell adhesive ability. Zein protein enhances the electrospinnability and fiber tensile strength, while the addition of collagen is functional for the improvement of surface wettability, in vitro degradability, and cell adhesion. The resulting membrane was observed to induce fast tissue regeneration when used as a dressing covering on skin wounds in mice.
1 had a sustained drug release profile, which can be further exploited as wound dressing material and tissue engineering scaffold. In a similar way, novel antibacterial structures have been obtained by co-electrospinning of zein/collagen blends with different weight ratio in aqueous acetic acid solution by means of berberine as drug model.117 Modifying the blending ratio, the electrospun membrane shows tunable fiber diameter, surface wettability, mechanical, and in vitro degradable properties as well as cell adhesive ability. Zein protein enhances the electrospinnability and fiber tensile strength, while the addition of collagen is functional for the improvement of surface wettability, in vitro degradability, and cell adhesion. The resulting membrane was observed to induce fast tissue regeneration when used as a dressing covering on skin wounds in mice.
In addition, an antioxidant electrospun zein nanofibrous network has been created by the encapsulation of the quercetin/cyclodextrin (c-CD) inclusion complex (IC) (Fig. 6).118
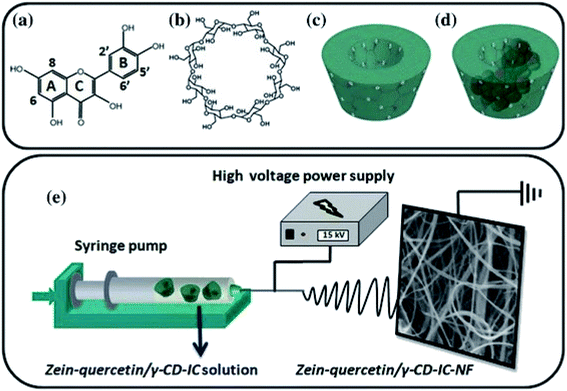 | ||
| Fig. 6 Chemical structure of (a) quercetin and (b) cyclodextrin (c-CD); schematic representation of (c) c-CD and (d) quercetin/c-CD- IC formation, and (e) electrospinning of nanofibers from zein-quercetin/c-CD-IC solution. This figure has been adapted/reproduced from ref. 118 with permission from Springer, 2021. | ||
As reported by Aytac and coworkers, non-covalent cyclodextrin inclusion complexes are formed in order to enhance quercetin's therapeutic bioavailability, bypassing its low aqueous solubility and fast breakdown. Another possible approach involves the use of this kind of substrate for the simultaneous delivery of two or multiple drugs to perform a synergic action in the treatment of certain pathologies. For instance, the co-administration of proton pump inhibitors is crucial for patients consuming non-steroidal anti-inflammatory drugs. Karthikeyan and coworkers developed an electrospun zein/eudragit nanofiber for the simultaneous delivery of aceclofenac and pantoprazole.119 In vitro tests demonstrated the release of both the drugs up to 8 h. In vivo animal experiments further confirmed that the co-administration of pantoprazole and aceclofenac reduced the gastrointestinal toxicity induced by the latter. A few years later, the same research group extended the use of zein-based NFs to gene silencing agents such as siRNA in order to facilitate its uptake and cytosolic delivery.120 In addition, Yao et al. described how zein electrospun NFs induce the biomineralization of hydroxyapatite (HA) nanocrystallites, mimicking both the architecture and composition of natural bone.121 The substrate was treated with acetic acid in order to induce the formation of carboxyl groups, which in turn are coordinated by calcium ions after immersion in CaCl2 aqueous solution. The nucleation and growth of randomly oriented apatite crystals were observed as a result of subsequent incubating cycles on phosphate and calcium-containing solutions. Bio-inspired approaches for mimicking the mineralization process of hydroxyapatite constitute a key point for this kind of material to be exploited in bone repair and regeneration. The potential use of CdS quantum dots-encapsulated fluorescent nanofibers synthesized via electrospinning as the bioimaging tool must also be underlined.122 In vitro biological evaluation confirmed their biocompatibility in terms of attachment and proliferation of mouse mesenchymal stem cell and fibroblast cells. The fluorescence properties of QD are not compromised after manufacturing and cells cultured on the fibrous scaffolds exhibit normal morphology, resulting in complete integration with the surrounding fibers.
Concluding this overview on zein electrospun fibers, a very recent work by Wang et al. has also to be mentioned.123 In this study, Janus zein-PVP medicated nanofibers were fabricated via side-by-side electrospinning using zein as a key filament-forming matrix and a drug carrier. Ferulic acid, as a poorly water-soluble model drug, was loaded on both sides of the Janus fibers. SEM and TEM investigations revealed a linear morphology without beads-on-a-string phenomena, smooth surface, and confirming the side-by-side structure. The drug was present in the fibers in an amorphous state due to its compatibility with zein and PVP. In vitro dissolution tests verified that the Janus fibers manipulated the release of FA in a two-stage manner: a fast release from the hydrophilic PVP matrix by erosion mechanism, followed by a second stage involving sustained release from the hydrophobic zein side via the diffusion mechanism. Based on this combined application of zein and PVP, this is a clear example of a process-structure–performance relationship: the protocols reported by Wang and colleagues can be used to expand the applications of several further food hydrocolloids for developing advanced functional nanomaterials.
Drug-loaded hydrogels have been paid increasing attention in tissue engineering and regenerative medicine.
The ability of zein to form gels is well known. Zein solutions and dispersions may gel with time and heat, and this tendency increases as the concentration of zein in the solution increases.
In the early 2000s literature,91 it was reported that alcohol-water zein solutions may easily form hydrogels and that the kinetics of the process could be controlled by adjusting a few parameters: solutions containing a lower concentration of water gelled at a slower rate, and that the addition of acid resins, such as rosin or shellac, also reduced the rate of zein gelation. It was also demonstrated that the addition of an aldehyde to the solution may stabilize the zein and prevent gelation. These earlier studies demonstrated that zein, in suitable binary or ternary solvent mixtures, could easily and in a controlled manner form gels. More recently, it was reported125 that 22 to 30% w/v zein solutions in water/ethanol mixtures (55–90% v/v ethanol) were able to gel and that the formation of the gel depended on the concentration of the zein and the fraction of ethanol in the mixture. The study revealed that the viscoelastic properties of zein dispersed in aqueous ethanol could be attributed to the partial dissolution of zein and swelling of partially solvated zein particles, both of which were affected by ethanol concentration, temperature, and zein concentration.
Sessa et al. demonstrated that zein could form gels in acetic acid (25% of zein in weight) only on the modification of zein with an appropriate amount of glutaraldehyde (GDA) occurred.126 In this study, it was found that only zein systems modified with 4 or 8% GDA gelled completely, while the ones with a lower amount of GDA did not form gels.
More recently, Vries and co-workers exploited the hydrophobicity of zein in order to get a thermoresponsive gel by self-assembly onto hydrophobic nuclei.127 They classified the zein protein as a Janus particle, which indicates particles with two sides, a hydrophobic and a hydrophilic one, because of zein significant amphiphilicity. In order to obtain thermoresponsive gels, Vries used a specific aggregation mechanism: they added oil droplets in the solution as spherical hydrophobic particles. The hydrophobic particles acted as nucleation sites: the Janus particles were dissolved in a hot glycerol solution, self-assembled, and the process was initiated at the surface of the nuclei, then adsorption takes place in an orderly fashion, leading to a specific network formation, thus leading to the gelation process.
More recently, an oil in glycerol zein emulgel was developed and the incorporation of β-carotene was achieved.128 As a polar solvent, glycerol was found to prevent the aggregation of zein by reducing strong hydrophobic intermolecular forces by hydrogen bonding interaction. This increased the mobility of the zein chains preventing their aggregation, leading to a soft network able to host foreign molecules; thus, the zein-based emulgels resulted in promising vehicles for the incorporation of antioxidant molecules.
The behavior of biopolymer-based gels has been a subject of interest for a long time due to their versatility and economic importance in many applications; recently, rheological studies were carried out by Gagliardi and co-workers in order to characterize various formulations that could be useful for alimentary, biomedical, topical, and drug delivery applications.129 Raw zein dispersed in a water/ethanol mixture was used to develop and characterize various formulations prepared as a function of the protein concentration to provide a panel of different dispersions. The study highlighted that gels containing 20% w/v of raw zein were promising for relevant and low-cost formulations.
In the medical field, the ability of zein to form gels was exploited by Gao et al. to study the release of pingyangmycin hydrochloride (PYM), a sclerosant to treat venous malformations.130 Zein was dissolved in glycerol formal (GF) and then used as the matrix for injectable biodegradable gels. A PYM-loaded zein/zein–sucrose acetate isobutyrate (SAIB) in situ gel was prepared and it was found that SAIB, a sucrose derivative employed as a plasticizer, could avoid the initial burst of PYM from the in situ gels. These PYM-loaded zein and zein-SAIB in situ gels were originally injected as a liquid and then formed a semisolid embolic agent, which blocked blood flow and sustained the release of PYM. The gel forming efficacy and the viscosity of the in situ gel solutions were dependent on the concentration of zein; thus, it was possible to choose a concentration in which the embolic efficacy of the in situ gel was effective and the viscosity not excessive. Overall, PYM-loaded injectable zein and zein-SAIB based in situ gels were evaluated as promising new drug delivery systems.
More recently, Cao and Shen independently developed a zein-based in situ gel suitable for the controlled release of doxorubicin: a liquid state was transformed into a semi-solid after intratumoral injection, in order to retard the initial burst of the drug.131,132 Specifically, Shen et al. developed sustained-release DOX-loaded in situ gel with enhanced anti-cancer efficacy for colorectal tumor in vivo, and reduced off-target side effects were observed, with an efficient accumulation of DOX in the tumor and low drug concentration in blood and normal organs.
As already mentioned, the predominant hydrophobicity of zein represented a major obstacle for forming hydrogels. Recently, this was overcome by chemically modifying the zein protein. Qi and co-workers were able to obtain zein-based hydrogels after chemically modifying zein using citric acid and acetic anhydride.133 The zein-based fibers were crosslinked by sodium hexametaphosphate, forming a hydrogel membrane and displaying stimuli-responsive behavior toward pH and ionic strength. The hydrogel membrane exhibited better protein adsorption, selectivity, and sustained release profile for positively charged proteins such as cytochrome C, fast biodegradation behavior, and low cytotoxicity for mouse embryonic; thus, they were considered to be promising biomaterials in the biomedical field.
A different approach in order to obtain zein-based hydrogels was used by Ni and co-workers: they carried out hydrolysis treatment in acidic conditions of the protein, followed by grafting hydrophilic groups on the backbone of the protein.134 They reported the synthesis of cryogels by graft copolymerization between hydrolyzed zein proteins and acrylic acid, using potassium persulfate (KPS) and sodium bisulfite (SBS) as redox initiators, followed by a freeze-drying step. The objective of the study was to demonstrate the effect of the chemical composition on the freezing temperature of the obtained cryogel. It was observed that the percentage of water absorbed by the gel changed by changing the chemical composition, while cryogels freezing at different temperatures had strong structural differences. The results of the dynamic mechanical analysis showed that the glass transition RH values increased with the increase in the acrylic acid ratio. The highest water absorbance (119.5 g g−1) and oil absorbance (45.79 g g−1) were achieved by cryogels synthesized with 1 g hydrolyzed zein, 3.3 g acrylic acid, 0.75 g potassium persulfate (KPS), 0.375 g sodium bisulfite (SBS), and 0.03 g N,N′-methylenebisacrylamide.
In another work, zein superabsorbent hydrogels were synthesized by graft polymerization on vinylic monomers on the protein backbone using N,N′-methylenebisacrylamide as the crosslinker and KPS/SBS as the redox initiators.135 The crosslinking density could be controlled by changing the ratio between the vinylic monomers and the crosslinker. It was observed that the addition of vinylic monomers could increase the hydrophilicity of zein, which resulted in an increased absorption capacity. The swelling ability of the hydrogels was highly sensitive to the pH and could reach a high swelling ratio in neutral or mild acid/alkaline conditions (pH 5–9). By optimizing the formulation of the hydrogel, it was possible to reach the maximum water absorbency of 239.6 g g−1 hydrogel. In both the works, the obtained hydrogels were found to be interesting as absorbent materials.
In recent literature, zein has been mainly used in combination with other biopolymers, proteins, or polysaccharides to form blended gel or hydrogels with improved properties for several applications.124,136–138
In the medical field, the zein/pectin mixture was used to form complex hydrogel beads, which were examined for potential use in colon-specific drug delivery.139 It was hypothesized that the combination of zein and pectin could suppress the swelling of pectin in the stomach and limit zein degradation in the small intestine. In this study, the pectin/zein complex hydrogel beads were prepared by pumping a pectin solution into an ethanol solution containing calcium chloride and different concentrations of zein. The resultant beads were characterized for their structure and swelling properties in simulated physiological environments. The resultant beads were also examined for controlled drug release under conditions mimicking transit from the mouth to the colon and were found to be resistant to the upper gastrointestinal environment.
More recently, in the medical field, a hydrogel blend of propylene glycol alginate (PGA)-zein has been proposed by Liu and co-workers.137 The composite hydrogel provided the controlled sustained release of curcumin in simulated gastrointestinal fluid, which was hypothesized to be attributed to the interaction of the two different polymers. The presence of zein not only allowed hydrogel formation but also had a beneficial effect in slowing down the dissolution of PGA under simulated gastrointestinal conditions. The cited work represented an interesting starting point in the preparation of composite hydrogels with controlled release properties to be exploited in the medical field.
Wang and co-workers used two proteins, zein, and hordein, which are both largely available biomaterials from renewable biomass, to obtain double-cross-linked hordein/zein protein hydrogels with strong mechanical properties.124 These hydrogels were the result of a double crosslinking process, a chemical one with glutaraldehyde, and a physical one induced by post-treatment with water, which allows hydrophobic interaction and hydrogen bonding (Fig. 1). It seemed that hordein greatly participated in the formation of chemical networks, whereas physically cross-linked domains consisted of beadlike particles with a diameter of ∼80 nm, mainly constituted of zein that acted as the “load carriers”. The study could be an attractive starting point for the design and construction of strong hydrogels from other plant protein resources, thus unlocking their potential as biodegradable and biocompatible materials, and exploiting other abundant and renewable resources. A more complex example of protein/polysaccharide-based hydrogel was very recently reported by Lai et al.138 In this work, a hydrogel blend of zein/konjac glucomannan (KGM), a water-soluble neutral plant polysaccharide with a high molecular weight, was obtained. The different ratios of the two materials affected the structure and properties of the hydrogel itself and by adding different amounts of zein, an improvement in the KGM gel properties could be achieved. The storage modulus (G′), as well as the initial decomposition temperature of the KGM gel, were found to be significantly improved even when the zein content was below 20%, while partial phase separation occurred when the zein content exceeded 50%. When zein dominated, the KGM and zein tended to form a phase-separated structure, which limits the possibility to investigate this kind of a structure. Anyway, the possibility of blending carbohydrates and protein represents a powerful innovation that may lead to high-performance networks.
Three-dimensionality in the biomedical field is an extremely important feature, especially when we think about targeted delivery or tissue regeneration.140 It is currently known that cells in the body grow within an organized 3D Extra-Cellular Matrix (ECM), surrounded by other cells. The cell–cell and cell–ECM interactions can determine whether a cell can proliferate, differentiate, reach out to apoptosis, or invasion.141–143 However, cell experiments are generally performed on 2D cultures; in this case, cells are polarized in a not-physiological way by keeping one side in close contact to a stiff/flat substrate and the other one facing the culture media, without any cell–cell and cell–ECM interactions. Thus, the 2D cultures are not able to properly mimic in vivo systems in terms of cellular communication, gene and protein expression pattern, and diffusion of soluble molecules.144,145 3D cultures are thus currently being developed to achieve the third dimension, which is crucial for the integration of mechanical and chemical signals, better mimicking the in vivo microenvironment.
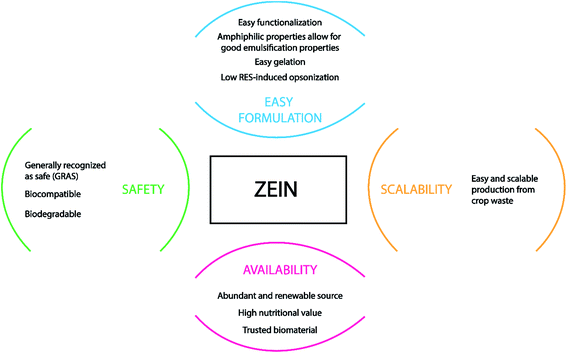
As previously described, zein is well suited for being converted into different shapes and structures including films, micro-beads, gels, and fibers, and it is a GRAS food material.146 Some of its unfavorable features, such as low mechanical strength, can be bypassed and improved by blending zein with other natural materials or plasticizers during the fabrication workflow, avoiding the impairment of the devices' integrity.147 Fig. 7 sums up the advantages of using zein protein as a drug carrier and the reasons for its high interest in biomedical applications, mostly in tissue engineering.
The current chapter focuses on three-dimensional objects zein-based that find a specific role in the biomedical field, as porous scaffolds for tissue engineering or beads suitable for drug delivery applications. These latter have been thoroughly investigated in the last decades by different research groups using zein alone or combined with other polymers, exploiting several methods for microbead preparation.
Hurtado-Lopez et al. in 2005 describe an interesting formulation of non-porous microspheres composed of zein for drug/vaccine delivery.148 These microbeads were prepared by a simple coacervation technique, based on the solubility properties of zein and, aiming to understand the effects of experimental variables on the particles' formation, a factorial design was applied. Ovalbumin (OVA), as a model antigen, was loaded into zein microspheres with a loading efficiency of 52%. The optimal experimental conditions resulted in good particle morphology, low aggregation, and a high amount of protein precipitation. The release of OVA was reported as the result of a slow erosion of the zein microsphere that starts to take place after 5 days in PBS:SDS-PAGE analysis confirmed that the process of microsphere preparation did not exert any deleterious effect on the structural integrity of released ovalbumin. Thus, these zein microspheres, formulated by a fast and safe method, could be further investigated as drug/vaccine delivery systems.
Another potential attractive drug delivery system made of zein and pectin beads was formulated by a research group of the U.S. Department of Agriculture in Pennsylvania. In vitro experiments showed pectin/zein complex hydrogel beads able to protect incorporated drugs from premature release into physiological environments, similar to the stomach and small intestines. The inclusion of a small portion of zein into pectin effectively stabilized the pectin networks in terms of the structural properties, overwhelming the swelling behavior of pectin. In turn, the pectin networks protected the bound zein from protease digestion.
In the same year, Liu et al. reported another zein-based microsphere drug delivery system of ivermectin (IVM), a highly effective parasiticide.149 The particles have been prepared by the phase separation method dissolving zein and IVM in 66.7% ethanol. SEM characterization showed that the microspheres were homogeneous in size (between 0.3 and 1.2 mm), pointing that the beads can be effectively taken up by macrophages because, as is known, the size plays a great role in controlling drug delivery to the target tissues.150 IVM loading and encapsulation efficiency increased with the increasing concentrations of IVM and zein at a given ratio. The release from zein microspheres and pepsin degradation of microspheres were also performed in vitro, showing zero-order release; this formulation can be thus used to administer drugs orally for maintaining a constant plasma drug concentration in vivo. Furthermore, this system could also be adapted for building 3D scaffolds made up of zein microspheres, able to release bioactive components for improving cell differentiation and proliferation in tissue engineering.
For scaffolds composed of zein microspheres, a research group from Shanghai has conducted an interesting study that implemented their previously published zein film made of particles (diameter of 100–2500 nm)96 loaded with heparin for cardiovascular devices (e.g., stent).107 In vitro MTT test on human umbilical veins endothelial cells (HUVECs) showed that both zein film and its degraded product had better biocompatibility compared with the Corning culture plate and the effect of the zein degraded product on HUVECs was dose-dependent. The encapsulation efficiency and the loading of heparin change with the amount of both zein and heparin. SEM characterization indicated that the zein film was made of microspheres in diameter from nano to micrometer, which could be tuned; the size of the microspheres changed before and after the release of heparin due to the conglutination among zein microspheres. The release rate of heparin from the microsphere film reached 33.5% within 12 h and began to get into subsequent slow-release phase; about 55% of the entrapped heparin was released after 20 days. Platelet adhesion was suppressed by both film types, and the heparin-loaded film showed better anticoagulation. These results suggest that the zein film could be used directly as a novel coating material for its better biocompatibility with HUVECs. The improvement of the hemocompatibility exerted by the heparin-loaded zein microsphere film, which means superior anticoagulation and inhibition of platelet adhesion, was worthwhile for continuing the research, with the aim of preventing acute or subacute thrombosis and endowing cardiovascular devices, e.g., stents, with biocompatibility.
The aforementioned group has been very prolific in the years from 2004 to 2009, working on zein as a novel resource in the tissue engineering field. In 2006, they published a work exploring mechanical properties and in vitro biocompatibility of porous zein scaffold made by the salt-leaching method.151 They studied scaffold degradation in collagenase and pepsin during 14 days. Mesenchymal stem cells from rats (MSCs) were able to adhere, proliferate, and differentiate in osteoblasts onto the scaffold. The alkaline phosphatase (ALP) activity was also assessed in the presence of dexamethasone. Later, in 2007, the researchers deepened the knowledge about tissue compatibility of these scaffolds in vivo in a rabbit subcutaneous implantation model, revealing good tissue response and degradability.152 With the aim of improving the mechanical properties (especially the brittleness), the formulation was prepared to add the club-shaped mannitol as the porogen, together with stearic acid or oleic acid. The scaffolds obtained had an interconnected tubular pore structure and about 80% total porosity, tunable by modulating the size of the club-shaped mannitol and its ratio with zein. The mechanical properties of the scaffolds were improved significantly by adding fatty acids, especially in terms of tensile modulus and flexural and tensile strength value. Cytotoxic testing showed that the MSCs could adhere, proliferate, and differentiate on all the porous scaffolds, and no signs of toxicity could be detected; moreover, blood vessels could form in the scaffold, and the degradation could be completed within 8 months in vivo.
Encouraged by these results, they decided to investigate the effect of the zein/inorganic composite on the physical and biological properties of the porous zein scaffolds previously fabricated.153 The study was based on the concept that composite scaffold materials, especially when combined with inorganic materials, better fit the mechanical and physiological demands of the host tissue compared to individual scaffold materials. The HA-coated zein scaffold showed the same porosity (over 75%) and good pore interconnectivity as the zein scaffold. However, the compressive Young's modulus and the compressive strength decreased in the presence of HA, remaining generally acceptable for the final application. In this study, human bone marrow stromal cells (hBMSCs) adhered and spread well on the HA-coated zein scaffolds. The HA-coated zein scaffolds also achieved a higher ALP activity and marker gene expression level than those achieved with the zein scaffolds, although, on the latter, the cells showed greater proliferation. Given all these mechanical and biological evaluations, zein porous scaffolds achieved better osteogenic potential after HA coating, making the HA-coated zein scaffold an optimal biomaterial for bone tissue engineering. As naturally occurs, the following step has been the in vivo demonstration of bone formation by mesenchymal stem cells in zein scaffolds.154 The scaffolds made of zein have been prepared and characterized as previously published using the salt-leaching method; the leap now is testing the complexes of zein scaffolds and rabbit MSCs and investigating ectopic bone formation in nude mice. Furthermore, they performed implantation into the radius defects of rabbits and assessed whether they could be helpful in the repair of critical-sized bone defects. The results demonstrated that the zein–MSCs complexes could undergo ectopic bone formation in the thigh muscle pouches of nude mice (Fig. 8). In addition, the complexes could promote the repair of rabbits' critical-sized radius defects with the successful formation of blood vessels, which establishes promise for the treatment of bone defects through tissue engineering.
 | ||
| Fig. 8 X-ray picture of thigh muscle pouches of nude mice at week 12 after implantation of zein with (C and D) or without (A and B) rabbit MSCs. The white arrows indicate ectopic bone formation. This figure has been adapted/reproduced from ref. 154 with permission from Elsevier, 2021. | ||
Riding the wave of bone regeneration is important to cite three works that present zein and poly(ε-caprolactone) (PCL) as co-protagonists of the 3D porous scaffold formation. The first was published in 2010 by Salerno et al.;155 they aimed to design novel biodegradable porous scaffolds for bone tissue engineering via a supercritical CO2 foaming process. The results showed that tuning both HA concentration and foaming temperature significantly affected the microstructural features of the scaffolds, such as the porosity. The biocompatibility of these scaffolds was assessed in vitro by verifying the adhesion and colonization of pre-osteoblast MG63 and hMSCs cells.156 Two years later, Wu et al. presented a zein-PCL biocomposite for porous scaffold fabrication using solvent casting-particulate leaching method with sodium chloride particles as the porogen. Porous biocomposite scaffolds with porosity of about 70% and a well-interconnected network were obtained. Tuning the hydrophilicity of the biomaterial surface has a strong influence on the adhesion and proliferation of cells.34 Zein/PCL composite scaffolds exhibited higher hydrophilic character than the PCL scaffold, as indicated by a decrease in the water contact angle; it is well known that tuning the hydrophilicity of the biomaterial surface has a strong influence on the adhesion and proliferation of cells,157 although no cytocompatibility experiments have been performed in this work. The degradation of the scaffold was studied in terms of the changes in pH, weight loss, and scaffold morphology by dipping the objects in PBS for 28 days. The gradual degradation of the zein/PCL composite scaffold is favorable for the objective of tissue engineering strategy, which is the substitution of the temporary matrix by the native tissue.158 By adjusting the content of zein in the composite, the degradation rate of the zein/PCL composite scaffolds could be tailored to match with the rate of tissue regeneration. Despite the lack of biocompatibility and biodegradability tests with the cells, the results of the in vitro degradation can provide an approximate prediction of the in vivo degradation behavior of the scaffolds.
More recently, in 2015, Fereshteh et al. presented the fabrication of a novel bioactive glass-based scaffold by the foam replication method159 and besides, they introduced a subsequent double PCL/zein coating in order to achieve improved mechanical properties and impart a local drug release function using tetracycline as a model drug (Fig. 9).160 The high macroporosity was checked by SEM observation (average pore size 300 μm and total porosity ∼96%). The compressive strength and mechanical stability were significantly increased after the coating. Both uncoated and PCL/zein coated scaffolds possessed appropriate bioactivity to form bone-like apatite layers after 2 weeks of immersion in simulated body fluid (SBF). The PCL/zein composite coating was also developed to enhance the biological activity of bioactive glass scaffolds, e.g., inducing faster HA formation. It was found that the presence of zein accelerates the degradation rate of the coating in the time period investigated, providing a way to control the in vitro degradation behavior of the coating by tuning the concentration of zein.
 | ||
| Fig. 9 Digital photographs of coated scaffolds: (a) top surface and (b) cross section of the scaffold coated by PCL (5% w/v), (c) top surface and (d) cross section of the scaffold coated by PCL/zein (2.5%:2.5% w/v) and (e) top surface and (f) cross section of the scaffold coated by zein (5% w/v). This figure has been adapted/reproduced from ref. 160 with permission from Elsevier, 2021. | ||
From the drug delivery side, the sustained release of tetracycline was confirmed, and the proportion of zein in the coating had a great impact on the drug release behavior.
Bioactive glass 3D scaffolds for bone tissue engineering applications were also successfully prepared by Zhou et al. in 2013 by harnessing two coating strategies with hydroxypropyl trimethyl ammonium chloride chitosan (HACC-) and HACC-Zein.161 Both HACC and HACC-Zein could improve the mechanical strength of bio-glass, which was brittle and had many unconnected pore walls. The bioactive glass was not found to have any antibacterial activity, and the scaffold modified with HACC showed only short-term antibacterial activity (24 h) but the coating with HACC-Zein showed both short-term and long-term antibacterial activity (72 h). Human mesenchymal stem cells were found to adhere to the pore network of all three types of scaffolds.
Close to bone regeneration, skin and wound dressing are widely investigated fields in tissue engineering. Time is the key factor in managing wound healing, mainly because of the risk of infections; thus, rapid care of skin wounds is important for the prevention of microbial infection and trans-epidermal water loss, leading to the acceleration of wound regeneration.162 Zein protein has been found to be very useful in the fabrication of scaffolds and materials in general for handling the needs in this field.
Unnithan et al. in 2014 successfully fabricated a drug-loaded composite nanofiber blend made of polyurethane (PU), cellulose acetate (CA), and zein by electrospinning.163 Blending of CA and zein with PU resulted in improved physiological and biological characteristics, checked by SEM characterization, FTIR analysis, contact angle, and tensiometer measurements. PU-CA-zein-drug composite nanofibrous scaffolds showed enhanced blood clotting and excellent platelet activation ability. Cytocompatibility studies in vitro indicated that the scaffolds could promote cell viability and infiltration; on the other hand, in vitro antibacterial activity studies proved the antibacterial potential of the prepared composite scaffold. All these shreds of evidence indicated that these novel PU-CA-zein-drug composite nanofibrous scaffolds can be used for burn, chronic, and diabetic wound infections.
Three years later, another electrospun fiber scaffold has been prepared by Zhijiang et al. by blending zein with poly(3-hydroxybutyrate-co-4-hydroxybutyrate) P(3HB-co-4HB).164 The ultra-fine fibers (diameter = 60–650 nm) show a circular and uniform morphology with random distribution. High porosity (>80%) with interconnected pores and large specific surface area were generated by the ultra-fine fibers. The blend with P(3HB-co-4HB) considerably improves the mechanical properties of the zein electrospun fiber scaffold; the tensile strength increases as well as the elongation at break. The cytocompatibility of the zein/P(3HB-co-4HB) blend fiber scaffolds has been evaluated by cell culture in vitro using NIH3T3 fibroblast cells and MG-63 osteoblast cells; the MTT assay showed no cytotoxic effects. The cells incubated for 72 h were able to spread and proliferate, a clear sign of good biocompatibility.
A couple of works by Rad et al. in 2018–2019 have presented and discussed a nanofiber scaffold made of PCL, zein, and gum arabic (GA) fabricated again via the electrospinning method.165,166 The structure showed an interconnected fibrous network with a porous framework; interestingly, the concentrations of PCL, zein, and GA in the solution could affect the fiber morphology of the composite scaffolds. Each component of the scaffold gave a specific effort; the presence of PCL in the blends improved mechanical properties of zein and GA; GA enhanced the hydrophilicity of scaffold; zein moderated the degradation behavior of the composite scaffolds. Also, the composite scaffold showed antibacterial properties due to the intrinsic antibacterial properties of GA. Fibroblast cells L929 exhibited good proliferation and adhesion to the PCL/zein/GA scaffolds. To closely mimic the skin ECM, the researchers developed a further PCL/zein/GA nanocomposite scaffold, incorporating Calendula officinalis extract as a potential substrate for skin regeneration. Nanofibrous composite scaffolds showed a high porosity (83%), moderated degradability behavior, as well as controlled C. officinalis release. The cell culture tests carried out with L929 cells indicated that C. officinalis and GA promote the adhesion, proliferation, and growth of fibroblasts. Compared with PCL/zein/GA scaffold, the antibacterial activity and biocompatibility of PCL/zein/GA/C. officinalis scaffolds were significantly improved. The results demonstrate that C. officinalis-loaded PCL/zein/GA scaffolds can be used as promising biomaterial candidates for skin tissue engineering applications.
It is very common, once a good scaffold fabrication protocol is established, to publish a subsequent work adding some modifications or improvements. In this regard, Li et al. in 2018 fabricated a zein/poly(L-lactic acid) (PLLA) NF scaffold based on their previous study167 and loaded with bone morphogenetic protein 2 (BMP-2) and dexamethasone (DEX) for dual controlled-release for bone tissue engineering applications.168 The release profile was different for the two molecules; a large quantity of DEX was released in the first 3 days, while the release of BMP-2 lasted for more than 21 days; this was probably due to the location of DEX in the shell layer, resulting in burst release during the initial stage. All the NF scaffolds 12 h after inoculation with MSCs demonstrated very good cell adhesion, with a noteworthy more pronounced viability on the zein/PLLA coaxial electrospun NF with dual controlled delivery of DEX and BMP-2 (Fig. 10). BMP-2 and DEX exerted a synergistic effect that, combined with the intrinsic ability of the surface topography of zein-DEX/PLLA-BMP-2 NF scaffold, provided effective osteoinduction. These results establish that the controlled dual release of BMP-2 and DEX by zein/PLLA core–shell electrospun scaffolds stimulates not only early cell adhesion but also osteogenic protein expression during differentiation and are therefore suitable for use in bone tissue engineering applications.
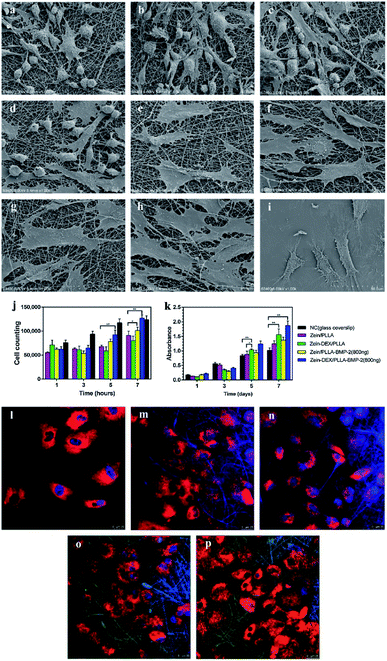 | ||
| Fig. 10 SEM images of MSCs adhesion 12 h after inoculation on: (a) Zein/PLLA; (b) Zein-DEX/PLLA; (c) Zein/PLLA-BMP-2; and (d) Zein-DEX/PLLA-BMP-2. Proliferative MSCs 7 days after inoculation on: (e) Zein/PLLA; (f) Zein-DEX/PLLA; (g) Zein/PLLA-BMP-2; (h) Zein-DEX/PLLA-BMP-2; and (i) glass coverslips. (j) Adhesion behavior of MSCs seeded onto different nanofiber scaffolds and glass coverslips (NC) after incubation for 1, 3, 5, and 7 h. (k) Viability and proliferation profiles of MSCs on different nanofiber scaffolds and glass coverslips (NC) after incubation for 1, 3, 5, and 7 days. Confocal microscopy images showing the attachment and spreading of MSCs on (l) glass coverslips; (m) Zein/PLLA; (n) Zein-DEX/PLLA; (o) Zein/PLLA-BMP-2 (800 ng); and (p) Zein-DEX/PLLA-BMP-2 (800 ng) scaffold 36 h after inoculation. The cytoskeleton was stained using PKH26 (red), nuclei with DAPI (blue), and BMP-2 is conjugated with 5-FITC (green). (*p < 0.05,**p < 0.01). This figure has been adapted/reproduced from ref. 168 with permission from Elsevier, 2021. | ||
In order to satisfy the complex and diverse regenerative requirements of osteochondral tissue and enhance osteogenic differentiation, Tamburaci et al. used Si-substituted nanohydroxyapatite particles (Si-nHap) and silica-based polyhedral oligomeric silsesquioxanes (POSS) nanocages as reinforcements in different polymer layers to mimic a cartilage-bone tissue interface.169 Chitosan and zein were used as biopolymers for developing a bilayer scaffold produced via the fabrication of two different nanocomposite layers with different polymer-inorganic composites: the chitosan/Si-nHap microporous layer and the zein/POSS NF layer. The POSS-incorporated bilayer scaffolds were appropriate for cell adhesion, growth, and proliferation and can be applied effectively in the bone-cartilage tissue systems. Metabolic activity and fluorescence microscopy images of cells seeded on chitosan/Si-nHap and zein/POSS porous NF layers evidence that both fiber and porous layers can mimic the cartilage and bone sections, exhibiting homogeneous cell distribution, matrix formation, and improved cellular activity due to the silica content of bioactive agents incorporated in the layers of the scaffold. Nanohydroxyapatite (nHAp) is well known to be crucial for osteoinduction in vitro but mostly in vivo, as demonstrated in 2019 by Ou et al.170 In a previous study 2 years before, they fabricated a zein/gelatin electrospinning scaffold with good biocompatibility but the low osteoinductive ability for human periodontal ligament stem cells (hPDLSCs).171 Therefore, they decided to create novel zein/gelatin/nanohydroxyapatite (zein/gelatin/nHAp) nanofibrous membranes to overcome the drawbacks of the zein/gelatin scaffold. The results showed that the surface wettability of the zein/gelatin/nHAp NF membranes was increased. Furthermore, the presence of nHAp facilitated the attachment, proliferation, but most of all, the osteogenic differentiation of hPDLSCs. The zein/gelatin/nHAp NF membranes biocompatibility was very good, demonstrating an osteoinductive activity for hPDLSCs in vitro and in vivo using Sprague-Dawley rats' cranium as the animal model; all these evidences together strongly suggested the potential applications of these membranes in bone tissue engineering.
A final mention for concluding this chapter is dedicated to a scaffold made of zein fabricated via additive manufacturing techniques as 3D bioprinting. In 2018, Jing et al. published the development of a series of PCL/zein composite inks suitable for electrohydrodynamic printing technique (EHDP) for scaffold fabrication (Fig. 11).172
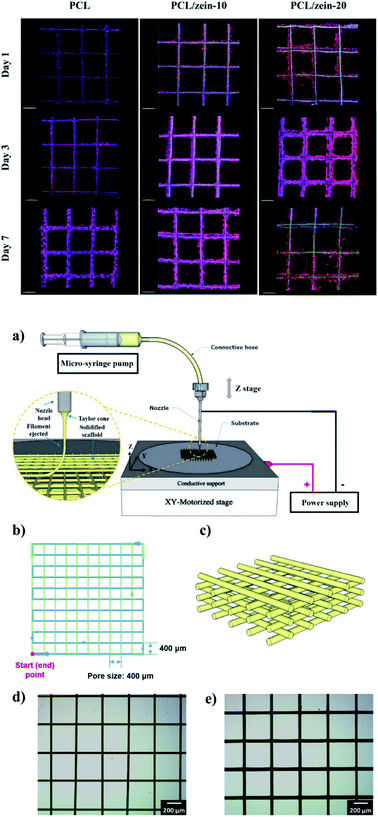 | ||
| Fig. 11 Left: (a) Schematic diagram of home-built 3D EHDP printer (inset is the enlarged view of the printing process). (b) Motion path of stage in a single layer. (c). 3D image of simulated grid scaffolds with multiple layers. d), e) Optical images of printed PCL/zein-20 scaffolds (Single layer and 24 layers). Right: Confocal laser scanning microscope (CLSM) of H1299 cell seeding on PCL and PCL/zein scaffolds after 1-, 3-, and 7 day cultures. Object lens: 10×; scale bar 200 μm. This figure has been adapted/reproduced from ref. 172 with permission from the American Chemical Society, 2021. | ||
The in vitro degradation test showed that the zein portion in the blend can significantly accelerate the degradation of PCL/zein; furthermore, PCL/zein scaffolds exhibited improved the mechanical strength (Young's modulus and yield stress values) compared with the PCL scaffolds. Zein-containing scaffolds also exhibit improved cell adhesion efficiency and proliferation rate, which could be altered by changing the portion of zein in the composite ink. This paper surely represents a valuable element in the field of additive manufacturing for the controlled deposition of zein and it is realistically foreseeable as a rise in scientific production over the next years.
Conclusions
Biomedicine based on protein-derived materials is nowadays a very great and inspiring aim. Proteins, especially from plants, have tuneable properties, safety, and biocompatibility characteristics that make them suitable for biomedical purposes.From the very beginning, zein protein has been mainly employed in the pharmaceutical field for edible coatings. However, in the last years, its applications strongly multiplied thanks to zein's unique properties, such as solubility and filming ability. This fully natural, biodegradable, and cheap material has found strong confirmations in several areas as drug delivery, target therapy, imaging, theranostics, and tissue regeneration.
Zein's capability of being shaped and chemically handled provides an incredible tool to the biomedical world. In the micro and nanoparticles field, in particular, zein could progressively replace metal particles that are currently adopted in the majority of therapeutic/diagnostic applications. Thanks to its excellent physicochemical properties, zein protein has a glowing future in the intracellular delivery of therapeutic peptides/genetic materials, a valid alternative to the currently employed synthetic polymers. The investigation on the fabrication parameters and surfactants usage is plentiful but, on the other hand, literature is lacking significant experiments on storage and stability of zein colloidal preparation, e.g., for avoiding aggregation, together as convincing in vivo trials. In general, the chemical modification of zein is a key point that could be enriched in every scope, which is crucial to work on the zein-based blends for improving the mechanical properties of the structure as fibers, films, and hydrogel, for instance, and, at once, additionally investigate on the release mechanisms of therapeutic agents from the zein matrix, especially in vivo.
We saw that the additional aptitude to create 3D structures also gives zein a very promising role in regenerative medicine and tissue engineering through hydrogel preparation, despite its intrinsic hydrophobic nature. The chemical structure modification of zein is well studied and discussed by several scientific contributions but the protocols need to be scaled up in order to project its application from a pharmaceutical/industrial perspective. Moreover, the majority of 3D scaffolds papers are primarily focused on the rheological and microscopic properties of the scaffolds, definitely a key factor for the whole characterization but it is time to better dedicate in-depth analysis about cytocompatibility and in vivo degradation, not only simulation and subsequent predictions.
All things considered, there are still many sides of this protein to be further explored, mostly concerning targeting in drug and vaccine delivery; nevertheless, the quickly increasing number of papers and reports in the literature is very promising.
Author contributions
Conceptualization: M. C. F., L. S.; formal analysis: M. M., E. L., V. V. B.; funding acquisition: M. C. F.; supervision: M. C. F., L. S.; writing – original draft: G. V., V. V. B, S. T.; writing – review and editing: M. M., E. L.Conflicts of interest
There are no conflicts to declare.Acknowledgements
University of Bologna is gratefully acknowledged.Notes and references
- Z. Song, Z. Han, S. Lv, C. Chen, L. Chen, L. Yin and J. Cheng, Chem. Soc. Rev., 2017, 46, 6570–6599 RSC.
- S. Tortorella, V. Vetri Buratti, M. Maturi, L. Sambri, M. Comes Franchini and E. Locatelli, Int. J. Nanomed., 2020, 15, 9909–9937 CrossRef CAS PubMed.
- H. Malekzad, H. Mirshekari, P. Sahandi Zangabad, S. M. Moosavi Basri, F. Baniasadi, M. Sharifi Aghdam, M. Karimi and M. R. Hamblin, Crit. Rev. Biotechnol., 2018, 38, 47–67 CrossRef CAS PubMed.
- T. D. Tavares, J. C. Antunes, F. Ferreira and H. P. Felgueiras, Biomolecules, 2020, 10, 148 CrossRef CAS PubMed.
- F. U. Akharume, R. E. Aluko and A. A. Adedeji, Compr. Rev. Food Sci. Food Saf., 2021, 20, 198–224 CrossRef CAS PubMed.
- N. Reddy and Y. Yang, Trends Biotechnol., 2011, 29, 490–498 CrossRef CAS PubMed.
- E. P. Winters and D. L. Deardorff, J. Am. Pharm. Assoc., Sci. Ed., 1958, 47, 608–612 CrossRef CAS PubMed.
- N. T. Weissmueller, H. D. Lu, A. Hurley and R. K. Prud’homme, Biomacromolecules, 2016, 17, 3828–3837 CrossRef CAS PubMed.
- Grand View Research, Corn Fiber Market Size, Share & Trends Analysis Report By Application (Textile, Animal Nutrition, Pharmaceutical, Food & Beverage), By Region, And Segment Forecasts, 2020–2027, 2019 Search PubMed.
- A. Esen, J. Cereal Sci., 1987, 5, 117–128 CrossRef CAS.
- J. W. Lawton, Cereal Chem. J., 2002, 79, 1–18 CrossRef CAS.
- H. Maeda, H. Nakamura and J. Fang, Adv. Drug Deliv. Rev., 2013, 65, 71–79 CrossRef CAS PubMed.
- R. Paliwal and S. Palakurthi, J. Controlled Release, 2014, 189, 108–122 CrossRef CAS PubMed.
- R. Shukla and M. Cheryan, Ind. Crops Prod., 2001, 13, 171–192 CrossRef CAS.
- Q. X. Zhong and M. F. Jin, Food Hydrocolloids, 2009, 23, 2380–2387 CrossRef CAS.
- R. Nunes, A. Baião, D. Monteiro, J. das Neves and B. Sarmento, Drug Delivery Transl. Res., 2020, 10, 826–837 CrossRef CAS PubMed.
- Y. Zhang, L. Cui, F. Li, N. Shi, C. Li, X. Yu, Y. Chen and W. Kong, Int. J. Pharm., 2016, 513, 191–210 CrossRef CAS PubMed.
- M. Pascoli, R. de Lima and L. F. Fraceto, Front. Chem., 2018 DOI:10.3389/fchem.2018.00006.
- H. Xu, Q. Jiang, N. Reddy and Y. Yang, J. Mater. Chem., 2011, 21, 18227–18235 RSC.
- J. Gomez-Estaca, M. P. Balaguer, R. Gavara and P. Hernandez-Munoz, Food Hydrocolloids, 2012, 28, 82–91 CrossRef CAS.
- S. Kang, Y. He, D.-G. Yu, W. Li and K. Wang, Colloids Surf. B Biointerfaces, 2021, 201, 111629 CrossRef CAS PubMed.
- P. Franco and I. De Marco, Processes, 2020, 8, 938 CrossRef CAS.
- M. T. M. G. Rosa, V. H. Alvarez, J. Q. Albarelli, D. T. Santos, M. A. A. Meireles and M. D. A. Saldaña, J. Supercrit. Fluids, 2020, 159, 104499 CrossRef CAS.
- P. Girotra, S. K. Singh and K. Nagpal, Pharm. Dev. Technol., 2013, 18, 22–38 CrossRef CAS PubMed.
- F. Rodríguez-Félix, C. L. Del-Toro-Sánchez and J. A. Tapia-Hernández, Food Sci. Biotechnol., 2020, 29, 619–629 CrossRef PubMed.
- J. M. Irache and C. J. González-Navarro, Nanomedicine, 2017, 12, 1209–1211 CrossRef CAS PubMed.
- A. Patel, Y. Hu, J. K. Tiwari and K. P. Velikov, Soft Matter, 2010, 6, 6192–6199 RSC.
- G. Liu, D. Wei, H. Wang, Y. Hu and Y. Jiang, Chem. Eng. J., 2016, 284, 1094–1105 CrossRef CAS.
- A. Anand David, R. Arulmoli and S. Parasuraman, Pharmacogn. Rev., 2016, 10, 84 CrossRef PubMed.
- R. Penalva, C. J. González-Navarro, C. Gamazo, I. Esparza and J. M. Irache, Nanomedicine, 2017, 13, 103–110 CrossRef CAS PubMed.
- A. Rauf, M. Imran, T. Abu-Izneid, I. Ul-Haq, S. Patel, X. Pan, S. Naz, A. Sanches Silva, F. Saeed and H. A. Rasul Suleria, Biomed. Pharmacother., 2019, 116, 108999 CrossRef CAS PubMed.
- T. Zou, Z. Li, S. S. Percival, S. Bonard and L. Gu, Food Hydrocolloids, 2012, 27, 293–300 CrossRef CAS.
- B. A. Teicher, Drug Resist. Updates, 2000, 3, 67–73 CrossRef CAS PubMed.
- G. W. Sledge and K. D. Miller, Eur. J. Cancer, 2003, 39, 1668–1675 CrossRef PubMed.
- C. G. da Rosa, M. V. de Oliveira Brisola Maciel, S. M. de Carvalho, A. P. Z. de Melo, B. Jummes, T. da Silva, S. M. Martelli, M. A. Villetti, F. C. Bertoldi and P. L. M. Barreto, Colloids Surf., A, 2015, 481, 337–344 CrossRef.
- L. F. Lai and H. X. Guo, Int. J. Pharm., 2011, 404, 317–323 CrossRef CAS PubMed.
- F. Dong, X. Dong, L. Zhou, H. Xiao, P. Y. Ho, M. S. Wong and Y. Wang, Colloids Surf. B Biointerfaces, 2016, 140, 324–331 CrossRef CAS PubMed.
- R. K. Thapa, H. T. Nguyen, J. H. Jeong, B. S. Shin, S. K. Ku, H. G. Choi, C. S. Yong and J. O. Kim, Nanomedicine, 2017, 13, 885–896 CrossRef CAS PubMed.
- E. T. L. Lau, S. K. Johnson, D. Mikkelsen, P. J. Halley and K. J. Steadman, J. Microencapsul., 2012, 29, 706–712 CrossRef CAS PubMed.
- E. T. L. Lau, S. J. Giddings, S. G. Mohammed, P. Dubois, S. K. Johnson, R. A. Stanley, P. J. Halley and K. J. Steadman, Pharmaceutics, 2013, 5, 277–293 CrossRef CAS PubMed.
- A. Bernstein, E. Morrel, E. Mathiowitz, K. Schwaller, and T. R. Beck, US Pat., 5679377, 1994 Search PubMed.
- S. Lee, N. S. A. Alwahab and Z. M. Moazzam, Int. J. Pharm., 2013, 454, 388–393 CrossRef CAS PubMed.
- C. L. Hardee, L. M. Arévalo-Soliz, B. D. Hornstein and L. Zechiedrich, Genes, 2017, 8(2), 65 CrossRef PubMed.
- M. C. Regier, J. D. Taylor, T. Borcyk, Y. Yang and A. K. Pannier, J. Nanobiotechnol., 2012, 10, 44 CrossRef CAS PubMed.
- S. Podaralla, R. Averineni, M. Alqahtani and O. Perumal, Mol. Pharm., 2012, 9, 2778–2786 CrossRef CAS PubMed.
- K. Hu and D. J. McClements, Food Res. Int., 2014, 64, 329–335 CrossRef CAS PubMed.
- X. Wang and X. Chu, Colloids Surf., A, 2018, 558, 110–116 CrossRef CAS.
- A. Gagliardi, S. Voci, M. C. Salvatici, M. Fresta and D. Cosco, Colloids Surf. B Biointerfaces, 2021, 201, 111647 CrossRef CAS PubMed.
- A. Gagliardi, S. Bonacci, D. Paolino, C. Celia, A. Procopio, M. Fresta and D. Cosco, Heliyon, 2019, 5, e02422 CrossRef PubMed.
- T. Chuacharoen and C. M. Sabliov, Colloids Surf., A, 2016, 503, 11–18 CrossRef CAS.
- S. Podaralla and O. Perumal, J. Biomed. Nanotechnol., 2010, 6, 312–317 CrossRef CAS PubMed.
- K. K. Li, S. W. Yin, Y. C. Yin, C. H. Tang, X. Q. Yang and S. H. Wen, J. Food Eng., 2013, 119, 343–352 CrossRef CAS.
- C. Chang, T. Wang, Q. Hu and Y. Luo, Int. J. Biol. Macromol., 2017, 104, 117–124 CrossRef CAS PubMed.
- J. Chen, J. Zheng, D. J. McClements and H. Xiao, Food Chem., 2014, 158, 466–472 CrossRef CAS PubMed.
- K. Hu, X. Huang, Y. Gao, X. Huang, H. Xiao and D. J. McClements, Food Chem., 2015, 182, 275–281 CrossRef CAS PubMed.
- R. Penalva, I. Esparza, E. Larraneta, C. J. González-Navarro, C. Gamazo and J. M. Irache, J. Agric. Food Chem., 2015, 63, 5603–5611 CrossRef CAS PubMed.
- D. Lucio, M. C. Martínez-Ohárriz, G. Jaras, P. Aranaz, C. J. González-Navarro, A. Radulescu and J. M. Irache, Eur. J. Pharm. Biopharm., 2017, 121, 104–112 CrossRef CAS PubMed.
- D. J. McClements, Curr. Opin. Colloid Interface Sci., 2012, 17, 235–245 CrossRef CAS.
- G. Heep, A. Almeida, R. Marcano, D. Vieira, R. M. Mainardes, N. M. Khalil and B. Sarmento, Int. J. Biol. Macromol., 2019, 138, 244–251 CrossRef CAS PubMed.
- K. Yao, W. Chen, F. Song, D. J. McClements and K. Hu, Food Hydrocolloids, 2018, 79, 262–272 CrossRef CAS.
- H. Liang, B. Zhou, L. He, Y. An, L. Lin, Y. Li, S. Liu, Y. Chen and B. Li, RSC Adv., 2015, 5, 13891–13900 RSC.
- Y. Zhang, Y. Niu, Y. Luo, M. Ge, T. Yang, L. Yu and Q. Wang, Food Chem., 2014, 142, 269–275 CrossRef CAS PubMed.
- S. Chen, Y. Han, C. Sun, L. Dai, S. Yang, Y. Wei, L. Mao, F. Yuan and Y. Gao, Carbohydr. Polym., 2018, 201, 599–607 CrossRef CAS PubMed.
- L. Dai, R. Li, Y. Wei, C. Sun, L. Mao and Y. Gao, Food Hydrocolloids, 2018, 77, 617–628 CrossRef CAS.
- S. Zhang and Y. Han, PLoS One, 2018, 13, e0194951 CrossRef PubMed.
- A. Gagliardi, D. Paolino, N. Costa, M. Fresta and D. Cosco, Mater. Sci. Eng. C, 2021, 118, 111538 CrossRef CAS PubMed.
- H. Zhang, Y. Fu, Y. Xu, F. Niu, Z. Li, C. Ba, B. Jin, G. Chen and X. Li, Food Funct., 2019, 10, 635–645 RSC.
- G. Davidov-Pardo, I. J. Joye, M. Espinal-Ruiz and D. J. McClements, J. Agric. Food Chem., 2015, 63, 8510–8518 CrossRef CAS PubMed.
- Y. Luo, B. Zhang, M. Whent, L. L. Yu and Q. Wang, Colloids Surf. B Biointerfaces, 2011, 85, 145–152 CrossRef CAS PubMed.
- Y. Luo, Z. Teng and Q. Wang, J. Agric. Food Chem., 2012, 60, 836–843 CrossRef CAS PubMed.
- Y. Luo, T. T. Y. Wang, Z. Teng, P. Chen, J. Sun and Q. Wang, Food Chem., 2013, 139, 224–230 CrossRef CAS PubMed.
- D. Pauluk, A. K. Padilha, N. M. Khalil and R. M. Mainardes, Food Hydrocolloids, 2019, 94, 411–417 CrossRef CAS.
- R. Girija Aswathy, B. Sivakumar, D. Brahatheeswaran, T. Fukuda, Y. Yoshida, T. Maekawa and D. Sakthi Kumar, Adv. Nat. Sci. Nanosci. Nanotechnol., 2012, 3, 025006 CrossRef.
- L. Wang, G. Qin, S. Geng, Y. Dai and J. Y. Wang, J. Biomed. Nanotechnol., 2013, 9, 367–376 CrossRef CAS PubMed.
- H. Wang, W. Zhu, Y. Huang, Z. Li, Y. Jiang and Q. Xie, Acta Biomater., 2017, 61, 88–100 CrossRef CAS PubMed.
- D. S. Chauhan, P. Arunkumar, R. Prasad, S. K. Mishra, B. P. K. Reddy, A. De and R. Srivastava, Mater. Sci. Eng. C, 2018, 90, 539–548 CrossRef CAS PubMed.
- H. Liang, B. Zhou, J. Li, W. Xu, S. Liu, Y. Li, Y. Chen and B. Li, Colloids Surf. B Biointerfaces, 2015, 136, 1224–1233 CrossRef CAS PubMed.
- Y. Zhang, L. Cui, X. Che, H. Zhang, N. Shi, C. Li, Y. Chen and W. Kong, J. Controlled Release, 2015, 206, 206–219 CrossRef CAS PubMed.
- Y. Guo, Z. Liu, H. An, M. Li and J. Hu, J. Cereal Sci., 2005, 41, 277–281 CrossRef CAS.
- J. Bouman, P. Belton, P. Venema, E. Van Der Linden, R. De Vries and S. Qi, Pharm. Res., 2015, 32, 2775–2786 CAS.
- K. Shi, J. L. Kokini and Q. Huang, J. Agric. Food Chem., 2009, 57, 2186–2192 CrossRef CAS PubMed.
- S. Subramanian and S. Sampath, Biomacromolecules, 2007, 8, 2120–2128 CrossRef CAS PubMed.
- B. Ghanbarzadeh and A. R. Oromiehi, Int. J. Biol. Macromol., 2008, 43, 209–215 CrossRef CAS PubMed.
- T. Yoshino, S. Isobe and T. Maekawa, J. Am. Oil Chem. Soc., 2000, 77, 699–704 CrossRef CAS.
- T. Yoshino, S. Isobe and T. Maekawa, J. Am. Oil Chem. Soc., 2002, 79, 345–349 CrossRef CAS.
- J. W. Lawton, Cereal Chem., 2004, 81, 1–5 CrossRef CAS.
- H. Xu, Y. Chai and G. Zhang, J. Agric. Food Chem., 2012, 60, 10075–10081 CrossRef CAS PubMed.
- T. Bourtoom, Int. Food Res. J., 2009, 16, 1–9 CAS.
- K. Shi, H. Yu, S. Lakshmana Rao and T. C. Lee, J. Agric. Food Chem., 2012, 60, 5988–5993 CrossRef CAS PubMed.
- M. G. A. Vieira, M. A. Da Silva, L. O. Dos Santos and M. M. Beppu, Eur. Polym. J., 2011, 47, 254–263 CrossRef CAS.
- J. W. Lawton, Cereal Chem., 2002, 79, 1–18 CrossRef CAS.
- S. Kim, D. J. Sessa and J. W. Lawton, Ind. Crops Prod., 2004, 20, 291–300 CrossRef CAS.
- E. A. Soliman, A. A. Khalil, S. F. Deraz, G. El-Fawal and S. A. Elrahman, J. Food Sci. Technol., 2014, 51, 2425–2434 CrossRef CAS PubMed.
- A. A. Khalil, S. F. Deraz, S. A. Elrahman and G. El-Fawal, Prep. Biochem. Biotechnol., 2015, 45, 551–567 CrossRef CAS PubMed.
- C. Xia, W. Wang, L. Wang, H. Liu and J. Xiao, Food Packag. Shelf Life, 2019, 19, 76–85 CrossRef.
- J. Dong, Q. Sun and J. Y. Wang, Biomaterials, 2004, 25, 4691–4697 CrossRef CAS PubMed.
- A. Berardi, L. Bisharat, H. S. AlKhatib and M. Cespi, AAPS PharmSciTech, 2018, 19, 2009–2022 CrossRef CAS PubMed.
- A. Raza, N. Shen, J. Li, Y. Chen and J. Y. Wang, Eur. J. Pharm. Sci., 2019, 132, 163–173 CrossRef CAS PubMed.
- M. N. U. Nguyen, P. H. L. Tran and T. T. D. Tran, Int. J. Pharm., 2019, 559, 402–409 CrossRef CAS PubMed.
- L. Bisharat, S. A. Barker, A. Narbad and D. Q. M. Craig, Int. J. Pharm., 2019, 556, 311–319 CrossRef CAS PubMed.
- Y. Luo, B. Zhang, W. H. Cheng and Q. Wang, Carbohydr. Polym., 2010, 82, 942–951 CrossRef CAS.
- Y. L. Han, Q. Xu, Z. Lu and J. Y. Wang, Colloids Surf. B Biointerfaces, 2013, 111, 479–485 CrossRef CAS PubMed.
- I. Arcan and A. Yemenicioǧlu, J. Agric. Food Chem., 2014, 62, 8238–8246 CrossRef CAS PubMed.
- P. L. Dawson, D. E. Hirt, J. R. Rieck, J. C. Acton and A. Sotthibandhu, Food Res. Int., 2003, 36, 959–968 CrossRef CAS.
- M. Mastromatteo, G. Barbuzzi, A. Conte and M. A. Del Nobile, Innovative Food Sci. Emerging Technol., 2009, 10, 222–227 CrossRef CAS.
- T. Marín, P. Montoya, O. Arnache, R. Pinal and J. Calderón, J. Magn. Magn. Mater., 2018, 458, 355–364 CrossRef.
- H. J. Wang, Z. X. Lin, X. M. Liu, S. Y. Sheng and J. Y. Wang, J. Controlled Release, 2005, 105, 120–131 CrossRef CAS PubMed.
- L. Wang, J. Xue and Y. Zhang, Ind. Crops Prod., 2019, 130, 71–80 CrossRef CAS.
- H. V Ngo, P. H. L. Tran, B.-J. Lee and T. T. D. Tran, Nanotechnology, 2019, 30, 415102 CrossRef PubMed.
- R. Rošic, P. Kocbek, J. Pelipenko, J. Kristl and S. Baumgartner, Acta Pharm., 2013, 63, 295–304 Search PubMed.
- K. DeFrates, R. Moore, J. Borgesi, G. Lin, T. Mulderig, V. Beachley and X. Hu, Nanomaterials, 2018, 8, 457 CrossRef PubMed.
- S. Babitha, L. Rachita, K. Karthikeyan, E. Shoba, I. Janani, B. Poornima and K. Purna Sai, Int. J. Pharm., 2017, 523, 52–90 CrossRef CAS PubMed.
- K. Zhao, S.-X. Kang, Y.-Y. Yang and D.-G. Yu, Polymers, 2021, 13, 226 CrossRef CAS PubMed.
- W. Huang, T. Zou, S. Li, J. Jing, X. Xia and X. Liu, AAPS PharmSciTech, 2013, 14, 675–681 CrossRef CAS PubMed.
- Q. Jiang, N. Reddy and Y. Yang, Acta Biomater., 2010, 6, 4042–4051 CrossRef CAS PubMed.
- H. Lu, Q. Wang, G. Li, Y. Qiu and Q. Wei, Mater. Sci. Eng. C, 2017, 74, 86–93 CrossRef CAS PubMed.
- J. Lin, C. Li, Y. Zhao, J. Hu and L. M. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 1050–1057 CrossRef CAS PubMed.
- Z. Aytac, S. Ipek, E. Durgun and T. Uyar, J. Mater. Sci., 2018, 53, 1527–1539 CrossRef CAS.
- K. Karthikeyan, S. Guhathakarta, R. Rajaram and P. S. Korrapati, Int. J. Pharm., 2012, 438, 117–122 CrossRef CAS PubMed.
- K. Karthikeyan, V. R. Krishnaswamy, R. Lakra, M. S. Kiran and P. S. Korrapati, J. Mater. Sci. Mater. Med., 2015, 26, 101 CrossRef CAS PubMed.
- C. Yao, Y. Li and F. Wu, Polym. Compos., 2013, 34, 1163–1171 CrossRef CAS.
- B. Dhandayuthapani, A. C. Poulose, Y. Nagaoka, T. Hasumura, Y. Yoshida, T. Maekawa and D. S. Kumar, Biofabrication, 2012 DOI:10.1088/1758-5082/4/2/025008.
- M. Wang, D. Li, J. Li, S. Li, Z. Chen, D.-G. Yu, Z. Liu and J. Z. Guo, Mater. Des., 2020, 196, 109075 CrossRef CAS.
- J. J. Wang, Y. Wang, Q. Wang, J. Yang, S.-Q. Hu and L. Chen, ACS Appl. Polym. Mater., 2019, 1, 1272–1279 CrossRef CAS.
- Q. Zhong and S. Ikeda, Food Hydrocolloids, 2012, 28, 46–52 CrossRef CAS.
- D. J. Sessa, A. Mohamed and J. A. Byars, J. Agric. Food Chem., 2008, 56, 7067–7075 CrossRef CAS PubMed.
- A. De Vries, C. V. Nikiforidis and E. Scholten, Europhys. Lett., 2014, 107(5), 58003 CrossRef.
- X.-W. Chen, S.-Y. Fu, J.-J. Hou, J. Guo, J.-M. Wang and X.-Q. Yang, Food Chem., 2016, 211, 836–844 CrossRef CAS PubMed.
- A. Gagliardi, F. Froiio, M. C. Salvatici, D. Paolino, M. Fresta and D. Cosco, Food Hydrocolloids, 2020, 101, 105555 CrossRef CAS.
- Z. Gao, P. Ding, L. Zhang, J. Shi, S. Yuan, J. Wei and D. Chen, Int. J. Pharm., 2007, 328, 57–64 CrossRef CAS PubMed.
- N. Shen, J. Hu, L. Zhang, L. Zhang, Y. Sun, Y. Xie, S. Wu, L. Liu and Z. Gao, Acta Pharm. Sin. B, 2012, 2, 610–614 CrossRef.
- X. Cao, J. Geng, W. Su, L. Zhang, Q. Xu, L. Zhang, Y. Xie, S. Wu, Y. Sun and Z. Gao, Chem. Pharm. Bull., 2012, 60, 1227–1233 CrossRef CAS PubMed.
- H. Qi, J. Cao, Y. Xin, X. Mao, D. Xie, J. Luo and B. Chu, Mater. Sci. Eng. C, 2017, 70, 347–356 CrossRef CAS PubMed.
- N. Ni, D. Duquette and M.-J. Dumont, Eur. Polym. J., 2017, 91, 420–428 CrossRef CAS.
- N. Ni, D. Zhang and M. J. Dumont, Polym. Bull., 2018, 75, 31–45 CrossRef CAS.
- N. D. B. Castilhos, N. M. F. M. Sampaio, B. C. da Silva, I. C. Riegel-Vidotti, M. T. Grassi and B. J. G. Silva, Carbohydr. Polym., 2017, 174, 507–516 CrossRef CAS PubMed.
- F. Liu, R. Li, L. Mao and Y. Gao, Food Chem., 2018, 255, 390–398 CrossRef CAS PubMed.
- R. Lai, Y. Liu and J. Liu, Food Hydrocolloids, 2021, 117, 106700 CrossRef CAS.
- L. S. Liu, M. L. Fishman, K. B. Hicks, M. Kende and G. Ruthel, Drug Deliv., 2006, 13, 417–423 CrossRef CAS PubMed.
- H. Rastin, N. Mansouri, T. T. Tung, K. Hassan, A. Mazinani, M. Ramezanpour, P. L. Yap, L. Yu, S. Vreugde and D. Losic, Adv. Healthcare Mater., 2021, 2101439 CrossRef CAS PubMed.
- P. Lu, K. Takai, V. M. Weaver and Z. Werb, Cold Spring Harbor Perspect. Biol., 2011, 3, a005058 Search PubMed.
- A. Sainio and H. Järveläinen, Cell. Signalling, 2020, 66, 109487 CrossRef CAS PubMed.
- W. P. Daley, S. B. Peters and M. Larsen, J. Cell Sci., 2008, 121, 255–264 CrossRef CAS PubMed.
- F. Pampaloni, E. G. Reynaud and E. H. K. Stelzer, Nat. Rev. Mol. Cell Biol., 2007, 8, 839–845 CrossRef CAS PubMed.
- K. S. M. Smalley, M. Lioni and M. Herlyn, In Vitro Cell. Dev. Biol.: Anim., 2006, 42, 242 CrossRef CAS PubMed.
- T. Lin, C. Lu, L. Zhu and T. Lu, AAPS PharmSciTech, 2011, 12, 172–176 CrossRef CAS PubMed.
- E. Corradini, E. Souto de Medeiros, A. J. F. Carvalho, A. A. S. Curvelo and L. H. C. Mattoso, J. Appl. Polym. Sci., 2006, 101, 4133–4139 CrossRef CAS.
- P. Hurtado-López and S. Murdan, J. Drug Deliv. Sci. Technol., 2005, 15, 267–272 CrossRef.
- X. Liu, Q. Sun, H. Wang, L. Zhang and J. Y. Wang, Biomaterials, 2005, 26, 109–115 CrossRef CAS PubMed.
- K. Makino, N. Yamamoto, K. Higuchi, N. Harada, H. Ohshima and H. Terada, Colloids Surf. B Biointerfaces, 2003, 27, 33–39 CrossRef CAS.
- S. Gong, H. Wang, Q. Sun, S. T. Xue and J. Y. Wang, Biomaterials, 2006, 27, 3793–3799 CrossRef CAS PubMed.
- H. J. Wang, S. J. Gong, Z. X. Lin, J. X. Fu, S. T. Xue, J. C. Huang and J. Y. Wang, Biomaterials, 2007, 28, 3952–3964 CrossRef CAS PubMed.
- Z. H. Qu, H. J. Wang, T. T. Tang, X. L. Zhang, J. Y. Wang and K. R. Dai, Acta Biomater., 2008, 4, 1360–1368 CrossRef CAS PubMed.
- J. Tu, H. Wang, H. Li, K. Dai, J. Wang and X. Zhang, Biomaterials, 2009, 30, 4369–4376 CrossRef CAS PubMed.
- A. Salerno, S. Zeppetelli, E. Di Maio, S. Iannace and P. A. Netti, Compos. Sci. Technol., 2010, 70, 1838–1846 CrossRef CAS.
- A. Salerno, M. Oliviero, E. Di Maio, P. A. Netti, C. Rofani, A. Colosimo, V. Guida, B. Dallapiccola, P. Palma, E. Procaccini, A. C. Berardi, F. Velardi, A. Teti and S. Iannace, J. Mater. Sci. Mater. Med., 2010, 21, 2569–2581 CrossRef CAS PubMed.
- C. Liu, Y. Chen, X. Wang, J. Huang, P. R. Chang and D. P. Anderson, J. Mater. Sci., 2010, 45, 6775–6785 CrossRef CAS.
- M. E. Gomes, H. S. Azevedo, A. R. Moreira, V. Ellä, M. Kellomäki and R. L. Reis, J. Tissue Eng. Regener. Med., 2008, 2, 243–252 CrossRef CAS PubMed.
- Q. Z. Chen, I. D. Thompson and A. R. Boccaccini, Biomaterials, 2006, 27, 2414–2425 CrossRef CAS PubMed.
- Z. Fereshteh, P. Nooeaid, M. Fathi, A. Bagri and A. R. Boccaccini, Mater. Sci. Eng. C, 2015, 54, 50–60 CrossRef CAS PubMed.
- P. Zhou, Y. Xia, J. Wang, C. Liang, L. Yu, W. Tang, S. Gu and S. Xu, J. Mater. Chem. B, 2013, 1, 685–692 RSC.
- R. Jayakumar, M. Prabaharan, P. T. Sudheesh Kumar, S. V. Nair and H. Tamura, Biotechnol. Adv., 2011, 29, 322–337 CrossRef CAS PubMed.
- A. R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y. S. Lee and C. S. Kim, Carbohydr. Polym., 2014, 102, 884–892 CrossRef CAS PubMed.
- C. Zhijiang, Z. Qin, S. Xianyou and L. Yuanpei, Mater. Sci. Eng. C, 2017, 71, 797–806 CrossRef PubMed.
- Z. Pedram Rad, J. Mokhtari and M. Abbasi, Mater. Sci. Eng. C, 2018, 93, 356–366 CrossRef CAS PubMed.
- Z. Pedram Rad, J. Mokhtari and M. Abbasi, Int. J. Biol. Macromol., 2019, 135, 530–543 CrossRef CAS PubMed.
- M. Zhang, X. Li, S. Li, Y. Liu and L. Hao, J. Mater. Sci. Mater. Med., 2016, 27, 136 CrossRef PubMed.
- R. Li, Y. Ma, Y. Zhang, M. Zhang and D. Sun, Colloids Surf. B Biointerfaces, 2018, 169, 384–394 CrossRef CAS PubMed.
- S. Tamburaci, B. Cecen, O. Ustun, B. U. Ergur, H. Havitcioglu and F. Tihminlioglu, ACS Appl. Bio Mater., 2019, 2, 1440–1455 CrossRef CAS.
- Q. Ou, Y. Miao, F. Yang, X. Lin, L.-M. Zhang and Y. Wang, Biomater. Sci., 2019, 7, 1973–1983 RSC.
- F. Yang, Y. Miao, Y. Wang, L.-M. Zhang and X. Lin, Materials, 2017, 10, 1168 CrossRef PubMed.
- L. Jing, X. Wang, H. Liu, Y. Lu, J. Bian, J. Sun and D. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 18551–18559 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |