Open Access Article
Open Access ArticleChemoselective synthesis of multifunctional ferrocene-containing derivatives by the cross Rauhut–Currier reaction†
Dragana Stevanović *a,
Jovana Bugarinović
*a,
Jovana Bugarinović a,
Marko Pešić
a,
Marko Pešić a,
Anka Todosijevićb,
Goran A. Bogdanović
a,
Anka Todosijevićb,
Goran A. Bogdanović c and
Ivan Damljanović
c and
Ivan Damljanović a
a
aFaculty of Science, University of Kragujevac, 34000 Kragujevac, Serbia. E-mail: dragana.stevanovic@pmf.kg.ac.rs
bFaculty of Agriculture, University of Niš, 37000 Kruševac, Serbia
cVINČA Institute of Nuclear Sciences - National Institute of the Republic of Serbia, University of Belgrade, 11001 Belgrade, Serbia
First published on 10th November 2021
Abstract
A simple protocol has been developed for the chemoselective synthesis of ferrocene-containing Rauhut–Currier adducts from 1-ferrocenyl-2-nitroethene and vinyl ketones using 20 mol% of triphenylphosphine. Multifunctional ferrocene derivatives were obtained in moderate to high yields (51–92%) by the coupling between the α-position of vinyl ketones and the β-position of the nitroalkene. The study of the Rauhut–Currier reaction under the described conditions showed that the strong electron-donating group at the β-position of nitroalkenes plays a significant role in the reaction outcome due to prevention of polymerization and stabilization of the zwitterionic intermediate. Additionally, a preparative synthesis of 4-ferrocenyl-3-methylene-5-nitropentan-2-one was carried out and its synthetic transformations showed easy conversion to other useful building blocks.
Introduction
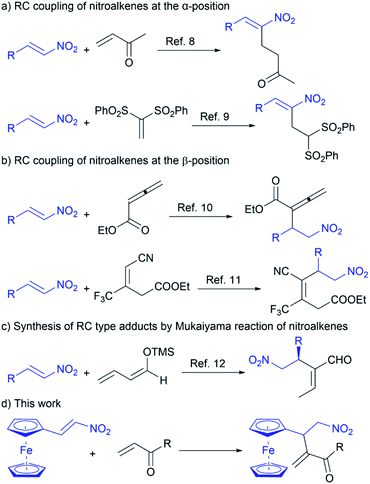
The cross Rauhut–Currier (RC) reaction provides a new carbon–carbon bond formation between two different electron-deficient alkenes (Michael acceptors) in the presence of nucleophilic catalysts (Lewis bases).1,2 The RC transformation involves the coupling between the α-position of the alkene activated by the nucleophilic catalyst and the β-position of another Michael acceptor. According to the literature,1,2 the nucleophilic catalyst participates in the conjugate addition to one of Michael acceptors to give zwitterion. This zwitterionic intermediate, acting as a nucleophile, further undergoes the conjugate addition to the second electron-deficient alkene forming a new carbon–carbon bond. Although the RC reaction yields multifunctional compounds that are used for further synthetic transformation and/or total synthesis of natural products,1a,3–5 its application is limited due to problematic selectivity control and low reaction efficiency. In fact, the zwitterion can be trapped with other electrophiles present in the reaction, so the self-condensation of the alkenes is often a concurrent reaction under the same conditions.6 Therefore, the development of efficient chemoselective synthetic cross RC protocols is a key challenge and of particular importance for organic chemistry.Nitroalkenes are well-known electron-deficient olefins that are employed in the cross RC reaction.1b,7 Depending on the reaction conditions, the cross RC coupling is directed at the α-position or the β-position of nitroalkenes (Scheme 1).8–11 Accordingly, the reaction of nitroalkenes and methyl vinyl ketone (MVK) in the presence of an imidazole–LiCl catalytic system is described.8 This catalytic system is also able to promote the cross RC reaction between nitroalkenes and vinyl sulfones.9 Under the described conditions, the RC adducts are obtained by the coupling of nitroalkenes at the α-position with methyl vinyl ketone and vinyl sulfones (Scheme 1a). The intermolecular RC coupling at the β-position of nitroalkenes with ethyl allenoate is achieved under mild conditions in the presence of quinidine-derived β-isocupreidine (β-ICD) (Scheme 1b).10 The phosphine-catalyzed cross RC coupling at the β-position of nitroalkenes with trifluoromethyl-containing acrylonitrile is also reported (Scheme 1b).11 Since the nitro RC products are useful building blocks for the synthesis of complex molecules,7 the synthesis of RC type adducts was achieved by the Mukaiyama reaction between nitroalkenes and silyl-diene enol ethers in the presence of bifunctional organocatalyst (Scheme 1c).12 Although the synthetic protocols to the nitro RC adducts are reported, lack of substrate scope, as well as an explanation of the reactivity of electron-deficient alkenes in the cross RC reaction, requires more comprehensive research with an emphasis on chemoselectivity. In addition, the main problem to employ nitroalkenes in the RC reaction is their easy polymerization in the presence of Lewis bases.13 Therefore, constant efforts are made to develop chemoselective methods for the cross RC reaction.
Our constant research interest in the functionalization of ferrocene, the synthetic transformation of its derivatives as well as the role of this metallocene in the reaction outcomes,14 has inspired us to employ ferrocene-containing electron-deficient olefin in the cross RC reaction. Herein we report a useful method for the cross RC reaction of ferrocene-containing nitroalkene with vinyl ketones affording multifunctional ferrocene-containing RC products in good yield with complete chemoselectivity (Scheme 1d).
Results and discussion
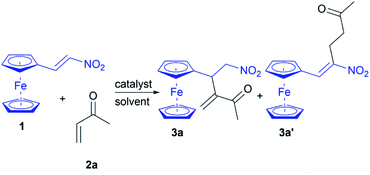
Our investigation began with a screening of nucleophilic catalysts for their ability to promote the RC reaction between nitroalkene 1 and MVK (2a) in DCM (Table 1, entries 1–7). When the nucleophilic tertiary amines (30 mol%) were used as a Lewis base catalyst, the results were not encouraging (Table 1, entries 1–5). Actually, the reactions catalyzed by triethylamine, diazabicyclo[2.2.2]octane (DABCO), and imidazole afforded the mixtures without the products (Table 1, entries 1, 2, and 5). The use of 4-(dimethylamino)pyridine (DMAP) as the nucleophilic catalyst of the RC reaction resulted in traces of product 3a after stirring at room temperature for 48 hours (Table 1, entry 3), while the reactions with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,1,3,3-tetramethylguanidine (TMG) gave the complex mixtures where are observed the product 3a′ in traces (Table 1, entries 4 and 6). An optimistic yield of 48% is obtained in the triphenylphosphine-catalyzed reaction after stirring for 24 hours at room temperature (Table 1, entry 7). Therefore, triphenylphosphine was selected as a convenient catalyst for the RC reaction, after which solvent screening was performed (Table 1, entries 8–12). Further attempts to increase the yield performing reactions in several solvents afforded 3a in 90% yield in CHCl3 (Table 1, entry 12), while the other solvents gave a poor yield (Table 1, entries 8–11). The catalyst loading was also investigated (Table 1, entries 12–14). We found that the use of 20 mol% of triphenylphosphine in CHCl3 led to a high yield of RC adduct 3a in the intermolecular reaction between nitroalkene 1 and 2a (Table 1, entry 13). RC adduct 3a′ was not observed in the phosphine-catalyzed reactions indicating that the phosphine carries out the nucleophilic attack on 2a generating the corresponding zwitterion for the RC coupling with 1. The established protocol offers the chemoselective synthesis of ferrocene-containing RC adduct 3a, and the reaction is conducted under an air atmosphere demonstrating the ease of operation and practicality.| Entry | Catalyst (mol%) | Solvent | Time (h) | 3a yieldb (%) |
|---|---|---|---|---|
| a All reactions were carried out with 1 (0.2 mmol) and 2a (0.24 mmol) in solvent (1 mL) at room temperature.b Isolated yield by flash column chromatography on silica gel.c NR: no reaction.d 1 is completely recovered by flash column chromatography.e 3a′ was observed and isolated in traces. | ||||
| 1 | Et3N (30) | DCM | 24 | NRc,d |
| 2 | DABCO (30) | DCM | 24 | NRc,d |
| 3 | DMAP (30) | DCM | 48 | <5 |
| 4 | DBU (30) | DCM | 24 | Complex mixturee |
| 5 | Imidazole (30) | DCM | 24 | NRc,d |
| 6 | TMG (30) | DCM | 24 | Complex mixturee |
| 7 | PPh3 (30) | DCM | 24 | 48 |
| 8 | PPh3 (30) | MeCN | 48 | 18 |
| 9 | PPh3 (30) | Toluene | 72 | 28 |
| 10 | PPh3 (30) | THF | 120 | 23 |
| 11 | PPh3 (30) | AcOEt | 120 | 20 |
| 12 | PPh3 (30) | CHCl3 | 24 | 90 |
| 13 | PPh3 (20) | CHCl3 | 24 | 92 |
| 14 | PPh3 (10) | CHCl3 | 24 | 75 |
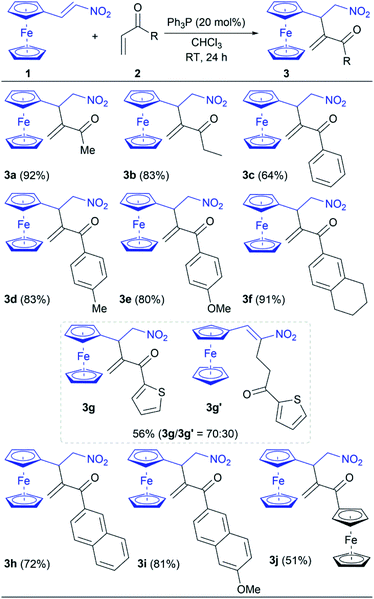
After optimization of reaction conditions (Table 1, entry 13), the scope of phosphine-catalyzed intermolecular RC reaction between nitroalkene 1 and vinyl ketones 2 was investigated (Table 2). The obtained results, listed in Table 2, show that the reaction of 1 with vinyl ketones 2 is easily completed under the established conditions giving the corresponding product 3 in 51–92% yields. These conditions provide the synthetic pathway with complete chemoselectivity since the cross RC coupling at the β-position of nitroalkene 1 was only achieved, except for the reaction with 2g. While products 3a and 3b were obtained in high yields (92% and 83%, respectively) employing the corresponding alkyl vinyl ketones 2a and 2b, the yields of reactions with aromatic vinyl ketones 2c–j are varied from moderate to excellent due to the different electron-donating and steric effects of aromatic groups. Thus, aryl vinyl ketones 2c–f reacted with 1 under the optimal conditions giving the corresponding products 3c–f in 64–91% yields (Table 2). Notably, substituents on the phenyl group of 2c–f affected the reaction outcomes. Utilizing phenyl vinyl ketone (2c) in the RC reaction with 1 led to 3c in a 64% yield, while vinyl ketones 2d–f with the electron-donating substituted phenyl group increased the yield up to 91% (Table 2). Heteroaryl vinyl ketone 2g smoothly gave a product mixture with lower efficiency. It is found that the reaction of 2g provided the desired product 3g as well as product 3g′ in a total yield of 56% (the ratio was 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for 3g and 3g′, respectively; see ESI†). Therefore, the catalyst participated in the conjugate addition to both of the Michael acceptors, whereby nucleophilic coupling partners were generated and subsequently cross-reacted with the present electrophile. The outcome indicated that the electron-donating effect of the 2-thienyl group significantly reduces the electrophilicity of 2g leading to the unfavoured addition to 1. Hence, the result of the reaction between 1 and 2g was the cross RC coupling at the β-position of nitroalkene 1 as well as the coupling at α-position. In the case of vinyl ketones 2h and 2i, the corresponding products 3h and 3i were obtained in a good yield of 72% and 81%, respectively. The influence of the steric effect was evident in the reaction between 1 and acryloylferrocene (2j) due to the presence of two bulky ferrocene units which definitely affect the reaction outcome. Product 3j was suitable for single-crystal X-ray diffraction analysis (Fig. 1).15 The analysis showed that two ferrocene units in the compound are mutually almost perfectly orthogonal. Thus a dihedral angle between two adjacent cyclopentadienyl rings (the C11–C15 and C16–C20 rings) is 86.4(2)°. This molecular geometry is clearly caused by a relatively strong intramolecular C–H⋯π interaction16 shown in Fig. 1 with the dotted green line. Namely, the C11–H group is directed to the midpoint (Cg) of the C16–C20 ring with an H⋯Cg distance of 2.84 Å (this distance for the position of H atoms normalized to neutron values is only 2.70 Å). The C11–H⋯Cg angle also has a desirable value of 151°. Selected bond lengths in 3j are given in Table S1† (see ESI†).
30 for 3g and 3g′, respectively; see ESI†). Therefore, the catalyst participated in the conjugate addition to both of the Michael acceptors, whereby nucleophilic coupling partners were generated and subsequently cross-reacted with the present electrophile. The outcome indicated that the electron-donating effect of the 2-thienyl group significantly reduces the electrophilicity of 2g leading to the unfavoured addition to 1. Hence, the result of the reaction between 1 and 2g was the cross RC coupling at the β-position of nitroalkene 1 as well as the coupling at α-position. In the case of vinyl ketones 2h and 2i, the corresponding products 3h and 3i were obtained in a good yield of 72% and 81%, respectively. The influence of the steric effect was evident in the reaction between 1 and acryloylferrocene (2j) due to the presence of two bulky ferrocene units which definitely affect the reaction outcome. Product 3j was suitable for single-crystal X-ray diffraction analysis (Fig. 1).15 The analysis showed that two ferrocene units in the compound are mutually almost perfectly orthogonal. Thus a dihedral angle between two adjacent cyclopentadienyl rings (the C11–C15 and C16–C20 rings) is 86.4(2)°. This molecular geometry is clearly caused by a relatively strong intramolecular C–H⋯π interaction16 shown in Fig. 1 with the dotted green line. Namely, the C11–H group is directed to the midpoint (Cg) of the C16–C20 ring with an H⋯Cg distance of 2.84 Å (this distance for the position of H atoms normalized to neutron values is only 2.70 Å). The C11–H⋯Cg angle also has a desirable value of 151°. Selected bond lengths in 3j are given in Table S1† (see ESI†).
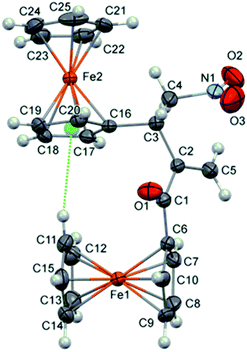 | ||
| Fig. 1 Crystal structure of molecules 3j (thermal ellipsoids are shown at the 40% probability level). Dotted green line represents intramolecular C–H⋯π interaction. | ||
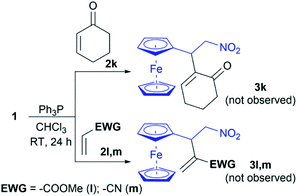
The potential impact of this method would be expanded if other α,β-unsaturated compounds with an electron-withdrawing group (Michael acceptors) could be used as a partner in the cross RC reaction with nitroalkenes 1. Thus, our investigation is directed to employ Michael acceptors 2k–m in the RC reaction under the optimal conditions (Scheme 2). Unfortunately, cyclic enone 2k did not cross-react with 1 under the conditions as well as vinyl Michael acceptors – methyl acrylate (2l) and acrylonitrile (2m). Therefore, we can define that the developed method of the intermolecular RC reaction with 1 is specific for vinyl ketones 2a–j.
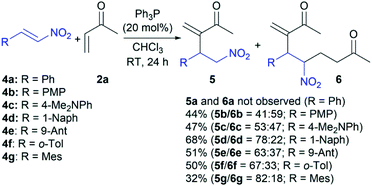
In the next step, we submitted various β-aryl nitroalkenes 4a–g in the RC reaction with vinyl ketone 2a under the established conditions (Scheme 3). The reaction of β-nitrostyrene (4a) and MVK (2a) in chloroform in the presence of 20 mol% of Ph3P at room temperature for 24 h resulted in a complete polymerization of nitroalkene 4a13 and self-condensation of MVK (2a).6 Actually, the product of the self-condensation was only isolated in a yield of 95%. The same reaction conditions were applied in the RC reaction between nitroalkene 4b and 2a. Since the ferrocenyl group has a stronger electron-donating effect than the phenyl group,17 we expected that the methoxy group at the para-position on the benzene ring of nitroalkene 4b could affect the reaction outcome. In fact, the strong electron-donating effect of the methoxy group should prevent rapid polymerization due to decreasing electrophilicity at the β-position of the nitroalkene13 and allow cross-reacting of 4b with partner 2a. Indeed, the reaction gave the product mixture followed by the polymerization of 4b, as well as the self-condensation of 2a (ca. 40%). The products were identified as desired RC adduct 5b and another 6b with a total yield of 44% (Scheme 3). In this case, the reaction conditions favored the formation of product 6b (the ratio is 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 for 5b and 6b, respectively). A similar trend was remarked in the reactions of nitroalkenes bearing stronger electron-donating groups at the β-position such as 4c and 4d because the product mixture was formed in a variable product ratio (Scheme 3). Also, polymerization of 4 and self-condensation of 2a were observed in these reactions, but to a lesser extent. Nitroalkene 4c with the dimethylamino group at the para-position on the benzene ring in the reaction with 2a gave both 5c and 6c in a total yield of 47% with an almost equal ratio of the products (Scheme 3). On the other hand, nitroalkene 4d reacted with 2a giving both products with a good efficacy (68% yields) and dominantly RC adduct 5d (Scheme 3). The steric effect of the bulky substituent at the β-position of nitroalkene 4d could affect this reaction outcome. Hence, nitroalkenes 4e–g with large aryl groups (9-anthracenyl, o-tolyl, and mesityl) at the β-position were employed to determine the influence of steric effect on the reaction efficiency. The reaction outcomes indicated that the efficiency decreases with increasing the group voluminosity and that nitroalkenes 4e–g mostly form RC product 5. While nitroalkenes 4e and 4f provided similar results in terms of the reaction efficiencies (51% and 50%, respectively) and product distribution (the ratios are 63
59 for 5b and 6b, respectively). A similar trend was remarked in the reactions of nitroalkenes bearing stronger electron-donating groups at the β-position such as 4c and 4d because the product mixture was formed in a variable product ratio (Scheme 3). Also, polymerization of 4 and self-condensation of 2a were observed in these reactions, but to a lesser extent. Nitroalkene 4c with the dimethylamino group at the para-position on the benzene ring in the reaction with 2a gave both 5c and 6c in a total yield of 47% with an almost equal ratio of the products (Scheme 3). On the other hand, nitroalkene 4d reacted with 2a giving both products with a good efficacy (68% yields) and dominantly RC adduct 5d (Scheme 3). The steric effect of the bulky substituent at the β-position of nitroalkene 4d could affect this reaction outcome. Hence, nitroalkenes 4e–g with large aryl groups (9-anthracenyl, o-tolyl, and mesityl) at the β-position were employed to determine the influence of steric effect on the reaction efficiency. The reaction outcomes indicated that the efficiency decreases with increasing the group voluminosity and that nitroalkenes 4e–g mostly form RC product 5. While nitroalkenes 4e and 4f provided similar results in terms of the reaction efficiencies (51% and 50%, respectively) and product distribution (the ratios are 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 for 5e and 6e and 67
37 for 5e and 6e and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 for 5f and 6f, respectively), nitroalkene 4g gave the low efficiency (32%) with dominant RC adduct 5g (the ratio is 82
33 for 5f and 6f, respectively), nitroalkene 4g gave the low efficiency (32%) with dominant RC adduct 5g (the ratio is 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 for 5g and 6g, respectively). These results showed that both the electronic and steric effects of the substituent at the β-position of nitroalkenes impact the outcome of the cross RC coupling, and the nitroalkenes with stronger electron-donating substituents at the β-position allow to obtain the corresponding RC adducts 5.
18 for 5g and 6g, respectively). These results showed that both the electronic and steric effects of the substituent at the β-position of nitroalkenes impact the outcome of the cross RC coupling, and the nitroalkenes with stronger electron-donating substituents at the β-position allow to obtain the corresponding RC adducts 5.
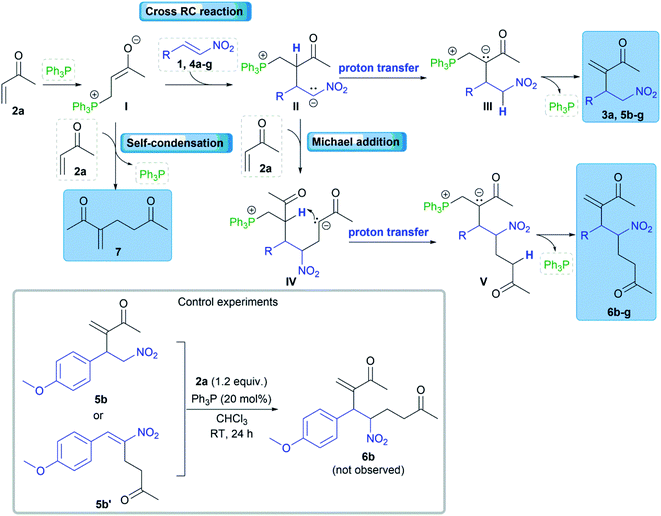
A plausible reaction mechanism could be explained by the influence of electro-donating substituents at the β-position of nitroalkenes (Scheme 4). The cross RC reaction certainly includes the generation of zwitterion I by the conjugate addition of nucleophilic catalyst to methyl vinyl ketone (2a) and the subsequent addition of I to the corresponding nitroalkene 1, 4a–g to form a new zwitterion II (Scheme 4).1 An alternative pathway of zwitterion I is the addition to another molecule of methyl vinyl ketone (2a). The corresponding cross RC adduct 3a, 5a–g as well as homo RC adduct 7 are formed by proton transfer following the regeneration of the nucleophilic catalyst (Scheme 4). The study showed that zwitterion II generated from 1 (ferrocenyl group at the β-position of the nitroalkene) exclusively undergoes proton transfer leading to the RC adduct. The reactions with β-aryl nitroalkenes 4a–g gave the product mixtures. The RC adduct 5 is obtained together with the product 6 formed by the Michael addition of the zwitterion II to another molecule of 2a (Scheme 4). The key intermediate in the mechanism is zwitterion II which can follow two possible pathways. The first pathway is proton transfer followed by elimination of the phosphine providing RC adduct 5, and the second is the Michael addition of formed II to 2a which processes to product 6 via intramolecular proton transfer and the elimination of the catalyst (Scheme 4). The second pathway is defined by control experiments that confirmed the formation of product 6 via zwitterion II (Scheme 4). The first control experiment was performed between RC adduct 5b and 2a and showed that product 5b could not be converted to 6b under the RC reaction conditions. Although product 5b′ was not observed after the reaction between 4b and 2a in the presence of the phosphine, a possible synthetic pathway to 6 could be double RC reaction. The second control experiment was performed assuming that product 5b′ is formed by the RC coupling at the α-position of 4b and then 5b′ cross-reacts with 2a as a partner in the subsequent RC reaction (Scheme 4).18 This experiment eliminated the double RC reaction as the pathway to product 6 due to the absence of 6b in the reaction mixture after 24 hours. Based on the obtained results, zwitterion II generated from nitroalkenes with a stronger electron-donating substituent at the β-position has favored the formation of RC adducts rather than the products of the Michael addition. In addition, nitroalkenes with the large aryl groups at the β-position predominantly provided the cross RC adducts presumably due to the steric hindrance that blocked the Michael addition. Therefore, the substituents affect the stability of zwitterion II, and their electron-donating properties could be crucial for the chemoselectivity and regioselectivity of the cross RC reactions.
We obtained comparable results in terms of electron and steric effects of substituents at the β-position of nitroalkenes and their ability to be used in the cross RC reaction (Fig. 2). The results indicated that the electron-donating effect of the ferrocenyl group is significantly stronger than the effects of p-anisyl, 4-dimethylamino phenyl, and 1-naphthyl groups, while the weakest effect shows the phenyl group. These observations are consistent with the previous reports.17 Nitroalkenes 4a, 4b, and 4f with weak electron-donating substituents at the β-position easily undergo polymerization, while nitroalkenes 1, 4d, 4e, and 4g, with stronger electron-donating effect of substituents and/or large aryl groups, are more stable under the reaction conditions. Thus, the reaction between 4a and 2a does not give the cross-product due to an easy polymerization of 4a. The generation of zwitterion I is probably a slower reaction than polymerization of 4a and thus zwitterion I only has the opportunity to react with another molecule of 2a giving 7 (Scheme 4). On the other hand, more stable nitroalkenes smoothly react with 2a in the cross RC reaction. The results showed that the reaction efficiency, as well as the distribution of products, depends on the electronic and steric properties of nitroalkenes (Fig. 2). The reactions of nitroalkene 4b–g gave the RC product 5 together with the product 6 while 1 only formed the corresponding RC adduct. Therefore, the reactivity of 1 in the RC reaction is directly related to the strong electron-donating and steric effects of the ferrocenyl group. The ratio of 5 and 6 suggests that fine-tuning the electronic and/or steric properties of nitroalkenes can achieve the chemoselective protocol. Briefly, the electron-donating and steric effects of the substituents at the β-position of nitroalkenes could play a double role in the cross RC reaction: (i) prevent the polymerization of nitroalkenes and (ii) contribute to the stabilization of zwitterion II during the RC transformation.
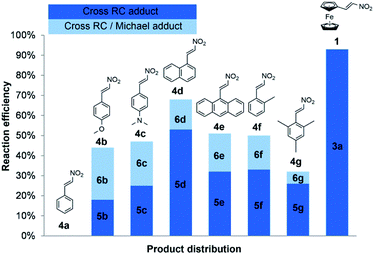 | ||
| Fig. 2 Influence of electronic and steric effects of substituents at the β-position of nitroalkenes on chemoselectivity. | ||
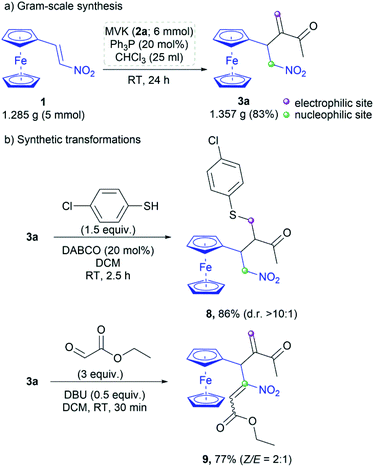
The developed protocol is validated through the gram-scale synthesis of 3a (Scheme 5a). RC adduct 3a was obtained in 83% yield by the reaction of 1.285 g (5 mmol) of nitroalkene 1 under the optimal conditions. Further synthetic transformations of 3a were also performed to demonstrate the importance of the RC product as a building block due to the presence of both nucleophilic and electrophilic centers (Scheme 5b). The RC product was subjected to the thia-Michael addition with 4-chlorothiophenol in the presence of DABCO (20 mol%).19 The corresponding product 8 was obtained in 86% yield with high diastereoselectivity (d. r. > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Under Henry conditions,20 3a was reacted with ethyl glyoxylate to afford product 9 in 77% yield as a mixture of Z/E isomers (Scheme 5b). The isomers were separated by column chromatography on silica gel and the ratio was 2
1). Under Henry conditions,20 3a was reacted with ethyl glyoxylate to afford product 9 in 77% yield as a mixture of Z/E isomers (Scheme 5b). The isomers were separated by column chromatography on silica gel and the ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Z and E isomers, respectively.
1 for Z and E isomers, respectively.
Conclusions
The chemoselective synthesis of multifunctional ferrocene derivatives by the cross Rauhut–Currier reaction between 1-ferrocenyl-2-nitroethene and vinyl ketones has been reported. The established reaction conditions provided the RC coupling of the nitroalkene at the β-position giving the corresponding products in moderate to high yields with complete chemoselectivity, except for the reaction with 2-thienyl vinyl ketone. Based on the results, we observed that the ferrocenyl group plays a significant role in this coupling process. The present study showed that the reaction efficiency directly depends on the electronic and steric properties of substituents at the β-position of nitroalkenes and the strong electron-donating bulky ferrocenyl group at the β-position certainly contributes to a more successful protocol. Reducing electrophilicity at the β-position of nitroalkenes ensures the conjugate addition of the catalyst to the vinyl ketone that is the more electrophilic partner in the reaction. The developed method overcomes the typical challenges associated with nitroalkene instability, limited selectivity, and low reaction efficiency, providing building blocks that can be used in further synthesis.Experimental
General procedure for the cross Rauhut–Currier reaction
To a solution of nitroalkene 1 (51.4 mg, 0.2 mmol, 1 equiv.) and the corresponding vinyl ketone 2a–j (0.24 mmol, 1.2 equiv.) in chloroform (1 mL) at room temperature, triphenylphosphine (10.5 mg, 20 mol%) was added. The reaction mixture was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the crude product was purified by flash column chromatography on silica gel with n-hexane/ethyl acetate as eluent. Following the general procedure for the RC reaction, 3a was obtained as a yellow solid in 92% yield (60.2 mg), after flash chromatography on silica gel (n-hexane/ethyl acetate = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp 82–83 °C; IR (KBr, cm−1) 1674 (C
1); mp 82–83 °C; IR (KBr, cm−1) 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1549 (NO2), 1381 (NO2); 1H NMR (200 MHz, CDCl3) δ 6.12 (s, 1H, CH2
O), 1549 (NO2), 1381 (NO2); 1H NMR (200 MHz, CDCl3) δ 6.12 (s, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 5.81 (s, 1H, CH2
C), 5.81 (s, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 5.06–4.74 (m, 2H, CH2), 4.64–4.42 (m, 1H, CH), 4.17–4.13 (overlapped m, 2H, C5H4), 4.15 (overlapped s, 5H, C5H5), 4.11–4.06 (m, 1H, C5H4), 4.06–4.01 (m, 1H, C5H4), 2.34 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 198.0, 147.8, 127.6, 87.2 78.7, 69.3, 68.3, 68.2, 66.8, 39.5, 26.2. Anal. calcd for C16H17FeNO3: C, 58.74; H, 5.24; Fe, 17.07; N, 4.28; O, 14.67. Found: C, 58.81; H, 5.25; N, 4.28.
C), 5.06–4.74 (m, 2H, CH2), 4.64–4.42 (m, 1H, CH), 4.17–4.13 (overlapped m, 2H, C5H4), 4.15 (overlapped s, 5H, C5H5), 4.11–4.06 (m, 1H, C5H4), 4.06–4.01 (m, 1H, C5H4), 2.34 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 198.0, 147.8, 127.6, 87.2 78.7, 69.3, 68.3, 68.2, 66.8, 39.5, 26.2. Anal. calcd for C16H17FeNO3: C, 58.74; H, 5.24; Fe, 17.07; N, 4.28; O, 14.67. Found: C, 58.81; H, 5.25; N, 4.28.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Serbian Ministry of Education, Science and Technological Development (Agreement No. 451-03-9/2021-14/200122).Notes and references
- (a) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (b) C. E. Aroyan, A. Dermenci and S. J. Miller, Tetrahedron, 2009, 65, 4069 CrossRef CAS; (c) P. Xie and Y. Huang, Eur. J. Org. Chem., 2013, 6213 CrossRef CAS; (d) K. C. Bharadwaj, RSC Adv., 2015, 5, 75923 RSC; (e) H. Li and Y. Lu, Asian J. Org. Chem., 2017, 6, 1130 CrossRef CAS.
- A. M. Wensley, N. T. McDougal and S. E. Schaus, Morita–Baylis–Hillman, Vinylogous Morita–Baylis–Hillman, and Rauhut–Currier Reactions in Lewis Base Catalysis in Organic Synthesis, ed. E. Vedejs and S. E. Denmark, Wiley-VCH Verlag GmbH & Co. KGaA, 2016 Search PubMed.
- S. M. Winbush, D. J. Mergott and W. R. Roush, J. Org. Chem., 2008, 73, 1818 CrossRef CAS PubMed.
- P. Webber and M. J. Krische, J. Org. Chem., 2008, 73, 9379 CrossRef CAS PubMed.
- S. Jeon and S. Han, J. Am. Chem. Soc., 2017, 139, 6302 CrossRef CAS PubMed.
- J. D. McClure, J. Org. Chem., 1970, 35, 3045 CrossRef CAS.
- D. K. Nair, T. Kumar and I. N. N. Namboothiri, Synlett, 2016, 27, 2425 CrossRef CAS.
- (a) M. Dadwal, R. Mohan, D. Panda, S. M. Mobin and I. N. N. Namboothiri, Chem. Commun., 2006, 338 RSC; (b) P. Shanbhag, P. R. Nareddy, M. Dadwal, S. M. Mobin and I. N. N. Namboothiri, Org. Biomol. Chem., 2010, 8, 4867 RSC.
- R. Kumar, T. Kumar, S. M. Mobin and I. N. N. Nambothiri, J. Org. Chem., 2013, 78, 5073 CrossRef CAS PubMed.
- S. Takizawa, M. Sako, K. Kishi, M. Shigenobu, G. Vo-Thanh and H. Sasai, Chem. Pharm. Bull., 2017, 65, 997 CrossRef CAS PubMed.
- X.-H. He, L. Yang, Y.-L. Ji, Q. Zhao, M.-C. Yang, W. Huang, C. Peng and B. Han, Chem. - Eur. J., 2018, 24, 1947 CrossRef CAS PubMed.
- M. Frias, R. Mas-Ballesté, S. Arias, C. Alvarado and J. Alemán, J. Am. Chem. Soc., 2017, 139, 672 CrossRef CAS PubMed.
- (a) X. Sun, S. Sengupta, J. L. Petersen, H. Wang, J. P. Lewis and X. Shi, Org. Lett., 2007, 9, 4495 CrossRef CAS PubMed; (b) V. Barbier, F. Couty and O. R. P. David, Eur. J. Org. Chem., 2015, 3679 CrossRef CAS.
- (a) A. Minić, D. Stevanović, I. Damljanović, A. Pejović, M. Vukićević, G. A. Bogdanović, N. S. Radulović and R. D. Vukićević, RSC Adv., 2015, 5, 24915 RSC; (b) A. Minić, D. Stevanović, M. Vukićević, G. A. Bogdanović, M. D’hooghe, N. S. Radulović and R. D. Vukićević, Tetrahedron, 2017, 73, 6268 CrossRef; (c) A. Minić, J. P. Bugarinović, A. Pejović, D. Ilić Komatina, G. A. Bogdanović, I. Damljanović and D. Stevanović, Tetrahedron Lett., 2018, 59, 3499 CrossRef; (d) D. Stevanović, G. Bertuzzi, A. Mazzanti, M. Fochi and L. Bernardi, Adv. Synth. Catal., 2018, 360, 893 CrossRef; (e) M. Pešić, J. Bugarinović, A. Minić, S. B. Novaković, G. A. Bogdanović, A. Todosijević, D. Stevanović and I. Damljanović, Bioelectrochemistry, 2020, 132, 107412 CrossRef PubMed.
- Crystallographic data for the structural analysis of compound 3j have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2095955. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, U.K., 1999 Search PubMed.
- (a) A. N. Nesmeyanov, E. G. Perovalova, S. P. Gubin, K. I. Grandberg and A. G. Kozlovsky, Tetrahedron Lett., 1966, 7, 2381 CrossRef; (b) M. Marijan, S. Jurić, Z. Mihalić and O. Kronja, Eur. J. Org. Chem., 2019, 2019, 537 CrossRef CAS.
- Compound 5b′ was prepared according to reported procedure.8.
- M. Tao, W. Zhou and J. Zhang, Adv. Synth. Catal., 2017, 359, 3347 CrossRef CAS.
- N. C. Barua, B. Saikia, P. Borah and G. Baishya, Process for the preparation of chiral amines and its application in the synthesis of sitagliptin, WO 2015/189862 A1, 2014.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, spectral characterisation (including copies of 1H and 13C NMR spectra) of all new compounds, crystallographic data and CIF file. CCDC 2095955. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra07619a |
| This journal is © The Royal Society of Chemistry 2021 |