Open Access Article
Open Access ArticleConcise synthesis of α-cyano tetrahydroisoquinolines with a quaternary center via Strecker reaction†
Yue Ji *,
Xue Zhang,
Weiwei Han,
Sichang Wang,
Ya Wu,
Keliang Zhang,
Penghui Yang,
Pei Xiao and
Yitao Wei
*,
Xue Zhang,
Weiwei Han,
Sichang Wang,
Ya Wu,
Keliang Zhang,
Penghui Yang,
Pei Xiao and
Yitao Wei
College of Chemistry & Chemical Engineering, Xi'an Shiyou University, Xi'an 710065, China. E-mail: jiyue@xsyu.edu.cn
First published on 1st December 2021
Abstract
A concise synthesis of α-cyano tetrahydroisoquinolines with a quaternary center via the Strecker reaction was successfully realized by employing TMSCN as cyano source and KF as fluoride source, furnishing the products with up to 99% yield. An isomerization of α-cyano tetrahydroisoquinoline was observed under alkaline conditions to give the isomer via [1,3]-H shift.
Tetrahydroisoquinoline is an important molecular skeleton widely distributed in natural alkaloids.1 The abundant biological activity of tetrahydroisoquinoline alkaloids, such as anti-tumor, anti-inflammatory, anti-convulsant and anti-epileptic activity, promotes its potential application in pharmaceutical chemistry and clinical medicine.2 Besides, tetrahydroisoquinolines with a quaternary center at the C-1 position also have diverse biological activities (Scheme 1). For example, MK801, also known as dizocilpine, was an effective and highly selective NMDA receptor (N-methyl-D-aspartic acid receptor).3 As a kind of central nervous system drug, MK801 has anaesthetic, anticonvulsant and antiepileptic effects. In addition, quaternary substituted tetrahydroisoquinoline carboxylic acids DNLCA and NLCA are potent noncompetitive dopamine hydroxylase inhibitors, which play an important role in the control of adrenaline synthesis.4 Therefore, the development of synthetic methodology for α-substituted tetrahydroisoquinolines with a quaternary center is important and desirable in organic synthesis.
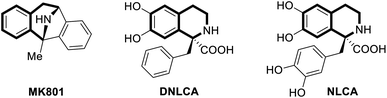 | ||
| Scheme 1 Representative biologically active molecules of tetrahydroisoquinolines with a quaternary center. | ||
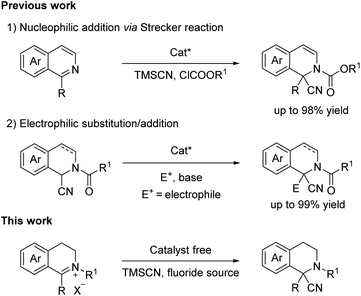
Although the synthesis of tetrahydroisoquinolines have been widely developed,5 the research on quaternary substituted tetrahydroisoquinolines synthesis was rarely explored in the past decades. It still remains a great challenge for the construction of quaternary carbon center attributed to steric hindrance and poor reactivity of substrates.6 Only a few researches focused on the study of tetrahydroisoquinolines with quaternary center. Currently, the most frequently used two strategies for the diverse synthesis were as follows: (1) the Lewis acid or enzyme catalysed classical Pictet–Spengler cyclization which provides a concise and straightforward methodology towards tetrahydroisoquinolines;7 (2) nucleophilic addition and electrophilic addition/substitution reaction of isoquinoline type substrates. The direct addition of nucleophiles to isoquinolines/isoquinoline type imines was restricted due to the poor reactivity of substrates. With acylating reagents as activator, the reactivity would be effectively enhanced.5e,h,8 With regard to isoquinoline type amines which was activated by acylating reagents to enhance the acidity of C-1 position proton of substrate, base is also essential for the electrophilic addition/substitution to the synthesis of quaternary substituted tetrahydroisoquinolines.9 Furthermore, Streuff group reported a concise and direct synthesis via titanium(III)-catalyzed reductive umpolung reaction of isoquinoline type imines with nitriles act as effective acylation agents without substrate activation.10
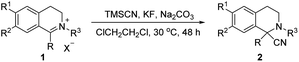
The Strecker reaction was an efficient and convenient strategy for the synthesis of quaternary amino nitrile adduct with a tetrahydroisoquinoline core via the cyanation of prochiral imine or iminium, which could be easily transformed into the corresponding carboxylic acid (Scheme 2).11 In 2001, Shibasaki and co-workers initially reported the bifunctional Al catalysed nucleophilic addition of isoquinolines using an acylating reagent as substrate activator via Strecker reaction.8 And then Maruoka group9b & Zhang group9c employed α-cyano tetrahydroisoquinolines possessing an N-acyl group as substrate in the base conditions, giving the products via electrophilic substitution/addition, respectively. And very recently, the Sbei group reported an electrosynthesis of 1,2,3,4-tetrahydroisoquinoline-1-carbonitriles via the electrogenerated cyanide anions from acetonitrile.12 Herein, we present a concise synthesis of α-cyano tetrahydroisoquinolines with a quaternary center via Strecker reaction, providing the quaternary α-aminonitriles with up to 99% yield.
At the outset of our study, 2-methyl-1-phenyl-3,4-dihydro-isoquinolin-2-ium iodide 1a, was selected as the model substrate for investigation (Table 1, entry 1). To our delight, with TMSCN as the cyano source, the desired product 2-methyl-1-phenyl-tetrahydroisoquinoline-1-carbonitrile 2a was obtained in the presence of 50 mol% Na2CO3 in dichloromethane with 80% yield. With the promising result, further experiments focused on solvents were conducted and 1,2-dichloroethane was proved to be the best choice in 86% isolated yield (entries 2–5). A survey of base revealed that both inorganic base (entries 5–8) and organic base Et3N (entry 9) was performed well in the reaction with moderate to good yield. With the temperature decreased to 30 °C, only a slight decrease in activity was observed (entry 10). The nucleophilic ability of TMSCN was much weaker compared with NaCN as the cyano source. And the addition of fluoride source could increase the nucleophilic ability of TMSCN to a great extent due to the strong chemical bond interaction between silicon and fluorine atom, which could promote the generation of cyano. To our delight, the addition of KF to the reaction system improved the reactivity efficiently and a 92% isolated yield of product was obtained (entry 11). Thus, we established the optimal condition for the synthesis of 2-methyl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile 2a: using 2 equiv. of TMSCN as the cyano source, 0.5 equiv. of Na2CO3 as base, 2 equiv. of KF as additive, ClCH2CH2Cl as solvent to perform reaction at 30 °C.
| Entry | Solvent | Base (mol%) | T (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), TMSCN (1.0 mol), 3 mL of solvents, 24 h.b Isolated yield.c KF (1.0 mol) was added as additive. | ||||
| 1 | CH2Cl2 | Na2CO3 (50 mol%) | 60 | 80 |
| 2 | Toluene | Na2CO3 (50 mol%) | 60 | 54 |
| 3 | 1,4-Dioxane | Na2CO3 (50 mol%) | 60 | 81 |
| 4 | MeOH | Na2CO3 (50 mol%) | 60 | Mixture |
| 5 | ClCH2CH2Cl | Na2CO3 (50 mol%) | 60 | 86 |
| 6 | ClCH2CH2Cl | K2CO3 (50 mol%) | 60 | 72 |
| 7 | ClCH2CH2Cl | Li2CO3 (50 mol%) | 60 | 79 |
| 8 | ClCH2CH2Cl | K3PO4 (34 mol%) | 60 | 67 |
| 9 | ClCH2CH2Cl | Et3N (100 mol%) | 60 | 83 |
| 10 | ClCH2CH2Cl | Na2CO3 (50 mol%) | 30 | 83 |
| 11c | ClCH2CH2Cl | Na2CO3 (50 mol%) | 30 | 92 |
With the optimized condition established, we then set out to demonstrate the generality and practicality of this Strecker reaction of iminiums with a tetrahydroisoquinoline core. A variety of 1-substituted 2-alkyl 3,4-dihydroisoquinolin-2-ium salts were examined under the standard condition, and the results were summarized in Table 2. Generally, the reactions could perform smoothly and effectively, giving the corresponding products in good to excellent yields. The results revealed that the position of the substituents on the aryl ring at C-1 position of tetrahydroisoquinoline core significantly affected the reactivity. For 1-aryl substituted substrate, the increase of sterically hindered effect of substituent resulted in relatively lower yield (entry 2). And it was also noted that the best result of 99% yield was obtained with substrate baring a methyl group at the meta-position of 1-aryl (entry 3). The electronic properties of aryl group at C-1 position also affected the reactivity with marginal effect (entries 4–7). However, the electronic properties of the substituent at C-7 position had great effect on the reactivity and the strongly electron-donating group gave a slightly lower yield (entries 10 vs. 8 and 9). For 1-methyl substituted substrate, the reaction performed well in 87% yield (entry 11). Iminiums bearing a long linear alkyl chain could be proceeded without impediments (entries 12 and 13). With the increasing of the carbon number of alkyl chains of substrate, the reactivity decreased gradually in the meanwhile. Moreover, the benzyl bromide substrate was also investigated and proceeded smoothly in the standard system, giving the desired product in 85% yield (entry 14).
| Entry | R1/R2 | R3/X | R | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), TMSCN (1.0 mol), KF (1.0 mol), Na2CO3 (0.25 mmol), 3 mL of ClCH2CH2Cl, 48 h.b Isolated yield. | ||||
| 1 | H/H | CH3/I | C6H5 | 93 (2a) |
| 2 | H/H | CH3/I | 2-CH3C6H4 | 85 (2b) |
| 3 | H/H | CH3/I | 3-CH3C6H4 | 99 (2c) |
| 4 | H/H | CH3/I | 4-CH3C6H4 | 94 (2d) |
| 5 | H/H | CH3/I | 4-FC6H4 | 88 (2e) |
| 6 | H/H | CH3/I | 4-ClC6H4 | 94 (2f) |
| 7 | H/H | CH3/I | 4-CH3OC6H4 | 92 (2g) |
| 8 | H/CH3 | CH3/I | C6H5 | 95 (2h) |
| 9 | CH3/CH3 | CH3/I | C6H5 | 93 (2i) |
| 10 | H/CH3O | CH3/I | C6H5 | 86 (2j) |
| 11 | H/H | CH3/I | CH3 | 87 (2k) |
| 12 | H/H | Et/I | C6H5 | 85 (2l) |
| 13 | H/H | CH3(CH2)9/I | C6H5 | 80 (2m) |
| 14 | H/H | Bn/Br | C6H5 | 85 (2n) |
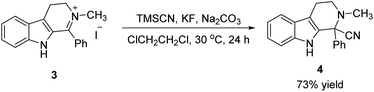
In order to further estimate the application possibility, iminium salt with a β-carboline core (3) was subjected to the standard condition (Scheme 3). To our satisfaction, the desired product (4) was obtained in 73% yield. It also provided a concise and efficient methodology for the synthesis of α-cyano tetrahydro-β-carboline with a quaternary center.
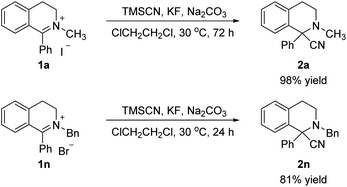
To demonstrate the practical utility, the gram scale reactions were conducted under the optimal condition, providing the corresponding products 2-methyl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (2a, 98% yield) and 2-benzyl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (2n, 81% yield), respectively (Scheme 4).
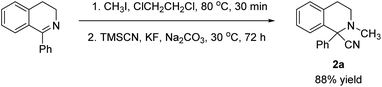
With 1-phenyl-3,4-dihydroisoquinoline as substrate, the cascade reaction for the synthesis of 2-methyl-1-phenyl-tetrahydroisoquinoline-1-carbonitrile (2a) via an iminium intermediate generated in situ was subsequently investigated (Scheme 5). The reaction was performed well, giving the product in 88% yield.
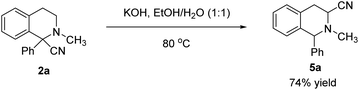
To further highlight the practical utility of the addition product α-cyano tetrahydroisoquinolines, the hydrolysis of 2-methyl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (2a) was conducted under alkaline conditions in the presence of potassium hydroxide (Scheme 6). To our surprise, the reaction performed smoothly to provide the isomerization product 2-methyl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-3-carbonitrile (5a) in good yield instead of hydrolysis product.13
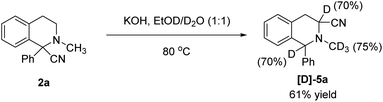
Then, to gain insight to the isomerization reaction mechanism, an isotopic labelling experiment was conducted with EtOD/D2O as solvent (Scheme 7). The product was obtained in 61% yield and with 70% deuterium incorporation in C-1 and C-3 positions and 75% deuterium incorporation on the methyl group.
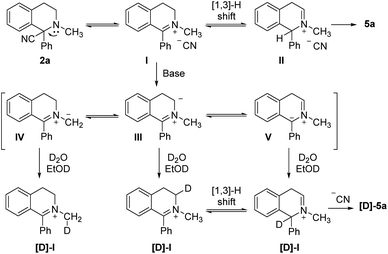
Based on the experimental results and structure of the product, the mechanism was proposed that the reaction experienced the process of base promoted tautomerization of iminium isomers and the [1,3]-H shift via a six-membered cyclic transition state under alkaline condition as depicted in Scheme 8. Considering the steric hindrance effect and stability of the iminium intermediate, the reaction was then followed by a nucleophilic addition of CN at C-3 position toward the isomerized product.
Conclusions
In summary, by employing TMSCN as the cyano source, we have successfully developed an efficient and convenient method for synthesis of α-cyano tetrahydroisoquinolines with a quaternary center via Strecker reaction in good to excellent yields. Meanwhile, a compatible one-pot, single-operation cascade reaction for the synthesis of quaternary α-cyano tetrahydroisoquinolines was realized, furnishing the desired product in 88% yield. Interestingly, an isomerization of α-cyano tetrahydroisoquinoline was observed under alkaline condition via [1,3]-H shift. Further studies to expand the scope to other amines with a quaternary center are ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (No. 21801204, 22005242), the Young Talent Fund of University Association for Science and Technology in Shaanxi Province of China (No. 20200606), the Research on the Training Plan of Innovation, Practice Ability of Postgraduates of Xi’an Shiyou University (No. YCS20211027), Scientific Research Program Funded by Shaanxi Provincial Education Department (20JK0830) and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2021JQ-584). The authors would like to thank RuiJun Ren from Shiyanjia Lab (www.shiyanjia.com) for the support of NMR test.Notes and references
- (a) J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102, 1669 CrossRef CAS PubMed; (b) T. Kametani, H. Sugi and S. Shibuya, Tetrahedron, 1971, 27, 2409 CrossRef CAS; (c) S. Peng, M. Guo and E. Winterfeldt, Liebigs Ann. Chem., 1993, 137 CAS.
- (a) V. G. Kartsev, Med. Chem. Res., 2004, 13, 325 CrossRef CAS; (b) M. Ohkubo, A. Kuno, K. Katsuta, Y. Ueda, K. Shirakawa, H. Nakanishi, I. Nakanishi, T. Kinoshita and H. Takasugi, Chem. Pharm. Bull., 1996, 44, 95 CrossRef CAS PubMed.
- Selected reportss: (a) W. J. Thompson, P. S. Anderson, S. F. Britcher, T. A. Lyle, J. E. Thies, C. A. Magill, S. L. Varga, J. E. Schwering, P. A. Lyle, M. E. Christy, B. E. Evans, C. D. Colton, M. K. Holloway, J. P. Springer, J. M. Hirshfield, R. G. Ball, J. S. Amato, R. D. Larsen, E. H. F. Wong, J. A. Kemp, M. D. Tricklebank, L. Singh, R. Oles, T. Priestly, G. R. Marshall, A. R. Knight, D. N. Middlemiss, G. N. Woodruff and L. L. Iversen, J. Med. Chem., 1990, 33, 789 CrossRef CAS PubMed; (b) J. A. Monn, A. Thurkauf, M. V. Mattson, A. E. Jacobson and K. C. Rice, J. Med. Chem., 1990, 33, 1069 CrossRef CAS PubMed; (c) J. P. Rung, A. Carlsson, K. R. Markinhuhta and M. L. Carlsson, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2005, 29, 827 CrossRef CAS PubMed.
- (a) J. C. Crscia, W. Burke, G. Jamroz, J. M. Lasala, J. Mcfarlane, J. Mitchell, M. M. O'Toole and M. L. Wilson, Nature, 1977, 269, 617 CrossRef PubMed; (b) J. M. Lasala and J. C. Crscia, Science, 1979, 203, 283 CrossRef CAS PubMed.
- For reviews, see: (a) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341 CrossRef CAS PubMed; (b) Y.-G. Zhou, Acc. Chem. Res., 2007, 40, 1357 CrossRef CAS PubMed; (c) W. Liu, S. Liu, R. Jin, H. Guo and J. Zhao, Org. Chem. Front., 2015, 2, 288 RSC; (d) D. Li, X. Chen and W. Gao, Synthesis, 2020, 52, 3337 CrossRef CAS . Selected reports on synthesis of tetrahydroisoquinolines: ; (e) S.-M. Lu, Y.-Q. Wang, X.-W. Han and Y.-G. Zhou, Angew. Chem., Int. Ed., 2006, 45, 2260 CrossRef CAS PubMed; (f) P.-C. Yan, J.-H. Xie, G.-H. Hou, L.-X. Wang and Q.-L. Zhou, Adv. Synth. Catal., 2009, 351, 3243 CrossRef CAS; (g) M. Chang, W. Li and X. Zhang, Angew. Chem., Int. Ed., 2011, 50, 10679 CrossRef CAS PubMed; (h) Z.-S. Ye, R.-N. Guo, X.-F. Cai, M.-W. Chen, L. Shi and Y.-G. Zhou, Angew. Chem., Int. Ed., 2013, 52, 3685 CrossRef CAS PubMed; (i) Q.-H. Zheng, W. Meng, G.-J. Jiang and Z.-X. Yu, Org. Lett., 2013, 15, 5928 CrossRef CAS PubMed; (j) Z.-Y. Ding, T. Wang, Y.-M. He, F. Chen, H.-F. Zhou, Q.-H. Fan, Q. Guo and A. S. C. Chan, Adv. Synth. Catal., 2013, 355, 3727 CrossRef CAS; (k) A. Iimuro, K. Yamaji, S. Kandula, T. Nagano, Y. Kita and K. Mashima, Angew. Chem., Int. Ed., 2013, 52, 2046 CrossRef CAS PubMed; (l) W. Lin, T. Cao, W. Fan, Y. Han, J. Kuang, H. Luo, B. Miao, X. Tang, Q. Yu, W. Yuan, J. Zhang, C. Zhu and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 277 CrossRef CAS PubMed; (m) Y. Ji, L. Shi, M.-W. Chen, G.-S. Feng and Y.-G. Zhou, J. Am. Chem. Soc., 2015, 137, 10496 CrossRef CAS PubMed; (n) M.-W. Chen, Y. Ji, J. Wang, Q.-A. Chen, L. Shi and Y.-G. Zhou, Org. Lett., 2017, 19, 4988 CrossRef CAS PubMed; (o) A. N. Kim, A. Ngamnithiporn, E. R. Welin, M. T. Daiger, C. U. Grünanger, M. D. Bartberger, S. C. Virgil and B. M. Stoltz, ACS Catal., 2020, 10, 3241 CrossRef CAS PubMed.
- Review for the construction of quaternary centers: (a) K. Fuji, Chem. Rev., 1993, 93, 2037 CrossRef CAS . Selected examples: ; (b) H. R. Tan, H. F. Ng, J. Chang and J. Wang, Chem.–Eur. J., 2012, 18, 3865 CrossRef CAS PubMed; (c) Z. Yan, B. Wu, X. Gao and Y.-G. Zhou, Chem. Commun., 2016, 52, 10882 RSC; (d) Z. Yan, X. Gao and Y. -G. Zhou, Chin. J. Catal., 2017, 38, 784 CrossRef CAS; (e) X.-W. Wang, M.-W. Chen, B. Wu, B. Wang and Y.-G. Zhou, J. Org. Chem., 2019, 84, 8300 CrossRef CAS PubMed; (f) X.-W. Wang, M.-W. Chen, B. Wu, B. Wang, B. Wan and Y.-G. Zhou, Tetrahedron Lett., 2021, 62, 152793 CrossRef; (g) X.-W. Wang, X. Li, M.-W. Chen, B. Wu and Y.-G. Zhou, J. Org. Chem., 2021, 86, 6897 CrossRef CAS PubMed.
- Selected reports on Pictet–Spengler cyclization: (a) Y. Horiguchi, H. Kodama, M. Nakamura, T. Yoshimura, K. Hanezi, H. Hamada, T. Saitoh and T. Sano, Chem. Pharm. Bull., 2002, 50, 253 CrossRef CAS PubMed; (b) A. Hegedus and Z. Hell, Tetrahedron Lett., 2004, 45, 8553 CrossRef; (c) M. J. V. Eynden, K. Kunchithapatham and J. P. Stambuli, J. Org. Chem., 2010, 75, 8542 CrossRef PubMed; (d) B. R. Lichman, J. Zhao, H. C. Hailes and J. M. Ward, Nat. Commun., 2017, 8, 14883 CrossRef CAS PubMed.
- K. Funabashi, H. Ratni, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2001, 123, 10784 CrossRef CAS PubMed.
- (a) A. I. Meyers, M. A. Gonzalez, V. Struzka, A. Akahane, J. Guiles and J. S. Warmus, Tetrahedron Lett., 1991, 32, 5501 CrossRef CAS; (b) S. Shirakawa, K. Liu, H. Ito, T. N. Le and K. Maruoka, Adv. Synth. Catal., 2011, 353, 2614 CrossRef CAS; (c) T.-Y. Qin, W.-W. Liao, Y.-J. Zhang and S. X.-A. Zhang, Org. Biomol. Chem., 2013, 11, 984 RSC; (d) X. Li and I. Coldham, J. Am. Chem. Soc., 2014, 136, 5551 CrossRef CAS PubMed.
- H.-T. Luu, S. Wiesler, G. Frey and J. Streuff, Org. Lett., 2015, 17, 2478 CrossRef CAS PubMed.
- Select reviews for Strecker reactions: (a) H. Gröger, Chem. Rev., 2003, 103, 2795 CrossRef PubMed; (b) J. Wang, X. Liu and X. Feng, Chem. Rev., 2011, 111, 6947 CrossRef CAS PubMed; (c) N. Kurono and T. Ohkuma, ACS Catal., 2016, 6, 989 CrossRef CAS . Select examples: ; (d) H.-W. Bersch, R. RiRmann and D. Schon, Arch. Pharm., 1982, 315, 749 CrossRef CAS; (e) H. Moehrle and H. Breves, Pharmazie, 2005, 60, 23 CAS; (f) N. G. Voznesenskaya, O. I. Shmatova, A. A. Sosnova and V. G. Nenajdenko, Eur. J. Org. Chem., 2019, 625 CrossRef.
- N. Sbei, A. A. Titov, E. B. Markova, M. N. Elinson and L. G. Voskressensky, ChemistrySelect, 2020, 5, 4493 CrossRef CAS.
- Selected researches for rearrangement of isoquinoline-tape substrates: (a) M. Sainseury, D. W. Brown, S. F. Dyke, R. G. Kinsman and B. J. Moon, Tetrahedron, 1968, 24, 6695 CrossRef; (b) J. Knabe and R. Dörr, Arch. Pharm., 1973, 306, 784 CrossRef CAS PubMed; (c) E. Langhals, H. Langhals and C. Riichardt, Chem. Ber., 1984, 117, 1436 CrossRef CAS; (d) N. Blank, B. F. Straub and T. Opatz, Eur. J. Org. Chem., 2011, 7355 CrossRef CAS; (e) J. C. O. Pacheco, G. Lahm and T. Opatz, J. Org. Chem., 2013, 78, 4985 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08469k |
| This journal is © The Royal Society of Chemistry 2021 |