Open Access Article
Open Access ArticleStable monovalent aluminum(I) in a reduced phosphomolybdate cluster as an active acid catalyst†
Ya-Qi
Zhang‡
a,
Lai-Yun
Zhou‡
a,
Yuan-Yuan
Ma
 *a,
Kamran
Dastafkan
*a,
Kamran
Dastafkan
 b,
Chuan
Zhao
b,
Chuan
Zhao
 b,
Lan-Zhi
Wang
b,
Lan-Zhi
Wang
 *a and
Zhan-Gang
Han
*a and
Zhan-Gang
Han
 *a
*a
aHebei Key Laboratory of Organic Functional Molecules, National Demonstration Center for Experimental Chemistry Education, College of Chemistry and Material Science, Hebei Normal University, Shijiazhuang, Hebei 050024, People's Republic of China. E-mail: mayy334@hebtu.edu.cn; wanglanzhi@126.com; hanzg116@126.com; hanzg116@hebtu.edu.cn
bSchool of Chemistry, The University of New South Wales, Sydney, NSW 2052, Australia. E-mail: chuan.zhao@unsw.edu.au
First published on 8th December 2020
Abstract
Low-valent aluminum Al(I) chemistry has attracted extensive research interest due to its unique chemical and catalytic properties but is limited by its low stability. Herein, a hourglass phosphomolybdate cluster with a metal-center sandwiched by two benzene-like planar subunits and large steric-hindrance is used as a scaffold to stabilize low-valent Al(I) species. Two hybrid structures, (H3O)2(H2bpe)11[AlIII(H2O)2]3{[AlI(P4MoV6O31H6)2]3·7H2O (abbr. Al6{P4Mo6}6) and (H3O)3(H2bpe)3[AlI(P4MoV6O31H7)2]·3.5H2O (abbr. Al{P4Mo6}2) (bpe = trans-1,2-di-(4-pyridyl)-ethylene) were successfully synthesized with Al(I)-sandwiched polyoxoanionic clusters as the first inorganic-ferrocene analogues of a monovalent group 13 element with dual Lewis and Brønsted acid sites. As dual-acid catalysts, these hourglass structures efficiently catalyze a solvent-free four-component domino reaction to synthesize 1,5-benzodiazepines. This work provides a new strategy to stabilize low-valent Al(I) species using a polyoxometalate scaffold.
Low- or sub-valence aluminum compounds are increasingly growing into a significant frontier subject in coordination and modern organic synthetic chemistry owing to their unique singlet carbene character, Lewis acid/base properties and catalytic reactivity.1 However, low-valence aluminum(I) compounds have inherent electron deficiency and exhibit thermodynamic instability, making them prone to self-polymerization with metal–metal bonds2 or disproportionation3 to metallic Al and Al(III) species. Inspired by the special stabilizing effect of metallocene compounds, a ligand stabilization strategy has recently been undertaken to stabilize the low-valence aluminum center.4,5 In this regard, the utilized ligand should satisfy two key criteria: (i) sufficient steric hindrance is required to inhibit monomer polymerization; and (ii) a suitable electronic effect is needed to stabilize the aluminum(I) center. A few organometallic Al(I) compounds protected by bulky organic groups have been prepared such as [(Cp*Al)4] (Cp* = C5Me5),6 and [(CMe3)3SiAl4].7 However, despite having the ligand effect, most of these Al(I) compounds still decompose in aqueous solutions or heating conditions. In contrast to organometallic Al(I) compounds, inorganic Al(I) structures, i.e. monomeric monohalides, only exist in gaseous form at high temperature8 and to the best of our knowledge, no stable inorganic Al(I) compound is known at room temperature due to thermodynamic instability. Therefore, exploring efficient strategies to synthesize stable inorganic Al(I) compounds remains highly desired but a great challenge.
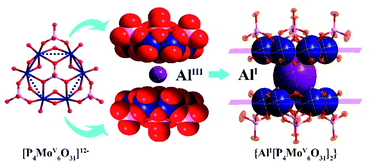
Polyoxometalates (POMs), a diverse family of inorganic molecular clusters based on early-transition metals (W, Mo, V, Nb, and Ta), have extensively attracted attention in research in various fields of materials science, coordination chemistry, medicinal chemistry and catalysis science.9–11 Owing to their adjustable constituent elements and well-defined structures, POMs have been considered as promising inorganic ligands to stabilize high- and low-valent metal ions. For instance, Rompel et al.12 reported one Keggin-type [α-CrW12O40]5− anion in which a labile {CrIIIO4} tetrahedral unit was assembled at the center of the cluster. Li and co-workers employed a monolacunary Keggin-type inorganic ligand to stabilize a high-valent Cu3+ ion.13 As a unique member of the POM family, the hourglass-type phosphomolybdate cluster {M[P4MoV6O31]2}n− (abbr. M{P4Mo6}2), consisting of two [P4MoV6O31]12− (abbr. {P4Mo6}2) subunits bridged by one metal (M) center, represents a fully reduced metal-oxo cluster. With all Mo atoms in the oxidation state of (+5), a more negative charge is endowed to the cluster surface.14,15 Such high electron density of {M[P4MoV6O31]2}n− polyoxoanions provides an electron-rich local environment for the possible stabilization of unusual-valence metals. It is worth noting that the [P4MoV6O31]12− subunit presents near-planar triangular structures with the side sizes ranging from 7.50–7.92 Å (Fig. S1†). The structural feature can supply sufficient steric hindrance to restrain the polymerization of low-valence metal species. Moreover, the six Mo atoms in each [P4MoV6O31]12− subunit arrange in a planar hexagonal-ring structure like a benzene ring, implying that such {M[P4MoV6O31]2}n− clusters may have a similar delocalized electron structure to conjugated benzene or cyclopentadiene. These features make [P4MoV6O31]12− a promising candidate with respect to organic protecting groups to construct an inorganic ‘ferrocene’ analogue of Al(I) (Scheme 1). Therefore, we hypothesize that hourglass-type polyoxoanion clusters are promising to stabilize the labile Al(I) center and isolate inorganic Al(I) species.
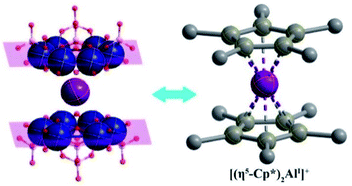 | ||
| Scheme 1 Similar ferrocene-like sandwich structure features of an inorganic hourglass-type [AlI(P4MoV6O31)2]23− polyanion to an organometallic [(η5-Cp*)2AlI]+ cation. | ||
Herein, we show a [P4MoV6O31]12− cluster as an inorganic scaffold to stabilize the Al(I) center in two hybrid compounds, (H3O)2(H2bpe)11[AlIII(H2O)2]3{[AlI(P4MoV6O31H6)2]3·7H2O (abbr. Al6{P4Mo6}6) and (H3O)3(H2bpe)3[AlI(P4MoV6O31H7)2]·3.5H2O (abbr. Al{P4Mo6}2) (bpe = trans-1,2-di-(4-pyridyl)-ethylene), in which the labile Al(I) center is sandwiched by two [P4MoV6O31]12− sides, forming an inorganic moiety of a ‘ferrocene’ analogue. Both Al6{P4Mo6}6 and Al{P4Mo6}2 are experimentally determined at room temperature for the first time, and prepared by hydrothermal reactions of Na2MoO4·2H2O, H3PO4, AlCl3·6H2O, ethanol and N-containing bpe at 160 °C with slightly different pH values. Notably, the combination of ethanol, N-containing bpe and high hydrothermal temperature is a prerequisite to the isolation of Al(I) species. First, both ethanol and N-containing bpe were used to provide a reducing environment under hydrothermal conditions. By combining high temperature and pressure, sufficient energy is supplied to reduce Mo6+ and Al3+ ions to Mo5+ and Al+ species, respectively. Then, Mo5+ species and phosphoric acid molecules are assembled to form [P4Mo6O31]12− subunits, which are subsequently combined with Al+ ions to form hourglass-type [Al(P4Mo6O31)2]23−, hence effectively stabilizing Al(I) species (Fig. 1). From the perspective of stereochemistry, two highly negative [P4Mo6O31]12− fragments, resembling the methyl cyclopentadiene organic group, sandwich one low-valent metal Al(I) center. Hence, the construction of a strong reducing hourglass-like skeleton makes it possible to stabilize the existing Al+ species.
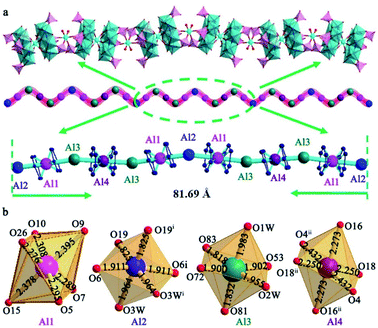
Single crystal X-ray diffraction revealed the hourglass-type {Al(P4Mo6)2} cluster in Al6{P4Mo6}6 and Al{P4Mo6}2 (Table S1†), in which the [P4Mo6O31]12− subunits have a C3 symmetry and display a near-planar structure formed by six edge-sharing {MoO6} octahedra with alternating short Mo–Mo single bonds and long non-bonding Mo⋯Mo contacts. The side sizes of the {P4Mo6} subunit range from 7.50–7.92 Å, which supplies sufficient steric hindrance to restrain the polymerization or disproportionation of low-valence Al(I) species. All Mo atoms are in a reduced oxidation state of +5 and the central Al atoms are in the +1 oxidation state, as confirmed by bond valence calculations (Table S2†). Thus, the synthesized Al{P4Mo6}2 represents a fully reduced metal–oxygen cluster. Moreover, the six Mo atoms in each {P4Mo6} subunit present a benzene-like planar hexagonal-ring structure with a similar π-type delocalization electron interaction with Al(I) instead of organic bulky groups. Such π-type delocalization electron interaction constructs an inorganic ‘ferrocene’ analogue of Al(I) and produces sufficient delocalization energy to stabilize Al(I) species. Considering the formation mechanism of traditional metallocenes, {P4Mo6} subunits with a similar strong electron-donating ability and suitable steric-hindrance effect on Cp rings, augment the stability of Al(I) species. Al6{P4Mo6}6 and Al{P4Mo6}2 compounds also present the first isolation of aluminum-sandwiched hourglass-type clusters in POM chemistry. Importantly, regarding the inherent and strong hydrolysis of aluminum species in water, these low-valent Al(I)-containing clusters represent the first example of stable solid-state inorganic sub-valent Al(I) compounds at room temperature.
The asymmetric structure of Al6{P4Mo6}6 consists of two crystallographically independent {Al(P4Mo6)2} clusters sandwiched by central Al(1) and Al(4) atoms, two bridging [Al(H2O)2]3+ (Al(2) and Al(3)) cations and six protonated bpe cations (Fig. S2†). Aluminum centers involve two kinds of oxidation states: the central Al(1) and Al(4) are in the +1 state, while the bridging Al(2) and Al(3) are in the +3 state. Both Al(1) and Al(4) display the six-coordinated octahedral configuration and bridge two {P4Mo6} subunits to form two {AlI(P4Mo6)2} clusters. The average lengths of Al–O bonds are 2.318–2.324 Å for Al(1) and Al(4) (Table S3†), which are slightly longer than those of classic Al–O bonds (1.90 Å) for Al(2) and Al(3), but close to that of the Al–O bond in silica-supported alkylaluminum(I) composites.16–20 The long Al–O lengths for Al(1) and Al(4) centers may be ascribed to the lower electron cloud density located at the surface of the Al(I) cation, resulting in slightly longer bonds with the surrounding oxygen donors.5,21 Moreover, the small distorted extents (sum((dij − dave)/dave)2/coordination number) of {Al(1)O6} (3.86 × 10−4) and {Al(4)O6} (1.89 × 10−3) indicate that they are in regular octahedral geometry. Moreover, another structural feature of Al6{P4Mo6}6 is that {AlI(P4Mo6)2} clusters are connected by bridging [Al(H2O)2]3+ cationic fragments (Al(2) and Al(3)), forming an unusual chain-like arrangement (Fig. 2a). It is worth noting that the 1-D chain contains a large repeating monomer with the maximum spacing of 81.69 Å, consisting of twelve Al-containing fragments ({–Al2–Al1–Al3–Al4–Al3–Al1–Al2–Al1–Al3–Al4–Al3–Al1–}). Such a long repeating monomer is rare. Each repeating monomer has two types of symmetric systems: Al(2) in the middle of the monomer plays a center of mirror symmetry and divides the whole repeating monomer into two equidistant half-units of {–Al1–Al3–Al4–Al3–Al1–}; Al(4) in each half-unit further acts as the reverse symmetric center of two {–Al3–Al1–Al2–} subunits. The two types of symmetrical systems form the infinitely extending chain-like structure in Al6{P4Mo6}6. Since bpe is a rigid and conjugated molecular structure, an effective π⋯π stacking interaction emerges and results in a honeycomb-like supramolecular organic moiety, which accommodates these 1-D inorganic chains and stabilizes the whole Al6{P4Mo6}6 framework (Fig. S3 and S4†).
Al{P4Mo6}2 has a similar structure to Al6{P4Mo6}6 (Table S4†), wherein the most obvious difference is that {AlI[P4Mo6]2} clusters exist in isolated form and interact with the surrounding protonated bpe cations via hydrogen bonding to form into a 3-D supramolecular framework (Fig. S5 and S6†). The different peripheral environment around the {AlI[P4Mo6]2} cluster can affect its acidity and catalytic activity.
The solid-state 27Al NMR spectrum of Al6{P4Mo6}6 depicts two distinct resonances at δ = −22.34 and 27.33 ppm due to the octahedrally coordinated AlIII and AlI sites, respectively (Fig. 3a), indicating two types of Al local environments in Al6{P4Mo6}6. In contrast, Al{P4Mo6}2 displays only one sharp signal at δ = 7.20 ppm due to the octahedrally coordinated AlI sites (Fig. 3b). The observed narrow peak-width corresponds to the highly symmetric charge distribution at the aluminum nucleus, similar to the ferrocene analogue [(η5-Cp*)2AlI]+.5 Noticeably, AlI resonance in Al6{P4Mo6}6 appears at a lower magnetic field compared to Al{P4Mo6}2, due to the different peripheral environment around the hourglass {Al(P4Mo6)2} cluster. XPS spectra of Al6{P4Mo6}6 and Al{P4Mo6}2 further affirm the valence states of Al and Mo elements (Fig. S7 and Table S5†). The Al 2p XPS profile of Al6{P4Mo6}6 reveals two peaks at 74.39 and 73.75 eV ascribed to AlIII and AlI, respectively (Fig. 3c). The area ratio of the two peaks is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in consistence with the chemical structure of Al6{P4Mo6}6. The high-resolution Al 2p XPS spectrum of Al{P4Mo6}2 displays a weaker broad peak attributed to the low amount of Al+ (Fig. 3d). Moreover, the structural stabilities of Al6{P4Mo6}6 and Al{P4Mo6}2 were investigated by soaking them in water for 24 hours. Fig. S9–S11† show the comparison of XRD, IR and XPS spectra of Al6{P4Mo6}6 and Al{P4Mo6}2 before and after soaking in water. It can be found that the characteristic diffraction peaks in XRD after soaking for 24 hours still show good agreement with the simulated data (Fig. S9†). The characterized absorption bands in IR spectra also exhibit good match with the original Al6{P4Mo6}6 and Al{P4Mo6}2 (Fig. S10†). The XPS spectra of Al6{P4Mo6}6 after soaking in water were also obtained. There is basically no change in the high-resolution spectra of Al 2p with the AlI/AlIII atomic ratios of ca. 1
1, in consistence with the chemical structure of Al6{P4Mo6}6. The high-resolution Al 2p XPS spectrum of Al{P4Mo6}2 displays a weaker broad peak attributed to the low amount of Al+ (Fig. 3d). Moreover, the structural stabilities of Al6{P4Mo6}6 and Al{P4Mo6}2 were investigated by soaking them in water for 24 hours. Fig. S9–S11† show the comparison of XRD, IR and XPS spectra of Al6{P4Mo6}6 and Al{P4Mo6}2 before and after soaking in water. It can be found that the characteristic diffraction peaks in XRD after soaking for 24 hours still show good agreement with the simulated data (Fig. S9†). The characterized absorption bands in IR spectra also exhibit good match with the original Al6{P4Mo6}6 and Al{P4Mo6}2 (Fig. S10†). The XPS spectra of Al6{P4Mo6}6 after soaking in water were also obtained. There is basically no change in the high-resolution spectra of Al 2p with the AlI/AlIII atomic ratios of ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S11†). The spectroscopic and theoretical observations verify that the low valence Al(I) species can stably exist in the reduced phosphomolybdates in the solid state (Fig. S12 and Table S6†). Moreover, the acidities of Al6{P4Mo6}6 and Al{P4Mo6}2 were measured to be 0.27 and 0.442 mmol g−1, respectively, demonstrating the promising potential of Al6{P4Mo6}6 and Al{P4Mo6}2 as dual-acid catalysts.
1 (Fig. S11†). The spectroscopic and theoretical observations verify that the low valence Al(I) species can stably exist in the reduced phosphomolybdates in the solid state (Fig. S12 and Table S6†). Moreover, the acidities of Al6{P4Mo6}6 and Al{P4Mo6}2 were measured to be 0.27 and 0.442 mmol g−1, respectively, demonstrating the promising potential of Al6{P4Mo6}6 and Al{P4Mo6}2 as dual-acid catalysts.
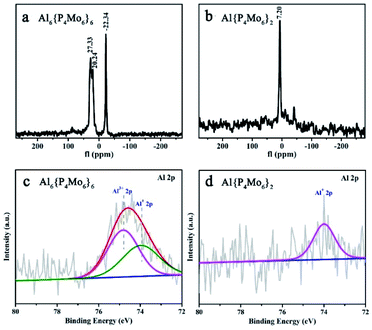 | ||
| Fig. 3 (a and b) 27Al NMR spectra of solid Al6{P4Mo6}6 and Al{P4Mo6}2; (c and d) XPS spectra of Al in Al6{P4Mo6}6 and Al{P4Mo6}2. | ||
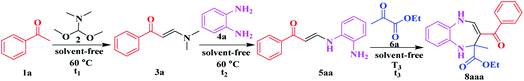
The catalytic performance of Al6{P4Mo6}6 and Al{P4Mo6}2 was evaluated via a solvent-free four-component domino reaction for the synthesis of pharmaceutical intermediate 1,5-benzodiazepine (Table 1). With Al6{P4Mo6}6 and Al{P4Mo6}2 as catalysts, the yields of the final product 8aaa reach 83% and 75%, respectively (Table 1, entries 1 and 2). Almost no 8aaa is observed without the acid catalysts, even when the reaction is set for a long time (Table 1, entry 3). This clarifies the excellent catalytic performance of Al6{P4Mo6}6 and Al{P4Mo6}2. Typical Brønsted acid p-TsOH and Lewis acid AlCl3 as control samples yield only 43% and 29% 8aaa, respectively (Table 1, entries 4 and 5), much lower than those attained by Al6{P4Mo6}6 and Al{P4Mo6}2 catalysts. Moreover, (H2en)12[{Na0.8K0.2(H2O)}2{Na[Mo6O12(OH)3(HPO4)2(PO4)2]2}2]·7H2O22,23 (abbr. {Na[P4Mo6]2}) in contrast achieved 72% yield of 8aaa in 30 min, slower than that of Al6{P4Mo6}6 and Al{P4Mo6}2. This indicates the advantage of the unique dual-acid features of Al(I)-stabilized reduced phosphomolybdate clusters with multiple Lewis and Brønsted acid active centers, in which the synergistic effect between the Al species and reduced phosphomolybdate cluster contributes to the catalytic activity.
| Entry | Catalystb | t 1 (h) | Yieldc (%) 3a | t 2 (h) | Yieldd (%) 5aa | T 3 (°C) | t 3 (min) | Yielde (%) 8aaa |
|---|---|---|---|---|---|---|---|---|
| a One-pot reaction conditions: acetophenone 1a (1.00 mmol), N,N-dimethylformamide dimethyl acetal 2 (1.00 mmol), 1,2-phenylenediamine 4a (1.00 mmol), ethyl pyruvate 6a (1.00 mmol) and catalyst (10.00 mg) for the four-component domino reaction. b Catalyst (10.00 mg). c Isolated yield in the first step. d Total isolated yield for the first two steps. e Overall isolated yield for the 3 steps. f The time taken for the reaction to complete. | ||||||||
| 1 | Al6{P4Mo6}6 | 3.0 | 98 | 1.8 | 92 | 25 | 20 | 83 |
| 2 | Al{P4Mo6}2 | 3.2 | 97 | 2.0 | 89 | 25 | 20 | 75 |
| 3 | No catalyst | 7.0 | 98 | 5.5 | 62 | 25 | 120 | Trace |
| 4 | p-TsOH | 4.0 | 92 | 3.0 | 73 | 25 | 26 | 43 |
| 5 | AlCl3 | 4.5 | 94 | 3.0 | 82 | 25 | 58 | 29 |
| 6 | {Na[P4Mo6]2} | 3.5 | 95 | 2.5 | 86 | 25 | 30 | 72 |
Furthermore, the Al6{P4Mo6}6 catalyst displays a wide substrate scope of auto-tandem catalytic reactions. A series of functional groups including carboxyl, ester and acyl groups on the 2-position of the seven-membered rings can be smoothly converted into the desired 1,5-benzodiazepine products with high and even excellent yields (Table S7†). 1,2-Phenylenediamines 4 which contain both electron-deficient (p-Cl and p-Br) and electron-rich (p-Me and 3,4-di(Me)) 1,2-phenylenediamines also undergo the reaction smoothly, providing the corresponding products in high yields within the given reaction times (Table S7†).
Additionally, the Al6{P4Mo6}6 catalyst can be easily recovered by simple filtration. No significant decay in the catalytic activity or selectivity was observed even after 5 recycles of Al6{P4Mo6}6 (Fig. S14†). The acquired XRD pattern, and IR and XPS spectra after 5 runs further revealed the good structural integrity and high solid-state stability of Al6{P4Mo6}6 (Fig. S15–S17†). Accordingly, the Al6{P4Mo6}6 cluster coupled with dual-acid sites presents great potential application towards the four-component domino reaction.
In summary, two cases of low valence Al-centered hourglass-type phosphomolybdates have been reported for the first time. {P4Mo6} subunits with highly negative charge and a benzene-like planar hexagonal-ring structure, display a similar π-type electron interaction with Al(I) to construct inorganic ‘ferrocene’ analogues of Al(I), thus effectively stabilizing Al(I) species. Al(I)-POM structures are confirmed and characterized using 27Al NMR and XPS spectra. When used as acid catalysts, both Al6{P4Mo6}6 and Al{P4Mo6}2 efficiently catalyze a solvent-free domino reaction to synthesize 1,5-benzodiazepines with high yield and selectivity. The Al(I)-stabilized reduced POM structures also exhibit excellent substrate compatibility and cycle stability. The design, synthesis and successful stabilization of the subvalent metallic aluminum compounds in the solid state unravel the significance of this study. This work is also important to develop highly active and multifunctional catalysts for organic reactions.
Author contributions
Y. Y. Ma, L. Z. Wang, C. Zhao and Z. G. Han proposed and supervised the project. Y. Y. Ma, L. Z. Wang and Z. G. Han conceived and designed the experiments. Y. Q. Zhang carried out the synthesis and structural characterization of the samples. L. Y. Zhou performed the catalytic experiments. K. Dastafkan, Y. Q. Zhang and L. Y. Zhou co-wrote the original draft. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21871076, 21776060, and 21901060), Natural Science Foundation of Hebei Province (Grants B2016205051, B2020205008 and B2019205074), Natural Science Foundation of Hebei Education Department (Grant BJ2020037) and Graduate Student Innovation Funding Project of Hebei Normal University (CXZZSS2020041). C. Z. is grateful for the award of Future Fellowship from Australian Research Council (FT170100224, DP210103892).References
- (a) S. G. Gallardo, T. Bollermann and R. A. Fischer, Chem. Rev., 2012, 112, 3136–3170 CrossRef PubMed; (b) Y. Liu, J. Li, X. Ma, Z. Yang and H. W. Roesky, Coord. Chem. Rev., 2018, 374, 387–415 CrossRef CAS; (c) A. Dmitrienko, M. Pilkington, J. F. Britten, B. M. Gabidullin, A. Est and G. I. Nikonov, Angew. Chem., Int. Ed., 2020, 59, 16147–16153 CrossRef CAS PubMed; (d) S. Grams, J. Eyselein, J. Langer, C. Farber and S. Harder, Angew. Chem., Int. Ed., 2020, 59, 15982–15986 CrossRef CAS PubMed.
- C. Dohmeier, D. Loos and H. Schnoeckel, Angew. Chem., Int. Ed. Engl., 1996, 35, 129–149 CrossRef CAS.
- U. Werner, Rev. Inorg. Chem., 1998, 18, 239–282 Search PubMed.
- S. Harder and M. H. Prosenc, Angew. Chem., Int. Ed. Engl., 1994, 33, 1744–1746 CrossRef.
- C. Dohmeier, H. Schnoeckel, C. Robl, U. Schneider and R. Ahlri-chs, Angew. Chem., Int. Ed. Engl., 1993, 32, 1655–1657 CrossRef.
- C. Dohmeier, C. Robl, M. Tacke and H. Schnoeckel, Angew. Chem., 1991, 103, 594–595 CrossRef CAS.
- (a) C. Schnitter, H. W. Roesky, C. Ropken, R. Herbst-Irmer, H.-G. Schmidt and M. Moltemeyer, Angew. Chem., Int. Ed., 1998, 37, 1952–1955 CrossRef CAS; (b) A. Purath and H. Schnockel, J. Organomet. Chem., 1999, 579, 373–375 CrossRef CAS; (c) C. Cui, H. W. Roesky, M. Noltemeyer, H. G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., 2000, 39, 4444–4446 CrossRef; (d) P. Henke and H. Schnoeckel, Chem.–Eur. J., 2009, 15, 13391–13398 CrossRef CAS PubMed.
- M. Tacke and H. Schnoeckel, Inorg. Chem., 1989, 28, 2895–2896 CrossRef CAS.
- (a) S. Omwoma, C. T. Gore, Y. Ji, C. Hu and Y.-F. Song, Coord. Chem. Rev., 2015, 286, 17–29 CrossRef CAS; (b) K. Yu, B. Zhou, Y. Yu, Z. Su, C. Wang, C. Wang, S. Gao and Y. Chen, Inorg. Chim. Acta, 2012, 384, 8–17 CrossRef CAS.
- (a) S. Kim, J. Yeo and W. Choi, Appl. Catal., B, 2008, 84, 148–155 CrossRef CAS; (b) Z. Han, X. Xin, R. Zheng and H. Yu, Dalton Trans., 2018, 47, 3356–3365 RSC; (c) H. Yang, D. Yang and X. Wang, Angew. Chem., Int. Ed., 2020, 59, 15527–15531 CrossRef CAS PubMed.
- (a) Q. Liu, P. He, H. Yu, L. Gu, B. Ni, D. Wang and X. Wang, Sci. Adv., 2019, 5, eaax1081 CrossRef CAS PubMed; (b) Y. Benseghir, A. Lemarchand, M. Duguet, P. Mialane, M. Gomez-Mingot, C. Roch-Marchal, T. Pino, M.-H. Ha-Thi, M. Haouas, M. Fontecave, A. Dolbecq, C. Sassoye and C. Mellot-Draznieks, J. Am. Chem. Soc., 2020, 142, 9428–9438 CrossRef CAS PubMed.
- N. I. Gumerova, A. Roller, G. Giester, J. Krzystek, J. Cano and A. Rompel, J. Am. Chem. Soc., 2020, 142, 3336–3339 CrossRef CAS PubMed.
- X. Li, Y. Ren, Z. Weng, B. Yue and H. He, Chem. Commun., 2020, 56, 2324–2327 RSC.
- (a) L. Xu, Y. Sun, E. Wang, E. Shen, Z. Liu, C. Hu, Y. Xing, Y. Lin and H. Jia, Inorg. Chem. Commun., 1998, 1, 382–385 CrossRef CAS; (b) H. Fu, W. Chen, E. Wang, J. Liu and S. Chang, Inorg. Chim. Acta, 2009, 362, 1412–1420 CrossRef CAS.
- X. Xin, N. Hu, Y. Ma, Y. Wang, L. Hou, H. Zhang and Z. Han, Dalton Trans., 2020, 49, 4570–4577 RSC.
- Y. Kikukawa, S. Yamaguchi, Y. Nakagawa, K. Uehara, S. Uch-ida, K. bYamaguchi and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 15872–15878 CrossRef CAS PubMed.
- C. N. Kato, Y. Makino, M. Yamasaki, Y. Kataoka, Y. Kitagawa and M. Okumura, Adv. Cryst. Processes, 2012, 23, 593–611 Search PubMed.
- Z.-G. Han, X.-Q. Chang, J.-S. Yan, K.-N. Gong, C. Zhao and X.-L. Zhai, Inorg. Chem., 2014, 53, 670–672 CrossRef CAS PubMed.
- C. Coperet, I. Moroz, P. Florian, J. Viger Gravel, C. P. Gordon and A. Lesage, Angew. Chem., Int. Ed., 2020, 59, 16167–16172 CrossRef PubMed.
- G. Nikonov, A. Dmitrienko, M. Pilkington, J. F. Britten, B. M. Gabidullin and A. van der Est, Angew. Chem., Int. Ed., 2020, 59, 16147–16153 CrossRef PubMed.
- D. C. Hutchison, R. D. Stern, L. N. Zakharov, K. A. Persson and M. Nyman, Inorg. Chem., 2020, 59, 2900–2909 CrossRef CAS PubMed.
- J. Du, Y.-Y. Ma, X. Xin, H. Na, Y.-N. Zhao, H.-Q. Tan, Z.-G. Han, Y.-G. Li and Z.-H. Kang, Chem. Eng. J., 2020, 398, 125518–125526 CrossRef CAS.
- X.-R. Tian, L. Hou, J.-J. Wang, X. Xin, H. Zhang, Y.-Y. Ma, Y.-L. Wang, L.-N. Zhang and Z.-G. Han, Dalton Trans., 2018, 47, 15121–15130 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1970140 for Al{P4Mo6}2 and 1970141 for Al6{P4Mo6}6. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc05277a |
| ‡ Y. Q. Zhang and L. Y. Zhou contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |