Open Access Article
Open Access ArticleEncapsulation of tricopper cluster in a synthetic cryptand enables facile redox processes from CuICuICuI to CuIICuIICuII states†
Weiyao
Zhang
 ,
Curtis E.
Moore
and
Shiyu
Zhang
,
Curtis E.
Moore
and
Shiyu
Zhang
 *
*
Department of Chemistry and Biochemistry, The Ohio State University, 100 W. 18th Ave, Columbus, OH, USA. E-mail: zhang.8941@osu.edu
First published on 26th December 2020
Abstract
One-pot reaction of tris(2-aminoethyl)amine (TREN), [CuI(MeCN)4]PF6, and paraformaldehyde affords a mixed-valent [TREN4CuIICuICuI(μ3-OH)](PF6)3 complex. The macrocyclic azacryptand TREN4 contains four TREN motifs, three of which provide a bowl-shape binding pocket for the [Cu3(μ3-OH)]3+ core. The fourth TREN caps on top of the tricopper cluster to form a cryptand, imposing conformational constraints and preventing solvent interaction. Contrasting the limited redox capability of synthetic tricopper complexes reported so far, [TREN4CuIICuICuI(μ3-OH)](PF6)3 exhibits several reversible single-electron redox events. The distinct electrochemical behaviors of [TREN4CuIICuICuI(μ3-OH)](PF6)3 and its solvent-exposed analog [TREN3CuIICuIICuII(μ3-O)](PF6)4 suggest that isolation of tricopper core in a cryptand enables facile electron transfer, allowing potential application of synthetic tricopper complexes as redox catalysts. Indeed, the fully reduced [TREN4CuICuICuI(μ3-OH)](PF6)2 can reduce O2 under acidic conditions. The geometric constraints provided by the cryptand are reminiscent of Nature's multicopper oxidases (MCOs). For the first time, a synthetic tricopper cluster was isolated and fully characterized at CuICuICuI (4a), CuIICuICuI (4b), and CuIICuIICuI (4c) states, providing structural and spectroscopic models for many intermediates in MCOs. Fast electron transfer rates (105 to 106 M−1 s−1) were observed for both CuICuICuI/CuIICuICuI and CuIICuICuI/CuIICuIICuI redox couples, approaching the rapid electron transfer rates of copper sites in MCO.
Introduction
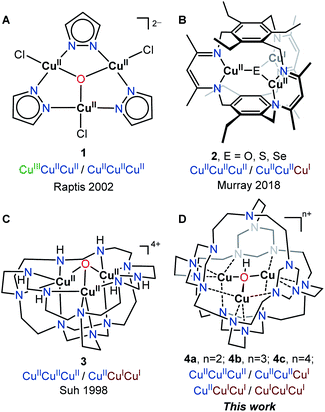
Synthetic tricopper clusters have been a prominent synthetic target for the (bio)inorganic community over the past few decades,1–9 since tricopper centers were identified/proposed as essential active sites for biological reduction of O2 to H2O in multicopper oxidase (MCO)10,11 and aerobic hydroxylation of methane in particulate methane monooxygenase (pMMO).12 Although a tricopper active site in pMMO has been disputed, the conversion of methane to methanol was demonstrated with small-molecule tricopper complexes.13,14 In both oxygen reduction and hydrocarbon hydroxylation, the synergy of three CuII/CuI redox couples in tricopper clusters is essential to harness the oxidative power of O2. Therefore, understanding factors governing the redox of tricopper clusters as a single unit is fundamental to their development as biomimetic catalysts in fuel cell technology and functionalization of alkanes.An ideal tricopper redox catalyst should be able to engage all three CuII/CuI couple and accommodate four redox states from CuICuICuI to CuIICuIICuII. However, the majority of synthetic tricopper clusters reported to date have limited redox capability, and only isolated at a single oxidation state (primarily CuIICuIICuII or CuICuICuI).15,16 Copper(I) center (d10) and copper(II) center (d9) prefer distinct geometry due to the Jahn–Teller effect. The redox of CuII/CuI, in an unconstrained solvent-exposed environment, often results in significant geometric rearrangement associated with high reorganization energy.17 Notably, among ca. 186 crystallographically characterized molecular tricopper μ3-E, (E = O, OH, S) clusters, 1 and 2 (Fig. 1A and B) represents the only two redox-active examples.18,19 Even 1 and 2, however, cannot be further reduced to the CuICuICuI state,16 which is crucial for the activation of O2 during catalytic oxygen reduction reaction (ORR) and hydrocarbon hydroxylation. In sharp contrast, Nature's trinuclear copper cluster (TNC) in MCOs has been observed in three different oxidation states: fully reduced (CuICuICuI, FR), alternative resting (CuIICuICuI, AR), and native intermediate (CuIICuIICuII, NI). Unlike synthetic tricopper complexes, TNC is embedded in a protein matrix, which provides conformational strains and site isolation that reduce reorganization energy during electron transfer (ET).20,21 According to Marcus theory, electron transfer in solution is strongly influenced by both inner sphere ligand interactions and outer sphere solvent environments.17 We posited that the encapsulation of a synthetic tricopper cluster in a cryptand could constrain the coordination environment, hence lowing the barriers for reorganization and allowing access to different redox states of tricopper clusters. Instead of using bulky ligands to simulate the protein environment, we leveraged a multicyclic azacryptand ligand to restrict the conformational freedom and limit solvent interactions. The electrochemical property of 4 was compared to its solvent-exposed analog [TREN3CuIICuIICuII(μ3-O)]Cl4, 322 to understand how compartmentalization of tricopper center impacts the redox behavior of CuII/CuI.
Results and discussion
The tricopper complex [TREN3CuIICuIICuII(μ3-O)](PF6)4, 3 (Fig. 1C) has been considered as a structural22 and spectroscopic model23,24 for TNC. However, 3 does not exhibit the multi-electron redox function of TNC. The ca. 550 mV separation of the redox couple (Fig. 4C), which is typical for synthetic tricopper complexes, indicates a substantial barrier for reorganization during ET. Notably, the reduction of CuIICuICuI to CuICuICuI was not observed within the voltage window of water, suggesting 3 is not suitable to activate dioxygen. To construct an isolated microenvironment, we sought to install an additional TREN cap on 3 to afford 4 (Fig. 1D). The desired azacryptand TREN4 has been synthesized previously,25 however, no transition metal complex has been reported thus far. We found that metalation of TREN4 with Cu(I) and Cu(II) salts only afforded complicated mixtures in low yields. Inspired by the metal-templated synthesis reported by Suh et al.,22 we explored the one-pot reaction of copper salts, TREN, and paraformaldehyde. Reaction of [CuI(MeCN)4]PF6, TREN (3.3 equivalents), and paraformaldehyde (20 equivalents based on CH2O units) resulted in the formation of a blue solid (Fig. 2A). Workup and recrystallization under our optimized conditions afforded complex 4b in 18% yield (see ESI†).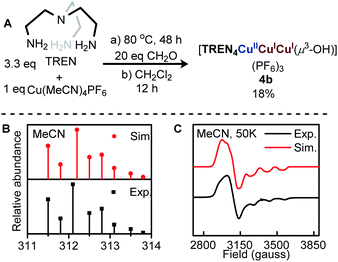 | ||
| Fig. 2 (A) Synthesis of [TREN4CuIICuICuI(μ3-OH)](PF6)3 (4b). (B) ESI-MS (positive mode) of 4b, (C) X-band EPR spectrum (frozen MeCN, 0.5 mM) of 4b, gx = 2.25 gy = 2.14, gz = 2.01, Az(Cu) = 146 MHz. | ||
The Electrospray Ionization Mass Spectrum (ESI-MS, Fig. 2B) of 4b exhibited a prominent peak at 312.2 m/z, and the isotope distribution pattern matches that of [TREN4CuIICuICuI(μ3-OH)]3+ (molecular mass = 938.75 g mol−1, C36H75Cu3N16O), supporting the presence of a [Cu3(μ3-OH)]3+ core. The frozen solution electron paramagnetic resonance (EPR) spectrum of 4b (Fig. 2C) displayed a rhombic signal (gx = 2.25, gy = 2.14, gz = 2.01) with hyperfine couplings to one 63/65Cu (I = 3/2) nucleus (Az(Cu) = 146 MHz), suggesting a valence-localized one-hole CuIICuICuI electronic structure. The high gx and gy values indicated that the unpaired electron resides in the dz2 orbital, consistent with a trigonal bipyramidal geometry.26
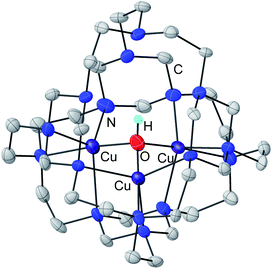
Single crystal X-ray diffraction analysis of 4b confirmed its assignment as mixed-valent [TREN4CuIICuICuI(μ3-OH)](PF6)3 (Fig. 3). Cryptate 4b shares the macrocyclic tricopper assembly of 3 but features a fully encapsulated tricopper core. Three of four TREN moieties in TREN4 serve as bowl-shape binding sites for the tricopper core, while the remaining one forms a cryptand that isolates the Cu3(μ3-OH) core in a capsule. With its one-hole CuIICuICuI oxidation state, complex 4b is the first synthetic model for the alternative resting (AR) state in MCO (CuIICuICuI).27,28 The Cu⋯Cu and Cu–O bond metrics within 4b (Cu⋯Cu: 2.973–3.260 Å; Cu–O: 1.878–1.971 Å) are similar to those in 3 (Cu⋯Cu: 3.095 Å; Cu–O: 1.863 Å) but vary greater from each other, perhaps as a result of its localized electronic structure.
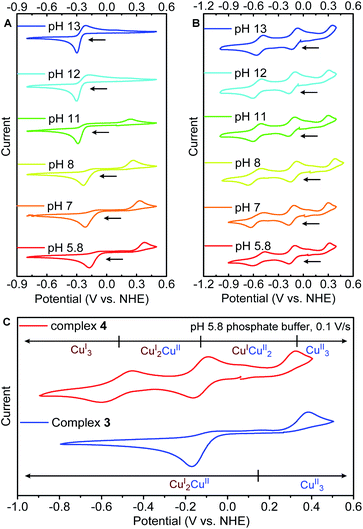
To evaluate the impact of tricopper encapsulation on the redox capability, we performed cyclic voltammetry (CV) studies of 3 and 4 in aqueous environments with pH values range from 5.6 to 13. Dissolution of 3 in water produces an equilibrium mixture of 3 and its conjugate acid (pKa = 4.6).22 Cyclic voltammogram of 3 at pH = 5.6 showed an irreversible reduction at −0.18 V (vs. NHE), which gradually shifts to −0.30 V (vs. NHE) at pH = 13. This cathodic peak is assigned to the two-electron reduction of [CuIICuIICuII(μ3-OH)]5+ to [CuIICuICuI(μ3-OH)]3+ based on variable scan rate CV study (see ESI, Fig. S19†). During the reverse scan, an anodic peak was observed at 0.37 V (vs. NHE, pH = 5.8), which is attributed to the oxidation of [CuIICuICuI(μ3-OH)]3+ back to [CuIICuIICuII(μ3-OH)]5+ (Fig. 4A). The ca. 550 mV separation of the redox couple indicates a substantial geometric difference at CuIICuIICuII and CuIICuICuI states. As the tricopper center in 3 is exposed to bulk exterior, it can lose/gain interactions with solvents during redox processes, further raising the reorganization energy. Notably, further reduction of [CuIICuICuI(μ3-OH)]3+ to [CuICuICuI(μ3-OH)]2+ was not observed within the voltage window of water. The CV of 3 becomes more reversible as the pH increases from 5.6 to 13, perhaps due to the deprotonation of central μ3-OH to μ3-O, a stronger ligand that rigidifies the coordination environment (see ESI, Scheme S2†).
In contrast to the irreversible redox behavior of 3, the cyclic voltammogram of 4 shows two reversible (E1/2 = −0.55 V and −0.13 V vs. NHE) and one irreversible (Eox = 0.33 V vs. NHE) redox events, allowing access to all four oxidation states of the tricopper cluster electrochemically (Fig. 4B). The three redox events of complex 4 are insensitive to the pH of the buffer (Fig. 4B), implicating that the cryptand of TREN4 isolates the tricopper core from direct solvent interactions. Additionally, the CVs of 4 in aqueous and non-aqueous environments, e.g. dimethylformamide (DMF) or acetonitrile, are essentially the same (Fig. S17†), further supporting that the redox of tricopper center in 4 is unaffected by the bulk exterior. As shown in the Fig. 4C, the reduction potentials required to reach CuIICuICuI state (−0.17 V for 3, −0.17 V for 4) and the oxidation potentials required for CuIICuIICuII state (0.38 V for 3, 0.32 V for 4) are similar for 3 and 4. Given the less electron-donating nature of TREN3vs.TREN4, the reduction and oxidation peaks of 3 should be more anodic than those of 4. The fact that 3 and 4 share the similar redox potentials suggests that the high solvent accessibility of 3 has shifted its redox potentials cathodically. Solvent molecules may serve as ligands to tricopper core and make 3 more electron-rich, while the tricopper core in 4 is completely shielded from such solvent coordination. This is also consistent with the cathodic shift of the reduction and oxidation peak of 3 when the pH of the solvent increases (Fig. 4A).
The highly reversible oxidation and reduction couples of 4b prompted us to pursue the isolation of fully reduced [TREN4CuICuICuI(μ3-OH)]2+ (4a, Fig. 5A) and two-hole [TREN4CuIICuIICuI(μ3-OH)]4+ (4c, Fig. 5A). Consistent with its electrochemical behavior, chemical oxidation of 4b with decamethylferrocenium hexafluorophosphate (Me10FcPF6, E1/2 = −0.49 V vs. Fc+/Fc) afforded a dark blue compound [TREN4CuIICuIICuI(μ3-OH)](PF6)4 (4c) in 50% yield (Fig. 5A). 1H NMR spectrum of 4c shows nineteen broad resonances from 0.13 to 122.5 ppm (see ESI, Fig. S8†), indicating an S = 1 ground state. Complex 4c is a rare example of a tricopper cluster with a CuIICuIICuI oxidation state with the only other example being 2 reported by Murray et al.19 Chemical reduction of 4b with cobaltocene (Cp2Co, E1/2 = −1.3 V vs. Fc+/Fc) led to a rapid color change from blue to yellow (Fig. 5B), and colorless crystals of fully reduced [TREN4CuICuICuI(μ3-OH)](PF6)2 (4a) were obtained by recrystallization. 1H NMR characterization of 4a was hampered by its low solubility. Therefore, we synthesized an analog of 4a with (3,5-bis(trifluoromethyl)phenyl)borate (BArF4) counter anions (4a-BArF4) by treating 4b-BArF4 with Cp2Co. All five 1H NMR resonances of 4a-BArF4 between 2 to 4.5 ppm are quite broad, perhaps because of the dynamic behavior of CH2 groups on TREN4 (see ESI, Fig. S6†).
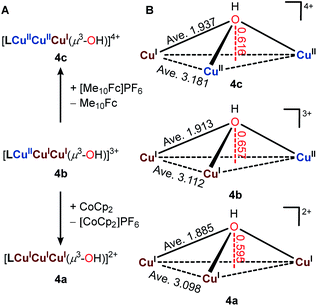 | ||
| Fig. 5 (A) Synthesis of 4a and 4c from 4b. (B) Geometric comparison of the Cu3[μ3-O(H)] core in 4a, 4b, and 4c. All bond metrics (Å) were determined based on X-ray single-crystal diffraction. | ||
Both 4a and 4c were characterized by X-ray single crystallography (Fig. 5B, S12 and S13†). The geometric features of Cu3(μ3-OH) are maintained throughout the redox processes. Complex 4a has a slightly contracted [CuICuICuI(μ3-OH)]2+ core as evidenced by the shortened Cu⋯Cu distance (average 3.098 Å in 4a comparing to 3.112 Å in 4b) and Cu–O distances (average 1.885 Å in 4a comparing to 1.913 Å in 4b). In contrast, the [CuIICuIICuI(μ3-OH)]4+ core in 4c expanded (average Cu⋯Cu distance 3.181 Å, average Cu–O distance 1.937 Å) because of the increased coulombic repulsion. The minimal geometric differences of complex 4a, 4b, and 4c agree with their highly reversible redox behaviors. Importantly, the trigonal bipyramidal geometry of the Cu centers is preserved, even at the CuICuICuI state, highlighting the extraordinary constraints imposed by the multicyclic ligand TREN4, a feature not shared by TREN3.
Infrared spectra of complexes 4a, 4b, 4c reveal that the O–H stretches of μ3-OH progressively blueshifts from 3372 cm−1 (CuIICuIICuI) to 3440 cm−1 (CuIICuICuI) to 3516 cm−1 (CuICuICuI), indicating increasing O–H bond strength as Cu oxidation state decreases (Fig. 6A). The UV-vis spectra of 4b and 4c both exhibit two broad intervalence charge-transfer bands (4b: 655 nm (800 M−1 cm−1) and 790 nm (870 M−1 cm−1), 4c: 680 nm (1000 M−1 cm−1) and 850 nm (1300 M−1 cm−1), Fig. 6B). Time-dependent density functional theory (TD-DFT) calculations at the TPSSh/TZVP level showed that these absorptions originate from charge transfers from combinations of copper d orbitals to the LUMO, which features the σ* interaction of copper dz2 and the μ3-O pz (see ESI, Tables S5 and S6†). Moreover, the calculated UV-vis spectra reflected the red-shifting trend from 4b to 4c (see ESI, Fig. S22†).
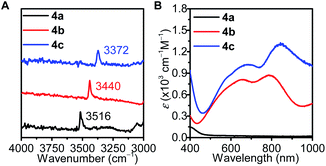 | ||
| Fig. 6 (A) Infrared and (B) UV-vis spectra of 4a (CuICuICuI, black), 4b (CuIICuICuI, red), and 4c (CuIICuIICuI, blue). | ||
The reorganization energies (λ) of tricopper clusters during ET have important implications in oxygen reduction reaction catalyzed by MCO.29 The reorganization energies of TNC have been used to rationalize the various rates of intermolecular ET between type 1 Cu sites and TNC21 as well as the inhibition30 or acceleration31 of ORR by halides. To further understand the kinetic factors that govern the redox of 4, we evaluated the self-exchange rates (k11) and reorganization energy (λ) of CuICuICuI/CuIICuICuI and CuIICuICuI/CuIICuIICuI redox couples. Determine of k11 using 1H NMR line broadening experiment32 was not feasible due to the dynamic behavior of CH2 resonances in 4a and paramagnetic nature of 4c. As an alternative, we employed an electrochemical method reported by Nicholson;33,34 the self-exchange rates of 4a/4b and 4b/4c were determined to be 7.4(2) × 105 M−1 s−1 and 7.2(2) × 105 M−1 s−1, respectively (see ESI, Fig. S20†). These fast ET rates approach the fastest synthetic mono- and dicopper system (105 to 106 M−1 s−1)33,35–43 as well as Nature's blue copper ET protein 105 to 106 M−1 s−1.44,45 The corresponding reorganization energies for 4a/4b and 4b/4c were calculated to be 1.21(1) eV and 1.21(1) eV using eqn (1) (Z = 1011 M−1 s−1, T = 298 K).39,46
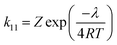 | (1) |
The reorganization energy (λ) can be further partitioned into inner-sphere reorganization energy (λi, ligands and coordinated/hydrogen-bonded solvent molecules) and outersphere reorganization energy (λo, free solvent molecules, eqn (2)). The outer sphere reorganization energy can be estimated with eqn (3), which is based on a spherical model that separates the inner sphere and the outer sphere contribution. Solvent molecules association and dissociation with the inner sphere during the electron transfer will affect the accuracy in the reorganization energy calculation. In order to minimize the inaccuracy in determining the reorganization energy caused by direct hydrogen bonding of solvent with 3 and 4, acetonitrile was utilized, instead of water, in the electrochemical study and the following calculations. The outersphere reorganization energy was calculated to be 0.68 eV for 4a/4b and 4b/4c using eqn (3), where Δe is the elementary charge, a1 and a2 are the radii of redox partners, and Dopt and Dstat are optical- and static-dielectric constants of acetonitrile47 at 298.15 K. Thus, the inner-sphere reorganization energy for 4a/4b and 4b/4c are estimated to be 0.53(1) eV and 0.53(1) eV using eqn (2).
| λ = λi + λo | (2) |
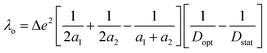 | (3) |
| λi = λox + λred | (4) |
Determination of k11 of 3 was not viable, since the redox of CuIICuIICuII/CuIICuICuI was not reversible, and CuIICuICuI/CuICuICuI was not observed experimentally. Therefore, we turned to computational study to compare the reorganization energies of 3 and 4. The inner-sphere reorganization energy λi can be calculated by eqn (4), where λox and λred are the reorganization energy of the reduced and oxidized complexes. λox and λred can be computationally estimated by the energy required to distort the equilibrium geometries to their redox-partners' geometries (see ESI, Fig. S21 and Scheme S3†).29,30,48
Single-point energies of 3 and 4 at all four redox states were calculated at TPSSh/def2-TZVP(Cu)/def2-SVP(C, N, O, H) level with a continuum solvation model49 and dispersion corrections.50,51 The Gibbs free energy of each species was determined by adding the solvated single point SCF energy to the thermal correction from the respective frequency calculation.52 The frequency calculations were conducted with BP86 functional due to the conversion issues of a few species in high spin states with other hybrid functionals. There are several competing spin states for the CuIICuIICuII and CuIICuIICuI oxidation states. Since our goal is to compare the reorganization energies of 3 and 4, we have only studied the electronic states with the lowest energies, that is quartet states for CuIICuIICuII with three ferromagnetically coupled Cu(II) centers and triplet states for CuIICuIICuI with two ferromagnetically coupled Cu(II) centers (see ESI, Table S3†). We compared the optimized geometry of 3 and 4 at different redox states (Table S7 and Fig. S23†). Consistent with their solid-state structure, no significant geometric distortion was observed for 4 at all four oxidation states. In contrast, there was substantial geometric changes of 3 from CuIICuICuI to CuICuICuI state, which may results in large reorganization energy for CuIICuICuI/CuICuICuI couple (Fig. S23†).
The inner-sphere reorganization energies (λi) of 3 and 4 were summarized in Table 1 and Scheme S3.† The λi values of 3 for each redox couple are generally greater than those of 4. The especially high λi value for CuIICuICuI/CuICuICuI redox couple of 3 (2.1 eV) agrees with the large degree of geometry distortion. Consequently, a large separation between the reduction and oxidation peak may be expected, which explains why the fully reduced CuICuICuI state for 3 cannot be achieved within the chemical window of water.53 The large inner-sphere reorganization energy for [TREN3CuIICuICuI(μ3-O)]2+/[TREN3CuICuICuI(μ3-O)]+ (2.1 eV) may be rationalized by its trigonal bipyramidal Cu centers, a geometry highly preferred by Cu(II). As a result, the reduction of CuIICuICuI to CuICuICuI, in an unconstrained environment, could be coupled with chemical reactions, e.g. ligand dissociation/reorganization. In contrast, multicyclic ligand TREN4 prevents such ligand dissociation and restrict solvent access to the tricopper centers, synergistically yielding small reorganization energy and fast ET rates.
The generation of the fully reduced CuICuICuI state is crucial for oxygen reduction reaction at MCO. Encouraged by the redox capability of 4, we studied its ability to reduce O2. As followed by UV-vis spectroscopy (see ESI, Fig. S10†), fully reduced 4a slowly reduced O2, while 4b and 4c did not react with O2, consistent with the fact that generation of CuICuICuI state is required to harness the oxidative power of O2. Addition of proton sources, e.g. acetic acid, significantly accelerated the rate of ORR. Complex 4b was regenerated in 96(2)% spectroscopic yield. The product of the O2 reduction was assigned as H2O2 in 97(2)% yield based on iodometric titration (see ESI, Table S1†).54 To elucidate the mechanism of ORR, we performed the reaction 4a with 18O2 in the presence of acetic acid. The resulting solution of 4b was analyzed by ESI-MS for 18O incorporation. Spectrum simulations show 10% of 18O incorporation (Fig. S15†), indicating that the ORR most likely proceeds through an outer-sphere mechanism,55 consistent with the predominant H2O2 selectivity.
Conclusions
In conclusion, we report that the encapsulation of tricopper core in a cryptand allows the redox of three CuII/CuI to be harnessed effectively in a homogeneous synthetic system. The tricopper complex was isolated and characterized in three of four oxidation states, showing that the redox-induced geometric changes were minimal. The encapsulated tricopper complex has self-exchange ET rates of 105 to 106 M−1 s−1, approaching Nature's copper electron transfer sites (blue copper protein 105 to 106 M−1 s−1). Marcus analysis indicates that the reorganization energy required for the reduction of 4b to 4a was 0.70 eV, much smaller than that for solvent-exposed 3 (2.1 eV). These results are particularly consistent with the minimal structural difference of 4 at CuICuICuI, CuIICuICuI, and CuIICuIICuI state and suggest that multicyclic cryptands TREN4 is effective at providing an isolated microenvironment. The geometric constraints and exclusion of solvent interactions synergistically reduce the reorganization energy of electron transfer. At last, we show that the fully reduced CuICuICuI cryptate can participate in oxygen reduction reaction. Taken together, our work provides insights into Nature strategy in leveraging the cooperativity of multimetallic sties in multielectron transformation. Our results show that redox processes not operational in bulk solution could potentially be accomplished by full encapsulation of the active site in an isolated environment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial supports from OSU, ACS-PRF (59036-DNI3), and National Science Foundation (CHE-1904560). The authors thank The Ohio State University Department of Chemistry and Biochemistry for additional financial support. High-performance computing resources were provided by the Ohio Supercomputer Center.References
- A. K. Gupta and W. B. Tolman, Inorg. Chem., 2012, 51, 1881–1888 CrossRef CAS.
- A. P. Cole, D. E. Root, P. Mukherjee, E. I. Solomon and T. D. P. Stack, Science, 1996, 273, 1848–1850 CrossRef.
- M. Taki, S. Teramae, S. Nagatomo, Y. Tachi, T. Kitagawa, S. Itoh and S. Fukuzumi, J. Am. Chem. Soc., 2002, 124, 6367–6377 CrossRef CAS.
- B. J. Cook, G. N. Di Francesco, M. T. Kieber-Emmons and L. J. Murray, Inorg. Chem., 2018, 57, 11361–11368 CrossRef CAS.
- D. Lionetti, M. W. Day and T. Agapie, Chem. Sci., 2013, 4, 785–790 RSC.
- X. Engelmann, E. R. Farquhar, J. England and K. Ray, Inorg. Chim. Acta, 2018, 481, 159–165 CrossRef CAS.
- E. Y. Tsui, M. W. Day and T. Agapie, Angew. Chem., Int. Ed., 2011, 50, 1668–1672 CrossRef CAS.
- K. D. Karlin, G. Qingfen, A. Farooq, L. Shuncheng and J. Zubieta, Inorg. Chim. Acta, 1989, 165, 37–39 CrossRef CAS.
- E. C. Brown, B. Johnson, S. Palavicini, B. E. Kucera, L. Casella and W. B. Tolman, Dalton Trans., 2007, 3035–3042 RSC.
- E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Chem. Rev., 2002, 96, 2563–2606 CrossRef.
- N. Mano, H.-H. Kim and A. Heller, J. Phys. Chem. B, 2002, 106, 8842–8848 CrossRef CAS.
- V. C.-C. Wang, S. Maji, P. P.-Y. Chen, H. K. Lee, S. S.-F. Yu and S. I. Chan, Chem. Rev., 2017, 117, 8574–8621 CrossRef CAS.
- P. P.-Y. Chen, R. B.-G. Yang, J. C.-M. Lee and S. I. Chan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14570–14575 CrossRef CAS.
- S. I. Chan, Y.-J. Lu, P. Nagababu, S. Maji, M.-C. Hung, M. M. Lee, I.-J. Hsu, P. D. Minh, J. C.-H. Lai, K. Y. Ng, S. Ramalingam, S. S.-F. Yu and M. K. Chan, Angew. Chem., Int. Ed., 2013, 52, 3731–3735 CrossRef CAS.
- J. Yoon and E. I. Solomon, Coord. Chem. Rev., 2007, 251, 379–400 CrossRef CAS.
- E. Salvadeo, L. Dubois and J. Latour, Coord. Chem. Rev., 2018, 374, 345–375 CrossRef CAS.
- R. A. Marcus, J. Chem. Phys., 1956, 24, 966–978 CrossRef CAS.
- M. Rivera-Carrillo, I. Chakraborty, G. Mezei, R. D. Webster and R. G. Raptis, Inorg. Chem., 2008, 47, 7644–7650 CrossRef CAS.
- B. J. Cook, G. N. Di Francesco, R. B. Ferreira, J. T. Lukens, K. E. Silberstein, B. C. Keegan, V. J. Catalano, K. M. Lancaster, J. Shearer and L. J. Murray, Inorg. Chem., 2018, 57, 11382–11392 CrossRef CAS.
- S. M. Jones and E. I. Solomon, Cell. Mol. Life Sci., 2015, 72, 869–883 CrossRef CAS.
- D. E. Heppner, C. H. Kjaergaard and E. I. Solomon, J. Am. Chem. Soc., 2013, 135, 12212–12215 CrossRef CAS.
- M. P. Suh, M. Y. Han, J. H. Lee, K. S. Min and C. Hyeon, J. Am. Chem. Soc., 1998, 120, 3819–3820 CrossRef CAS.
- J. Yoon, L. M. Mirica, T. D. P. Stack and E. I. Solomon, J. Am. Chem. Soc., 2005, 127, 13680–13693 CrossRef CAS.
- J. Yoon and E. I. Solomon, Inorg. Chem., 2005, 44, 8076–8086 CrossRef CAS.
- Z. Bing-Guang, M. Hong, D. Chun-Ying, H. Cheng, M. Qing-Jin, W. Zhe-Ming and Y. Chun-Hua, Chem. Commun., 2001, 24, 2652–2653 RSC.
- E. Garribba and G. Micera, J. Chem. Educ., 2006, 83, 1229–1232 CrossRef CAS.
- C. H. Kjaergaard, S. M. Jones, N. Mano and E. I. Solomon, J. Am. Chem. Soc., 2015, 137, 8783–8794 CrossRef CAS.
- C. H. Kjaergaard, F. Durand, F. Tasca, M. F. Qayyum, B. Kauffmann, S. Gounel, E. Suraniti, K. O. Hodgson, B. Hedman, N. Mano and E. I. Solomon, J. Am. Chem. Soc., 2012, 134, 5548–5551 CrossRef CAS.
- L. Hu, M. Farrokhnia, J. Heimdal, S. Shleev, L. Rulíšek and U. Ryde, J. Phys. Chem. B, 2011, 115, 13111–13126 CrossRef CAS.
- K. P. Kepp, Inorg. Chem., 2015, 54, 476–483 CrossRef CAS.
- S. Tian, S. M. Jones, A. Jose and E. I. Solomon, J. Am. Chem. Soc., 2019, 141, 10736–10743 CrossRef CAS.
- E. W. Dahl and N. K. Szymczak, Angew. Chem., 2016, 128, 3153–3157 CrossRef.
- M. Gennari, J. Pécaut, S. DeBeer, F. Neese, M.-N. Collomb and C. Duboc, Angew. Chem., Int. Ed., 2011, 50, 5662–5666 CrossRef CAS.
- R. S. Nicholson, Anal. Chem., 1965, 37, 1351–1355 CrossRef CAS.
- E. W. Dahl and N. K. Szymczak, Angew. Chem., Int. Ed., 2016, 55, 3101–3105 CrossRef CAS.
- H. Doine, Y. Yano and T. W. Swaddle, Inorg. Chem., 1989, 28, 2319–2322 CrossRef CAS.
- K. Krylova, C. P. Kulatilleke, M. J. Heeg, C. A. Salhi, L. A. Ochrymowycz and D. B. Rorabacher, Inorg. Chem., 1999, 38, 4322–4328 CrossRef CAS.
- E. J. Pulliam and D. R. McMillin, Inorg. Chem., 1984, 23, 1172–1175 CrossRef CAS.
- T. J. Zerk, C. T. Saouma, J. M. Mayer and W. B. Tolman, Inorg. Chem., 2019, 58, 14151–14158 CrossRef CAS.
- B. Xie, T. Elder, L. J. Wilson, D. M. Stanbury and R. V. August, Inorg. Chem., 1999, 38, 12–19 CrossRef CAS.
- B. Xie, L. J. Wilson, D. M. Stanbury and R. V. April, Inorg. Chem., 2001, 40, 3606–3614 CrossRef CAS.
- M. Lee, L. Maigre, M. Ibrahim, M. Tahir, E. R. T. Tiekink, T. Begum, R. Rosli, C. Policar, N. Delsuc, K. A. Crouse and J. Pag, Eur. J. Med. Chem., 2016, 120, 1–12 CrossRef.
- J. Stanek, M. Konrad, J. Mannsperger, A. Hoffmann and S. Herres-Pawlis, Eur. J. Inorg. Chem., 2018, 4997–5006 CrossRef CAS.
- C. E. Slutter, R. Langen, D. Sanders, S. M. Lawrence, P. Wittung, A. J. Di Bilio, M. G. Hill, J. A. Fee, J. H. Richards, J. R. Winkler and B. G. Malmström, Inorg. Chim. Acta, 1996, 243, 141–145 CrossRef CAS.
- E. T. Adman, G. W. Canters, H. A. O. Hill and N. A. Kitchen, Inorg. Chim. Acta, 1983, 79, 127–128 CrossRef.
- L. E. Eberson, Electron transfer reactions in organic chemistry, Springer-Verlag, Berlin, 1987 Search PubMed.
- C. Moreau and D. Gérard, J. Chem. Thermodyn., 1976, 8, 403–410 CrossRef CAS.
- E. Sigfridsson, M. H. M. Olsson and U. Ryde, Inorg. Chem., 2001, 40, 2509–2519 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS.
- E. R. Johnson and A. D. Becke, J. Chem. Phys., 2006, 124, 174104 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS.
- S. J. Koepke, K. M. Light, P. E. Vannatta, K. M. Wiley and M. T. Kieber-Emmons, J. Am. Chem. Soc., 2017, 139, 8586–8600 CrossRef CAS.
- M. C. Henstridge, E. Laborda, N. V. Rees and R. G. Compton, Electrochim. Acta, 2012, 84, 12–20 CrossRef CAS.
- I. Garcia-Bosch, R. E. Cowley, D. E. Díaz, M. A. Siegler, W. Nam, E. I. Solomon and K. D. Karlin, Chem.–Eur. J., 2016, 22, 5133–5137 CrossRef CAS.
- M. L. Pegis, C. F. Wise, D. J. Martin and J. M. Mayer, Chem. Rev., 2018, 118, 2340–2391 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, theoretical calculations, and supporting figures. CCDC 1987932 and 1984893–1984895. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc05441k |
| This journal is © The Royal Society of Chemistry 2021 |