Open Access Article
Open Access ArticleElucidating dissociation activation energies in host–guest assemblies featuring fast exchange dynamics†
Ronit
Shusterman-Krush
 a,
Laura
Grimm
b,
Liat
Avram
a,
Laura
Grimm
b,
Liat
Avram
 c,
Frank
Biedermann
c,
Frank
Biedermann
 b and
Amnon
Bar-Shir
b and
Amnon
Bar-Shir
 *a
*a
aDepartment of Organic Chemistry, Weizmann Institute of Science, Rehovot, 7610001, Israel. E-mail: amnon.barshir@weizmann.ac.il
bInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
cDepartment of Chemical Research Support, Weizmann Institute of Science, Rehovot, 7610001, Israel
First published on 8th December 2020
Abstract
The ability to mediate the kinetic properties and dissociation activation energies (Ea) of bound guests by controlling the characteristics of “supramolecular lids” in host–guest molecular systems is essential for both their design and performance. While the synthesis of such systems is well advanced, the experimental quantification of their kinetic parameters, particularly in systems experiencing fast association and dissociation dynamics, has been very difficult or impossible with the established methods at hand. Here, we demonstrate the utility of the NMR-based guest exchange saturation transfer (GEST) approach for quantifying the dissociation exchange rates (kout) and activation energy (Ea,out) in host–guest systems featuring fast dissociation dynamics. Our assessment of the effect of different monovalent cations on the extracted Ea,out in cucurbit[7]uril:guest systems with very fast kout highlights their role as “supramolecular lids” in mediating a guest's dissociation Ea. We envision that GEST could be further extended to study kinetic parameters in other supramolecular systems characterized by fast kinetic properties and to design novel switchable host–guest assemblies.
Introduction
For many supramolecular host–guest systems, elucidating their kinetic characteristics is critical for thoroughly understanding their performance and further improving their design as synthetic channels,1 receptors,2 transporters,3 drug carriers,4 catalysts,5 stimuli-responsive materials6,7 and more. Controlling the kinetic properties in such systems can be obtained through an external stimulus that changes the system's activation energy (Ea) so as to yield an “open” or “closed” state of the host and, thus, govern the exchange dynamics of the bound guest. Considerable advances have been made in the design of such switchable open/closed molecular hosts and their response to a variety of external stimuli, such as pH,8 light,9 heat,10 redox11 and more. However, a robust and accessible tool for studying their effect on the dissociation activation energy Ea (Ea,out) in a quantitative manner, which is crucial for the further development and improved performance of such systems, has yet to be offered. Indeed, well-established classical methods, such as stopped-flow experiments,12 UV-Vis measurements13 and exchange spectroscopy (EXSY)-NMR,14 are useful for characterizing slow dynamic processes. Nevertheless, these analytical tools are less favourable when it comes to supramolecular complexes with fast exchange dynamics, including the evaluation of such systems' Ea values.Applying the chemical exchange saturation transfer (CEST) NMR method to the study of host–guest systems using a hyperpolarized 129Xe gas guest15 has opened new opportunities for quantifying exchange dynamics in supramolecular assemblies. Indeed, hyperCEST was applied to a wide array of molecular hosts, such as cryptophanes,16,17 cucurbit[n]urils (CBn),18–20 pillar[n]arenes21 and paramagnetic-capsules,22 demonstrating its applicability to a range of exchange regimes that are dependent on the host properties.23 The combination of CEST and 19F-NMR24,25 and its extension to host–guest systems26–28 have expanded the arsenal of molecular guests that are suitable for CEST-based studies beyond that of 129Xe. This approach, termed guest exchange saturation transfer (GEST),29 allows – as we introduce herein – now also the use of conventional NMR-setups to quantitatively study host–guest dissociation rates (kout) and Ea,out. In fact, most host–guest systems that we are aware of display such low activation energies (and thus very fast complex formation and dissociation rate constants) that their study is simply infeasible by the available methods. In several cases, direct binding assays can be used in combination with stopped-flow experiments,30–34 particularly, when a chromophoric or emissive guest considerably alters their spectroscopic properties upon binding to the host. However, often in these cases a too fast complex formation is observed which is completed within the mixing time (“dead time”) of the technique. Thus, alternative methods for quantifying kinetic parameters applicable to the study of fast equilibrating host–guest systems are still in need.
We demonstrate here how the GEST-NMR method can be used to quantify relatively fast kout values, which makes it complementary to other tools,35,36 including those based on NMR (i.e., EXSY),37 which are better suited for studying slow exchange rates. By exploring the relationship between kout rates and the applied temperature, we demonstrate GEST's use as an analytical method for the study of Ea,out in host–guest systems.
Specifically, we show that GEST-NMR can be used to quantitatively elucidate Ea,out values of fluorinated guests (G) from cucurbit[7]uril (CB7). The CB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38–41 molecular host has a broad range of applications through host–guest inclusion complex formation,42–46 but also shows an unprecedented affinity to cations through ion–dipole interactions forming “supramolecular-lids”47–51 that mediate both thermodynamic52 and kinetic properties31,53,54 of CB7:G systems. Herein, we demonstrate the capability of GEST-NMR to quantify the effect of cationic-CB7 “lids” on the Ea,out values of fast-exchanging guests, thus establishing it as an accessible analytical tool for future kinetic studies in supramolecular systems.
38–41 molecular host has a broad range of applications through host–guest inclusion complex formation,42–46 but also shows an unprecedented affinity to cations through ion–dipole interactions forming “supramolecular-lids”47–51 that mediate both thermodynamic52 and kinetic properties31,53,54 of CB7:G systems. Herein, we demonstrate the capability of GEST-NMR to quantify the effect of cationic-CB7 “lids” on the Ea,out values of fast-exchanging guests, thus establishing it as an accessible analytical tool for future kinetic studies in supramolecular systems.
Results and discussion
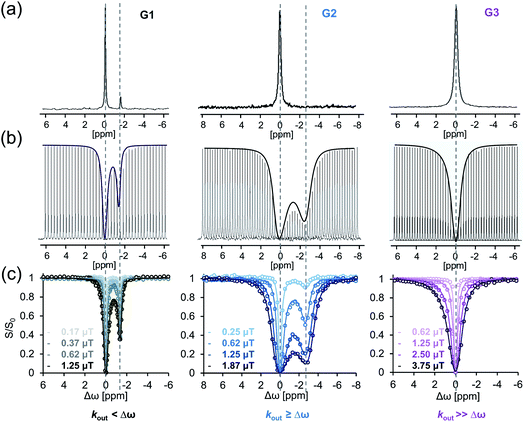
In the here presented study, we characterized the following three host–guest systems regarding their kout values by GEST-NMR: CB7 as host with halothane (G1), 5-fluorotryptophan (G2), and fluroxene (G3) as guests (Fig. 1). As a first step, we acquired 19F-NMR spectra for each system to classify them roughly as either slow or fast exchanging on the NMR timescale (Fig. 2a and S1†). While CB7:G1 clearly exhibited the typical additional peak of a bound guest (upfield shifted to that of free G1), the spectra of CB7:G2 and CB7:G3 featured only a single peak, assigned to the non-bound guest. The clear, sharp, distinct peaks in the CB7:G1 spectrum are typical for a relatively slow exchange regime on the NMR timescale. However, faster exchange processes, as in the cases of CB7:G2 and CB7:G3 (Fig. 2a), lead to NMR-line broadening and peak coalescence, which prevent one from distinguishing between free and bound guests in the 19F-NMR spectra.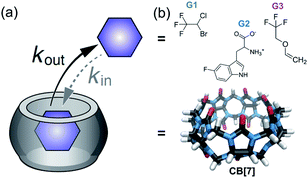 | ||
| Fig. 1 Dynamic CB7:guest systems. (a) Schematic representation of the studied dynamic process. (b) Chemical structures of CB7 and guests G1–G3. | ||
To further elaborate on this observation, GEST experiments were carried out on solutions containing the studied CB7:G systems. When performing GEST-NMR experiments of a CB7:G1 complex at room temperature (298 K), a well-defined saturation transfer effect was observed at the frequency of the bound peak (Fig. 2a, left). Interestingly, in the equivalent GEST-NMR experiment of CB7:G2, we found a clear GEST effect (Fig. 2b, middle), marked by the lack of the characteristic CB7:G2 peak in the 19F-NMR spectrum (Fig. 2a, middle). In the CB7:G3 solution at 298 K, there was no observable asymmetry in the GEST-spectrum (Fig. 2b, right). Nonetheless, the isothermal titration calorimetry (ITC) experiments indicated the formation of CB7:G3 with an association constant Ka of 7 × 104 M−1 (Fig. S2†), which indicates a system with fast exchange kinetics that is accompanied by symmetric GEST spectrum (Fig. 2b, right).
In order to quantify the guest dissociation rates of each of the CB7:G systems, we set up a series of GEST experiments with varied pre-saturation pulses (B1, Fig. 2c). Fitting the experimental data to the Bloch–McConnell equations55 allowed us to quantitatively evaluate the kout of the studied CB7:G systems. As expected, CB7:G1 (Fig. 2, left) exhibited relatively slow dissociation kinetics, with kout = 15 ± 1 s−1; this kout value fits in the slow exchange rate regime on the NMR timescale, with a kout ≪ Δω for a Δω of 1.3 ppm (equal to 490 Hz at 9.4 T NMR) offset between free and bound G1 in the 19F-NMR spectrum. Note that such a slow kout value could also be quantified with the established EXSY-NMR method,56 which, as noted above, is not applicable to host–guest systems with faster dissociation rates where two distinct NMR peaks are not detected (as shown for G2 and G3 in Fig. 2). Ideally suited for the study of faster exchange regimes,57 GEST-NMR was used to quantify the kout of CB7:G2, found to be 2000 ± 100 s−1 (for Δω of ∼1200 Hz; Fig. 2c, middle). Nevertheless, we were unable to determine the exchange rate by which G3 is excluded from its CB7:G3 complex (kout >4000 s−1, 298 K, Fig. 2c, right), as a very broad z-spectrum was obtained (Fig. 2b, right). Thus, we can use GEST-NMR to differentiate between the kinetic regimes of each of the abovementioned host–guest systems – CB7:G1, CB7:G2 and CB7:G3, representing slow-, intermediate-, and fast-exchange processes on the NMR timescale at room temperature (298 K), respectively.
For the elucidation of the binding mechanism, and thus for obtaining deeper insights into non-covalent interactions and supramolecular principles, the knowledge of the activation energies is of utmost benefit.
Having identified two CB7:G systems that experience intermediate-to-fast kout rates (>2000 s−1), we turned to evaluate the capability of GEST-NMR to determine the Ea,out. To this end, the kout values for both CB7:G2 and CB7:G3 were determined at a series of temperatures and then correlated to the inverse temperature using the Arrhenius equation (eqn (1)):
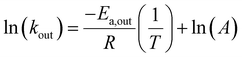 | (1) |
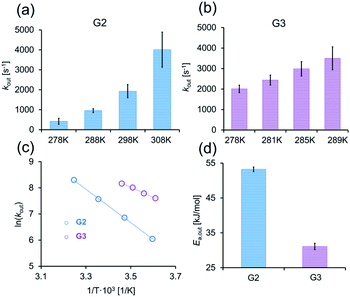
The linear relationship between ln(kout) and T−1 in eqn (1) can be used to evaluate Ea,out values even for host–guest systems that experience fast exchange (kout ≫ Δω) at a given temperature (e.g., for CB7:G3 at 298 K, Fig. 2b, right). This can be achieved by simply performing a series of GEST experiments at lower temperatures where the condition kout ≤ Δω is fulfilled. Therefore, we conducted GEST-NMR experiments of CB7 with either G2 or G3 in a phosphate buffer solution (5 mM sodium phosphate, pH = 7) at different temperatures (Fig. 3a, b, S6 and S7†), from which different kout values were extracted and plotted as a function of T−1 (Fig. 3c). The obtained linear relationships allowed the estimation of the dissociation Ea values (eqn (1)) from the slope of these plots.
Our findings clearly demonstrate that the Ea,out value of CB7:G2 (53 ± 1 kJ mol−1) is much higher than that of CB7:G3 (Ea,out = 32 ± 1 kJ mol−1), which is in good correlation with the observed differences in the extracted kout values. In comparison, dissociation activation energies of the fluorescent guest berberine and other fluorescent alkaloids are much larger, i.e. Ea,out > 65 ± 1 kJ mol−1,58 as was determined by direct binding assays. Neither faster equilibrating guests for CB7 with lower Ea,out barriers nor non-chromophoric guests can be investigated by established stopped-flow-coupled direct binding assays. Indeed, we thoroughly attempted to obtain the binding kinetics and activation energies for the CB7:G3 complex by fluorescent-based stopped-flow measurements at a range of different temperatures, pH and salt concentrations. However, in all cases the low emission signal change upon binding and the very fast guest inclusion kinetics prevented the extraction of any meaningful data with this established protocol. Likewise, even with the newly introduced kinetic versions of the indicator- and guest-displacement assays (kinGDA and kinIDA) that are applicable also to non-chromophoric guest,59 we did not succeed in fitting reliable rate constants for these fast equilibrating guests (Fig. S3–S5†).
These results show that by applying GEST-NMR on supramolecular systems with fast exchange dynamic, one can directly quantify Ea,out values, providing for the first time access to activation energies of host–guest systems with fast formation and dissociation kinetics. It is important to mention, that CEST-based approaches are less suited for systems with a slow exchange dynamic. In this regard, the slow kout of G1 at 298 K and its relatively low boiling temperature prevented us from performing GEST at higher temperatures to obtain faster kout and thus did not allow to accurately evaluating the Ea,out value of CB7:G1.
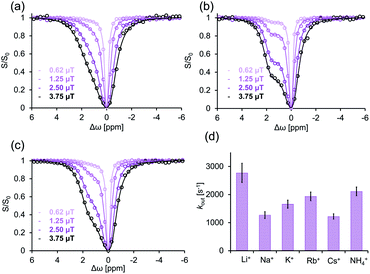
To investigate GEST-NMR's applicability to systems where the Ea,out is mediated also by external factors, we utilized the “supramolecular-lidding” capabilities of monovalent cations known to increase the binding affinities of guests to CBn in systems with very slow kout characteristics.31,53,60,61 As a first step, we used GEST-NMR to determine and quantify the effect of different monovalent cations on the kout of the studied CB7:G3 system (Fig. 4). In contrast to the very fast kout of G3 from its CB7:G3 complex and no asymmetry in the z-spectrum in phosphate buffer solution (Fig. 2b right, 298 K), an increase in the salt concentration resulted in a significant observable asymmetry of the z-spectrum plot. Altering the added cation (i.e., 140 mM of Li+, Na+, K+, Rb+, Cs+ or NH4+) resulted in different z-spectrum profiles (Fig. 4a–c and S8†), indicating different kout values (Fig. 4d). Specifically, fitting of the experimental GEST data revealed that the fastest dissociation rate constant occurred in the presence of Li+ (kout = 2800 ± 300 s−1, at 298 K), and the slowest one, in the presence of Na+ (kout = 1300 ± 100 s−1, at 298 K), with an intermediate kout value in the presence of Rb+ (kout = 2000 ± 150 s−1, at 298 K). The reproducibility of this observation was examined and the difference between the evaluated kout values was found to be statistically significant (Fig. S9†). Note here, that this effect and the obtained dissociation rates were not affected by changes in the pH (Fig. S10†), with similar kout values extracted for the same Na+ containing solution but with a variety of pH values, i.e., pH = 3 (1100 ± 80 s−1), pH = 5 (1300 ± 80 s−1), and pH = 7.2 (1300 ± 100 s−1). This observation indicates that the dissociation process of the guest from the CB7 cavity is governed primarily by the cation content in the system. To validate that the obtained exchange process is indeed between bound (CB7:G3) and free G3 in solution, a guest that strongly binds to CB7 (i.e., 1-aminoadamantane) was used as a competitor (Fig. S11†). The preferable binding of 1-aminoadamantane to CB7 completely eliminates the GEST effect, confirming that the observed exchange dynamics depend on the availability of the CB7 cavity to accommodate G3. After determining various cation effects on the kout rates in the fast exchanging system CB7:G3 (Fig. 4), we turned to study their effect on the Ea,out values.
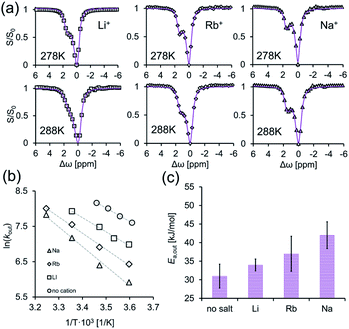
To this end, we performed GEST-NMR experiments at different temperatures (Fig. 5a and S12†) on CB7:G3 in 5 mM phosphate buffer solution to which LiCl (fast exchange, Fig. 4a), RbCl (intermediate exchange, Fig. 4c) or NaCl (slow exchange, Fig. 4b) was added. The obtained Ea,out values (evaluated from the slopes of the linear plots in Fig. 5b) are shown in Fig. 5c. We found that the Ea,out value, which was found to be 32 ± 1 kJ mol−1 in the absence of cations (Fig. 3d), increased in the presence of Li+, Rb+ and Na+ to 34 ± 2, 37 ± 5 and 42 ± 3 kJ mol−1, respectively (Fig. 5c). This observed dependency of the dissociation activation energy for guest exclusion on the cationic content manifests its role in mediating the dissociation process even in systems with fast exchange kinetics. By combining Ea,out values with the enthalpy change of the reaction (extracted from the ITC data for each system, Fig. S13†), the association activation energy (Ea,in) values were accessible. Furthermore, assuming a one-step reaction, we used the correlation of the Eyring equation to calculate the dissociation activation free energy (ΔG#out) as summarized in Table 1.
| CB7:G3 +Li+ | CB7:G3 +Rb+ | CB7:G3 +Na+ | |
|---|---|---|---|
| a Calculated using the Eyring equation. | |||
| k out [s−1] (T = 298 K) | 2800 ± 300 | 2000 ± 150 | 1300 ± 100 |
| E a,out [kJ mol−1] | 34 ± 2 | 37 ± 5 | 42 ± 3 |
| E a,in [kJ mol−1] | 15 ± 2 | 17 ± 5 | 33 ± 3 |
| ΔH#out [kJ mol−1] | 36 ± 2 | 40 ± 5 | 45 ± 3 |
| TΔS#out [kJ mol−1] | −22 ± 1 | −20 ± 5 | −16 ± 4 |
| ΔG#out [kJ mol−1]a | 58 ± 1 | 59 ± 4 | 60 ± 3 |
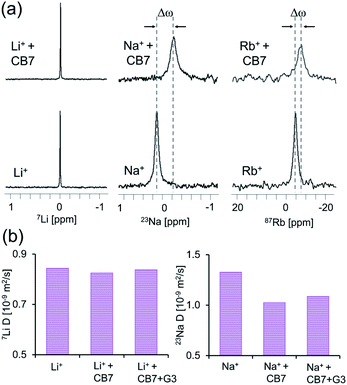
To confirm the formation of supramolecular CB7 “capsules” with M+·CB7:G3·M+ and to assure that M+-CB7 capping indeed occurred and mediated the obtained Ea,out values, 1D-NMR (7Li-NMR, 23Na-NMR and 87Rb-NMR) and 7Li- and 23Na-diffusion NMR experiments were performed.62,63 This entailed the direct measurement of the NMR-characteristics of the cations (Li+, Na+ or Rb+) in aqueous solutions of LiCl, NaCl and RbCl with and without CB7. From the obtained 1D 7Li-NMR, 23Na-NMR and 87Rb-NMR spectra, it is evident that CB7 has no effect on the chemical shift of 7Li+ (Fig. 6a, left), which is in contrast to the pronounced effect on the chemical shift of 23Na+ (Fig. 6a, middle) and 87Rb+ (Fig. 6a, right).
These observations indicate a stronger interaction of CB7 with the Rb+ and Na+ cations as compared to that with the Li+ cations and correlate with previous studies showing that different cations have different affinities to the portals of CBns.47,52,60,61,64–67 Our 7Li- and 23Na-diffusion NMR experiments are in agreement with previous reports68 and further corroborate these observation (Fig. 6b). Notably, we found no significant change in the diffusion coefficient of Li+ upon the addition of CB7, further corroborating that this cation does not bind to the portals of the host (Fig. 6b, left). In contrast, we noted a significant reduction in the diffusion coefficient of Na+ upon the addition of the CB7 host, either with or without G3 (Fig. 6b, right). The shift in the 133Cs-NMR spectrum (Fig. S14a†) and the decrease in the diffusion coefficient of 133Cs+ in the presence of CB7 (Fig. S14b†) were similar to those obtained for Na+. This correlates with the slowest and similar kout values calculated for CB7:G3 in the presence of either Na+ or Cs+ (Fig. 4d). Such a reduction in Na+ (or Cs+, Fig. S14b†) diffusivity confirms that these cations strongly bind to the CB7 portals and serve as an active “lid”, in comparison to the in size smaller Li+ cation. These observed different affinities of various cations to the host portals govern the changes in the transition energy barrier of the host–guest complex and, therefore, mediate guest egression kinetics.61
Conclusions
In summary, GEST-NMR was used to elucidate dissociation activation energies in host–guest assemblies featuring fast exchange dynamics highlighting the role of “supramolecular lids” in mediating guests' dissociation Ea. Our results emphasize GEST's ability to quantify exchange rates that cannot be measured by other approaches used for the study of kinetics in host–guest systems, in general, and in particular in CBn–guest systems.31,53,69,70 Performing GEST-NMR at a range of temperatures and plotting the quantified kout values as a function of the (inverse) experimental temperature allowed the evaluation of different dissociation activation energies with various kinetic profiles. Finally, we demonstrated the role of monovalent cations in mediating kout and the energetic barrier of guest dissociation by their supramolecular capping features for a fast-exchanging system. Thus, GEST can serve as an important analytical tool in designing supramolecular systems where controlling the Ea is crucial, such as switchable molecular host systems.9–11 The fact that GEST can be applied with a conventional NMR setup, which is available at any research institute, offers new opportunities to explore dissociation dynamics and Ea in a variety of supramolecular systems and should provide insights into less-studied mechanisms. The extension of CEST-NMR experiments to 15N- and 13C- and its implementation in other dynamic molecular systems, such as proteins,71,72 emphasizes the potential of the proposed approach to be further developed. Because state-of-the-art experimental methods for determining the rate constants of host–guest complexes were so far limited to comparably slow binding systems (<10 s−1), having now a method at avail that can also be applied to rapidly unbinding guests (>1000 s−1) may open new possibilities for shedding light on fundamental questions in host–guest complexation kinetics. GEST can thus provide an additional insight into binding mechanisms in host–guest systems, as for example in the influence of guest and host desolvation that remains hidden so far. Therefore, we envision that using GEST-NMR for the study of binding kinetics and dissociation Ea will advance the knowledge of supramolecular systems toward better understanding their kinetic properties and allow their further development as functional materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 677715). We thank Dr Moritz Zaiss from the Max Planck Institute for Biological Cybernetics for providing the CEST data-fitting tool and consultations.Notes and references
- R. Wang, Y. Sun, F. Zhang, M. Song, D. Tian and H. Li, Angew. Chem., Int. Ed., 2017, 56, 5294–5298 CrossRef CAS.
- Z. Rodriguez-Docampo, S. I. Pascu, S. Kubik and S. Otto, J. Am. Chem. Soc., 2006, 128, 11206–11210 CrossRef CAS PubMed.
- S. Peng, A. Barba-Bon, Y. C. Pan, W. M. Nau, D. S. Guo and A. Hennig, Angew. Chem., Int. Ed., 2017, 56, 15742–15745 CrossRef CAS PubMed.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS.
- S. R. Shenoy, F. R. Pinacho Crisostomo, T. Iwasawa and J. Rebek Jr, J. Am. Chem. Soc., 2008, 130, 5658–5659 CrossRef CAS PubMed.
- E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195–6214 RSC.
- X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC.
- A. K. Chan, W. H. Lam, Y. Tanaka, K. M. Wong and V. W. Yam, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 690–695 CrossRef CAS PubMed.
- D. H. Qu, Q. C. Wang, Q. W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543–7588 CrossRef CAS PubMed.
- Y. Liu, A. H. Flood and J. F. Stoddart, J. Am. Chem. Soc., 2004, 126, 9150–9151 CrossRef CAS PubMed.
- I. Pochorovski and F. Diederich, Acc. Chem. Res., 2014, 47, 2096–2105 CrossRef CAS PubMed.
- Z. Miskolczy, L. Biczok and I. Jablonkai, Phys. Chem. Chem. Phys., 2016, 19, 766–773 RSC.
- S. Sánchez Carrera, J.-L. Kerdelhué, K. J. Langenwalter, N. Brown and R. Warmuth, Eur. J. Org. Chem., 2005, 2005, 2239–2249 CrossRef.
- T. Brotin and J.-P. Dutasta, Eur. J. Org. Chem., 2003, 2003, 973–984 CrossRef.
- L. Schroder, T. J. Lowery, C. Hilty, D. E. Wemmer and A. Pines, Science, 2006, 314, 446–449 CrossRef PubMed.
- Y. Bai, P. A. Hill and I. J. Dmochowski, Anal. Chem., 2012, 84, 9935–9941 CrossRef CAS.
- M. Kunth, J. Dopfert, C. Witte, F. Rossella and L. Schroder, Angew. Chem., Int. Ed., 2012, 51, 8217–8220 CrossRef CAS.
- M. Kunth, C. Witte, A. Hennig and L. Schroder, Chem. Sci., 2015, 6, 6069–6075 RSC.
- M. Schnurr, J. Sloniec-Myszk, J. Dopfert, L. Schroder and A. Hennig, Angew. Chem., Int. Ed., 2015, 54, 13444–13447 CrossRef CAS PubMed.
- Y. Wang and I. J. Dmochowski, Chem. Commun., 2015, 51, 8982–8985 RSC.
- M. Schnurr, R. Joseph, A. Naugolny-Keisar, D. Kaizerman-Kane, N. Bogdanoff, P. Schuenke, Y. Cohen and L. Schroder, ChemPhysChem, 2019, 20, 246–251 CrossRef CAS.
- K. Du, S. D. Zemerov, S. Hurtado Parra, J. M. Kikkawa and I. J. Dmochowski, Inorg. Chem., 2020, 59, 13831–13844 CrossRef CAS PubMed.
- Y. Wang and I. J. Dmochowski, Acc. Chem. Res., 2016, 49, 2179–2187 CrossRef CAS PubMed.
- A. Bar-Shir, A. A. Gilad, K. W. Chan, G. Liu, P. C. van Zijl, J. W. Bulte and M. T. McMahon, J. Am. Chem. Soc., 2013, 135, 12164–12167 CrossRef CAS PubMed.
- A. Bar-Shir, N. N. Yadav, A. A. Gilad, P. C. van Zijl, M. T. McMahon and J. W. Bulte, J. Am. Chem. Soc., 2015, 137, 78–81 CrossRef CAS.
- L. Avram, V. Havel, R. Shusterman-Krush, M. A. Iron, M. Zaiss, V. Sindelar and A. Bar-Shir, Chem.–Eur. J., 2019, 25, 1687–1690 CrossRef CAS.
- L. Avram, M. A. Iron and A. Bar-Shir, Chem. Sci., 2016, 7, 6905–6909 RSC.
- L. Avram, A. D. Wishard, B. C. Gibb and A. Bar-Shir, Angew. Chem., Int. Ed., 2017, 56, 15314–15318 CrossRef CAS.
- L. Avram and A. Bar-Shir, Org. Chem. Front., 2019, 6, 1503–1512 RSC.
- M. Megyesi, L. Biczók and I. Jablonkai, J. Phys. Chem. C, 2008, 112, 3410–3416 CrossRef CAS.
- H. Tang, D. Fuentealba, Y. H. Ko, N. Selvapalam, K. Kim and C. Bohne, J. Am. Chem. Soc., 2011, 133, 20623–20633 CrossRef CAS PubMed.
- E. A. Appel, F. Biedermann, D. Hoogland, J. Del Barrio, M. D. Driscoll, S. Hay, D. J. Wales and O. A. Scherman, J. Am. Chem. Soc., 2017, 139, 12985–12993 CrossRef CAS PubMed.
- E. Masson, M. Raeisi and K. Kotturi, Isr. J. Chem., 2018, 58, 413–434 CrossRef CAS.
- Z. Miskolczy and L. Biczok, J. Phys. Chem. B, 2014, 118, 2499–2505 CrossRef CAS PubMed.
- C. Bohne, Chem. Soc. Rev., 2014, 43, 4037–4050 RSC.
- M. D. Pluth and K. N. Raymond, Chem. Soc. Rev., 2007, 36, 161–171 RSC.
- C. L. Perrin and T. J. Dwyer, Chem. Rev., 1990, 90, 935–967 CrossRef CAS.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS PubMed.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- D. Shetty, J. K. Khedkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747–8761 RSC.
- K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394–418 RSC.
- D. W. Lee, K. M. Park, M. Banerjee, S. H. Ha, T. Lee, K. Suh, S. Paul, H. Jung, J. Kim, N. Selvapalam, S. H. Ryu and K. Kim, Nat. Chem., 2011, 3, 154–159 CrossRef CAS PubMed.
- Z. Hirani, H. F. Taylor, E. F. Babcock, A. T. Bockus, C. D. Varnado Jr, C. W. Bielawski and A. R. Urbach, J. Am. Chem. Soc., 2018, 140, 12263–12269 CrossRef CAS PubMed.
- S. K. Samanta, J. Quigley, B. Vinciguerra, V. Briken and L. Isaacs, J. Am. Chem. Soc., 2017, 139, 9066–9074 CrossRef CAS PubMed.
- A. I. Lazar, F. Biedermann, K. R. Mustafina, K. I. Assaf, A. Hennig and W. M. Nau, J. Am. Chem. Soc., 2016, 138, 13022–13029 CrossRef CAS PubMed.
- A. Palma, M. Artelsmair, G. Wu, X. Lu, S. J. Barrow, N. Uddin, E. Rosta, E. Masson and O. A. Scherman, Angew. Chem., Int. Ed., 2017, 56, 15688–15692 CrossRef CAS PubMed.
- S. Zhang, L. Grimm, Z. Miskolczy, L. Biczok, F. Biedermann and W. M. Nau, Chem. Commun., 2019, 55, 14131–14134 RSC.
- A. L. Koner, C. Marquez, M. H. Dickman and W. M. Nau, Angew. Chem., Int. Ed., 2011, 50, 545–548 CrossRef CAS PubMed.
- K. A. Kellersberger, J. D. Anderson, S. M. Ward, K. E. Krakowiak and D. V. Dearden, J. Am. Chem. Soc., 2001, 123, 11316–11317 CrossRef CAS PubMed.
- Y. Miyahara, K. Abe and T. Inazu, Angew. Chem., Int. Ed., 2002, 41, 3020–3023 CrossRef CAS PubMed.
- S. He, F. Biedermann, N. Vankova, L. Zhechkov, T. Heine, R. E. Hoffman, A. De Simone, T. T. Duignan and W. M. Nau, Nat. Chem., 2018, 10, 1252–1257 CrossRef CAS PubMed.
- W. Ong and A. E. Kaifer, J. Org. Chem., 2004, 69, 1383–1385 CrossRef CAS PubMed.
- S. S. Thomas, H. Tang and C. Bohne, J. Am. Chem. Soc., 2019, 141, 9645–9654 CrossRef CAS PubMed.
- Z. Miskolczy, M. Megyesi, L. Biczok, A. Prabodh and F. Biedermann, Chem.–Eur. J., 2020, 26, 7433–7441 CrossRef CAS PubMed.
- M. Zaiss, G. Angelovski, E. Demetriou, M. T. McMahon, X. Golay and K. Scheffler, Magn. Reson. Med., 2018, 79, 1708–1721 CrossRef CAS PubMed.
- L. Perez, B. G. Caulkins, M. Mettry, L. J. Mueller and R. J. Hooley, Chem. Sci., 2018, 9, 1836–1845 RSC.
- P. C. van Zijl and N. N. Yadav, Magn. Reson. Med., 2011, 65, 927–948 CrossRef CAS PubMed.
- Z. Miskolczy, M. Megyesi, O. Toke and L. Biczók, Phys. Chem. Chem. Phys., 2019, 21, 4912–4919 RSC.
- A. Prabodh, S. Sinn, L. Grimm, Z. Miskolczy, M. Megyesi, L. Biczok, S. Brase and F. Biedermann, Chem. Commun., 2020, 56, 12327–12330 RSC.
- Y.-M. Jeon, J. Kim, D. Whang and K. Kim, J. Am. Chem. Soc., 1996, 118, 9790–9791 CrossRef CAS.
- C. Marquez, R. R. Hudgins and W. M. Nau, J. Am. Chem. Soc., 2004, 126, 5806–5816 CrossRef CAS PubMed.
- L. Avram and Y. Cohen, Chem. Soc. Rev., 2015, 44, 586–602 RSC.
- Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520–554 CrossRef CAS PubMed.
- M. Morisue, S. Ueda, M. Kurasawa, M. Naito and Y. Kuroda, J. Phys. Chem. A, 2012, 116, 5139–5144 CrossRef CAS.
- H.-J. Buschmann, E. Cleve, L. Mutihac and E. Schollmeyer, J. Inclusion Phenom. Macrocyclic Chem., 2009, 65, 293 CrossRef CAS.
- X. Shi, W. Gu, C. Zhang, L. Zhao, L. Li, W. Peng and Y. Xian, Chem.–Eur. J., 2016, 22, 5643–5648 CrossRef CAS PubMed.
- L. L. Liang, X. L. Ni, Y. Zhao, K. Chen, X. Xiao, Y. Q. Zhang, C. Redshaw, Q. J. Zhu, S. F. Xue and Z. Tao, Inorg. Chem., 2013, 52, 1909–1915 CrossRef CAS PubMed.
- N. J. Wheate, P. G. Kumar, A. M. Torres, J. R. Aldrich-Wright and W. S. Price, J. Phys. Chem. B, 2008, 112, 2311–2314 CrossRef CAS PubMed.
- A. Hennig, H. Bakirci and W. M. Nau, Nat. Methods, 2007, 4, 629–632 CrossRef CAS.
- F. Biedermann and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef CAS.
- G. Bouvignies and L. E. Kay, J. Biomol. NMR, 2012, 53, 303–310 CrossRef CAS PubMed.
- V. P. Tiwari, S. Pandit and P. Vallurupalli, J. Biomol. NMR, 2019, 73, 43–48 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: For experimental details and supporting figures. See DOI: 10.1039/d0sc05666a |
| This journal is © The Royal Society of Chemistry 2021 |