Open Access Article
Open Access ArticleIntermolecular oxidative amination of unactivated alkenes by dual photoredox and copper catalysis†
Xiangli
Yi
and
Xile
Hu
 *
*
Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne 1015, Switzerland. E-mail: xile.hu@epfl.ch
First published on 8th December 2020
Abstract
Oxidative amination of alkenes via amidyl radical addition is potentially an efficient method to generate allylic amines, which are versatile synthetic intermediates to bioactive compounds and organic materials. Here by combining photochemical generation of amidyl radicals with Cu-mediated β-H elimination of alkyl radicals, we have developed an intermolecular oxidative amination of unactivated alkenes. The reaction relies on tandem photoredox and copper catalysis, and works for both terminal and internal alkenes. The radical nature of the reaction and the mild conditions lead to high functional group tolerance.
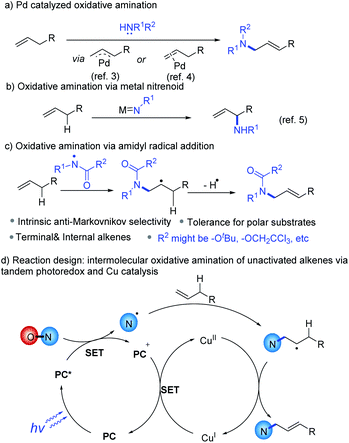
Oxidative amination of alkenes1 is a versatile method to synthesize allylic amines and related nitrogen-containing compounds, which are prevalent in bioactive molecules and organic materials.2 Pd-catalyzed allylic C–H activation is an efficient approach to synthesize allylic amines (Scheme 1a), yet so far only mono-substituted terminal alkenes are suitable substrates.3 Anti-Markovnikov oxidative amination via aminopalladation4 is another potentially versatile method (Scheme 1a), but at this stage chain-walking is a problem for many substrates. Allylic amines can also be prepared by allylic C–H insertion of metal-nitrenoid of alkenes typically with a branch selectivity (Scheme 1b).5 These methods have generally limited tolerance for substrates containing highly polar or nucleophilic moieties. Addition of amidyl radical, typically stabilized by an electron-withdrawing group, to an alkene followed by elimination of β-H provides an alternative strategy to effect the oxidative amination (Scheme 1c). This strategy has intrinsic anti-Markovnikov selectivity, is potentially suitable for internal alkenes and the radical nature of the reaction is compatible with polar substrates, which are important for industrial applications.6
 | ||
| Scheme 1 Different approaches to oxidative amination of alkenes (a–c) and reaction design of this work (d). | ||
Amidyl radical addition to alkenes for intramolecular cyclization7 and the intermolecular addition to activated alkenes8 have been well explored. Unactivated alkenes are more challenging substrates for intermolecular amidyl radical addition because the carbon radical formed upon addition are not stabilized by a heteroatom or aryl group as in the case of activated alkenes. The high reactivity of these radical intermediates makes selective further functionalization harder. Elegant approaches have been developed to trap such C-radicals by a hydrogen donor9 or a SOMOphile10 or a Cu(II)–CF3 species11 for further functionalization. However, oxidative amination of unactivated alkenes via amidyl radical addition is rarely applied for the synthesis of allylic amines. During the preparation of this manuscript, the group of Ritter reported an elegant method for allylic amination through addition of aminium radicals generated from energy transfer between a photocatalyst and iminothianthrenes under illumination to alkenes.12 Radical–radical cross-coupling between the resulting carbon radical and persistent thianthrenium radical followed by elimination, or oxidation of the carbon radical by thianthrenium radical followed by deprotonation furnishes the products. Here we report an alternative approach based on synergetic photoredox and Cu catalysis.
Cu has been reported to mediate β-H elimination of alkyl radicals to produce alkenes.13 Recent reports described facile generation of an amidyl radical via reduction of a hydroxylamine precursor by an excited state of a photoredox catalyst.7f,14 In this context, we envisioned synergetic photoredox and Cu catalysis for oxidative amination of unactivated alkenes (Scheme 1d). The photochemically generated amidyl radical adds to an alkene to give an alkyl radical, which is trapped by a Cu species. The latter undergoes β-H elimination to give the desired allylic amine and a reduced Cu species, which can be oxidized back to the initial Cu catalyst by the oxidized photocatalyst. Here we describe the successful development of such a process.
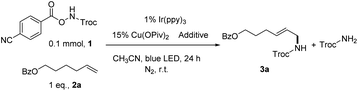
Studer and co-workers showed that oxidation of α-amido-oxy acids by a photoexcited catalyst gave a carboxyl radical, which transformed into an amidyl radical after CO2 and aldehyde/ketone fragmentation.9a,10b,e They also showed that these electrophilic amidyl radicals could add to unactivated alkenes for eventual amidofluorination and carboamination.9a,10b,e Inspired by these studies we explored various N-(benzoyloxy)carbamates as sources of amidyl radicals under photoredox conditions (Scheme S1†). We found that 1, with a Troc-protection (Troc = 2,2,2-trichloroethoxycarbonyl), could react with alkene 2a to afford the allylic amine derivative 3a in 38% yield (Table 1, Entry 1), when 1% of Ir(ppy)3 (ppy = 2-(2-pyridinyl)phenyl) was used as a photocatalyst and 15% of Cu(2-ethylhexanoate)2 was used as a metal catalyst. Troc-NH2 was the main side product and diamination was not observed. The reaction conditions were then optimized (Tables S1–S4, ESI†). A summary is provided in Table 1. The use of Cu(OPiv)2 as metal catalyst improved the yield to 42% (Entry 2), while Cu(OTf)2 was not able to catalyze the reaction (Entry 3). Addition of 1 equiv. of Zn(OPiv)2 (Entry 4) or Mg(OPiv)2 (Entry 5) decreased the yield, yet 1 equiv. of Mg(OPiv)2 and H2O slightly increased the yield to 44% (Entry 6). Notably, the addition of H2O resulted in a precipitate during the reaction, which was analyzed as mainly Mg(4-cyanobenzoate)2·xH2O. The replacement of Ir(ppy)3 by Ir (dfppy)2(ppy) (dfppy = 2-(2-pyridinyl)-3,5-difluorophenyl) further increased the yield to 49% (Entry 7). Increasing the amount of alkene to 2 equiv. and 3 equiv. increased the yield to 59% and 64%, respectively (Entries 8 and 9). A slow generation of the amidyl radical by slow addition of 1 to the reaction mixture improved further the yield of 3a to 77% (70% after isolation, Entry 10).
| Entry | Variations | Additives | Yieldb/% |
|---|---|---|---|
| a Conditions: all reagents of indicated amount dissolved in 1 mL CH3CN in a glass vial (d = 2 cm), blue LED radiation for 24 h. b NMR yield with mesitylene as an internal standard. c Slow addition procedure: 0.02 mmol 1 and all other reagents were dissolved in 0.2 mL CH3CN; the rest of 1 (0.08 mmol) was dissolved in 0.4 mL CH3CN; the latter was added at 1.2 μL min−1 to the former after reaction starts. d Isolated yield. | |||
| 1 | Cu(2-ethyl hexanoate)2 instead of Cu(OPiv)2 | — | 38 |
| 2 | — | — | 42 |
| 3 | Cu(OTf)2 instead of Cu(OPiv)2 | — | 0 |
| 4 | — | 1 eq. Zn(OPiv)2 | 21 |
| 5 | — | 1 eq. Mg(OPiv)2 | 38 |
| 6 | — | 1 eq. Mg(OPiv)2 + 1 eq. H2O | 44 |
| 7 | Ir(dfppy)2(ppy) instead of Ir(ppy)3 | 1 eq. Mg(OPiv)2 + 1 eq. H2O | 49 |
| 8 | Ir(dfppy)2(ppy) instead of Ir(ppy)3, 2 eq. 2a | 1 eq. Mg(OPiv)2 + 1 eq. H2O | 59 |
| 9 | Ir(dfppy)2(ppy) instead of Ir(ppy)3, 3 eq. 2a | 1 eq. Mg(OPiv)2 + 1 eq. H2O | 64 |
| 10c | Ir(dfppy)2(ppy) instead of Ir(ppy)3, 3 eq. 2a, 10% Cu(OPiv)2 | 1 eq. Mg(OPiv)2 + 1 eq. H2O | 77 (70d) |
Based on the optimal conditions, we examined the scope of terminal alkenes (Fig. 1). Remote electrophilic functionalities such as bromide (2b), iodide (2c), and terminal epoxide (2d) were all tolerated to afford the products in good yields (3b–3d, 66–75%). Alcohol (2e) and amide (2f) were also compatible. A range of alkenes containing a terminal carboxylic acid group were tested. 9-Decenoic acid (2g), 5-hexenoic acid (2h) and 4-pentenoic acid (2i), but not 3-butenoic acid, were successfully aminated. The products were isolated after methylation as methyl esters in moderate yields (50–53%). Polar substrates (2j, 2k), derived from L-phenylalanine and L-threonine, respectively, were also viable for this reaction. When a more electron-deficient alkenyl group was present in the internal position (2l, 2m), amination of the terminal alkenyl group was dominant, consistent with electrophilic nature of the amidyl radical.14a
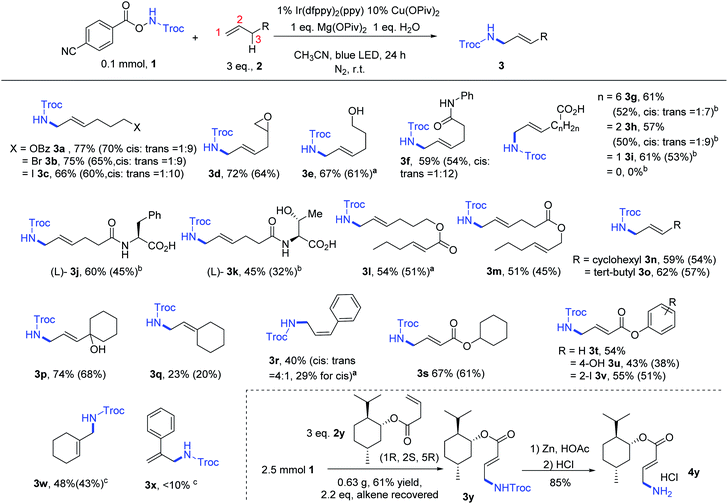 | ||
| Fig. 1 Scope of terminal alkenes for the oxidative amination. Reaction conditions: same as Table 1, Entry 10. NMR yields and isolation yields (in the bracket) are shown. a Reaction conditions: same as Table 1, Entry 9. b Reaction conditions: same as Table 1, Entry 2. Products were isolated after methylation as esters. c Reaction conditions: based on Table 1, Entry 9, acetone as solvent. | ||
Next, alkenes with various substitutions at the C3 position were examined. A bulky substituent, such as cyclohexyl (2n), tert-butyl (2o) and 1-hydroxycyclohexyl (2p), didn't deteriorate the reaction yield. However, double substitutions at C3 decreased the yield (3q, 23%). In the case of a phenyl substitution (2r), 3r was obtained in 40% yield, with the cis-alkene as the major isomer.15 Although 3-butenoic acid was not a suitable substrate, the esters of 3-butenoic acid could be used for the generation of α,β-unsaturated esters (3s–3v). 4-Hydroxyl (2u) and 2-iodo (2v) groups on the phenyl substituent were tolerated against potential oxidation or dehalogenation. The reactions of 1,1-disubstituted alkenes gave lower yields of the target products (48% for 3w, <10% for 3x). The reaction of 2y was successfully scaled up to 2.5 mmol, giving 0.63 g of 3y as product (61% yield). The latter can be easily deprotected to give the primary amine 4y. Notably, the majority of the excess alkene 2y could be recovered (1.23 g, 2.2 equiv.).
The oxidative amination method was then applied on internal alkenes (Fig. 2). The transformation was effective when the alkenes were symmetrically substituted, such as cyclohexene (2z), cyclopentene (2aa), and 4-octene (2ab). The reactions of trans-4-octene and cis-4-octene gave similar yields of 3ab. The reaction of an unsymmetrically substituted alkene, e.g., 2-octene, had a good yield but poor regioselectivity (3ac–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ac–2 = 1.4
3ac–2 = 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When 2-hexen-1-ol was used as the substrate, amination at the 2 position (3ad) was dominant (58% yield) compared to amination at the 3 position (5ad, 16% yield). Analogous allylic alcohols (2ae–2ag) were aminated, with modest to good yields (33% to 61%). The diastereoselectivity was low despite having a bulky substituent adjacent to the alcohol group. The reaction of a trisubstituted alkene 2ah gave 3ah in 51% yield. The allylamine derivative 2ai reacted to give 3ai in 60% yield. Esters (2aj and 2ak) reacted to give both allylic amines (3aj and 3ak) and aziridines (6aj, 6ak) in similar yields.
1). When 2-hexen-1-ol was used as the substrate, amination at the 2 position (3ad) was dominant (58% yield) compared to amination at the 3 position (5ad, 16% yield). Analogous allylic alcohols (2ae–2ag) were aminated, with modest to good yields (33% to 61%). The diastereoselectivity was low despite having a bulky substituent adjacent to the alcohol group. The reaction of a trisubstituted alkene 2ah gave 3ah in 51% yield. The allylamine derivative 2ai reacted to give 3ai in 60% yield. Esters (2aj and 2ak) reacted to give both allylic amines (3aj and 3ak) and aziridines (6aj, 6ak) in similar yields.
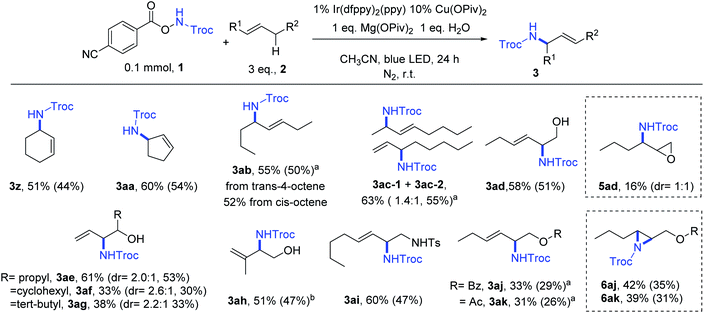 | ||
| Fig. 2 Scope of internal alkenes for oxidative amination. Reaction conditions: same as Table 1, Entry 10. NMR yields and isolation yields (in the bracket) are shown. a Reaction conditions: same as Table 1, Entry 9. b Reaction conditions: based on Table 1, Entry 9, acetone as solvent. | ||
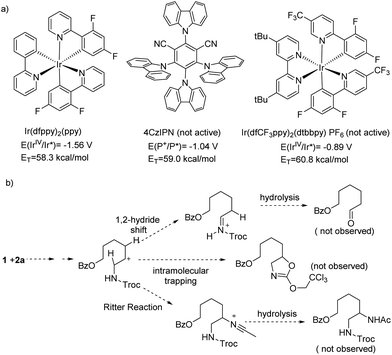
A preliminary mechanistic study was conducted for the reaction. First, the fluorescence quenching of Ir(dfppy)2(ppy) by different reagents was probed (Fig. S1†). Among all reagents used in the reaction, only 1 and Cu(OPiv)2 could significantly quench the excited photocatalyst. In the corresponding Stern–Volmer plots (Fig. S2 and S3†), the slopes, which are proportional to the quenching coefficients, are 9955 M−1 and 2438 M−1 for 1 and Cu(OPiv)2, respectively, suggesting 1 as a more efficient quencher. Additionally, according to cyclic voltammetry (Fig. S5†), the reduction potential of 1 (Ered(1) = −1.43 V vs. SCE) is less negative than the oxidation potential of the excited photocatalyst (E(IrIV/Ir*) = −1.56 V), indicating that oxidative quenching of the excited state of Ir(dfppy)2(ppy) by 1 is thermodynamically downhill. To probe the possibility of energy transfer between 1 and the excited photocatalyst, we tested the oxidative amination using Ir(dfCF3ppy)2(dtbbpy) PF6 (dfCF3ppy = 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl, dtbbpy = 4,4′-di-tert-butyl-bipyridine) or 4CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) as the photocatalyst (Scheme 2a). These complexes have similar triplet energies to Ir(dfppy)2(ppy) but they have a less reducing excited state (−0.89 V (ref. 16) and −1.04 V,17 respectively). No oxidative amination was observed using these two photosensitizers. This result indicates energy transfer is not the likely pathway for the generation of the amidyl radical.
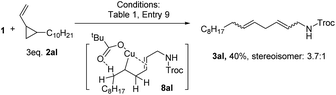
To verify the radical nature of this reaction, 1 equiv. of TEMPO (TEMPO = (2,2,6,6-tetramethylpiperidin-1-yl)oxyl) was added to the reaction mixture of 1 and 2a (eqn (1)). The oxidative amination product 3a was not obtained, but the TEMPO adduct 7a was isolated in 17% yield. Additionally, the reaction of substrate 2al containing a cyclopropyl substituent, which served as a radical clock, yielded product 3al where the cyclopropyl ring was opened (eqn (2)). These results indicate the formation of a carbon radical upon amidyl radical addition. It is conceivable that this carbon radical is further oxidized to a carbon cation en route to alkene. Nevertheless, in the reaction of 1 with 2a, we did not observe products that would arise from 1,2-hydride shift,18 intramolecular nucleophilic trapping, or Ritter reaction of a carbon cation intermediate (Scheme 2b). This result suggests a free carbon cation is an unlikely intermediate.
 | (1) |
 | (2) |
During the investigation of reaction scope, we observed a competition between elimination of β-H and cyclization for substrates with a pendant nucleophilic group (–OH or –NHTs, 2am–2ar; Scheme 3). When there is one carbon between the alkene and nucleophilic groups, only cyclization was observed (5am, 5ap). When there are two carbons, only elimination was observed (3an, 3aq). When there are 3 carbons, cyclization was favored (5ao, 5ar), yet the product of elimination (3ar) was still obtained if the nucleophilic group was –OH. CuII species have been reported to mediate cyclization of alkyl radical with a nucleophile.19 In the present case, we envisage a Cu–alkyl species 8 in which the nucleophilic group coordinates to Cu as the intermediate to cyclization after reductive elimination. The observation of cyclization products supports the formation of a Cu–alkyl intermediate upon trapping of the carbon radical by a Cu species.
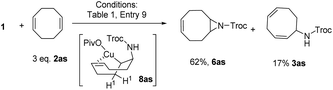
Interestingly, when cyclooctadiene 2as was applied as a substrate (eqn (3)), aziridine 6as was obtained as the main product (62%) instead of 3as (17%). This result might be rationalized by the coordination of Cu in 8as by the alkene moiety. The coordination could shield H1 from accessing the Cu center so that elimination of H1 is suppressed. Consequently C–N reductive elimination to give the aziridine product 6as is favored. This rational might be applicable to explain the generation of 6aj and 6ak as in these cases the ester group of the alkenes could act as a coordinating group. The coordination of Cu by an internal alkene moiety as in intermediate 8al (eqn (2)) might rationalize the generation of a skipped diene 3al instead of a conjugated diene in the reaction of 1 with 2al.
 | (3) |
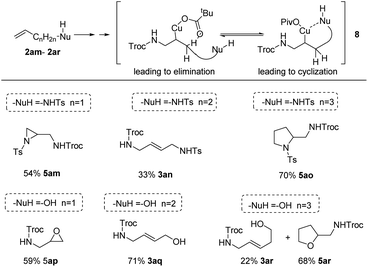 | ||
| Scheme 3 Reactions of substrates with a tethered nucleophilic group: competition between cyclization and elimination. Conditions: same as Table 1, Entry 9. | ||
Taking into account the above results, we proposed a plausible catalytic cycle (Scheme 4). The reaction starts from the oxidative quenching of the excited photocatalyst by 1, which generates the amidyl radical 9 while releasing a carboxylate. The addition of 9 to the alkene 2 leads to the alkyl radical 10, which is then trapped by a CuII–pivolate species to give a formal CuIII–alkyl species 8. The latter undergoes elimination of β-H to form the allylic amine 3 and a CuI species13 which is oxidized back to the starting CuII by the oxidized photocatalyst IrIV. Note that the alkene formation has an exclusive E-selectivity. This selectivity can be attributed to the higher stability of the A conformer over the B conformer of the Cu–alkyl intermediate (Scheme 4, right).13a Based on precedents,13b,13e we propose that the elimination step is assisted by the pivolate ligand. To explain the regioselectivity for H elimination in 8, we propose that the coordination of the Troc group to CuIII prevents the H2 to be accessed by the carboxylate so that elimination of H1 dominates.13b The influence of intramolecular coordination to the reaction selectivity was evidenced by the products of several substrates (e.g., 2al, 2as).
Conclusion
In summary, by integrating photochemical generation of amidyl radicals with Cu-mediated β-H elimination of alkyl radical via a tandem photoredox and copper catalysis, we developed an intermolecular oxidative amination of unactivated alkenes. The method can be used to synthesize a wide range of allylic amines, from readily available alkenes, with high functional group tolerance. The work broadens the scope of oxidative amination of alkenes via amidyl radical addition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the EPFL. We thank Zhikun Zhang and Abdusalom Suleymanov for their help in fluorescence quenching experiments.Notes and references
- (a) R. I. McDonald, G. S. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981–3019 CrossRef CAS; (b) S. R. Chemler, S. D. Karyakarte and Z. M. Khoder, J. Org. Chem., 2017, 82, 11311–11325 CrossRef CAS; (c) J. H. Lee, S. Choi and K. B. Hong, Molecules, 2019, 24, 2634 CrossRef.
- (a) M. Johannsen and K. A. Jorgensen, Chem. Rev., 1998, 98, 1689–1708 CrossRef CAS; (b) Y. Shen, W. J. Liang, Y. N. Shi, E. J. Kennelly and D. K. Zhao, Nat. Prod. Rep., 2020, 37, 763–796 RSC; (c) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS.
- (a) S. A. Reed and M. C. White, J. Am. Chem. Soc., 2008, 130, 3316–3318 CrossRef CAS; (b) G. S. Liu, G. Y. Yin and L. Wu, Angew. Chem., Int. Ed., 2008, 47, 4733–4736 CrossRef CAS; (c) S. A. Reed, A. R. Mazzotti and M. C. White, J. Am. Chem. Soc., 2009, 131, 11701–11706 CrossRef CAS.
- (a) D. G. Kohler, S. N. Gockel, J. L. Kennemur, P. J. Waller and K. L. Hull, Nat. Chem., 2018, 10, 333–340 CrossRef CAS; (b) J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 2868–2869 CrossRef CAS.
- (a) H. H. Lei and T. Rovis, J. Am. Chem. Soc., 2019, 141, 2268–2273 CrossRef CAS; (b) N. S. Dolan, R. J. Scamp, T. Yang, J. F. Berry and J. M. Schomaker, J. Am. Chem. Soc., 2016, 138, 14658–14667 CrossRef CAS; (c) T. Knecht, S. Mondal, J. H. Ye, M. Das and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 7117–7121 CrossRef CAS.
- D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Nat. Chem., 2018, 10, 383–394 CrossRef CAS.
- (a) J. Davies, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2017, 56, 13361–13365 CrossRef CAS; (b) L. ElKaim and C. Meyer, J. Org. Chem., 1996, 61, 1556–1557 CrossRef; (c) G. J. Choi and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 9226–9229 CrossRef CAS; (d) P. Xiong, H. H. Xu and H. C. Xu, J. Am. Chem. Soc., 2017, 139, 2956–2959 CrossRef CAS; (e) X. L. Yi and X. L. Hu, Angew. Chem., Int. Ed., 2019, 58, 4700–4704 CrossRef CAS; (f) L. Angelina, J. Davies, M. Simonetti, L. M. Sanz, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2019, 58, 5003–5007 CrossRef.
- (a) X. D. An, Y. Y. Jiao, H. Zhang, Y. X. Gao and S. Y. Yu, Org. Lett., 2018, 20, 401–404 CrossRef CAS; (b) D. H. Wang, L. Q. Wu, F. Wang, X. L. Wan, P. H. Chen, Z. Y. Lin and G. S. Liu, J. Am. Chem. Soc., 2017, 139, 6811–6814 CrossRef CAS; (c) H. W. Zhang, W. Y. Pu, T. Xiong, Y. Li, X. Zhou, K. Sun, Q. Liu and Q. Zhang, Angew. Chem., Int. Ed., 2013, 52, 2529–2533 CrossRef CAS; (d) K. Miyazawa, T. Koike and M. Akita, Chem.–Eur. J., 2015, 21, 11677–11680 CrossRef CAS.
- (a) J. Guin, R. Frohlich and A. Studer, Angew. Chem., Int. Ed., 2008, 47, 779–782 CrossRef CAS; (b) Q. L. Zhu, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 741–747 CrossRef CAS.
- (a) Y. Zhang, H. D. Liu, L. N. Tang, H. J. Tang, L. Wang, C. Zhu and C. Feng, J. Am. Chem. Soc., 2018, 140, 10695–10699 CrossRef CAS; (b) H. Jiang and A. Studer, Angew. Chem., Int. Ed., 2018, 57, 10707–10711 CrossRef CAS; (c) Z. Liu and Z. Q. Liu, Org. Lett., 2017, 19, 5649–5652 CrossRef CAS; (d) H. Jiang and A. Studer, Chem.–Eur. J., 2019, 25, 516–520 CAS; (e) H. Jiang, G. Seidler and A. Studer, Angew. Chem., Int. Ed., 2019, 58, 16528 CrossRef CAS.
- H. W. Xiao, H. G. Shen, L. Zhu and C. Z. Li, J. Am. Chem. Soc., 2019, 141, 11440–11445 CrossRef CAS.
- Q. Cheng, J. Chen, S. Lin and T. Ritter, J. Am. Chem. Soc., 2020, 142, 17287–17293 CrossRef CAS.
- (a) J. K. Kochi, J. Am. Chem. Soc., 1963, 85, 1958–1968 CrossRef CAS; (b) X. S. Wu, J. Riedel and V. M. Dong, Angew. Chem., Int. Ed., 2017, 56, 11589–11593 CrossRef CAS; (c) A. Tlahuext-Aca, L. Candish, R. A. Garza-Sanchez and F. Glorius, ACS Catal., 2018, 8, 1715–1719 CrossRef CAS; (d) J. K. Kochi, A. Bemis and C. L. Jenkins, J. Am. Chem. Soc., 1968, 90, 4616–4625 CrossRef CAS; (e) J. Xu, Y. Fu, D. F. Luo, Y. Y. Jiang, B. Xiao, Z. J. Liu, T. J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300–15303 CrossRef CAS.
- (a) J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Chem.–Eur. J., 2018, 24, 12154–12163 CrossRef CAS; (b) Q. X. Qin, Y. Y. Han, Y. Y. Jiao, Y. Y. He and S. Y. Yu, Org. Lett., 2017, 19, 2909–2912 CrossRef CAS; (c) L. J. Allen, P. J. Cabrera, M. Lee and M. S. Sanford, J. Am. Chem. Soc., 2014, 136, 5607–5610 CrossRef CAS; (d) T. D. Svejstrup, A. Ruffoni, F. Julia, V. M. Aubert and D. Leonori, Angew. Chem., Int. Ed., 2017, 56, 14948–14952 CrossRef CAS.
- G. L. Dai, S. Z. Lai, Z. Z. Luo and Z. Y. Tang, Org. Lett., 2019, 21, 2269–2272 CrossRef CAS.
- Q. Q. Zhou, Y. Q. Zou, L. Q. Lu and W. J. Xiao, Angew. Chem., Int. Ed., 2019, 58, 1586–1604 CrossRef CAS.
- Y. Sugihara, S. Iimura and J. Nakayama, Chem. Commun., 2002, 134–135 RSC.
- T. Y. Shang, L. H. Lu, Z. Cao, Y. Liu, W. M. He and B. Yu, Chem. Commun., 2019, 55, 5408–5419 RSC.
- (a) J. S. Lin, X. Y. Dong, T. T. Li, N. C. Jiang, B. Tan and X. Y. Liu, J. Am. Chem. Soc., 2016, 138, 9357–9360 CrossRef CAS; (b) Z. J. Liu, X. Lu, G. Wang, L. Li, W. T. Jiang, Y. D. Wang, B. Xiao and Y. Fu, J. Am. Chem. Soc., 2016, 138, 9714–9719 CrossRef CAS; (c) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462–12465 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc05952h |
| This journal is © The Royal Society of Chemistry 2021 |