Open Access Article
Open Access ArticleCatalytic enantioselective synthesis of macrodiolides and their application in chiral recognition†
Wanlong
Xiao
 ,
Yuhao
Mo
,
Yuhao
Mo
 ,
Jing
Guo
,
Jing
Guo
 ,
Zhishan
Su
,
Zhishan
Su
 ,
Shunxi
Dong
,
Shunxi
Dong
 * and
Xiaoming
Feng
* and
Xiaoming
Feng
 *
*
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: dongs@scu.edu.cn; xmfeng@scu.edu.cn
First published on 27th December 2020
Abstract
New types of C2-symmetric chiral macrodiolides are readily obtained via chiral N,N′-dioxide-scandium(III) complex-promoted asymmetric tandem Friedel–Crafts alkylation/intermolecular macrolactonization of ortho-quinone methides with C3-substituted indoles. This protocol provides an array of enantioenriched macrodiolides with 16, 18 or 20-membered rings in moderate to good yields with high diastereoselectivities and excellent enantioselectivities through adjusting the length of the tether at the C3 position of indoles. Density functional theory calculations indicate that the formation of macrocycles is more favorable than that of 9-membered-ring lactones in terms of kinetics and thermodynamics. The potential utility of these intriguing chiral macrodiolide molecules is demonstrated in the enantiomeric recognition of aminols and chemical recognition of metal ions.
Introduction
Macrocycles are one of the most popular structural motifs in many natural products and commercial drugs.1 In particular, macrolactones have received considerable and continuous attention due to their unique structure and remarkable biological activities (Scheme 1a).2 In addition, some recent examples illustrated that macrolactones are good candidates as host molecules in supramolecular chemistry, further highlighting the importance of this type of compound.3 In the above context, the development of feasible and facile routes to macrocycles holds a pivotal position. Therefore, tremendous efforts have been devoted over the past few decades and many kinds of methods have been established including macrolactonization, ring-closing metathesis, intramolecular cross-coupling, the Nozaki–Hiyama–Kishi reaction, the Horner–Wadsworth–Emmons reaction, the click reaction, etc.4 Among them, the most frequently used synthetic strategy toward macrolactones is macrolactonization, which mainly relies on acyl activation with special requirements involving high dilution conditions, structural pre-organization and the use of exotic coupling reagents.5 Nevertheless, the exploration of concise and efficient synthetic methods to construct new kinds of macrolactones not only expands their chemical diversity, but also accelerates the discovery of new functions of such compounds.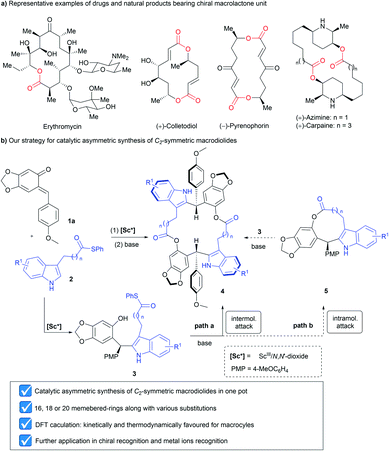 | ||
| Scheme 1 (a) Representative examples of drugs and natural products bearing a chiral macrolactone unit; (b) our strategy for catalytic asymmetric synthesis of C2-symmetric macrodiolides. | ||
Molecular chirality plays a crucial role in chemistry, biology, and drug development since enantiomeric molecules have distinct interactions with another chiral object.6 Accordingly, the chirality of macrolactones is closely associated with their biological activities and enantiomeric recognition properties.7 However, most of the known synthetic strategies towards C2-symmetric chiral macrodiolides are linear multistep synthesis approaches, which need orthogonally protected building blocks and/or previously constructed stereogenic centers.8 Although asymmetric catalysis9 is the most effective and powerful method to construct chiral molecules, this strategy has been seldom used in the synthesis of chiral macrolactones probably due to the lack of reliable methods.10
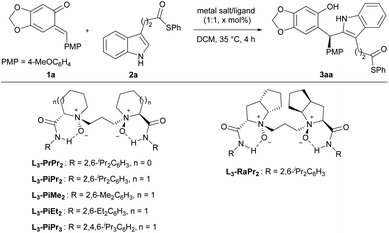
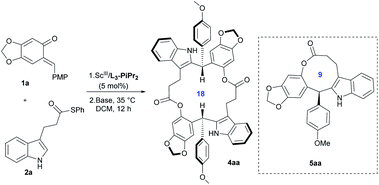
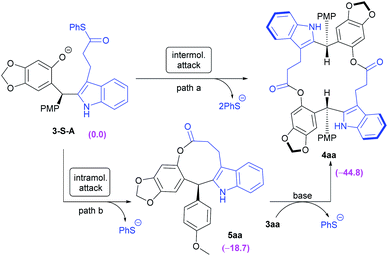
As part of our continuous efforts in expanding the utility of chiral N,N′-dioxide-metal complexes11 and inspired by the versatile roles played by ortho-quinone methides (o-QMs)12 in organic synthesis, we envisioned that rational design of a substrate would provide a potential solution to the above issue. As depicted in Scheme 1b, indoles 2 bearing an alkyl thioester unit at the C3-position were chosen. We envisaged that they can react with ortho-quinone methide 1a to afford chiral diarylindolylmethane intermediate 3via Friedel–Crafts alkylation.13 Then, under basic conditions, the forming phenol group in compound 3 undergoes either intramolecular or intermolecular nucleophilic attack of the alkyl thioester to form 9 or 18-membered-ring lactones 5 or 4. Arising from the large strain14 of the 9-membered ring with the rigid indole skeleton, we assumed that 18-membered-ring lactone 4 was more likely to form in this process.15 Herein, we wish to report our endeavor along this line. The chiral N,N′-dioxide-scandium(III) complex was found to be efficient in promoting the tandem Friedel–Crafts alkylation/intermolecular macrolactonization reaction, providing chiral 16, 18 or 20-membered-ring macrolactones in moderate to good yields with high diastereoselectivities and excellent enantioselectivities. Theoretical calculations disclosed that the formation of macrocycles 4 was kinetically and thermodynamically favored compared to the generation of lactones 5 with the 9-membered ring. The constantly excellent ee values of chiral macrodiolides 4 were rationalized by the formation of their meso counterparts in the macrolactonization step. The application of these optically active macrodiolides was demonstrated in the enantiomeric recognition16 of aminols and chemical recognition of metal ions.17
Results and discussion
Initially, we chose ortho-quinone methide 1a and 3-indolepropanethioate 2a as model substrates to optimize the Friedel–Crafts alkylation reaction conditions (Table 1). The screening of a series of metal salts combined with chiral N,N′-dioxide L3-PiPr2 (Table 1, entries 1–4) indicated that many Lewis acids enabled accelerating the Friedel–Crafts alkylation reaction except Zn(OTf)2 (see Tables S1–S5 in the ESI,† pages 6–8). The use of Al(OTf)3 as the central metal led to a racemic product 3aa in 30% yield (Table 1, entry 2). In comparation, rare-earth metals afforded good enantioselectivities, and Sc(OTf)3 was a good choice, providing the desired product 3aa in 96% yield with 93% ee (Table 1, entry 4). Next, the influence of chiral N,N′-dioxide ligands was studied (Table 1, entries 4–9). Compared with L3-PiPr2 derived from S-pipecolic acid, L-proline-derived L3-PrPr2 and L-ramipril-based L3-RaPr2 produced adduct 3aa in lower enantioselectivities (Table 1, entries 4–6, 73% ee and 84% ee vs. 93% ee). Reducing the steric hindrance at the 2,6-position of aniline in the ligands resulted in an obvious decrease in enantioselectivity (Table 1, entries 7 and 8 vs. entry 4). Installing another isopropyl substitution at the para-position of the phenyl group of the amide moiety had an adverse effect on chiral control (Table 1, entry 9, 86% ee). Both the yield and enantioselectivity were maintained when the reaction was carried out with 5 mol% catalyst loading (Table 1, entry 10). However, upon further decreasing the catalyst loading from 5 mol% to 2.5 mol%, the desired product 3aa was obtained in poor reactivity and enantioselectivity (Table 1, entry 11 vs. entry 10). Consequently, the optimized reaction conditions for the Friedel–Crafts alkylation reaction involved the use of 5 mol% L3-PiPr2/Sc(OTf)3 as the catalyst in DCM at 35 °C for 4 hours. Under such conditions, the desired product 3aa was isolated in 99% yield and 93% ee (Table 1, entry 10).| Entry | Metal salt | Ligand | x (mol%) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
a All reactions were carried out with 1a (0.1 mmol), 2a (0.1 mmol), and metal salt/ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, x mol%) in dichloromethane (DCM, 1.0 mL) at 35 °C for 4 h.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase. n.r. = no reaction. 1, x mol%) in dichloromethane (DCM, 1.0 mL) at 35 °C for 4 h.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase. n.r. = no reaction.
|
|||||
| 1 | Zn(OTf)2 | L3-PiPr2 | 10 | n.r. | — |
| 2 | Al(OTf)3 | L3-PiPr2 | 10 | 30 | 0 |
| 3 | Y(OTf)3 | L3-PiPr2 | 10 | 72 | 71 |
| 4 | Sc(OTf)3 | L3-PiPr2 | 10 | 96 | 93 |
| 5 | Sc(OTf)3 | L3-PrPr2 | 10 | 95 | 73 |
| 6 | Sc(OTf)3 | L3-RaPr2 | 10 | 83 | 84 |
| 7 | Sc(OTf)3 | L3-PiEt2 | 10 | 95 | 90 |
| 8 | Sc(OTf)3 | L3-PiMe2 | 10 | 99 | 83 |
| 9 | Sc(OTf)3 | L3-PiPr3 | 10 | 95 | 86 |
| 10 | Sc(OTf)3 | L3-PiPr2 | 5 | 99 | 93 |
| 11 | Sc(OTf)3 | L3-PiPr2 | 2.5 | 39 | 56 |
After establishing the optimized reaction conditions for the Friedel–Crafts alkylation reaction (Table 1, entry 10), we carried out the study on the lactonization reaction using the same substrates 1a and 2a with a one-pot procedure. It was found that bases showed a crucial influence on the reactivity of the ring-closing reaction (see Tables S6–S9 in the ESI,† pages 8–11). In this ring-closing process, the 18-membered-ring macrodiolide product 4aa was preferably generated instead of the 9-membered ring product 5aa in terms of kinetics and thermodynamics, which was proved by the density functional theory calculations in the following part. 4-Dimethylamino-pyridine (DMAP) promoted the ring-closing process smoothly, delivering 18-membered-ring macrodiolide 4aa as the major product (Table 2, entry 1, 47% yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr and 99% ee). It should be noted that the other diastereomer was a meso compound, which was identified by X-ray crystal structure analysis.18a Comparably, the lactonization reaction didn't occur in the presence of DIPEA (Table 2, entry 2). An inorganic base, for instance Cs2CO3, provided the best results, and the 18-membered-ring product 4aa was isolated in 55% yield with comparable dr and ee values (Table 2, entry 3). The addition of 2 equivalents of Cs2CO3 led to a higher yield (Table 2, entry 4, 67%). No better result was obtained by further increasing the amount of Cs2CO3 (Table 2, entry 5). The subsequent investigation indicated that the use of a mixture of Cs2CO3 (1.5 equiv.) and DIPEA (0.5 equiv.) afforded a slightly higher yield (Table 2, entry 6, 70%). Therefore, the optimal reaction conditions for the overall reaction were established as 5 mol% L3-PiPr2/Sc(OTf)3 as a catalyst in DCM at 35 °C for 4 hours, and then 1.5 equivalents of Cs2CO3 and 0.5 equivalents of DIPEA were added in DCM at 35 °C for 12 hours.
8 dr and 99% ee). It should be noted that the other diastereomer was a meso compound, which was identified by X-ray crystal structure analysis.18a Comparably, the lactonization reaction didn't occur in the presence of DIPEA (Table 2, entry 2). An inorganic base, for instance Cs2CO3, provided the best results, and the 18-membered-ring product 4aa was isolated in 55% yield with comparable dr and ee values (Table 2, entry 3). The addition of 2 equivalents of Cs2CO3 led to a higher yield (Table 2, entry 4, 67%). No better result was obtained by further increasing the amount of Cs2CO3 (Table 2, entry 5). The subsequent investigation indicated that the use of a mixture of Cs2CO3 (1.5 equiv.) and DIPEA (0.5 equiv.) afforded a slightly higher yield (Table 2, entry 6, 70%). Therefore, the optimal reaction conditions for the overall reaction were established as 5 mol% L3-PiPr2/Sc(OTf)3 as a catalyst in DCM at 35 °C for 4 hours, and then 1.5 equivalents of Cs2CO3 and 0.5 equivalents of DIPEA were added in DCM at 35 °C for 12 hours.
| Entry | Base | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|
a All reactions were carried out with 1a (0.1 mmol), 2a (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mol%) in DCM (1.0 mL) at 35 °C for 4 h, and then base was added, and the resulting reaction mixture was stirred at 35 °C for 12 h.
b Yield of isolated product 4aa.
c Determined by 1H NMR analysis.
d Determined by UPC2 analysis on a chiral stationary phase. DMAP = 4-dimethylaminopyridine, DIPEA = N,N-diisopropylethylamine. 1, 5 mol%) in DCM (1.0 mL) at 35 °C for 4 h, and then base was added, and the resulting reaction mixture was stirred at 35 °C for 12 h.
b Yield of isolated product 4aa.
c Determined by 1H NMR analysis.
d Determined by UPC2 analysis on a chiral stationary phase. DMAP = 4-dimethylaminopyridine, DIPEA = N,N-diisopropylethylamine.
|
||||
| 1 | DMAP (1.0 equiv.) | 47 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 2 | DIPEA (1.0 equiv.) | Trace | — | — |
| 3 | Cs2CO3 (1.0 equiv.) | 55 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 4 | Cs2CO3 (2.0 equiv.) | 67 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 5 | Cs2CO3 (3.0 equiv.) | 58 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 6 | Cs2CO3 (1.5 equiv.) | 70 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| DIPEA (0.5 equiv.) | ||||
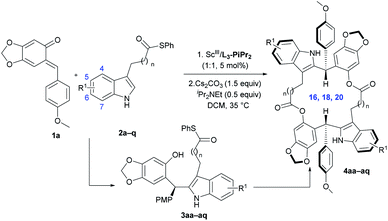
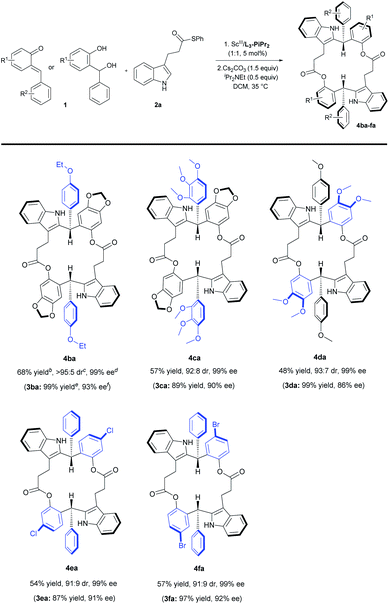
With the optimized reaction conditions (Table 2, entry 6) in hand, the substrate scope of indoles was explored (Table 3). A series of indoles 2 with different substituents could react with ortho-quinone methide 1a smoothly, regardless of the electronic nature and the position of substituents at the aromatic ring (Table 3, entries 1–17). In detail, both electron-withdrawing and electron-donating substituents on the 4, 5 or 6-positions of indoles could give the corresponding 18-membered-ring products 4ab–4ao in moderate to good yields (47–75%), high diastereoselectivities (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 94
9 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr) and excellent enantioselectivity (in all cases, 99% ee). We performed two parallel reactions for each substrate, and thereby Friedel–Crafts alkylation products 3 were isolated and characterized as well. The configuration of chiral product 3as (see Table S10, entry 19 in the ESI,† page 13) was determined to be (S) by X-ray crystal structure analysis.18b Notably it's interesting to find that regardless of the enantioselectivities (90–97% ee) of the intermediates 3, the enantiomeric excess of 18-membered-ring products 4 was always 99% ee. Generally, indoles with electron-donating substituents provided slightly higher yields than those with electron-withdrawing substituents (Table 3, entry 4 vs. entries 2 and 3; entries 8 and 9 vs. entries 5–7). In contrast, the yield of 6-methyl substituted indole 2m was lower (Table 3, entry 13 vs. entries 10–12). Besides, moderate yields with excellent diastereoselectivities and enantioselectivities were obtained for 7-chlorine and methyl substituted indoles (Table 3, entries 14 and 15). To our delight, upon increasing or decreasing the carbon chain length in indole substrates 2p and 2q, the reactions were equally good, generating the corresponding 20-membered-ring product 4ap and 16-membered-ring product 4aq in decent yields with high diastereo- and enantioselectivities (Table 3, entries 16 and 17). The absolute configuration of product 4ab was confirmed to be (S,S) by X-ray diffraction analysis18c and the configurations of all other examples were assigned by comparing their CD spectra with that of 4ab. Subsequently, the substrate scope of ortho-quinone methides was tested (Table 4). Representative ortho-quinone methides 1 were subjected to the standard conditions, and the reaction occurred smoothly to afford the corresponding 18-membered-ring products (4ba–4da) in good yields (48–68%), high diastereoselectivities (92
6 dr) and excellent enantioselectivity (in all cases, 99% ee). We performed two parallel reactions for each substrate, and thereby Friedel–Crafts alkylation products 3 were isolated and characterized as well. The configuration of chiral product 3as (see Table S10, entry 19 in the ESI,† page 13) was determined to be (S) by X-ray crystal structure analysis.18b Notably it's interesting to find that regardless of the enantioselectivities (90–97% ee) of the intermediates 3, the enantiomeric excess of 18-membered-ring products 4 was always 99% ee. Generally, indoles with electron-donating substituents provided slightly higher yields than those with electron-withdrawing substituents (Table 3, entry 4 vs. entries 2 and 3; entries 8 and 9 vs. entries 5–7). In contrast, the yield of 6-methyl substituted indole 2m was lower (Table 3, entry 13 vs. entries 10–12). Besides, moderate yields with excellent diastereoselectivities and enantioselectivities were obtained for 7-chlorine and methyl substituted indoles (Table 3, entries 14 and 15). To our delight, upon increasing or decreasing the carbon chain length in indole substrates 2p and 2q, the reactions were equally good, generating the corresponding 20-membered-ring product 4ap and 16-membered-ring product 4aq in decent yields with high diastereo- and enantioselectivities (Table 3, entries 16 and 17). The absolute configuration of product 4ab was confirmed to be (S,S) by X-ray diffraction analysis18c and the configurations of all other examples were assigned by comparing their CD spectra with that of 4ab. Subsequently, the substrate scope of ortho-quinone methides was tested (Table 4). Representative ortho-quinone methides 1 were subjected to the standard conditions, and the reaction occurred smoothly to afford the corresponding 18-membered-ring products (4ba–4da) in good yields (48–68%), high diastereoselectivities (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to >95
8 to >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and excellent enantioselectivity (99% ee). When ortho-hydroxybenzyl alcohols were employed as the precursor of o-QMs, they could transform into the o-QMs in situ and react with indole 2a efficiently, yielding the desired macrodiolides 4ea–4fa in medium yields (54% and 57%) with a high dr value (91
5 dr) and excellent enantioselectivity (99% ee). When ortho-hydroxybenzyl alcohols were employed as the precursor of o-QMs, they could transform into the o-QMs in situ and react with indole 2a efficiently, yielding the desired macrodiolides 4ea–4fa in medium yields (54% and 57%) with a high dr value (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and excellent ee value (99% ee).
9) and excellent ee value (99% ee).
| Entry | R1, n | 3 | 4 | |||
|---|---|---|---|---|---|---|
| Yieldb (%) | eec (%) | Yieldd (%) | dre | eef (%) | ||
a Reaction conditions for products 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (0.1 mmol), 2 (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1 1a (0.1 mmol), 2 (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mol%) in DCM (1.0 mL) at 35 °C. Reaction conditions for products 4 1, 5 mol%) in DCM (1.0 mL) at 35 °C. Reaction conditions for products 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (0.1 mmol), 2 (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1 1a (0.1 mmol), 2 (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mol%) in DCM (1.0 mL) at 35 °C, and then Cs2CO3 (1.5 equiv.) and iPr2NEt (0.5 equiv.) were added, and the resulting reaction mixture was stirred at 35 °C.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase.
d Yield of the isolated product over two steps.
e Determined by 1H NMR analysis.
f Determined by UPC2 analysis on a chiral stationary phase. 1, 5 mol%) in DCM (1.0 mL) at 35 °C, and then Cs2CO3 (1.5 equiv.) and iPr2NEt (0.5 equiv.) were added, and the resulting reaction mixture was stirred at 35 °C.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase.
d Yield of the isolated product over two steps.
e Determined by 1H NMR analysis.
f Determined by UPC2 analysis on a chiral stationary phase.
|
||||||
| 1 | H, n = 1 | 99 (3aa) | 93 | 70 (4aa) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 2 | 4-Cl, n = 1 | 95 (3ab) | 93 | 63 (4ab) | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
99 |
| 3 | 4-F, n = 1 | 91 (3ac) | 91 | 58 (4ac) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 4 | 4-Me, n = 1 | 99 (3ad) | 93 | 63 (4ad) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 5 | 5-Br, n = 1 | 99 (3ae) | 91 | 48 (4ae) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
99 |
| 6 | 5-Cl, n = 1 | 97 (3af) | 92 | 47 (4af) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
99 |
| 7 | 5-F, n = 1 | 90 (3ag) | 91 | 57 (4ag) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 8 | 5-Me, n = 1 | 99 (3ah) | 93 | 60 (4ah) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
99 |
| 9 | 5-OMe, n = 1 | 93 (3ai) | 92 | 69 (4ai) | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 10 | 6-Br, n = 1 | 96 (3aj) | 91 | 75 (4aj) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 11 | 6-Cl, n = 1 | 99 (3ak) | 94 | 66 (4ak) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 12 | 6-F, n = 1 | 98 (3al) | 94 | 60 (4al) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 13 | 6-Me, n = 1 | 95 (3am) | 92 | 59 (4am) | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 14 | 7-Cl, n = 1 | 97 (3an) | 92 | 70 (4an) | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
99 |
| 15 | 7-Me, n = 1 | 97 (3ao) | 90 | 50 (4ao) | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
99 |
| 16 | H, n = 2 | 97 (3ap) | 94 | 45 (4ap) | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
99 |
| 17 | H, n = 0 | 99 (3aq) | 97 | 58 (4aq) | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
99 |
a Reaction conditions of products 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.1 mmol), 2a (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1 1 (0.1 mmol), 2a (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mol%) in DCM (1.0 mL) at 35 °C. Reaction conditions of products 4 1, 5 mol%) in DCM (1.0 mL) at 35 °C. Reaction conditions of products 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.1 mmol), 2a (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1 1 (0.1 mmol), 2a (0.1 mmol), and Sc(OTf)3/L3-PiPr2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mol%) in DCM (1.0 mL) at 35 °C, and then Cs2CO3 (1.5 equiv.) and iPr2NEt (0.5 equiv.) were added, and the resulting reaction mixture was stirred at 35 °C.
b Yield of the isolated product over two steps.
c Determined by 1H NMR analysis.
d Determined by UPC2 analysis on a chiral stationary phase.
e Yield of the isolated product.
f Determined by HPLC analysis on a chiral stationary phase. 1, 5 mol%) in DCM (1.0 mL) at 35 °C, and then Cs2CO3 (1.5 equiv.) and iPr2NEt (0.5 equiv.) were added, and the resulting reaction mixture was stirred at 35 °C.
b Yield of the isolated product over two steps.
c Determined by 1H NMR analysis.
d Determined by UPC2 analysis on a chiral stationary phase.
e Yield of the isolated product.
f Determined by HPLC analysis on a chiral stationary phase.
|
|---|
|
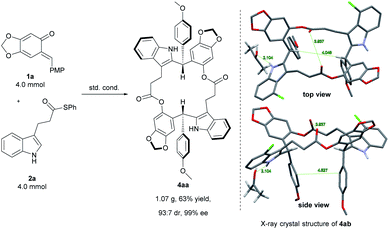
In order to evaluate the synthetic utility of this catalytic system, a gram-scale synthesis of 4aa was carried out (Scheme 2, left). Under the optimized reaction conditions, ortho-quinone methide 1a (4.0 mmol, 1.03 g) reacted smoothly with 3-indolepropanethioate 2a (4.0 mmol, 1.13 g), affording the desired product 4aa in 63% yield (1.07 g) with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr and 99% ee.
7 dr and 99% ee.
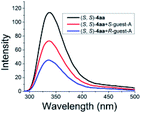
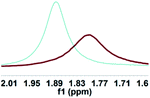
As illustrated in Scheme 2, right, the chiral macrodiolide product (S,S)-4ab possesses a C2-symmetric structure, wherein two carbonyl oxygens orient up with a distance of 3.94 Å, and two PMP groups point down. The distance between the two hydrogens located at stereogenic centers is approximately 4.05 Å. In view of such a chiral macrocyclic structure bearing hydrogen-bond donor and acceptor sites, we subsequently investigated its potential application in chiral recognition. Firstly, the chiral discrimination abilities of chiral macrodiolide 4aa (99% ee) were evaluated with enantiomers of chiral amines, alcohols, amino alcohols, alcohol esters, etc. Because the strength of interaction between enantiomers of chiral objects and chiral macrocycles is different, the chiral recognition effect is significantly different (see Tables S13 and S14 in the ESI,† pages 98–114). The preliminary results indicated that chiral macrodiolide 4aa showed enantiodifferentiation of the enantiomers of amines and amino alcohols (Table 5, entries 1–4, left). The best result was obtained with 36 mM chiral macrodiolide 4aa and 72 mM 2-aminobutanol in deuterated chloroform, and the corresponding Δδ value was 0.087 ppm (Table 5, entry 4). The subsequently enantioselective fluorescence recognition studies suggested that chiral macrodiolide 4aa could serve as a chiral receptor to discriminate the enantiomers of 2-aminobutanol, and the addition of chiral macrodiolide 4aa into the solution of (S)-2-aminobutanol in CH3CN led to a slight fluorescence quenching. In sharp contrast, an obvious fluorescence quenching was observed in the case of (R)-2-aminobutanol. The enantiomeric fluorescence difference ratio, ef = (I0 − IR)/(I0 − IS), was as high as 5.46. Comparably, the acyclic intermediate 3aa showed much less discriminating ability (1.45 ef value) under the same conditions (see Table S14 in the ESI,† pages 113 and 114), indicating the beneficial effect of a relatively more rigid macrocyclic structure. For the fluorescence quenching chiral recognition probe, many fluorescence quenching processes followed the Stern–Volmer equation: I0/I = 1 + KSV[Q]. Here I and I0 are the fluorescent intensity of the fluorophore (S,S)-4aa with and without the quencher, [Q] is the concentration of the quencher, and KSV is the Stern–Volmer constant.19 For guest-B (1,2-diphenyl-1,2-ethanediamine), the Stern–Volmer constant of (S,S)-4aa was 326.53 M−1 [KSV(S − S)] in the presence of S-guest-B and 275.09 M−1 [KSV(S − R)] in the presence of R-guest-B. The ratio of the Stern–Volmer constant [KSV(S − S)]/[KSV(S − R)] was 1.19 (see Table S15 in the ESI,† page 125). Thus, S-guest-B quenched the fluorescence of (S,S)-4aa more efficiently than R-guest-B. In addition, considering the potential recognition ability of macrocycles towards metal ions, we investigated the recognition effect of macrodiolide (S,S)-4aa in acetonitrile by UV-vis absorption spectroscopy and fluorescence spectrum analysis (see Fig. S1–S4 in the ESI,† pages 129 and 130). The addition of Fe(OTf)2 to the solution of (S,S)-4aa lowered the fluorescence intensity of (S,S)-4aa (Fig. 1). Furthermore, the addition of Fe(OTf)3 or Cu(OTf)2 to the solution of (S,S)-4aa led to a redshift and enhancement of fluorescence. This process was accompanied by color changes visible to the naked eye (see Fig. S5–S7 in the ESI,† page 131). The experimental results showed that macrodiolide product (S,S)-4aa could selectively recognize Fe2+, Fe3+, Cu2+.
| Entry | Guest | Chiral recognition by 1H NMR analysisa | Chiral recognition by fluorescence analysisd | ||
|---|---|---|---|---|---|
| Spectrumb (1H NMR) | Δδc (ppm) | Spectrum (fluorescence)e | eff | ||
| a All samples were prepared by mixing (S,S)-4aa and guests A–D in NMR tubes [36 mM (S,S)-4aa and 72 mM guests A–D in 0.7 mL of CDCl3]. 1H NMR data were collected on an NMR spectrometer (400 MHz) at 25 °C. b The N–H signals of S-guests A–D with (S,S)-4aa (blue) vs. R-guests A–D with (S,S)-4aa (red). c Δδ of the N–H group of guests A–D. d Fluorescence responses of (S,S)-4aa (0.5 mM) toward guests A–D (1 mM) in CH3CN (λex = 265 nm, slits: 2.5/20 nm). e Fluorescence spectra of (S,S)-4aa (black) vs. (S,S)-4aa with S-guests-A–D (red) vs. (S,S)-4aa with R-guests-A–D (blue). f The enantiomeric fluorescence difference ratio, ef = (I0 − IR)/(I0 − IS). (λem = 335.2 nm). g The configuration of S-guest-B is (1S,2S) and the configuration of R-guest-B is (1R,2R). | |||||
| 1 |
|
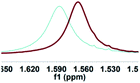
|
0.021 |
|
1.68 (0.60) |
| 2g |
|
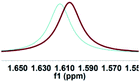
|
0.008 |
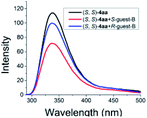
|
3.02 (0.33) |
| 3 |
|
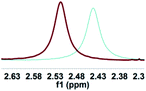
|
0.076 |
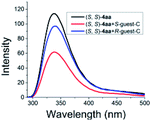
|
2.92 (0.34) |
| 4 |
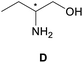
|
|
0.087 |
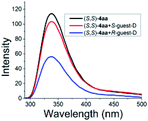
|
5.46 (0.18) |
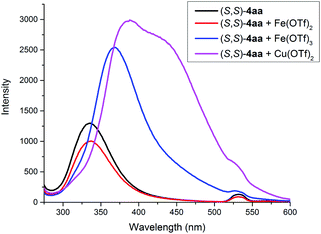 | ||
| Fig. 1 Fluorescence spectra of (S,S)-4aa at 5 × 10−5 M with or without a metal precursor (5 × 10−5 M) in CH3CN. (λex = 265 nm, slits: 2.5/20 nm). | ||
To gain insight into the formation of the eighteen-membered-ring product (S,S)-4aa, DFT calculation was performed at the B3LYP-D3(BJ)/6-31G(d,p) (SMD, CH2Cl2) theoretical level. Two pathways (Scheme 3, path a and b) were investigated, wherein the intramolecular or intermolecular cyclization reactions were involved, respectively. As shown in Scheme 3, (S)-3aa underwent a deprotonation process with a basic reagent (e.g. Cs2CO3), producing the corresponding anion 3-S-A. The eighteen-membered-ring product (S,S)-4aa could be generated through intermolecular attack of the phenoxy anion at the C atom of the carbonyl group in another molecule (path a). In addition, if an intramolecular cyclization reaction occurs, the nine-membered-ring intermediate (S)-5aa was afforded with the release of a SPh anion (path b). Molecular modeling indicated that the nine-membered-ring (S)-5aa adopted a boat-like conformation (see Fig. S10 in the ESI,† page 133), in which two aromatic rings were connected by the planar ester group as well as a –(CH2)2– chain. This less stable rigid structure rendered (S)-5aa prone to undergoing a ring-opening reaction with another (S)-3aa to yield the final eighteen-membered-ring product (S,S)-4aa. Compared to (S)-5aa with a nine-membered ring (ΔG = −18.7 kcal mol−1), (S,S)-4aa has a lower relative Gibbs free energy of formation (ΔG = −44.8 kcal mol−1), suggesting that the eighteen-membered-ring product (S,S)-4aa was more stable thermodynamically (see Fig. S9 and S10 in the ESI,† pages 132 and 133).
In terms of the activation energy barrier, the activation energy barrier of the initial intermolecular attack in path a was 7.0 kcal mol−1 lower than that of the ring-closure reaction for nine-membered-ring product (S)-5aa in path b (2.4 vs. 9.4 kcal mol−1). These results indicated that it was more possible for (S)-3aa to undergo intermolecular reaction via path a, producing the eighteen-membered-ring product (S,S)-4aa. However, path b cannot be ruled out.
Moreover, to gain insights into the product distribution, the formation of mesoisomer (R,S)-4aavia path a was studied at the same theoretical level. The calculation results showed that the formation of mesoisomer (R,S)-4aa in the second C–O bond construction step was slightly favored over that of chiral (S,S)-4aa in terms of kinetics (the activation energy barrier, 6.8 kcal mol−1vs. 13.9 kcal mol−1) and thermodynamics (relative Gibbs free energy of formation ΔG: −48.4 kcal mol−1vs. −44.8 kcal mol−1), which provide a rational explanation for the excellent ee value (99% ee) of eighteen-membered-ring products 4 (see Fig. S11–S13 in the ESI,† page 134).
Conclusions
In summary, we have developed a concise and facile route to C2-symmetric chiral macrodiolides through chiral N,N′-dioxide-scandium(III) complex-promoted asymmetric tandem Friedel–Crafts alkylation/intermolecular macrolactonization of ortho-quinone methides with C3-substituted indoles. An array of enantioenriched macrodiolides with 16, 18 or 20-membered rings was readily afforded in moderate to good yields with high diastereoselectivities and excellent enantioselectivities by adjusting the length of the tether at the C3 position in indoles. DFT calculation was performed to elucidate the formation process of chiral macrodiolide products and the observed excellent enantiomeric excess. The potential application of this type of chiral macrodiolide molecule in enantiomeric recognition of aminols was confirmed by NMR study and enantioselective fluorescence recognition experiments. And their chemical recognition ability of metal ions was also studied through UV-vis absorption spectroscopy and fluorescence spectrum analysis. Further bioactivity testing of macrocyclic products and the exploration of other reactions with the chiral N,N′-dioxide-metal complex are being carried out in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the National Natural Science Foundation of China (No. 21890723 and 21801175) for financial support.Notes and references
- For selected reviews: (a) L. A. Wessjohann, E. Ruijter, D. Garcia-Rivera and W. Brandt, Mol. Diversity, 2005, 9, 171–186 CrossRef CAS; (b) E. Marsault and M. L. Peterson, J. Med. Chem., 2011, 54, 1961–2004 CrossRef CAS; (c) J. Mallinson and I. Collins, Future Med. Chem., 2012, 4, 1409–1438 CrossRef CAS.
- For a leading review and a selected example: (a) L. Katz and G. W. Ashley, Chem. Rev., 2005, 105, 499–527 CrossRef CAS; (b) I. B. Seiple, Z. Y. Zhang, P. Jakubec, A. Langlois-Mercier, P. M. Wright, D. T. Hog, K. Yabu, S. R. Allu, T. Fukuzaki, P. N. Carlsen, Y. Kitamura, X. Zhou, M. L. Condakes, F. T. Szczypiński, W. D. Green and A. G. Myers, Nature, 2016, 533, 338–345 CrossRef CAS.
- For selected recent examples: (a) J. D. Wu, J. R. Lu, J. G. Liu, C. H. Zheng, Y. X. Gao, J. Hu and Y. Ju, Sens. Actuators, B, 2017, 241, 931–937 CrossRef CAS; (b) Z. Zhang, C. Q. Deng, Y. Zou and L. Chen, J. Photochem. Photobiol., A, 2018, 356, 7–17 CrossRef CAS; (c) H. X. Chai, L.-P. Yang, H. Ke, X.-Y. Pang and W. Jiang, Chem. Commun., 2018, 54, 7677–7680 RSC.
- For selected reviews: (a) C. M. Madsen and M. H. Clausen, Eur. J. Org. Chem., 2011, 2011, 3107–3115 CrossRef; (b) V. Martí-Centelles, M. D. Pandey, M. I. Burguete and S. V. Luis, Chem. Rev., 2015, 115, 8736–8834 CrossRef; (c) K. T. Mortensen, T. J. Osberger, T. A. King, H. F. Sore and D. R. Spring, Chem. Rev., 2019, 119, 10288–10317 CrossRef CAS; (d) S. Sengupta and G. Mehta, Org. Biomol. Chem., 2020, 18, 1851–1876 RSC.
- For selected reviews: (a) K. C. Nicolaou, Tetrahedron, 1977, 33, 683–710 CrossRef CAS; (b) A. Parenty, X. Moreau, G. Niel and J.-M. Campagne, Chem. Rev., 2013, 113, PR1–PR40 CrossRef CAS; for selected recent examples: (c) M. de Léséleuc and S. K. Collins, ACS Catal., 2015, 5, 1462–1467 CrossRef; (d) B. Jiang, M. Zhao, S.-S. Li, Y.-H. Xu and T.-P. Loh, Angew. Chem., Int. Ed., 2018, 57, 555–559 CrossRef CAS; (e) C. S. Higman, D. L. Nascimento, B. J. Ireland, S. Audörsch, G. A. Bailey, R. McDonald and D. E. Fogg, J. Am. Chem. Soc., 2018, 140, 1604–1607 CrossRef CAS; (f) Q. Zeng, K. Y. Dong, C. Pei, S. L. Dong, W. H. Hu, L. H. Qiu and X. F. Xu, ACS Catal., 2019, 9, 10773–10779 CrossRef CAS; (g) M. Yang, X. W. Wang and J. F. Zhao, ACS Catal., 2020, 10, 5230–5235 CrossRef CAS.
- For selected books: (a) A. N. Collins, G. N. Sheldrake and J. Crosby, Chirality in Industry: The Commercial Manufacture and Applications of Optically Active Compounds, John Wiley & Sons Ltd., Chichester, 1992 Search PubMed; (b) A. N. Collins, G. N. Sheldrake and J. Crosby, Chirality in Industry II: Developments in the Commercial Manufacture and Applications of Optically Active Compounds, John Wiley & Sons Ltd., Chichester, 1997 Search PubMed; (c) K. Mislow, in Topics in Stereochemistry, ed. S. E. Denmark, John Wiley & Sons Inc., New York, 1999, vol. 22, pp. 1–82 Search PubMed.
- For selected reviews: (a) S. D. Appavoo, S. Huh, D. B. Diaz and A. K. Yudin, Chem. Rev., 2019, 119, 9724–9752 CrossRef CAS; (b) K. Zheng and R. Hong, Nat. Prod. Rep., 2019, 36, 1546–1575 RSC; for selected examples: (c) M. Z. Gao, J. Gao, Z. L. Xu and R. A. Zingaro, Tetrahedron Lett., 2002, 43, 5001–5003 CrossRef CAS; (d) S. Şeker, D. Barış, N. Arslan, Y. Turgut, N. Pirinççioğlu and M. Toğrul, Tetrahedron: Asymmetry, 2014, 25, 411–417 CrossRef.
- E. J. Kang and E. Lee, Chem. Rev., 2005, 105, 4348–4378 CrossRef CAS.
- For selected books: (a) New Frontiers in Asymmetric Catalysis, ed. K. Mikami and M. Lautens, John Wiley & Sons Inc., New York, 2007 Search PubMed; (b) Catalytic Asymmetric Synthesis, ed. I. Ojima, John Wiley & Sons Inc., New York, 2010 Search PubMed.
- For a selected recent review and examples: (a) Y. Li, X. L. Yin and M. J. Dai, Nat. Prod. Rep., 2017, 34, 1185–1192 RSC; (b) A. M. Haydl and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 15530–15534 CrossRef CAS; (c) S. Ganss and B. Breit, Angew. Chem., Int. Ed., 2016, 55, 9738–9742 CrossRef CAS; (d) P. Steib and B. Breit, Angew. Chem., Int. Ed., 2018, 57, 6572–6576 CrossRef CAS; (e) C. Gagnon, É. Godin, C. Minozzi, J. Sosoe, C. Pochet and S. K. Collins, Science, 2020, 367, 917–921 CrossRef CAS.
- For reviews on chiral N,N′-dioxides: (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574–587 CrossRef CAS; (b) X. H. Liu, L. L. Lin and X. M. Feng, Org. Chem. Front., 2014, 1, 298–302 RSC; (c) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621–2631 CrossRef CAS; (d) X. H. Liu, S. X. Dong, L. L. Lin and X. M. Feng, Chin. J. Chem., 2018, 36, 791–797 CrossRef CAS; (e) Z. Wang, X. H. Liu and X. M. Feng, Aldrichimica Acta, 2020, 53, 3–10 Search PubMed.
- For selected reviews of o-QMs: (a) W.-J. Bai, J. G. David, Z.-G. Feng, M. G. Weaver, K.-L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655–3664 CrossRef CAS; (b) M. S. Singh, A. Nagaraju, N. Anand and S. Chowdhury, RSC Adv., 2014, 4, 55924–55959 RSC; (c) B. C. Yang and S. H. Gao, Chem. Soc. Rev., 2018, 47, 7926–7953 RSC.
- For selected reviews of Friedel–Crafts alkylation of indoles: (a) M. Bandini, A. Melloni, S. Tommasi and A. Umani-Ronchi, Synlett, 2005, 8, 1199–1222 CrossRef; (b) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742–778 RSC; (c) I. P. Beletskaya and A. D. Averin, Curr. Organocatal., 2016, 3, 60–83 CrossRef CAS; (d) J.-B. Chen and Y.-X. Jia, Org. Biomol. Chem., 2017, 15, 3550–3567 RSC.
- For a selected review and book on medium-sized rings: (a) I. Shiina, Chem. Rev., 2007, 107, 239–273 CrossRef CAS; (b) T. Janecki, in Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity, Wiley-VCH, Weinheim, 2013, pp. 193–227 CrossRef; for selected examples on medium-sized rings: (c) Z. J. Wu and J. Wang, ACS Catal., 2017, 7, 7647–7652 CrossRef CAS; (d) Y. Wei, S. Liu, M.-M. Li, Y. Li, Y. Lan, L.-Q. Lu and W.-J. Xiao, J. Am. Chem. Soc., 2019, 141, 133–137 CrossRef CAS.
- For a selected review on the formation of macrocycles instead of medium rings, see: (a) J. S. Bradshaw, G. E. Maas, R. M. Izatt and J. J. Christensen, Chem. Rev., 1979, 79, 37–52 CrossRef CAS, for selected examples of the formation of macrocycles instead of medium rings, see: (b) B. M. Trost and A. Quintard, Angew. Chem., Int. Ed., 2012, 51, 6704–6708 CrossRef CAS; (c) A. W. H. Speed, T. J. Mann, R. V. O'Brien, R. R. Schrock and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 16136–16139 CrossRef CAS; (d) Y. J. Zhou, P. Prediger, L. C. Dias, A. C. Murphy and P. F. Leadlay, Angew. Chem., Int. Ed., 2015, 54, 5232–5235 CrossRef CAS; (e) W. Fu, L. H. Wang, Z. Yang, J.-S. Shen, F. Tang, J. Y. Zhang and X. L. Cui, Chem. Commun., 2020, 56, 960–963 RSC.
- For selected reviews and examples of chiral discrimination: (a) X. X. Zhang, J. S. Bradshaw and R. M. Izatt, Chem. Rev., 1997, 97, 3313–3361 CrossRef CAS; (b) L. Pu, Chem. Rev., 2004, 104, 1687–1716 CrossRef CAS; (c) G. Uccello-Barretta, F. Balzano and P. Salvadori, Curr. Pharm. Des., 2006, 12, 4023–4045 CrossRef CAS; (d) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591–10596 CrossRef CAS; (e) L.-L. Wang, Z. Chen, W.-E. Liu, H. Ke, S.-H. Wang and W. Jiang, J. Am. Chem. Soc., 2017, 139, 8436–8439 CrossRef CAS; (f) H. X. Chai, Z. Chen, S.-H. Wang, M. Quan, L.-P. Yang, H. Ke and W. Jiang, CCS Chem., 2020, 2, 440–452 CrossRef.
- For selected reviews: (a) R. P. Peng, Y. L. Xu and Q. Y. Cao, Chin. Chem. Lett., 2018, 29, 1465–1474 CrossRef CAS; (b) Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim and J. L. Sessler, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS.
- (a) CCDC 2016020 (4aa); ; (b) CCDC 2016694 (3as); ; (c) CCDC 2016695 (4ab) contains the ESI.†.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum, New York, 2nd edn, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C{1H} and 19F{1H} NMR, HPLC spectra, and CD spectra (PDF). X-ray crystallographic data for 4aa, 3as and 4ab (CIF). CCDC 2016020, 2016694 and 2016695. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc06162j |
| This journal is © The Royal Society of Chemistry 2021 |