Open Access Article
Open Access ArticleA generator of peroxynitrite activatable with red light†
Cristina
Parisi‡
 a,
Mariacristina
Failla‡
a,
Mariacristina
Failla‡
 b,
Aurore
Fraix‡
b,
Aurore
Fraix‡
 a,
Luca
Menilli
a,
Luca
Menilli
 c,
Francesca
Moret
c,
Francesca
Moret
 c,
Elena
Reddi
c,
Elena
Reddi
 c,
Barbara
Rolando
b,
Francesca
Spyrakis
b,
Loretta
Lazzarato
c,
Barbara
Rolando
b,
Francesca
Spyrakis
b,
Loretta
Lazzarato
 *b,
Roberta
Fruttero
b,
Alberto
Gasco
b and
Salvatore
Sortino
*b,
Roberta
Fruttero
b,
Alberto
Gasco
b and
Salvatore
Sortino
 *a
*a
aPhotoChemLab, Department of Drug and Health Sciences, University of Catania, I-95125, Italy. E-mail: ssortino@unict.it
bDepartment of Science and Drug Technology, University of Torino, I-10125 Torino, Italy. E-mail: Loretta.lazzarato@unito.it
cDepartment of Biology, University of Padova, I-35131 Padova, Italy
First published on 4th March 2021
Abstract
The generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) as “unconventional” therapeutics with precise spatiotemporal control by using light stimuli may open entirely new horizons for innovative therapeutic modalities. Among ROS and RNS, peroxynitrite (ONOO−) plays a dominant role in chemistry and biology in view of its potent oxidizing power and cytotoxic action. We have designed and synthesized a molecular hybrid based on benzophenothiazine as a red light-harvesting antenna joined to an N-nitroso appendage through a flexible spacer. Single photon red light excitation of this molecular construct triggers the release of nitric oxide (˙NO) and simultaneously produces superoxide anions (O2˙−). The diffusion-controlled reaction between these two radical species generates ONOO−, as confirmed by the use of fluorescein-boronate as a highly selective chemical probe. Besides, the red fluorescence of the hybrid allows its tracking in different types of cancer cells where it is well-tolerated in the dark but induces remarkable cell mortality under irradiation with red light in a very low concentration range, with very low light doses (ca. 1 J cm−2). This ONOO− generator activatable by highly biocompatible and tissue penetrating single photon red light can open up intriguing prospects in biomedical research, where precise and spatiotemporally controlled concentrations of ONOO− are required.
Introduction
Peroxynitrite (ONOO−) is a reactive nitrogen species (RNS) with a half-life of 1.9 s at pH 7.4, arising from the diffusion-controlled reaction between nitric oxide (˙NO) and the superoxide anion (O2˙−) (k ∼ 1010 M−1 s−1).1 ONOO− is simultaneously a potent oxidant and a nucleophile and these features determine its fate in a biological environment.2 In particular, ONOO− is in equilibrium with its conjugate acid, ONOOH (pKa = 6.8),3 which may either isomerize (∼70%) or undergo homolytic cleavage to produce ˙OH and ˙NO2 radicals (∼30%)4 which are, in turn, mainly responsible for its oxidizing and nitrating activity.5 In view of this double ability to act as a nucleophile and generator of very reactive secondary free radical species, ONOO− is labelled as a strong cytotoxic agent.6 Analogously to singlet oxygen (1O2), the ROS playing a dominant role in photodynamic therapy,7 ONOO− is a multitarget cytotoxic agent able to react with lipids, DNA, and proteins as demonstrated by its short biological half-life (<20 ms).2,4,5 However, in contrast to 1O2, ONOO− offers the additional advantage of radical-mediated nitration of the protein tyrosines,5,8 a process at the basis of the inhibition of the cell efflux pumps mainly responsible for multidrug resistance (MDR).9 As a consequence, ONOO− can be exploited not only as a cytotoxic agent but also as a powerful tool to reduce the cell extrusion of conventional chemotherapeutics, and thus enhancing their anticancer activity.10This scenario makes molecules able to generate ONOO− potential anticancer prodrugs. However, due to the lack of selectivity of ONOO− for bio-substrates, precise spatiotemporal control in the ONOO− delivery is highly desirable in view of practical applications. Light represents a powerful remote activation tool in this regard, offering high potential for innovative “unconventional” therapeutic approaches, which can open entirely new horizons in cancer treatment.11 Photons are in fact ideal external stimuli for the rapid introduction of cytotoxic agents at the target site starting from appropriate photoprecursors, mimicking an “optical syringe” with superb control of sites, time and dosage of the released species, all these factors being determining for a positive therapeutic outcome. Moreover, light-triggering provides the unique advantage of not altering the physiological values of temperature, pH and ionic strength, which is fundamental for biomedical applications.11
The first examples of ONOO− generators exclusively controlled by light stimuli were reported by Nakagawa and Miyata almost a decade ago.12 However, they require poorly biocompatible and scarcely tissue penetrating UVA light for their activation. Therefore, the development of ONOO− photoprecursors operating with more biocompatible and highly tissue penetrating visible or near infrared (NIR) light is very challenging in view of bio-applications. Very recently, Wang et al. reported a molecular construct able to generate ONOO−, as a result of tandem cascade reactions, and to emit green fluorescence upon excitation with visible violet light.13 The large two-photon absorption cross section of this compound allows also its activation through two-photon excitation in the NIR region.13
In this paper we report the design, synthesis, photochemical characterization and biological evaluation of a ONOO− generator, the molecular hybrid BPT-NO (Scheme 1), activatable with single photon red light excitation and which also offers the additional advantage of red fluorescence emission, a useful tool for its cellular tracking. It consists of benzophenothiazine as a suitable light-harvesting antenna, joined to an N-nitroso appendage through a flexible spacer. Excitation of this molecular construct with 670 nm light leads to the generation of ONOO− and produces the analogue non-nitrosate derivative BPT as the main stable photoproduct, through the mechanism proposed in Scheme 1 and discussed later.
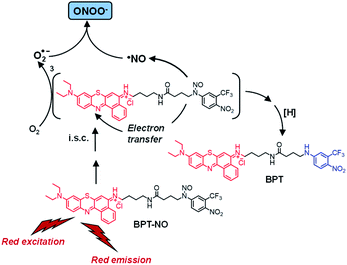 | ||
| Scheme 1 Proposed molecular mechanism leading to the generation of ONOO− and the main stable photoproduct BPT upon red light excitation of the molecular hybrid BPT-NO. | ||
Results and discussion
Rational design and syntheses
The design and fabrication of photoactivatable ONOO− generators is not an easy task, as proven by the scarcity of ONOO− photoprecursors developed to date.12,13 In fact, since ONOO− is the result of a very fast reaction between ˙NO and O2˙−, these two species need to be simultaneously generated under light input from suitable chromogenic units. The hybrid BPT-NO was inspired by the merging of two different findings: (i) our recently developed ˙NO photodonors based on green-light antennae covalently linked to the same N-nitroso unit of BPT-NO;14 (ii) the recent studies by Peng et al. demonstrating that the same phenothiazine unit of BPT-NO is a suitable photogenerator of O2˙− under red light excitation.15 In particular, ˙NO release from our ˙NO photodonors proceeds through a mechanism involving an intramolecular photoinduced electron transfer from the nitroso appendage to the excited state (i.e. singlet or triplet) of the light-harvesting antennae;14 on the other hand, the phenothiazine-based system of Peng et al. generates O2˙−via an intermolecular photoinduced electron transfer involving the lowest triplet state of the red-absorbing chromophore and the molecular oxygen.15 On the basis of these considerations we thought that the hybrid BPT-NO may, in principle, exploit the red excitation energy to trigger the ˙NO release from the N-nitroso unit through a mechanism similar to that reported for other molecular hybrids13 and, at the same time, produce O2˙− analogously to what was already reported for the benzophenothiazine derivatives.15BPT-NO and its analogue non-nitrosate derivative BPT, as a suitable model compound, were synthesized according to the procedures reported in the ESI.† They involve at first the synthesis of the N-nitroso and the related non-nitrosate appendage followed by their condensation with the phenothiazine scaffold through an amide linkage.
Spectroscopic and photochemical properties
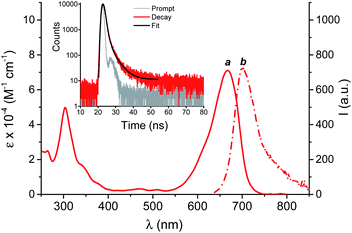
Fig. 1 shows the UV-Vis spectroscopic features of BPT-NO in phosphate buffer (PB)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), where the molecular hybrid dissolves quite well. The absorption spectrum is dominated by the benzophenothiazine chromophore, with an intense band with a maximum at 670 nm and extending into the whole red region.15,16 The fluorescence emission spectrum shows the typical band arising from the benzophenothiazine fluorogenic center, with a maximum at 700 nm and extending up to the near infrared region.15,16 The fluorescence quantum yield is Φf = 0.024 and the related fluorescence decay (see the inset of Fig. 1) exhibits a dominant component (ca. 92%) with a lifetime τf = 0.90 ns.
1 v/v), where the molecular hybrid dissolves quite well. The absorption spectrum is dominated by the benzophenothiazine chromophore, with an intense band with a maximum at 670 nm and extending into the whole red region.15,16 The fluorescence emission spectrum shows the typical band arising from the benzophenothiazine fluorogenic center, with a maximum at 700 nm and extending up to the near infrared region.15,16 The fluorescence quantum yield is Φf = 0.024 and the related fluorescence decay (see the inset of Fig. 1) exhibits a dominant component (ca. 92%) with a lifetime τf = 0.90 ns.
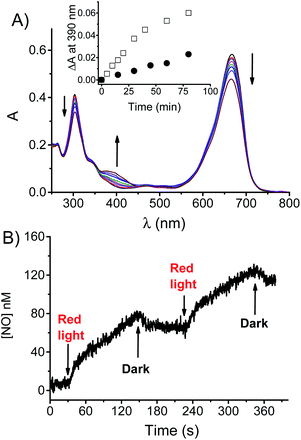
Irradiation of a N2-saturated solution of BPT-NO with red light leads to the absorption spectral changes illustrated in Fig. 2A. They show bleaching of the main absorption bands in the visible and UV region, and the formation of a new absorption band at 390 nm accompanied by the presence of isosbestic points at 583 and 324 nm, which are indicative of the occurrence of a quite clean photochemical reaction. Such a spectral evolution is in excellent agreement with the formation of the non-nitrosate compound BPT characterized by a lower molar absorptivity in the Vis and UV regions and by the characteristic band at 390 nm arising from the push–pull character of the nitroaniline moiety17 (Fig. S1†). This band is suppressed in BPT-NO because of the presence of the electron withdrawing nitroso group attached to the amino functionality. HPLC analysis carried out with the authentic model compound BPT as a reference confirmed it to be the only stable photoproduct (Fig. S2†). The absorption profile observed in air-equilibrated solution was identical in nature but slower when compared to that observed under anaerobic conditions (see the inset of Fig. 2A). This finding provides an unambiguous indication that the photoreactivity of BPT-NO is more likely mediated by a long-lived excited triplet state rather than the singlet one. This proposal accords well with the values of Φf and τf found for the non-nitrosate compound BPT (Fig. S3†) which were identical to those of BPT-NO. This rules out any other deactivation process from the excited singlet state of BPT-NO competitive with fluorescence and intersystem crossing (i.s.c.) to the triplet state. Furthermore, the short τf, makes any diffusional quenching of the excited singlet state by molecular oxygen non-competitive with the singlet decay.
Formation of BPT as the only stable photoproduct clearly indicates the occurrence of ˙NO photorelease from BPT-NO upon red light excitation. This was demonstrated by the direct detection of this radical through an amperometric technique using an ultrasensitive ˙NO electrode. Fig. 2B shows that ˙NO release by BPT-NO takes place exclusively under stimuli of red light, stops in the dark, and restarts as the red light is turned on again. Since the benzophenothiazine chromophore is the sole antenna absorbing the red excitation light, loss of ˙NO from the N-nitroso moiety must necessarily involve an electronic communication between these two components. Population of the N-nitroso appendage by photoinduced energy transfer from the excited benzophenothiazine is, of course, not possible because it is highly endergonic.18 Therefore, uncaging of ˙NO from BPT-NO seems to be triggered by a photoinduced electron transfer from the N-nitrosoaniline-derivative moiety, as an electron donor, to the excited triplet state of benzophenothiazine, as an electron acceptor, similarly to what was recently observed for a doxorubicin molecular hybrid.14a According to literature, oxidation of the N-nitroso functionality strongly encourages the ˙NO detachment.14,19 Our proposal is also supported by the negative free energy value for the photoinduced triplet electron transfer processes, ΔG ∼ 0.2 eV, estimated using the Rehm–Weller equation:20
| ΔG = e[Eox − Ered] − ET |
The proposed photoinduced intramolecular electron transfer is strongly facilitated by the proximity of the electron donor and electron acceptor units due to the U-shaped conformation adopted by BPT-NO, as confirmed by molecular dynamics simulations (Fig. S4†).
Taken together, all these results account well for the ˙NO release mechanism proposed in Scheme 1. ˙NO release after triplet mediated electron transfer involves, of course, the generation of an anilino radical derivative after a back electron transfer. This species is strongly stabilized by the presence of the two electron drawing nitro and trifluoromethyl groups. Besides, it is expected to be basically insensitive to the presence of oxygen and to evolve to the stable photoproduct BPT, after i.s.c. and H-transfer from the solvent, the latter typically observed for the other N-nitrosamine after loss of ˙NO.14,24
Quenching of the lowest triplet state by molecular oxygen usually occurs through type I or type II mechanisms.7,25 The former leads to O2˙− through intermolecular electron transfer and the latter generates 1O2 through a collisional energy transfer process.7,25 As outlined above, the benzophenothiazine unit integrated in the structure of BPT-NO was appropriately chosen due to its capability to generate O2˙− under red light excitation.15 In this case, if O2˙− is generated simultaneously to ˙NO, it is expected to react with the latter at a diffusion-controlled rate to produce ONOO−.1
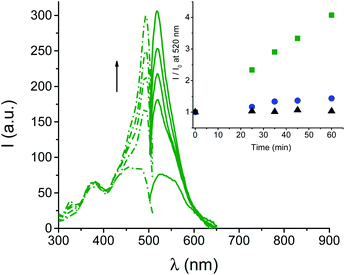
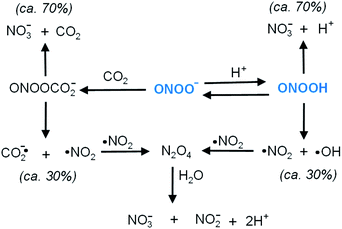
Generation of ONOO− was demonstrated by experiments carried out in the presence of fluorescein-boronate (Fl-B), a very selective and sensitive probe for ONOO− recently developed by Radi et al.26 Fl-B is weakly fluorescent but it is rapidly (k ∼ 106 M−1 s−1) and effectively (yield = 99%) oxidized by ONOO−, liberating the highly fluorescent fluorescein.25Fig. 3 shows the appearance of the typical fluorescence excitation and emission spectra of fluorescein with λmax = 490 nm and 520 nm, respectively, upon irradiation of an air-equilibrated solution of BPT-NO. In contrast, negligible fluorescence emission was observed when BPT-NO was irradiated under N2-saturated conditions where the O2˙− required for the ONOO− is, of course, not generated (inset of Fig. 3).§ Besides, very low emission increase was observed in the case of the model compound BPT as a suitable control (see the inset of Fig. 3), according to the absence of ˙NO photorelease occurring in this compound. This low, but no negligible emission increase in the case of this compound might arise from the very slow oxidation (k ∼ 1–2 M−1 s−1) of Fl-B by the H2O2 (ref. 25) derived by the slow dismutation of O2˙−, which in BPT is expected to be produced analogously to BPT-NO, but not consumed in the reaction with ˙NO. This hypothesis was confirmed by using the dihydrorhodamine 123 probe which is non-fluorescent but becomes strongly green fluorescent after reaction with O2˙− (Fig. S5†).15a Quantitative comparison between the amount of the photoproduct BPT and the fluorescein formed in the early stage of the photoreaction was then performed by HPLC which revealed the % (mol mol−1) for these compounds to be 9.8 ± 1.3 and 7.4 ± 1.1, respectively. Since ONOO− quantitatively oxidizes Fl-B to fluorescein, this finding suggests that NO is produced only slightly in excess with respect to O2˙− of ca. 1.3 fold (9.8/7.4).
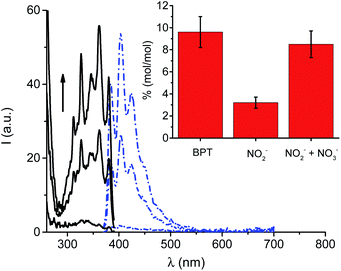
Additional confirmation for the formation of ONOO− was provided by analysis of the nitrite (NO2−) formed after irradiation and its quantitative comparison with the only stable photoproduct BPT. In fact, in the absence of other reactive species, ˙NO is almost quantitatively oxidized to NO2− but not to NO3− by molecular oxygen.27 Therefore, in such a case NO2− and BPT are expected to be found in an equimolar ratio. NO2− was detected by using both the very sensitive spectrofluorimetric and spectrophotometric assays based on 2,3-diaminonaphthalene (DAN)28 and Griess29 reactants, respectively. Fig. 4 shows the characteristic well-structured fluorescence emission and excitation spectra of the highly fluorescent 2,3-diaminonaphthotriazole (DANT) formed after reaction of DAN with NO2−, only in the irradiated sample and with intensity strictly dependent on the irradiation time.
Quantitative comparison between NO2− and BPT was then carried out in the early stage of the photoreaction by the Griess assay. The inset of Fig. 4 clearly shows that the amount of NO2− was much lower than that of BPT, with the related % (mol mol−1) being 3.2 ± 0.5 and 9.6 ± 1.4, respectively. This result in good agreement with the generation of O2˙−, which rapidly converts the ˙NO photogenerated into ONOO− before it is slowly oxidized to NO2− by molecular oxygen. This view was well supported by the NO2− analysis performed after treating the irradiated mixture of BPT-NO with VCl3. In fact, ONOO− leads to a large amount of nitrate (NO3−) according to the well-known decomposition pathways of ONOO− and its conjugate acid ONOOH recalled in Scheme 2, for the sake of clarity. The inset of Fig. 4 shows that the total NO2− detected after treatment of the irradiated solution with VCl3, which is able to convert NO3− into NO2−,30 remarkably increased reaching a value very close to that of the photoproduct BPT.
A specific point that needs to be clarified is related to the photodegradation mechanism proposed in Scheme 1. The type I reaction involved in the generation of O2˙− involves, of course, the formation of a benzophenothiazine-centered radical cation, which is expected to evolve to products besides BPT. Therefore, at first sight, the observation of BPT as the sole stable product of photolysis can seem quite surprising. A possible hypothesis to explain this finding might consist of a typical deprotonation of the benzophenothiazine-centered radical cation, leading to neutral radical species which would restore the starting benzophenothiazine chromophore by H-transfer from the solvent.
Cell internalization and toxicity
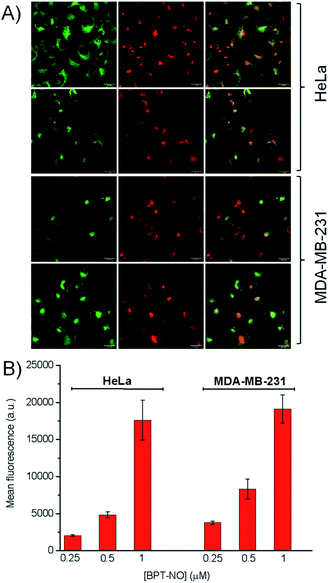
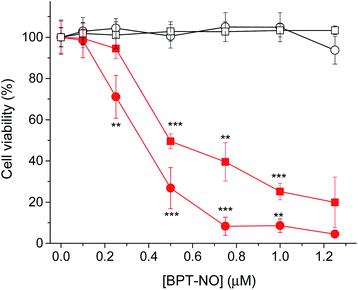
The biological activity of BPT-NO was evaluated with two different cancer cell lines, HeLa (derived from a human cervix carcinoma) and MDA-MB-231 (derived from a human breast carcinoma). The intracellular localization of internalized BPT-NO was determined by confocal fluorescence microscopy after 2 h incubation of cell monolayers. Fifteen minutes before completing the incubation, the cells were stained with either BODIPY® FL C5-ceramide or ER-Tracker™ Green used as markers for Golgi apparatus and endoplasmic reticulum, respectively. Fluorescence images of the cells were then acquired using 633 nm and 488 nm lasers as excitation sources for BPT-NO and organelle markers, respectively. The overlapping of green (organelle markers) and red fluorescence (BPT-NO) (Fig. 5A) shows clear-cut evidence for the localization of the molecular hybrid in the endoplasmic reticulum and Golgi apparatus of HeLa and MDA-MB-231 cells. In contrast, poor localization in lysosomes and mitochondria was observed (Fig. S6†). Flow cytometry analysis showed that the intracellular uptake was very efficient and strictly dependent on the concentration of BPT-NO (Fig. 5B).To assess the efficacy of the molecular hybrid as a phototherapeutic agent, the cancer cells were incubated for 2 h with increasing concentrations of BPT-NO, and either kept in the dark or irradiated with very low doses of red light (1 J cm−2). Fig. 6 shows that no relevant toxicity was observed in the dark in a concentration range below 2 μM. In contrast, in the same concentration range a remarkable decrease of cell viability was observed, with both cell lines upon irradiation, with the loss of cell viability being strictly dependent on the concentration of BPT-NO. A remarkable point which deserves to be stressed is that the strong phototoxic effects are induced by using very low concentrations of the molecular hybrid combined with very low doses of red light irradiation. This is in excellent agreement with the high toxicity of the ONOO− and its secondary free radical species, as also confirmed by the lower phototoxic effects displayed by the model compound BPT (Fig. S7†).
Conclusions
In summary, we have developed a novel molecular hybrid which represents a rare example of an ONOO− generator exclusively controlled by visible light. The present compound offers remarkable advantages compared with the few existing ONOO− photodonors such as (i) ONOO− generation triggered by highly biocompatible and tissue penetrating single photon red light, corresponding to an improvement of ca. 250 nm in terms of excitation wavelength; (ii) very high extinction coefficient in the red region, allowing the absorption of red photons even in a very low concentration range; (iii) satisfactory red fluorescence, useful for cellular tracking. Besides, the only generated stable photoproduct does not suffer from the disadvantage of undergoing undesired reactions with ONOO−. Finally, it is very important to underline that ˙NO and O2˙−, responsible for the formation of ONOO−, are generated in comparable amounts, with the former being in slight excess with respect to the latter. It is well known that in biological systems, while oxidative pathways involving a direct reaction with ONOO− are not altered, oxidative processes initiated by the ˙NO2/CO3˙−/˙OH secondary free radicals, formed from the small fraction of spontaneous decomposition of ONOO− (see Scheme 2), are inhibited by an excess production of either ˙NO or O2˙−.31 On the basis of these results we expect that BPT-NO can open up intriguing prospects in chemical and biomedical research for studies where precise and spatiotemporally concentrations of ONOO− are required.Author contributions
M. F. and C. P. carried out the synthesis, C. P. and A. F. performed the spectroscopic and photochemical experiments, B. R. performed the HPLC analysis, F. S. performed the molecular dynamics calculations, L. M. performed the fluorescence microscopy analysis, F. M. and E. R. performed the cell toxicity experiments, L. L. supervised the synthesis and contributed to the discussion and editing, R. F. contributed to the discussion and editing, A. G. conceived the synthesis and contributed to the discussion, S. S. devised the work, coordinated the experiments and wrote the paper.Conflicts of interest
We have no conflict of interest to declare.Acknowledgements
We thank AIRC – Italian Association for Cancer Research (IG-19859) for financial support.Notes and references
- (a) N. V. Blough and O. C. Zafiriou, Inorg. Chem., 1985, 24, 3502–3504 CrossRef; (b) R. Kissner, T. Nauser, P. Bugnon, P. G. Lye and W. H. Koppenol, Chem. Res. Toxicol., 1997, 10, 1285–1292 Search PubMed; (c) S. Goldstein and G. Czapski, Free Radical Biol. Med., 1995, 19, 505–510 CrossRef.
- R. Radi, G. Peluffo, M. N. Alvarez, M. Naviliat and A. Cayota, Free Radical Biol. Med., 2001, 30, 463–488 CrossRef.
- G. Ferrer-Sueta and R. Radi, ACS Chem. Biol., 2009, 4, 161–177 CrossRef.
- (a) R. M. Uppu and W. A. Pryor, J. Am. Chem. Soc., 1999, 121, 9738–9739 CrossRef; (b) G. Merényi, J. Lind, S. Goldstein and G. Czapski, Chem. Res. Toxicol., 1998, 11, 712–713 Search PubMed.
- (a) G. Ferrer-Sueta, N. Campolo, M. Trujillo, S. Bartesaghi, S. Carballal, N. Romero, B. Alvarez and R. Radi, Chem. Rev., 2018, 118, 1338–1408 CrossRef; (b) S. Bartesaghia and R. Radi, Redox Biol., 2018, 14, 618–625 CrossRef PubMed; (c) R. Radi, J. Biol. Chem., 2013, 288, 26464–26472 CrossRef PubMed.
- (a) C. Szabó, H. Ischiropoulos and R. Radi, Nat. Rev. Drug Discovery, 2007, 6, 662–679 CrossRef PubMed; (b) J. S. Beckman and W. H. Koppenol, Am. J. Physiol., 1996, 271, 1424–1437 CrossRef PubMed; (c) N. Hogg, V. M. Darley-Usmar, M. T. Wilson and S. Moncada, Biochem. J., 1992, 291, 419–424 CrossRef PubMed.
- (a) A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef PubMed; (b) J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 12, 2795–2838 CrossRef.
- (a) H. Gunaydin and K. N. Houk, Chem. Res. Toxicol., 2009, 22, 894–898 Search PubMed; (b) R. Radi, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 4003–4008 CrossRef.
- (a) M. M. Gottesman, Annu. Rev. Med., 2002, 53, 615–627 CrossRef PubMed; (b) N. A. Colabufo, F. Berardi, M. Contino, M. Niso and R. Perrone, Curr. Top. Med. Chem., 2009, 9, 119–129 CrossRef PubMed.
- (a) A. Fraix, C. Conte, E. Gazzano, C. Riganti, F. Quaglia and S. Sortino, Mol. Pharmaceutics, 2020, 17, 2135–2144 CrossRef PubMed; (b) C. Riganti, E. Miraglia, D. Viarisio, C. Costamagna, G. Pescarmona, D. Ghigo and A. Bosia, Cancer Res., 2005, 65, 516–525 Search PubMed; (c) K. Chegaev, A. Fraix, E. Gazzano, G. E. F. Abd-Ellatef, M. Blangetti, B. Rolando, S. Conoci, C. Riganti, R. Fruttero, A. Gasco and S. Sortino, ACS Med. Chem. Lett., 2017, 8, 361–365 CrossRef PubMed.
- (a) S. Sortino, J. Mater. Chem., 2012, 22, 301–318 RSC; (b) Topics in Curr. Chem., Light-responsive nanostructured systems for applications in nanomedicine, ed. S. Sortino, 2016, vol. 370 Search PubMed; (c) S. Swaminathan, J. Garcia-Amoròs, A. Fraix, N. Kandoth, S. Sortino and F. M. Raymo, Chem. Soc. Rev., 2014, 43, 4167–4178 RSC.
- (a) N. Ieda, H. Nakagawa, T. Peng, D. Yang, T. Suzuki and N. Miyata, J. Am. Chem. Soc., 2012, 134, 2563–2568 CrossRef PubMed; (b) N. Ieda, H. Nakagawa, T. Horinouchi, T. Peng, D. Yang, H. Tsumoto, T. Suzuki, K. Fukuhara and M. Naoki, Chem. Commun., 2011, 47, 6449–6451 RSC.
- J. Sun, X. Cai, C. Wang, K. Du, W. Chen, F. Feng and S. Wang, J. Am. Chem. Soc., 2021, 143, 868–878 CrossRef PubMed.
- (a) A. Fraix, C. Parisi, M. Failla, K. Chegaev, F. Spyrakis, L. Lazzarato, R. Fruttero, A. Gasco and S. Sortino, Chem. Commun., 2020, 56, 6332–6335 RSC; (b) C. Parisi, M. Failla, A. Fraix, B. Rolando, E. Gianquinto, F. Spyrakis, E. Gazzano, C. Riganti, L. Lazzarato, R. Fruttero, A. Gasco and S. Sortino, Chem.–Eur. J., 2019, 25, 11080–11084 CrossRef PubMed; (c) C. Parisi, A. Fraix, S. Guglielmo, F. Spyrakis, B. Rolando, L. Lazzarato, R. Fruttero, A. Gasco and S. Sortino, Chem.–Eur. J., 2020, 26, 13627–13633 CrossRef PubMed; (d) C. Parisi, M. Failla, A. Fraix, A. Rescifina, B. Rolando, L. Lazzarato, V. Cardile, A. C. E. Graziano, R. Fruttero, A. Gasco and S. Sortino, Bioorg. Chem., 2019, 85, 18–22 CrossRef PubMed.
- (a) M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef PubMed; (b) B. Gurram, M. Li, M. Li, K. H. Gebremedhin, W. Sun, J. Fan, J. Wang and X. Peng, J. Mater. Chem. B, 2019, 7, 4440–4450 RSC.
- S. Verma, U. W. Sallum, H. Athar, L. Rosenblum, J. W. Foley and T. Hasan, Photochem. Photobiol., 2009, 85, 111–118 CrossRef PubMed.
- (a) E. B. Caruso, S. Petralia, S. Conoci, S. Giuffrida and S. Sortino, J. Am. Chem. Soc., 2007, 129, 480–481 CrossRef PubMed; (b) S. Conoci, S. Petralia and S. Sortino, EP 2051935A1/US Pat.20090191284, 2006 Search PubMed; (c) S. Sortino, Chem. Soc. Rev., 2010, 39, 2903–2913 RSC.
- The lowest excited singlet state of the N-nitroso unit is ca. 1.5 eV higher than the lowest excited singlet state of the benzophenothazine chromophore..
- (a) N. Ieda, Y. Hotta, N. Miyata, K. Kimura and H. Nakagawa, J. Am. Chem. Soc., 2014, 136, 7085–7091 CrossRef PubMed; (b) H. Okuno, N. Ieda, Y. Hotta, M. Kawaguchi, K. Kimura and H. Nakagawa, Org. Biomol. Chem., 2017, 15, 2791–2796 RSC.
- D. Rehm and A. Weller, Isr. J. Chem., 1970, 8, 259–262 CrossRef.
- (a) M. Jonsson, J. Lind, T. E. Eriksen and G. Merényi, J. Am. Chem. Soc., 1994, 116, 1423–1427 CrossRef; (b) Y. L. Chow, Acc. Chem. Res., 1973, 6, 354–360 CrossRef.
- T. Ohno and N. N. Lictin, J. Am. Chem. Soc., 1980, 102, 4636–4643 CrossRef.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochemistry, CRC Press, Boca Raton, 3rd edn, 2006 Search PubMed.
- (a) H. He, Y. Xia, Y. Qi, H.-Y. Wang, A. Wang, J. Bao, Z. Zhang, F.-G. Wu, H. D. Chen, D. Yang, X. Liang, J. Chen, S. Zhou, X. Liang, X. Qian and Y. Yang, Bioconjugate Chem., 2018, 29, 1194–1198 CrossRef PubMed; (b) H. He, Z. Ye, Y. Xiao, W. Yang and Y. Yang, Anal. Chem., 2018, 90, 2164–2169 CrossRef PubMed.
- (a) I. J. McDonald and T. Doughery, J. Porphyrins Phthalocyanines, 2001, 5, 105 CrossRef; (b) T. Hasan, A. C. E. Moor and B. Ortel, Cancer Medicine, Decker BC Inc., Hamilton, Ontario, Canada, 5th edn, 2000 Search PubMed.
- N. Riosa, L. Piacenza, M. Trujillo, A. Martíneza, V. Demichelia, C. Prolo, M. N. Álvareza, G. V. Lópezb and R. Radi, Free Radical Biol. Med., 2016, 101, 284–295 CrossRef PubMed.
- L. J. Ignarro, J. M. Fukuto, J. M. Griscavage, N. E. Rogers and R. E. Byrns, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 8103–8107 CrossRef PubMed.
- T. P. Misko, R. J. Schilling, D. Salvemini, W. M. Moore and M. G. Currie, Anal. Biochem., 1993, 214, 11–16 CrossRef PubMed.
- N. S. Bryan and M. B. Grisham, Free Radical Biol. Med., 2007, 43, 645–657 CrossRef PubMed.
- K. M. Miranda, M. G. Espey and D. A. Wink, Nitric Oxide, 2001, 5, 62–71 CrossRef PubMed.
- D. Jourd'heuil, F. L. Jourd'heuil, P. S. Kutchukian, R. A. Musah, D. A. Winki and M. B. Grisham, J. Biol. Chem., 2001, 276, 28799–28805 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc06970a |
| ‡ These authors contributed equally to this work. |
| § Experiments performed in O2-saturated solution revealed that the amount of ONOO− photogenerated is ca. 25% smaller than that observed in air-equilibrated solution. This result is in good agreement with the mechanism proposed and involved the long-lived excited triplet state of BPT-NO as the key intermediate in the photodecomposition. In fact, an increase of the O2 concentration on one hand could lead to an increase of the concentration of O2˙−via a bimolecular reaction with the triplet state; on the other hand it is expected to increase the triplet quenching efficiency, reducing the amount of NO generated by a unimolecular reaction from this excited state. |
| This journal is © The Royal Society of Chemistry 2021 |