 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Rational design of a “dual lock-and-key” supramolecular photosensitizer based on aromatic nucleophilic substitution for specific and enhanced photodynamic therapy
Kun-Xu
Teng
,
Li-Ya
Niu
*,
Yan-Fei
Kang
and
Qing-Zheng
Yang
*
Key Laboratory of Radiopharmaceuticals, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, P. R. China. E-mail: niuly@bnu.edu.cn; qzyang@bnu.edu.cn
First published on 8th December 2020
Abstract
Correction for ‘Rational design of a “dual lock-and-key” supramolecular photosensitizer based on aromatic nucleophilic substitution for specific and enhanced photodynamic therapy’ by Kun-Xu Teng et al., Chem. Sci., 2020, 11, 9703–9711, DOI: 10.1039/D0SC01122C.
The authors regret an error in Fig. 1f. The correct image is shown below.
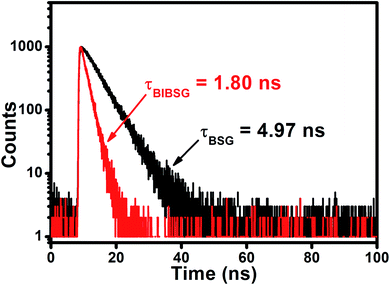 | ||
| Fig. 1 (f) Fluorescence decay curves of BSG and BIBSG, and the detection wavelength is 600 nm in DMSO. | ||
Additionally, there was a minor error in Fig. 4a. The correct image is shown below.
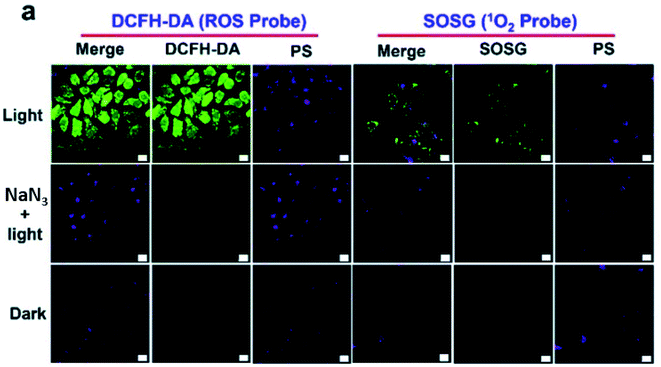 | ||
| Fig. 4 (a) Evaluation of 1O2 generation in HepG2 cells with DCFH-DA and SOSG. The scale bar represents 20 μm. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2021 |
