Open Access Article
Open Access ArticleFormal [4 + 4]-, [4 + 3]-, and [4 + 2]-cycloaddition reactions of donor–acceptor cyclobutenes, cyclopropenes and siloxyalkynes induced by Brønsted acid catalysis†
Haifeng
Zheng
,
Rui
Wang
,
Kan
Wang
,
Daniel
Wherritt
 ,
Hadi
Arman
and
Michael P.
Doyle
,
Hadi
Arman
and
Michael P.
Doyle
 *
*
Department of Chemistry, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, USA. E-mail: michael.doyle@utsa.edu
First published on 18th February 2021
Abstract
Brønsted acid catalyzed formal [4 + 4]-, [4 + 3]-, and [4 + 2]-cycloadditions of donor–acceptor cyclobutenes, cyclopropenes, and siloxyalkynes with benzopyrylium ions are reported. [4 + 2]-cyclization/deMayo-type ring-extension cascade processes produce highly functionalized benzocyclooctatrienes, benzocycloheptatrienes, and 2-naphthols in good to excellent yields and selectivities. Moreover, the optical purity of reactant donor–acceptor cyclobutenes is fully retained during the cascade. The 1,3-dicarbonyl product framework of the reaction products provides opportunities for salen-type ligand syntheses and the construction of fused pyrazoles and isoxazoles that reveal a novel rotamer-diastereoisomerism.
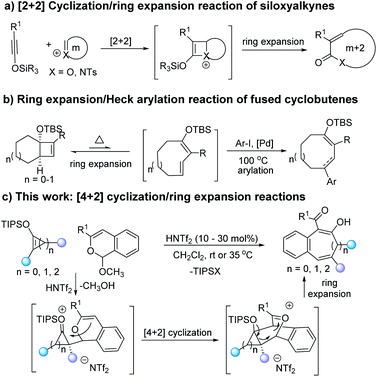
Medium-sized rings are the core skeletons of many natural products and bioactive molecules,1 and a growing number of strategies have been developed for their synthesis.2 Because of their enthalpic and entropic advantages, ring expansion is a highly efficient methodology for these constructions.3–6 For example, Sun and co-workers have developed acid promoted ring extensions of oxetenium and azetidinium species formed from siloxyalkynes with cyclic acetals and hemiaminals (Scheme 1a).4 Takasu and co-workers have reported an elegant ring expansion with a palladium(II) catalyzed 4π-electrocyclic ring-opening/Heck arylation cascade with fused cyclobutenes (Scheme 1b).5 Each transformation is initiated by the formation of fused bicyclic units followed by ring expansion or rearrangement to give medium-sized rings.
Strategies for the formation of fused bicyclic compounds rely on cycloaddition of dienes or dipoles with unsaturated cyclic compounds7 and, if the reactant cyclic compound is strained and chiral, the resulting bicyclic compound is activated toward ring opening that results in retention of chirality. We have recently reported access to donor–acceptor cycloalkenes by [3 + n] cycloaddition that have the prerequisites of unsaturated cyclic compounds suitable for cycloaddition.8 Donor–acceptor (D–A) cyclopropenes9 and cyclobutenes10 have sufficient strain in the resulting bicyclic compounds to undergo ring opening. We envision that the selection of a diene or dipolar reactant and suitable reaction conditions could realize cycloaddition and subsequent ring expansion. Benzopyrylium species,11,12 which are generated by metal or acid catalysis, have attracted our attention. We anticipated that their high reactivity would overcome the conventional unfavorable kinetic and/or thermodynamic factors that typically impede medium-sized ring formation. From a mechanistic perspective, benzopyrylium species are often formed by transition metal catalyzed reactions with 2-alkynylbenzaldehydes.11 Recently, the reaction between 1H-isochromene acetal and Brønsted acid catalyst forms the 2-benzopyrylium salts that could react with functional alkenes to give same cycloaddition products.12 Consequently, we believed that the [4 + 2]-cyclization between benzopyrylium species and donor–acceptor cyclobutenes, cyclopropenes, or siloxyalkynes would give bridged oxetenium intermediates, which contain high strain energy that should provide the driving force for ring expansion. The “push and pull” electronic effect of donor–acceptor functional groups facilitates deMayo-type ring-opening of the cyclobutane or cyclopropane skeletons (Scheme 1c).13
Here we report bis(trifluoromethanesulfonyl)imide (HNTf2) catalyzed formal [4 + 4]-, [4 + 3]- and [4 + 2]-cycloaddition reactions of D–A cyclobutenes, cyclopropenes, and siloxyalkynes with benzopyrylium salts. Polysubstituted benzo-cyclooctatrienes, benzocycloheptatrienes and 2-naphthols, are produced in good to excellent yields and selectivities. Complete retention of configuration occurs using chiral cyclobutenes, and opportunities for further functionalization are built into these constructions.
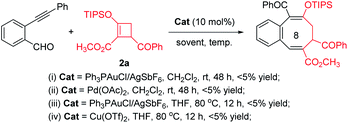
Initially, we conducted transition metal catalyzed reactions with 2-alkynylbenzaldehyde intending to produce the corresponding benzopyrylium ion and explore the possibility of cycloaddition/ring opening with donor–acceptor cyclobutene 2a. Use of Ph3PAuCl/AgSbF6, Pd(OAc)2 and Cu(OTf)2, which were efficient catalysts in previous transformations,11 gave only a trace amount of cycloaddition product (Fig. 1). Spectral analysis showed that mostly starting material remained (Fig. 1-i and ii). Increasing the reaction temperature led only to decomposition of 2-alkynylbenzaldehyde (Fig. 1-iv) or donor–acceptor cyclobutene 2a (Fig. 1-iv).
From these disappointments we turned our attention to 1H-isochromene acetal 1a as the benzopyrylium ion precursor. Various Lewis acid catalysts were employed with limited success, but we were pleased to observe the formation of the desired formal [4 + 4]-cycloaddition benzocyclooctatriene product 3aa, albeit in low yields (Table 1, entries 1–6). Selection of the Brønsted super acid HNTf2 (ref. 14) proved to be the most promising, producing 3aa in 40% yield (Table 1, entry 7). Increasing the amount of D–A cyclobutene 2a by 30% gave a much higher yield of 3aa (Table 1, entry 8 vs. 7). Optimization of the stoichiometric reaction between 1a and 2a by increasing the reaction temperature from rt to 35 °C led to the formation of the desired product in 76% isolated yield (Table 1, entry 9 vs. 8). However, a further increase in the reaction temperature (Table 1, entry 10) or reducing the catalyst loading to 5 mol% did not improve the yield of 3aa (Table 1, entry 11).
| Entry | Cat (10 mol%) | Temp. (°C) | Yieldb |
|---|---|---|---|
| a Reactions were performed by adding the catalyst (10 mol%) to 1a (0.1 mmol) and 2a (0.1 mmol) in CH2Cl2 (2 mL) at the corresponding temperature for 24 h. b Yields were determined by 1H NMR spectroscopic analysis with CH2Br2 as the internal standard. c 1.3 equiv. 2a was used. d Isolated yield. e Reaction performed at 60 °C for 12 h. f 5 mol% catalyst loading. | |||
| 1 | Sc(OTf)3 | rt | 23 |
| 2 | Yb(OTf)3 | rt | Trace |
| 3 | In(OTf)3 | rt | 16 |
| 4 | TiCl4 | rt | Trace |
| 5 | BF3·OEt2 | rt | 20 |
| 6 | TMSOTf | rt | 17 |
| 7 | HNTf2 | rt | 40 |
| 8c | HNTf2 | rt | 67 |
| 9c | HNTf2 | 35 | 78(76)d |
| 10c,e | HNTf2 | 60 | 62 |
| 11c,f | HNTf2 | 35 | 50 |
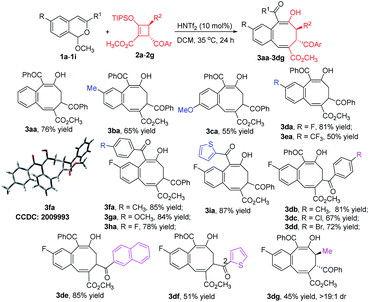
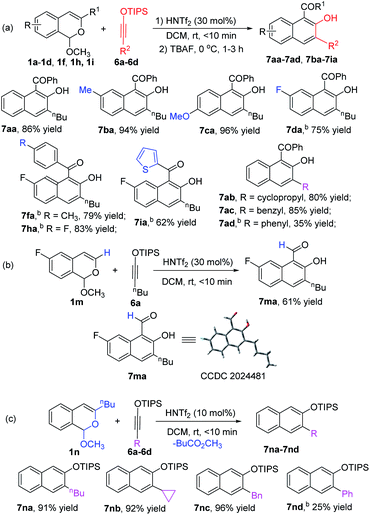
With optimized conditions using 1a in hand, we examined the scope of the formal [4 + 4]-cycloaddition reactions of D–A cyclobutenes 2 with a diverse set of acetal compounds 1. As shown in Scheme 2, a wide range of acetal substrates (1a–1i) with different substituents at different positions all reacted smoothly with D–A cyclobutene 2a to form the corresponding benzocyclooctatriene products 3 in good to excellent yields. Structural variations in the acetals produced only modest changes in product yields which ranged from 55 to 87%. Similarly, both electron-withdrawing and electron-donating substituents at the 4-position of the cyclobutene phenyl ring produced the corresponding products (3db–3dd) in good yields, and 2-naphthyl (2e) and 2-thienyl (2f) substituted cyclobutenes were suitable substrates (85% and 51% product yields, respectively). trans-1,2,3,4-Tetrasubstituted (R2 = CH3) 2-sil-oxycyclobutenecarboxylate 2g also underwent [4 + 4]-cycloaddition with 1d in good yield and fully retained its diastereoselectivity. The structure of 3fa was confirmed by X-ray diffraction (Scheme 2).15
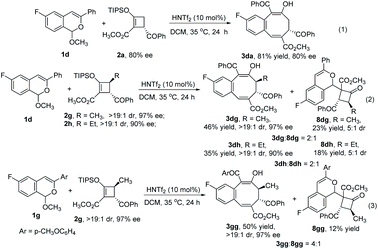
Chiral donor–acceptor cyclobutenes with high enantiomeric excess and diastereoselectivity are conveniently obtained by catalytic [3 + 1]-cycloaddition of enoldiazoacetates with acyl ylides of sulfur.10 To determine if optical purity is retained, chiral D–A cyclobutene 2a (80% ee) was reacted with 1d under the optimized conditions, and the corresponding benzocyclo-octatriene product 3da was obtained in good yield with complete retention of configuration (Scheme 3, eqn (1)). With trans-disubstituted 2g and 2h that have higher optical purity, however, 3dg and 3dh were obtained in moderate yields with full retention of diastereo- and enantioselectivities, but addition products 8dg and 8dh were formed competitively (Scheme 3, eqn (2)). These compounds resulted from initial addition then desilylation, indicating that the [4 + 2]-cyclization is a stepwise reaction. Attempts to suppress the competing pathway by changing solvents or using the isopropyl acetal substrate to form a bulky 2-propanol nucleophile (1j) failed (for details, see ESI†). However, p-methoxy (R1) substituted 1g that would stabilize the incipient benzopyrylium ion gave higher selectivity (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 2
1 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the desired [4 + 4] cycloaddition product 3gg was isolated in 50% yield with 97% ee and >19
1), and the desired [4 + 4] cycloaddition product 3gg was isolated in 50% yield with 97% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 3, eqn (3)).
1 dr (Scheme 3, eqn (3)).
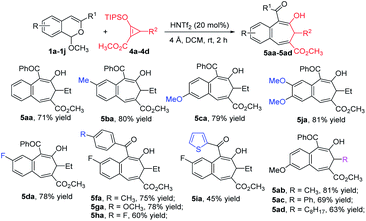
To further expand the generality of this strategy, we investigated its use with donor–acceptor cyclopropenes 4. The desired formal [4 + 3]-cycloaddition products 5 were obtained in good to excellent yields (Scheme 4). Optimized conditions used 20 mol% HNTf2 catalyst with 4 Å molecular sieves at room temperature for 2 h. The scope of this [4 + 3]-cycloaddition reaction with cyclopropenes 4 showed that acetals bearing both electron-donating and electron-withdrawing substituents on the aromatic ring were tolerated. However, as with [4 + 4]-cycloaddition reactions, the [4 + 3] reactions of 1 with R1 = alkyl or H did not produce any of the desired products. Furthermore, 3-substituted cyclopropenes 4b–4d participated in this reaction, and their products (5ab–5ad) were obtained in 63%–81% yields.
Siloxyalkynes 6, as electron-rich alkynes, have been widely used in diverse cyclization reaction.4,16 We expected that 6 could also participate in [4 + 2]-cyclization/ring-expansion cascade processes, giving substituted 2-naphthol products. Interestingly, substituent controlled diverse products were obtained in good to excellent reactivity and selectivity (Scheme 5). Aryl (R1) substituted acetals (1a–1d, 1f, 1h, 1i) reacted with siloxyalkynes 6a–6d, giving 2-naphthols (7aa–7ad and 7ba–7ia) in 35%–96% yields. With electron-donating group (EDG) substituents (7ba and 7ca) on the aromatic ring, higher reactivity was observed relative to those with electron-withdrawing groups (7da–7ia). In addition, the acetal with R1 = H (1m) reacted with siloxyalkynes 6 to form the 2-naphthol-1-carboxaldehyde derivative in good yield, and the structure of 7ma was confirmed by X-ray diffraction (Scheme 5b).15 Intriguingly, the n-butyl (R1) substituted acetal 1n reacted with siloxyalkynes via a [4 + 2]-cyclization with loss of methyl pentanoate (BuCO2CH3), affording siloxy naphthalenes 7na–7nd that are important precursors to the widely used axially chiral 2,2′-binols.17 The substrate scope of siloxyalkynes 6 for their formal [4 + 2]-cycloaddition reaction with n-butyl substituted acetal 1n was also explored (Scheme 5c). In all cases methyl pentanoate was eliminated to form 2,3-disubstituted naphthalene products (7na–7nd). Alkyl substituted siloxyalkynes (6a–6c) showed higher reactivity compared with phenyl substituted siloxyalkynes 6d. It should be mentioned that, recently, a similar transformation using BF3·OEt2 as catalyst or in excess (2 equiv.) with 2,4,6-collidine (1 equiv.) was reported,18 and HNTf2 was stated to be much less effective. To clarify this discrepancy, we carefully repeated these transformations (7ma and 7na) and found that all starting materials are completely consumed in less than 10 min to deliver [4 + 2]-cycloaddition products in good yields. Prolonging the reaction time to 12 hours, which was the reaction time used by the authors, results in their decomposition to a complex mixture of materials.
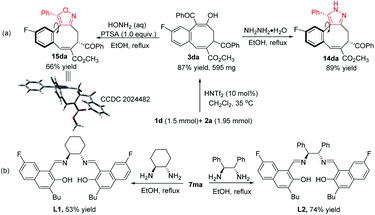
To illustrate the utility of this process, a large scale catalytic [4 + 4] cycloaddition was performed, and adduct 3da was obtained in 87% yield. Further transformations were conducted for the synthesis of pyrazole and isoxazole structures based on its 1,3-dicarbonyl skeleton (Fig. 2a). Compound 3da reacted with hydrazine and hydroxylamine in refluxed ethanol, affording pyrazole 14da and isoxazole 15da in 89% yield or 66% yield, respectively. The structure of 15da was confirmed by X-ray diffraction.15 Interestingly, two NMR distinguishable interconvertible diastereoisomers were detected for each of these eight-membered cyclic products (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr for 14da and 15da, respectively, in CDCl3). These diastereoisomers are rotamers (for details, see ESI†) that exist at equilibrium with each other in solution but form one crystalline product (X-ray structure of 15da). In addition, the cycloaddition product 7ma reacted with chiral 1,2-cyclohexanediamine and 1,2-diphenylethylenediamine to give salen-type ligands L1 and L2 in 53% yield and 74% yield, respectively, which provides new opportunities for ligand screening (Fig. 2b).19
1 dr for 14da and 15da, respectively, in CDCl3). These diastereoisomers are rotamers (for details, see ESI†) that exist at equilibrium with each other in solution but form one crystalline product (X-ray structure of 15da). In addition, the cycloaddition product 7ma reacted with chiral 1,2-cyclohexanediamine and 1,2-diphenylethylenediamine to give salen-type ligands L1 and L2 in 53% yield and 74% yield, respectively, which provides new opportunities for ligand screening (Fig. 2b).19
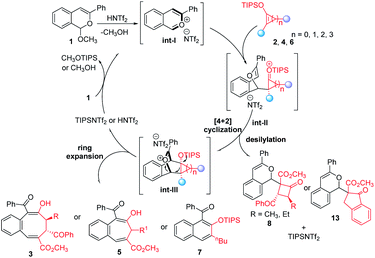
In the mechanistic possibility considered for these HNTf2 catalyzed cycloaddition reactions (Fig. 3), protonation of acetal 1 with HNTf2 gives the corresponding highly reactive benzopyrylium intermediate int-I, which reacts with donor–acceptor cyclobutenes 2, cyclopropenes 4, or siloxyalkynes 6 affording addition intermediates int-II that undergo ring closure to int-III. Ring expansion then occurs to deliver 3, 5 and 7 in good to excellent yields with fully retained stereoselectivities. Furthermore, the formed TIPSNTf2 ([4 + 4]- and [4 + 3]-cycloaddition) or HNTf2 {[4 + 2] cycloaddition} are active acid catalysts for the conversion of 1 to benzopyrylium intermediate int-I that continues the catalytic cycle. With sterically larger D–A cyclobutenes or when a less ring-strained benzocyclopentene 12 is employed (for details, see ESI†), the competing direct desilylation of int-II occurs, delivering addition byproducts 8 or 13. Compounds 7na–7nd arise from the analog to int-III from which ketene formation or methanol displacement effects 1,4-elimination.
Conclusions
In summary, we have realized formal [4 + 4]-, [4 + 3]-, and [4 + 2]-cycloaddition reactions of D–A cyclobutenes, cyclopropenes, and silyloxyalkynes via Brønsted acid catalysis that are not feasible via the alternative metal catalysed process. The success of these transformations is attributed to the design of the [4 + 2]-cyclization/deMayo-type ring-expansion cascade processes in which various benzocyclooctatrienes, benzocycloheptatrienes and 2-naphthols are obtained in good to excellent yields and selectivities. In addition, the optical purity of the reactant donor–acceptor cyclobutenes is fully retained. The cycloaddition products provide additional opportunities in salen-type ligand synthesis and heterocyclic synthesis exemplified by the formation of pyrazole and isoxazole products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the U.S. National Science Foundation (CHE-1763168) for funding this research. The X-ray diffractometer used in this research was supported by a grant from the U.S. National Science Foundation (CHE-1920057).Notes and references
-
(a) I. M. Fellows, D. E. Kaelin and S. F. Martin, J. Am. Chem. Soc., 2000, 122, 10781 CrossRef CAS
; (b) B. M. Trost and M. K. Ameriks, Org. Lett., 2004, 6, 1745 CrossRef CAS
; (c) I. Shiina, Chem. Rev., 2007, 107, 239 CrossRef CAS
; (d) H. M. C. Ferraz, F. I. Bombonato, M. K. Sano Jr and L. S. Longo, Quim. Nova, 2008, 31, 885 CrossRef CAS
; (e) J.-X. Pu, L.-M. Yang, W.-L. Xiao, R.-T. Li, C. Lei, X.-M. Gao, S.-X. Huang, S.-H. Li, Y.-T. Zheng, H. Huang and H.-D. Sun, Phytochemistry, 2008, 69, 1266 CrossRef CAS
; (f) Natural Lactones and Lactams: Synthesis Occurrence and Biological Activity, ed. T. Janecki, Wiley-VCH, Weinheim, 2013 Search PubMed
.
-
(a) G. Mehta and V. Singh, Chem. Rev., 1999, 99, 881 CrossRef CAS
; (b) G. A. Molander, Acc. Chem. Res., 1998, 31, 603 CrossRef CAS
; (c) A. Parenty, X. Moreau, G. Niel and J. M. Campagne, Chem. Rev., 2013, 113, PR1 CrossRef CAS
; (d) M. Murakami, S. Ashida and T. Matsuda, J. Am. Chem. Soc., 2006, 128, 2166 CrossRef CAS
; (e) Z. X. Yu, Y. Wang and Y. Y. Wang, Chem.–Asian J., 2010, 5, 1072 CrossRef CAS
.
- Reviews on ring expansion synthetic strategies, see:
(a) E. Leemans, M. D'hooghe and N. De Kimpe, Chem. Rev., 2011, 111, 3268 CrossRef CAS
; (b) J. Christoffers, Catal. Today, 2011, 159, 96 CrossRef CAS
; (c) G. Fumagalli, S. Stanton and J. F. Bower, Chem. Rev., 2017, 117, 9404 CrossRef CAS
; (d) J. R. Donald and W. P. Unsworth, Chem.–Eur. J., 2017, 23, 8780 CrossRef CAS
Selected examples: (e) A. Klapars, S. Parris, K. W. Anderson and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 3529 CrossRef CAS
; (f) C. Kitsiou, J. J. Hindes, P. I'Anson, P. Jackson, T. C. Wilson, E. K. Daly, H. R. Felstead, P. Hearnshaw and W. P. Unsworth, Angew. Chem., Int. Ed., 2015, 54, 15794 CrossRef CAS
; (g) J. E. Hall, J. V. Matlock, J. W. Ward, K. V. Gray and J. Clayden, Angew. Chem., Int. Ed., 2016, 55, 11153 CrossRef CAS
; (h) R. Costil, Q. Lefebvre and J. Clayden, Angew. Chem., Int. Ed., 2017, 56, 14602 CrossRef CAS
; (i) N. Wang, Q. S. Gu, Z. L. Li, Z. Li, Y. L. Guo, Z. Guo and X. Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 14225 CrossRef CAS
Enantioselective synthesis of chiral medium-sized cyclic compounds: (j) H. Z. Ni, X. D. Tang, W. R. Zheng, W. J. Yao, N. Ullah and Y. X. Lu, Angew. Chem., Int. Ed., 2017, 56, 14222 CrossRef CAS
; (k) G. W. Kang, M. Yamagami, S. Vellalath and D. Romo, Angew. Chem., Int. Ed., 2018, 57, 6527 CrossRef CAS
; (l) X. Gao, M. R. Xia, C. H. Yuan, L. J. Zhou, W. Sun, C. L. B. Wu, D. Y. Zhu, C. Zhang, B. Zheng, D. Q. Wang and H. C. Guo, ACS Catal., 2019, 9, 1645 CrossRef CAS
; (m) C. R. Xu, K. X. Wang, D. W. Li, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2019, 58, 18438 CrossRef CAS
.
-
(a) W. X. Zhao, Z. G. Li and J. W. Sun, J. Am. Chem. Soc., 2013, 135, 4680 CrossRef CAS
; (b) W. X. Zhao, H. Qian, Z. G. Li and J. W. Sun, Angew. Chem., Int. Ed., 2015, 54, 10005 CrossRef CAS
.
- N. Arichi, K. I. Yamada, Y. Yamaoka and K. Takasu, J. Am. Chem. Soc., 2015, 137, 9579 CrossRef CAS
.
- Y. R. Zhou, Y. L. Wei, J. Rodriguez and Y. Coquerel, Angew. Chem., Int. Ed., 2019, 58, 456 CrossRef CAS
.
-
(a) M. H. Filippini and J. Rodriguez, Chem. Rev., 1999, 99, 27 CrossRef CAS
; (b) X. X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS
; (c) G. Masson, C. Lalli, M. Benohoud and G. Dagousset, Chem. Soc. Rev., 2013, 42, 902 RSC
; (d) T. Hashimoto and K. J. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS
; (e) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621 CrossRef CAS
; (f) N. De and E. J. Yoo, ACS Catal., 2018, 8, 48 CrossRef CAS
.
-
(a) Q. Q. Chen, Y. M. Deng, M. Lankelma and M. P. Doyle, Chem. Soc. Rev., 2017, 46, 5425 RSC
; (b) K. O. Marichev and M. P. Doyle, Org. Biomol. Chem., 2019, 17, 4183 RSC
; (c) X. F. Xu and M. P. Doyle, Acc. Chem. Res., 2014, 47, 1396 CrossRef CAS
.
-
(a) Y. M. Deng, C. Jing and M. P. Doyle, Chem. Commun., 2015, 51, 12924 RSC
; (b) Q.-Q. Chen, J. Yedoyan, H. Arman and M. P. Doyle, Angew. Chem., Int. Ed., 2016, 55, 5573 CrossRef
.
-
(a) Y. M. Deng, L. A. Massey, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2017, 56, 7479 CrossRef CAS
; (b) Q. Yao, H. Yu, H. Zhang, S. X. Dong, F. Z. Chang, L. L. Lin, X. H. Liu and X. M. Feng, Chem. Commun., 2018, 54, 3375 RSC
; (c) Y. Luo, H. Zhang, S. Y. Wang, Y. Q. Zhou, S. X. Dong and X. M. Feng, Org. Lett., 2020, 22, 2645 CrossRef CAS
.
- Reviews on isobenzopyrylium ions:
(a) E.-V. Kuznetsov, I.-V. Shcherbakova and A.-T. Balaban, Adv. Heterocycl. Chem., 1990, 50, 157 CrossRef CAS
; (b) M. Nogradi, Sci. Synth., 2003, 14, 201 CAS
; (c) J. Santamaría and C. Valdés, Mod. Heterocycl. Chem., 2011, 3, 1631 Search PubMed
; (d) J.-R. Chen, X.-Q. Hu and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 4038 CrossRef CAS
; (e) S. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo and W. R. Dithtel, Acc. Chem. Res., 2017, 50, 2776 CrossRef CAS
; (f) L. Chen, K. Chen and S. F. Zhu, Chem, 2018, 1208 CrossRef CAS
Selected examples: (g) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650 CrossRef CAS
; (h) N. Asao, T. Nogami, S. Lee and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 10921 CrossRef CAS
; (i) J. Barluenga, H. Vázquez-Villa, A. Ballesteros and J. M. González, Org. Lett., 2003, 5, 4121 CrossRef CAS
; (j) H. Kusama, H. Funami, M. Shido, Y. Hara, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2005, 127, 2709 CrossRef CAS
; (k) J. Zhu, N. P. Grigoriadis, J. P. Lee and J. A. Porco, J. Am. Chem. Soc., 2005, 127, 9342 CrossRef CAS
; (l) J. Barluenga, H. Vazquez-Villa, I. Merino, A. Ballesteros and J. M. Gonzalez, Chem.–Eur. J., 2006, 12, 5790 CrossRef CAS
; (m) D. Yue, N. D. Cá and R. C. Larock, J. Org. Chem., 2006, 71, 3381 CrossRef CAS
; (n) Y.-C. Hsu, C.-M. Ting and R.-S. Liu, J. Am. Chem. Soc., 2009, 131, 2090 CrossRef CAS
; (o) X. Zhao, X.-G. Zhang, R.-Y. Tang, C.-L. Deng and J.-H. Li, Eur. J. Org. Chem., 2010, 4211 CrossRef CAS
; (p) D. Lehnherr, J. M. Alzola, E. B. Lobkovsky and W. R. Dichtel, Chem.–Eur. J., 2015, 21, 18122 CrossRef CAS
; (q) R. Liang, J. Zhang, L. Chen and S. F. Zhu, Asian J. Org. Chem., 2018, 7, 545 CrossRef CAS
; (r) A. S. K. Raj and R.-S. Liu, Angew. Chem., Int. Ed., 2019, 58, 10980 CrossRef CAS
Catalytic asymmetric reactions of isobenzopyrylium ions: (s) H. Suga, K. Inoue, S. Inoue and A. Kakehi, J. Am. Chem. Soc., 2002, 124, 14836 CrossRef CAS
; (t) D. Hojo, K. Noguchi and K. Tanaka, Angew. Chem., Int. Ed., 2009, 48, 8129 CrossRef CAS
; (u) S. Handa and L. M. Slaughter, Angew. Chem., Int. Ed., 2012, 51, 2912 CrossRef CAS
; (v) M. Terada and Y. Toda, Angew. Chem., Int. Ed., 2012, 51, 2093 CrossRef CAS
; (w) K. Saito, Y. Kajiwara and T. Akiyama, Angew. Chem., Int. Ed., 2013, 52, 13284 CrossRef CAS
; (x) S.-Y. Yu, H. Zhang, Y. Gao, L. Mo, S. Z. Wang and Z.-J. Yao, J. Am. Chem. Soc., 2013, 135, 11402 CrossRef CAS
.
- H. Qian, W. X. Zhao, Z. B. Wang and J. W. Sun, J. Am. Chem. Soc., 2015, 137, 560 CrossRef CAS
.
- P. DeMayo, Acc. Chem. Res., 1971, 4, 41 CrossRef CAS
.
- W. X. Zhao and J. W. Sun, Chem. Rev., 2018, 118, 10349 CrossRef CAS
.
- CCDC 2009993 (3fa); CCDC 2024481 (7ma); CCDC 2024482 (15da).
- Reviews on siloxy alkynes:
(a) M. Shindo, Tetrahedron, 2007, 63, 10 CrossRef CAS
; (b) H. Qian, W. Zhao and J. Sun, Chem. Rec., 2014, 14, 1070 CrossRef CAS
Selected examples: (c) C. J. Kowalski and G. S. Lal, J. Am. Chem. Soc., 1988, 110, 3693 CrossRef CAS
; (d) R. L. Danheiser, R. G. Brisbois, J. L. Kowalczyk and R. F. Miller, J. Am. Chem. Soc., 1990, 112, 3093 CrossRef CAS
; (e) R. F. Sweis, M. P. Schramm and S. A. Kozmin, J. Am. Chem. Soc., 2004, 126, 7442 CrossRef CAS
; (f) M. Movassaghi, M. D. Hill and O. K. Ahmad, J. Am. Chem. Soc., 2007, 129, 10096 CrossRef CAS
; (g) Y. E. Türkmen, T. J. Montavon, S. A. Kozmin and V. H. Rawal, J. Am. Chem. Soc., 2012, 134, 9062 CrossRef
; (h) W. X. Zhao, Z. B. Wang and J. W. Sun, Angew. Chem., Int. Ed., 2012, 51, 6209 CrossRef CAS
; (i) J. R. Cabrera-Pardo, D. I. Chai, S. Liu, M. Mrksich and S. A. Kozmin, Nat. Chem., 2013, 5, 423 CrossRef CAS
; (j) A. Wu, Q. Feng, H. H. Y. Sung, I. D. Williams and J. W. Sun, Angew. Chem., Int. Ed., 2019, 58, 6776 CrossRef CAS
.
-
(a) H. Wang, Chirality, 2010, 22, 827 CrossRef CAS
; (b) J. M. Brunel, Chem. Rev., 2005, 105, 857 CrossRef CAS
.
- A. Wu, H. Qian, W. X. Zhao and J. W. Sun, Chem. Sci., 2020, 11, 7957 RSC
.
-
(a) M. Viciano, B. K. Muõoz, C. Godard, S. Castillón, M. L. Reyes, M. García-Ruiz and C. Claver, ChemCatChem, 2017, 9, 3974 CrossRef CAS
; (b) C. A. Discolo, E. E. Touney and S. V. Pronin, J. Am. Chem. Soc., 2019, 141, 17527 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, constraints for cycloaddition analytical and spectral characterizations, X-ray structures. CCDC 2009993 (3fa), 2024481 (7ma) and 2024482 (15da). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc00158b |
| This journal is © The Royal Society of Chemistry 2021 |