Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective synthesis of unnatural α-amino acid derivatives through photoredox catalysis†
Andrey
Shatskiy
 a,
Anton
Axelsson
a,
Elena V.
Stepanova
a,
Anton
Axelsson
a,
Elena V.
Stepanova
 bc,
Jian-Quan
Liu
bc,
Jian-Quan
Liu
 a,
Azamat Z.
Temerdashev
a,
Azamat Z.
Temerdashev
 d,
Bhushan P.
Kore
e,
Björn
Blomkvist
a,
James M.
Gardner
d,
Bhushan P.
Kore
e,
Björn
Blomkvist
a,
James M.
Gardner
 e,
Peter
Dinér
e,
Peter
Dinér
 a and
Markus D.
Kärkäs
a and
Markus D.
Kärkäs
 *a
*a
aDivision of Organic Chemistry, Department of Chemistry, KTH Royal Institute of Technology, SE-100 44, Stockholm, Sweden. E-mail: karkas@kth.se
bTomsk Polytechnic University, Lenin Avenue 30, 634050 Tomsk, Russia
cZelinsky Institute of Organic Chemistry of the Russian Academy of Sciences, Leninsky Prospect 47, 119991 Moscow, Russia
dDepartment of Analytical Chemistry, Kuban State University, Stavropolskaya St. 149, 350040 Krasnodar, Russia
eDivision of Applied Physical Chemistry, Department of Chemistry, KTH Royal Institute of Technology, SE-100 44, Stockholm, Sweden
First published on 3rd March 2021
Abstract
A protocol for stereoselective C-radical addition to a chiral glyoxylate-derived N-sulfinyl imine was developed through visible light-promoted photoredox catalysis, providing a convenient method for the synthesis of unnatural α-amino acids. The developed protocol allows the use of ubiquitous carboxylic acids as radical precursors without prior derivatization. The protocol utilizes near-stoichiometric amounts of the imine and the acid radical precursor in combination with a catalytic amount of an organic acridinium-based photocatalyst. Alternative mechanisms for the developed transformation are discussed and corroborated by experimental and computational studies.
Introduction
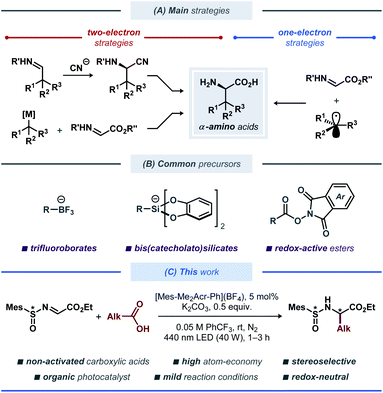
Unnatural α-amino acids constitute an important class of biologically relevant compounds that are widely used in both pharmaceutical industry and for fundamental research within molecular and structural biology.1 A number of pharmaceuticals based on unnatural α-amino acids are currently available, including angiotensin-converting enzyme (ACE) inhibitors for the treatment of cardiovascular and renal diseases,2 antiviral medicines,3 and others.4 Recently, peptidomimetic α-ketoamide inhibitors based on unnatural α-amino acids have received increased attention as drug candidates for treatment of COVID-19 disease caused by the SARS-CoV-2 coronavirus,5 highlighting the high demand for such building blocks.A variety of synthetic strategies to access unnatural amino acid derivatives have been developed over the years, with some notable methods being the catalytic asymmetric Strecker-type reactions, asymmetric hydrogenation of dehydroamino acids, and electrophilic and nucleophilic alkylation of glycine derivatives (Fig. 1A).6 Among these, functionalization or reduction of α-imino esters offers a straightforward route to various enantiomerically enriched α-amino acids.7 Traditionally, these strategies have employed polar retrosynthetic disconnections, which often require the use of (super)stoichiometric amounts of toxic and highly sensitive reagents at low temperatures, thereby limiting the substrate scope and practicality for scale up of these reactions. These limitations have recently been challenged by re-introduction of free-radical reaction manifolds, aided by the developments in base-metal catalysis,8 electrosynthesis9 and photoredox catalysis,10 leading to a vast array of strategies for light-induced modification and synthesis of amino acids and peptides.11 Among these, radical addition to imines through photoredox catalysis was demonstrated in symmetric12 and asymmetric13 fashion (Fig. 1B). In 2017, Alemán and co-workers reported a protocol for asymmetric radical addition to imines mediated by visible light.13a The developed catalytic system made use of a chiral sulfoxide auxiliary group, commonly employed in the synthesis of chiral amines.14 Here, the C-centered radical was generated through visible light-mediated reductive cleavage of the N–O bond in a redox-active phthalimide ester, followed by radical addition to the N-sulfinyl imine. The reductive nature of this protocol required the use of a stoichiometric amount of a reducing agent (Hanztsch ester). More recently, a related Ni-based catalytic system was described by Baran and co-workers.15 This protocol employed a tetrachloro-substituted redox-active ester as the radical precursor, with Zn as a stoichiometric reducing agent and a Ni-based catalyst for mediating the C–C bond formation. Although this protocol displayed an impressive substrate scope, it is associated with moderate atom-economy, limiting its applicability for large-scale synthesis.
Results and discussion
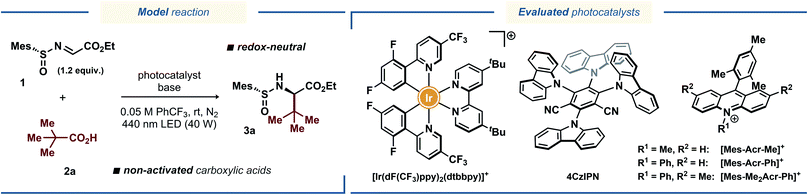
Inspired by the catalytic systems developed by the Alemán13a and Baran15 groups, we sought to realize a protocol for diastereoselective decarboxylative radical addition to chiral N-sulfinyl imines that would utilize ubiquitous non-activated carboxylic acids as radical precursors.16 A related direct decarboxylative addition process was attempted by the Alemán group for a benzaldehyde-derived N-sulfinyl imine under reaction conditions reported by MacMillan;17 however, no formation of the desired product was observed (see the ESI to ref. 13a). Similarly, we observed no desired product with pivalic acid 2a as the radical precursor and N-sulfinyl imine 1 as the radical acceptor when the reaction was conducted in DMSO with [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) as photocatalyst (Table 1, entry 1), presumably due to fast decomposition of N-sulfinyl imine 1. Gratifyingly, changing the solvent to α,α,α-trifluorotoluene (PhCF3) furnished the desired product 3a in fairly good yield of 65%, although with poor diastereoselectivity (Table 1, entry 2). Using other bases in place of Cs2CO3 completely prohibited the reaction (for details on the optimization studies, see the ESI†), and the highly-oxidizing photocatalyst 4CzIPN18 failed to deliver the radical addition product (Table 1, entry 3). Fortunately, the highly-oxidizing organic acridinium-based photocatalyst [Mes-Acr-Me](BF4) delivered product 3a with excellent diastereoselectivity, although in poor yield (Table 1, entry 4). Increasing the catalyst loading from 1 to 5 mol% and switching to the more stable N-phenyl-substituted photocatalysts [Mes-Acr-Ph](BF4) and [Mes-Me2Acr-Ph](BF4)19 dramatically increased the yield of the stereoselective radical addition product up to 78% (Table 1, entries 5–7). Conducting the reaction in more conventional solvents, such as MeCN, CH2Cl2, and 2,2,2-trifluoroethanol (TFE) in place of PhCF3 resulted in diminished yields (Table S1, see the ESI†), highlighting the documented inertness of PhCF3 towards free-radical intermediates.20 Changing the base to K2CO3 and increasing the base loading further improved the yield up to 85% (Table 1, entry 11). Finally, utilizing a slight excess of the acid radical precursor 2a delivered the desired product 3a in excellent yields (91% and 95% for 1.2 and 1.5 equiv. of 2a, respectively; Table 1, entries 13 and 14). Consistently with the previous reports on radical additions to N-sulfinyl imines, the tert-butyl- and para-tolyl-substituted N-sulfinyl imines 4 and 5 proved to be inefficient as radical acceptors (Table 1, entries 15 and 16).13a,15 In case of tert-butyl-substituted N-sulfinyl imine 4, it is likely that the transiently formed aminyl radical intermediate underwent decomposition to form an iminosulfanone (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), thereby disrupting the catalytic cycle.21
O), thereby disrupting the catalytic cycle.21
| Entry | Photocatalyst | Base | Time | Yieldb | drb |
|---|---|---|---|---|---|
| a The reactions were performed on 0.1 mmol scale: stock solutions of pivalic acid 2 and the photocatalyst (each in 1 mL of the solvent) were mixed with N-sulfinyl imine 1 and the base under anhydrous conditions, and stirred under irradiation with 440 nm blue LED light at room temperature (for details, see the ESI). b Determined by 1H NMR of crude reaction mixture with 1,3,5-trimethoxybenzene as an internal standard. c 0.1 M DMSO. d 0.1 M PhCF3. e 1.0 equiv. of N-sulfinyl imine 1 and 1.2 equiv. of pivalic acid 2. f 1.0 equiv. of N-sulfinyl imine 1 and 1.5 equiv. of pivalic acid 2. | |||||
| 1c | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | — | — |
| 2 | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | 65% | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 4CzIPN, 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | — | — |
| 4 | [Mes-Acr-Me](BF4), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | 27% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 5 | [Mes-Acr-Me](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 20 min | 48% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 60 min | 66% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|||
| 6 | [Mes-Acr-Ph](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 60 min | 73% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7 | [Mes-Me2Acr-Ph](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 60 min | 78% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 8 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K3PO4, 0.2 equiv. | 60 min | 80% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 9 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.2 equiv. | 60 min | 84% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 10 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.05 equiv. | 20 min | <5% | — |
| 11 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 85% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 12d | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 77% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 13 | [Mes-Me 2 Acr-Ph](BF 4 ), 5 mol% | K 2 CO 3 , 0.5 equiv. | 60 min | 91% |
>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
| 14f | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 95% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 15 | As entry 13, but with tBu-sulfinyl imine 4 | 60 min | — | — | |
| 16 | As entry 13, but with p-Tol-sulfinyl imine 5 | 60 min | 50% | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Deviations from the conditions in entry 13 | |||||
| 17 | Under air | 60 min | 12% | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|
| 18 | No photocatalyst | 60 min | — | — | |
| 19 | No light | 60 min | — | — | |
The substrate scope of the developed transformation was evaluated with a variety of non-functionalized and functionalized tertiary, secondary, and primary carboxylic acids (Fig. 2). For all of the amino acid derivatives excellent diastereoselectivity at the α-position was observed (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr). The radical precursors producing tertiary and secondary alkyl-substituted radicals provided the expected products in generally good to high yields (3a–e and 3k–n). The highly reactive primary alkyl radicals displayed lower selectivity for the addition reaction (3o and 3p), consistent with previous reports featuring unstable free-radical intermediates under related conditions.22 Further optimization of the reaction conditions for the primary acids 2o and 2p did not result in improved yields (Tables S2 and S3†), illustrating the intrinsic instability of the respective radical intermediates and/or the photocatalyst under the employed conditions. Benzylic-type radicals were generally inefficient (see Fig. 2, unsuccessful substrates); however, a cyclopropyl-substituted benzylic radical and an indole-derived benzylic-type radical provided the expected products 3f and 3q, respectively, in satisfactory yields.
5 dr). The radical precursors producing tertiary and secondary alkyl-substituted radicals provided the expected products in generally good to high yields (3a–e and 3k–n). The highly reactive primary alkyl radicals displayed lower selectivity for the addition reaction (3o and 3p), consistent with previous reports featuring unstable free-radical intermediates under related conditions.22 Further optimization of the reaction conditions for the primary acids 2o and 2p did not result in improved yields (Tables S2 and S3†), illustrating the intrinsic instability of the respective radical intermediates and/or the photocatalyst under the employed conditions. Benzylic-type radicals were generally inefficient (see Fig. 2, unsuccessful substrates); however, a cyclopropyl-substituted benzylic radical and an indole-derived benzylic-type radical provided the expected products 3f and 3q, respectively, in satisfactory yields.
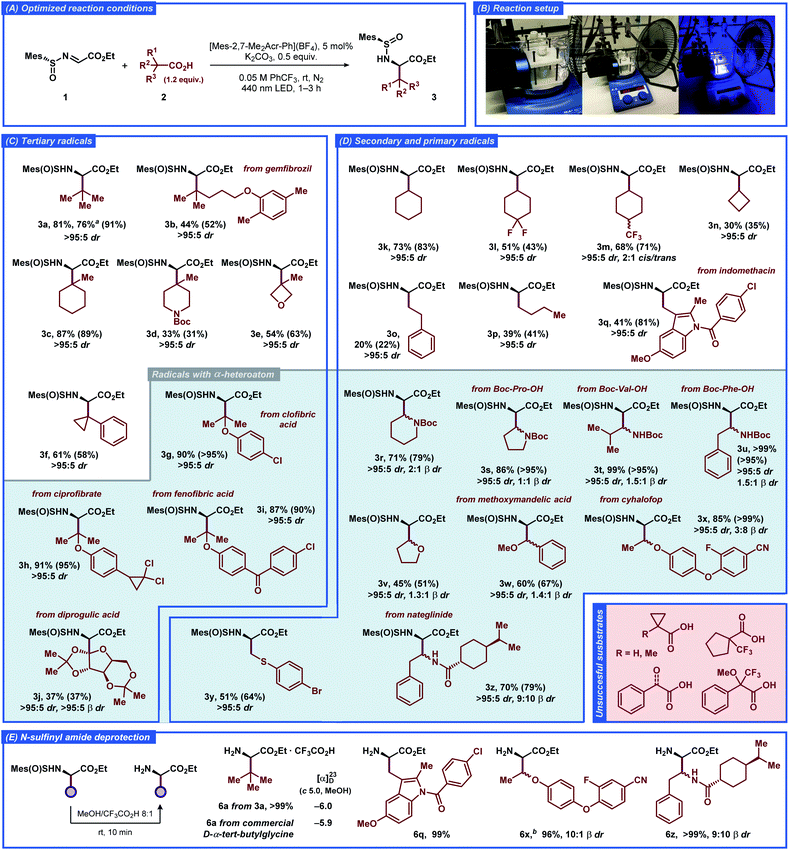 | ||
| Fig. 2 Substrate scope for the decarboxylative radical addition to glyoxylate-derived N-sulfinyl imine 1 to furnish the unnatural α-amino acid derivatives 3. The isolated yields of the products and NMR-yields from the crude reaction mixtures (in parenthesis) are reported (for details, see the ESI†). a1 mmol scale reaction. bSynthesized from the 3x-2 β-diastereomer of 3x (see the ESI†). | ||
The carboxylic acid radical precursors that furnish stabilized α-heteroatom C-radicals generally provided the addition products in good to excellent yields. Gratifyingly, N-Boc-protected α-amino acid radical precursors based on pipecolic acid, proline, valine, and phenylalanine furnished the expected amino acid derivatives 3r–u in generally excellent yields, exemplifying a prominent synthetic route to biologically active α,β-diamino acids.23 The α-O-substituted radicals derived from dialkyl (3v, 3w) and alkyl aryl ethers (3g–i, 3x) provided the expected products in moderate and excellent yields, respectively. To our delight, a primary α-S-substituted radical containing an aryl bromide functionality afforded the expected product 3y in satisfactory yield despite combining several structural features that can be deleterious under free-radical conditions. The sterically-demanding carbohydrate-based radical derived from diprogulic acid 2j delivered the monosaccharide-amino acid conjugate product 3j in satisfactory yield and excellent diastereoselectivity at both the α- and β-stereocenters (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 α dr, >95
5 α dr, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 β dr).
5 β dr).
The developed transformation was successfully applied for late-stage derivatization of a number of complex biologically-active compounds, including gemfibrozil (2b), ciprofibrate (2h), indomethacin (2q), cyhalofop (2x), nateglinide (2z), and clofibric (2g), diprogulic (2j) and fenofibric acids (2i). Besides the aforementioned functional groups, the reaction tolerated alkyl and aryl chloro- (3g–i, 3q) and fluorosubstituted substrates (3l, 3m, 3x), diaryl ether (3x), diaryl ketone (3i), chiral carboxamide (3z), and aryl cyanide (3x) functionalities, demonstrating the high versatility of the developed approach for stereoselective synthesis of unnatural α-amino acids. Notably, some of the products also displayed slight diastereoselectivity at the β-position, up to ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 β dr for products 3r and 3x. Isolation of a single α,β-diastereomer could be achieved for several products with conventional chromatography (3j, 3m, 3n, 3r, 3x). In general, the acids with strong electron-withdrawing (CF3, carbonyl) or aromatic (phenyl) substituents at the α-position did not provide the desired products, likely due to the insufficient nucleophilic character of the formed C-radicals (see Fig. 2, unsuccessful substrates). The α-H- and α-methyl-substituted cyclopropanecarboxylic acids were also inefficient, despite successful reaction with the analogous α-phenyl-substituted substrate 2f.
1 β dr for products 3r and 3x. Isolation of a single α,β-diastereomer could be achieved for several products with conventional chromatography (3j, 3m, 3n, 3r, 3x). In general, the acids with strong electron-withdrawing (CF3, carbonyl) or aromatic (phenyl) substituents at the α-position did not provide the desired products, likely due to the insufficient nucleophilic character of the formed C-radicals (see Fig. 2, unsuccessful substrates). The α-H- and α-methyl-substituted cyclopropanecarboxylic acids were also inefficient, despite successful reaction with the analogous α-phenyl-substituted substrate 2f.
The N-sulfinyl amide functionality in product 3a could be conveniently removed under mild acidic conditions in quantitative yield (Fig. 2E). The absolute configuration at the α-stereocenter in the obtained α-amino ester was then determined as (R), in complete agreement with the previous observations and the results of computational studies (for details, see the ESI†). Similarly, removal of the N-sulfinyl amide functionality was carried out for more complex products 3q, 3x and 3z, and the corresponding α-amino esters 6q, 6x and 6z were isolated in excellent yields (>95%).
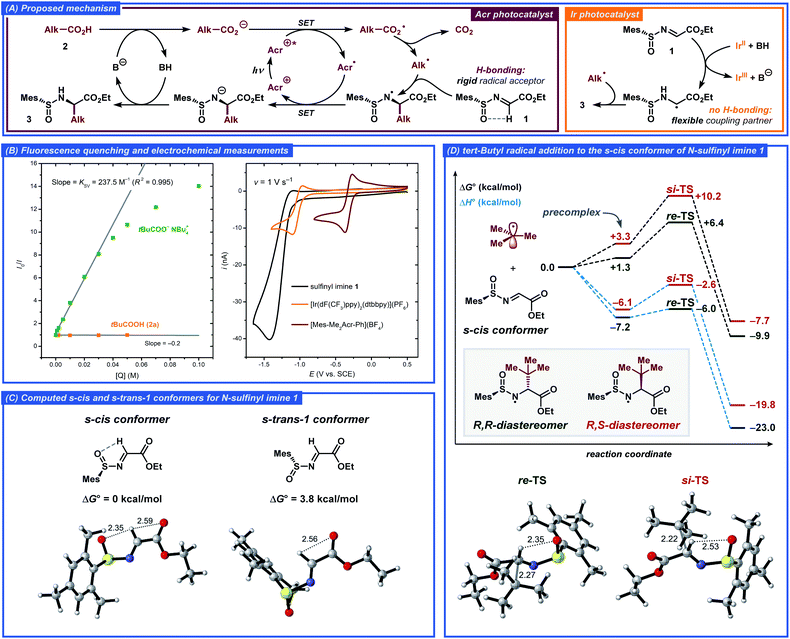
Based on literature precedents, a mechanism for the developed transformation was proposed and corroborated by fluorescence quenching and computational studies (Fig. 3).13a,24 Initially, the acridinium photocatalyst [Mes-Me2Acr-Ph]+ (Acr+) is excited by visible light (λmax ≈ 425 nm) to a highly oxidizing excited state Acr+* (E(Acr+*/Acr˙) ≈ 2.09 V vs. SCE).25 In this state, the photocatalyst can abstract an electron from the deprotonated carboxylic acid via a single-electron transfer (SET) event to generate a carboxylate radical while being reduced to the acridinium radical Acr˙. The steady-state and time-resolved fluorescence quenching measurements for tetrabutylammonium pivalate as the model radical precursor demonstrated efficient quenching of the excited acridinium photocatalyst with Stern–Volmer quenching constant KSV = 237.5 M−1 and bimolecular quenching constant kq = 6.8 × 109 M−1 s−1, while no quenching was observed for the free pivalic acid (Fig. 3B, S3 and S4†). The carboxyl radical formed via SET then extrudes CO2 to yield a C-centered radical, which undergoes addition to the N-sulfinyl imine 1 in the key step of the reaction, forming an α-alkylated N-centered radical. Finally, the N-centered radical is reduced by acridinium radical Acr˙, closing the photocatalytic cycle and furnishing the desired product 3 upon protonation (Fig. 3A).
In order to gain better understanding of the stereodetermining C–C bond forming step in the proposed mechanism, DFT calculations were performed on the M062X-D3/6-311+G(d,p) level of theory (for details, see the ESI†). First, the structure of the N-sulfinyl imine radical acceptor 1 was evaluated. Previously, Alemán and co-workers tentatively suggested an s-cis conformation around the N–S bond as being more stable in such compounds due to the hydrogen bonding between the imine proton and the sulfoxide oxygen.13a Such a conformational preference would then lead to the α-(R) product when the S(R)-sulfinyl imine is employed as the radical acceptor. This stereochemical outcome was indeed observed for both Alemán's and our catalytic system. The calculations confirmed that the s-cis conformer is more stable compared to the s-trans-1 conformer by 3.8 kcal mol−1, corresponding to >99.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 ratio between the conformers from the Boltzmann distribution at room temperature (Fig. 3C). In the s-cis conformer, the hydrogen bonding between the imine hydrogen and the sulfoxide oxygen could be observed from the non-covalent interaction (NCI) plots, while no hydrogen bonding was present in the s-trans-1 conformer (for a detailed discussion, see the ESI†).
0.2 ratio between the conformers from the Boltzmann distribution at room temperature (Fig. 3C). In the s-cis conformer, the hydrogen bonding between the imine hydrogen and the sulfoxide oxygen could be observed from the non-covalent interaction (NCI) plots, while no hydrogen bonding was present in the s-trans-1 conformer (for a detailed discussion, see the ESI†).
Subsequently, the radical addition step was evaluated for the tert-butyl radical donor and the N-sulfinyl imine radical acceptor 1. The computed Gibbs free energy and enthalpy diagrams for the reaction are presented in Fig. 3D. The formation of the (R,R)-diastereomer of 3a was found to be favored both kinetically and thermodynamically and the computed activation barrier was found to be 3.8 kcal mol−1 smaller for the re-addition compared to the si-addition, while the (R,R)-diastereomer product is 2.5 kcal mol−1 more stable compared to the (R,S)-diastereomer. Interestingly, the difference in the computed activation barriers for the re- and si-addition reactions originated almost exclusively from the enthalpic terms (ΔΔG‡ = 3.8 kcal mol−1, ΔΔH‡ = 3.4 kcal mol−1). The better stabilization of the re-TS is in part due to the stronger hydrogen bonding between the imine hydrogen and the sulfoxide oxygen for this transition state, as evident from the calculated bond distances and the NCI plots (Fig. 3D and S7†). Additionally, significant steric crowding occurs in the si-TS, where the incoming tert-butyl radical requires the mesityl group to become almost completely coplanar to the sulfoxide S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. In contrast, the mesityl group and the S
O bond. In contrast, the mesityl group and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the re-TS are out of plane by 50° while the incoming tert-butyl radical experiences no steric crowding.
O bond in the re-TS are out of plane by 50° while the incoming tert-butyl radical experiences no steric crowding.
An alternative mechanism for a related radical addition to imine derivatives was proposed by Ooi and co-workers.26 In this mechanism, the key C–C bond-forming step was found to proceed through radical–radical coupling between a C-centered radical and an α-amino radical formed by one-electron one-proton reduction of an imine substrate. However, under our conditions such a mechanistic pathway seems unlikely due to weak reducing ability of the one-electron reduced form of the employed acridinium photocatalyst (E1/2(Acr+/Acr˙) ≈ −0.32 V vs. SCE, Fig. 3B). As opposed to the conditions reported by Ooi and co-workers, where strongly reducing [Ir(ppy)2(bpy)]+-type photocatalysts (E(IrIII/IrII) ≈ −1.5 V vs. SCE) were used, electron transfer from Acr˙ to N-sulfinyl imine 1 (Ep/2 ≈ −1.24 V vs. SCE, Fig. 3B) should not be favored. However, a contribution from the radical–radical coupling pathway would explain the low diastereoselectivity (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) during formation of product 3a when the reaction was conducted with the [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) photocatalyst (Table 1, entry 2). Indeed, this photocatalyst displayed relatively low reduction potential (E1/2(IrIII/IrII) = −1.10 V vs. SCE, Fig. 3B), sufficient to reduce the N-sulfinyl imine substrate 1 to the corresponding α-amino radical. The conformation analysis of this radical then revealed nearly free rotation around the N–S bond with a barrier of ca. 2.5 kcal mol−1, while N-sulfinyl imine 1 displayed a significantly higher rotation barrier of ca. 8.0 kcal mol−1 and only one dominant conformer. Addition of the tert-butyl radical to the α-amino radical would therefore be expected to proceed with low, if any, diastereoselectivity. The low diastereoselectivity could also be explained by product epimerization during the reaction; however, no epimerization was observed when product 3a was subjected to the comparable reaction conditions with the Ir-based photocatalyst.
1 dr) during formation of product 3a when the reaction was conducted with the [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) photocatalyst (Table 1, entry 2). Indeed, this photocatalyst displayed relatively low reduction potential (E1/2(IrIII/IrII) = −1.10 V vs. SCE, Fig. 3B), sufficient to reduce the N-sulfinyl imine substrate 1 to the corresponding α-amino radical. The conformation analysis of this radical then revealed nearly free rotation around the N–S bond with a barrier of ca. 2.5 kcal mol−1, while N-sulfinyl imine 1 displayed a significantly higher rotation barrier of ca. 8.0 kcal mol−1 and only one dominant conformer. Addition of the tert-butyl radical to the α-amino radical would therefore be expected to proceed with low, if any, diastereoselectivity. The low diastereoselectivity could also be explained by product epimerization during the reaction; however, no epimerization was observed when product 3a was subjected to the comparable reaction conditions with the Ir-based photocatalyst.
Conclusions
In conclusion, a practical protocol for stereoselective synthesis of various α-amino acids has been developed, employing ubiquitous carboxylic acids as radical precursors and an organic photocatalyst under visible light irradiation. This protocol allows for synthesis of highly functionalized α-amino acids, which are challenging to prepare through traditional two-electron reaction manifolds. The protocol utilizes near-stoichiometric amounts of reagents and does not produce large quantities of waste, which is an intrinsic disadvantage of the previously described systems utilizing redox-active esters as radical precursors.Author contributions
A. S. performed optimization studies, major part of substrate scope investigation, electrochemical and spectroscopic studies, and wrote the manuscript. A. A. performed the computational studies and part of the substrate scope investigation. E. V. S., J.-Q. L., and B. B. performed part of the substrate scope investigation. A. Z. T. performed part of the analytic measurements. B. P. K. and J. M. G. assisted during data acquisition and analysis of the spectroscopical studies. M. D. K. conceived and directed the project. P. D. and M. D. K. supervised the project. All authors discussed the results and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Formas (grant no. 2019-01269), Swedish Research Council (grant no. 2020-04764), Magnus Bergvall foundation, and KTH Royal Institute of Technology to M. D. K. are gratefully acknowledged. The Olle Engkvist Foundation and the Wenner-Gren Foundations are kindly acknowledged for postdoctoral fellowships to A. S. and J. L., respectively. P. D. acknowledges financial support from the Carl Trygger Foundation for a postdoctoral fellowship to A. A. E. V. S. acknowledges Tomsk Polytechnic University Competitiveness Enhancement Program grant No 203\2020. A. Z. T. acknowledges financial support from the Ministry of Education and Science of the Russian Federation (project no. FZEN-2020-0022), and the Ecological Analytical Core Facility Center of the Kuban State University for providing the necessary equipment.Notes and references
- (a) Y. Ravikumar, S. P. Nadarajan, T. Hyeon Yoo, C. Lee and H. Yun, Trends Biotechnol., 2015, 33, 462–470 CrossRef CAS PubMed; (b) M. A. T. Blaskovich, J. Med. Chem., 2016, 59, 10807–10836 CrossRef CAS PubMed; (c) P. Neumann-Staubitz and H. Neumann, Curr. Opin. Struct. Biol., 2016, 38, 119–128 CrossRef CAS PubMed.
- P. K. Arora and A. Chauhan, Int. J. Pharm. Sci. Res., 2013, 3, 532–549 Search PubMed.
- E. De Clercq and G. Li, Clin. Microbiol. Rev., 2016, 29, 695–747 CrossRef PubMed.
- (a) L. M. Miller, J. M. Pritchard, S. J. F. Macdonald, C. Jamieson and A. J. B. Watson, J. Med. Chem., 2017, 60, 3241–3251 CrossRef CAS PubMed; (b) I. Giraud, M. Rapp, J.-C. Maurizis and J.-C. Madelmont, J. Med. Chem., 2002, 45, 2116–2119 CrossRef CAS PubMed; (c) M. Ankersen, N. L. Johansen, K. Madsen, B. S. Hansen, K. Raun, K. K. Nielsen, H. Thøgersen, T. K. Hansen, B. Peschke, J. Lau, B. F. Lundt and P. H. Andersen, J. Med. Chem., 1998, 41, 3699–3704 CrossRef CAS PubMed.
- (a) D. E. Gordon, G. M. Jang, M. Bouhaddou, J. Xu, K. Obernier, K. M. White, M. J. O'Meara, V. V. Rezelj, J. Z. Guo, D. L. Swaney, T. A. Tummino, R. Hüttenhain, R. M. Kaake, A. L. Richards, B. Tutuncuoglu, H. Foussard, J. Batra, K. Haas, M. Modak, M. Kim, P. Haas, B. J. Polacco, H. Braberg, J. M. Fabius, M. Eckhardt, M. Soucheray, M. J. Bennett, M. Cakir, M. J. McGregor, Q. Li, B. Meyer, F. Roesch, T. Vallet, A. Mac Kain, L. Miorin, E. Moreno, Z. Z. C. Naing, Y. Zhou, S. Peng, Y. Shi, Z. Zhang, W. Shen, I. T. Kirby, J. E. Melnyk, J. S. Chorba, K. Lou, S. A. Dai, I. Barrio-Hernandez, D. Memon, C. Hernandez-Armenta, J. Lyu, C. J. P. Mathy, T. Perica, K. B. Pilla, S. J. Ganesan, D. J. Saltzberg, R. Rakesh, X. Liu, S. B. Rosenthal, L. Calviello, S. Venkataramanan, J. Liboy-Lugo, Y. Lin, X.-P. Huang, Y. Liu, S. A. Wankowicz, M. Bohn, M. Safari, F. S. Ugur, C. Koh, N. S. Savar, Q. D. Tran, D. Shengjuler, S. J. Fletcher, M. C. O'Neal, Y. Cai, J. C. J. Chang, D. J. Broadhurst, S. Klippsten, P. P. Sharp, N. A. Wenzell, D. Kuzuoglu-Ozturk, H.-Y. Wang, R. Trenker, J. M. Young, D. A. Cavero, J. Hiatt, T. L. Roth, U. Rathore, A. Subramanian, J. Noack, M. Hubert, R. M. Stroud, A. D. Frankel, O. S. Rosenberg, K. A. Verba, D. A. Agard, M. Ott, M. Emerman, N. Jura, M. von Zastrow, E. Verdin, A. Ashworth, O. Schwartz, C. d'Enfert, S. Mukherjee, M. Jacobson, H. S. Malik, D. G. Fujimori, T. Ideker, C. S. Craik, S. N. Floor, J. S. Fraser, J. D. Gross, A. Sali, B. L. Roth, D. Ruggero, J. Taunton, T. Kortemme, P. Beltrao, M. Vignuzzi, A. García-Sastre, K. M. Shokat, B. K. Shoichet and N. J. Krogan, Nature, 2020, 583, 459–468 CrossRef CAS PubMed; (b) L. Zhang, D. Lin, X. Sun, U. Curth, C. Drosten, L. Sauerhering, S. Becker, K. Rox and R. Hilgenfeld, Science, 2020, 368, 409–412 CrossRef CAS PubMed; (c) L. Zhang, D. Lin, Y. Kusov, Y. Nian, Q. Ma, J. Wang, A. von Brunn, P. Leyssen, K. Lanko, J. Neyts, A. de Wilde, E. J. Snijder, H. Liu and R. Hilgenfeld, J. Med. Chem., 2020, 63, 4562–4578 CrossRef CAS PubMed; (d) C. Liu, Q. Zhou, Y. Li, L. V. Garner, S. P. Watkins, L. J. Carter, J. Smoot, A. C. Gregg, A. D. Daniels, S. Jervey and D. Albaiu, ACS Cent. Sci., 2020, 6, 315–331 CrossRef CAS PubMed.
- (a) C. Nájera and J. M. Sansano, Chem. Rev., 2007, 107, 4584–4671 CrossRef PubMed; (b) J.-A. Ma, Angew. Chem., Int. Ed., 2003, 42, 4290–4299 CrossRef CAS PubMed.
- B. Eftekhari-Sis and M. Zirak, Chem. Rev., 2017, 117, 8326–8419 CrossRef CAS PubMed.
- (a) D. J. Weix, Acc. Chem. Res., 2015, 48, 1767–1775 CrossRef CAS PubMed; (b) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed; (c) A. Kaga and S. Chiba, ACS Catal., 2017, 7, 4697–4706 CrossRef CAS; (d) M. Milan, M. Salamone, M. Costas and M. Bietti, Acc. Chem. Res., 2018, 51, 1984–1995 CrossRef CAS PubMed.
- For leading reviews on organic electrosynthesis, see: (a) A. Shatskiy, H. Lundberg and M. D. Kärkäs, ChemElectroChem, 2019, 6, 4067–4092 CrossRef CAS; (b) S. Möhle, M. Zirbes, E. Rodrigo, T. Gieshoff, A. Wiebe and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 6018–6041 ( Angew. Chem. , 2018 , 130 , 6124–6149 ) CrossRef PubMed; (c) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed; (d) M. D. Kärkäs, Chem. Soc. Rev., 2018, 47, 5786–5865 RSC.
- For leading reviews on photoredox catalysis, see: (a) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed; (b) M. D. Kärkäs, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef PubMed; (c) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- For leading reviews on light-promoted synthesis and modification of amino acids and peptides, see: (a) J.-Q. Liu, A. Shatskiy, B. S. Matsuura and M. D. Kärkäs, Synthesis, 2019, 51, 2759–2791 CrossRef CAS; (b) T. A. King, J. Mandrup Kandemir, S. J. Walsh and D. R. Spring, Chem. Soc. Rev., 2021, 50, 39–57 RSC; (c) V. A. Larionov, N. V. Stoletova and V. I. Maleev, Adv. Synth. Catal., 2020, 362, 4325–4367 CrossRef CAS; (d) F. J. Aguilar Troyano, K. Merkens, K. Anwar and A. Gómez‐Suárez, Angew. Chem., Int. Ed., 2021, 60, 1098–1115 ( Angew. Chem. , 2021 , 133 , 1112–1130 ) CrossRef CAS PubMed; (e) C. Bottecchia and T. Noël, Chem.–Eur. J., 2019, 25, 26–42 CrossRef CAS PubMed.
- (a) G. Wu, J. Wang, C. Liu, M. Sun, L. Zhang, Y. Ma, R. Cheng and J. Ye, Org. Chem. Front., 2019, 6, 2245–2249 RSC; (b) Y. Yoshimi, K. Kobayashi, H. Kamakura, K. Nishikawa, Y. Haga, K. Maeda, T. Morita, T. Itou, Y. Okada and M. Hatanaka, Tetrahedron Lett., 2010, 51, 2332–2334 CrossRef CAS; (c) S. T. J. Cullen and G. K. Friestad, Org. Lett., 2019, 21, 8290–8294 CrossRef CAS PubMed; (d) P. Ji, Y. Zhang, Y. Wei, H. Huang, W. Hu, P. A. Mariano and W. Wang, Org. Lett., 2019, 21, 3086–3092 CrossRef CAS PubMed; (e) D. P. Plasko, C. J. Jordan, B. E. Ciesa, M. A. Merrill and J. M. Hanna, Photochem. Photobiol. Sci., 2018, 17, 534–538 CrossRef CAS PubMed; (f) N. R. Patel, C. B. Kelly, A. P. Siegenfeld and G. A. Molander, ACS Catal., 2017, 7, 1766–1770 CrossRef CAS PubMed; (g) J. Guo, Q.-L. Wu, Y. Xie, J. Weng and G. Lu, J. Org. Chem., 2018, 83, 12559–12567 CrossRef CAS PubMed; (h) J. Wang, Z. Shao, K. Tan, R. Tang, Q. Zhou, M. Xu, Y.-M. Li and Y. Shen, J. Org. Chem., 2020, 85, 9944–9954 CrossRef CAS PubMed; (i) G. Wu, J. Wang, C. Liu, M. Sun, L. Zhang, Y. Ma, R. Cheng and J. Ye, Org. Chem. Front., 2019, 6, 2245–2249 RSC; (j) J. Jia, Q. Lefebvre and M. Rueping, Org. Chem. Front., 2020, 7, 602–608 RSC.
- (a) A. F. Garrido-Castro, H. Choubane, M. Daaou, M. C. Maestro and J. Alemán, Chem. Commun., 2017, 53, 7764–7767 RSC; (b) Y. Li, K. Zhou, Z. Wen, S. Cao, X. Shen, M. Lei and L. Gong, J. Am. Chem. Soc., 2018, 140, 15850–15858 CrossRef CAS PubMed.
- For selected reviews on the use of chiral sulfoxides in asymmetric synthesis, see: (a) J. Han, V. A. Soloshonok, K. D. Klika, J. Drabowicz and A. Wzorek, Chem. Soc. Rev., 2018, 47, 1307–1350 RSC; (b) M. Carmen Carreño, G. Hernández-Torres, M. Ribagorda and A. Urbano, Chem. Commun., 2009, 6129–6144 RSC.
- S. Ni, A. F. Garrido-Castro, R. R. Merchant, J. N. de Gruyter, D. C. Schmitt, J. J. Mousseau, G. M. Gallego, S. Yang, M. R. Collins, J. X. Qiao, K.-S. Yeung, D. R. Langley, M. A. Poss, P. M. Scola, T. Qin and P. S. Baran, Angew. Chem., Int. Ed., 2018, 57, 14560–14565 ( Angew. Chem. , 2018 , 130 , 14768–14773 ) CrossRef CAS PubMed.
- Y. Yoshimi, J. Photochem. Photobiol., A, 2017, 342, 116–130 CrossRef CAS.
- L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886–10889 CrossRef CAS PubMed.
- T.-Y. Shang, L.-H. Lu, Z. Cao, Y. Liu, W.-M. He and B. Yu, Chem. Commun., 2019, 55, 5408–5419 RSC.
- K. A. Margrey and D. A. Nicewicz, Acc. Chem. Res., 2016, 49, 1997–2006 CrossRef CAS PubMed.
- J. J. Maul, P. J. Ostrowski, G. A. Ublacker, B. Linclau and D. P. Curran, in Modern Solvents in Organic Synthesis, ed. P. Knochel, Springer Berlin Heidelberg, Berlin, Heidelberg, 1999, vol. 206, pp. 79–105 Search PubMed.
- (a) W. Huang, J.-L. Ye, W. Zheng, H.-Q. Dong and B.-G. Wei, J. Org. Chem., 2013, 78, 11229–11237 CrossRef CAS PubMed; (b) J. L. M. Matos, S. Vásquez-Céspedes, J. Gu, T. Oguma and R. A. Shenvi, J. Am. Chem. Soc., 2018, 140, 16976–16981 CrossRef CAS PubMed.
- (a) L. Kammer, A. Rahman and T. Opatz, Molecules, 2018, 23, 764 CrossRef PubMed; (b) J. Davies, L. Angelini, M. Alkhalifah, L. Sanz, N. Sheikh and D. Leonori, Synthesis, 2018, 50, 821–830 CrossRef CAS.
- (a) A. Viso, R. Fernández de la Pradilla, A. García and A. Flores, Chem. Rev., 2005, 105, 3167–3196 CrossRef CAS PubMed; (b) J. Wang, L. Zhang, H. Jiang and H. Liu, Curr. Pharm. Des., 2010, 16, 1252–1259 CrossRef CAS PubMed.
- J. A. Fernández-Salas, M. C. Maestro, M. M. Rodríguez-Fernández, J. L. García-Ruano and I. Alonso, Org. Lett., 2013, 15, 1658–1661 CrossRef PubMed.
- Note that the excited state of the acridinium photocatalyst is a biradical and is better represented as Acr˙–Mes˙*, see the following paper for details: S. Fukuzumi and K. Ohkubo, Org. Biomol. Chem., 2014, 12, 6059–6071 RSC.
- (a) D. Uraguchi, N. Kinoshita, T. Kizu and T. Ooi, J. Am. Chem. Soc., 2015, 137, 13768–13771 CrossRef CAS PubMed; (b) T. Kizu, D. Uraguchi and T. Ooi, J. Org. Chem., 2016, 81, 6953–6958 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc00658d |
| This journal is © The Royal Society of Chemistry 2021 |