Open Access Article
Open Access ArticleUnexpected formation of 1,2- and 1,4-bismethoxyl Sc3N@Ih-C80 derivatives via regioselective anion addition: an unambiguous structural identification and mechanism study†
Yajing
Hu
a,
Yang-Rong
Yao
b,
Xuechen
Liu
a,
Ao
Yu
a,
Xiaoming
Xie
b,
Laura
Abella
 c,
Antonio
Rodríguez-Fortea
c,
Antonio
Rodríguez-Fortea
 c,
Josep M.
Poblet
c,
Josep M.
Poblet
 c,
Takeshi
Akasaka
c,
Takeshi
Akasaka
 a,
Ping
Peng
a,
Qianyan
Zhang
*b,
Su-Yuan
Xie
b,
Fang-Fang
Li
a,
Ping
Peng
a,
Qianyan
Zhang
*b,
Su-Yuan
Xie
b,
Fang-Fang
Li
 *a and
Xing
Lu
*a and
Xing
Lu
 *a
*a
aState Key Laboratory of Material Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan, Hubei 430074, China. E-mail: ffli@hust.edu.cn; lux@hust.edu.cn
bState Key Laboratory for Physical Chemistry of Solid Surfaces, Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: xmuzhangqy@xmu.edu.cn
cDepartament de Química Física i Inorgànica, Universitat Rovira i Virgili, Marcel·lí Domingo 1, 43007 Tarragona, Spain
First published on 4th May 2021
Abstract
An attempt to achieve heterocyclic cycloadducts of Sc3N@Ih-C80via reaction with Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in the presence of tetrabutylammonium hydroxide (TBAOH) stored in CH3OH led to the formation of the unexpected bismethoxyl adducts of Sc3N@Ih-C80 (1 and 2). Further studies reveal that TBAOH in CH3OH can boost the CH3O− addition efficiently, regardless of the presence of other reagents. Single-crystal X-ray diffraction results firmly assign the molecular structures of 1 and 2 as respective 1,4- and 1,2-bismethoxyl adducts, and reveal unusual relationships between the internal Sc3N cluster and the addition modes, in addition to the unusual packing mode in view of the orientation of the methoxyl groups. Electrochemical results demonstrate smaller electrochemical gaps for 1 and 2, relative to that of Sc3N@Ih-C80, confirming their better electroactive properties. Finally, a plausible reaction mechanism involving anion addition and a radical reaction was proposed, presenting new insights into the highly selective reactions between the methoxyl anion and metallofullerenes. 1 and 2 represent the first examples of methoxyl derivatives of metallofullerenes. This work not only presents a novel and facile strategy for the controllable synthesis of alkoxylated metallofullerene derivatives, but also provides new non-cycloadducts for the potential applications of EMFs.
N in the presence of tetrabutylammonium hydroxide (TBAOH) stored in CH3OH led to the formation of the unexpected bismethoxyl adducts of Sc3N@Ih-C80 (1 and 2). Further studies reveal that TBAOH in CH3OH can boost the CH3O− addition efficiently, regardless of the presence of other reagents. Single-crystal X-ray diffraction results firmly assign the molecular structures of 1 and 2 as respective 1,4- and 1,2-bismethoxyl adducts, and reveal unusual relationships between the internal Sc3N cluster and the addition modes, in addition to the unusual packing mode in view of the orientation of the methoxyl groups. Electrochemical results demonstrate smaller electrochemical gaps for 1 and 2, relative to that of Sc3N@Ih-C80, confirming their better electroactive properties. Finally, a plausible reaction mechanism involving anion addition and a radical reaction was proposed, presenting new insights into the highly selective reactions between the methoxyl anion and metallofullerenes. 1 and 2 represent the first examples of methoxyl derivatives of metallofullerenes. This work not only presents a novel and facile strategy for the controllable synthesis of alkoxylated metallofullerene derivatives, but also provides new non-cycloadducts for the potential applications of EMFs.
Introduction
Endohedral metallofullerenes (EMFs) are a collection of hybrid molecules with metallic species trapped inside fullerene cages which show novel structures, fascinating properties and promising applications in energy storage/conversion, materials science and biomedicine.1–4 Exohedral functionalization of EMFs has shown its effectiveness to broaden their application by generation of a variety of useful derivatives. During the last few decades, many synthetic methods have been applied to modify EMFs, among which cycloadditions, such as the Bingel–Hirsch reaction,5,6 [2+2] benzyne addition,7,8 the 1,3-dipolar reaction,9–12 the Diels–Alder reaction,13 carbene addition14 and so on,5,15,16 have been widely utilized. The results show that the chemical properties of EMFs are much different from those of empty fullerenes, resulting in the fact that some chemical reactions which are effective for C60 do not work on EMFs.In contrast, non-cycloadditions of EMFs are relatively rare, and the addition is generally uncontrollable and has low selectivity. Radical reactions, such as trifluoromethylation and benzylation, represent the most effective strategies that afforded singly-bonded EMF-derivatives with identified structures. However, the high reactivity of radical species normally produced a mixture of numerous isomers, bringing challenges in selectivity and the subsequent isolation of pure adducts. For instance, trifluoromethylation of Sc3N@Ih-C80, Y2C2@Cs(6)-C82 and M@C60 (M = Gd, La) afforded plenty of isomers for the respective EMFs, i.e. Sc3N@Ih-C80(CF3)2–20,17–19 Y2C2@C82(CF3)16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and M@C60(CF3)3–5 (M = Gd, La),21 respectively, in spite that benzyl radical additions to Sc3N@Ih-C80
20 and M@C60(CF3)3–5 (M = Gd, La),21 respectively, in spite that benzyl radical additions to Sc3N@Ih-C80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and La2@Ih-C80
22 and La2@Ih-C80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 showed much better selectivity. Unexpectedly, the Bingel–Hirsch reactions of the paramagnetic M@C82 (M = La, Y and so on)6,24 also afforded singly-bonded adducts, and Lewis acid–base pairs of Sc3N@Ih-C80/Lu2@C82 and N-heterocyclic carbenes commonly bear the singly-bonded nature.25,26 Additionally, the η1-complex of Y@C2v(9)-C82Re(CO)5 stands as an example with a metal–carbon single bond.27 However, each of the above strategies requires a specific category of EMFs. For example, the Bingel–Hirsch reactions of diamagnetic EMFs always produce cycloadducts and Lewis acid–base pairs of mono-EMFs have never been obtained. Accordingly, it is currently urgent and meaningful to search for new synthetic methods to achieve non-cycloadducts of EMFs in a controllable manner so as to obtain useful compounds with novel structures and fascinating properties.
23 showed much better selectivity. Unexpectedly, the Bingel–Hirsch reactions of the paramagnetic M@C82 (M = La, Y and so on)6,24 also afforded singly-bonded adducts, and Lewis acid–base pairs of Sc3N@Ih-C80/Lu2@C82 and N-heterocyclic carbenes commonly bear the singly-bonded nature.25,26 Additionally, the η1-complex of Y@C2v(9)-C82Re(CO)5 stands as an example with a metal–carbon single bond.27 However, each of the above strategies requires a specific category of EMFs. For example, the Bingel–Hirsch reactions of diamagnetic EMFs always produce cycloadducts and Lewis acid–base pairs of mono-EMFs have never been obtained. Accordingly, it is currently urgent and meaningful to search for new synthetic methods to achieve non-cycloadducts of EMFs in a controllable manner so as to obtain useful compounds with novel structures and fascinating properties.
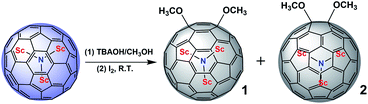
Herein, we report the unexpected addition of the methoxyl anion to Sc3N@Ih-C80via reaction with the methanol solution of tetrabutylammonium hydroxide (TBAOH) which produced the methoxyl anion (CH3O−) in situ. Only two derivatives are obtained (1 and 2), whose structures have been established by single-crystal X-ray diffraction as 1,4- and 1,2-bismethoxyl adducts, respectively. Further studies reveal an anion-radical relay mechanism for the reaction process. To the best of our knowledge, this is the first report on alkoxylated metallofullerenes and the current method differs from those available for the alkoxylation of fullerenes, which provide new insights into the controlled synthesis and formation mechanism of alkoxyl metallofullerene derivatives.
Results and discussion
In fact, our first attempt was to achieve heterocyclic cycloadducts of Sc3N@Ih-C80 through the reaction with Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
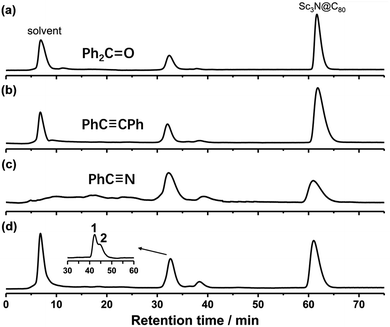
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in the presence of TBAOH, following the procedures for C60 previously reported by Gao and coworkers.28 3 mg Sc3N@Ih-C80 was dissolved in 10 ml anhydrous o-DCB, and then 10 equivalent of TBAOH (stored in CH3OH) was added into the solution, the color of which rapidly changed from light brown to dark green (Fig. S1, ESI†). The in situ UV-Vis-NIR spectra of the reaction mixture in Fig. S1† showed that the absorption of Sc3N@Ih-C80 is red-shifted from 371 to 391 nm, and a new peak appears at about 568 nm, indicating the formation of an anionic intermediate. 100 equivalent of Ph2C
N in the presence of TBAOH, following the procedures for C60 previously reported by Gao and coworkers.28 3 mg Sc3N@Ih-C80 was dissolved in 10 ml anhydrous o-DCB, and then 10 equivalent of TBAOH (stored in CH3OH) was added into the solution, the color of which rapidly changed from light brown to dark green (Fig. S1, ESI†). The in situ UV-Vis-NIR spectra of the reaction mixture in Fig. S1† showed that the absorption of Sc3N@Ih-C80 is red-shifted from 371 to 391 nm, and a new peak appears at about 568 nm, indicating the formation of an anionic intermediate. 100 equivalent of Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N was then added into the green solution which was quenched by adding 50 equivalent of I2 after stirring for 2 h. The obtained solid products from all three reactions were dissolved in toluene and analyzed by HPLC (Fig. 1a–c). To our surprise, the reactions do not afford any cycloadducts which have been found for C60 (according to mass spectrum data). As a matter of fact, we eventually realized that Ph2C
N was then added into the green solution which was quenched by adding 50 equivalent of I2 after stirring for 2 h. The obtained solid products from all three reactions were dissolved in toluene and analyzed by HPLC (Fig. 1a–c). To our surprise, the reactions do not afford any cycloadducts which have been found for C60 (according to mass spectrum data). As a matter of fact, we eventually realized that Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N did not participate in the reaction as we expected. Therefore, the reaction of Sc3N@Ih-C80 and the CH3OH solution of TBAOH without the addition of Ph2C
N did not participate in the reaction as we expected. Therefore, the reaction of Sc3N@Ih-C80 and the CH3OH solution of TBAOH without the addition of Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
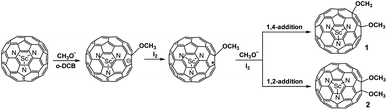
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N was conducted according to the same procedure as described above (Scheme 1, Fig. 1d). As shown in Fig. 1, the HPLC profiles of the reaction mixtures, either with or without Ph2C
N was conducted according to the same procedure as described above (Scheme 1, Fig. 1d). As shown in Fig. 1, the HPLC profiles of the reaction mixtures, either with or without Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/PhC
O/PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh/PhC
CPh/PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, showed that the adduct peaks emerged at around 32 min after stirring for 2 hours. Further separation gave the pure isomers of 1 and 2 (Fig. S2, ESI†), which have singly-bonded structures (vide infra).
N, showed that the adduct peaks emerged at around 32 min after stirring for 2 hours. Further separation gave the pure isomers of 1 and 2 (Fig. S2, ESI†), which have singly-bonded structures (vide infra).
 | ||
| Scheme 1 Reaction of Sc3N@Ih-C80 with a methanol solution of TBAOH in o-DCB at room temperature, forming the unexpected bismethoxyl derivatives 1 and 2. | ||
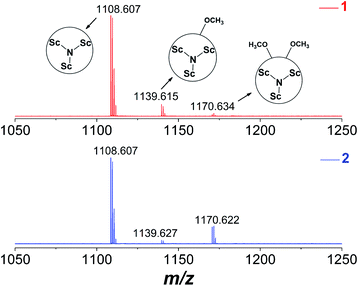
1 and 2 are fully characterized by MALDI-TOF MS, NMR, Vis-NIR spectroscopy and single-crystal X-ray diffraction. Fig. 2 displays the mass spectra of 1 and 2 where the peaks at around m/z 1170.6 correspond to the dimethoxylated adducts Sc3N@Ih-C80(OCH3)2. The peaks at around 1139.6 and 1108.6 are ascribed to the fragments of Sc3N@Ih-C80(OCH3) and Sc3N@Ih-C80 due to the loss of one or two OCH3 groups, respectively, confirming the successful attachment of two methoxyl groups on to Sc3N@Ih-C80.
1H NMR spectra (Fig. 3a) provide additional structural features of 1 and 2. Only one single signal for the two OCH3 groups in either 1 or 2 is observed, in detail, 3.76 ppm in 1 and 3.84 ppm in 2, indicative of the highly symmetric placement of the two equivalent OCH3 groups. Since there are more than 40 possible isomers for Sc3N@Ih-C80 bisadducts, the formation of merely two derivatives reveals high regioselectivity. The 13C NMR spectrum of product 1 is shown in Fig. 3b. 38 (including one doublet signal) resonances for the 78 sp2Ih-C80 cage-carbons are detected from 120.26 to 170.56 ppm, confirming a symmetrical addition pattern of 1. Resonance for the two sp3Ih-C80 cage-carbons bonded to the OCH3 groups appears at 53.76 ppm, while the peak for the two sp3 OCH3 carbons appears at 29.15 ppm. Although, the 13C NMR data collection of 2 was not successful due to its relatively low yield, the 1H NMR spectra of the two products could alternatively elucidate the symmetry of the products, and its X-ray structure provided the absolute structure.
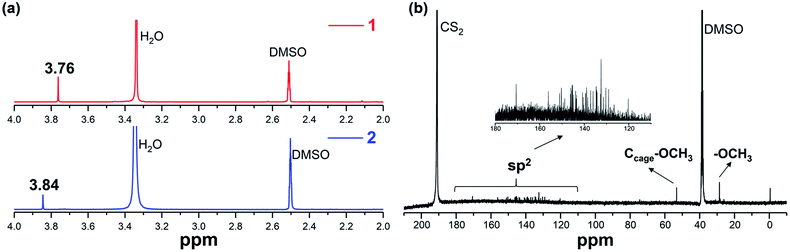 | ||
| Fig. 3 (a) 1H NMR spectra of 1 and 2 and (b) 13C NMR spectrum of 1 recorded in CS2 with DMSO-d6 as the external lock solvent. | ||
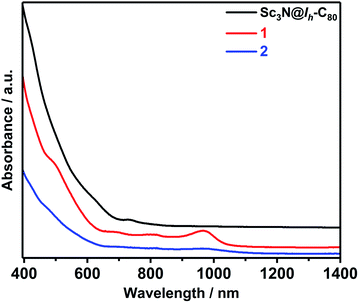
Vis-NIR spectroscopy is a diagnostic tool to estimate the addition position on fullerenes, especially for the highly symmetric C60 and Sc3N@C80 cages. Fig. 4 shows the absorption spectra of 1 and 2. The characteristic peak at 968 nm of 1 is very similar to those of the previously reported 1,4-Sc3N@C80(CH2Ph)2 (898 nm)22 and 1,4-Sc3N@C80(CF3)2 (920 nm),17 suggesting a 1,4-addition pattern. However the absorption curve of 2 is analogous to those of the [5,6]-adducts of Sc3N@C80,7,8,29 indicative of a 1,2-[5,6]-fashion.
Finally, the molecular structures of 1 and 2 are unambiguously established by single crystal X-ray diffraction. The co-crystals of [1]·2DPC and 2[2]·4DPC·2toluene were obtained by slow evaporation of the toluene/CS2 solution of the corresponding derivatives and decapyrrylcorannulene (DPC).30 Both crystals fall into the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with variations in the asymmetric unit. 1 shows disorder of both the C80 cage and the Sc3N cluster. Two orientations of the C80 cage with equal occupancy and four orientations of the Sc3N cluster with occupancies of 0.40, 0.35, 0.15 and 0.10 are observed. Interestingly, the C80 cage of 2 is highly ordered and only two positions of the Sc3N cluster with occupancies of 0.82 and 0.18, respectively, are presented.
space group with variations in the asymmetric unit. 1 shows disorder of both the C80 cage and the Sc3N cluster. Two orientations of the C80 cage with equal occupancy and four orientations of the Sc3N cluster with occupancies of 0.40, 0.35, 0.15 and 0.10 are observed. Interestingly, the C80 cage of 2 is highly ordered and only two positions of the Sc3N cluster with occupancies of 0.82 and 0.18, respectively, are presented.
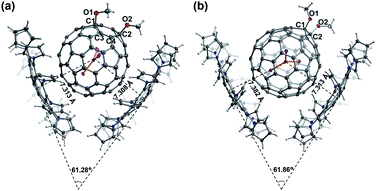
Fig. 5 shows the molecular structures of 1 and 2 with only the major components together with the cocrystallized DPC molecules. The pairs of DPC and 1 or 2 are assembled into a similar V-shaped configuration with an angle of 61.28° for [1]·2DPC (Fig. 5a) and 61.86° for [2]·2DPC (Fig. 5b). The distance between the centroid of the C80 cage and the central five-membered ring of DPC is 7.313 and 7.308 Å for 1 and 7.382 and 7.313 Å for 2. The cage-plane distance in 1 and 2 is ca. 3.3–3.6 Å, indicating π–π interactions between the fullerene cages and DPC.
It is rather evident that two OCH3 groups are located at the para-position of a six-membered ring, i.e., 1,4-bisaddition on [566]-carbons in 1, while in 2 are on a [5,6]-ring junction, namely, 1,2-bisaddition. It is noteworthy that the addition patterns show somehow influence on the orientation of the inner cluster. The planar Sc3N cluster in 1 is orthogonal with the plane crossing the sites of addition, namely, C1–O1–O2–C2, but the metal cluster in 2 is nearly parallel to the plane of C1–O1–O2–C2. Thus, it is proposed that exohedral modification, even merely the addition pattern of bisaddition, is practical to control the cluster orientation.
Notably, the conformations of the two OCH3 groups in 1 and 2 also differ significantly. The two OCH3 groups in 1 are unexpectedly arranged towards the same direction, which is unusual and different from the situations observed in the previously reported 1,4-Sc3N@C80(CH2Ph)2 and 1,4-Sc3N@C80(CF3)2 where the two addition groups are aligned in the opposite position to release steric hindrance.17,22 We speculate that only the intermolecular steric effect between DPC and 1, instead of any electronic influence, is responsible for the abnormal alignment of the two OCH3 groups since there are no C–H⋯π interactions between OCH3 and DPC.
The packing mode of [1]·2DPC (Fig. 6a) reveals that one of the OCH3 groups (labeled as A) points to three DPC molecules with long intermolecular distances (red circle). Accordingly, the other OCH3 group (labeled as B) is not allowed to point to the opposite position relative to group A, because it will cause large steric hindrance with the other two adjacent DPC molecules. Besides, the addition pattern of the two OCH3 groups in 2 is also abnormal since they show a back-and-forth arrangement to decrease both the intramolecular and intermolecular repulsive forces. One OCH3 group is trapped into the cavity of two DPC molecules (red circle in Fig. 6b) so that the other OCH3 group can only choose the back orientation, facing three DPC molecules without obvious C–H⋯π interactions. It is thus concluded that the abnormal configurations of the two OCH3 groups in both 1 and 2 are possibly caused by the intermolecular steric effects of the cocrystallized DPC molecules, which is proved by our theoretical calculations. The optimized structures of 1 and 2 without DPC show that the two OCH3 are arranged oppositely (Fig. S4, ESI†). In addition, both 1 and 2 are aligned with DPC molecules to form a one-dimensional zigzag supramolecular chain along the c and b axis, respectively. The fullerenes are arranged in a head-to-tail mode in the packing structures of 1 (along the b axis) and 2 (along the c axis), respectively (Fig. 6).
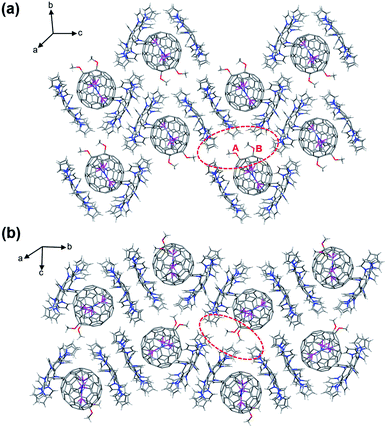 | ||
| Fig. 6 Packing structures of [1]·2DPC (a) and [2]·2DPC (b). Only one cage orientation and the major Sc3N cluster are shown and the solvent molecules are omitted for clarity. | ||
The electrochemical properties of 1 and 2 are studied by cyclic voltammetry (CV) and the results are listed in Fig. 7 and Table 1. The 1,4-adduct 1 displays two irreversible oxidations at +0.40 and +0.59 V and three reversible reductions at −1.17, −1.33 and −1.74 V, respectively, a behavior slightly different from that of the 1,4-Sc3N@Ih-C80(CF3)2 which displays only one reversible anodic process.17 Similarly, the 1,2-bisadduct 2 also exhibits two irreversible oxidations at +0.43 and +0.98 V and three reversible reductions at −1.03, −1.40 and −1.72 V, respectively, which is similar to those of the previous reported Sc3N@Ih-C80 [5,6]-monoadducts.7,31,32 Compared with the corresponding redox potentials of pristine Sc3N@Ih-C80 (Table 1), the first reduction potentials of 1 and 2 are less negative and their first oxidation potentials are less positive, resulting in smaller electrochemical gaps of the derivatives (1.57 V for 1 and 1.46 V for 2) than that of Sc3N@Ih-C80 (1.88 V) and accordingly higher reactivities, which are beneficial to their future application in photovoltaics and electronics.
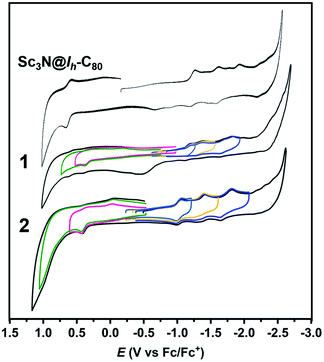 | ||
| Fig. 7 Cyclic voltammograms of Sc3N@Ih-C80, 1 and 2 recorded in a 0.05 M solution of TBAPF6 in o-DCB at a scan rate of 100 mV s−1. | ||
Currently, conversion of halofullerenes to alkoxy fullerenes by substituting Cl/Br groups is the most common approach to obtain alkoxy fullerenes,33–39 which is a complex and multi-step process, requiring the synthesis of halofullerenes first. However, neither halo-EMFs nor alkoxyl-EMFs have been reported due to the unrecognized chemical properties of EMFs. Accordingly, our finding presents a novel solution to the controllable synthesis of alkoxylated fullerene derivatives. We then try to understand the reaction mechanism of this unexpected bis-methoxylation process.
In fact, our initial purpose was to synthesize heterocyclic cycloadducts of Sc3N@Ih-C80 following the method reported by Gao and co-workers where C60, tetrabutylammonium hydroxide (TBAOH) and benzonitrile (PhCN) were involved.28 In the case of C60, OH− from TBAOH acts as an oxygen nucleophile to form a dianionic intermediate C60−–O− which then attacks the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond of PhCN to produce an O,N-heterocyclic adduct. However, the same process does not occur on Sc3N@Ih-C80 even when the more reactive Ph2C
N triple bond of PhCN to produce an O,N-heterocyclic adduct. However, the same process does not occur on Sc3N@Ih-C80 even when the more reactive Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhCN were used, and no any cycloadducts have been detected in the reaction mixture, indicating that the reaction mode was changed by the Sc3N cluster.
CPh or PhCN were used, and no any cycloadducts have been detected in the reaction mixture, indicating that the reaction mode was changed by the Sc3N cluster.
Based on the experimental facts, we propose an anion-radical relay mechanism for the formation of 1 and 2 (Scheme 2). First, TBAOH, as a strong organic base, deprotonates CH3OH to generate CH3O− in the less polar solvent o-DCB. Due to the better miscibility of CH3OH with o-DCB than TBAOH, CH3O− prevails in the addition to Sc3N@C80 over OH− (from TBAOH) in the o-DCB solution. Then, the monoanion [Sc3N@C80(OCH3)]− is oxidized to the [Sc3N@C80(OCH3)]˙ radical by I2 which accepts another CH3O− to form the dimethoxyfullerene anion [Sc3N@C80(OCH3)2]−. Final oxidation by I2 gives the final products of Sc3N@C80(OCH3)2 with either 1,2- or 1,4-addition patterns. This anion radical relay process ensures the high selectivity and controllability of the reaction. The possibility of forming dianionic methoxyfullerene intermediates before I2-oxidation could be reasonably excluded by considering the high charge density on the cage and thus the low stability in solution.
Most electrochemical reactions involving fullerenes or EMF anions follow the “electron transfer-SN2 reaction” mechanism.15,16,40 But there is an exception that the electrochemical reaction of a C60 derivative reported by Wang's group follows a “stepwise one-electron reduction and protonation” mechanism, in which the monoanionic intermediate was protonated to generate a free radical on the adjacent carbon.41 The anion radical relay mechanism of methoxylation of Sc3N@C80 follows the electron transfer process proposed in most electrochemical reactions, but is followed by a process in which the anion is directly oxidized to a free radical.
It is known that the hydroxylation reactions of C60 and EMFs were conducted by adding strong base NaOH containing TBAOH as a catalyst to the fullerene solution. The OH− as a nucleophile was added on the fullerene cage and a negative charge was transferred to the carbon cage. Then, the negative charge was neutralized by H+ addition.42,43 Moreover, the reactions proceeded in an uncontrolled manner and generated multi-hydroxy derivatives,42,43 which makes it difficult to perform unambiguous structural identification. However, in our methoxylation reaction of Sc3N@C80, TBAOH (1.0 M in CH3OH) acted as a deprotonating agent of CH3OH rather than a nucleophile to participate in the reaction. Therefore, no hydroxyl derivatives were formed, and instead methoxyl derivatives were formed. More importantly, the methoxylation reaction was carried out in a controlled manner. The unique stepwise addition pattern ensured high regioselectivity of the reaction, thereby promoting the separation, purification and structure determination of the products.
Conclusions
In summary, we discovered for the first time that the methoxyl anion (CH3O−) could react with Sc3N@Ih-C80 in a highly regioselective manner to produce merely two isomers (1,4-adduct for 1 and 1,2-adduct for 2) out of more than 40 possibilities, representing the first examples of bismethoxyl derivatives of metallofullerenes. Their molecular structures and electrochemical properties are systematically investigated by a collection of experimental techniques. Based on the experimental evidence, an anion-radical relay mechanism for the formation of 1 and 2 has been proposed which is highly dependent on the type of solvents used, revealing an unprecedented reaction process for metallofullerenes. Significantly, we provide a facile synthetic strategy for alkoxylation of metallofullerenes in a highly regioselective manner under mild conditions.Experimental
Materials and methods
Sc3N@Ih-C80 was synthesized by the direct current arc discharge method and isolated by high performance liquid chromatography (HPLC).7 Tetrabutylammonium hydroxide (TBAOH) (1.0 M in CH3OH), anhydrous o-DCB, Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh, anhydrous PhC
CPh, anhydrous PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, I2, toluene, DMSO-d6 and CS2 were purchased from Sigma-Aldrich and were used as received. Tetra-n-butylammonium-hexafluorophosphate (TBAPF6) was recrystallized from absolute ethanol and dried in a vacuum before use. The purity of the products 1 and 2 was verified by HPLC (LaboACE LC-5060, Japan Analytical Industry Co., Ltd., Japan) equipped with Buckyprep and 5PBB columns with toluene as the eluent. Vis-NIR spectra were recorded using a SHIMADZU UV-3600 spectrophotometer. MALDI-TOF MS was conducted on a Bruker autoflex speed mass spectrometer. The 1H NMR spectra were recorded using a Bruker Ascend™ 600 MHz NMR spectrometer. The 13C NMR spectrum was recorded using a Bruker Ascend™ 400 MHz NMR spectrometer. CV studies were conducted in a one-compartment cell connected to a CHI 760E workstation in a solution of o-DCB containing 0.05 M TBAPF6. A 2 mm diameter glassy carbon disk was used as the working electrode, and a Pt wire and a Ag wire as the counter and pseudo-reference electrodes, respectively. Ferrocene (Fc) was added to the solution at the end of each experiment as an internal standard. All reactions were carried out in a glove box with Ar protection.
N, I2, toluene, DMSO-d6 and CS2 were purchased from Sigma-Aldrich and were used as received. Tetra-n-butylammonium-hexafluorophosphate (TBAPF6) was recrystallized from absolute ethanol and dried in a vacuum before use. The purity of the products 1 and 2 was verified by HPLC (LaboACE LC-5060, Japan Analytical Industry Co., Ltd., Japan) equipped with Buckyprep and 5PBB columns with toluene as the eluent. Vis-NIR spectra were recorded using a SHIMADZU UV-3600 spectrophotometer. MALDI-TOF MS was conducted on a Bruker autoflex speed mass spectrometer. The 1H NMR spectra were recorded using a Bruker Ascend™ 600 MHz NMR spectrometer. The 13C NMR spectrum was recorded using a Bruker Ascend™ 400 MHz NMR spectrometer. CV studies were conducted in a one-compartment cell connected to a CHI 760E workstation in a solution of o-DCB containing 0.05 M TBAPF6. A 2 mm diameter glassy carbon disk was used as the working electrode, and a Pt wire and a Ag wire as the counter and pseudo-reference electrodes, respectively. Ferrocene (Fc) was added to the solution at the end of each experiment as an internal standard. All reactions were carried out in a glove box with Ar protection.
Synthetic procedures of 1 and 2
3 mg Sc3N@Ih-C80 was dissolved in 10 ml anhydrous o-DCB, and then 10 equivalent of TBAOH (stored in CH3OH) was added into the solution, which was quenched by the addition of 50 equivalent of I2 after stirring for 2 h. The obtained solid products from the reaction were dissolved in toluene and analyzed by HPLC (Fig. 1d). Products 1 and 2 were isolated and purified by two-step HPLC.Compound 1 (yield: 29.7%): MALDI-TOF MS (negative ionization mode): m/z 1170.634; Vis-NIR 968 nm; 1H NMR (600 MHz, CS2/DMSO, 25 °C, TMS): δ = 3.76 ppm (s, 6H, –OCH3). 13C NMR (400 MHz, CS2/DMSO, 25 °C, TMS): δ = 170.56 (2C), 163.87 (2C), 156.13 (2C), 151.09 (2C), 150.25 (2C), 149.37 (2C), 148.79 (2C), 146.27 (2C), 146.05 (2C), 145.66 (2C), 145.45 (2C), 145.24 (2C), 144.90 (2C), 143.89 (2C), 143.63 (2C), 143.44 (2C), 140.82 (2C), 140.42 (2C), 140.27 (2C), 139.40 (2C), 139.33 (2C), 138.98 (2C), 138.93 (2C), 138.47 (2C), 137.13 (2C), 136.05 (2C), 134.67 (2C), 134.59 (2C), 134.47 (2C), 134.33 (2C), 134.25 (2C), 132.72 (2C), 132.45 (4C), 131.45 (2C), 130.32 (2C), 129.12 (2C), 126.60 (2C), 120.26 (2C), 53.76 (2C, sp3, Ccage–OCH3), 29.15 ppm (2C, sp3, –OCH3).
Compound 2 (yield: 10.9%): MALDI-TOF MS (negative ionization mode): m/z 1170.622; Vis-NIR 992 nm; 1H NMR (600 MHz, CS2/DMSO, 25 °C, TMS): δ = 3.84 ppm (s, 6H, –OCH3).
Single crystal X-ray crystallography
0.5 mg of 1 was dissolved in 0.5 ml carbon disulfide in a 5.0 ml centrifuge tube. Then, a toluene solution of DPC was added into the tube. The mixed solution, in which DPC and fullerene have a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was kept undisturbed at room temperature for slow evaporation. After two weeks, black sheet-like crystals were formed. A crystal of 0.12 mm × 0.08 mm × 0.01 mm dimension was mounted in the 100 K nitrogen cold stream on an XtaLAB PRO MM007HF diffractometer with Cu-Kα radiation (λ = 1.5406 Å). The CrystalClear software package (Rigaku) was used for data collection, cell refinement, and data reduction. The crystal structure of 1 was solved by direct methods and refined by the full-matrix method based on F2 using the SHELXLTL software package.44 All the non-hydrogen atoms were refined anisotropically and the positions of the hydrogen atoms were generated geometrically.
1, was kept undisturbed at room temperature for slow evaporation. After two weeks, black sheet-like crystals were formed. A crystal of 0.12 mm × 0.08 mm × 0.01 mm dimension was mounted in the 100 K nitrogen cold stream on an XtaLAB PRO MM007HF diffractometer with Cu-Kα radiation (λ = 1.5406 Å). The CrystalClear software package (Rigaku) was used for data collection, cell refinement, and data reduction. The crystal structure of 1 was solved by direct methods and refined by the full-matrix method based on F2 using the SHELXLTL software package.44 All the non-hydrogen atoms were refined anisotropically and the positions of the hydrogen atoms were generated geometrically.
Crystal data of [1]·2DPC (CCDC-2049232): C202H86N21O2Sc3, Mw = 2973.79 amu, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 14.4545(3) Å, b = 17.5373(5) Å, c = 30.8351(5) Å, α = 96.365(2)°, β = 101.776(2)°, γ = 96.180(2)°, V = 7536.3(3) Å3, T = 100 K, Z = 2, R indices (all data) R1 = 0.1158, wR2 = 0.3470, GOF (on F2) = 1.045.
, a = 14.4545(3) Å, b = 17.5373(5) Å, c = 30.8351(5) Å, α = 96.365(2)°, β = 101.776(2)°, γ = 96.180(2)°, V = 7536.3(3) Å3, T = 100 K, Z = 2, R indices (all data) R1 = 0.1158, wR2 = 0.3470, GOF (on F2) = 1.045.
Black sheet-like crystals of 2 were obtained similarly. Crystallographic characterization of a piece of cocrystal (0.03 mm × 0.03 mm × 0.02 mm) was performed at 100 K by using synchrotron radiation (0.65250 Å) with a MarCCD detector at the beamline BL17B station of Shanghai Synchrotron Radiation Facility. The multiscan method was used for absorption corrections. The crystal structure of 2 was solved by the direct methods and refined by the full-matrix method based on F2 using the SHELXLTL software package.44 All the non-hydrogen atoms were refined anisotropically and the positions of the hydrogen atoms were generated geometrically.
Crystal data of [2]·2DPC·toluene (CCDC-2049233): C209H94N21O2Sc3, Mw = 3065.93 amu, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 14.4947(8) Å, b = 31.8931(18) Å, c = 32.660(2) Å, α = 91.077(1)°, β = 95.165(2)°, γ = 101.899(1)°, V = 14
, a = 14.4947(8) Å, b = 31.8931(18) Å, c = 32.660(2) Å, α = 91.077(1)°, β = 95.165(2)°, γ = 101.899(1)°, V = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703.0(15) Å3, T = 100 K, Z = 4, R indices (all data) R1 = 0.1613, wR2 = 0.4173, GOF (on F2) = 1.025.
703.0(15) Å3, T = 100 K, Z = 4, R indices (all data) R1 = 0.1613, wR2 = 0.4173, GOF (on F2) = 1.025.
Computational details
All computations were performed using the density functional theory (DFT) methodology with the ADF 2016 program. The exchange-correlation functionals of Becke and Perdew (BP86) and the Slater triple-ζ + polarization (TZP) basis sets were selected for geometry optimizations.45,46Author contributions
Y. Hu: data collection, writing-original draft; Y.-R. Yao: crystal structure analysis; X. Liu and A. Yu: synthesis of raw materials; X. Xie, Q. Zhang and S.-Y. Xie: synthesis of DPC; L. Abella, A. Rodríguez-Fortea, and J. M. Poblet: theoretical calculations; T. Akasaka: writing-commenting and editing; P. Peng: project management; F.-F. Li: conceptualization, supervision, writing-commenting and editing; X. Lu: conceptualization, writing-commenting and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant No. 22071070, 21925104, 21971077, 51772111 and 21672076) and the Spanish Ministry of Science (grant CTQ2017-87269-P) is gratefully acknowledged.Notes and references
- S. Yang, T. Wei and F. Jin, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
- K. Wang, X. Liu, R. Huang, C. Wu, D. Yang, X. Hu, X. Jiang, J. C. Duchamp, H. Dorn and S. Priya, ACS Energy Lett., 2019, 4, 1852–1861 CrossRef CAS.
- K. Zhang, C. Wang, M. Zhang, Z. Bai, F.-F. Xie, Y.-Z. Tan, Y. Guo, K.-J. Hu, L. Cao, S. Zhang, X. Tu, D. Pan, L. Kang, J. Chen, P. Wu, X. Wang, J. Wang, J. Liu, Y. Song, G. Wang, F. Song, W. Ji, S.-Y. Xie, S.-F. Shi, M. A. Reed and B. Wang, Nat. Nanotechnol., 2020, 15, 1019–1024 CrossRef PubMed.
- T. Wang and C. Wang, Small, 2019, 15, 1901522 CrossRef CAS PubMed.
- Y. Hu, A. Solé-Daura, Y.-R. Yao, X. Liu, S. Liu, A. Yu, P. Peng, J. M. Poblet, A. Rodríguez-Fortea, L. Echegoyen and F.-F. Li, Chem.–Eur. J., 2020, 26, 1748–1753 CrossRef CAS PubMed.
- W. Shen, L. Yang, B. Li, P. Jin, B. Yu, H. Cong, T. Akasaka and X. Lu, Chem. Commun., 2020, 56, 14357–14360 RSC.
- F.-F. Li, J. R. Pinzón, B. Q. Mercado, M. M. Olmstead, A. L. Balch and L. Echegoyen, J. Am. Chem. Soc., 2011, 133, 1563–1571 CrossRef CAS PubMed.
- G.-W. Wang, T.-X. Liu, M. Jiao, N. Wang, S.-E. Zhu, C. Chen, S. Yang, F. L. Bowles, C. M. Beavers, M. M. Olmstead, B. Q. Mercado and A. L. Balch, Angew. Chem., Int. Ed., 2011, 50, 4658–4662 CrossRef CAS PubMed.
- O. Semivrazhskaya, S. Aroua, M. Yulikov, A. Romero-Rivera, S. Stevenson, M. Garcia-Borràs, S. Osuna and Y. Yamakoshi, J. Am. Chem. Soc., 2020, 142, 12954–12965 CrossRef CAS PubMed.
- Y. Chai, X. Liu, B. Wu, L. Liu, Z. Wang, Y. Weng and C. Wang, J. Am. Chem. Soc., 2020, 142, 4411–4418 CrossRef CAS PubMed.
- O. Semivrazhskaya, A. Romero-Rivera, S. Aroua, S. I. Troyanov, M. Garcia-Borràs, S. Stevenson, S. Osuna and Y. Yamakoshi, J. Am. Chem. Soc., 2019, 141, 10988–10993 CrossRef CAS PubMed.
- S. Yang, M. Chen, R. Guan, B. Li, L. Yang, C. Niu, P. Jin and G.-W. Wang, Angew. Chem., Int. Ed., 2021, 60, 7880–7886 CrossRef PubMed.
- Y. García-Rodeja, M. Solà and I. Fernández, J. Org. Chem., 2018, 83, 3285–3292 CrossRef PubMed.
- M. Yamada, Y. Tanabe, J.-S. Dang, S. Sato, N. Mizorogi, M. Hachiya, M. Suzuki, T. Abe, H. Kurihara, Y. Maeda, X. Zhao, Y. Lian, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2016, 138, 16523–16532 CrossRef CAS PubMed.
- F.-F. Li, A. Rodríguez-Fortea, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2011, 133, 2760–2765 CrossRef CAS PubMed.
- F.-F. Li, A. Rodríguez-Fortea, P. Peng, G. A. Campos Chavez, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2012, 134, 7480–7487 CrossRef CAS PubMed.
- N. B. Shustova, A. A. Popov, M. A. Mackey, C. E. Coumbe, J. P. Phillips, S. Stevenson, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2007, 129, 11676–11677 CrossRef CAS PubMed.
- N. B. Shustova, D. V. Peryshkov, I. V. Kuvychko, Y.-S. Chen, M. A. Mackey, C. E. Coumbe, D. T. Heaps, B. S. Confait, T. Heine, J. P. Phillips, S. Stevenson, L. Dunsch, A. A. Popov, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2011, 133, 2672–2690 CrossRef CAS PubMed.
- S. Yang, C. Chen, M. Jiao, N. B. Tamm, M. A. Lanskikh, E. Kemnitz and S. I. Troyanov, Inorg. Chem., 2011, 50, 3766–3771 CrossRef CAS PubMed.
- F. Jin, N. B. Tamm, S. I. Troyanov and S. Yang, J. Am. Chem. Soc., 2018, 140, 3496–3499 CrossRef CAS PubMed.
- A. Nakagawa, M. Nishino, H. Niwa, K. Ishino, Z. Wang, H. Omachi, K. Furukawa, T. Yamaguchi, T. Kato, S. Bandow, J. Rio, C. Ewels, S. Aoyagi and H. Shinohara, Nat. Commun., 2018, 9, 3073 CrossRef PubMed.
- C. Shu, C. Slebodnick, L. Xu, H. Champion, T. Fuhrer, T. Cai, J. E. Reid, W. Fu, K. Harich, H. C. Dorn and H. W. Gibson, J. Am. Chem. Soc., 2008, 130, 17755–17760 CrossRef CAS PubMed.
- L. Bao, M. Chen, C. Pan, T. Yamaguchi, T. Kato, M. Olmstead, A. Balch, T. Akasaka and L. Xing, Angew. Chem., Int. Ed., 2016, 55, 4242–4246 CrossRef CAS PubMed.
- L. Feng, T. Nakahodo, T. Wakahara, T. Tsuchiya, Y. Maeda, T. Akasaka, T. Kato, E. Horn, K. Yoza, N. Mizorogi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 17136–17137 CrossRef CAS PubMed.
- M. Chen, L. Bao, M. Ai, W. Shen and X. Lu, Chem. Sci., 2016, 7, 2331–2334 RSC.
- W. Shen, L. Yang, Y. Wu, L. Bao, Y. Li, P. Jin, H. Fang, Y. Xie and X. Lu, J. Org. Chem., 2019, 84, 606–612 CrossRef CAS PubMed.
- Y.-P. Xie, C. Pan, L. Bao, Z. Slanina, T. Akasaka and X. Lu, Organometallics, 2019, 38, 2259–2263 CrossRef CAS.
- W.-W. Chang, Z.-J. Li, W.-W. Yang and X. Gao, Org. Lett., 2012, 14, 2386–2389 CrossRef CAS PubMed.
- T. Cai, C. Slebodnick, L. Xu, K. Harich, T. E. Glass, C. Chancellor, J. C. Fettinger, M. M. Olmstead, A. L. Balch, H. W. Gibson and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 6486–6492 CrossRef CAS PubMed.
- Y.-Y. Xu, H.-R. Tian, S.-H. Li, Z.-C. Chen, Y.-R. Yao, S.-S. Wang, X. Zhang, Z.-Z. Zhu, S.-L. Deng, Q. Zhang, S. Yang, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Nat. Commun., 2019, 10, 485 CrossRef CAS PubMed.
- Y. Maeda, M. Kimura, C. Ueda, M. Yamada, T. Kikuchi, M. Suzuki, W.-W. Wang, N. Mizorogi, N. Karousis, N. Tagmatarchis, T. Hasegawa, M. M. Olmstead, A. L. Balch, S. Nagase and T. Akasaka, Chem. Commun., 2014, 50, 12552–12555 RSC.
- J. R. Pinzón, D. C. Gasca, S. G. Sankaranarayanan, G. Bottari, T. Torres, D. M. Guldi and L. Echegoyen, J. Am. Chem. Soc., 2009, 131, 7727–7734 CrossRef PubMed.
- A. G. Avent, P. R. Birkett, A. D. Darwish, S. Houlton, R. Taylor, K. S. T. Thomson and X.-W. Wei, J. Chem. Soc., Perkin Trans. 2, 2001, 782–786 RSC.
- L.-L. Deng, S.-L. Xie, C. Yuan, R.-F. Liu, J. Feng, L.-C. Sun, X. Lu, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Sol. Energy Mater. Sol. Cells, 2013, 111, 193–199 CrossRef CAS.
- G. A. Olah, I. Bucsi, C. Lambert, R. Aniszfeld, N. J. Trivedi, D. K. Sensharma and G. K. S. Prakash, J. Am. Chem. Soc., 1991, 113, 9385–9387 CrossRef CAS.
- E. A. Khakina, O. g. A. Kraevaya, M. L. Popova, A. S. Peregudov, S. I. Troyanov, A. V. Chernyak, V. M. Martynenko, A. V. Kulikov, D. Schols and P. A. Troshin, Org. Biomol. Chem., 2017, 15, 773–777 RSC.
- H. Ueno, K. Uchiyama, Y. Ma, K. Watanabe, K. Yoza, Y. Matsuo and H. Moriyama, J. Org. Chem., 2018, 83, 10655–10659 CrossRef CAS PubMed.
- N. Lou, Y. Li and L. Gan, Angew. Chem., Int. Ed., 2017, 56, 2403–2407 CrossRef CAS PubMed.
- Z. Jia, Q. Zhang, Y. Li, L. Gan, B. Zheng, G. Yuan, S. Zhang and D. Zhu, Tetrahedron, 2007, 63, 9120–9123 CrossRef CAS.
- K. M. Kadish, X. Gao, O. Gorelik, E. Van Caemelbecke, T. Suenobu and S. Fukuzumi, J. Phys. Chem. A, 2000, 104, 2902–2907 CrossRef CAS.
- X.-X. Yan, B. Li, H.-S. Lin, F. Jin, C. Niu, K.-Q. Liu, G.-W. Wang and S. Yang, Research, 2020, 2020, 2059190 CAS.
- M. Mikawa, H. Kato, M. Okumura, M. Narazaki, Y. Kanazawa, N. Miwa and H. Shinohara, Bioconjugate Chem., 2001, 12, 510–514 CrossRef CAS PubMed.
- J. Zhang, Y. Ye, Y. Chen, C. Pregot, T. Li, S. Balasubramaniam, D. B. Hobart, Y. Zhang, S. Wi, R. M. Davis, L. A. Madsen, J. R. Morris, S. M. LaConte, G. T. Yee and H. C. Dorn, J. Am. Chem. Soc., 2014, 136, 2630–2636 CrossRef CAS PubMed.
- G. M. Sheldrick, Program for crystal structure solution, University of Göttingen, Germany, 1997 Search PubMed.
- N. Alegret, A. Rodríguez-Fortea and J. M. Poblet, Chem.–Eur. J., 2013, 19, 5061–5069 CrossRef CAS PubMed.
- A. Rodríguez-Fortea, J. M. Campanera, C. M. Cardona, L. Echegoyen and J. M. Poblet, Angew. Chem., Int. Ed., 2006, 45, 8176–8180 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: In situ UV-Vis-NIR spectra of the reaction mixture of Sc3N@Ih-C80 with TBAOH (in CH3OH) in o-DCB probed at different times, recycling HPLC profiles of the products, HPLC profiles of pure 1 and 2, full 1H NMR spectra of 1 and 2, theoretical optimized structures of 1 and 2, and optimized xyz coordinates of 1 and 2. CCDC 2049232 and 2049233. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01178b |
| This journal is © The Royal Society of Chemistry 2021 |