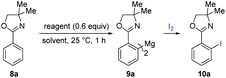Open Access Article
Open Access ArticleDirected regioselective ortho,ortho′-magnesiations of aromatics and heterocycles using sBu2Mg in toluene†
Andreas
Hess
a,
Jan P.
Prohaska
a,
Sabrina B.
Doerrich
a,
Florian
Trauner
a,
Ferdinand H.
Lutter
a,
Sébastien
Lemaire
b,
Simon
Wagschal
 c,
Konstantin
Karaghiosoff
a and
Paul
Knochel
c,
Konstantin
Karaghiosoff
a and
Paul
Knochel
 *a
*a
aLudwig-Maximilians-Universität München, Department Chemie, Butenandtstrasse 5-13, Haus F, 81377 München, Germany. E-mail: paul.knochel@cup.uni-muenchen.de
bJanssen Pharmaceutica, Chemical Process Research & Development, Turnhoutseweg 30, B-2340 Beerse, Belgium
cJanssen Pharmaceutica, Chemical Process Research & Development, Hochstrasse 201, 8200 Schaffhausen, Switzerland
First published on 18th May 2021
Abstract
Aryl azoles are ubiquitous as bioactive compounds and their regioselective functionalization is of utmost synthetic importance. Here, we report the development of a toluene-soluble dialkylmagnesium base sBu2Mg. This new reagent allows mild and regioselective ortho-magnesiations of various N-arylated pyrazoles and 1,2,3-triazoles as well as arenes bearing oxazoline, phosphorodiamidate or amide directing groups. The resulting diarylmagnesium reagents were further functionalized either by Pd-catalyzed arylation or by trapping reactions with a broad range of electrophiles (aldehydes, ketones, allylic halides, acyl chlorides, Weinreb amides, aryl halides, hydroxylamine benzoates, terminal alkynes). Furthermore, several double ortho,ortho′-magnesiations were realized in the case of aryl oxazolines, N-aryl pyrazoles as well as 2-aryl-2H-1,2,3-triazoles by simply repeating the magnesiation/electrophile trapping sequence allowing the preparation of valuable 1,2,3-functionalized arenes.
Introduction
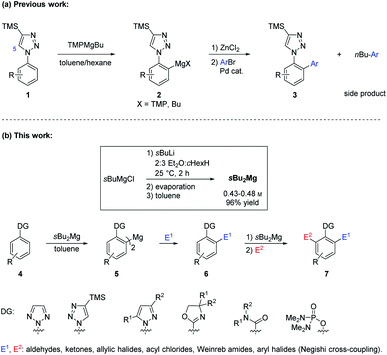
The directed magnesiation of arenes and heteroarenes is an important synthetic tool for the preparation of polyfunctional aryl- and heteroaryl-magnesium organometallics.1 Mixed magnesium and lithium amides R2NMgX·LiCl are usually the most efficient reagents for such metalations.2 Recently, we have examined the regioselective metalation of various pharmaceutically relevant aryl azoles such as 1.3 We found that standard metal amides such as LDA or TMPLi (TMP = 2,2,6,6-tetramethylpiperidyl) gave the lithiated products 2 with poor regioselectivity, due to a competitive deprotonation at the 5-position of the triazole ring of 1. The best result was achieved in toluene4 using the alkylmagnesium amide TMPMgBu5 prepared from commercial Bu2Mg, which provided after cross-coupling with aryl bromides various products of type 3. Although this base was highly regioselective in toluene, an excess of ArBr was required to compensate the formation of the Ar-nBu side-product, originating from a faster cross-coupling of the nBu moiety compared to the metalated azole 2 (Scheme 1).While commercially available Bu2Mg contained a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mixture of nBu2Mg and sBu2Mg, we have found only small amounts of the branched coupling side-product Ar-sBu, suggesting that the secondary alkyl moiety was reacting much slower than the primary one.
40 mixture of nBu2Mg and sBu2Mg, we have found only small amounts of the branched coupling side-product Ar-sBu, suggesting that the secondary alkyl moiety was reacting much slower than the primary one.
Herein, we report the preparation of sBu2Mg,6 which avoided these side reactions and significantly increased the metalation scope. Thus, we showed that sBu2Mg was an improved magnesiation reagent, which allowed a highly ortho-regioselective magnesiation of arenes 4 bearing various directing groups (DG), leading after trapping of the resulting diarylmagnesium species 5 with various electrophiles E1 to products of type 6. These polyfunctional arenes were in several cases magnesiated again using sBu2Mg producing, after addition of a second different electrophile E2, valuable 1,2,3-polyfunctional arenes of type 7.
Results and discussion
The reaction of sBuMgCl in diethyl ether with sBuLi (1.0 equiv.) in cyclohexane at 25 °C (2 h) gave, after solvent evaporation under vacuum, redissolution in toluene and filtration, a 0.43–0.48 M solution of sBu2Mg in 96% yield.7,8In preliminary experiments, we have observed a smooth magnesiation of oxazoline 8a with a toluene solution of 0.6 equiv. of sBu2Mg leading to the diarylmagnesium 9a (Table 1). A full conversion to the diarylmagnesium species was achieved within 1 hour and the iodolyzed product 10a was isolated in 80% yield (entry 1). sBu2Mg gave also good results in cyclohexane or THF, albeit in lower yields (entries 2 and 3). cHex2Mg in toluene delivered the desired product 10a in only 46% yield and other bases such as Ph2Mg, (TMSCH2)2Mg and tBu2Mg or sBuMgCl9 did not give any conversion (entries 4–8).
| Entry | Reagent | Solvent | Yielda |
|---|---|---|---|
| a Calibrated GC-yield using undecane as internal standard. b 1.2 equiv. of sBuMgCl were used. c Isolated yield. | |||
| 1 | sBu2Mg | Toluene | 91% (80)c |
| 2 | sBu2Mg | THF | 73% |
| 3 | sBu2Mg | Cyclohexane | 64% |
| 4 | cHex2Mg | Toluene | 46% |
| 5 | tBu2Mg | Toluene | 0% |
| 6 | (TMSCH2)2Mg | Toluene | 0% |
| 7 | Ph2Mg | Toluene | 0% |
| 8 | sBuMgClb | Ether/toluene | 0% |
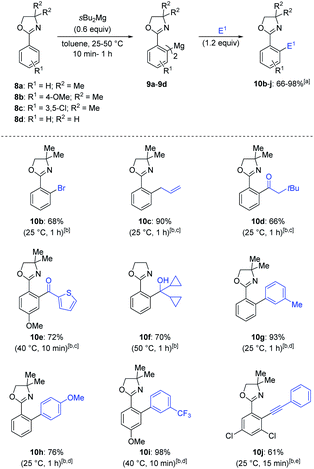
Therefore, a range of oxazolines (8a–d) were magnesiated selectively on the aryl ring and the resulting diarylmagnesiums (9a–d) underwent Negishi cross-couplings,10 copper-catalyzed allylation or acylation,11in situ Sonogashira cross-coupling12 or trapping reactions with tetrachlorodibromoethane or dicyclopropylketone, leading to the mono–ortho substituted oxazolines 10b–j in 68–98% yield (Scheme 2).
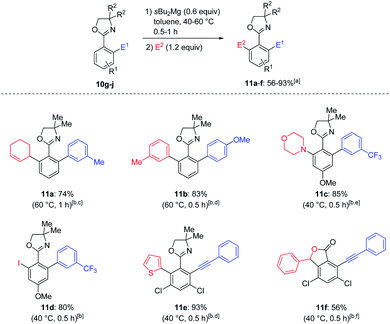
Most of the C–H activation methods currently available for the arylation of aryl azoles were performed by using transition metal catalysts and suffered from the unwanted formation of symmetrical bis-arylated products and the selective preparation of unsymmetrical ortho–ortho′-bis-functionalized13 aryl azoles remained challenging.14 We have found that various oxazolines 10g–j were again magnesiated at 40–60 °C with sBu2Mg in toluene (Scheme 3).15 The intermediate diarylmagnesium species were further functionalized by a copper-catalyzed allylation, Negishi cross-coupling, cobalt-catalyzed electrophilic amination16 and iodolysis furnishing the desired products 11a–e in 74–93% yield. Interestingly, magnesiation of 10j followed by trapping with benzaldehyde and subsequent treatment with 6 M HCl provided lactone 11f in 56% yield.17
To demonstrate the versatility of the oxazoline directing group, the strongly sterical hindered ortho,ortho′-functionalized oxazoline 11b was successfully converted to the corresponding nitrile 11g using thionyl chloride and DMF18 in 92% yield (Scheme 4).19
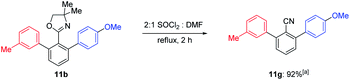 | ||
| Scheme 4 Transformation of ortho,ortho′-functionalized oxazoline 11b to the corresponding nitrile 11g. a Isolated yield. | ||
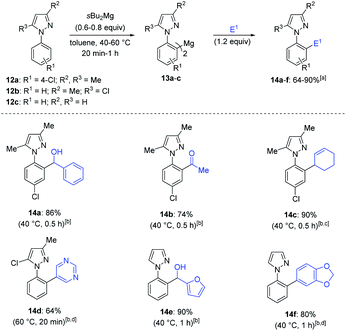
We then turned our attention to the magnesiation of various N-aryl pyrazoles (12a–c). sBu2Mg proved also to be an excellent base for the regioselective magnesiation of N-aryl pyrazole 12a, affording the corresponding bis-arylmagnesium species 13a after 0.5 h at 40 °C. After addition of benzaldehyde or Weinreb amide MeCON(OMe)Me, alcohol 14a and ketone 14b were obtained in 74–86% yield (Scheme 5). Copper-catalyzed allylation with 3-bromocyclohex-1-ene produced the pyrazole 14c (90% yield). Interestingly, N-aryl pyrazoles 12b and 12c although bearing relatively acidic protons at the heterocyclic ring were selectively magnesiated at the ortho-position of the phenyl ring. In particular, unsubstituted pyrazole 12c was metalated in 94% yield and >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity, as determined by deuterolysis of a reaction aliquot.20 These results further confirm the key role of the coordination at the N(2)-atom of the pyrazole to direct the metalation selectively on the aryl ring in a non-polar solvent like toluene. Thus, the functionalized pyrazoles 14d–f were obtained after Negishi cross-coupling with 5-bromopyrimidine, 5-bromobenzo[d][1,3]dioxole or addition of furfural in 64–90% yield. We also achieved an unsymmetrical ortho,ortho′-functionalization and mono-substituted pyrazole 14f was selectively magnesiated at 60 °C (0.5 h) and trapped by Negishi cross-coupling with 6-iodoquinoline and 4-iododibenzo[b,d]thiophene providing the products 15a–b in 67–84% yield (Scheme 6).
1 selectivity, as determined by deuterolysis of a reaction aliquot.20 These results further confirm the key role of the coordination at the N(2)-atom of the pyrazole to direct the metalation selectively on the aryl ring in a non-polar solvent like toluene. Thus, the functionalized pyrazoles 14d–f were obtained after Negishi cross-coupling with 5-bromopyrimidine, 5-bromobenzo[d][1,3]dioxole or addition of furfural in 64–90% yield. We also achieved an unsymmetrical ortho,ortho′-functionalization and mono-substituted pyrazole 14f was selectively magnesiated at 60 °C (0.5 h) and trapped by Negishi cross-coupling with 6-iodoquinoline and 4-iododibenzo[b,d]thiophene providing the products 15a–b in 67–84% yield (Scheme 6).
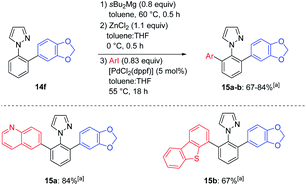 | ||
| Scheme 6 Regioselective magnesiation of mono-functionalized N-aryl pyrazole 14f with sBu2Mg leading to ortho,ortho′-functionalized N-aryl pyrazoles 15a–b. a All yields refer to isolated compounds. | ||
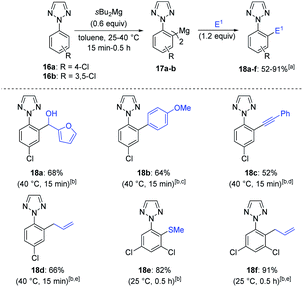
The functionalization of less common heterocycles is of key importance for pharmaceutical applications.21 Thus, the metalation of symmetrical 2-aryl-2H-1,2,3-triazoles 16a–b was then investigated (Scheme 7).22,23 After metalation of 16a with 0.6 equiv. of sBu2Mg for 15 min at 40 °C, the resulting bis-arylmagnesium species 17a was then trapped with furfural, affording the functionalized 1,2,3-triazole 18a in a 68% yield. Further trapping reactions such as Negishi cross-coupling, copper-catalyzed allylation and oxidative alkynylation with (phenylethynyl)lithium24 lead to 2-aryl-1,2,3-triazoles 18b–d in 52–66% yield. Similarly, 1,2,3-triazole 16b was readily magnesiated at 25 °C (0.5 h) as shown by the quantitative formation of a single regioisomer by NMR-analysis of a deuterolyzed reaction aliquot.20 Further quenching reactions of 17b like thiomethylation and allylation furnished triazoles 18e–f in 82–91% yield. A second functionalization was performed on N-aryl triazoles 18d–f using again sBu2Mg in toluene, followed by quench with a different electrophile (E2) (Scheme 8). We observed a complete magnesiation of 18d with sBu2Mg within 15 min at 40 °C and a subsequent reaction with benzaldehyde produced the mixed bis-functionalized 1,2,3-triazole 19a in 42% yield. Similarly, 18e and 18f were magnesiated under the standard conditions and the resulting bis-arylmagnesium species were trapped with a different electrophile (E2) leading to a range of unsymmetrical functionalized 1,2,3-triazoles 19b–f in 70–88% yield.
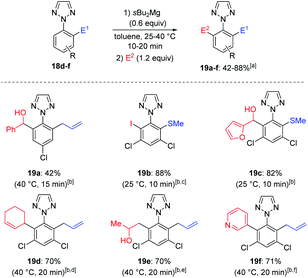 | ||
| Scheme 8 Regioselective magnesiation of mono-functionalized 2-aryl-2H-1,2,3-triazoles 18d–f with sBu2Mg leading to ortho,ortho′-functionalized 2-aryl-2H-1,2,3-triazoles 19a–f. a All yields refer to isolated compounds. b Magnesiation conditions. c The regioselectivity was determined by crystal structure analysis, see ESI.†d The reaction was catalyzed by CuCN·2LiCl (20 mol%). e The reaction was catalyzed by CuI (10 mol%). f Obtained after transmetalation with ZnCl2 (1.1 equiv.) and a palladium-catalyzed cross-coupling with [PdCl2(dppf)] (5 mol%) and an aryl bromide (0.83 equiv.). | ||
Finally, we examined the metalation of 1-aryl-1H-1,2,3-triazoles such as 20a–e and found that sBu2Mg led to a highly regioselective magnesiation at the ortho-position of the aryl ring in toluene (25–40 °C, 0.5–1 h), affording the bis-aryl-magnesium species 21a in 75% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 regioselectivity (Scheme 9).20
3 regioselectivity (Scheme 9).20
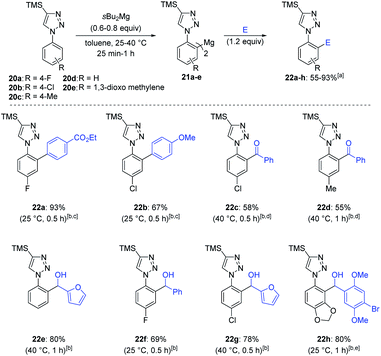 | ||
| Scheme 9 Regioselective magnesiation of 1-aryl-2H-1,2,3-triazoles 20a–e with sBu2Mg leading, via diarylmagnesium species 21a–e, to functionalized 1-aryl-2H-1,2,3-triazoles 22a–h. a All yields refer to isolated compounds. b Magnesiation conditions. c Obtained after transmetalation with ZnCl2 (1.1 equiv.) and a palladium-catalyzed cross-coupling with [PdCl2(dppf)] (5 mol%) and an aryl halide (0.83 equiv.).d The reaction was catalyzed by CuCN·2LiCl (20 mol%). e The regioselectivity was determined by crystal structure analysis, see ESI.† | ||
This new metalation procedure occurred twice as fast as the previously reported TMPMgBu base.3 1,2,3-Triazoles 22a and 22b were isolated in 93% and 67% yields respectively after Negishi cross-couplings with only 0.83 equiv. of aryl bromide.3 Copper-catalyzed acylation11 with benzoyl chloride lead to products 22c and 22d in 55–58% yield and quenching with various aldehydes afforded compounds 22e–h in 69–80% yield.
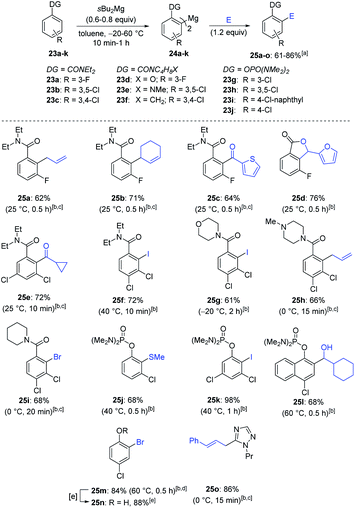
Remarkably, sBu2Mg was also an excellent base for the magnesiation of various arenes bearing directing groups such as a tertiary amide or phosphorodiamidate (23a–j; Scheme 10).25 The addition of sBu2Mg to the aromatic amide 23a in toluene led to a clean magnesiation within 0.5 h at room temperature. The resulting diarylmagnesium species 24a was then further allylated with allyl and cyclohexenyl bromides, leading to 25a and 25b in 62% and 71% yield respectively. Copper-catalyzed acylation of 23a with thiophene-2-carbonyl chloride or trapping with furfural furnished the ketone 25c (64% yield) and the lactone 25d (76% yield).26 Similarly, the amides 23b–f afforded with the same magnesiation/trapping sequence the polyfunctional amides (25e–i) in 61–72% yield. Various phosphorodiamidates (23g–j) were also metalated with sBu2Mg at 40–60 °C (0.5–1 h) providing the diarylmagnesiums 24g–j, which were trapped with a range of electrophiles (MeSSO2Me, I2, cHexCHO and (BrCCl2)2) furnishing the phenol derivatives 25j–m in 68–98% yield. Removal of the phosphorodiamidate group27 in 25m was achieved with a 2 M HCl treatment in dioxane (105 °C, 1 h) leading to phenol 25n in 88% yield. Interestingly, 1-propyl-1,2,4-triazole (23k) was magnesiated with sBu2Mg and allylated with cinnamyl bromide providing the N-heterocycle 25o in 86% yield.28
We performed some further transformations leading to polyfunctionalized 1,2,3-trisubstituted arenes to show the utility of these magnesiations. The newly prepared amide 25b was thus selectively reduced with Cp2Zr(H)Cl29 (25 °C, 15 min) to the aldehyde 26a in 90% yield. A two–step transformation consisting of a reduction with the complex borohydride LiH3BPyrr (Pyrr = pyrrolidino)30 followed by a treatment with ethyl chloroformate31 provided the benzylic chloride 26b in 85% overall yield (Scheme 11).
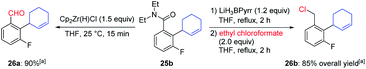 | ||
| Scheme 11 Synthetic transformations of magnesiated product 25b. a All yields refer to isolated compounds. | ||
Conclusions
In summary, we have developed a new preparation of sBu2Mg in toluene and showed its utility for the directed magnesiation of various aromatic and heterocyclic systems including pharmaceutically relevant N-arylated pyrazoles as well as N-arylated 1,2,3-triazoles. This method provides a unique access to varius diarylmagnesium reagents in toluene. Furthermore, a range of arenes bearing various directing groups such as an oxazoline, phosphorodiamidate or an amide were magnesiated with sBu2Mg. Remarkably, a second unsymmetrical ortho,ortho′-functionalization was achieved in the case of aryl oxazolines, N-aryl pyrazoles as well as N-aryl triazoles, leading to valuable synthetic intermediates of potential pharmaceutical relevance. Further investigations of the use of sBu2Mg as metalating agent are currently underway in our laboratory.Author contributions
A. H., J. P. P, S. B. D., F. T. and K. K. performed and analyzed the experiments. A. H., F. H. L., S. L., S. W. and P. K. designed the experiments. A. H., S. L., S. W., and P. K. prepared the manuscript with contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft and the Ludwig-Maximilians-Universität for financial support. We also thank Albemarle Lithium GmbH (Frankfurt) and the BASF AG (Ludwigshafen) for the generous gift of chemicals.Notes and references
-
(a) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3803 CrossRef PubMed
; (b) K. W. Henderson and W. J. Kerr, Chem.–Eur. J., 2001, 7, 3430 CrossRef CAS
; (c) C. R. Hauser and H. G. Walker, J. Am. Chem. Soc., 1947, 69, 295 CrossRef CAS
; (d) J. Wei, W.-X. Zhang and Z. Xi, Org. Chem. Front., 2014, 1, 983 RSC
.
-
(a) A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 2958 CrossRef CAS PubMed
; (b) G. C. Clososki, C. J. Rohbogner and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7681 CrossRef CAS PubMed
; (c) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794 CrossRef CAS PubMed
; (d) P. E. Eaton, C. H. Lee and Y. Xiong, J. Am. Chem. Soc., 1989, 111, 8016 CrossRef CAS
; (e) P. E. Eaton and K. A. Lukin, J. Am. Chem. Soc., 1993, 115, 11370 CrossRef CAS
.
- F. H. Lutter, L. Grokenberger, L. A. Perego, D. Broggini, S. Lemaire, S. Wagschal and P. Knochel, Nat. Commun., 2020, 11, 4443 CrossRef CAS PubMed
.
- D. S. Ziegler, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2018, 57, 6701 CrossRef CAS PubMed
.
-
(a) E. Hevia, A. R. Kennedy, R. E. Mulvey and S. Weatherstone, Angew. Chem., Int. Ed., 2004, 43, 1709 CrossRef CAS PubMed
; (b) B. Conway, E. Hevia, A. R. Kennedy, R. E. Mulvey and S. Weatherstone, Dalton Trans., 2005, 1532 RSC
.
-
(a) L. J. Bole, N. R. Judge and E. Hevia, Angew. Chem., Int. Ed., 2021, 60, 7626 CrossRef CAS PubMed
; (b) C. W. Kamienski and J. F. Eastham, J. Organomet. Chem., 1967, 8, 542 CrossRef CAS
; (c) N. D. R. Barnett, W. Clegg, R. E. Mulvey, P. A. O'Neil and D. Reed, J. Organomet. Chem., 1996, 510, 297 CrossRef CAS
.
- This reagent still contained 0.5 equiv. of Et2O, having the formula sBu2Mg0.5Et2O (determined by 1H-NMR analysis) that we abbreviated sBu2Mg for the sake of clarity.
- The toluene solution of sBu2Mg can be stored at −30 °C for at least 1 week.
- A. Marxer and M. Siegrist, Helv. Chim. Acta, 1974, 57, 1988 CrossRef CAS
.
- A. O. King, N. Okukado and E.-I. Negishi, J. Chem. Soc., Chem. Commun., 1977, 19, 683 RSC
.
- P. Knochel, M. C. P. Yeh, S. C. Berk and J. Talbert, J. Org. Chem., 1988, 53, 2390 CrossRef CAS
.
-
(a) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 50, 4467 CrossRef
; (b) M. Mosrin, T. Bresser and P. Knochel, Org. Lett., 2009, 11, 3406 CrossRef CAS PubMed
.
-
(a) A. J. Martinez-Martinez, A. R. Kennedy, R. E. Mulvey and C. T. O'Hara, Science, 2014, 346, 834 CrossRef CAS PubMed
; (b) A. J. Martinez-Martinez, S. Justice, B. J. Fleming, A. R. Kennedy, I. D. H. Oswald and C. T. O'Hara, Sci. Adv., 2017, 3, e1700832 CrossRef PubMed
.
-
(a) M. Simonetti, D. M. Cannas, X. Just-Baringo, I. J. Vitorica-Yrezabal and I. Larrosa, Nat. Chem., 2018, 10, 724 CrossRef CAS PubMed
; (b) L. Ackermann, A. Althammer and R. Born, Tetrahedron, 2008, 64, 6115 CrossRef CAS
; (c) S. Oi, H. Sato, S. Sugawara and Y. Inoue, Org. Lett., 2008, 10, 1823 CrossRef CAS PubMed
; (d) C. J. Teskey, S. M. A. Sohel, D. L. Bunting, S. G. Modha and M. F. Greaney, Angew. Chem., Int. Ed., 2017, 56, 5263 CrossRef CAS PubMed
; (e) O. Daugulis and V. G. Zaitsev, Angew. Chem., Int. Ed., 2005, 44, 4046 CrossRef CAS PubMed
.
- A. I. Meyers and W. B. Avila, J. Org. Chem., 1981, 46, 3881 CrossRef CAS
.
-
(a) A. M. Berman and J. S. Johnson, J. Am. Chem. Soc., 2004, 126, 5680 CrossRef CAS PubMed
; (b) A. M. Berman and J. S. Johnson, J. Org. Chem., 2005, 70, 364 CrossRef CAS PubMed
; (c) M. Campbell and J. S. Johnson, Org. Lett., 2007, 9, 1521 CrossRef CAS PubMed
; (d) Y.-H. Chen, S. Graßl and P. Knochel, Angew. Chem., Int. Ed., 2018, 57, 1108 CrossRef CAS PubMed
; (e) S. Graßl, Y.-H. Chen, C. Hamze, C. P. Tüllmann and P. Knochel, Org. Lett., 2019, 21, 494 CrossRef PubMed
.
- C. A. Boulet and G. A. Poulton, Heterocycles, 1989, 28, 405 CrossRef CAS
.
-
(a) O. Fischer, A. Muller and A. Vilsmeier, J. Prakt. Chem., 1925, 109, 69 CrossRef CAS
; (b) G. Jones and S. P. Stanforth, Org. React., 2000, 56, 355 CAS
.
-
(a) I. M. Dordor and J. M. Mellor, Tetrahedron Lett., 1983, 24, 1437 CrossRef CAS
; (b) H. Petersen, US Pat.US2003/13895, A1, 2003 Search PubMed
.
- See Supporting Information for further details.†.
-
(a) V. M. Ahrens, K. Bellmann-Sickert and A. G. Beck-Sickinger, Future Med. Chem., 2012, 4, 1567 CrossRef CAS PubMed
; (b) C. R. Dass and P. F. M. Choong, Peptides, 2006, 27, 3020 CrossRef CAS PubMed
.
-
(a) J. L. Riebsommer, J. Org. Chem., 1948, 13, 815 CrossRef PubMed
; (b) G. F. Myachina, T. G. Ermakova, N. P. Kuznetsova, R. G. Sultangareev, L. I. Larina, L. V. Klyba, G. T. Suchanov and B. A. Trofimov, Chem. Heterocycl. Compd., 2010, 46, 79 CrossRef CAS
.
- S. Shi, W. Liu, P. He and C. Kuang, Org. Biomol. Chem., 2014, 12, 3576 RSC
.
- S. R. Dubbaka, M. Kienle, H. Mayr and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 9093 CrossRef CAS PubMed
.
-
(a) P. Beak, G. R. Brubaker and R. J. Farney, J. Am. Chem. Soc., 1976, 98, 3621 CrossRef CAS
; (b) P. Beak and R. A. Brown, J. Org. Chem., 1977, 42, 1823 CrossRef CAS
; (c) P. Bowles, J. Clayden, M. Helliwell, C. McCarthy, M. Tomkinson and N. Westlund, J. Chem. Soc., Perkin Trans. 1, 1997, 2607 RSC
; (d) J.-C. Cuevas, P. Patil and V. Snieckus, Tetrahedron Lett., 1989, 30, 5841 CrossRef CAS
; (e) W. H. Puterbaugh and C. R. Hauser, J. Org. Chem., 1964, 29, 853 CrossRef CAS
; (f) M. Watanabe and V. Snieckus, J. Am. Chem. Soc., 1980, 102, 1457 CrossRef CAS
; (g) F. Hintze and D. Hoppe, J. Org. Chem., 1990, 55, 1375 CrossRef
; (h) H. Gilman and R. L. Bebb, J. Am. Chem. Soc., 1939, 61, 109 CrossRef
; (i) G. Wittig and G. Fuhrman, Chem. Ber., 1940, 73, 1197 CrossRef
; (j) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS
.
- W. Lin, O. Baron and P. Knochel, Org. Lett., 2006, 24, 5673 CrossRef PubMed
.
-
(a) M. Watanabe, M. Date, K. Kawanishi, M. Tsukazaki and S. Furukawa, Chem. Pharm. Bull., 1989, 37, 2564 CrossRef CAS
; (b) C. J. Rohbogner, S. Wirth and P. Knochel, Org. Lett., 2010, 9, 1984 CrossRef PubMed
.
- K. Schwärzer, C. P. Tüllmann, S. Graßl, B. Górski, C. E. Brocklehurst and P. Knochel, Org. Lett., 2020, 5, 1899 CrossRef PubMed
.
-
(a) J. M. White, A. R. Tunoori and G. I. Georg, J. Am. Chem. Soc., 2000, 122, 11995 CrossRef CAS
; (b) Y. Zhao and V. Snieckus, Org. Lett., 2014, 2, 390 CrossRef PubMed
.
- G. B. Fisher, J. C. Fuller, J. Harrison, C. T. Goralski and B. Singaram, Tetrahedron Lett., 1993, 34, 1091 CrossRef CAS
.
- H. Kapnang and G. Charles, Tetrahedron Lett., 1983, 24, 3233 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2061336 and 2061337. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01777b |
| This journal is © The Royal Society of Chemistry 2021 |