 Open Access Article
Open Access ArticleRecent advances in heterogeneous catalysis for the nonoxidative conversion of methane
Tianyu
Zhang
 *
*
Department of Chemistry, Joint Institute for Advanced Materials, University of Tennessee, Knoxville, TN 37996, USA. E-mail: tzhang29@utk.edu
First published on 22nd September 2021
Abstract
The direct conversion of methane to high-value chemicals is an attractive process that efficiently uses abundant natural/shale gas to provide an energy supply. The direct conversion of methane to high-value chemicals is an attractive process that efficiently uses abundant natural/shale gas to provide an energy supply. Among all the routes used for methane transformation, nonoxidative conversion of methane is noteworthy owing to its highly economic selectivity to bulk chemicals such as aromatics and olefins. Innovations in catalysts for selective C–H activation and controllable C–C coupling thus play a key role in this process and have been intensively investigated in recent years. In this review, we briefly summarize the recent advances in conventional metal/zeolite catalysts in the nonoxidative coupling of methane to aromatics, as well as the newly emerging single-atom based catalysts for the conversion of methane to olefins. The emphasis is primarily the experimental findings and the theoretical understanding of the active sites and reaction mechanisms. We also present our perspectives on the design of catalysts for C–H activation and C–C coupling of methane, to shed some light on improving the potential industrial applications of the nonoxidative conversion of methane into chemicals.
1. Introduction
The conversion of the abundant amount of available methane into high-value chemicals is becoming more attractive owing to the growing depletion of petroleum reserves and proliferation of the availability of natural and shale gas.1–3 Currently, the indirect route is the most widely used process to produce valuable chemicals from methane.4 In this method the methane is first converted into syngas (CO and H2) either by partial oxidation or reforming, and then the products of the long-chain hydrocarbons or alcohols are made from the syngas using Fischer–Tropsch (FT) synthesis.5 This process causes an additional energy loss and aggravates concerns regarding greenhouse gases, therefore, the direct conversion of methane to high-value chemicals has been considered as an important goal to reduce costs and energy consumption.6 However, the selective activation of C–H bonds, which has been regarded as a “Holy Grail” in the chemical community, still faces significant challenges owing to the completely symmetrical tetrahedral structure of methane with extremely stable C–H bonds.To date, many routes, including the oxidative coupling of methane (OCM), partial oxidation of methane (POM), and the nonoxidative coupling of methane (NOCM), have been proposed for the direct conversion of methane into the desired chemical products.7–9 Although the oxidative methane conversion is thermodynamically feasible, methyl radicals are present in low concentrations and react more easily in the presence of a much higher concentration of oxygen to form CH3O2·, which is a precursor for the deeper oxidation of methane. Therefore, C2 hydrocarbons and C3 in trace amounts are the main products in the OCM depending on the reaction conditions, which reduces the selectivity to desired chemicals and makes the processes uneconomical. In contrast, the nonoxidative conversion of methane is noteworthy owing to its high selectivity to target products such as olefins and aromatics.10 Nevertheless, a very high reaction temperature is generally required for NOCM as a result of the tremendous thermodynamic barrier, which causes severe coking deactivation of the catalyst.11 Therefore, significant efforts have been devoted to exploring efficient catalytic systems to enable activation of the methane C–H bond and C–C coupling to improve the NOCM reaction for the efficient utilization of methane.7,12–14
Among the catalysts for NOCM, the metal/zeolite based catalysts, such as molybdenum exchanged zeolites, have been extensively studied and show great potential for the aromatization of methane to aromatics.8 Many properties of the catalyst, including the kind of active metal species, the physical/chemical properties of the supports, as well as the interaction between the metal species and supports, have been proven to be crucial in determining the catalytic performance for the NOCM to aromatics. More recently, the silica matrix-confined single-Fe-atom catalyst (Fe@SiO2) was reported to realize direct methane conversion to olefins, which is also viewed as a landmark discovery for the transformation of methane.15 The results of these findings highlight the importance of engineering active sites on supports for modulating the selectivity in C–C coupling products. In this review, we summarize the recent experimental advances and theoretical understanding of the conventional metal/zeolite (primarily Mo/zeolite based catalysts) and the newly emerged single-atom catalysts, focusing on the active species, reaction mechanism, and catalytic deactivation for NOCM. Combined with recent progress, prospects for future research on the nonoxidative conversion of methane are also presented.
2. Metal/zeolite catalysts for NOCM
2.1 Mo/zeolite-based catalysts
Since the first report of Mo/HZSM-5 as an efficient catalyst for methane dehydroaromatization (MDA) in 1993,16 extensive efforts have been devoted to improving the Mo-based catalysts to promote the overall catalytic performances for the NOCM to aromatics by modulating the nature of the metal species, changing the type of zeolite supports, as well as introducing different promoters into the catalysts.The structure of the Mo species supported on HZSM-5 with various calcination temperatures was investigated in detail by Iglesia et al.18–20. The results of their work indicate that the MoOx species were mainly distributed on the external surface at a low temperature (∼350 °C) and migrated into the zeolite channels between 500 and 700 °C. The ion exchange of the mononuclear MoOx species at the Brønsted acid sites (BASs) leads to the formation of dinuclear [Mo2O52+] species, with the extraction of Al ions and the disappearance of the two BASs. The active MoCx species were formed during the initial stages of methane conversion with the reduction of the [Mo2O52+] species. However, the higher content of the MoOx species over the external zeolite surface results in the formation of (MoO3)n oligomers or Al2(MoO4)3, which were identified as the main reason for the poor catalytic performance. Similar results were also observed by Tessonnier et al.21,22 As evidenced, when the number of BASs is too low to adopt dinuclear [Mo2O52+] species, the formation of Al2(MoO4)3 or extra-framework (MoO3)n cannot be avoided. The results of these works suggest that both the high dispersion of [Mo2O52+] species inside the zeolite channels and the formation of MoCx during the induction period are critical for methane conversion.
In the work performed by Kosinov et al.,23 the structural and textural stability of the Mo/HZSM-5 catalyst was investigated as a function of the different Mo loadings. As shown in Fig. 1a and b, the low Mo/Al ratios lead to the dispersion of monomeric and dimeric Mo-oxo species in the zeolite micropores, even under an increased calcination temperature. Therefore, an almost unchanged catalytic performance with respect to the calcination temperature was observed for catalysts with low Mo loading contents (1–2 wt%). However, for Mo/HZSM-5 with a Mo loading of 5 wt%, the reaction of the Mo-oxo species with the framework Al causes the formation of Al2(MoO4)3 and destroys the main framework of the zeolite, resulting in a rapid reduction of its initial activity. A similar conclusion was also demonstrated by Julian et al.24 in Fig. 2a and b, that is, the catalyst with a low loading of 5% Mo on MCM-22 exhibited the best benzene selectivity and stability as compared to the samples with Mo loadings of 8% and 10%. With the application of in situ X-ray absorption spectroscopy (XAS), the impact of the Mo coordination environment in the MFI zeolites towards the MDA performance was recently systematically studied by Agote-Aran et al.25. As shown in Fig. 2c and d, when the calcination temperature is above 600 °C, the peaks for the Mo/Silicalite-1 in the post-edge region of the near edges spectra disappeared, along with the gradually decreased Mo–Mo peak intensity in the extended X-ray absorption fine structure (EXAFS), indicating the loss of the long-range order with the formation of Mo-oxo species in the microporous framework. By comparing with Mo/H-ZSM-5, the presence of BASs was found to be important for the stabilization of the Mo species, but not essential for benzene generation. The results from these works proved the dispersion of the MoOx species as a key factor for improving the catalytic performance for methane conversion. In recent work performed by Julian et al.,26 supercritical fluid was used to enhance the dispersion of Mo species within the zeolite channels. After 15 h MDA, the catalyst prepared under supercritical conditions revealed well dispersed MoCx clusters at the zeolite surface, while carbon nanotubes and nanofibers were observed for the catalyst prepared using the conventional impregnation (IMP) method (Fig. 2e). Owing to the excellent Mo dispersion, the long-term catalytic stability of Mo/ZSM-5 was achieved and the formation of coke species on the Mo active sites was alleviated.
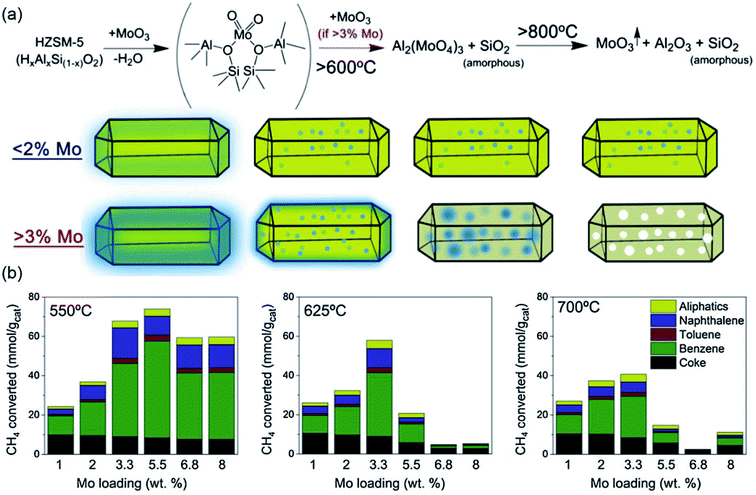 | ||
| Fig. 1 (a) Schematic representation of the dispersed monomeric and dimeric Mo-oxo species in the MoO3-HZSM-5 catalysts. (b) Cumulative product yields of the Mo/HZSM-5 catalysts pre-calcined at different temperatures during 16 h MDA tests. Reproduced with permission from ref. 23. Copyright 2017 Elsevier. | ||
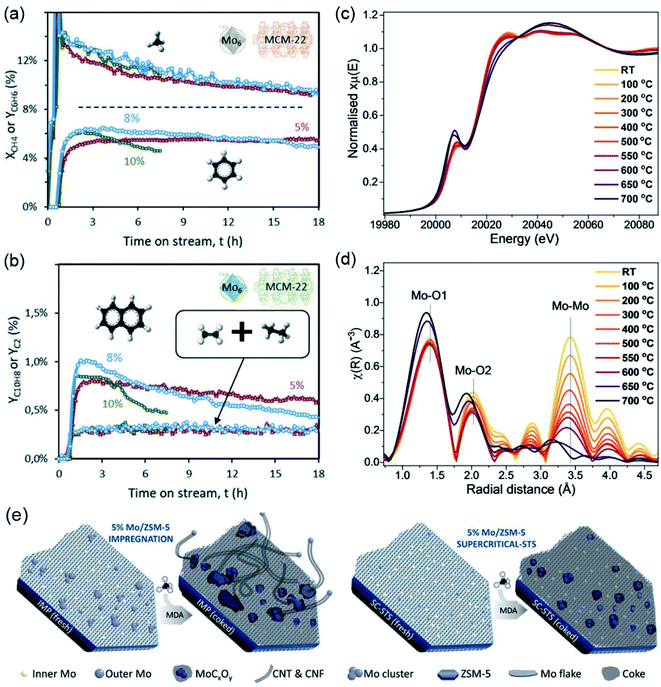 | ||
| Fig. 2 (a) Transient benzene yield, (b) naphthalene and C2 (ethane, ethylene) yield obtained for the Mo/MCM-22 catalysts with different Mo loadings. Reproduced with permission from ref. 24. Copyright 2019 Royal Society of Chemistry. (c) XANES spectra, and (d) FT-EXAFS of Mo/silicalite-1 collected during in situ calcination. Reproduced with permission from ref. 25. Copyright 2020 John Wiley and Sons. (e) Conceptual drawing of the distribution of Mo species for fresh and spent Mo/ZSM-5 prepared using different methods. Reproduced with permission from ref. 26. Copyright 2020 Elsevier. | ||
The Mo carbide species (such as Mo2C and MoOxCy), formed from the reduction of the Mo species by methane during the reaction induction period, have generally been considered as active sites for methane activation. The induction period in MDA over Mo/HZSM-5 was first mentioned in the work conducted by Lunsford et al.27 The results of this work suggest that the Mo6+ species are reduced to Mo2C during the induction period. In addition, compared to the clean surface of Mo2C, which is too reactive for the formation of higher hydrocarbons, the coke modified Mo2C was identified as being critical for the formation of ethylene.28 Further studies indicate that the Mo species on the external surface of the zeolite could be easily reduced to β-Mo2C, and it is partially transformed to MoOxCy in the zeolite channels, which was identified to be critical for the MDA.29 Taking advantage of the operando time-resolved combined X-ray diffraction (XRD) and X-ray absorption near-edge spectroscopy (XANES), Beale et al. demonstrated that the metastable MoOxCy species converted from Mo-oxo species are primarily responsible for the formation of C2Hx/C3Hx.30 Recently, thermochemical kinetic analyses were employed by Bhan et al.31 to ascertain the catalytic roles of the BASs and Mo carbide species. The results of this work suggest that the active carbidic Mo content is the only catalyst descriptor that can be used to predict the steady state and activity of MDA catalysis over Mo/HZSM-5, while the zeolitic acid sites were found to be beneficial for dispersing Mo species to catalyze the equilibrated reaction steps. In the work conducted by Rahman et al.,32 the catalytic activity and stability over MoCy/HZSM-5, with ex situ prepared Mo carbides, was found to be much higher than that of MoOx/HZSM-5. All of these works highlight the critical role of BASs and Mo carbide species in MDA. However, to avoid the formation of Al2(MoO4)3 and extra-framework (MoO3) species, the optimal contents of BASs and Mo carbide species should be considered in future studies.
A bifunctional mechanism, including the conversion of methane into ethylene on Mo carbide sites and subsequent oligomerization into aromatics over BASs, has been widely proposed for the Mo/zeolites catalytic systems.33 However, the mechanism emphasized both the activation of methane to acetylene and the subsequent aromatization over the Mo carbide phase have also been reported in many previously published works.18,34,35 In addition, the Mo-based carbide plays an important role in the C–H activation and C–C coupling to C2 intermediates. Theoretically, we have performed a systematic study of the methane activation and conversion over the Mo-terminated surfaces derived from different phases of the Mo2C carbides (Fig. 3). Our results show that Mo-terminated orthorhombic β-Mo2C, with a lower carburization in its subsurface, possesses a superior reactivity toward methane C–H activation, resulting in the complete dissociation of methane to the carbon adatom on the surface. This carbon adatom causes further carburization of the surface, reducing the reactivity toward methane activation.36 Moreover, although carburization reduces the activities for methane activation, it promotes C–C coupling for dimerization of the (CH)ad species, resulting in (C2H2)ad on the Mo-terminated surfaces. On the deep carburized molybdenum carbide (MoC) surfaces, the Mo-terminated MoC surfaces derived from different bulk phases (hexagonal α-MoC and cubic δ-MoC) of MoC possess a similar mechanism to that on the noble-metal surfaces for methane dissociation, that is, CH4 dissociates sequentially to (CH)ad with both kinetic and thermodynamic feasibilities while breaking the last C–H bond in (CH)ad requires a high activation barrier. As such, C–C coupling through dimerization of the (CH)ad species occurs more readily, resulting in (C2H2)ad on the Mo-terminated surfaces. These (C2H2)ad species can dehydrogenate easily to other C2 adsorbates such as (C2H)ad and (C2)ad. Consequently, these C2 species from CH4 dissociation will likely be precursors for producing long chain hydrocarbons and/or aromatics on molybdenum carbide based catalysts or oligomerization over BASs.37
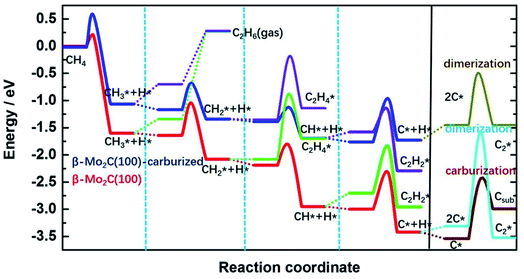 | ||
| Fig. 3 Energy profile of the elementary steps for methane dissociation, the dimerization of CHx* species and the carburization on carburized β-Mo2C(100) surfaces. Reproduced with permission from ref. 36. Copyright 2020 Elsevier. | ||
It is generally agreed that the BASs plays an important role in MDA, which serve as both the anchoring centers for the metal species and oligomerization sites for C2Hx to benzene or coke.39,40 The strength and density of the BASs, which are related to the Si/Al ratio and pore architecture of the zeolite supports, thus determine the location and nature of the Mo species, as well as the overall catalytic performance. For instance, the partial removal of the framework Al from the zeolite lattice, with decreases in both the strength and number of BASs, results in a noticeable improvement in the benzene yield and the durability of the catalyst for the Mo/HZSM-5 catalyst.41 A recent study conducted by Kosinov et al. suggests that the embedding of the Mo oxide into properly sized micropores is critical for the conversion of methane into benzene (Fig. 4a), and the role of BASs in promoting the dispersion of Mo species.34 In addition, by varying the ratios of Si/Al2, monomeric Mo oxide species could be stabilized on the MCM-22 zeolite as a result of the promoted migration of Mo oxides into the micropore (Fig. 4b). The microchannels of the zeolite were observed to be critical for providing a shape-selective environment, thus enhancing the rate of benzene formation.42 The effect of the Si/Al ratio on the activity of the Mo/HZSM-5 catalysts was further studied by Rahman et al.,43 and their results indicate that a higher channel occupation of Mo species, resulting from a lower Si/Al ratio and higher Mo loading over HZSM-5 zeolite, is directly correlated with the higher selectivity and yield towards benzene. In the work performed by Tan et al.,44 by impregnating the HZSM-5 zeolite with NH3 heptamolybdate solution, the catalytic performance of Mo/HZSM-5 was significantly improved owing to the larger amount of highly dispersed Mo species on the HZSM-5 zeolite.
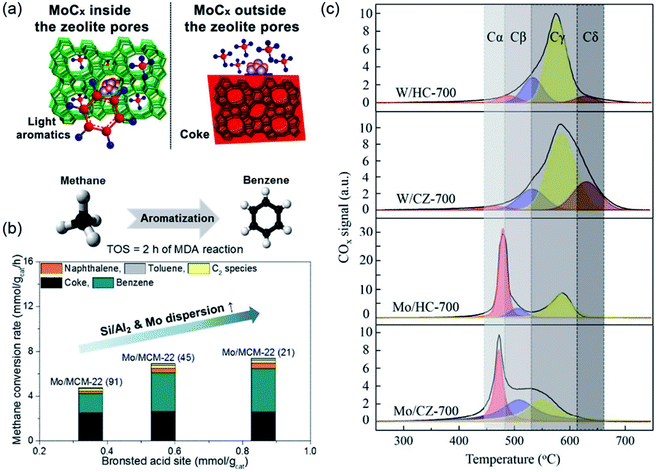 | ||
| Fig. 4 (a) Mo carbide dispersed inside and outside of the zeolite pores for MDA. Reproduced with permission from ref. 34, Copyright 2016 American Chemical Society. (b) Effect of Si/Al2 ratios in Mo/H-MCM-22 on MDA. Reproduced with permission from ref. 42, Copyright 2018 Elsevier. (c) Gaussian deconvolution of the TPO profiles for a metal supported on a hollow capsule (HC) or commercial HZSM-5 (CZ). Reproduced with permission from ref. 46, Copyright 2018 Royal Society of Chemistry. | ||
The cooperative effects of the zeolite structure and metal species also affect the catalytic performance for MDA. For instance, a hollow silicalite-1-HZSM-5 zeolite with a capsule structure as a support for Mo nanoparticles (Mo/HZSM-5) was developed by Zhu et al.45. The hollow structure of the zeolite was suggested to accelerate the mass-transfer rate, which significantly improved the rate of benzene formation and the catalytic stability. Recently, in the work performed by Huang et al.,46 a hollow HZSM-5 capsule zeolite (HC) was prepared and used as the MDA supports. As shown in Fig. 4c, the different coke types were clearly discriminated, which depend greatly on the structure of the zeolites and the metals, and the hollow capsule structure is important for suppressing the external coke (Cβ) formation in Mo-based catalysts (Mo/HC-700), leading to an excellent catalytic stability in the MDA reaction.
In work reported by Cheng et al.,50 a series of metal modified Mo/ZSM-5 were prepared as MDA catalysts. With Mg as a promoter, both the catalytic activity and selectivity to benzene were improved with an inhibited carbon deposition. X-ray photoelectron spectroscopy results demonstrated that the promotion effect in generating Mo2C active species by the presence of Mg was the main reason for the improved catalytic performance. Recently, Sridhar et al. found that with modification using Co and Ni additives, Zeolite-supported Mo carbide catalysts could increase the yield of benzene along with a better stability.51 However, the effect of the additives was only beneficial for catalysts with ex situ formed Mo carbide species by pre-carburization. As shown in Fig. 5, the MoO3 peak completely disappeared with the appearance of Mo2C as the active phase for the spent catalysts pre-treated using helium. With Co- and Ni-modification, a much stronger Mo2C peak was observed for the pre-carburized catalysts, indicating sintering of the Mo2C phases over the external surface of the zeolite. The synergy effect between the additive and Mo thus changed both the reducibility and mobility of the Mo species, which increases the retention of the Mo species within the channels of zeolite and benefits the catalytic selectivity towards benzene.
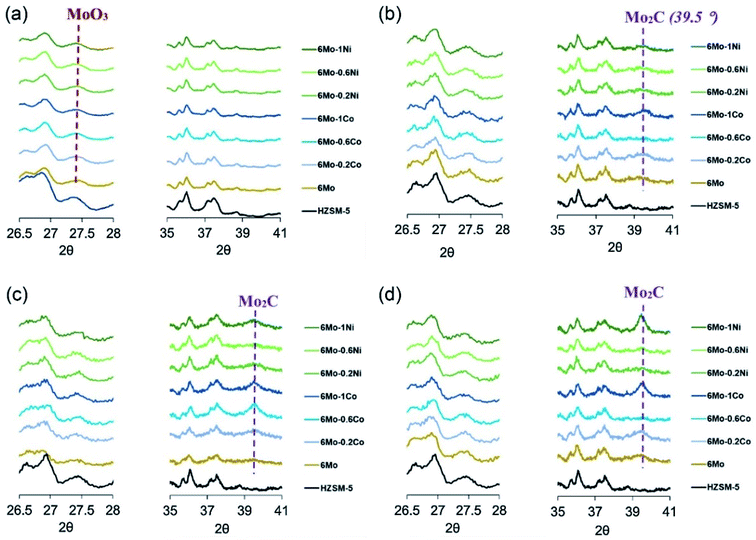 | ||
| Fig. 5 XRD patterns for (a) He pre-treated, (b) pre-carburized, (c) spent He pre-treated and (d) spent pre-carburized Co- and Ni-modified Ni/ZSM-5 catalysts. Reproduced with permission from ref. 51. Copyright 2019 Elsevier. | ||
The addition of metal promoters is also beneficial for reducing the formation of coke in zeolite channels. For instance, in the work performed by Bajec et al.,52 the addition of Fe to Mo/HZSM-5 catalysts reduces the amount of coke formation, which results in a better stability for the coupling of methane to ethane/ethylene synthesis. The effect of the addition of Fe, Rh, and K on MDA over Mo/HZSM-5 was studied by Ramasubramanian et al.,53 in which the K-promoted catalyst was found to have a better selectivity for benzene (∼50%) after 255 min of reaction (Fig. 6a). The temperature programmed oxidation results (Fig. 6b) indicated that the formation of coke was largely reduced by the addition of K, which modified the acidic strength of Mo/HZSM-5 and restricted the agglomeration of C6H6 to coke. Recently, the promoting effect of nano-Fe on the MDA performance over the Mo/HZSM-5 catalyst was studied by Sun et al.,49 and a mechanism for the formation of carbon nanotubes was suggested during the nonoxide dehydroaromatization. In their proposed mechanism, the coke formation over both the external surfaces and channels of zeolite could be suppressed owing to the competitive consumption of coke precursors by the formation of carbon nanotubes over nano-Fe. Meanwhile, these carbon nanotubes created by Fe lead to appropriate cavities with a kinetic diameter of about 10.0 Å. The activation of methane was speculated to facilely occur over the Brønsted sites inside the carbon nanotubes, thus enhancing both the rate and stability for the production of aromatics.
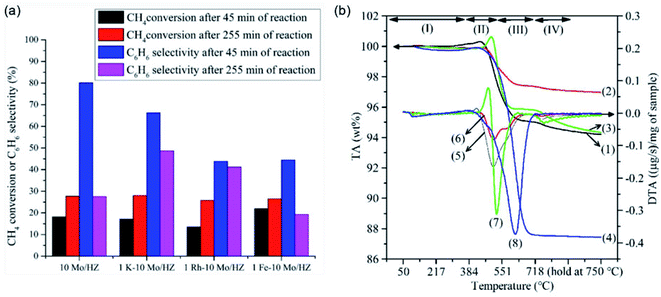 | ||
| Fig. 6 (a) Effect of addition of Fe, Rh, and K on MDA over Mo/HZSM-5. (b) TPO profiles of Rh (green line), Fe (blue line), and K (red line) promoted, as well as parent (black line), Mo/HZSM-5 catalysts. Reproduced with permission from ref. 53. Copyright 2019 Springer. | ||
2.2 Non-Mo based catalysts
In addition to the studies on Mo-based catalysts, non-Mo based catalysts such as Pt-containing catalysts, transition metals (Fe, Zn, Cr, Ni, Cu, and Co) and metals in group IIIB (Ga and Al) supported on SiO2 and H-ZSM-5 have also been extensively investigated to enable improved catalytic activity and stability for methane activation and conversion.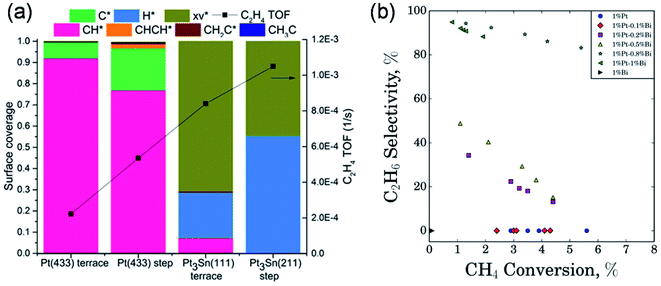 | ||
| Fig. 7 (a) Surface coverage and ethylene turnover frequency values of the PtSn/H-ZSM-5 surfaces under conditions of 5% H2 cofeeding with methane predicted using the microkinetic model. Reproduced with permission from ref. 55. Copyright 2017 American Chemical Society. (b) Performance of different Pt–Bi/ZSM-5 catalysts at various methane conversions for ethane selectivity over ZSM-5 zeolite supported bimetallic Pt–Bi catalysts. Reproduced with permission from ref. 56. Copyright 2018 American Chemical Society. | ||
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2Ta–H is known to catalyze the hydrogenolysis of ethane into methane. Basset et al. reported the first example of the transformation of methane into ethane via nonoxidative coupling57 over a heterogeneous Ta-based catalyst (
SiO)2Ta–H is known to catalyze the hydrogenolysis of ethane into methane. Basset et al. reported the first example of the transformation of methane into ethane via nonoxidative coupling57 over a heterogeneous Ta-based catalyst (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2Ta–H. A high selectivity (>98%) of ethane was achieved among the hydrocarbon products with a methane conversion degree of approximately 0.1% at a temperature of 375 °C in the continuous flow reactor. As shown in Fig. 8a, the reaction mechanism involved the migration of the methyl ligand onto tantalum-methylidene, which was proposed as the key intermediate for ethane formation in the NOCM. Recently, free Ta8O2+ cluster ions were also found to be capable of NOCM reactions.58 A similar catalytic cycle for the C–C coupling step was proposed on the Ta8O2+ cluster ions and the Ta-based catalyst (Fig. 8b), and the difference lies in the final step of the product formation. As shown in Fig. 8c, the activation of an extra methane molecule was required for the formation of ethane over tantalum hydride catalysts, while the formation of ethane was completed with the previously formed carbene and methane on Ta8O2+. The results of these works prove the great potential of tantalum-based catalysts for the NOCM.
SiO)2Ta–H. A high selectivity (>98%) of ethane was achieved among the hydrocarbon products with a methane conversion degree of approximately 0.1% at a temperature of 375 °C in the continuous flow reactor. As shown in Fig. 8a, the reaction mechanism involved the migration of the methyl ligand onto tantalum-methylidene, which was proposed as the key intermediate for ethane formation in the NOCM. Recently, free Ta8O2+ cluster ions were also found to be capable of NOCM reactions.58 A similar catalytic cycle for the C–C coupling step was proposed on the Ta8O2+ cluster ions and the Ta-based catalyst (Fig. 8b), and the difference lies in the final step of the product formation. As shown in Fig. 8c, the activation of an extra methane molecule was required for the formation of ethane over tantalum hydride catalysts, while the formation of ethane was completed with the previously formed carbene and methane on Ta8O2+. The results of these works prove the great potential of tantalum-based catalysts for the NOCM.
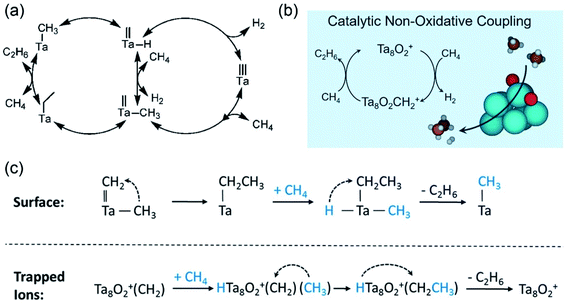 | ||
Fig. 8 (a) Proposed mechanism for NOCM over silica-supported tantalum hydride (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2Ta–H. Reproduced with permission from ref. 57. Copyright 2008 American Chemical Society. (b) NOCM catalyzed by Ta8O2+. (c) Comparison of the mechanisms for ethane formation in NOCM. Reproduced with permission from ref. 58. Copyright 2020 American Chemical Society. SiO)2Ta–H. Reproduced with permission from ref. 57. Copyright 2008 American Chemical Society. (b) NOCM catalyzed by Ta8O2+. (c) Comparison of the mechanisms for ethane formation in NOCM. Reproduced with permission from ref. 58. Copyright 2020 American Chemical Society. | ||
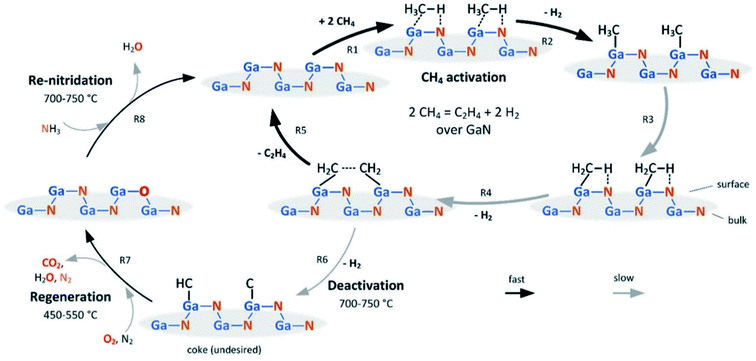 | ||
| Fig. 9 Proposed mechanism for the activation/regeneration/renitridation cycle of GaN/SBA-15 in NOCM. Reproduced with permission from ref. 61. Copyright 2020 American Chemical Society. | ||
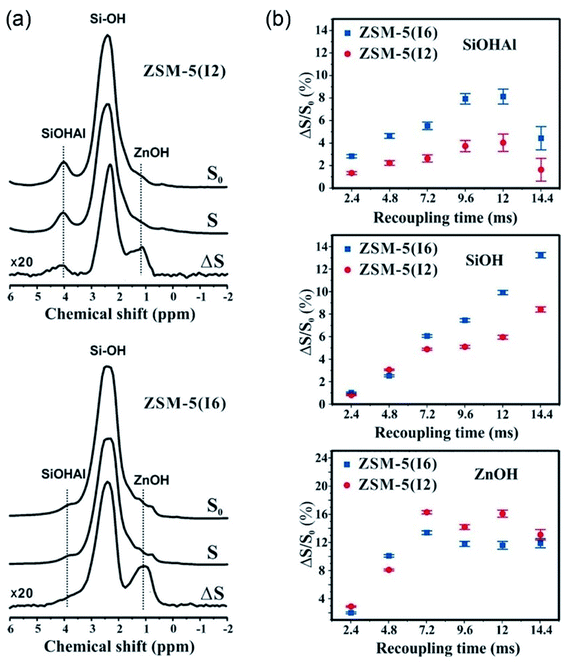 | ||
| Fig. 10 (a) NMR spectra of ZSM-5(I2) and ZSM-5(I6) recorded at 18.8 T with a recoupling time of 9.6 ms. (b) ΔS/S0 signal fraction versus the total recoupling time. S and S0 denote the 1H spectrum with and without 67Zn irradiation, respectively. Reproduced with permission from ref. 68. Copyright 2016 John Wiley and Sons. | ||
The co-aromatization mechanism of methane with other hydrocarbons on Zn-modified zeolites has also been widely explored. It is proposed that methane was first converted to a methoxy species when co-fed with higher hydrocarbons. By undergoing methyl formation with the aid of aromatic species in the zeolite, it was then transferred to the phenyl rings.64 Recently, He et al. studied the impact of the Al sites of Zn/HZSM-5 on the methane conversion mechanism during the co-aromatization with alkanes.69 The diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and NH3-TPD characterization results of Zn/HZSM-5 suggest that the extra-framework and framework Al sites are related to the Lewis acid and Brönsted acid of the zeolite, respectively. Both the extra-framework and framework Al sites were demonstrated to be vital for methane activation, and the catalytic activity could be enhanced by converting some of the framework Al atoms to the extra-framework Al sites. Meanwhile, the effect of the Zn location in Zn/HZSM-5 was also studied for the co-aromatization of methane/alkanes, in which the Zn species located on the external surface of HZSM-5 were related to the generation of the alkyl substitution group while the Zn within the inner pores was responsible for the formation of the phenyl rings of the aromatics.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), which dimerizes into ethylene and is then oligomerized into the aromatics on the acid sites of the zeolite. On the other hand, the degree of carburization of the Fe species can be enhanced in the presence of promoters, which promote the reactivity of NOCM over the Fe-based catalyst. For example, the promotional effect of Au toward the carburization of Fe on Fe/HZSM-5 for an improved NOCM was recently reported by Sim et al.74.
CH2), which dimerizes into ethylene and is then oligomerized into the aromatics on the acid sites of the zeolite. On the other hand, the degree of carburization of the Fe species can be enhanced in the presence of promoters, which promote the reactivity of NOCM over the Fe-based catalyst. For example, the promotional effect of Au toward the carburization of Fe on Fe/HZSM-5 for an improved NOCM was recently reported by Sim et al.74.
3. Newly developed single-atom based catalysts
A single-atom catalyst (SAC), defined as a catalyst composed of only isolated single atom active sites on supports,75 provides significant advantages for optimizing the catalytic performance owing to the well-defined structure of the catalytic sites. Even though highly dispersed Fe cations supported on HZSM-5 were shown to be active in the NOCM, they suffered from severe coke deposition.73,76,77 Novel catalytic systems are thus greatly desired to make the nonoxidative conversion of methane more selective and efficient. In this regard, coordinatively unsaturated iron sites show significant potential owing to their high activity towards C–H activation.78,79 In 2014, Bao and colleagues reported single iron atoms embedded in the lattice of silica (Fe@SiO2) as a highly efficient catalyst for the nonoxidative conversion of methane to ethylene and benzene (Fig. 11a).15 As shown in Fig. 11b and c, these coordinatively unsaturated iron atom species interact extensively with the SiO2 through bonding to the Si and C atoms, leading to an excellent stability, even after a prolonged reaction time of 60 h. This study was viewed as a landmark discovery for the transformation of methane, and it thus opened up a new avenue for the application of single atom catalysts (SACs) in NOCM.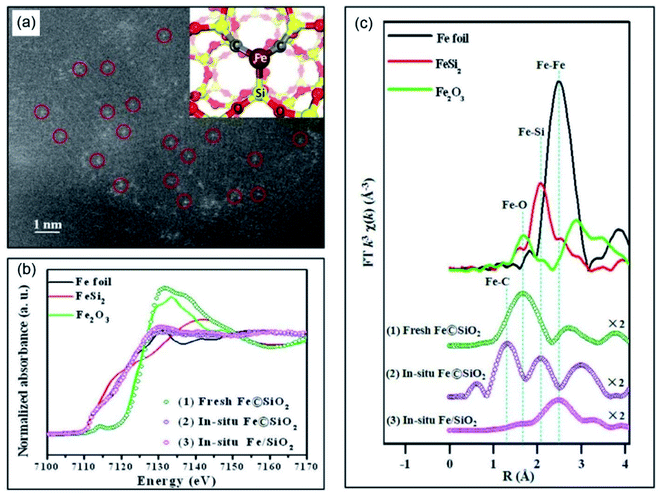 | ||
| Fig. 11 (a) Scanning transmission electron microscopy high-angle annular dark-field (STEM-HAADF) image of Fe@SiO2 (inset shows the computational model of single iron atoms embedded in the lattice of silica). (b) In situ XANES, and (c) Fourier transformed (FT) EXAFS spectra upon activation. Reproduced with permission from ref. 15. Copyright 2014, American Association for the Advancement of Science. | ||
Over the past few years, a number of studies have been carried out using SACs as catalysts for NOCM.80–85 For instance, the effect of the catalytic surface of silica-based iron catalysts (Fe©CRS) on MTOAH was studied by Han et al.81. The highly dispersed confined Fe sites with Fe–Si coordination were identified to be more favorable for the formation of the methyl radical compared to the Fe3C clusters, and the optimized Fe©CRS catalyst can achieve a 6.9–5.8% methane conversion with a 86.2% C2 selectivity at 1080 °C for 100 h. Recently, nanoceria-supported single Pt atom catalysts (Pt1@CeO2) were demonstrated with a higher C2 product selectivity than that of Fe@SiO2 at 950 °C.85 Based on the DRIFTS analysis results, the methane conversion over Fe@SiO2 was proposed through catalytic coupling of dehydrogenated *CH3 and *CH2 adsorbates on the single Pt sites. In the work carried out by Sakbodin et al.,82 a significant improvement in methane conversion (∼30% C2+ single-pass yield with 99% selectivity to C2 products at 30% methane conversion) was achieved by integrating the Fe@SiO2 catalyst in a hydrogen (H2) permeable tubular membrane reactor. A comparison of the catalytic performance for recently reported catalysts for the NOCM is shown in Table 1. As shown, the results from recent advances have demonstrated the isolation of single-Fe-atom sites as a promising way to maintain the catalytic activity and long-term stability, enabling more sophisticated studies to be performed to elucidate the underlying mechanism and optimize the catalysts, and even improve the design of the reactor to achieve an optimized performance for the NOCM.
| Catalyst (metal loading) | Reaction conditions | Methane conversion, % | Selectivity for indicated products | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | Feed composition | C6H6 | C10H8 | Coke | Others | |||
| Mo6/MCM-22 (5 wt%) | 700 °C | 1500 mL g−1 h−1 | 9% | 66% | 9% | 16% | 9% | 24 |
| CH4/N2 80/20 | 5 h | |||||||
| Mo/ZSM-5 | 700 °C | 1500 mL g−1 h−1 | 11.6% | C6+: 8.8% | 26 | |||
| CH4/N2 80/20 | 15 h | |||||||
| 10MoCy/HZSM-5 (10 wt%) | 700 °C | 1550 mL g−1 h−1 | 11.6% | 80% | — | — | — | 32 |
| CH4/N2 91/9 | 15 h | |||||||
| Mo/HZSM-5 | 700 °C | WHSV 2 h−1 | 22.7% | 51.5% | 21.8% | C7H8: 3.2 | 34 | |
| CH4/N2 95/5 | 16 h | C8H10: 17.3 | ||||||
| C2: 6.1 | ||||||||
| Mo5/MCM-22 (21) | 680 °C | 1500 mL g−1 h−1 | 7.2% | 51.9% | ∼7% | 5.6% | 34.4% | 42 |
| (5 wt%) | CH4/N2 90/10 | 2 h | ||||||
| Mo5/MCM-22 (91) | 680 °C | 1500 mL g−1 h−1 | 4.5% | 36.1% | ∼7% | 5% | 51.1% | 42 |
| (5 wt%) | CH4/N2 90/10 | 2 h | ||||||
| MoOx/ZSM-5 (3 wt%) | 700 °C | 1550 mL h−1 g−1 | 8% | 62% | — | 22% | 16% | 43 |
| 9% N2/CH4 | 12 h | |||||||
| MoOx/ZSM-5 (10 wt%) | 700 °C | 1550 mL h−1 g−1 | 9.5% | 85% | — | 2% | 13% | 43 |
| 9% N2/CH4 | 12 h | |||||||
| Mo/HZSM-5 (MA) | 700 °C | 1500 mL g−1 h−1 | 11.8% | 70.7% | 12.9% | 9.3% | 7.1% | 44 |
| (10 wt%) | CH4/N2 90/10 | 10 h | ||||||
| Mo/H-S-Z (6 wt%) | 700 °C | 1500 mL g−1 h−1 | 6.29% | 55.6% | — | 6.6% | 37.8% | 45 |
| CH4/Ar 90.3/9.7 | 3 h | |||||||
| Mo/HZSM-5 | 700 °C | 1500 mL g−1 h−1 | 12% | 83.3% | — | — | — | 46 |
| CH4/N2 90/10 | 10 h | |||||||
| 0.5% Re-4.0%Mo/ZSM-5 | 750 °C | 1000 mL g−1 h−1 | 14.8% | 54.05% | 30.4% | 13% | 2% | 47 |
| CH4/99.99% | 20 min | |||||||
| 0.5% Cr-5% Mo–SZ | 700 °C | 600 mL g−1 h−1 | 13.5% | 55% | — | 11.49% | 27.5% | 48 |
| CH4/N2 90/10 | ||||||||
| 1.0% Fe (nano)-5% Mo/HZSM-5 | 700 °C | 1650 mL g−1 h−1 | 14% | 22% | 3% | 52.3% | 22.7% | 49 |
| CH4/Ar 10/1 | ||||||||
| 1.5% Fe (nano)-5% Mo/HZSM-5 | 700 °C | 1650 mL g−1 h−1 | 12% | 17% | 6% | 57% | 20% | 49 |
| CH4/Ar 10/1 | ||||||||
| Mg–Mo/HZSM-5 | 800 °C | 1440 mL g−1 h−1 | 12.3% | 68.4% | — | 10.1% | — | 50 |
| CH4/Ar/CO2 90/8/2 | 1 h | |||||||
| 6Mo-0.2Ni/ZSM-5 | 700 °C | 1500 mL g−1 h−1 | 9.8%, 10 h | 35% | 2.5% | 59% | 3.5% | 51 |
| 6Mo-0.6Co/ZSM-5 | CH4/N2 91/9 | 5%, 10 h | 30% | 2.1% | 57% | 10.9% | ||
| 10 Mo -1K/HZSM-5 | 750 °C | — | 28% | 50% | — | 47% | — | 53 |
| 255 min | ||||||||
| PtSn/SiO2–5%H2 | 700 °C | 5% CH4 introduce to 25% H2/He tank | 0.05% | C2H4: 63% | 55 | |||
| 6 h | C2H6: 36% | |||||||
| Pt–Bi/ZSM-5 | 600–700 °C | — | 2% 8 h | C2H4 + C2H6: >90% | 56 | |||
| GaN/SBA15 | 700 °C | 567 h−1 | 2.5% 8 h | C2H4: 97.4% C3H6: 2.04% C6 + 0.6% | 61 | |||
| Fe/HMCM-22 | 750 °C | 1500 mL g−1 h−1 | 896 nmol/(gcat. s) | 74.6% | 15.1% | — | 10.3% | 73 |
| CH4/Ar 90/10 | ||||||||
| Fe/HZSM-5 | 700 °C | 3750 mL g−1 h−1 | 1.3% 10 hours | 22.5% | — | 42% | 35.5% | 76 |
| CH4/He 50/50 | ||||||||
| Fe©CRS (0.41 wt%) | 1080 °C | 8000 mL g−1 h−1 | 6.9–5.8% | C2: 86.2%, C3–C5: ∼6% | 81 | |||
| CH4/H2 50/50 | 100 h | Aromatics: ∼8%, coke: ∼1% | ||||||
| Fe©SiO2 (0.5 wt%) | 1030 °C | 3200 mLg−1 h−1 | 30% | C2 and aromatic | 82 | |||
| CH4/Ar 90/10 | 99% | |||||||
| Fe©SiO2 (0.5 wt%) | 1090 °C | 21.4 L g−1 h−1 | 48.1% | 28% | 24% | C2H4: 48% | 15 | |
| CH4/N2 90/10 | ||||||||
| Pt1@CeO2 (0.5 wt%) | 975 °C | 6 L g−1 h−1 | 14.4% | ∼13% | ∼2% | C2: 74.6% | 85 | |
| 1% CH4/He | C3: <10% | |||||||
On the other hand, considerable efforts have also been devoted to uncovering the catalytic mechanism of the NOCM on SACs. Results from recent theoretical studies provided an in-depth mechanistic understanding of the elementary steps.86–90 In the work conducted by Bao and colleagues,15 a gas-phase reaction mechanism was proposed based on preliminary theoretical calculations and VUV-SPI-MBMS analysis. It was proposed that methane was first activated over the single Fe atom center, resulting in the C–H bond cleavage of CH4 with the dissociated H and methyl being adsorbed at the C and Fe sites, respectively. The target ethylene and aromatics were then generated from the released gas phase of methyl via gas-phase radical/molecule collision. Further quantum chemistry calculations performed by He et al. suggest a monofunctional mechanism occurs over the surface of the Fe@SiO2 catalysts and metal/zeolites for the NOCM.35 It was suggested in this work that both the activation of methane and its further C–C coupling occurred on the active FeC3− cluster. Recently, by using DFT calculation methods and ab initio molecular dynamics (AIMD) simulations, we have also systematically studied the reaction mechanisms of methane conversion over Fe1@SiC2.87 As shown in Fig. 12a, a new quasi Mars–van Krevelen mechanism is revealed for NOCM over the Fe1@SiC2 active center. The activation of the C–H bond of methane occurs at the Fe single sites to produce Fe–CH3, and this dissociated methyl was found to facilely transfer to the adjacent carbon site of SiC2 by realizing the C–C coupling. Following this, hydrogen transfer occurs and ethylene is then produced via a key –CH-CH2 intermediate on the Fe single-atom site and results in a carbon vacancy on the SiC2 surface. Meanwhile, this carbon defect on the SiC2 surface can be recovered by the activation of another methane on the Fe single-atom site. It thus involves the withdrawal and regeneration of the surface C atom on the support during methane conversion. The involvement of both C and Fe suggests the importance of the dual site synergetic interaction for the nonoxidative conversion of methane on the SACs. The dynamic formation mechanism of the FeSiC2@SiO2 from FeO3©SiO2 was further explored.88 As shown in Fig. 12b, eight different active centers evolving from FeO3©SiO2 were monitored, which demonstrated different catalytic performances for methane conversion. The Fe active centers coordinated with unsaturated C atoms were revealed to be more active for methane dissociation, these prefer to transfer the dissociated methyl group to adjacent C atoms and follow a surface coupling reaction mechanism. Meanwhile, the Fe active centers coordinated with saturated C/O/Si were shown to have a reduced activity for methane conversion, which leads to the desorption of the methyl group into the gas phase and follows a gas-phase reaction mechanism. This highlights the importance of engineering active sites in determining the activity and selectivity in the NOCM, which should be considered in the design of further catalysts for methane conversion.
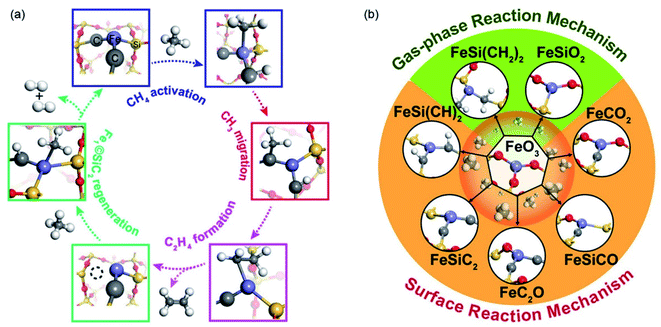 | ||
| Fig. 12 (a) Proposed quasi Mars–van Krevelen mechanism for methane conversion over the Fe1@SiC2 active center. Reproduced with permission from ref. 87. Copyright 2020 John Wiley and Sons. (b) Theoretically revealed active centers evolving from FeO3©SiO2 during methane conversion. Reproduced with permission from ref. 88. Copyright 2020 American Chemical Society. | ||
4. Summary and outlook
The direct conversion of methane to high-value chemicals is a highly economical way to utilize methane. In terms of the conventional zeolite supported Mo-based catalysts for the nonoxidative conversion of methane to aromatics, significant progress has been made, including identification of the active site, an explanation of the function of the zeolite, and the design of promoters. The nature of Mo species under real-time conditions, the pore structure with the acidity of the zeolite supports, as well as the synergy effect of promoters, play a crucial role in determining the catalytic activity and stability of methane nonoxidative coupling over Mo-based conventional catalysts. On the other hand, various non-Mo-based catalysts have been considered recently. In particular, SACs as a novel topic in heterogeneous catalysis have been demonstrated to possess significant potential in the nonoxidative conversion of methane. The single-atom active site of the SACs with the synergistic support enables an unusual catalytic behavior providing the olefins as a primary product in methane nonoxidative coupling, which makes it a newly emerging candidate in the area of methane chemistry.Despite this, the activation of the C–H bond of methane and the selective controlling of C–C coupling is still a challenge in the nonoxidative conversion of methane. Further investigation of the structure–activity relationship in methane activation and coupling is greatly needed, not only for the conventional Mo-based catalysts but also in the development of novel catalysts. Recent experimental and theoretical results have demonstrated that the coordinatively unsaturated single-Fe-atom sites show significant potential for use in the NOCM. The activation of C–H bond occurs at the Fe single site and the adjacent carbon site is essential for C–C coupling. For example, elucidating the exact atomic structure of the active sites, especially in real-time reactions, with their associated electronic structure, acidic/basic properties and others, as well as the possible roles of neighboring chemical environments, would be important to correlate with the catalytic activity of the C–H bond activation. On the other hand, the catalysts also require functional sites for C–C coupling which determines the length of the carbon chain, and the dependency of the size and geometry of the active center seems to have a negligible effect on the product distributions. In this regard, the rational design of SACs with the desired coordination environment (such as C, Si, O coordination number) is considered as a promising route to achieving the efficient activation of the C–H bond and selective C–C coupling.
Furthermore, in-depth insights into the underlying mechanism of NOCM will require in situ characterization on the active phases and the experimental identification of the reaction intermediates under a high reaction temperature. To this end, techniques such as electron spin resonance (ESR) spectroscopy, Mössbauer spectroscopy, atomic resonance absorption spectroscopy, and so on can be employed. The development of spatially resolved reactors is also important for providing insights into the gas-phase reaction intermediates. Theoretical modelling is a powerful tool to access information such as C–H bond breaking and C–C coupling, as well as determining the energy barriers during the whole process. In addition, the carburization and accumulation of carbonaceous species on the surface of the catalyst remain the main challenges under nonoxidative conditions. Therefore, the development of novel catalysts with a high activity, selectivity, and stability is still a long-pursued target.
We anticipate that further advances will be achieved in this field. Meanwhile, the direct carbon chain growth of methane with other carbon resources such as CO2 is also a significant development in the area of methane conversion and application. Although the oxidative coupling of methane was studied extensively at the end of the last century, methane coupling using a modern catalytic technique is now welcoming in a new era in this field, and it also offers human beings a novel path for the rational utilization of natural resources.
Author contributions
Conceptualization, writing – original draft, writing – review & editing, T. Z.Conflicts of interest
There are no conflicts to declare.References
- V. R. Choudhary, A. K. Kinage and T. V. Choudhary, Low-temperature nonoxidative activation of methane over H-galloaluminosilicate (MFI) zeolite, Science, 1997, 275, 1286–1288 CrossRef CAS PubMed.
- S. H. Morejudo, R. Zanon, S. Escolastico, I. Yuste-Tirados, H. Malerod-Fjeld, P. K. Vestre, W. G. Coors, A. Martinez, T. Norby, J. M. Serra and C. Kjolseth, Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor, Science, 2016, 353, 563–566 CrossRef CAS PubMed.
- P. Tang, Q. J. Zhu, Z. X. Wu and D. Ma, Methane activation: the past and future, Energy Environ. Sci., 2014, 7, 2580–2591 RSC.
- J. J. Spivey and G. Hutchings, Catalytic aromatization of methane, Chem. Soc. Rev., 2014, 43, 792–803 RSC.
- L. L. Sun, Y. Wang, N. J. Guan and L. D. Li, Methane Activation and Utilization: Current Status and Future Challenges, Energy Technol., 2020, 8, 1900826 CrossRef CAS.
- X. G. Meng, X. J. Cui, N. P. Rajan, L. Yu, D. H. Deng and X. H. Bao, Direct Methane Conversion under Mild Condition by Thermo-, Electro-, or Photocatalysis, Chem, 2019, 5, 2296–2325 CAS.
- P. Schwach, X. Pan and X. Bao, Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects, Chem. Rev., 2017, 117, 8497–8520 CrossRef CAS PubMed.
- S. Ma, X. Guo, L. Zhao, S. Scott and X. Bao, Recent progress in methane dehydroaromatization: From laboratory curiosities to promising technology, J. Energy Chem., 2013, 22, 1–20 CrossRef CAS.
- S. D. Senanayake, J. A. Rodriguez and J. F. Weaver, Low Temperature Activation of Methane on Metal-Oxides and Complex Interfaces: Insights from Surface Science, Acc. Chem. Res., 2020, 53, 1488–1497 CrossRef CAS PubMed.
- K. D. Sun, D. M. Ginosar, T. He, Y. L. Zhang, M. H. Fan and R. P. Chen, Progress in Nonoxidative Dehydroaromatization of Methane in the Last 6 Years, Ind. Eng. Chem. Res., 2018, 57, 1768–1789 CrossRef CAS.
- M. A. A. Aziz, A. A. Jalil, S. Wongsakulphasatch and D. V. N. Vo, Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: a short review, Catal. Sci. Technol., 2020, 10, 35–45 RSC.
- I. Vollmer, I. Yarulina, F. Kapteijn and J. Gascon, Progress in Developing a Structure-Activity Relationship for the Direct Aromatization of Methane, Chemcatchem, 2019, 11, 39–52 CrossRef CAS.
- D. V. Golinskii, N. V. Vinichenko, E. V. Zatolokina, V. V. Pashkov, E. A. Paukshtis, T. I. Gulyaeva, P. E. Pavlyuchenko, O. V. Krol’ and A. S. Belyi, Modern Catalysts and Methods of Nonoxidative Methane Conversion, Russ. J. Gen. Chem., 2020, 90, 1104–1119 CrossRef CAS.
- R. Horn and R. Schlögl, Methane Activation by Heterogeneous Catalysis, Catal. Lett., 2015, 145, 23–39 CrossRef CAS.
- X. Guo, G. Fang, G. Li, H. Ma, H. Fan, L. Yu, C. Ma, X. Wu, D. Deng, M. Wei, D. Tan, R. Si, S. Zhang, J. Li, L. Sun, Z. Tang, X. Pan and X. Bao, Direct, Nonoxidative Conversion of Methane to Ethylene, Aromatics, and Hydrogen, Science, 2014, 344, 616–619 CrossRef CAS PubMed.
- L. Wang, L. Tao, M. Xie, G. Xu, J. Huang and Y. Xu, Dehydrogenation and aromatization of methane under non-oxidizing conditions, Catal. Lett., 1993, 21, 35–41 CrossRef CAS.
- P. L. Tan, The catalytic performance of Mo-impregnated HZSM-5 zeolite in CH4 aromatization: Strong influence of Mo loading and pretreatment conditions, Catal. Commun., 2018, 103, 101–104 CrossRef CAS.
- R. W. Borry, Y. H. Kim, A. Huffsmith, J. A. Reimer and E. Iglesia, Structure and Density of Mo and Acid Sites in Mo-Exchanged H-ZSM5 Catalysts for Nonoxidative Methane Conversion, J. Phys. Chem. B, 1999, 103, 5787–5796 CrossRef CAS.
- W. Li, G. D. Meitzner, R. W. Borry and E. Iglesia, Raman and X-Ray Absorption Studies of Mo Species in Mo/H-ZSM5 Catalysts for Non-Oxidative CH4 Reactions, J. Catal., 2000, 191, 373–383 CrossRef CAS.
- W. Ding, S. Li, G. D Meitzner and E. Iglesia, Methane Conversion to Aromatics on Mo/H-ZSM5:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Structure of Molybdenum Species in Working Catalysts, J. Phys. Chem. B, 2001, 105, 506–513 CrossRef CAS.
Structure of Molybdenum Species in Working Catalysts, J. Phys. Chem. B, 2001, 105, 506–513 CrossRef CAS. - J.-P. Tessonnier, B. Louis, S. Rigolet, M. J. Ledoux and C. Pham-Huu, Methane dehydro-aromatization on Mo/ZSM-5: About the hidden role of Brønsted acid sites, Appl. Catal., A, 2008, 336, 79–88 CrossRef CAS.
- J.-P. Tessonnier, B. Louis, S. Walspurger, J. Sommer, M.-J. Ledoux and C. Pham-Huu, Quantitative Measurement of the Brönsted Acid Sites in Solid Acids:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Toward a Single-Site Design of Mo-Modified ZSM-5 Zeolite, J. Phys. Chem. B, 2006, 110, 10390–10395 CrossRef CAS PubMed.
Toward a Single-Site Design of Mo-Modified ZSM-5 Zeolite, J. Phys. Chem. B, 2006, 110, 10390–10395 CrossRef CAS PubMed. - N. Kosinov, F. J. A. G. Coumans, G. Li, E. Uslamin, B. Mezari, A. S. G. Wijpkema, E. A. Pidko and E. J. M. Hensen, Stable Mo/HZSM-5 methane dehydroaromatization catalysts optimized for high-temperature calcination-regeneration, J. Catal., 2017, 346, 125–133 CrossRef CAS.
- I. Julian, J. L. Hueso, N. Lara, A. Sole-Daura, J. M. Poblet, S. G. Mitchell, R. Mallada and J. Santamaria, Polyoxometalates as alternative Mo precursors for methane dehydroaromatization on Mo/ZSM-5 and Mo/MCM-22 catalysts, Catal. Sci. Technol., 2019, 9, 5927–5942 RSC.
- M. Agote-Aran, R. E. Fletcher, M. Briceno, A. B. Kroner, I. V. Sazanovich, B. Slater, M. E. Rivas, A. W. J. Smith, P. Collier, I. Lezcano-Gonzalez and A. M. Beale, Implications of the Molybdenum Coordination Environment in MFI Zeolites on Methane Dehydroaromatisation Performance, Chemcatchem, 2020, 12, 294–304 CrossRef CAS.
- I. Julian, M. B. Roedern, J. L. Hueso, S. Irusta, A. K. Baden, R. Mallada, Z. Davis and J. Santamaria, Supercritical solvothermal synthesis under reducing conditions to increase stability and durability of Mo/ZSM-5 catalysts in methane dehydroaromatization, Appl. Catal., B, 2020, 263, 118360 CrossRef CAS.
- D. Wang, J. H. Lunsford and M. P. Rosynek, Catalytic conversion of methane to benzene over Mo/ZSM-5, Top. Catal., 1996, 3, 289–297 CrossRef CAS.
- D. Wang, J. H. Lunsford and M. P. Rosynek, Characterization of a Mo/ZSM-5 Catalyst for the Conversion of Methane to Benzene, J. Catal., 1997, 169, 347–358 CrossRef CAS.
- H. Liu, X. Bao and Y. Xu, Methane dehydroaromatization under nonoxidative conditions over Mo/HZSM-5 catalysts: Identification and preparation of the Mo active species, J. Catal., 2006, 239, 441–450 CrossRef CAS.
- I. Lezcano-González, R. Oord, M. Rovezzi, P. Glatzel, S. W. Botchway, B. M. Weckhuysen and A. M. Beale, Molybdenum Speciation and its Impact on Catalytic Activity during Methane Dehydroaromatization in Zeolite ZSM-5 as Revealed by Operando X-Ray Methods, Angew. Chem., Int. Ed., 2016, 55, 5215–5219 CrossRef PubMed.
- N. K. Razdan and A. Bhan, Carbidic Mo is the sole kinetically-relevant active site for catalytic methane dehydroaromatization on Mo/H-ZSM-5, J. Catal., 2020, 389, 667–676 CrossRef CAS.
- M. Rahman, A. Sridhar and S. J. Khatib, Impact of the presence of Mo carbide species prepared ex situ in Mo/HZSM-5 on the catalytic properties in methane aromatization, Appl. Catal., A, 2018, 558, 67–80 CrossRef CAS.
- Z. R. Ismagilov, E. V. Matus and L. T. Tsikoza, Direct conversion of methane on Mo/ZSM-5 catalysts to produce benzene and hydrogen: achievements and perspectives, Energy Environ. Sci., 2008, 1, 526–541 RSC.
- N. Kosinov, F. J. A. G. Coumans, E. A. Uslamin, A. S. G. Wijpkema, B. Mezari and E. J. M. Hensen, Methane Dehydroaromatization by Mo/HZSM-5: Mono- or Bifunctional Catalysis?, ACS Catal., 2017, 7, 520–529 CrossRef CAS.
- H. F. Li, L. X. Jiang, Y. X. Zhao, Q. Y. Liu, T. Zhang and S. G. He, Formation of Acetylene in the Reaction of Methane with Iron Carbide Cluster Anions FeC3- under High-Temperature Conditions, Angew. Chem., Int. Ed., 2018, 57, 2662–2666 CrossRef CAS PubMed.
- T. Zhang, X. Yang and Q. Ge, A DFT study of methane conversion on Mo-terminated Mo2C carbides: Carburization vs. CC coupling, Catal. Today, 2020, 368, 140–147 CrossRef.
- T. Zhang, X. Yang and Q. Ge, CH4 Dissociation and C-C Coupling on Mo-terminated MoC Surfaces: A DFT Study, Catal. Today, 2020, 339, 54–61 CrossRef CAS.
- C.-L. Zhang, S. Li, Y. Yuan, W.-X. Zhang, T.-H. Wu and L.-W. Lin, Aromatization of methane in the absence of oxygen over Mo-based catalysts supported on different types of zeolites, Catal. Lett., 1998, 56, 207–213 CrossRef CAS.
- H. Zheng, D. Ma, X. Bao, J. Z. Hu, J. H. Kwak, Y. Wang and C. H. F. Peden, Direct Observation of the Active Center for Methane Dehydroaromatization Using an Ultrahigh Field 95Mo NMR Spectroscopy, J. Am. Chem. Soc., 2008, 130, 3722–3723 CrossRef CAS PubMed.
- J. Z. Hu, J. H. Kwak, Y. Wang, C. H. F. Peden, H. Zheng, D. Ma and X. Bao, Studies of the Active Sites for Methane Dehydroaromatization Using Ultrahigh-Field Solid-State 95Mo NMR Spectroscopy, J. Phys. Chem. C, 2009, 113, 2936–2942 CrossRef CAS.
- D. Ma, Y. Lu, L. Su, Z. Xu, Z. Tian, Y. Xu, L. Lin and X. Bao, Remarkable Improvement on the Methane Aromatization Reaction:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Highly Selective and Coking-Resistant Catalyst, J. Phys. Chem. B, 2002, 106, 8524–8530 CrossRef CAS.
A Highly Selective and Coking-Resistant Catalyst, J. Phys. Chem. B, 2002, 106, 8524–8530 CrossRef CAS. - T. H. Lim, K. Nam, I. K. Song, K. Y. Lee and D. H. Kim, Effect of Si/Al2 ratios in Mo/H-MCM-22 on methane dehydroaromatization, Appl. Catal., A, 2018, 552, 11–20 CrossRef CAS.
- M. Rahman, A. Infantes-Molina, A. S. Hoffman, S. R. Bare, K. L. Emerson and S. J. Khatib, Effect of Si/Al ratio of ZSM-5 support on structure and activity of Mo species in methane dehydroaromatization, Fuel, 2020, 278, 118290 CrossRef CAS.
- P. L. Tan, Ammonia-basified 10 wt% Mo/HZSM-5 material with enhanced dispersion of Mo and performance for catalytic aromatization of methane, Appl. Catal., A, 2019, 580, 111–120 CrossRef CAS.
- P. Zhu, G. Yang, J. Sun, R. Fan, P. Zhang, Y. Yoneyama and N. Tsubaki, A hollow Mo/HZSM-5 zeolite capsule catalyst: preparation and enhanced catalytic properties in methane dehydroaromatization, J. Mater. Chem. A, 2017, 5, 8599–8607 RSC.
- X. Huang, X. Jiao, M. G. Lin, K. Wang, L. T. Jia, B. Hou and D. B. Lia, Coke distribution determines the lifespan of a hollow Mo/HZSM-5 capsule catalyst in CH4 dehydroaromatization, Catal. Sci. Technol., 2018, 8, 5753–5762 Search PubMed.
- A. A. Stepanov, V. I. Zaikovskii, L. L. Korobitsyna and A. V. Vosmerikov, Nonoxidative Conversion of Methane to Aromatic Hydrocarbons in the Presence of ZSM-5 Zeolites Modified with Molybdenum and Rhenium, Pet. Chem., 2019, 59, 91–98 CrossRef CAS.
- M. A. Abedin, S. Kanitkar, S. Bhattar and J. J. Spivey, Promotional Effect of Cr in Sulfated Zirconia-Based Mo Catalyst for Methane Dehydroaromatization, Energy Technol., 2020, 8, 1900555 CrossRef CAS.
- K. D. Sun, W. B. Gong, K. Gasem, H. Adidharma, M. H. Fan and R. P. Chen, Catalytic Methane Dehydroaromatization with Stable Nano Fe Doped on Mo/HZSM-5 Synthesized with a Simple and Environmentally Friendly Method and Clarification of a Perplexing Catalysis Mechanism Dilemma in This Field for a Period of Time, Ind. Eng. Chem. Res., 2017, 56, 11398–11412 CrossRef CAS.
- X. Cheng, P. Yan, X. Z. Zhang, F. Yang, C. Y. Dai, D. P. Li and X. X. Ma, Enhanced methane dehydroaromatization in the presence of CO2 over Fe- and Mg-modified Mo/ZSM-5, Mol. Catal., 2017, 437, 114–120 CrossRef CAS.
- A. Sridhar, M. Rahman, A. Infantes-Molina, B. J. Wylie, C. G. Borcik and S. J. Khatib, Bimetallic Mo-Co/ZSM-5 and Mo-Ni/ZSM-5 catalysts for methane dehydroaromatization: A study of the effect of pretreatment and metal loadings on the catalytic behavior, Appl. Catal., A, 2020, 589, 117247 CrossRef.
- D. Bajec, A. Kostyniuk, A. Pohar and B. Likozar, Nonoxidative methane activation, coupling, and conversion to ethane, ethylene, and hydrogen over Fe/HZSM-5, Mo/HZSM-5, and Fe-Mo/HZSM-5 catalysts in packed bed reactor, Int. J. Energy Res., 2019, 43, 6852–6868 CAS.
- V. Ramasubramanian, D. J. Lienhard, H. Ramsurn and G. L. Price, Effect of Addition of K, Rh and Fe Over Mo/HZSM-5 on Methane Dehydroaromatization Under Non-oxidative Conditions, Catal. Lett., 2019, 149, 950–964 CrossRef CAS.
- M. Belgued, P. Pareja, A. Amariglio and H. Amariglio, Conversion of methane into higher hydrocarbons on platinum, Nature, 1991, 352, 789–790 CrossRef CAS.
- D. Gerceker, A. H. Motagamwala, K. R. Rivera-Dones, J. B. Miller, G. W. Huber, M. Mavrikakis and J. A. Dumesic, Methane Conversion to Ethylene and Aromatics on PtSn Catalysts, ACS Catal., 2017, 7, 2088–2100 CrossRef CAS.
- Y. Xiao and A. Varma, Highly Selective Nonoxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts, ACS Catal., 2018, 8, 2735–2740 CrossRef CAS.
- D. Soulivong, S. Norsic, M. Taoufik, C. Coperet, J. Thivolle-Cazat, S. Chakka and J.-M. Basset, Non-Oxidative Coupling Reaction of Methane to Ethane and Hydrogen Catalyzed by the Silica-Supported Tantalum Hydride: (
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2Ta−H, J. Am. Chem. Soc., 2008, 130, 5044–5045 CrossRef CAS PubMed.
SiO)2Ta−H, J. Am. Chem. Soc., 2008, 130, 5044–5045 CrossRef CAS PubMed. - N. Levin, J. Lengyel, J. F. Eckhard, M. Tschurl and U. Heiz, Catalytic Non-Oxidative Coupling of Methane on Ta8O2+, J. Am. Chem. Soc., 2020, 142, 5862–5869 CrossRef CAS PubMed.
- M. V. Luzgin, A. A. Gabrienko, V. A. Rogov, A. V. Toktarev, V. N. Parmon and A. G. Stepanov, The “Alkyl” and “Carbenium” Pathways of Methane Activation on Ga-Modified Zeolite BEA: 13C Solid-State NMR and GC-MS Study of Methane Aromatization in the Presence of Higher Alkane, J. Phys. Chem. C, 2010, 114, 21555–21561 CrossRef CAS.
- K. Dutta, V. Chaudhari, C. J. Li and J. Kopyscinski, Methane conversion to ethylene over GaN catalysts. Effect of catalyst nitridation, Appl. Catal., A, 2020, 595, 117430 CrossRef.
- K. Dutta, M. Shahryari and J. Kopyscinski, Direct Nonoxidative Methane Coupling to Ethylene over Gallium Nitride: A Catalyst Regeneration Study, Ind. Eng. Chem. Res., 2020, 59, 4245–4256 CrossRef CAS.
- V. R. Choudhary, K. C. Mondal and S. A. Mulla, Simultaneous conversion of methane and methanol into gasoline over bifunctional Ga-, Zn-, In-, and/or Mo-modified ZSM-5 zeolites, Angew. Chem., Int. Ed., 2005, 44, 4381–4385 CrossRef CAS PubMed.
- T. Gong, L. Qin, J. Lu and H. Feng, ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion, Phys. Chem. Chem. Phys., 2016, 18, 601–614 RSC.
- M. V. Luzgin, V. A. Rogov, S. S. Arzumanov, A. V. Toktarev, A. G. Stepanov and V. N. Parmon, Understanding Methane Aromatization on a Zn-Modified High-Silica Zeolite, Angew. Chem., Int. Ed., 2008, 47, 4559–4562 CrossRef CAS PubMed.
- J. F. Wu, W. D. Wang, J. Xu, F. Deng and W. Wang, Reactivity of C1 Surface Species Formed in Methane Activation on Zn-Modified H-ZSM-5 Zeolite, Chem.–Eur. J., 2010, 16, 14016–14025 CrossRef CAS PubMed.
- A. A. Gabrienko, S. S. Arzumanov, A. V. Toktarev, I. G. Danilova, I. P. Prosvirin, V. V. Kriventsov, V. I. Zaikovskii, D. Freude and A. G. Stepanov, Different Efficiency of Zn2+ and ZnO Species for Methane Activation on Zn-Modified Zeolite, ACS Catal., 2017, 7, 1818–1830 CrossRef CAS.
- A. A. Gabrienko, S. S. Arzumanov, M. V. Luzgin, A. G. Stepanov and V. N. Parmon, Methane Activation on Zn2+-Exchanged ZSM-5 Zeolites. The Effect of Molecular Oxygen Addition, J. Phys. Chem. C, 2015, 119, 24910–24918 CrossRef CAS.
- G. Qi, Q. Wang, J. Xu, J. Trébosc, O. Lafon, C. Wang, J.-P. Amoureux and F. Deng, Synergic Effect of Active Sites in Zinc-Modified ZSM-5 Zeolites as Revealed by High-Field Solid-State NMR Spectroscopy, Angew. Chem., Int. Ed., 2016, 55, 15826–15830 CrossRef CAS PubMed.
- P. He, A. G. Wang, S. J. Meng, G. M. Bernard, L. J. Liu, V. K. Michaelis and H. Song, Impact of Al sites on the methane co-aromatization with alkanes over Zn/HZSM-5, Catal, Today, 2019, 323, 94–104 CrossRef CAS.
- B. M. Weckhuysen, D. Wang, M. P. Rosynek and J. H. Lunsford, Conversion of Methane to Benzene over Transition Metal Ion ZSM-5 Zeolites: I. Catalytic Characterization, J. Catal., 1998, 175, 338–346 CrossRef CAS.
- B. M. Weckhuysen, D. Wang, M. P. Rosynek and J. H. Lunsford, Conversion of Methane to Benzene over Transition Metal Ion ZSM-5 Zeolites: II. Catalyst Characterization by X-Ray Photoelectron Spectroscopy, J. Catal., 1998, 175, 347–351 CrossRef CAS.
- S. Takenaka, M. Serizawa and K. Otsuka, Formation of filamentous carbons over supported Fe catalysts through methane decomposition, J. Catal., 2004, 222, 520–531 CrossRef CAS.
- P. L. Tan, Active phase, catalytic activity, and induction period of Fe/zeolite material in nonoxidative aromatization of methane, J. Catal., 2016, 338, 21–29 CrossRef CAS.
- J. P. Sim, B. J. Lee, G. H. Han, D. H. Kim and K. Y. Lee, Promotional effect of Au on Fe/HZSM-5 catalyst for methane dehydroaromatization, Fuel, 2020, 274, 117852 CrossRef CAS.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Single-atom catalysis of CO oxidation using Pt1/FeOx, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- Y. Lai and G. Veser, The nature of the selective species in Fe-HZSM-5 for non-oxidative methane dehydroaromatization, Catal. Sci. Technol., 2016, 6, 5440–5452 RSC.
- B. R. Wood, J. A. Reimer, A. T. Bell, M. T. Janicke and K. C. Ott, Methanol formation on Fe/Al-MFI via the oxidation of methane by nitrous oxide, J. Catal., 2004, 225, 300–306 CrossRef CAS.
- H. Schwarz, Chemistry with Methane: Concepts Rather than Recipes, Angew. Chem., Int. Ed., 2011, 50, 10096–10115 CrossRef CAS PubMed.
- E. W. McFarland and H. Metiu, Catalysis by Doped Oxides, Chem. Rev., 2013, 113, 4391–4427 CrossRef CAS PubMed.
- P. Sot, M. A. Newton, D. Baabe, M. D. Walter, A. P. van Bavel, A. D. Horton, C. Coperet and J. A. van Bokhoven, Non-oxidative Methane Coupling over Silica versus Silica-Supported Iron(II) Single Sites, Chem.–Eur. J., 2020, 26, 8012–8016 CrossRef CAS PubMed.
- S. J. Han, S. W. Lee, H. W. Kim, S. K. Kim and Y. T. Kim, Nonoxidative Direct Conversion of Methane on Silica-Based Iron Catalysts: Effect of Catalytic Surface, ACS Catal., 2019, 9, 7984–7997 CrossRef CAS.
- M. Sakbodin, Y. Q. Wu, S. C. Oh, E. D. Wachsman and D. X. Liu, Hydrogen-Permeable Tubular Membrane Reactor: Promoting Conversion and Product Selectivity for Non-Oxidative Activation of Methane over an Fe (c) SiO2 Catalyst, Angew. Chem., Int. Ed., 2016, 55, 16149–16152 CrossRef CAS PubMed.
- A. T. Bell, Single iron sites for catalytic, nonoxidative conversion of methane, Sci. China: Chem., 2014, 57, 923 CrossRef CAS.
- D. Bajec, A. Kostyniuk, A. Pohar and B. Likozar, Micro-kinetics of non-oxidative methane coupling to ethylene over Pt/CeO2 catalyst, Chem. Eng. J., 2020, 396, 125182 CrossRef CAS.
- P. F. Xie, T. C. Pu, A. M. Nie, S. Hwang, S. C. Purdy, W. J. Yu, D. Su, J. T. Miller and C. Wang, Nanoceria-Supported Single-Atom Platinum Catalysts for Direct Methane Conversion, ACS Catal., 2018, 8, 4044–4048 CrossRef CAS.
- Z. Q. Huang, T. Y. Zhang, C. R. Chang and J. Li, Dynamic Frustrated Lewis Pairs on Ceria for Direct Nonoxidative Coupling of Methane, ACS Catal., 2019, 9, 5523–5536 CrossRef CAS.
- Y. Liu, J.-C. Liu, T.-H. Li, Z.-H. Duan, T.-Y. Zhang, M. Yan, W.-L. Li, H. Xiao, Y.-G. Wang, C.-R. Chang and J. Li, Unravelling the Enigma of Nonoxidative Conversion of Methane on Iron Single-Atom Catalysts, Angew. Chem., Int. Ed., 2020, 132, 18745–18749 CrossRef.
- T. H. Li, M. Yan, Y. Liu, Z. Q. Huang, C. R. Chang and J. Li, Cooperative Catalysis by Multiple Active Centers in Nonoxidative Conversion of Methane, J. Phys. Chem. C, 2020, 124, 13656–13663 CrossRef CAS.
- T. Z. H. Gani and H. J. Kulik, Understanding and Breaking Scaling Relations in Single-Site Catalysis: Methane to Methanol Conversion by Fe-IV=O, ACS Catal., 2018, 8, 975–986 CrossRef CAS.
- X. F. Ma, K. J. Sun, J. X. Liu, W. X. Li, X. M. Cai and H. Y. Su, Single Ru Sites-Embedded Rutile TiO2 Catalyst for Non-Oxidative Direct Conversion of Methane: A First-Principles Study, J. Phys. Chem. C, 2019, 123, 14391–14397 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |
