 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nascent chain dynamics and ribosome interactions within folded ribosome–nascent chain complexes observed by NMR spectroscopy†
Charles
Burridge‡
 ,
Christopher A.
Waudby‡
,
Christopher A.
Waudby‡
 *,
Tomasz
Włodarski
*,
Tomasz
Włodarski
 ,
Anaïs M. E.
Cassaignau
,
Anaïs M. E.
Cassaignau
 ,
Lisa D.
Cabrita
,
Lisa D.
Cabrita
 and
John
Christodoulou
and
John
Christodoulou
 *
*
Institute of Structural and Molecular Biology, University College London, London WC1E 6BT, UK. E-mail: c.waudby@ucl.ac.uk; j.christodoulou@ucl.ac.uk
First published on 9th September 2021
Abstract
The folding of many proteins can begin during biosynthesis on the ribosome and can be modulated by the ribosome itself. Such perturbations are generally believed to be mediated through interactions between the nascent chain and the ribosome surface, but despite recent progress in characterising interactions of unfolded states with the ribosome, and their impact on the initiation of co-translational folding, a complete quantitative analysis of interactions across both folded and unfolded states of a nascent chain has yet to be realised. Here we apply solution-state NMR spectroscopy to measure transverse proton relaxation rates for methyl groups in folded ribosome–nascent chain complexes of the FLN5 filamin domain. We observe substantial increases in relaxation rates for the nascent chain relative to the isolated domain, which can be related to changes in effective rotational correlation times using measurements of relaxation and cross-correlated relaxation in the isolated domain. Using this approach, we can identify interactions between the nascent chain and the ribosome surface, driven predominantly by electrostatics, and by measuring the change in these interactions as the subsequent FLN6 domain emerges, we may deduce their impact on the free energy landscapes associated with the co-translational folding process.
Introduction
Co-translational folding is a fundamental mechanism for ensuring that nascent polypeptide chains (NCs) efficiently acquire and assemble their correct tertiary and quaternary structures following biosynthesis by the ribosome.1,2 It is increasingly apparent that the ribosome itself can play a role in regulating or modulating this process,3 and interactions between NCs and the nearby ribosomal surface have been suggested to provide a simple mechanism through which this may be achieved.4–6 However, the direct measurement of such intramolecular interactions, involving highly dynamic regions of a 2.3 MDa complex, presents a formidable experimental challenge that has only recently begun to be met.7We have previously studied the co-translational folding of FLN5, the fifth immunoglobulin domain from the tandem repeat protein filamin, using the SecM arrest peptide to generate translationally-arrested RNCs that are tethered to the peptidyl-transferase center by varying lengths of the subsequent FLN6 domain8,9 (Fig. 1A). Through a combination of NMR and biophysical methods, an offset was identified between the emergence of the FLN5 domain from the exit tunnel and the initiation of co-translational folding,8,10 due at least in part to strong interactions between the unfolded state and the ribosome surface.7 However, an understanding of the dynamics and interactions of both folded and unfolded species is essential to understand the co-translational folding equilibrium fully,3 and so in this work we complement measurements of interactions of the unfolded state of FLN5 (ref. 7) with a systematic survey of dynamics within folded states.
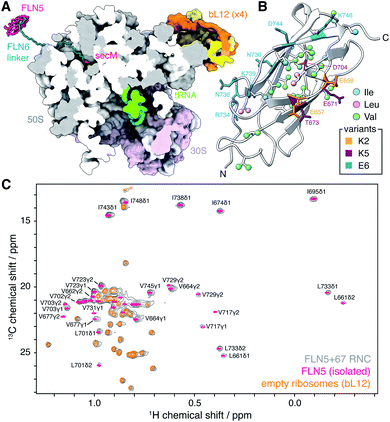 | ||
| Fig. 1 Methyl TROSY NMR spectroscopy of FLN5 RNCs. (A) Modelled structure of an FLN5+67 RNC, comprising the FLN5 domain, FLN6 linker and SecM arrest peptide, emerging from the E. coli 70S ribosome (pdb 3j9z12). The nascent chain has been built manually in SPDBV13 and energy minimised in Foldit.14 (B) Cartoon representation of the crystal structure of the FLN5 domain (pdb 1qfh15), showing isoleucine, leucine and valine methyls (ball and stick representation, labelled in Fig. S1†), and highlighting residues mutated in charged variants (stick representation). (C) Comparison of 1H,13C HMQC spectra (298 K, 900 MHz) of ILV-labelled FLN5+67 RNC, isolated FLN5, and unoccupied 70S ribosomes. | ||
Methods
Sample preparation
Wild-type FLN5 and variants were expressed and purified as previously described.8 bL12 was expressed and purified as previously described.11 FLN5 RNC samples were expressed and purified following an adaptation of previous protocols:8,9E. coli BL21 (DE3) cells were adapted progressively into MDG media (100% D2O, 2 g L−1 d7-glucose) and then grown to an OD600 of 3 (37 °C, 220 rpm). Cells were resuspended in an equal volume of EM9 media (100% D2O, 2 g L−1 d7-glucose, 80 mg L−1 2-ketobutyric-4-13C,3,3-d2 acid and 80 mg L−1 2-keto-3-(methyl-13C)-butyric-4-13C acid). Following 30 min incubation (37 °C, 200 rpm), expression was induced with 1 mM IPTG for 50 min at 37 °C. Cells were then harvested and RNCs purified using previously reported protocols.7 Purified RNCs were resuspended in Tico buffer (10 mM HEPES, 30 mM NH4Cl, 6 mM MgCl2, 0.1% (w/v) EDTA-free protease inhibitor tablet (Roche), pH 7.5), flash frozen and stored at −80 °C.Unprogrammed [2H, 13CH3-ILV]-labelled 70S ribosomes were prepared from E. coli BL21 (DE3) cells grown in MDG media (100% D2O, 2 g L−1 d7-glucose, 80 mg L−1 2-ketobutyric-4-13C,3,3-d2 acid and 80 mg L−1 2-keto-3-(methyl-13C)-butyric-4-13C acid) at 37 °C and harvested at an OD600 of 3. Ribosomes were purified following the same protocol as RNCs, omitting the metal affinity chromatography step.
NMR spectroscopy
NMR data were acquired at 298 K on Bruker Avance III HD spectrometers operating at 1H Larmor frequencies of 700, 800, 900 and 950 MHz. Data were processed with NMRPipe16 and analysed using NMRPipe, NMRFAM-Sparky17 and Fuda.18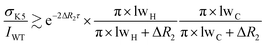 | (1) |
Results and discussion
We have previously found that structured FLN5 RNCs were most effectively observed using deuteration and selective 13CH3 labelling of isoleucine Cδ methyl groups.8 However, as this provides rather sparse coverage across the domain, to enable a more detailed structural analysis here we have expanded our labelling approach to include leucine and valine methyl groups, which are widely distributed throughout the domain (Fig. 1B and S1†). We selected the FLN5+67 RNC (Fig. 1A) as a starting point for our study, as this long linker sequence, comprising a fragment of the subsequent FLN6 domain together with the SecM arrest peptide, ensures that the FLN5 domain has emerged completely from the ribosome exit tunnel and can fold to a native-like state.8 The resulting 1H,13C correlation spectrum is shown in Fig. 1C, and from this approximately twenty FLN5 resonances can be resolved unambiguously. These resonances overlay closely with those of isolated FLN5, indicating that there are no structural perturbations within the folded nascent chain. Additional resonances are also observed in spectra of unprogrammed ILV-labelled ribosomes, and can be attributed to the mobile C-terminal domain of the bL12 stalk protein, previously observed using 15N-based methods11,22,23 (Fig. S2†). Despite the heightened sensitivity of the labelling scheme and methyl TROSY experiment, no additional ribosomal signals were detected in these measurements.To investigate the mobility of the NC we then acquired 1H R2 relaxation measurements, using an adapted methyl-SOFAST HMQC24 incorporating a Hahn echo and a filter for slowly relaxing inner transitions (Fig. S3†). These measurements were acquired in ca. 3 h blocks interleaved with control measurements and parallel biochemical assays to monitor sample integrity (Fig. S4†). The measured relaxation data fitted well to single exponential decays (Fig. 2A and S5†), and resonances in the FLN5+67 RNC showed significantly higher relaxation rates (36 ± 12 s−1, mean ± s.d.) than in the isolated protein (8.4 ± 2.5 s−1, mean ± s.d.) (Fig. 2B), indicating that the mobility of the NC is reduced. As the differences in R2 relaxation rates between the isolated domain and RNC varied significantly between methyl groups, we can exclude a constant lifetime line broadening effect due to interactions with the ribosome surface in slow chemical exchange.25 Instead, changes in relaxation rates are consistent either with a reduction in the rotational diffusion of the domain itself, or with transferred relaxation from the ribosome occurring in the fast chemical exchange limit. Relaxation rates for the FLN5 domain were also much greater than those of the bL12 CTD (Fig. 2B, 17 ± 6 s−1, mean ± s.d.). As the effective rotational correlation time, τc, of the bL12 CTD has previously been determined as ca. 14 ns,22 this provides an approximate lower bound for the mobility of the NC.
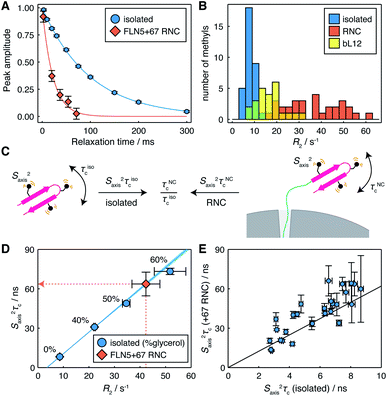 | ||
| Fig. 2 Measurement of methyl 1H relaxation and analysis of mobility for the FLN5+67 RNC. (A) 1H R2 measurements for the representative residue L661δ2 in isolated FLN5 and FLN5+67 RNC (298 K, 950 MHz). Measurements for other residues are plotted in Fig. S5.† (B) Distribution of R2 measurements in isolated FLN5, FLN5+67 RNC and the bL12 C-terminal domain (298 K, 950 MHz). (C) Illustration of the cancellation of Saxis2 terms through the comparison of dynamics in isolated FLN5 and FLN5 RNCs. (D) Correlation between 1H R2 rates and Saxis2τc values measured for L661δ2 in isolated FLN5 at varying concentrations of d8-glycerol, also illustrating the conversion from 1H R2 rates to Saxis2τc values for the FLN5+67 RNC. (E) Correlation between Saxis2τc values measured for methyl groups in isolated FLN5, and Saxis2τc values determined from 1H R2 rates for the FLN5+67 RNC. Error bars show standard errors propagated from R2 or cross-correlated relaxation measurements. | ||
The mobility of methyl groups within folded domains can be described by the product Saxis2τc, where Saxis2 is an order parameter for the three-fold methyl symmetry axis, representing motion on a ps–ns timescale, and τc is an effective rotational correlation time, which may depend on the orientation of the methyl symmetry axis relative to the rotational diffusion tensor, as well as transient interactions with the ribosome surface (Fig. 2C). However, each methyl R2 relaxation rate, measured here, will depend differently on changes in τc, due to a combination of effects: various dipolar relaxation pathways involving spectral densities at multiple frequencies, and chemical exchange. Taking an empirical approach, we therefore constructed calibration curves for each methyl group using the isolated protein at varying concentrations of glycerol in order to relate observed R2 rates (Table S2†) to the effective mobility, Saxis2τc (Table S3†), obtained using measurements of cross-correlated relaxation20 (Fig. 2D and S6†).
Using these calibration curves, measured R2 rates for RNCs (Table S4†) could be converted to Saxis2τc values (Fig. 2D and Table S5†) and subsequently compared with the isolated protein (Fig. 2E). As Saxis2 order parameters are a local property of the domain structure, and given that methyl chemical shifts, and 13C chemical shifts in particular, are highly sensitive to changes in structure and rotamer distribution,26 we argue that it is reasonable to assume that they do not change significantly between the isolated protein and the NC. Therefore, changes in Saxis2τc between a NC and the isolated protein may be interpreted purely in terms of the relative change in the effective correlation times, τNCc/τisoc (Fig. 2C).
In the case of the FLN5+67 RNC, we observe a strong correlation (r = 0.85 ± 0.06) between Saxis2τc values in isolated FLN5 and in the NC (Fig. 2E). Linear regression analysis indicates a ca. six-fold reduction in mobility of the NC (τNCc/τisoc = 6.2 ± 0.3, Table 1), in good agreement with an earlier estimate of 5.2 obtained from cross-correlated relaxation measurements of three isoleucine resonances.27 The strong correlation indicates that the rotational diffusion tensor is being scaled rather than deformed or rotated by the presence of the ribosome. This is most consistent with transferred relaxation due to transient interactions with the surface, although the deviations may indicate some additional degree of anisotropic motion due to the tethering of the domain by the FLN6 linker, giving an orientational dependence to the local τc of methyl groups. These results also indicate that broadened resonances previously reported for a uniformly 1H,13C-labelled FLN5 RNC28 correspond closely with those exhibiting high Saxis2τc values in the isolated domain (Fig. S6†), i.e. strongly ordered resonances are naturally relaxed more efficiently, and highlights the benefit of the selective methyl labelling scheme that we have applied in the present work.
| Sample | Relative τc | τ c/ns |
|---|---|---|
| Isolated FLN5 (0% glycerol) | 1 | 7.7 (ref. 29) |
| Isolated FLN5 (40% glycerol) | 3.4 ± 0.1 | 26.2 ± 0.8 |
| Isolated FLN5 (50% glycerol) | 5.1 ± 0.1 | 39.3 ± 0.8 |
| Isolated FLN5 (60% glycerol) | 7.5 ± 0.2 | 57.8 ± 1.5 |
| wt FLN5 +47 RNC | 10.6 ± 0.9 | 82 ± 7 |
| wt FLN5 +57 RNC | 10.4 ± 0.6 | 80 ± 5 |
| wt FLN5 +67 RNC | 6.2 ± 0.3 | 48 ± 2 |
| wt FLN5 +110 RNC | 4.2 ± 0.2 | 32 ± 2 |
| wt FLN5 +67 RNC, GS linker | 6.6 ± 0.4 | 51 ± 3 |
| E6 FLN5 +67 RNC | 5.6 ± 0.4 | 43 ± 3 |
| K2 FLN5 +67 RNC | 15.6 ± 1.5 | 120 ± 12 |
| K5 FLN5 +67 RNC | ≳100 | ≳770 |
| 70S ribosome | 430 | 3600 (ref. 30) |
Having established an effective measurement strategy, we sought to investigate systematically how basic physical properties of the NC affect its mobility and interactions with the ribosome surface. In particular, by comparison of engineered RNC variants (Table S1†) we sought to distinguish unambiguously between changes in the mobility of the NC due to tethering, interactions of the FLN5 domain with the ribosome surface, and intra- or inter-molecular interactions of the FLN6 linker itself (Fig. 3A).
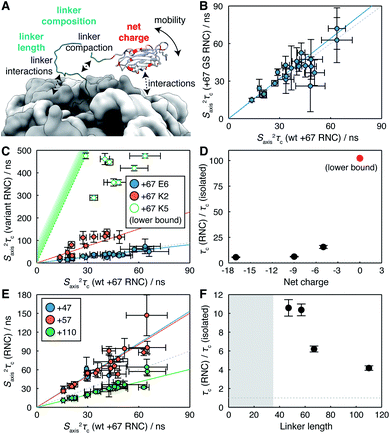 | ||
| Fig. 3 Analysis of the determinants of reduced NC mobility. (A) Illustration of the potential influences on NC mobility explored within this work. (B) Correlation between Saxis2τc values determined from 1H R2 rates for FLN5+67 RNCs comprising wild-type FLN6 and poly-GS linker sequences. (C) Correlation between Saxis2τc values determined from 1H R2 rates for FLN5+67 charge variants and wild-type FLN5+67. Open circles for the K5 variant indicate approximate lower bounds determined from an analysis of signal intensity. (D) Changes in rotational correlation times for FLN5+67 RNC charge variants, relative to the isolated domain. (E) Correlation between Saxis2τc values determined from 1H R2 rates for FLN5 RNCs as a function of FLN6 linker length, relative to wild-type FLN5+67. (F) Changes in rotational correlation times for FLN5 RNCs, relative to the isolated domain, as a function of linker length. Shading indicates the approximate extent of polypeptide enclosed within the exit tunnel.8 | ||
We first explored the effect of substituting the FLN6 linker for a poly-GS sequence that is neutral, non-hydrophobic, disordered, and that has previously been shown not to alter the onset of co-translational folding,8 or to perturb interactions of the unfolded state.71H R2 measurements were acquired for a FLN5 RNC containing a 67 residue GS linker (comprising 48 GS residues and the 19 residue SecM arrest peptide), and no significant differences were found with respect to the WT FLN5+67 RNC. This is presented in terms of estimated Saxis2τc values in Fig. 3B, and a regression analysis indicates a relative mobility τGSc/τWTc = 1.07 ± 0.03 (r = 0.85 ± 0.13). Thus, we conclude that neither the residual structure, compaction or interactions of the linker region are responsible for the reduced mobility of the FLN5 domain within RNCs.
We next examined the effect of varying the charge of the FLN5 domain (net charge Z = −9) on electrostatic interactions with the predominantly negatively charged ribosome surface.31 We have previously introduced the E6 variant for the analysis of interactions of the unfolded state,7 in which six surface residues were substituted with negatively charged glutamate residues (N730E/R734E/N736E/K739E/D7444E/K746E, Z = −17, Fig. 1C). Expanding on this, here we have generated two additional variants, K2 (E657K/E659K, Z = −5) and K5 (K2/E671K/T673K/D704K, Z = 0), in which a second cluster of surface residues were mutated to positively charged lysine residues (Fig. 1C). As the K5 variant was already found to interact strongly (see below), no further positively charged variants were generated. All variants remained fully folded under the conditions used in this work, and we observed very limited methyl chemical shift perturbations (Fig. S7†), indicating that the core structure of the variants was not affected by the surface mutations. On this basis we have used the same calibration curves for the interpretation of R2 measurements as for WT FLN5 (Fig. 2D).
RNC samples of the E6, K2 and K5 FLN5 variants were prepared using a constant linker length of 67 for comparison with the WT observations above. R2 measurements were successfully acquired for the E6 and K2 variants (Table S4†), although as a consequence of more rapid relaxation the intensity of resonances in the K2 variant were reduced ca. 4-fold relative to the WT. No NC resonances could be detected from the K5 variant. However, translational diffusion measurements of bL12 resonances confirmed the integrity of the core ribosome particle, and the presence of the NC was confirmed both by immunoblotting, and by observation of the released NC following treatment with RNase (Fig. S3†). In line with changes in intensity and relaxation rates observed between the WT and K2 variants, we therefore conclude that the mobility of the K5 NC is reduced further, likely due to stronger interactions following the elimination of the net negative charge.
S axis 2 τ c values estimated from measured relaxation rates are plotted in Fig. 3C. Strong linear correlations were observed for both E6 and K2 variants (r = 0.89 ± 0.10 and 0.85 ± 0.12 respectively), from which the relative changes in mobility may be determined. As discussed earlier for the WT RNC, such linear correlations are consistent with transferred relaxation from a bound state, and the reduction in mobility as the net charge on the NC decreases indicates the role of electrostatic interactions between positively charged patches on the NC and the negatively charged ribosome surface. However, given that the WT and E6 variant also exhibit similar behaviour (Fig. 3C), the net charge alone is clearly insufficient to fully characterise interactions, indicating that factors such as the distribution of charges across the surface of the domain may also be relevant. For the K5 variant, lower limits for relaxation rates and hence Saxis2τc could be estimated from the spectrum noise level. The strongest constraint on NC mobility was provided by the I743 resonance, the intensity of which was less than 4% of that of the WT RNC, from which we estimate that the R2 ≳480 s−1 and therefore τK5c/τisoc ≳ 100 (Fig. 3D).
Lastly, we have probed the effect of varying the length of the subsequent FLN6 tether, mimicking the progressive emergence of the domain from the ribosome exit tunnel during translation. This has previously been found to modulate interactions between the unfolded state and the ribosome surface by varying the effective concentration of binding sites on the ribosome surface.6 Here, four linker lengths were examined, from 47 to 110 residues (of which ca. 30–35 residues are enclosed within the exit tunnel7) (Fig. 3E). An increase in mobility was observed with increasing chain lengths (Fig. 3F), consistent with a decrease in the effective concentration of interaction sites on the ribosome surface.
Collectively, our observations of the effects of net charge, linker length and linker composition on the mobility of the FLN5 domain can be accounted for most simply by the transient interaction of the FLN5 domain with the ribosome surface. Given that the (isotropic) rotational correlation time of the isolated domain (in D2O at 298 K) is 7.7 ns,29 and the ribosome itself is ca. 3.6 μs,30 complete binding would correspond to a change in effective correlation time τriboc/τisoc ≈ 430. Thus, for the wild-type RNC from linker lengths of 47 to 110 (Fig. 3F), we estimate that the fraction of bound NC decreases from ca. 2.2% to 0.9%.
The interactions we observe here can be compared with the much stronger interaction of a C-terminal segment within unfolded NCs, which has a bound population of up to 90% at short tether lengths.7 This is plotted here in terms of the free energy of interaction, 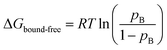 (Fig. 4A). The dependence of ΔGbound-free on linker length is similar for observed lengths of native and unfolded states, indicating that the changing linker modulates the effective concentration of the ribosome surface to a similar extent. However, interactions of the unfolded state are approximately 2 kcal mol−1 stronger than for the native state, corresponding to a ca. 30-fold difference in association constant. We may combine these data with previous measurements of co-translational folding energetics,10 to construct a free energy diagram illustrating for the first time the impact of interactions of both folded and unfolded states on the co-translational folding of a NC (Fig. 4B).
(Fig. 4A). The dependence of ΔGbound-free on linker length is similar for observed lengths of native and unfolded states, indicating that the changing linker modulates the effective concentration of the ribosome surface to a similar extent. However, interactions of the unfolded state are approximately 2 kcal mol−1 stronger than for the native state, corresponding to a ca. 30-fold difference in association constant. We may combine these data with previous measurements of co-translational folding energetics,10 to construct a free energy diagram illustrating for the first time the impact of interactions of both folded and unfolded states on the co-translational folding of a NC (Fig. 4B).
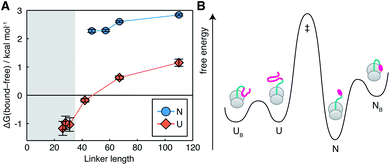 | ||
| Fig. 4 Impact of NC–ribosome interactions on energetics of co-translational folding. (A) Free energy of FLN5–ribosome interactions for folded (this work) and unfolded7 states as a function of polypeptide chain length. Shading indicates the approximate extent of polypeptide enclosed within the exit tunnel.8 (B) Free energy diagram for the FLN5+47 RNC, combining measurements of native state interactions in this work with measurements of folding energetics10 and unfolded state interactions.7 Barrier heights are illustrative only. | ||
We have previously shown that the effect of an interaction on the net energetics of folding is:7
ΔΔGfolding = ±RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 − pB) ln(1 − pB) | (2) |
Conclusions
The ribosome has now been shown to perturb the co-translational folding of many different proteins, generally leading to a reduction in stability relative to the isolated domain.4,5,8,32–34 This is usually ascribed to the effect of interactions, although given the diversity of the proteome, such interactions are likely to be variable in strength and significance. Ultimately, the ability to predict the strength of these interactions would be valuable in better understanding the general mechanisms of co-translational folding. The measurements we have undertaken in this work, together with analogous measurements of the unfolded state,7 provide initial steps in this direction. By determining the first complete description of interactions for both sides of the folding equilibrium, we may begin to progress studies of co-translational folding towards a quantitative standard comparable to that achieved for the reversible folding of isolated domains.Data availability
Relaxation and cross-correlation relaxation datasets supporting this article have been uploaded as part of the ESI.† Side-chain resonance assignments for FLN5 and bL12 have been deposited in the BMRB under accession numbers 51075 and 51076.Author contributions
Conceptualization: CAW, JC. Funding acquisition: JC. Investigation: CB, CAW. Methodology: CAW, TW. Supervision: CAW, AMEC, LDC, JC. Writing – original draft: CB, CAW, JC. Writing – review & editing: all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the use of the UCL Biomolecular NMR Centre and thank associated staff for their support. This work was supported by a Wellcome Trust Investigator Award (to J. C., 206409/Z/17/Z) and by the BBSRC (BB/T002603/1). This work was supported in part by the Wellcome Trust (208400/Z/17/Z) and we thank HWB-NMR staff at the University of Birmingham for providing open access to their Wellcome Trust-funded 900 MHz spectrometers. This work was supported by the Francis Crick Institute through provision of access to the MRC Biomedical NMR Centre. The Francis Crick Institute receives its core funding from Cancer Research UK (FC001029), the UK Medical Research Council (FC001029), and the Wellcome Trust (FC001029).References
- A. M. E. Cassaignau, L. D. Cabrita and J. Christodoulou, Annu. Rev. Biochem., 2020, 89, 389–415 CrossRef CAS PubMed.
- Y.-W. Shieh, P. Minguez, P. Bork, J. J. Auburger, D. L. Guilbride, G. Kramer and B. Bukau, Science, 2015, 350, 678–680 CrossRef CAS PubMed.
- C. A. Waudby, C. M. Dobson and J. Christodoulou, Trends Biochem. Sci., 2019, 44, 914–926 CrossRef CAS PubMed.
- C. M. Kaiser, D. H. Goldman, J. D. Chodera, I. Tinoco and C. Bustamante, Science, 2011, 334, 1723–1727 CrossRef CAS PubMed.
- A. J. Samelson, M. K. Jensen, R. A. Soto, J. H. D. Cate and S. Marqusee, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13402–13407 CrossRef CAS PubMed.
- A. M. Knight, P. H. Culviner, N. Kurt-Yilmaz, T. Zou, S. B. Ozkan and S. Cavagnero, ACS Chem. Biol., 2013, 8, 1195–1204 CrossRef CAS PubMed.
- A. M. E. Cassaignau, T. Wlodarski, S. H. S. Chan, L. F. Woodburn, I. V. Bukvin, J. O. Streit, L. D. Cabrita, C. A. Waudby and J. Christodoulou, Nat. Chem. Search PubMed , in press.
- L. D. Cabrita, A. M. E. Cassaignau, H. M. M. Launay, C. A. Waudby, T. Wlodarski, C. Camilloni, M.-E. Karyadi, A. L. Robertson, X. Wang, A. S. Wentink, L. Goodsell, C. A. Woolhead, M. Vendruscolo, C. M. Dobson and J. Christodoulou, Nat. Struct. Mol. Biol., 2016, 23, 278–285 CrossRef CAS PubMed.
- A. M. E. Cassaignau, H. M. M. Launay, M.-E. Karyadi, X. Wang, C. A. Waudby, A. Deckert, A. L. Robertson, J. Christodoulou and L. D. Cabrita, Nat. Protoc., 2016, 11, 1492–1507 CrossRef PubMed.
- C. A. Waudby, T. Wlodarski, M.-E. Karyadi, A. M. E. Cassaignau, S. H. S. Chan, A. S. Wentink, J. M. Schmidt-Engler, C. Camilloni, M. Vendruscolo, L. D. Cabrita and J. Christodoulou, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9744–9749 CrossRef CAS PubMed.
- X. Wang, J. P. Kirkpatrick, H. M. M. Launay, A. de Simone, D. Häussinger, C. M. Dobson, M. Vendruscolo, L. D. Cabrita, C. A. Waudby and J. Christodoulou, Sci. Rep., 2019, 9, 13528 CrossRef PubMed.
- W. Li, Z. Liu, R. K. Koripella, R. Langlois, S. Sanyal and J. Frank, Sci. Adv., 2015, 1, e1500169 CrossRef PubMed.
- N. Guex and M. C. Peitsch, Electrophoresis, 1997, 18, 2714–2723 CrossRef CAS PubMed , 9504803.
- R. Kleffner, J. Flatten, A. Leaver-Fay, D. Baker, J. B. Siegel, F. Khatib and S. Cooper, Bioinformatics, 2017, 33, 2765–2767 CrossRef CAS PubMed.
- A. J. McCoy, P. Fucini, A. A. Noegel and M. Stewart, Nat. Struct. Biol., 1999, 6, 836–841 CrossRef CAS PubMed.
- F. Delaglio, S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer and A. Bax, J. Biomol. NMR, 1995, 6, 277–293 CrossRef CAS PubMed.
- W. Lee, M. Tonelli and J. L. Markley, Bioinformatics, 2015, 31, 1325–1327 CrossRef PubMed.
- S. M. Kristensen and D. F. Hansen, FuDA: a function and data fitting and analysis package, https://www.ucl.ac.uk/hansen-lab/, 2006 Search PubMed.
- E. V. Bocharov, A. T. Gudkov and A. S. Arseniev, FEBS Lett., 1996, 379, 291–294 CrossRef CAS PubMed.
- H. Sun, L. E. Kay and V. Tugarinov, J. Phys. Chem. B, 2011, 115, 14878–14884 CrossRef CAS PubMed.
- T. Didenko, R. Boelens and S. G. D. Rüdiger, Protein Eng., Des. Sel., 2011, 24, 99–103 CrossRef CAS PubMed.
- J. Christodoulou, G. Larsson, P. Fucini, S. R. Connell, T. A. Pertinhez, C. L. Hanson, C. Redfield, K. H. Nierhaus, C. V. Robinson, J. Schleucher and C. M. Dobson, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 10949–10954 CrossRef CAS PubMed.
- F. A. A. Mulder, L. Bouakaz, A. Lundell, M. Venkataramana, A. Liljas, M. Akke and S. Sanyal, Biochemistry, 2004, 43, 5930–5936 CrossRef CAS PubMed.
- C. Amero, P. Schanda, M. A. Durá, I. Ayala, D. Marion, B. Franzetti, B. Brutscher and J. Boisbouvier, J. Am. Chem. Soc., 2009, 131, 3448–3449 CrossRef CAS PubMed.
- N. J. Anthis and G. M. Clore, Q. Rev. Biophys., 2015, 48, 35–116 CrossRef CAS PubMed.
- L. Siemons, B. Uluca-Yazgi, R. B. Pritchard, S. McCarthy, H. Heise and D. F. Hansen, Chem. Commun., 2019, 55, 14107–14110 RSC.
- C. A. Waudby, C. Burridge and J. Christodoulou, J. Magn. Reson., 2021, 326, 106937 CrossRef CAS PubMed.
- S.-T. D. Hsu, L. D. Cabrita, P. Fucini, J. Christodoulou and C. M. Dobson, J. Am. Chem. Soc., 2009, 131, 8366–8367 CrossRef CAS PubMed.
- S.-T. D. Hsu, L. D. Cabrita, P. Fucini, C. M. Dobson and J. Christodoulou, J. Mol. Biol., 2009, 388, 865–879 CrossRef CAS PubMed.
- D. Lavalette, B. Amand and F. Pochon, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 1407–1411 CrossRef CAS PubMed.
- J. Wang, C. Karki, Y. Xiao and L. Li, Biophys. J., 2020, 118, 1205–1212 CrossRef CAS PubMed.
- K. Liu, K. Maciuba and C. M. Kaiser, Mol. Cell, 2019, 74, 310–319.e7 CrossRef CAS.
- S. J. Kim, J. S. Yoon, H. Shishido, Z. Yang, L. A. Rooney, J. M. Barral and W. R. Skach, Science, 2015, 348, 444–448 CrossRef CAS PubMed.
- O. B. Nilsson, A. A. Nickson, J. J. Hollins, S. Wickles, A. Steward, R. Beckmann, G. von Heijne and J. Clarke, Nat. Struct. Mol. Biol., 2017, 24, 221–225 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc04313g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |
