Open Access Article
Open Access ArticleAdsorptive separation of cyclohexanol and cyclohexanone by nonporous adaptive crystals of RhombicArene†
Yongye
Zhao
,
Hongyan
Xiao
 ,
Chen-Ho
Tung
,
Chen-Ho
Tung
 ,
Li-Zhu
Wu
,
Li-Zhu
Wu
 and
Huan
Cong
and
Huan
Cong
 *
*
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Key Laboratory of Bio-inspired Materials and Interfacial Science, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences & School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100190, China. E-mail: hcong@mail.ipc.ac.cn
First published on 22nd November 2021
Abstract
As feedstock chemicals with similar boiling points, cyclohexanol (CHOL) and cyclohexanone (CHON) are often obtained as mixtures during production processes. Separation of mixed CHOL and CHON is important but energy-consuming by distillation. Here we report the development of a new macrocycle RhombicArene, which forms a host–guest complex with CHON through C–H⋯π interactions and hydrogen bonds. The nonporous adaptive crystals of RhombicArene exhibit excellent capability for rapid (30 minutes), exclusive (>99.9%), and recyclable vapor adsorption of CHON in the presence of CHOL under mild and user-friendly conditions.
Introduction
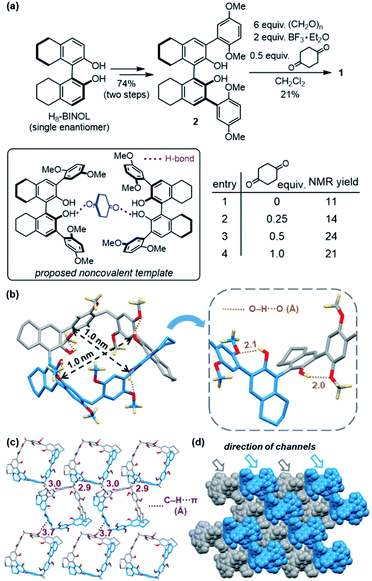
Cyclohexanol (CHOL) and cyclohexanone (CHON), precursors for caprolactam and adipic acid, are vital feedstock chemicals for the production of nylons. In addition, CHOL serves as an emulsion stabilizer and a raw material for plasticizers, and CHON is widely used as a solvent for resins and paints.1 Industrial preparations of CHOL and CHON entail oxidation of cyclohexane or hydrogenation of phenol.2 Alternatively, hydration of cyclohexene is developed to afford CHOL3 which can further be converted to CHON by oxidation (Fig. 1a).4 During the above production processes, CHOL and CHON are inevitably obtained as mixtures, which are known as KA-oil. Due to very close boiling points, mixed CHOL and CHON are energy-consuming to purify by distillation,5 thereby the development of alternative methods for separation is of significant importance.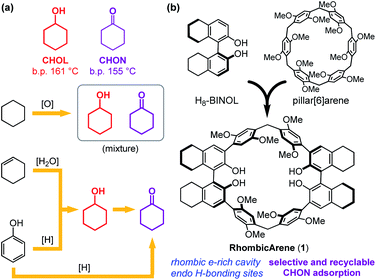 | ||
| Fig. 1 (a) Preparative routes of cyclohexanol and cyclohexanone. (b) Molecular design of RhombicArene (1). | ||
Recently, macrocycle-based nonporous adaptive crystals (NACs) have emerged as efficient and selective adsorbents for mixed organic vapors with similar boiling points.6 The high selectivity of the NAC strategy offers potential for economical and environmentally friendly separation of organic vapors, and stems from the deliberately designed macrocycles whose adaptive skeletons can provide noncovalent interactions to recognize certain guest molecules. Specifically, successful examples of NAC materials often employ electron-rich arene-based macrocycles, functioning as sources for C–H⋯π interactions.7 Although adsorptions of single-component CHOL or CHON have been reported using adsorbent materials, their adsorptive separation remains a challenging goal thus far.8 In view of the functional group difference between CHON and CHOL, we envisioned that the development of a hybrid macrocycle containing electron-rich arenes and endo hydrogen-bonding moieties9 would be helpful to differentiate these two molecules during adsorption.
Here we report a novel macrocycle named RhombicArene (1, Fig. 1b) as an adaptive and selective adsorbent for CHON. The structural design of the C2-symmetrical 1 features methylene-bridged para-dimethoxy arenes adopted from pillararenes,10 as well as two octahydrobinaphthol (H8-BINOL) subunits as linkers and in-cavity hydrogen-bonding sites.11 The crystal structure reveals a host–guest complex between 1 and CHON through C–H⋯π interactions and hydrogen bonds, demonstrating the adaptivity of 1 in the crystalline state. The activated crystals of 1 exhibit rapid, exclusive, and recyclable vapor adsorption of CHON in the presence of CHOL. Furthermore, treatment of a CHOL/CHON mixture (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with 1 at 30 °C for just 30 minutes can produce high-purity liquid CHOL (99.7% by GC analysis) through selective adsorption of CHON, indicating attractive potential toward efficient and greener purification.
2) with 1 at 30 °C for just 30 minutes can produce high-purity liquid CHOL (99.7% by GC analysis) through selective adsorption of CHON, indicating attractive potential toward efficient and greener purification.
Results and discussion
Synthesis and crystal structure
The concise synthesis of 1 entails three steps from H8-BINOL, a widely useful building block, both enantiomers of which are commercially available.12 Starting with a single enantiomer of H8-BINOL, iodination followed by cross-coupling smoothly installed the electron-rich aryl moieties, affording precursor 2. Initially, the desired macrocycle 1 was prepared, albeit with low yield, employing Friedel–Crafts-type condensation between 2 and paraformaldehyde promoted by Lewis acid (Table S1†).13 During the subsequent investigation, examination of the crystal structure of CHON@1 confirmed the host–guest interactions inside 1's cavity (cf.Fig. 3c), which inspired us to revisit the preparation. We reasoned that a CHON analogue bearing two ketone moieties could hold two molecules of 2 in proximity, and serve as a noncovalent template to facilitate the macrocyclization step.14 Indeed, improved yield could be obtained upon addition of 0.5 equivalent of 1,4-cyclohexanedione (Fig. 2a and Table S2†). The overall three-step yield of the macrocycle 1 is 16%.X-ray crystallographic analysis of 1 confirmed its rhombic-shaped cavity with the size of 1.0 nm, and indicated four intramolecular hydrogen bonds within one molecule (Fig. 2b). The hydrogen bond between each hydroxyl group and the methoxy group of the adjacent arene moiety could enable the para-dimethoxy arenes not only to maintain the upright conformation with enhanced rigidity, but also to adopt the same orientation because all H8-BINOL subunits carried single chiral configuration. The crystal structure of 1 would provide a clue to explain the unsuccessful macrocyclization employing racemic precursor 2 (Table S3†), which may lead to mismatched orientations of the para-dimethoxy arenes. Examination of the molecular packing of 1 in the crystalline state revealed that the macrocycles adopt two orientations linked by multiple intermolecular C–H⋯π interactions (Fig. 2c). Each unit cell contains a pair of macrocycles with complementary orientations, and further packs into staggered layers with brick-wall-like patterns. Although the neighboring layers block each other along the axes of the unit cell, long-range channels exist in the diagonal direction (Fig. 2d).
Single-component vapor adsorption
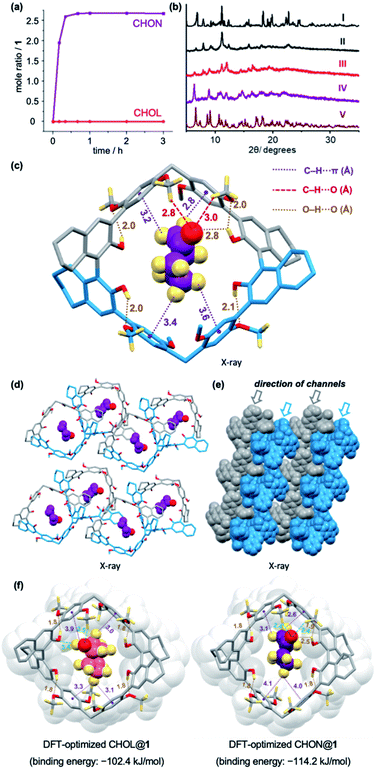
The crystallized macrocycle 1 was activated by heating at 50 °C under reduced pressure. The resulting desolvated white powder was proved to be nonporous, as indicated by its minimal Brunauer–Emmett–Teller (BET) surface area (Fig. S5†). Single-component solid–vapor uptake experiments using activated 1 confirmed the adsorptive capability of CHON, with negligible adsorption of CHOL even after prolonged time at 30 °C (Fig. 3a and S6†). Notably, it takes less than 30 min for the CHON adsorption to reach saturation, which is significantly faster compared to most NAC materials reported to date.6,15 Powder X-ray diffraction analysis (PXRD, Fig. 3b and S7†) revealed the crystallinity of the activated 1, whose PXRD patterns matched with the simulated results based on the crystal structure of 1, suggesting comparable molecular packing modes. Consistent with the single-component adsorption experiments, the PXRD patterns of 1 showed little changes after treatment with CHOL vapor for 3 hours at 30 °C, whereas distinct pattern changes were observed upon in contact with CHON vapor for 30 min at the same temperature. Moreover, the new PXRD patterns resemble the simulated patterns of the crystals of CHON@1, which were obtained by slow diffusion of diethyl ether into a cyclohexanone solution of 1.The crystal structure of CHON@1 showed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex (Fig. 3c). One CHON molecule, adopting a chair-like conformation, is located inside each cavity of 1. The host–guest supramolecular structure was stabilized by the hydrogen bond between one of the macrocycle's hydroxyl groups and the oxygen atom of the ketone, along with multiple C–H⋯π and C–H⋯O interactions contributed by the para-dimethoxy arene moieties. Induced by guest inclusion, slight distortions of the macrocyclic skeleton were observed (Fig. S3 and S4†). In addition, CHON@1 exhibits different molecular packing (Fig. 3d and e) compared to the crystals of 1 by itself, demonstrating the adaptive feature of the macrocyclic host. Besides CHON and macrocycle 1, each unit cell of CHON@1 also contains a Et2O molecule and a disordered molecule (which cannot be well resolved, likely another Et2O molecule, Fig. S4b†) occupying the voids outside the macrocyclic cavity. In comparison, the vapor adsorption experiments employ the desolvated solid of 1, in which the voids in the crystalline solid are unoccupied and available for adsorption.6g,i Such difference may account for the higher molar ratio of CHON
1 host–guest complex (Fig. 3c). One CHON molecule, adopting a chair-like conformation, is located inside each cavity of 1. The host–guest supramolecular structure was stabilized by the hydrogen bond between one of the macrocycle's hydroxyl groups and the oxygen atom of the ketone, along with multiple C–H⋯π and C–H⋯O interactions contributed by the para-dimethoxy arene moieties. Induced by guest inclusion, slight distortions of the macrocyclic skeleton were observed (Fig. S3 and S4†). In addition, CHON@1 exhibits different molecular packing (Fig. 3d and e) compared to the crystals of 1 by itself, demonstrating the adaptive feature of the macrocyclic host. Besides CHON and macrocycle 1, each unit cell of CHON@1 also contains a Et2O molecule and a disordered molecule (which cannot be well resolved, likely another Et2O molecule, Fig. S4b†) occupying the voids outside the macrocyclic cavity. In comparison, the vapor adsorption experiments employ the desolvated solid of 1, in which the voids in the crystalline solid are unoccupied and available for adsorption.6g,i Such difference may account for the higher molar ratio of CHON![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 observed in the vapor adsorption experiment than in the crystal structure of CHON@1. Indeed, similar observations regarding the guest/macrocycle molar ratios have been reported for other NAC materials in the literature.6o,r Accordingly, the preference for CHON adsorption would be contributed by both interstitial space and macrocyclic cavities of the adaptive crystals of 1. While the mechanism at the molecular level for the highly selective CHON adsorption would warrant future investigation, the observed similarities between the simulated and experimental PXRD patterns (Fig. 3b) implied the adaptive morphology transformation as a result of CHON vapor uptake. The structural details and molecular packing of CHON@1 would, in turn, be helpful to elucidate the noncovalent interactions accompanying CHON adsorption.
1 observed in the vapor adsorption experiment than in the crystal structure of CHON@1. Indeed, similar observations regarding the guest/macrocycle molar ratios have been reported for other NAC materials in the literature.6o,r Accordingly, the preference for CHON adsorption would be contributed by both interstitial space and macrocyclic cavities of the adaptive crystals of 1. While the mechanism at the molecular level for the highly selective CHON adsorption would warrant future investigation, the observed similarities between the simulated and experimental PXRD patterns (Fig. 3b) implied the adaptive morphology transformation as a result of CHON vapor uptake. The structural details and molecular packing of CHON@1 would, in turn, be helpful to elucidate the noncovalent interactions accompanying CHON adsorption.
In addition, DFT calculation was performed to optimize the structures of CHOL@1 and CHON@1 (Fig. 3f). Because the hydroxyl group of CHOL takes an equatorial position of the chair conformation of the cyclohexane ring, CHOL and CHON adopt different orientations inside the macrocyclic cavity. Hence, the DFT-optimized structure of CHOL@1 indicates a weaker hydrogen bond (relative to that in CHON@1) with the O–H⋯O length of 3.4 Å between the hydroxyl group of CHOL and one of the oxygen atoms located at the macrocycle's H8-BINOL subunit. The results indicated that the binding energy of CHOL@1 is 11.8 kJ mol−1 less than that of CHON@1, which is thermodynamically consistent with the higher selectivity for CHON. Additionally, in view of the calculated exothermic CHOL binding, the observed minimal CHOL adsorption shown in Fig. 3a may be due to kinetic barriers.
Mixed CHOL/CHON vapor adsorption
Encouraged by the single-component adsorption performance, we evaluated the capability of 1 as a selective adsorbent for mixed vapor (Fig. 4a). When subjected to the mixed vapor of CHOL and CHON at 30 °C (v/v 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
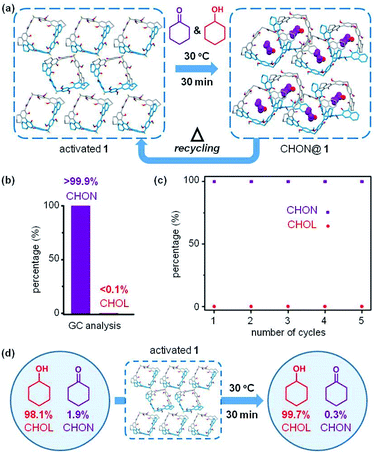
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 based on Antoine equation, Fig. S17†),16 the activated crystals of 1 exhibited exclusive adsorption of CHON, with the purity of >99.9% by GC analysis of the adsorbed CHON (Fig. 4b and S15†). Time-dependent vapor uptake plot indicated rapid saturation for CHON with minimal CHOL adsorption, maintaining the same features of the single-component adsorptions (Fig. S12†vs.Fig. 3a). In addition, PXRD analysis of 1 after adsorption of the mixed vapor also showed resembling patterns compared to those after single-component adsorption of CHON (Fig. S13†). After vapor adsorption, the activated crystals of 1 could be easily regenerated through heating under vacuum, and the recyclability test of 1 by five adsorption–activation cycles confirmed the robust separative performance for CHOL/CHON mixed vapor (Fig. 4c).
77 based on Antoine equation, Fig. S17†),16 the activated crystals of 1 exhibited exclusive adsorption of CHON, with the purity of >99.9% by GC analysis of the adsorbed CHON (Fig. 4b and S15†). Time-dependent vapor uptake plot indicated rapid saturation for CHON with minimal CHOL adsorption, maintaining the same features of the single-component adsorptions (Fig. S12†vs.Fig. 3a). In addition, PXRD analysis of 1 after adsorption of the mixed vapor also showed resembling patterns compared to those after single-component adsorption of CHON (Fig. S13†). After vapor adsorption, the activated crystals of 1 could be easily regenerated through heating under vacuum, and the recyclability test of 1 by five adsorption–activation cycles confirmed the robust separative performance for CHOL/CHON mixed vapor (Fig. 4c).
Since ultra-pure chemicals are of considerable practical and commercial value, we next investigated the utility of 1 to remove trace CHON from liquid CHOL through solid–vapor adsorption. Upon exposure of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 liquid CHOL/CHON (0.05 mL, containing 1.9% CHON impurity) to activated crystals of 1 (9 mg) in a sealed container at 30 °C, the CHON content dramatically reduced to 0.3% as revealed by GC analysis (Fig. 4d and S18†). During the experiment, the activated macrocycle 1 does not directly contact with the liquid CHOL, leading to user-friendly operations and easy separation of adsorbent. Notably, the purity enhancement of liquid CHOL could be achieved in 30 minutes, demonstrating the facile and selective CHON adsorption even at very low CHON content.
2 liquid CHOL/CHON (0.05 mL, containing 1.9% CHON impurity) to activated crystals of 1 (9 mg) in a sealed container at 30 °C, the CHON content dramatically reduced to 0.3% as revealed by GC analysis (Fig. 4d and S18†). During the experiment, the activated macrocycle 1 does not directly contact with the liquid CHOL, leading to user-friendly operations and easy separation of adsorbent. Notably, the purity enhancement of liquid CHOL could be achieved in 30 minutes, demonstrating the facile and selective CHON adsorption even at very low CHON content.
Conclusions
In summary, we have designed and synthesized a new macrocycle RhombicArene (1). The adaptive cavity of 1 is surrounded by electron-rich para-dimethoxy arenes and endo hydroxyl moieties, enabling the host–guest complexation with CHON through C–H⋯π interactions and hydrogen bonds, respectively. The activated crystals of 1 have been successfully used as a highly selective, recyclable, and facile adsorbent for CHON, at the same time showing minimal adsorption for CHOL. The properties and utility of 1 demonstrate the advantages of macrocycle-based adaptive adsorbent to distinguish organic vapors with minor structural differences, showing promising potential for energy-saving separation and high-grade chemical purification.Data availability
The crystallographic data for 1 and CHON@1 have been deposited at CCDC with deposition numbers 2067429 and 2067432, respectively. The data can be obtained viawww.ccdc.cam.ac.uk/structures.Author contributions
H. C. and Y. Z. proposed the project and designed the experiments. Y. Z. carried out the experiments. H. X. performed DFT calculations. H. C., Y. Z., C.-H. T. and L.-Z. W. analyzed data. H. C. and L.-Z. W. wrote the manuscript with inputs from all authors. H.C. directed the project with critical consultation from C.-H. T. and L.-Z. W.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (21672227, 21922113, 21988102, 22071257), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB17000000), the National Key Research and Development Program of China (2017YFA0206903), K. C. Wong Education Foundation and the TIPC Director's Fund is gratefully acknowledged. We thank Profs Congyang Wang (ICCAS), Zhaohui Wang, Shaoguang Zhang and Bi-Jie Li (Tsinghua University); Drs Xiaodi Yang (Shanghai University of Traditional Chinese Medicine) and Wen Zhou (Peking University); Mr Shu Niu and Lijie Zhan are acknowledged for the help with compound characterizations.References
- (a) W. B. Fisher and J. F. Van Peppen, in Kirk-Othmer Encyclopedia of Chemical Technology,John Wiley & Sons, Inc., New York, 2000 Search PubMed; (b) M. T. Musser, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- (a) U. Schuchardt, D. Cardoso, R. Sercheli, R. Pereira, R. S. Da Cruz, M. C. Guerreiro, D. Mandelli, E. V. Spinacé and E. L. Pires, Appl. Catal., A, 2001, 211, 1 CrossRef CAS; (b) I. Dodgson, K. Griffin, G. Barberis, F. Pignataro and G. Tauszik, Chem. Ind., 1989, 830 CAS; (c) H. Liu, T. Jiang, B. Han, S. Liang and Y. Zhou, Science, 2009, 326, 1250 CrossRef CAS PubMed.
- (a) O. Mitsui and Y. Fukuoka, US Pat., 4588846, 1986 Search PubMed; (b) F. Steyer and Z. W. Qi, Chem. Eng. Sci., 2002, 57, 1511 CrossRef CAS; (c) T. Qiu, C.-H. Kuang, C.-G. Li, X.-W. Zhang and X.-D. Wang, Ind. Eng. Chem. Res., 2013, 52, 8139 CrossRef CAS.
- (a) V. Z. Fridman and A. A. Davydov, J. Catal., 2000, 195, 20 CrossRef CAS; (b) B. M. Nagaraja, V. S. Kumar, V. Shashikala, A. H. Padmasri, S. S. Reddy, B. D. Raju and K. S. R. Rao, J. Mol. Catal. A: Chem., 2004, 223, 339 CrossRef CAS.
- H. Wang, C. Cui, H. Lyu and J. Sun, Sep. Purif. Technol., 2019, 211, 279 CrossRef CAS.
- (a) J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef CAS PubMed; (b) P. K. Thallapally, B. P. McGrail, S. J. Dalgarno, H. T. Schaef, J. Tian and J. L. Atwood, Nat. Mater., 2008, 7, 146 CrossRef CAS PubMed; (c) K. Jie, M. Liu, Y. Zhou, M. A. Little, S. Bonakala, S. Y. Chong, A. Stephenson, L. Chen, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2017, 139, 2908 CrossRef CAS PubMed; (d) T. Ogoshi, Y. Shimada, Y. Sakata, S. Akine and T.-a. Yamagishi, J. Am. Chem. Soc., 2017, 139, 5664 CrossRef CAS PubMed; (e) K. Jie, M. Liu, Y. Zhou, M. A. Little, A. Pulido, S. Y. Chong, A. Stephenson, A. R. Hughes, F. Sakakibara, T. Ogoshi, F. Blanc, G. M. Day, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2018, 140, 6921 CrossRef CAS PubMed; (f) K. Jie, Y. Zhou, E. Li, R. Zhao and F. Huang, Angew. Chem., Int. Ed., 2018, 57, 12845 CrossRef CAS PubMed; (g) J.-R. Wu and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 12280 CrossRef CAS PubMed; (h) Y. Wang, K. Xu, B. Li, L. Cui, J. Li, X. Jia, H. Zhao, J. Fang and C. Li, Angew. Chem., Int. Ed., 2019, 58, 10281 CrossRef CAS PubMed; (i) J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, J. Am. Chem. Soc., 2020, 142, 2228 CrossRef CAS PubMed; (j) X. Sheng, E. Li, Y. Zhou, R. Zhao, W. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 6360 CrossRef CAS PubMed; (k) Y. Zhou, K. Jie, R. Zhao, E. Li and F. Huang, J. Am. Chem. Soc., 2020, 142, 6957 CrossRef CAS PubMed; (l) J.-R. Wu, B. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59, 2251 CrossRef CAS PubMed; (m) H. Zuilhof, K. Samanta, W. Yang, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994 CrossRef PubMed; (n) Q. Li, K. Jie and F. Huang, Angew. Chem., Int. Ed., 2020, 59, 5355 CrossRef CAS PubMed; (o) H. Yao, Y.-M. Wang, M. Quan, M. U. Farooq, L.-P. Yang and W. Jiang, Angew. Chem., Int. Ed., 2020, 59, 19945 CrossRef CAS PubMed; (p) A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2021, 143, 4090 CrossRef CAS PubMed; (q) W. Zhu, E. Li and F. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 7370 CrossRef CAS PubMed; (r) D. Luo, J. Tian, J. L. Sessler and X. Chi, J. Am. Chem. Soc., 2021, 143, 18849 CrossRef CAS PubMed.
- (a) S. Tsuzuki, K. Honda, T. Uchimaru, M. Mikami and K. Tanabe, J. Am. Chem. Soc., 2000, 122, 3746 CrossRef CAS; (b) M. Nishio, Y. Umezawa, K. Honda, S. Tsuboyama and H. Suezawa, CrystEngComm, 2009, 11, 1757 RSC.
- (a) L. Duan, Z.-H. Wu, J.-P. Ma, X.-W. Wu and Y.-B. Dong, Inorg. Chem., 2010, 49, 11164 CrossRef CAS PubMed; (b) L. Fan, M. Xue, Z. Kang, G. Wei, L. Huang, J. Shang, D. Zhang and S. Qiu, Microporous Mesoporous Mater., 2014, 192, 29 CrossRef CAS.
- (a) G.-W. Zhang, P.-F. Li, Z. Meng, H.-X. Wang, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2016, 55, 5304 CrossRef CAS PubMed; (b) C.-F. Chen and Y. Han, Acc. Chem. Res., 2018, 51, 2093 CrossRef CAS PubMed; (c) L.-P. Yang, W.-E. Liu and W. Jiang, Tetrahedron Lett., 2016, 57, 3978 CrossRef CAS; (d) G.-B. Huang, W.-E. Liu, A. Valkonen, H. Yao, K. Rissanen and W. Jiang, Chin. Chem. Lett., 2018, 29, 91 CrossRef CAS; (e) L.-P. Yang, X. Wang, H. Yao and W. Jiang, Acc. Chem. Res., 2020, 53, 198 CrossRef CAS PubMed; (f) G. Huang, Z. Chen, X. Wei, Y. Chen, X. Li, H. Zhong and M. Tan, Chin. J. Org. Chem., 2020, 40, 614 CrossRef CAS.
- (a) J.-R. Wu, A. U. Mu, B. Li, C.-Y. Wang, L. Fang and Y.-W. Yang, Angew. Chem., Int. Ed., 2018, 57, 9853 CrossRef CAS PubMed; (b) P. Della Sala, R. Del Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. De Rosa, A. Spinella, A. Soriente, P. Neri and C. Gaeta, J. Am. Chem. Soc., 2020, 142, 1752 CrossRef CAS PubMed; (c) X.-N. Han, Y. Han and C.-F. Chen, J. Am. Chem. Soc., 2020, 142, 8262 CrossRef CAS PubMed; (d) S.-N. Lei, H. Xiao, Y. Zeng, C.-H. Tung, L.-Z. Wu and H. Cong, Angew. Chem., Int. Ed., 2020, 59, 10059 CrossRef CAS PubMed.
- (a) X. Wu, X. Han, Q. Xu, Y. Liu, C. Yuan, S. Yang, Y. Liu, J. Jiang and Y. Cui, J. Am. Chem. Soc., 2019, 141, 7081 CrossRef CAS PubMed; (b) Y. Ohishi, M. Murase, H. Abe and M. Inouye, Org. Lett., 2019, 21, 6202 CrossRef CAS PubMed; (c) R. Ning, H. Zhou, S.-X. Nie, Y.-F. Ao, D.-X. Wang and Q.-Q. Wang, Angew. Chem., Int. Ed., 2020, 59, 10894 CrossRef CAS PubMed; (d) T. Hong, Z. Zhang, Y. Sun, J.-J. Tao, J.-D. Tang, C. Xie, M. Wang, F. Chen, S.-S. Xie, S. Li and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 10244 CrossRef CAS PubMed.
- Both enantiomers of H8-BINOL are available with list prices of less than $5 (USD) per gram. For example, www.bldpharm.com, accessed May 2021.
- K. Xu, Z.-Y. Zhang, C. Yu, B. Wang, M. Dong, X. Zeng, R. Gou, L. Cui and C. Li, Angew. Chem., Int. Ed., 2020, 59, 7214 CrossRef CAS PubMed.
- T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937 CrossRef CAS PubMed.
- (a) K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064 CrossRef CAS PubMed; (b) J.-R. Wu and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 1690 CrossRef CAS PubMed.
- (a) D. R. Stull, Ind. Eng. Chem., 1947, 39, 517 CrossRef CAS; (b) M. C. Burguet, J. B. Monton, M. Sanchotello and M. I. Vazquez, J. Chem. Eng. Data, 1993, 38, 328 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2067429 and 2067432. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04728k |
| This journal is © The Royal Society of Chemistry 2021 |