A reductive ion exchange strategy using NaTi2(PO4)3 for metal removal/recovery from wastewater†
Shun
Dong
,
Bin
Wang
,
Xinrui
Liu
,
Ling
Wang
,
Jing
Zhan
,
Abdul
Qayum
,
Xiuling
Jiao
 ,
Dairong
Chen
and
Ting
Wang
,
Dairong
Chen
and
Ting
Wang
 *
*
National Engineering Research Center for Colloidal Materials, School of Chemistry & Chemical Engineering, Shandong University, China. E-mail: t54wang@sdu.edu.cn
First published on 25th November 2020
Abstract
With the increasing concerns about energy and environments, there are growing demands for efficient metal removal/recovery strategies for environmental protection and sustainable development. Here, a novel reductive ion exchange (RIE) strategy is proposed to use faradaic electrode materials, such as NaTi2(PO4)3 (NTP), for metal removal/recovery from wastewater. For a pre-charged Na3Ti2(PO4)3 in metal-bearing water, the potential differences between the NTP film and the reducible metal ions (such as Pb, Cu, Ag and Au) drive a rapid metal reduction process, which produces metallic nanoparticles, while the NTP film is discharged by the release of Na ions. Compared to the common adsorption-based methods, the NTP film provides a large electrical force to capture and reduce the specific metal ions, which highly increases its efficiency in removing trace amounts of metal ions from water. We show that by using an as-assembled NTP-based device the reducible metal concentrations all drop by two orders of magnitude at both the ppm and ppb levels. More interestingly, the reduced nanoparticles are loosely attached on the surface of the NTP films and can be facilely separated and recycled, converting metal pollutants into valuable materials without utilizing reductive agents or high temperature thermal treatments. Also, the charging of NTP is in Na-bearing water instead of wastewater; therefore, the film contamination by the organic matter and other unreducible cations in wastewater can be avoided, and the NTP film can be recharged and reused for hundreds of times. Our research brings a new strategy for recycling trace amounts of valuable noble metal ions from wastewater. It may also bring new insights in the application of the traditional faradaic electrode materials.
Introduction
Water pollution affects millions of people, and it is one of the most pervasive environmental problems afflicting people's life around the world. Water is contaminated by toxic metals, such as Cr, Hg and Pb, which are notorious owing to their accumulation in human body and long-term toxicity. For example, there are about 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of Pb emission globally every year, which raised great concerns for biological and environmental safety.1–4 Moreover, noble metals, such as Au and Ag, are essential for electronic industry owing to their outstanding chemical and physical properties. Unfortunately, owing to their scarcity, noble metals are expensive and their emission into the environment is a waste of valuable resources, leading to their future depletion. Industries, such as printed circuit board (PCB) manufacturing, solar cell industry and photographic operations all produce wastewater containing noble metals. It is essential to treat noble metal-containing wastewater prior to their discharge into the environment, thus removing noble metals from wastewater and recovering them as recyclable resources for both environmental protection and sustainable development.5–8
000 tons of Pb emission globally every year, which raised great concerns for biological and environmental safety.1–4 Moreover, noble metals, such as Au and Ag, are essential for electronic industry owing to their outstanding chemical and physical properties. Unfortunately, owing to their scarcity, noble metals are expensive and their emission into the environment is a waste of valuable resources, leading to their future depletion. Industries, such as printed circuit board (PCB) manufacturing, solar cell industry and photographic operations all produce wastewater containing noble metals. It is essential to treat noble metal-containing wastewater prior to their discharge into the environment, thus removing noble metals from wastewater and recovering them as recyclable resources for both environmental protection and sustainable development.5–8
Numerous technologies have been applied for aqueous metal removal, such as chemical precipitation and membrane filtration. However, the chemical precipitation strategy usually suffers from the drawbacks of sludge generation and large reagent consumption, and the membrane filtration usually suffers from the high operation cost due to membrane fouling.9 These two strategies are efficient for metal removal, but usually difficult to be used for metal recy3adsorbents has been effective for noble metal removal/recovery from wastewater, and the metal removal relies on the interactions of target metals with the metal-binding ligands or functional groups of the adsorbents.10 For example, amyloid–carbon hybrids, α-cyclodextrin (α-CD) and methionine-decorated metal–organic frameworks (MOFs) can effectively remove/recover Au from wastewater.11–14 However, the adsorbent regeneration is usually difficult and requires complex procedures. Also, gold collected by adsorbents is in an ionic form, which needs further high temperature thermal reduction or addition of extra reductive chemicals to obtain its metallic form.
Over the past decades, with the urgent demands in the energy storage field, hundreds of potential faradaic electrode materials have been synthesized with their work potential, storage capacity, cycle life and energy efficiency well characterized. This includes vast research activities in the electrodes of lithium, sodium and potassium batteries.15–17 Other than energy storage, these materials may also be used in water treatment. For example, with electrodes such as Na0.44MnO2, NaTi2(PO4)3 (NTP), Na3V2(PO4)3 for sodium ion removal, and chloride-selective materials as the counter electrode, water desalination could be achieved.18,19 Also, lithium-selective materials (such as LiFePO4) coupled with chloride-selective materials achieved a high lithium recovery in Li-bearing wastewater.20 In the current strategy, the selective metal removal relies on the specific ion adsorption/insertion into the electrode materials; however, the existence of organic matter and various types of cations in wastewater may influence the efficiency and regeneration of these materials.21,22 Also, it is not clear how to remove/recover noble metals from wastewater using these newly developed faradaic materials.
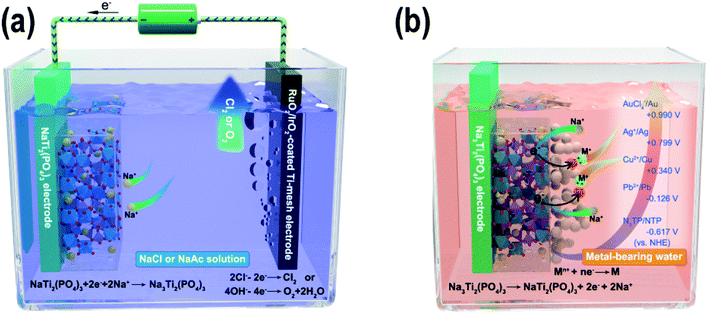
Here, we propose a novel reductive ion exchange (RIE) strategy for metal removal/recovery from wastewater. Instead of the traditional strategy using metal ion insertion/adsorption in the material for the metal removal, NASICON-type NaTi2(PO4)3 (NTP) was selected as the active material for the metal removal/recovery experiments. As shown in Fig. S1,† NTP exhibits a three-dimensional framework structure with TiO6 octahedra and PO4 tetrahedra sharing all their corner oxygen atoms, so there is enough space for the insertion and extraction of the Na-ions. In the first step, the NTP film was charged with Na-ion intercalated in the NaTi2(PO4)3 lattice in a Na-ion solution (Fig. 1a, eqn (1), the sodium solution can be NaCl solution or CH3COONa solution). The NTP worked as the cathode (negatively charged), while the RuO2/IrO2-coated Ti-mesh electrode was used as the anode (positively charged). While Na-ion intercalation happened in NTP, the RuO2/IrO2-electrode generated oxygen (for sodium acetate electrolyte) or chlorine (for sodium chloride electrolyte) gases (eqn (2) and (3)). In the second step, when immersed in metal-bearing water, owing to the potential difference between the pre-charged Na-ion intercalated NTP and the metal ions, various metal ions, such as Pb, Cu, Ag and Au could be spontaneously reduced into metallic nanoparticles (Fig. 1b, eqn (4)). Moreover, Na-ions were continuously released from the NTP film for charge compensation (eqn (5)). Compared to ion exchange materials, such as ion exchange resins, the RIE process provided a facile metal recovery process and high cycle stability. Compared to the common adsorption-based methods, the NTP film provided a large electrical force to capture and reduce the specific metal ions, which highly increased its efficiency in removing trace amounts of metal ions from water. A simple device using charged NTP films showed metal removal efficiencies up to ∼98–99% from ppm to ppb levels. More interestingly, the reduced nanoparticles were loosely attached on the surface of the NTP films and could be facilely separated and recycled, converting metal pollutants into valuable materials. Also, the charging of NTP was in Na-bearing water instead of wastewater, which avoided the film contamination and effectively promoted the cycle stabilities. Our research provides a new strategy to use faradaic electrode materials to recycle trace amounts of valuable noble metal ions from wastewater.
| NaTi2(PO4)3 + 2e− + 2Na+ → Na3Ti2(PO4)3 | (1) |
| 2Cl− → Cl2↑ + 2e− | (2) |
| 4OH− → O2↑ + 2H2O + 4e− | (3) |
| Mn+ + ne− → M↓ | (4) |
| Na3Ti2(PO4)3 → NaTi2(PO4)3 + 2e− + 2Na+ | (5) |
Results and discussion
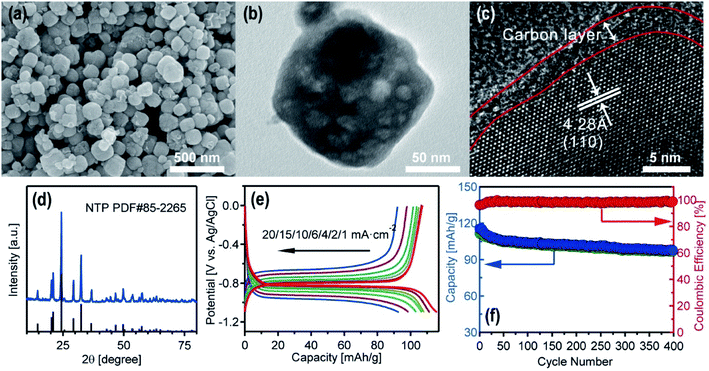
Among various faradaic electrode materials, NASICON-type NaTi2(PO4)3 was selected as the active material for the metal removal/recovery experiments. NTP was found to be a very attractive anode material for its high Na+ conductivity, very flat charge–discharge plateau, relatively high theoretical capacity of 133 mA h g−1 and superior thermal stability. Nano-sized NTP particles with carbon coating effectively improved their low intrinsic electronic conductivity, and promoted their application in aqueous Na-ion batteries.23 The NTP powder was synthesized according to previous reports with slight modifications (Experimental details are provided in the ESI†).24 As shown in Fig. 2a and b, the SEM and TEM images of the NTP showed a nanocube morphology with an average size of 100 nm (size distribution is provided in Fig. S2†). HRTEM image in Fig. 2c confirmed the surface carbon coating on NTP particles, and the marked d-spacing of 0.428 nm could be assigned to the (110) plane of the NTP lattice. Fig. 2d illustrates the XRD pattern of the as-formed NTP. It can be observed that the main peaks could all be indexed as a well-crystalline rhombohedral NaTi2(PO4)3 structure (PDF#85-2265).The NTP films were fabricated by loading NTP particles on stainless steel meshes, and the electrochemical performance of the NTP films was evaluated with aqueous sodium acetate as the electrolyte (details in the ESI†). Fig. 2e displays the charge–discharge curves of NTP in the voltage range of −1.1 V–0 V (vs. Ag/AgCl) at numerous current densities from 1 to 20 mA cm−2. The small polarization indicated its high rate performance. The long-cycle performance of the as-prepared NTP film measured in the voltage of −1.1 V–0 V (vs. Ag/AgCl) at 10 mA cm−2 is shown in Fig. 2f. The NTP film could deliver ∼98 mA h g−1 with a capacity retention of 85.7% after 400 cycles, confirming the good reversibility of the as-prepared NTP films.
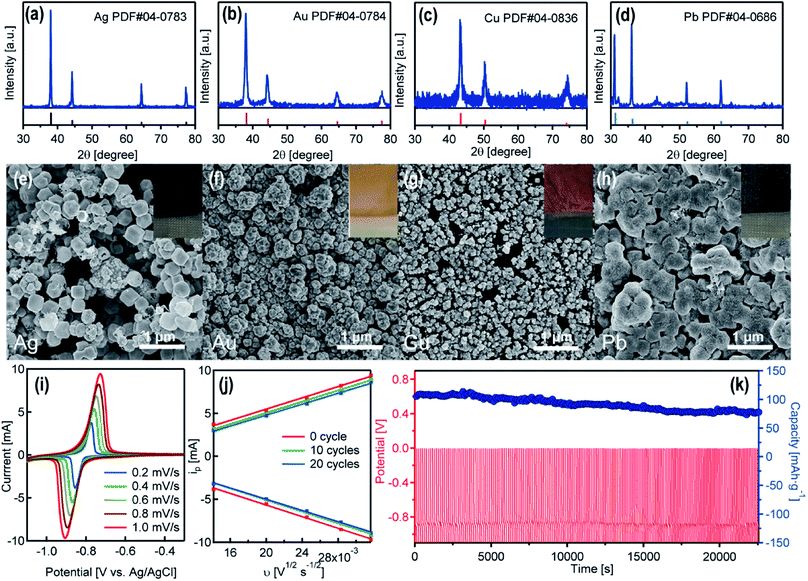
As showing in Video S1,† when immersing the pre-charged NTP film in an AgNO3 solution (0.1 M), the color of the black film turned to yellow-greyish immediately. The initially formed yellow colored powders on the edge of the film are Ag nanoparticles (Fig. 3e), and the grey products close to the center of the film are Ag nano-branches (Fig. S3†). As another example (Video S2†), when immersing the NTP film in the KAuCl4 solution (0.1 M), the surface of the film turns from black to a golden color within seconds. There are also color changes for Cu(NO3)2 and Pb(NO3)2 solutions (0.1 M) (color of the films with deposited nanoparticles presented in the insets in Fig. 3e–h). The XRD patterns of the attached nanoparticles show the existence of well-crystalline Ag, Au, Cu and Pb (Fig. 3a–d). The SEM images and the corresponding energy dispersive X-ray spectroscopy results confirm the Ag, Au, Cu and Pb nanoparticles on the surface of the substrates (Fig. 3e–h and S4†). All these nanoparticles are loosely attached on the NTP films and can be washed-off using deionized water. We have analyzed various metal ions, and the NTP film can only reduce metal ions with a redox potential more positive than that of NTP. Metal ions such as Ag+, Cu2+, Pb2+, Hg2+, Cd2+, AuCl4−, PtCl62−, PdCl42− can be reduced, and the metal ions with relatively negative redox potentials, such as Zn2+, Al3+, Mg2+, cannot be reduced. This phenomenon may also help the selective reduction/recovery of specific metal species.
With the spontaneous metal reduction, the NTP films discharged rapidly within minutes. After washing off the attached nanoparticles from the surface of the NTP film, the film could be recharged in an aqueous sodium acetate solution, and reused for the metal reduction. The cycle stability of the NTP films was measured by continuous recharging (Na-ion intercalation in NTP) in the sodium acetate solution and Na de-intercalation/Ag reduction in the AgNO3 solution. As displayed in Fig. 3i and S5,† CV curves of the initial NTP film and the film after 10 cycles and 20 cycles were measured at various scan rates ranging from 0.2 mV s−1 to 1.0 mV s−1. Two redox peaks were clearly observed, corresponding to the oxidation and reduction of Ti3+/Ti4+, which is in good agreement with previous studies. A linear relationship between the anodic/cathodic peak current and the square root of the scan rate can be observed (Fig. 3j), indicating the controlling step of the Na-ion intercalation is the Na-ion diffusion.25 The curve for the initial NTP film and the film after 10 cycles/20 cycles are almost identical, owing to the fact that the continuous Na-ion releasing from the NTP film avoids the tight attachment of the reduced Ag nanoparticles on the film, and the loosely attached Ag nanoparticles can all be facilely washed off using deionized water, which will not influence the Na-ion intercalation in the following cycles. The long-term cycling performance of the NTP film is demonstrated in Fig. 3k. After 200 cycles, the initial capacity of 105.3 mA h g−1 dropped to 77.9 mA h g−1 with a capacity retention of 74%. Such a cycle stability for metal reduction was not as good as the cycle stability in sodium acetate solution (capacity retention of 85.7% after 400 cycles), since a few NTP particles were also washed off from the film while washing the surface-attached metal nanoparticles.
In general, the reducible metal ions can be considered as ‘liquid cathodes’, and the charged NTP film can be considered as the anode. The metal ion migration/reduction towards the NTP film and the Na-ion releasing from the film promoted the formation of a current flow iRIE, which can be estimated by the equation: 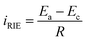 , here the Ea and Ec represent the redox potentials of the anodic NTP and the cathodic reducible ions and R represents the solution resistance. From this equation, the large potential differences between the metal ions and the NTP films were the main driving forces for the spontaneous and rapid metal reduction.
, here the Ea and Ec represent the redox potentials of the anodic NTP and the cathodic reducible ions and R represents the solution resistance. From this equation, the large potential differences between the metal ions and the NTP films were the main driving forces for the spontaneous and rapid metal reduction.
Compared to ion exchange materials, such as ion exchange resins, the RIE process provided a facile metal recovery process (metallic nanoparticles could be facilely recycled) and a high cycle stability. Compared to common electrochemical methods, RIE requires no counter electrodes in the metal reduction step, which promoted the flexibility in the device design for more efficient metal removal/recovery. For a limited number of reducible metal ions in water, the metal removal efficiency (η) of the NTP film was determined by the equation: 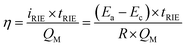 , where tRIE is the time for the RIE treatment, and QM is the overall charge of the reducible metal ions in water. For a specific metal ion with certain concentration, Ea − Ec and QM were all constant, and the metal removal efficiency was mainly determined by the time for the RIE treatment tRIE and the resistance R; the resistance was determined by the equation:
, where tRIE is the time for the RIE treatment, and QM is the overall charge of the reducible metal ions in water. For a specific metal ion with certain concentration, Ea − Ec and QM were all constant, and the metal removal efficiency was mainly determined by the time for the RIE treatment tRIE and the resistance R; the resistance was determined by the equation: 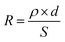 , here ρ is the resistivity of the metal-bearing water. For the electrochemical process in which metal ion migration/reduction occurred on the surface of the NTP films, S could be considered as the surface area of the NTP film, and d was the distance from reducible metal ions to the NTP film. By a proper device design to increase S and reduce d, the resistance could be reduced, and the metal removal efficiency could be effectively increased.26
, here ρ is the resistivity of the metal-bearing water. For the electrochemical process in which metal ion migration/reduction occurred on the surface of the NTP films, S could be considered as the surface area of the NTP film, and d was the distance from reducible metal ions to the NTP film. By a proper device design to increase S and reduce d, the resistance could be reduced, and the metal removal efficiency could be effectively increased.26
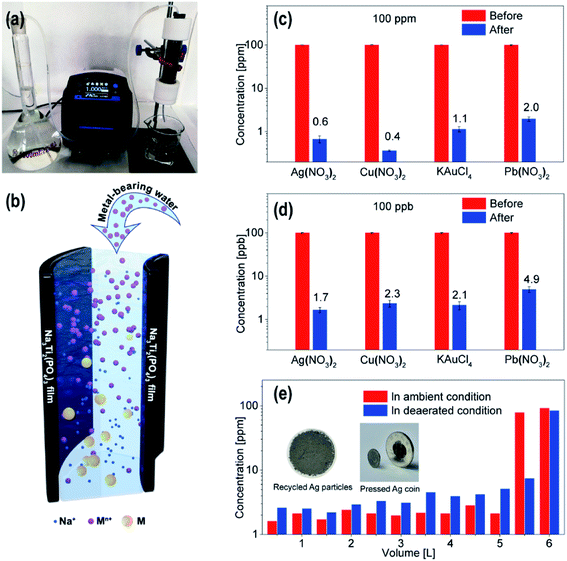
Here, we propose a new cell design using NTP films as the active materials for metal removal/recovery. As shown in Fig. 4a, metal-bearing water was prepared in a volumetric flask, which was pumped through a glass tube by peristaltic pump. NTP films were rolled up and placed in the tube (Fig. S6†) when metal-bearing water flowed through the NTP rolls, and the metal ions were reduced to metal nanoparticles, while Na ions were released into the water for charge compensation (Fig. 4b). A filtration membrane was placed at the bottom of the tube for metal nanoparticle collection. The concentration of the metal ions in water after the treatment were measured via inductively coupled plasma-mass spectrometry (ICP-MS), and the NTP films were pre-charged at a current of 200 mA for 10 min, and about 500 mL metal-bearing water was used for each measurement. In the NTP roll, the width of the NTP films was all 10 cm, while the NTP layer–layer distance could be tuned by tuning the length of the NTP film. As shown in Fig. S7a,† for initial Ag concentration of 10 ppm, with a layer–layer distance of ∼0.4 mm and at a flow rate of 3 mL min−1, only 0.12 ppm silver ions left in water after the treatment, which was reduced by two orders of magnitude as compared to the initial value, and more efficient as compared to a layer–layer distance of ∼1 mm. The short layer–layer distance limited the parameter d (the distance from the reducible ions to the NTP film), which reduced the solution resistance R and improved the metal removal efficiency. With a fixed size of the glass tube, the flow rate determined the time tRIE of the RIE process (time for the metal ions in the tube). As shown in Fig. S7b,† for AgNO3 with an initial concentration of 10 ppm at a flow rate of 5 mL min−1 and 1 mL min−1, the final concentrations after treatment are 0.14 ppm and 0.06 ppm. Here, we want to emphasize that the flow rates used are relatively low because a small tube was used in the test, and if the diameter of the tube was enlarged by 4 times and the length was increased by 5 times with larger NTP films, the flow rate could be increased by 80 times to obtain similar metal removal efficiency owing to the similar RIE time for the metal ions passed through the tube. In the following tests, the layer–layer distance of 0.4 mm was used in assembling the NTP rolls with a flow rate of 1 mL min−1.
As shown in Fig. 4c, the initial concentrations of AgNO3, Cu(NO3)2, KAuCl4 and Pb(NO3)2 were tuned to 100 ppm, after treatment, the concentrations of the metal ions dropped to 0.6 ppm, 0.4 ppm, 1.1 ppm and 2.0 ppm, corresponding to the metal removal efficiencies of 99.4% for Ag, 99.6% for Cu, 98.9% for Au and 98% for Pb. While most of the reduced metal nanoparticles are on the filtration membranes, there are also nanoparticles attached on the surface of the NTP films (Fig. S8†). These attached nanoparticles can be further removed by washing with deionized water or acidic water. The cycle stability of the NTP rolls has also been investigated, as shown in Fig. S9,† and the Ag removal efficiency was not influenced for 100 metal removal/recharging cycles owing to the fact that the metal removal is mainly driven by the metal reduction caused by the potential difference.
The effectiveness of the NTP film to remove trace amounts of metal ions in the ppb level was also investigated, and the results are presented in Fig. 4d. In the ppb level, the reduced metal ions are difficult to assemble into nanoparticles, and in our experiment, active carbon powder was filled between the layers of the NTP rolls to absorb the reduced metal species. For the AgNO3, Cu(NO3)2, KAuCl4 and Pb(NO3)2 precursors with initial concentration all tuned to 100 ppb, after treatment, the concentration of the metal ions dropped to 1.7 ppb, 2.3 ppb, 2.1 ppb and 4.9 ppb, corresponding to metal removal efficiencies of 98.3% for Ag, 97.7% for Cu, 97.9% for Au and 95.1% for Pb. Such a performance confirmed the high metal removal efficiency from ppm to ppb levels. As a comparison, the glass tube was also fully filled with active carbon to do the same experiment for the AgNO3 precursor with a concentration of 100 ppb. The result showed that up to 20 ppb Ag still left in the solution, indicating a metal removal efficiency of 80% for active carbon. The charged NTP provided a large potential difference for metal attraction/reduction, and compared to metal ions, the reduced metal species showed a large tendency to be adsorbed on solid surfaces, which greatly improved the metal removal efficiency in ppb level.
The precursor solutions in the above experiments were not degassed, the oxygen dissolved in the solutions may influence the experimental results. To further study the energy efficiency and the metal recovery efficiency, the NTP film in the device was pre-charged with a constant current of 200 mA for 2600 s, and 6 L of AgNO3 solution with a concentration of 100 ppm was also prepared and degassed with a vacuum pump. Extra Ar gas was purged into the AgNO3 solution in the volumetric flask to avoid oxygen diffusion into the solution. At a flow rate of 5 mL min−1, the AgNO3 solution passed through the tube. Every 500 mL, the residual Ag concentration after the treatment was checked by ICP (blue curve in Fig. 4e). The initial 5.5 L all showed Ag concentration below 10 ppm, for 5.5–6 L, the concentration increased to ∼80 ppm, indicating that the NTP film was discharged with no ability for the Ag removal. Based on these results, the calculated energy efficiency for the metal removal was ∼93%. The Ag particles attached on the film were washed off, together with the Ag nanoparticles on the filtration film, and the mass of the dried and recycled Ag was ∼526 mg. The recycled Ag nanoparticles could be pressed into an Ag coin with a purity of 98.8% compared with the Ag ions removed from the solution, and an Ag recovery efficiency of 97% could be obtained.
In the above experiment, the initial solution was pre-vacuum-pumped and degassed. For the AgNO3 (100 ppm) solution without the degassing process, the performance of the films was also recorded. As shown in the red bars in Fig. 4e, the metal removal efficiency of the film was much better for the solution under ambient conditions than the degassed solution. However, ∼5 L AgNO3 solution could be effectively treated compared to ∼5.5 L degassed AgNO3 solution. After treatment, the pH value of the aqueous solution increased to ∼9 for the AgNO3 solution without the degassing process owing to the competitive oxygen reduction reaction (ORR), which consumed oxygen and produced OH− in the process (eqn (6)). Owing to this ORR reaction, the amount of the treated metal-bearing water decreased without the degassing process; however, the increased pH value promoted the precipitation of metal species and greatly increased the metal removal efficiency.
| O2 + 4e− + 2H2O → 4OH− | (6) |
In application of the faradaic materials for practical water treatment, one concern is the organic impurities in wastewater, which may attach on the films and block/deactivate the films.27 We prepared AgNO3 (100 ppm) with humic acid (200 ppm) to simulate natural organic impurities, and the results indicated better metal removal abilities as compared to the pure AgNO3 solution. With humic acid in the AgNO3 solution, the NTP films were recharged and reused for 5 times without a noticeable performance decay (Fig. S10†). With large amounts of sodium ions released from NTP, the solution near NTP turned basic, which greatly increased the solubility of humic acid, and the attached humic acid had a large tendency to be detached and dissolved near the NTP film. The existence of these organic impurities may also promote the accumulation of the metal nanoparticles and increase the metal removal efficiency. The existence of unreducible metal ions, such as Ca2+ and Mg2+, has also been investigated. We added unreducible Ca2+ (100 ppm) and Mg2+ (100 ppm) ions in the AgNO3 solution (100 ppm) to study their influence, and the results showed no obvious differences in the metal removal performance. Also, with these unreducible metal ions in the AgNO3 solution, the NTP films could be recharged and reused for 5 times without a noticeable performance decay (Fig. S11†). Up to now, there are also lots of natural materials proposed for water purification, which are low-cost and may potentially be used in large-scale.28,29 Compared to these natural materials, the NTP films require an extra electrical power to be activated. However, the electrical power can be supplied by green energy, such as solar or wind. Considering the high metal removal efficiency of NTP in low metal concentrations, the high cycle stability and the selectivity, NTP materials may be used for noble metal recovery in the presence of unreducible species.
Conclusions
In conclusion, a novel reductive ion exchange strategy for metal removal/recovery from wastewater is proposed. Compared to ion exchange materials, such as ion exchange resins, the RIE process provides a facile metal recovery process and high cycle stability. Compared to common adsorption-based methods, the NTP film provides a large electrical force to capture and reduce specific metal ions, which highly increases its efficiency in removing trace amounts of metal ions from water. A simple device using charged NTP films showed metal removal efficiency up to ∼98–99% from ppm to ppb levels. More interestingly, the charging (metal intercalation) process was in Na-bearing water instead of wastewater, which effectively promoted the cycle stabilities. The as-prepared NTP films can be recharged in Na-bearing solutions for up to 100 times for the metal removal/recovery measurements without a performance decay. Our research provides a practical solution to use faradaic electrode materials to recycle trace amounts of valuable noble metal ions from wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Young Scholars Program of Shandong University (YSPSDU, No. 2015WLJH27) and Shandong Provincial Natural Science Foundation, China (2019GSF109029), and was also funded by the National Natural Science Foundation of China (no. 21771118, 21701098, and 21875128) and the Taishan Scholars Climbing Program of Shandong Province (tspd20150201).References
- F. Fu and Q. Wang, Removal of heavy metal ions from wastewaters: a review, J. Environ. Manage., 2011, 92(3), 407–418 CrossRef CAS.
- M. S. Islam, M. K. Ahmed, M. Raknuzzaman, M. Habibullah-Al-Mamun and M. K. Islam, Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country, Ecol. Indic., 2015, 48, 282–291 CrossRef CAS.
- S. T. Glassmeyer, E. T. Furlong, D. W. Kolpin, A. L. Batt, R. Benson, J. S. Boone, O. Conerly, M. J. Donohue, D. N. King, M. S. Kostich, H. E. Mash, S. L. Pfaller, K. M. Schenck, J. E. Simmons, E. A. Varughese, S. J. Vesper, E. N. Villegas and V. S. Wilson, Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States, Sci. Total Environ., 2017, 581–582, 909–922 CrossRef CAS PubMed.
- S. Kim, K. H. Chu, Y. A. J. Al-Hamadani, C. M. Park, M. Jang, D.-H. Kim, M. Yu, J. Heo and Y. Yoon, Removal of contaminants of emerging concern by membranes in water and wastewater: a review, Chem. Eng. J., 2018, 335, 896–914 CrossRef CAS.
- W. Jin and Y. Zhang, Sustainable Electrochemical Extraction of Metal Resources from Waste Streams: From Removal to Recovery, ACS Sustainable Chem. Eng., 2020, 8(12), 4693–4707 CrossRef CAS.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Science and technology for water purification in the coming decades, Nature, 2008, 452(7185), 301–310 CrossRef CAS.
- M. Ike, M. Yamashita and M. Kuroda, Microbial Removal and Recovery of Metals from Wastewater, Applied Bioengineering, 2017, 573–595 CAS.
- B. K. Reck and T. E. Graedel, Challenges in Metal Recycling, Science, 2012, 337(6095), 690 CrossRef CAS.
- M. A. Barakat, New trends in removing heavy metals from industrial wastewater, Arabian J. Chem., 2011, 4(4), 361–377 CrossRef CAS.
- L. Joseph, B.-M. Jun, M. Jang, C. M. Park, J. C. Muñoz-Senmache, A. J. Hernández-Maldonado, A. Heyden, M. Yu and Y. Yoon, Removal of contaminants of emerging concern by metal–organic framework nanoadsorbents: a review, Chem. Eng. J., 2019, 369, 928–946 CrossRef CAS.
- S. Bolisetty and R. Mezzenga, Amyloid–carbon hybrid membranes for universal water purification, Nat. Nanotechnol., 2016, 11(4), 365–371 CrossRef CAS.
- Z. Liu, M. Frasconi, J. Lei, Z. J. Brown, Z. Zhu, D. Cao, J. Iehl, G. Liu, A. C. Fahrenbach, Y. Y. Botros, O. K. Farha, J. T. Hupp, C. A. Mirkin and J. Fraser Stoddart, Selective isolation of gold facilitated by second-sphere coordination with α-cyclodextrin, Nat. Commun., 2013, 4(1), 1855 CrossRef PubMed.
- Z. Liu, A. Samanta, J. Lei, J. Sun, Y. Wang and J. F. Stoddart, Cation-Dependent Gold Recovery with α-Cyclodextrin Facilitated by Second-Sphere Coordination, J. Am. Chem. Soc., 2016, 138(36), 11643–11653 CrossRef CAS PubMed.
- M. Mon, J. Ferrando-Soria, T. Grancha, F. R. Fortea-Pérez, J. Gascon, A. Leyva-Pérez, D. Armentano and E. Pardo, Selective Gold Recovery and Catalysis in a Highly Flexible Methionine-Decorated Metal–Organic Framework, J. Am. Chem. Soc., 2016, 138(25), 7864–7867 CrossRef CAS.
- T. Perveen, M. Siddiq, N. Shahzad, R. Ihsan, A. Ahmad and M. I. Shahzad, Prospects in anode materials for sodium ion batteries – a review, Renewable Sustainable Energy Rev., 2020, 119, 109549 CrossRef CAS.
- J.-Y. Hwang, S.-T. Myung and Y.-K. Sun, Sodium-ion batteries: present and future, Chem. Soc. Rev., 2017, 46(12), 3529–3614 RSC.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Research Development on Sodium-Ion Batteries, Chem. Rev., 2014, 114(23), 11636–11682 CrossRef CAS PubMed.
- M. Pasta, C. D. Wessells, Y. Cui and F. La Mantia, A Desalination Battery, Nano Lett., 2012, 12(2), 839–843 CrossRef CAS PubMed.
- D.-H. Nam and K.-S. Choi, Bismuth as a New Chloride-Storage Electrode Enabling the Construction of a Practical High Capacity Desalination Battery, J. Am. Chem. Soc., 2017, 139(32), 11055–11063 CrossRef CAS.
- M. Pasta, A. Battistel and F. La Mantia, Batteries for lithium recovery from brines, Energy Environ. Sci., 2012, 5(11), 9487–9491 RSC.
- P. Srimuk, X. Su, J. Yoon, D. Aurbach and V. Presser, Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements, Nat. Rev. Mater., 2020, 5(7), 517–538 CrossRef CAS.
- M. E. Suss and V. Presser, Water Desalination with Energy Storage Electrode Materials, Joule, 2018, 2(1), 10–15 CrossRef.
- S. Chen, C. Wu, L. Shen, C. Zhu, Y. Huang, K. Xi, J. Maier and Y. Yu, Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries, Adv. Mater., 2017, 29(48), 1700431 CrossRef.
- Q. Hu, J.-Y. Liao, C.-T. Li, X.-D. He, X. Ding and C.-H. Chen, Synthesis of porous carbon-coated NaTi2(PO4)3 nanocubes with a high-yield and superior rate properties, J. Mater. Chem. A, 2018, 6(47), 24503–24508 RSC.
- B. Zhao, Q. Wang, S. Zhang and C. Deng, Self-assembled wafer-like porous NaTi2(PO4)3 decorated with hierarchical carbon as a high-rate anode for aqueous rechargeable sodium batteries, J. Mater. Chem. A, 2015, 3(22), 12089–12096 RSC.
- G. V. Akimov, Processes of Electrochemical Corrosion, Corrosion, 1958, 14(10), 33–53 CrossRef.
- X. Liu, J. F. Whitacre and M. S. Mauter, Mechanisms of Humic Acid Fouling on Capacitive and Insertion Electrodes for Electrochemical Desalination, Environ. Sci. Technol., 2018, 52(21), 12633–12641 CrossRef CAS PubMed.
- Z. Yang, H. Liu, J. Li, K. Yang, Z. Zhang, F. Chen and B. Wang, High-Throughput Metal Trap: Sulfhydryl-Functionalized Wood Membrane Stacks for Rapid and Highly Efficient Heavy Metal Ion Removal, ACS Appl. Mater. Interfaces, 2020, 12(13), 15002–15011 CrossRef CAS PubMed.
- S. He, C. Chen, G. Chen, F. Chen, J. Dai, J. Song, F. Jiang, C. Jia, H. Xie and Y. Yao, High-Performance, Scalable Wood-Based Filtration Device with a Reversed-Tree Design, Chem. Mater., 2020, 32(5), 1887–1895 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta09029h |
| This journal is © The Royal Society of Chemistry 2021 |