Improving quantum efficiency in organic solar cells with a small energetic driving force†
Haiqin
Liu
,
Mengyang
Li
,
Hongbo
Wu
,
Jie
Wang
,
Zaifei
Ma
 * and
Zheng
Tang
* and
Zheng
Tang
 *
*
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Center for Advanced Low-dimension Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China. E-mail: ztang@dhu.edu.cn; mazaifei@dhu.edu.cn
First published on 24th March 2021
Abstract
Organic solar cells based on a polymer donor (PM7) and a non-fullerene acceptor (Y5) with a very small energetic difference between the local excited state and the charge transfer (CT) state are investigated. We find that the small energetic difference (ΔECT) leads to a low voltage loss (0.44 eV). However, the short-circuit current density (Jsc) of the solar cell based on PM7:Y5 is very low, due to monomolecular recombination of the CT state excitons, limiting the internal quantum efficiency of the device. To solve the problem with the inefficient exciton dissociation, a polymer donor (PBDB-T) with a similar chemical structure to PM7 is employed as a second donor component for constructing ternary solar cells. We find that the frontier energy levels of the two donors are hybridized, allowing us to realize fine-tuning of the effective energy of the CT state and ΔECT of the ternary blend, by varying the PBDB-T content. As a result, a significantly improved CT state dissociation efficiency is achieved by adding a small amount of PBDB-T in the active layer. Meanwhile, the low voltage loss property of PM7:Y5 is very well maintained in the ternary solar cell, due to the energy level hybridization of the donor materials.
1. Introduction
The development of non-fullerene acceptor materials in the last few years has increased the power conversion efficiency (PCE) of the bulk-heterojunction (BHJ) organic solar cells up to 17–18%.1–4 Many of the highly efficient solar cells are realized based on the ternary concept consisting of adding an additional donor or acceptor into the binary BHJ active materials system:5–7 The use of the third component can often lead to improved spectral coverage for photon harvesting and a more desired phase separation in the active layer for a more efficient charge carrier collection. Today, high quantum efficiencies (QE) are observed in organic solar cells based on different donor–acceptor combinations, and the short-circuit current density (Jsc) of the most efficient organic solar cells has already exceeded 25 mA cm−2.8–10 Thus, it has become increasingly more important to increase the open-circuit voltage (Voc) to further improve the performance of organic solar cells.Minimizing the voltage lost in the indispensable charge transfer process in organic solar cells is crucial. Generally, voltage losses (Vloss) in organic solar cells can be divided into three parts.11–18 ΔECT, ΔVr, and ΔVnr. ΔECT (also known as the energetic driving force) is defined as the energetic difference between the charge transfer (CT) state and the local excited state of the donor or the acceptor material (S1). The typical value for ΔECT is in the range of 0.05 to 0.2 eV for the most efficient organic solar cells. ΔVr and ΔVnr are the voltage loss terms related to the radiative and non-radiative decay rate of CT states, respectively.15,19,20 Because the CT state can be regarded as the lowest energy of state in the blend photoactive layer,21,22 increasing the energy of CT state (ECT) for a reduced ΔECT can directly lead to a reduced Vloss in the solar cell. Besides, the higher ECT can result in a reduced ΔVnr, due to the weakened electronic coupling between the vibrational excited and ground states.17 Furthermore, the coupling between S1 and CT state is stronger in the blend system with a higher ECT and a smaller ΔECT. This can lead to a further weakened CT to ground state coupling.16,19 As a result, the non-radiative decay rate of CT states in the blend system with a smaller ΔECT is slower, and ΔVnr of the solar cell with a smaller ΔECT is lower.
However, the strong coupling between S1 and CT states in the blend active layer with a too-small ΔECT can lead to inefficient exciton dissociation, which limits QE and thus Jsc of the solar cell. This has been observed in solar cells based on several different BHJ systems.23–26 The inefficient exciton dissociation can be ascribed to a rapid back transfer of CT states to S1 states, enabled by the small ΔECT at the heterointerfaces.27 This reduces the probability for the CT state to dissociate into free charge carriers, since the energy of the free charge carrier state (EFC) is generally higher than that of the CT state.28 Therefore, there is always a tradeoff between QE and Vloss which must be balanced to construct efficient organic solar cells using the blends with small ΔECT. Currently, there is no solution for the poorly performing solar cells with QE limited by a too-small ΔECT, apart from designing new materials with different frontier energy levels.
In this work, a ternary blend strategy is employed to solve the high QE loss problem in the low Vloss organic solar cells based on low ΔECT systems. First, we perform a detailed investigation on the organic blend system based on poly[4,8-bis[5-(2-ethylhexyl)-4-chloro-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]-2,5-thiophenediyl[5,7-bis(2-ethylhexyl)-4,8-dioxo-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-1,3-diyl]-2,5-thiophenediyl] (PM7) and (2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro[1,2,5]thiadiazolo[3,4e]thieno[2′′,3′′:4′,5′]thieno[2′,3′:4,5] pyrrolo[3,2-g]thieno[2′,3′:4,5] thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile) (Y5) with a very small ΔECT (close to 0 eV), and we identify a very low Vloss (0.44 V) in the solar cell associated with the small ΔECT. However, the ΔECT is too-small, providing an insufficient driving force for CT states to dissociate. This limits QE and Jsc of the solar cell. To overcome this problem, poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b’]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′- bis(2-ethylhexyl)benzo [1′,2′-c:4′,5′-c’]dithiophene-4,8-dione) (PBDB-T) with a chemical structure similar to PM7 is introduced as a third component for constructing ternary solar cells. We find that the addition of PBDB-T can lead to a hybridization of the frontier orbitals of the donor materials. As a result, fine-tuning of the effective ECT and ΔECT of the ternary solar cell is achieved by varying the PBDB-T content: a significantly improved CT state dissociation efficiency, and thus an increased QE is realized by adding a small amount of PBDB-T ((PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio = 10 wt%)) in the photoactive layer. More importantly, we find that the low Vloss property of the PM7:Y5 blend is well kept in the ternary solar cell with a low PBDB-T content, due to the hybridization of the energy levels of the donor materials.
PM7 donor ratio = 10 wt%)) in the photoactive layer. More importantly, we find that the low Vloss property of the PM7:Y5 blend is well kept in the ternary solar cell with a low PBDB-T content, due to the hybridization of the energy levels of the donor materials.
2. Results and discussion
Fig. 1a shows the chemical structures of the active materials used in this work. First, we focus on investigating the Voc (0.98 V, Table 1) of the solar cell based on PM7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y5 (ref. 29 and 30) (1
Y5 (ref. 29 and 30) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, weight ratio), which is higher than any of the other solar cells based on PM7 and the Y-series acceptors reported in literature.31,32 To understand the reason for the high Voc, optical transitions in the BHJ system is investigated. The absorption properties of the blend and the pure acceptor material are studied using sensitive external quantum efficiency (EQE) and electroluminescence (EL) measurements, as shown in Fig. 1b. From the onset of the EQE spectrum, we determine ECT, using a Gaussian fit derived based on the Marcus theory:12,33,34
1.2, weight ratio), which is higher than any of the other solar cells based on PM7 and the Y-series acceptors reported in literature.31,32 To understand the reason for the high Voc, optical transitions in the BHJ system is investigated. The absorption properties of the blend and the pure acceptor material are studied using sensitive external quantum efficiency (EQE) and electroluminescence (EL) measurements, as shown in Fig. 1b. From the onset of the EQE spectrum, we determine ECT, using a Gaussian fit derived based on the Marcus theory:12,33,34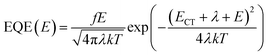 | (1) |
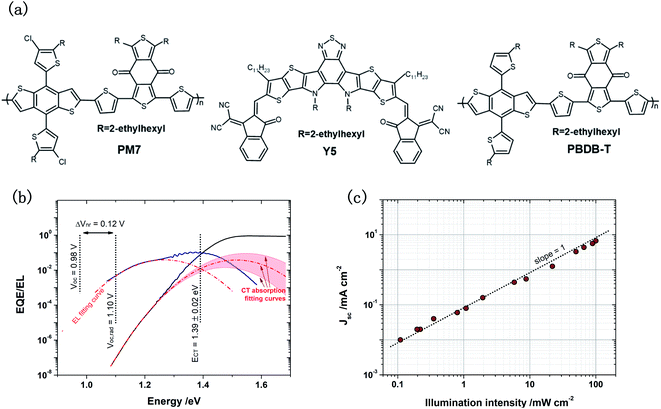 | ||
Fig. 1 (a) Chemical structures of the active materials used in this work. (b) EQE and EL of the solar cell based on PM7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y5 (1 Y5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 wt ratio). The tail of the EQE spectrum is calculated from the measured EL using the reciprocal relation, and it is used to calculated ECT based on the Marcus theory. Voc,rad is also calculated by integrating the product of EQE and ϕBB, and ΔVnr is equal to the difference between Voc,rad and Voc. To avoid an arbitrary fitting on the EQE spectrum, two boundary conditions are applied. Details are provided in ESI-2.† (c) Dependence of Jsc of the solar cell on illumination intensity. A slope of one is obtained indicating monomolecular recombination limiting Jsc of the solar cell. 1.2 wt ratio). The tail of the EQE spectrum is calculated from the measured EL using the reciprocal relation, and it is used to calculated ECT based on the Marcus theory. Voc,rad is also calculated by integrating the product of EQE and ϕBB, and ΔVnr is equal to the difference between Voc,rad and Voc. To avoid an arbitrary fitting on the EQE spectrum, two boundary conditions are applied. Details are provided in ESI-2.† (c) Dependence of Jsc of the solar cell on illumination intensity. A slope of one is obtained indicating monomolecular recombination limiting Jsc of the solar cell. | ||
| J sc (mA cm−2) | V oc (V) | PCE (%) | E S1,A (eV) | E CT (eV) | ΔECT (eV) | V loss (V) | J 0,rad (mA cm−2) | V oc,rad (V) | ΔVnra (V) | ΔVnrb (V) | EQEEL (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a ΔVnr calculated from J0,rad. b ΔVnr calculated from measured EQEEL. | ||||||||||||
| PM7:Y5 | 7.8 | 0.98 | 3.28 | 1.42 | 1.39 | 0.03 | 0.44 | 1.5 × 10−18 | 1.10 | 0.12 | 0.13 | 0.751 |
| PM7:PBDB-T(10%):Y5 | 12.0 | 0.95 | 5.27 | 1.42 | 1.39 | 0.03 | 0.47 | 1.3 × 10−18 | 1.10 | 0.15 | 0.16 | 0.142 |
| PBDB-T:Y5 | 17.2 | 0.88 | 9.04 | 1.42 | 1.37 | 0.05 | 0.54 | 2.7 × 10−18 | 1.08 | 0.21 | 0.22 | 0.015 |
More specifically, to avoid an arbitrary fitting on the EQE spectrum, a series of fittings are performed: radiative recombination limit of the voltage loss derived using the detailed balance theory35 is employed for determining the minimum value for ECT, while the upper limit for ECT is restricted by the assumption that the contribution of the weak CT state absorption to the EQE of the solar cell is at most 10%.12,36 These boundary conditions allow us to identify a range for the values of ECT with real physical meaning, and the upper and the lower boundary values are found to differ by only about 0.02–0.04 eV. For the analysis of the energetics and voltage losses in the solar cells studied in this work, the mean value of the range is used. More details regarding the fitting of the EQE spectrum are provided in ESI (SI-2).†
The value of ECT from the EQE spectrum for the solar cell based on PM7:Y5 is found to be very close to the energy of the optical bandgap (Eg) of the active layer, which is equivalent to the energy of the singlet excited state of the acceptor material (ES1,A) (ESI-3†).37 Therefore, ΔECT is very small, approaching zero.
Furthermore, the radiative recombination limit for the saturation current (J0,rad) is also calculated from the EQE spectrum using the detailed balance theory:11,38
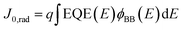 | (2) |
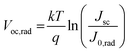 | (3) |
We find that Voc,rad of the solar cell based on PM7:Y5 is 1.10 V, thus ΔVnr is very low, only 0.12 V (ΔVnr = Voc,rad– Voc). Therefore, recombination of charge carriers in the solar cell is expected to be highly emissive. The low ΔVnr of the solar cell and the high emission efficiency of CT state recombination are also confirmed by directly measuring the external quantum efficiency of electroluminescence (EQEEL) of the solar cell, which is close to 1%, corresponding to a ΔVnr value of 0.13 V 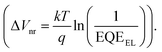 12,38 The value of ΔVnr from EQEEL agrees very well with that calculated from Voc,rad. Consequently, due to the small ΔECT and low ΔVnr, the total voltage loss defined as
12,38 The value of ΔVnr from EQEEL agrees very well with that calculated from Voc,rad. Consequently, due to the small ΔECT and low ΔVnr, the total voltage loss defined as 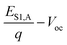 is also very low for the solar cell based on PM7:Y5 (0.44 V), considerably lower than the generally reported Vloss for organic solar cells (0.5–0.6 V).39,40
is also very low for the solar cell based on PM7:Y5 (0.44 V), considerably lower than the generally reported Vloss for organic solar cells (0.5–0.6 V).39,40
J sc of the solar cell based on PM7:Y5 is rather low, because IQE of the solar cell calculated using a transfer matrix model is only about 30% (ESI-4†).41,42 For organic solar cells, a low IQE is normally due to the bimolecular recombination loss of charge carriers.43–46 However, fast bimolecular recombination in the solar cell often leads to a low EQEEL and a high ΔVnr, contradictory to the experimental results discussed above. Therefore, light intensity dependent current density–voltage (J–V) (Fig. 1c) measurements are performed on the solar cell based on PM7:Y5, and we find a linear dependence of Jsc on the light intensity, which suggests that the IQE and Jsc of the solar cell are limited by inefficient charge carrier generation, a result of monomolecular recombination losses.47,48
The monomolecular recombination loss in BHJ solar cells can be associated with inefficient exciton dissociation in the active layer. This is indeed observed for the PM7:Y5 blend system in the PL quenching measurements. As shown in Fig. 2a, the characteristic PL emission spectra of the neat PM7 and the neat Y5 film are peaked at 680 and 920 nm, respectively. In the blend active layer of PM7:Y5, the PM7 emission peak is found completely quenched, but the Y5 emission peak is only slightly reduced. This suggests that the singlet excitons generated in the Y5 phase of the blend active layer do not dissociate into free charge carriers. However, the atomic force microscopic image reveals that there is no obvious phase separation in the active layer (ESI-5†), and the efficient quenching of the PM7 PL emission implies that the degree of phase separation in the active layer is low. Therefore, the reason for the inefficient dissociation of the singlet Y5 excited state (S1,A) is ascribed to the too-small ΔECT providing an insufficient driving force for the already formed CT state excitons to dissociate, which is very common for the BHJ systems with small ΔECT.23–26 In this case, an electric field-dependent PL quenching of the excited state is expected, because dissociation of the weakly bound CT excitons is often facilitated by an electric field, resulting in less populated S1,A states, and thus reduced S1,A emission intensity.23,24
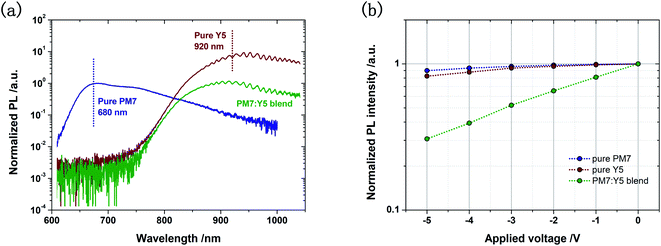 | ||
| Fig. 2 (a) PL spectra of pure PM7, pure Y5, and the PM7:Y5 blend, measured with laser excitation at 460 nm. The emission peaks of pure PM7 and pure Y5 are located at 680 and 920 nm, respectively, and the PL of the blend is dominated by the Y5 emission. (b) Emission intensity values of the PL peaks of the solar cells based on pure PM7, pure Y5, and the PM7:Y5 blend plotted as a function of applied voltage. The intensity values are normalized by the intensity value of the peak measured under short circuit conditions. Complete spectra are provided in ESI-6.† | ||
The field-dependent PL spectra are measured for the solar cell based on PM7:Y5, as well as the photovoltaic device based on pure Y5 (ESI-6†). In Fig. 2b, the intensities of the emission peak are plotted as a function of applied voltage. We find that for the solar cell based on the blend active layer, the Y5 emission peak at 920 nm is effectively quenched by the applied voltage, whereas the degree of the electric field induced quenching of the emission from the device based on pure Y5 is significantly smaller. These field-dependent PL results suggest that CT states are indeed formed in the solar cell based on PM7:Y5, but they do not easily dissociate into free charge carriers under a short-circuit condition.
The chemical structure of PBDB-T49 is similar to PM7, but the highest-occupied-molecular-orbital (HOMO) level of PBDB-T (−5.3 eV) is slightly upper laying compared to that of PM7 (−5.4 eV).29 Therefore, a higher ΔECT exists in the solar cell based on the blend of PBDB-T:Y5, allowing for more efficient dissociation of CT excitons and higher Jsc (Table 1). However, Vloss in the solar cell is significantly higher, not only because of the higher ΔECT but also a higher ΔVnr.
Therefore, to overcome the problem of inefficient CT dissociation in the low Vloss solar cell based on PM7:Y5, PBDB-T is employed as a third component, acting as an additional donor for constructing ternary solar cells. Note that the ternary solar cells based on PBDB-T:PM7:Y5 are with a fixed donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor weight ratio (1
acceptor weight ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2). The representative J–V characteristic curves of the solar cells are provided in ESI-7,† and Jsc are plotted as a function of the PBDB-T content in Fig. 3a. Surprisingly, we find that with the addition of a very small amount of PBDB-T (PBDB-T
1.2). The representative J–V characteristic curves of the solar cells are provided in ESI-7,† and Jsc are plotted as a function of the PBDB-T content in Fig. 3a. Surprisingly, we find that with the addition of a very small amount of PBDB-T (PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio = 10 wt%), Jsc of the solar cell is increased by 30%, from 7 to 10 mA cm−2, which is further doubled, when the donor ratio is increased to 20%. The Jsc of the solar cell does not noticeably increase when the donor ratio is increased to over 20%, and when the donor ratio exceeds 40%, Jsc is reduced.
PM7 donor ratio = 10 wt%), Jsc of the solar cell is increased by 30%, from 7 to 10 mA cm−2, which is further doubled, when the donor ratio is increased to 20%. The Jsc of the solar cell does not noticeably increase when the donor ratio is increased to over 20%, and when the donor ratio exceeds 40%, Jsc is reduced.
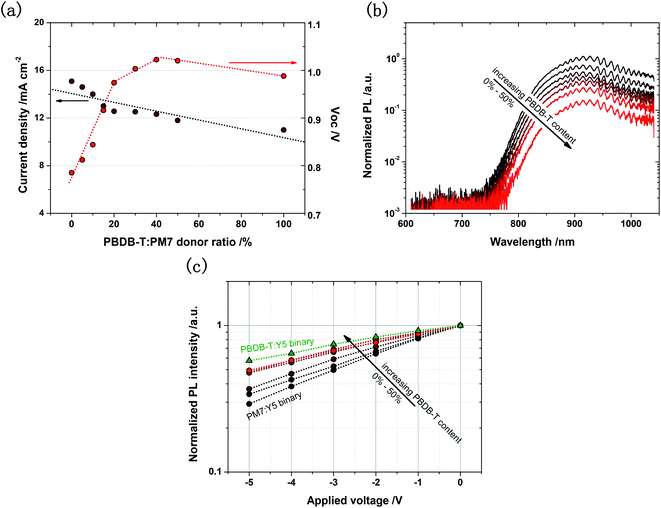 | ||
Fig. 3 (a) Jsc and Voc of the solar cells based on PBDB-T:PM7:Y5, plotted as a function of the PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio. (b) PL emission spectra of the solar cell based on different PBDB-T PM7 donor ratio. (b) PL emission spectra of the solar cell based on different PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratios. More efficient quenching of the Y5 emission is found in the solar cell with higher PBDB-T content. (c) Emission intensities of the PL peaks of the solar cells based on different PBDB-T PM7 donor ratios. More efficient quenching of the Y5 emission is found in the solar cell with higher PBDB-T content. (c) Emission intensities of the PL peaks of the solar cells based on different PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratios plotted as a function of applied voltage. The intensity values are normalized by the intensity value of the peak measured under short circuit conditions. The dependence of the PL quenching efficiency on the applied voltage reduces with the increasing PBDB-T content. Complete spectra are provided in ESI-6.† PM7 donor ratios plotted as a function of applied voltage. The intensity values are normalized by the intensity value of the peak measured under short circuit conditions. The dependence of the PL quenching efficiency on the applied voltage reduces with the increasing PBDB-T content. Complete spectra are provided in ESI-6.† | ||
These results indicate that the IQE of the ternary solar cell is increased significantly with the PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio exceeding 20%, which is remarkable as it suggests that adding PBDB-T into the PM7:Y5 blend can lead to substantially more efficient CT state dissociation. Indeed, from Fig. 3b, we find that the PL peak of the blend at 920 nm, corresponding to Y5 emission, is much more efficiently quenched, and the dependence of the PL intensity on the applied electric field is significantly reduced in the ternary solar cell with PBDB-T (Fig. 3c).
PM7 donor ratio exceeding 20%, which is remarkable as it suggests that adding PBDB-T into the PM7:Y5 blend can lead to substantially more efficient CT state dissociation. Indeed, from Fig. 3b, we find that the PL peak of the blend at 920 nm, corresponding to Y5 emission, is much more efficiently quenched, and the dependence of the PL intensity on the applied electric field is significantly reduced in the ternary solar cell with PBDB-T (Fig. 3c).
There are two possible mechanisms for the increased dissociation efficiency of the CT excitons in the ternary blend:50 (1) There is no interaction of the frontier orbitals of the different donor materials, and most of the holes generated in Y5 are transferred to PBDB-T to contribute to free charge carrier generation. (2) There is a hybridization of the energy levels of PBDB-T and PM7, leading to effectively reduced ECT and increased driving force for the whole system. This can result in a decreased electronic coupling strength between S1,A and CT states, and thus reducing the probability for CT states to back transfer to S1,A state, and increasing the dissociation probability of the CT states. Both mechanisms require a homogeneous distribution of PBDB-T in the blend active layer.
When there is no interaction between the orbitals of the donor materials in the ternary solar cells, the recombination dynamics of the photogenerated charge carriers should be primarily determined by the electronic property of the CT state formed at the PBDB-T:Y5 interfaces, even when the blend is with a low PBDB-T content since the PBDB-T:Y5 CT state is the lowest energy of state in the blend system. Therefore, with a small amount of PBDB-T in the ternary blend, Voc of the solar cell should be similar to that of the binary solar cell based on PBDB-T:Y5. In addition, the characteristics of the recombination current of the ternary solar cell should be the same as that of the binary solar cell based on PBDB-T:Y5. Because in the ternary solar cell, the electrically injected holes are expected to rapidly relax to the HOMO of PBDB-T, and then find electrons at the PBDB-T:Y5 interfaces to recombine. However, we observe that Voc of the ternary solar cell almost linearly decreases with the increasing PBDB-T content (Fig. 3a), and the recombination current in the solar cell gradually increases with the PBDB-T content (ESI-7†). These results indicate that the energy levels of PBDB-T and PM7 are hybridized to form an effective CT state in the ternary blend, which are also observed for other ternary blends reported in literature.50
To elucidate that the energy levels of the PBDB-T and PM7 in the blend are hybridized, we further investigate the charge generation process in the devices based on PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7. We find that the Jsc and EQE of the device based on PBDB-T
PM7. We find that the Jsc and EQE of the device based on PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 (1
PM7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%) are considerably higher than that of the device based on pure PBDB-T or pure PM7 (Fig. S11a and b, ESI†), but the absorption spectra of the two materials are very similar (Fig. S3b†). PL measurements, shown in Fig. S11c (ESI),† suggest that the emissions from PBDB-T and PM7 are significantly quenched, in the blend of PBDB-T:PM7. This suggests that the singlet excited states of PBDB-T and PM7 are hybridized, resulting in the formation of CT states at the interface between the two polymers, facilitating the dissociation of the excitons generated in the blend active layer. Sensitive electroluminescence and EQE measurements (Fig. S11d and e, ESI†) reveal that the absorption and emission spectra of the blend thin film are slightly red-shifted compared to those of the films based on pure PBDB-T or PM7, also confirming that the energy of the excited states, i.e., the energy levels of PBDB-T and PM7 are hybridized in the blend.
1 wt%) are considerably higher than that of the device based on pure PBDB-T or pure PM7 (Fig. S11a and b, ESI†), but the absorption spectra of the two materials are very similar (Fig. S3b†). PL measurements, shown in Fig. S11c (ESI),† suggest that the emissions from PBDB-T and PM7 are significantly quenched, in the blend of PBDB-T:PM7. This suggests that the singlet excited states of PBDB-T and PM7 are hybridized, resulting in the formation of CT states at the interface between the two polymers, facilitating the dissociation of the excitons generated in the blend active layer. Sensitive electroluminescence and EQE measurements (Fig. S11d and e, ESI†) reveal that the absorption and emission spectra of the blend thin film are slightly red-shifted compared to those of the films based on pure PBDB-T or PM7, also confirming that the energy of the excited states, i.e., the energy levels of PBDB-T and PM7 are hybridized in the blend.
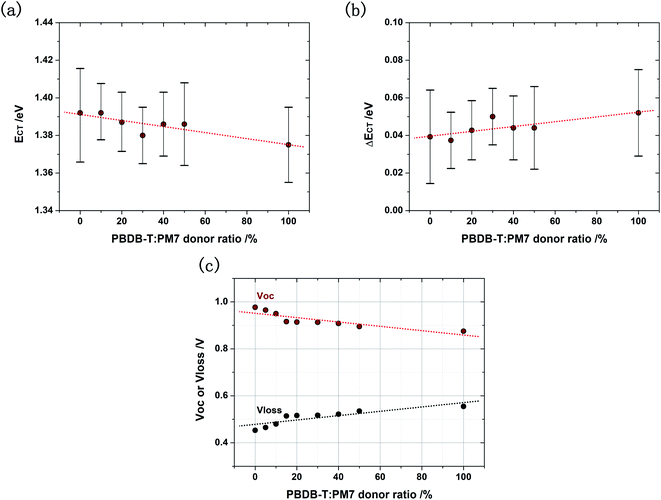
Therefore, we can also use the Marcus theory to evaluate the CT state property in the ternary solar cells (SI-2). We find that ECT is indeed gradually reduced with the increasing PBDB-T content (Fig. 4a), leading to a continuously tuned ΔECT (Fig. 4b). The electronic coupling between the S1,A and CT state is thus reduced.
Inevitably, the Voc of the ternary solar cell reduces with increasing PBDB-T content (Fig. 4c). However, due to the hybridization of the energy levels of PBDB-T and PM7, the Voc does not directly reduce to that of the binary solar cell based on PBDB-T:Y5. Therefore, the low Vloss property of the solar cell based on PM7:Y5 can be well kept in the ternary solar cell, while QE of the solar cell is significantly increased, with the addition of a small amount of PBDB-T. As shown in Fig. 4c, Vloss is about 0.47 V in the ternary solar cell with a PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio of 10%, which is slightly higher than that of the binary solar cell based on PM7:Y5 (0.44 eV), but the Jsc of the ternary solar cell is higher by a factor of 1.30; and the Vloss is increased to 0.50 V for the ternary solar cell with a donor ratio of 20%, while the Jsc is doubled. The gain of using the ternary blend strategy is remarkably high.
PM7 donor ratio of 10%, which is slightly higher than that of the binary solar cell based on PM7:Y5 (0.44 eV), but the Jsc of the ternary solar cell is higher by a factor of 1.30; and the Vloss is increased to 0.50 V for the ternary solar cell with a donor ratio of 20%, while the Jsc is doubled. The gain of using the ternary blend strategy is remarkably high.
The increased Vloss in the ternary solar cell is partially due to the reduced effective ECT, since ECT is the electronic bandgap of the blend active layer.12 Meanwhile, the reduced ECT can lead to increased ΔVnr due to a stronger vibrational coupling between the excited and ground state.17 In addition, the electronic coupling between the S1,A and CT state is reduced due to the reduced ECT: although the reduction in the electronic coupling is needed to prevent the back transfer of the CT state to the S1,A state, the reduced coupling can also lead to a reduced the effective dipole moment for the CT to ground state transition,19 resulting in the increased non-radiative decay rate of CT state, reduced EQEEL, and thus increased ΔVnr in the solar cell. EQEEL measurements (Fig. S8, ESI†) confirm that ΔVnr in the ternary solar cell is indeed higher than that in the binary solar cell based on PM7:Y5, and it gradually increases with the PBDB-T content, as shown in Fig. 5a, and transient photovoltage decay time plotted as a function of bias illumination intensity confirms that the increased ΔVnr is a result of the increased non-radiative decay rate of CT state (Fig. 5b).
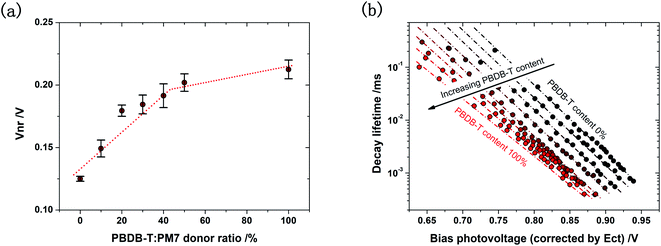 | ||
Fig. 5 (a) ΔVnr of the solar cells based on PBDB-T:PM7:Y5 with different PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratios. The ΔVnr values are calculated from the measured Voc and Voc,rad determined from the sensitive EQE spectra, and they are verified by EQEEL measurements. (b) Transient photovoltage decay lifetime measured with a pulsed LED and a bias illumination at different intensities for the solar cells with different PBDB-T PM7 donor ratios. The ΔVnr values are calculated from the measured Voc and Voc,rad determined from the sensitive EQE spectra, and they are verified by EQEEL measurements. (b) Transient photovoltage decay lifetime measured with a pulsed LED and a bias illumination at different intensities for the solar cells with different PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratios. The x-axis represents the photovoltage of the solar cell generated by the bias illumination. To compare the decay lifetime at the same charge carrier concentration, the x-axis is corrected for the difference in ECT of the solar cells based on different PBDB-T contents. Photovoltage vs. time plots for these solar cells are provided in ESI-9.† PM7 donor ratios. The x-axis represents the photovoltage of the solar cell generated by the bias illumination. To compare the decay lifetime at the same charge carrier concentration, the x-axis is corrected for the difference in ECT of the solar cells based on different PBDB-T contents. Photovoltage vs. time plots for these solar cells are provided in ESI-9.† | ||
3. Conclusion
To conclude, we investigated organic solar cells based on PM7:Y5, in which an extremely low Vloss (0.44 V) was found, and we showed that the low Vloss was a result of a small ΔECT and a low ΔVnr. However, the QE of the solar cell was low, due to the small ΔECT, leading to inefficient dissociation of CT state excitons. To solve this problem, a ternary blend strategy was employed. We demonstrated that the use of PBDB-T with slightly upper laying HOMO, compared to PM7, as a second donor in the blend active layer could effectively lead to a hybridization of the energy levels of PBDB-T and PM7. This resulted in reduced ECT and increased ΔECT in the ternary system, giving rise to a significantly improved CT state dissociation efficiency in the active layer, and thus an increased Jsc of the solar cell. More importantly, the low Vloss property of the PM7:Y5 based blend was very well maintained: Jsc of the ternary solar cell with a PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PM7 donor ratio of 20% was increased by two folds, compared to that of the binary solar cell based on PM7, but Vloss was only increased by 0.06 eV. Thus, we propose that the ternary blend strategy is highly effective for realizing a balance between QE and Vloss in organic solar cells with a small ΔECT. To achieve a high degree of hybridization of the energy levels of the active materials, and the tuning of ΔECT of the ternary blend, the second donor should have different energy levels compared to the primary donor, and the chemical structures of the donor materials should be similar to allow a high degree of intermixing in the ternary blend. This is a newly discovered advantage of the ternary blend strategy, which has not yet been fully explored.
PM7 donor ratio of 20% was increased by two folds, compared to that of the binary solar cell based on PM7, but Vloss was only increased by 0.06 eV. Thus, we propose that the ternary blend strategy is highly effective for realizing a balance between QE and Vloss in organic solar cells with a small ΔECT. To achieve a high degree of hybridization of the energy levels of the active materials, and the tuning of ΔECT of the ternary blend, the second donor should have different energy levels compared to the primary donor, and the chemical structures of the donor materials should be similar to allow a high degree of intermixing in the ternary blend. This is a newly discovered advantage of the ternary blend strategy, which has not yet been fully explored.
4. Experimental
Materials and devices
PEDOT:PSS (CLEVIOS P VP AL 4083) was purchased from Heraeus, PBDB-T, PM7, Y5 and PFN-Br were purchased from Solarmer. The solar cells are based on a standard architecture of ITO/PEDOT:PSS active layer/PFN-Br/Ag. The indium tin oxide (ITO) substrates were sonicated with acetone, isopropanol, and ethanol, and then rinsed with deionized water. PEDOT:PSS interlayers were spin-coated on the clean ITO substrates at a spin-coating speed of 5000 rpm for 60 s, and annealed on a hotplate at 150 °C for 20 min. The thickness of the PEDOT:PSS interlayers was about 30 nm. The organic photoactive layers (100 nm) were spin-coated onto the PEDOT:PSS coated substrate in a glovebox filled with nitrogen, at a speed of 2000 rpm for 30 s, from chloroform solutions with a total concentration of the active materials of 8 mg mL−1 (the donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor weight ratio is kept to 1
acceptor weight ratio is kept to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 for both the binary and the ternary systems). The solutions were stirred at 40 °C for 4 hours, prior to use, and the active layers were annealed at 110 °C for 10 min in the glove box. Then, the PFN–Br interlayers (1 nm) were spin-coated on the active layers at a spin-coating speed of 3000 rpm for 30 s. Construction of the solar cells was finalized by a thermal evaporation of 100 nm Ag electrode in a vacuum chamber at a pressure of 1 × 10−6 Pa. The photoactive area of the solar cells is about 4 mm2.
1.2 for both the binary and the ternary systems). The solutions were stirred at 40 °C for 4 hours, prior to use, and the active layers were annealed at 110 °C for 10 min in the glove box. Then, the PFN–Br interlayers (1 nm) were spin-coated on the active layers at a spin-coating speed of 3000 rpm for 30 s. Construction of the solar cells was finalized by a thermal evaporation of 100 nm Ag electrode in a vacuum chamber at a pressure of 1 × 10−6 Pa. The photoactive area of the solar cells is about 4 mm2.
Characterizations
Details regarding characterization of the materials and devices are provided in ESI (ESI-1).†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is financial supported by the National Natural Science Foundation of China (Grant No. 51973031, 51933001, 52073056), Shanghai Pujiang Program (Grant No. 19PJ1400500), the Natural Science Foundation of Shanghai (Grant No. 19ZR1401400) and the Fundamental Research Funds for the Central Universities (Grant No. 2232021A09).References
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272–275 Search PubMed.
- H. N. Tran, S. Park, F. T. A. Wibowo, N. V. Krishna, J. H. Kang, J. H. Seo, H. Nguyen-Phu, S. Jang and S. Cho, Adv. Sci., 2020, 7, 2002395 Search PubMed.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094–1098 Search PubMed.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 Search PubMed.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 Search PubMed.
- N. Gasparini, A. Salleo, I. McCulloch and D. Baran, Nat. Rev. Mater., 2019, 4, 229–242 Search PubMed.
- P. Bi and X. Hao, Sol. RRL, 2019, 3, 1800263 Search PubMed.
- P. Chao, H. Chen, Y. Zhu, H. Lai, D. Mo, N. Zheng, X. Chang, H. Meng and F. He, Adv. Mater., 2020, 32, 1907059 Search PubMed.
- W. Gao, H. Fu, Y. Li, F. Lin, R. Sun, Z. Wu, X. Wu, C. Zhong, J. Min, J. Luo, H. Y. Woo, Z. Zhu and A. K. -Y. Jen, Adv. Energy Mater., 2020, 2003177 Search PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 Search PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Nat. Mater., 2009, 8, 904–909 Search PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 125204 Search PubMed.
- Z. Tang, J. Wang, A. Melianas, Y. Wu, R. Kroon, W. Li, W. Ma, M. R. Andersson, Z. Ma, W. Cai, W. Tress and O. Inganäs, J. Mater. Chem. A, 2018, 6, 12574–12581 Search PubMed.
- K. Vandewal, K. Tvingstedt and O. Inganäs, in Semiconductors and Semimetals, Elsevier, 2011, vol. 85, pp. 261–295 Search PubMed.
- M. Azzouzi, J. Yan, T. Kirchartz, K. Liu, J. Wang, H. Wu and J. Nelson, Phys. Rev. X, 2018, 8, 031055 Search PubMed.
- D. Qian, Z. Zheng, H. Yao, W. Tress, T. R. Hopper, S. Chen, S. Li, J. Liu, S. Chen, J. Zhang, X.-K. Liu, B. Gao, L. Ouyang, Y. Jin, G. Pozina, I. A. Buyanova, W. M. Chen, O. Inganäs, V. Coropceanu, J.-L. Bredas, H. Yan, J. Hou, F. Zhang, A. A. Bakulin and F. Gao, Nat. Mater., 2018, 17, 703–709 Search PubMed.
- J. Benduhn, K. Tvingstedt, F. Piersimoni, S. Ullbrich, Y. Fan, M. Tropiano, K. A. McGarry, O. Zeika, M. K. Riede, C. J. Douglas, S. Barlow, S. R. Marder, D. Neher, D. Spoltore and K. Vandewal, Nat. Energy, 2017, 2, 17053 Search PubMed.
- K. D. Rosenthal, M. P. Hughes, B. R. Luginbuhl, N. A. Ran, A. Karki, S.-J. Ko, H. Hu, M. Wang, H. Ade and T.-Q. Nguyen, Adv. Energy Mater., 2019, 9, 1901077 Search PubMed.
- F. D. Eisner, M. Azzouzi, Z. Fei, X. Hou, T. D. Anthopoulos, T. J. S. Dennis, M. Heeney and J. Nelson, J. Am. Chem. Soc., 2019, 141, 6362–6374 Search PubMed.
- K. Vandewal, Annu. Rev. Phys. Chem., 2016, 67, 113–133 Search PubMed.
- V. Coropceanu, X.-K. Chen, T. Wang, Z. Zheng and J.-L. Brédas, Nat. Rev. Mater., 2019, 4, 689–707 Search PubMed.
- K. Vandewal, K. Tvingstedt, J. V. Manca and O. Inganäs, IEEE J. Sel. Top. Quantum Electron., 2010, 16, 1676–1684 Search PubMed.
- K. Vandewal, Z. Ma, J. Bergqvist, Z. Tang, E. Wang, P. Henriksson, K. Tvingstedt, M. R. Andersson, F. Zhang and O. Inganäs, Adv. Funct. Mater., 2012, 22, 3480–3490 Search PubMed.
- Z. Ma, W. Sun, S. Himmelberger, K. Vandewal, Z. Tang, J. Bergqvist, A. Salleo, J. W. Andreasen, O. Inganäs, M. R. Andersson, C. Müller, F. Zhang and E. Wang, Energy Environ. Sci., 2013, 7, 361–369 Search PubMed.
- C. Yang, J. Zhang, N. Liang, H. Yao, Z. Wei, C. He, X. Yuan and J. Hou, J. Mater. Chem. A, 2019, 7, 18889–18897 Search PubMed.
- Y. Xie and H. Wu, Materials Today Advances, 2020, 5, 100048 Search PubMed.
- L. Benatto, M. de J. Bassi, L. C. W. de Menezes, L. S. Roman and M. Koehler, J. Mater. Chem. C, 2020, 8, 8755–8769 Search PubMed.
- S. Ullbrich, J. Benduhn, X. Jia, V. C. Nikolis, K. Tvingstedt, F. Piersimoni, S. Roland, Y. Liu, J. Wu, A. Fischer, D. Neher, S. Reineke, D. Spoltore and K. Vandewal, Nat. Mater., 2019, 18, 459–464 Search PubMed.
- H. Zhang, H. Yao, J. Hou, J. Zhu, J. Zhang, W. Li, R. Yu, B. Gao, S. Zhang and J. Hou, Adv. Mater., 2018, 30, 1800613 Search PubMed.
- J. Yuan, Y. Zhang, L. Zhou, C. Zhang, T.-K. Lau, G. Zhang, X. Lu, H.-L. Yip, S. K. So, S. Beaupré, M. Mainville, P. A. Johnson, M. Leclerc, H. Chen, H. Peng, Y. Li and Y. Zou, Adv. Mater., 2019, 31, 1807577 Search PubMed.
- M.-A. Pan, T.-K. Lau, Y. Tang, Y.-C. Wu, T. Liu, K. Li, M.-C. Chen, X. Lu, W. Ma and C. Zhan, J. Mater. Chem. A, 2019, 7, 20713–20722 Search PubMed.
- R. Ma, T. Liu, Z. Luo, Q. Guo, Y. Xiao, Y. Chen, X. Li, S. Luo, X. Lu, M. Zhang, Y. Li and H. Yan, Sci. China: Chem., 2020, 63, 325–330 Search PubMed.
- R. A. Marcus, J. Phys. Chem., 1989, 93, 3078–3086 Search PubMed.
- I. R. Gould, D. Noukakis, L. Gomez-Jahn, R. H. Young, J. L. Goodman and S. Farid, Chem. Phys., 1993, 176, 439–456 Search PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 Search PubMed.
- Z. Tang, Z. Ma, A. Sánchez-Díaz, S. Ullbrich, Y. Liu, B. Siegmund, A. Mischok, K. Leo, M. Campoy-Quiles, W. Li and K. Vandewal, Adv. Mater., 2017, 29, 1702184 Search PubMed.
- K. Vandewal, J. Benduhn and V. C. Nikolis, Sustainable Energy Fuels, 2018, 2, 538–544 Search PubMed.
- U. Rau, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085303 Search PubMed.
- Y. Xu, H. Yao, L. Ma, J. Wang and J. Hou, Rep. Prog. Phys., 2020, 83, 082601 Search PubMed.
- J. Wang, H. Yao, Y. Xu, L. Ma and J. Hou, Mater. Chem. Front., 2021, 5, 709–722 Search PubMed.
- L. A. A. Pettersson, L. S. Roman and O. Inganäs, J. Appl. Phys., 1999, 86, 487–496 Search PubMed.
- G. F. Burkhard, E. T. Hoke, S. R. Scully and M. D. McGehee, Nano Lett., 2009, 9, 4037–4041 Search PubMed.
- J. Vollbrecht, V. V. Brus, S.-J. Ko, J. Lee, A. Karki, D. X. Cao, K. Cho, G. C. Bazan and T.-Q. Nguyen, Adv. Energy Mater., 2019, 9, 1901438 Search PubMed.
- G.-J. A. H. Wetzelaer, N. J. V. der Kaap, L. J. A. Koster and P. W. M. Blom, Adv. Energy Mater., 2013, 3, 1130–1134 Search PubMed.
- L. J. A. Koster, V. D. Mihailetchi and P. W. M. Blom, Appl. Phys. Lett., 2006, 88, 052104 Search PubMed.
- G. Lakhwani, A. Rao and R. H. Friend, Annu. Rev. Phys. Chem., 2014, 65, 557–581 Search PubMed.
- P. Hartnagel and T. Kirchartz, Adv. Theory Simul., 2020, 3, 2000116 Search PubMed.
- A. K. K. Kyaw, D. H. Wang, V. Gupta, W. L. Leong, L. Ke, G. C. Bazan and A. J. Heeger, ACS Nano, 2013, 7, 4569–4577 Search PubMed.
- D. Qian, L. Ye, M. Zhang, Y. Liang, L. Li, Y. Huang, X. Guo, S. Zhang, Z. Tan and J. Hou, Macromolecules, 2012, 45, 9611–9617 Search PubMed.
- W. Tress, B. Beyer, N. Ashari Astani, F. Gao, S. Meloni and U. Rothlisberger, J. Phys. Chem. Lett., 2016, 7, 3936–3944 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta00576f |
| This journal is © The Royal Society of Chemistry 2021 |