Recent advances and prospects of D1:D2:A non-fullerene ternary polymer solar cells
Xingyu
Zhang†
 ,
Qiao
Wang†
,
Wenfei
Shen
,
Qiao
Wang†
,
Wenfei
Shen
 *,
Chenyu
Han
,
Yuying
Shao
,
Laurence A.
Belfiore
and
Jianguo
Tang
*
*,
Chenyu
Han
,
Yuying
Shao
,
Laurence A.
Belfiore
and
Jianguo
Tang
*
Institute of Hybrid Materials, National Center of International Joint Research for Hybrid Materials Technology, National Base of International Science & Technology Cooperation on Hybrid Materials, College of Materials Science and Engineering, Qingdao University, 308 Ningxia Road, Qingdao 266071, China. E-mail: shenwenfei@qdu.edu.cn; jianguotangde@hotmail.com
First published on 4th November 2020
Abstract
Ternary polymer solar cells (PSCs) exhibit broader absorption bands, greater potential in micro-morphology regulation, energy level tuning, and other advantages compared with binary PSCs, and is a facile and efficient approach for further enhancing photovoltaic performances. Because of its much more complicated morphology-forming process, the ternary devices with two donors and one non-fullerene acceptor are difficult to regulate. In contrast, the ternary devices with one donor and two acceptors show greater potential in improving the morphology stability of the active layer film, which is one of the biggest obstacles that hinder the commercialization of PSCs. This review systematically summarizes the principles and functions of the third component of high-performance non-fullerenes ternary PSCs, and outlines the recent advancements of ternary PSCs with two donors and one non-fullerene acceptor, including two polymer donors and small molecular acceptor, all-polymer PSCs, and PSCs with small molecule donors. The relationship between the composition and property was carefully examined and outlined. In addition, the valuable guidelines in developing high-efficiency and high-stability non-fullerene ternary PSCs are demonstrated.
1. Introduction
Given the steady increase in fossil-fuel energy use and the ever-growing global environmental problem, green and sustainable energy options are inevitable.1–5 Thankfully, the sun supplies the earth with 321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024 joules of energy per year, which is just around 10
024 joules of energy per year, which is just around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times greater than the world's population actually absorbs.6 However, finding a way to use the massive energy resource efficiently remains a daunting task. Nowadays, not only has solar energy been the main clean energy source, but it is also the fastest increasing type of green energy.7 The research and engineering of solar cells currently focus primarily on: (i) mature silicone-based solar cells,8 which have sophisticated manufacturing processes, high energy consumption, and high cost. (ii) Thin-film solar cells,9 which have high efficiency and stability, although cadmium is toxic. (iii) Emerging solar cells, such as polymer solar cells (PSCs),10 dye-sensitized solar cells11 and perovskite solar cells.12 Interest in PSCs has been steadily increasing since it was known that polymer solar cells are lightweight, low pollution, low cost and easy to process (via a roll-to-roll printing technology).13–17 The production of a polymer solar cell's efficient photocurrent relies on the dissociation of excitons from the donor and acceptor interfaces of specific electron affinities (and/or potential for ionization).18 Hence, much of the latest work on solar cells focuses on how to boost exciton propagation and dissociation. Studies around the world have focused on achieving better performance of PSCs in the last few years.19–27
000 times greater than the world's population actually absorbs.6 However, finding a way to use the massive energy resource efficiently remains a daunting task. Nowadays, not only has solar energy been the main clean energy source, but it is also the fastest increasing type of green energy.7 The research and engineering of solar cells currently focus primarily on: (i) mature silicone-based solar cells,8 which have sophisticated manufacturing processes, high energy consumption, and high cost. (ii) Thin-film solar cells,9 which have high efficiency and stability, although cadmium is toxic. (iii) Emerging solar cells, such as polymer solar cells (PSCs),10 dye-sensitized solar cells11 and perovskite solar cells.12 Interest in PSCs has been steadily increasing since it was known that polymer solar cells are lightweight, low pollution, low cost and easy to process (via a roll-to-roll printing technology).13–17 The production of a polymer solar cell's efficient photocurrent relies on the dissociation of excitons from the donor and acceptor interfaces of specific electron affinities (and/or potential for ionization).18 Hence, much of the latest work on solar cells focuses on how to boost exciton propagation and dissociation. Studies around the world have focused on achieving better performance of PSCs in the last few years.19–27
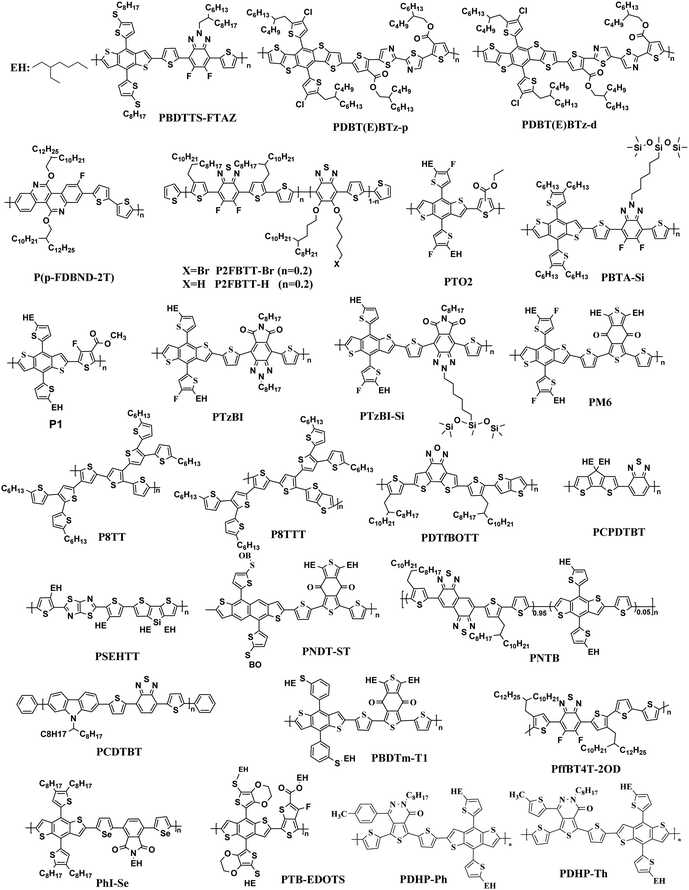
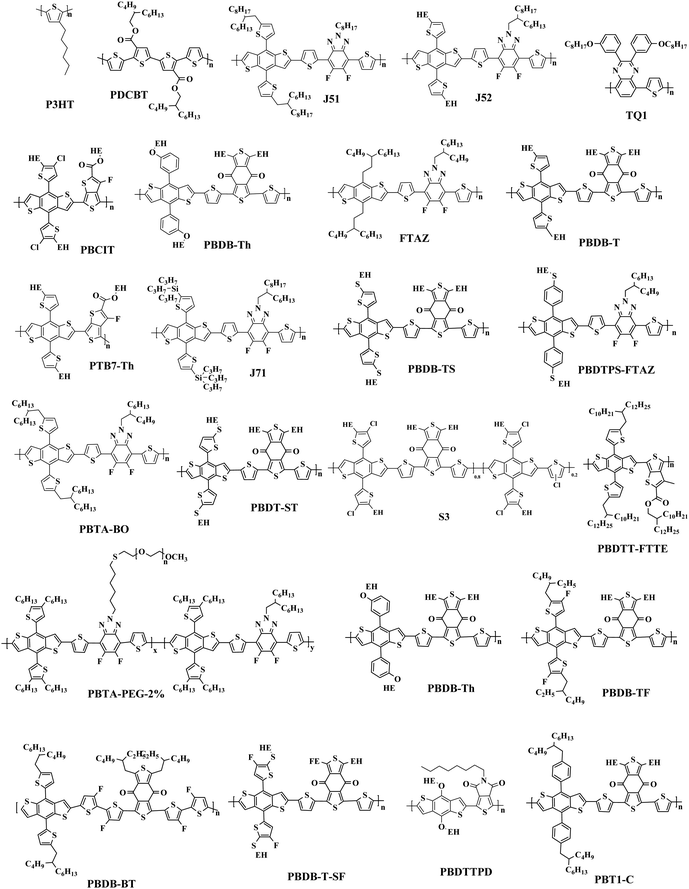
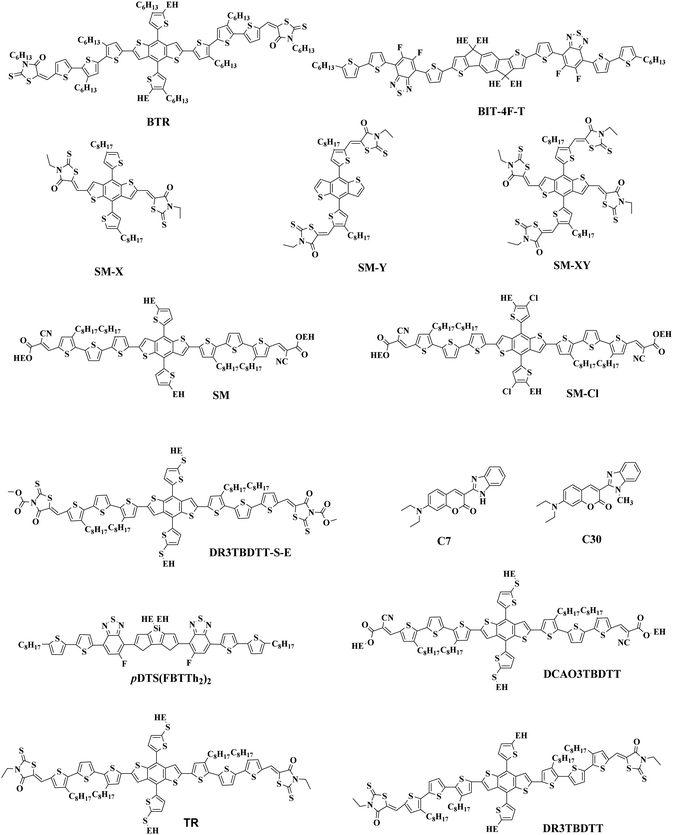
The most effective bulk heterojunction (BHJ) system consists of binary elements, in which a p-type electron donor and an n-type electron acceptor are mixed together to create a bi-continuous interpenetrating network to achieve highly efficient load separation and transportation.28–30 However, due to the narrow absorption of the organic semiconductors, the binary PSCs can only utilize a limited part of the solar spectrum, which limits further improvement of the efficiency of the single-junction PSCs.31–35 Furthermore, the efficiency of single-junction PSCs is over 17%, which is rapidly approaching the theoretical maximum efficiency of PSCs; so it is important to consider other more efficient approaches to further increase the conversion efficiency of PSCs.36 In these cases, various strategies with different principles have been developed. One potential strategy for development is tandem PSCs, where multiple single-junction active layers with different absorption bandgaps are stacked.37–39 Nevertheless, the multiple layers required make the production and optimization of tandem batteries difficult, as opposed to the roll-to-roll process of simple manufacturing. In this case, researchers focused their attention to ternary solar cells, which introduced a second donor or second acceptor to form three-component single active layer PSCs.40–43 The addition of a “third component” is a successful method, which not only has the advantages of a wide range of photon capture (as in tandem solar cells), but also the benefit of easy manufacturing conditions of single junction solar cells.44–47 Simultaneously, the third component plays a crucial role in optimizing the film morphology, facilitating exciton dissociation, and improving charge transport. The key parameters open-circuit voltage (Voc), short-circuit current densities (Jsc), and fill factor (FF) can be optimized to the limit by changing the donor or acceptor doping ratio, choosing the best absorbing complementary material, changing the phase separation and adopting the interface layer.48–50 One of the earliest ternary PSCs was reported by Koppe et al. in 2010.51 The low bandgap polymer PCPDTBT (Fig. 1) is added in P3HT/PCBM (Fig. 2 and 3) binary blends as a third component, which can provide a strong absorption in the near-infrared region, and obtained additional charge transfer to increase Jsc.
 | ||
| Fig. 3 Chemical structures of the small molecular acceptor and polymer acceptor materials used in D1:D2:A NF-PSCs. | ||
In the previous research, due to the broad variety of applications of fullerene materials and weak non-fullerene materials, most ternary PSCs choose fullerene derivatives (such as PC61BM, PC71BM) as an acceptor.52–59 However, such fullerene-based ternary PSCs performance is severely limited because of the weak absorption in the visible region, limited tunability of energy levels, and poor stability issues of fullerene.60,61 That is why the increasing growth of non-fullerene acceptors (NFAs), like both polymeric and small molecular organic acceptors, has occurred. Two different types of NFAs have evolved extremely rapidly, particularly in the last few years. Unlike fullerenes, NFAs have strong light absorption, facile synthesis, and readily tunable frontier orbital energy levels.62,63 So far, various types of outstanding non-fullerene acceptors have been designed and synthesized, such as arylene imide/imide-based,64,65 indacenodithiophene (IDT)-based,66,67 and diketopyrrolopyrrole-based small molecules.68,69 Notably, the best power conversion efficiencies (PCE) of ternary non-fullerene PSCs have recently exceeded 17%, and were fabricated by Ma and co-workers.70 The high efficiency of these ternary PSCs is due to the addition of the third component MeIC in PM6:BTP-4F-12 (Fig. 1 and 3), which not only increases light absorption, but also greatly reduces energy loss (Eloss). As a morphological regulator, MeIC can obtain a more orderly face-on orientation in the blend films, and provide more orderly charge transport pathways. In addition, the bimolecular recombination in the ternary blend films can be suppressed, which will reduce the Eloss in the ternary devices. The Eloss of the optimized ternary PSC is less than that of the two binary PSCs, and the reduced Eloss should help improve the performance of the ternary PSC.
The “third component” can be either an acceptor, donor or solid additives added to the binary donor/acceptor blend system to constitute the D:A1:A2 system or D1:D2:A system.71–73 The additional donor or acceptor component has multiple functions to increase PCE, such as extending the absorption range, preventing charge recombination, and improving the blend morphology. Ternary PSCs with multiple acceptor system are further divided into two types: with and without using the fullerene acceptor. Although fullerene derivatives have serious disadvantages, they also have the advantages of high electron mobility, large electron affinity, and isotropic charge transport. It has been demonstrated that introducing a small portion of NFA to polymer/fullerene blends will boost light absorption, optimizing the morphology and achieving energy level compatibility, resulting in more effective isolation and transition of charges. For example, Xiao and co-workers74 reported a low-bandgap non-fullerene acceptor (COi8DFIC) with strong near-infrared (NIR) absorption and high efficiency. The PTB7-Th (Fig. 2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COi8DFIC (1
COi8DFIC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary blends had a high PCE of 12.16% with a Jsc of 26.12 mA cm−2. When 30 wt% fullerene acceptors (PC71BM) were introduced to the binary system, the PCE increased to 14.08% due to the high Jsc and FF. Recently, ternary PSCs using two non-fullerene receptors also received attention. Jiang et al.75 designed and synthesized a novel electron acceptor ITCN, and employed it in a ternary PSCs based on PBDBT:ITM host blends. The devices with 20 wt% ITCN in PBDB-T (Fig. 2):IT-M system achieved the greatest Jsc of 17.79 mA cm−2 and a higher PCE of 12.06% with higher charge mobility. In addition, Yu et al.76 and Yang et al.77 synthesized two solid additives named SA-1 and PZ1, respectively. The solid additives not only enhanced the π–π stacking, aggregation and charge transport of the active layer, but also enhanced its thermal stability at high temperatures, such that the additive devices achieved a visually improved performance.
1) binary blends had a high PCE of 12.16% with a Jsc of 26.12 mA cm−2. When 30 wt% fullerene acceptors (PC71BM) were introduced to the binary system, the PCE increased to 14.08% due to the high Jsc and FF. Recently, ternary PSCs using two non-fullerene receptors also received attention. Jiang et al.75 designed and synthesized a novel electron acceptor ITCN, and employed it in a ternary PSCs based on PBDBT:ITM host blends. The devices with 20 wt% ITCN in PBDB-T (Fig. 2):IT-M system achieved the greatest Jsc of 17.79 mA cm−2 and a higher PCE of 12.06% with higher charge mobility. In addition, Yu et al.76 and Yang et al.77 synthesized two solid additives named SA-1 and PZ1, respectively. The solid additives not only enhanced the π–π stacking, aggregation and charge transport of the active layer, but also enhanced its thermal stability at high temperatures, such that the additive devices achieved a visually improved performance.
Overall, the ternary non-fullerene PSCs with two donors and one acceptor (the device is abbreviated as D1:D2:A NF-PSCs) often exhibit relatively low PCE due to the non-fullerene materials being very picky regarding the donor acceptor with matched energy levels and compatible ternary system morphology.15,78,79 Nam et al.80 introduced PTB7-Th and ITIC-Th (Fig. 3) in PBDB-T:PC71BM binary blends to construct two types of ternary systems: D1:D2:A and D:A1:A2. After a series of tests, they were surprised to find that both PSCs showed higher PCE than the PBDB-T:PC71BM blends, but the D:A1:A2 ternary system got a more obvious performance optimization due to better blend film morphology. This also confirms the reason for the development of ternary PSCs with two polymer donors being slower than PSCs with two acceptors. Therefore, in the selection of the second donor, the light absorption range and the formation of an optimized interface film morphology can be expanded.
Many previous reports have focused on ternary NF-PSCs and PSCs with two acceptors, but none have summarized the D1:D2:A NF-PSCs. In this review, we summarize the multiple functions of the third component in ternary PSCs, describe the fundamental mechanisms in D1:D2:A NF-PSCs, and list the present progress in D1:D2:A NF-PSCs from the last five years. Furthermore, we look forward to the future development direction of D1:D2:A NF-PSCs.
2. The functions of the third component
In ternary PSCs, the second donor enhances the PCE of PSCs in four main areas: (i) complementary absorption,81,82 (ii) tune the energy levels,83 (iii) optimize the morphology,84 and (iv) enhance the morphology stability. First, the light-harvesting capability of binary blends is regulated by the absorption range. The light absorption range of the active layer can naturally be increased by a large absorber with the third component's complementary absorption spectrum. In addition, the third component can tune the energy levels to achieve highly efficient separation of the charges by means of energy driving forces, cascaded energy level synchronization and/or separate transport networks.85 Furthermore, the third component will promote the creation of a further improved blend morphology that favors the separation of excitons and the transport processes of the devices.86,87 Therefore, the design and selection technique for most second donors is to balance energy levels between the two donors and acceptors to create an energy cascade system. Changing the morphology of the ternary blend film to shape more organized domains would be advantageous to further improve the efficiency of the PSCs.882.1 Complement the absorption
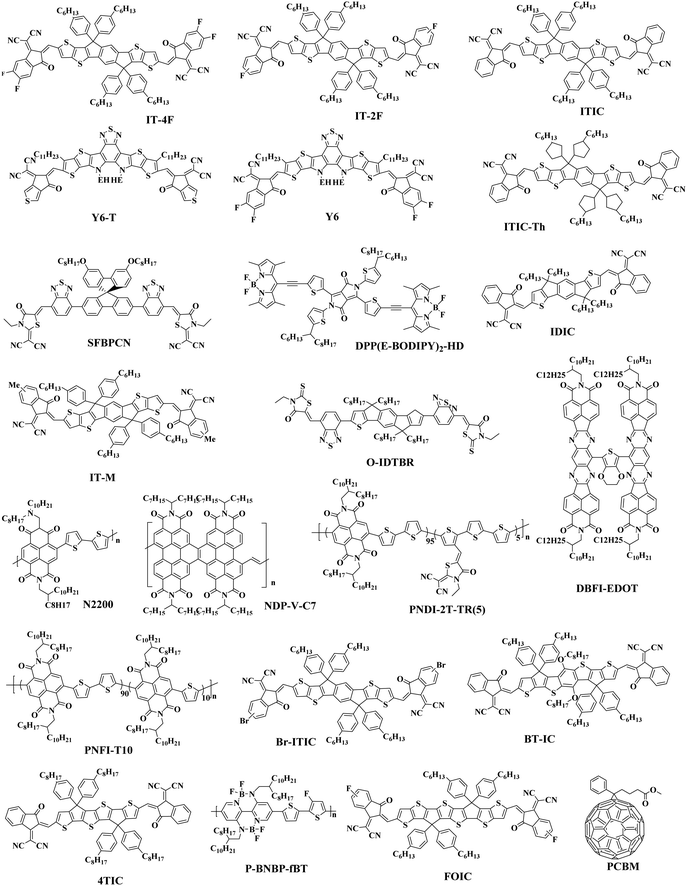
The Jsc is usually proportional to the number of photons absorbed in the active layer. However, the narrow absorption window of the organic semiconductors leads to insufficient photon capture. In these cases, the added second donor achieves a wider light absorption range compared to the binary blends, and increases the value of Jsc, enabling the ternary PSCs to achieve a higher PCE. Therefore, a straightforward design rule is to introduce a third compound whose absorption range is complementary to the host binary blends. Introducing a second donor or NFA into the ternary system always produces a wider absorption, and some ternary systems even achieve an entire visible light absorption. For example, the first ternary synthesized with two polymer donors and a non-fullerene acceptor was manufactured in 2016. Hwang et al.89 used the two polymer donors, PSEHTT (Fig. 1) and PTB7-Th, and a non-fullerene electron acceptor, DBFI-EDOT (Fig. 3). The new ternary PSCs showed a high PCE of 8.52%. In contrast, the PSEHTT:DBFI-EDOT and PBDTT-EFT:DBFI-EDOT binary PSCs only had PCE values of 8.10% and 6.70%, respectively. The EQE spectra (Fig. 4b) show that the photoresponse range is 300–720 nm for PSEHTT and 300–765 nm for PBDTT-FTTE. This largely resulted in the increase of Jsc from 13.82 and 13.99 to 15.67 mA cm−2, with the much broader light harvesting of PBDTT-EFT. Besides, in 2019, Liu and co-workers90 made ternary D:A1:A2 NF-PSCs with PM7:ITC-2Cl as the host blends, and a nonfullerene acceptor named IXIC-4Cl as the second acceptor. Because the IXIC-4Cl has an absorption edge reaching 1000 nm, the ternary blends PSCs had a wider absorption range than the binary system. Fig. 4c and d are normalized UV-Vis absorption spectra of the neat PM7, ITC-2Cl and IXIC-4Cl films, and the absorption spectra of the blend films. Compared with the binary devices, the maximum extinction coefficients of the PM7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITC-2Cl
ITC-2Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IXIC-4Cl (1
IXIC-4Cl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) blend film has a very obvious improvement at 619, 748 and 856 nm. The addition of the second receptor expands the absorption range from 350–820 nm to 350–1000 nm, which is beneficial for a high Jsc of 23.99 mA cm−2. Briefly, the addition of the third component expands the absorption range of the ternary PSCs for sunlight, and increases the PCE of NF-PSCs from 13.72% to 15.37%.
0.5) blend film has a very obvious improvement at 619, 748 and 856 nm. The addition of the second receptor expands the absorption range from 350–820 nm to 350–1000 nm, which is beneficial for a high Jsc of 23.99 mA cm−2. Briefly, the addition of the third component expands the absorption range of the ternary PSCs for sunlight, and increases the PCE of NF-PSCs from 13.72% to 15.37%.
 | ||
| Fig. 4 (a) Current density–voltage characteristics of DBFI-EDOT:PSEHTT and DBFI-EDOT:PBDTT-FTTE blend films, (b) EQE curves of DBFI-EDOT:PSEHTT and DBFI-EDOT:PBDTT-FTTE blend films,89 (c) normalized UV-Vis absorption spectra of neat PM7, ITC-2Cl and IXIC-4Cl films; (d) absorption spectra of the blend films of PM7:ITC-2Cl:IXIC-4Cl with different ITC-2Cl:IXIC-4Cl weight ratios.90 | ||
2.2 Tune the energy levels
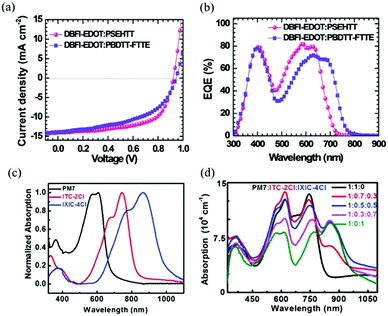
A successful exciton splitting and charging dissociation in the solar cells involves an appropriate energy offset between the donor and acceptor LUMO energy rates. Additionally, the Voc of PSCs is directly proportional to the gap between the donor's highest occupied molecular orbital energy level (HOMO) and the acceptor's lowest unoccupied molecular orbital energy level (LUMO).85 So, the donor–acceptor pair can improve the charging and energy transfer properties at the donor–acceptor interface by regulation of the HOMO–LUMO gap. The terpolymer approach is a simple method that integrates an additional third component, which could achieve a fine tuning of the polymers' energy level location.91–93 Zhang et al.94 used two conjugated polymer donor materials, PTB7-Th and PffBT4T-2OD (Fig. 1), and a non-fullerene acceptor material, IEICO-4F (Fig. 3), to fabricate a series of ternary NF-PSCs. The energy levels of the three optoelectronic materials are shown in Fig. 5b. The HOMO and LUMO values of the second donor PffBT4T-2OD are located between the host donor and the acceptor, respectively, and this cascade energy level structure arrangement is conducive to charge transfer and energy transfer. In addition, the second donor played an important role in the suppressed non-radiative recombination process and morphology optimization. The findings show that the configured PTB7-Th:PffBT4T-2OD:IEICO-4F ternary blend ratio of 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 hit the maximum PCE value of 12.12%, which is around 16% higher than that of the binary systems centered on PTB7-Th:IEICO-4F.
1.5 hit the maximum PCE value of 12.12%, which is around 16% higher than that of the binary systems centered on PTB7-Th:IEICO-4F.
 | ||
| Fig. 5 (a) Normalized absorption spectrum of PffBT4T-2OD, PTB7-Th, and IEICO-4F. (b) Energy levels of PffBT4T-2OD, PTB7-Th, and IEICO-4F.94 | ||
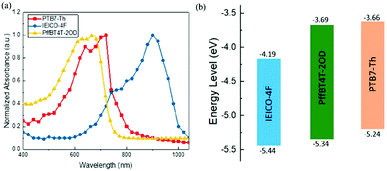
Actually, introducing a third component to form the ternary PSCs to tune the energy levels is simpler and more convenient than side chain engineering. As of now, there are several energy structures in the ternary non-fullerene PSCs that can effectively promote charge transfer. The first one is the cascade energy level, where the HOMO of the second donor is between the HOMO of the host donor and acceptor, and the LUMO of the second donor is also between the LUMO of the host donor and acceptor. It plays a bridging role in affording additional channels to allow for efficient charge transfer of both electrons and holes.95–97 For instance, Liu et al.98 blended PTB7-Th, PffBT4T-2OD and ITIC (Fig. 1 and 2) to fabricate ternary non-fullerene PSCs, which had a cascade energy level alignment, as schematically shown in Fig. 6a. A part of the electrons of PTB7-Th is first transferred to the second donor PffBT4T-2OD, and then the electrons on PffBT4T-2OD are transferred to the acceptor ITIC. Similarly, electron holes are transferred from ITIC via PffBT4T-2OD to PTB7-Th. The optimized ternary NF-PSCs received a higher PCE of 8.22% than the PTB7-Th:ITIC binary device's PCE of 6.48%. The second model is that the LUMO of the second donor is between the LUMO of the host donor and acceptor, yet the two donors have very close HOMO and it has a different transport mechanism. For instance, Li and co-workers99 combined the wide-bandgap polymer donor of PBTA-BO (Fig. 2) with narrow-bandgap polymer PNTB and polymer acceptor N2200 to fabricate the non-fullerene all-polymer PSCs (the structure shown in Fig. 1–3, energy levels shown in Fig. 6b). The electrons were delivered from PBTA-BO to PNTB, and then to N2200. It is worth noting that the majority of holes generated in N2200 may finally be transferred to the second donor PNTB, and then collected by the electrode. In addition, there is another model in which the energy structures are not cascade type. Chen and co-workers100 built polymer:polymer:non-fullerene based ternary-blend PSCs with PBDTPS-FTAZ, P(p-FDBND-2T) and ITIC (the structure shown in Fig. 1–3, energy levels shown in Fig. 6c). The offset of the LUMO energy levels of the two donors is too small to dissociate the excitons. In addition, the HOMO energy level of P(p-FDBND-2T) is lower than the HOMO energy level of PBDTPS-FTAZ, and even ITIC. It results in the transfer of the lower holes from PBDTPS-FTAZ or ITIC to P(p-FDBND-2T). After further research, Li found that the intense FRET energy transfer process of the ternary blend would be the main reason for the high photovoltaic performance.
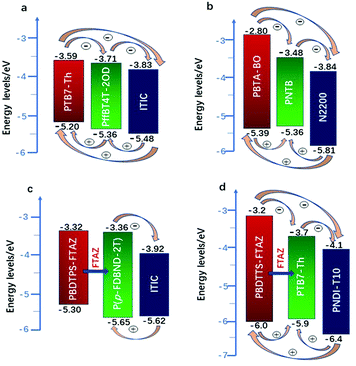 | ||
| Fig. 6 Energy levels of three types of PSCs: (a) is the PTB7-Th:PffBT4T-2OD:ITIC;98 (b) PBTA-BO:PNTB:N2200;99 (c) PBDTPS-FTAZ:P(P-FDBND-2T:ITIC;100 (d) PBDTTS-FTAZ:PTB7-Th:PNDI-T10.101 | ||
2.3 Morphology regulation of the blend film
Apart from the favourable influencing factors of efficient light absorption ability and tuning energy levels, as mentioned above, the BHJ blend morphology effect (including the domain sizes, crystallinity, and molecular stacking and orientation) of the BHJ blends plays a crucial role in the performance of the ternary PSCs devices, especially in improving the free charge carrier generation, charge transport properties, and FF values.85,102 The tuned crystallinity and suitable miscibility formed small domain sizes, which produced the desired nanoscale blend morphology with balanced hole and electron mobilities, and reduced the bimolecular recombination. Compared with the binary PSCs, the ternary system with two polymer martials usually observed a larger phase separation due to the significantly reduced entropic contribution by the two polymer chains that energetically disfavours mixing.103 Therefore, the interface morphology of the ternary NF-PSCs can be improved by using two donor materials with further improved compatibility and crystallinity or some other means, such as varying the processing solvent, thermal annealing conditions, and even modifying the chemical structures and molecular weight of the polymers. Experiments by Li Kim and co-workers103 proved that a smaller domain sizes contributed to improvements in both exciton dissociation and charge transport abilities, leading to an enhancement of the PCE. It is worth noting that the excessive aggregation of molecules might lead to a low Jsc and FF. To promote the phase separation in the non-fullerene PSCs, using the second donor with high crystallinity seemed to be useful. Unlike the spherical structure of the fullerene acceptor being easy to separate, the non-fullerene acceptor and the donor have a planar configuration, which makes them have good compatibility. The effect of the morphology of the ternary blend film properties has been fully studied in fullerene systems, such that it guides the improvement of the morphology in non-fullerene systems. For example, Liu et al.98 researched the morphological features of PTB7-Th:PffBT4T-2OD:ITIC ternary PSCs with different amounts of PffBT4T-2OD. The TEM images of the ternary blends, in comparison to the binary blends, are shown in Fig. 7. It can be seen from Fig. 7a and b that the PffB4T-2OD:ITIC binary blend exhibits a more obvious phase separation than the PtB7-Th:ITIC binary blend. When 20 wt% of PffB4T-2OD is added, the ternary hybrid film shows the interpenetrating network, as shown in Fig. 7c. This morphological transition is attributed to the low compatibility of the two polymer donors, which results in individual fibrillar PffBT4T-2OD-rich and amorphous PTB7-Th-rich phases, and facilitates exciton dissociation and transportation of charges. However, when the amount of the second donor increased to 40%, the casting sizes of the ternary blend films becomes remarkably large, as shown in Fig. 7d. Such findings suggest that the morphological modification of the ternary blend is primarily based on the equilibrium between the PtB7-Th and PffBT-4T-2OD crystallinity and stability. Thanks to the optimized film morphology, the ternary PSCs containing 20 wt% PffBT4T-2OD exhibit an impressive PCE of 8.22% with a Jsc of 15.36 mA cm−2, a Voc of 0.84 V, and a high FF of 62.6%.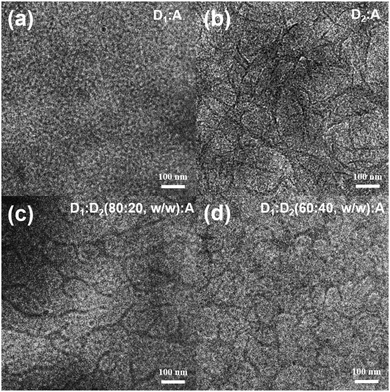 | ||
| Fig. 7 TEM images of (a and b) PTB7-Th:ITIC and PffBT4T-2OD:ITIC binary blend films and (c and d) PTB7-Th:PffBT4T-2OD:ITIC ternary blend films.98 | ||
Because of the high crystallinity of the blend material, there is often so much phase separation in some binary or ternary systems with limited molecular structures, so the function of the third variable in changing the morphology is especially significant. For example, Zhong and co-workers104 researched a ternary SOC with two polymer donors J51,PTB7-Th and a non-fullerene acceptor (ITIC). RSoXs explored the phase separation in the blend films, with findings shown in Fig. 8. The J51:ITIC as-cast blends displayed a strong combination, exhibiting a wide hump at 0.004–0.02 A−1 (30–150 nm). The annealed PTB7-Th ternary blends of 20 per cent displayed a significantly lower diffraction bump at 0.006–0.02 A−1 (30–100 nm). The larger phase separation of the binary blend membrane is because ITIC shows higher crystallinity after annealing. After adding PTB7-Th, which is compatible with ITIC, the phase separation of the ternary membrane is well balanced for better controlling the morphology of the film. He reported that the synergistic effects of enhanced light absorption, more balanced hole/electron stability, energy transfer between J51 and PTB7-Th, and improved morphology resulted in the enhanced Jsc being highly successful. The ternary devices with 10% PTB7-Th achieved a peak PCE of 9.7% and a higher Jsc of 17.75 mA cm−2.
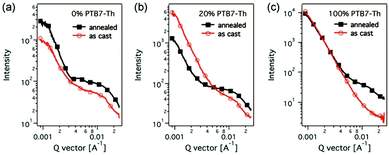 | ||
| Fig. 8 RSoXs of the blend films from different photon energies: (a) J51:ITIC binary as-cast film and annealed film, (b) J51:PTB7-Th:ITIC ternary as-cast film and annealed film, (c) PTB7-Th:ITIC binary as-cast film and annealed film.104 | ||
Adding small amounts of small-molecule additives into the casting solution prior to processing has proven to be a simple and effective way to further improve the nanoscale phase separation.105,106 Adil and co-workers107 reported a ternary strategy was used to effectively modify the phase separation between the J71:ITIC (Fig. 2 and 3) blend by incorporating tetraphenylethylene (TPE), a 3D aggregation-induced emission (AIE) small molecular material. AIE is a photophysical process in which the formed aggregates cause light emitting non-luminescent agents. This result is just the reverse of the result of quenching (ACQ) caused by aggregation. The added TPE plays a role in improving the load-bearing capacity and optimizing the morphology of the three-component devices, resulting in increased FF. The ternary system with 15 wt% TPE resulted in a 21.23% improvement in the PCE relative to the binary systems, delivering an impressive PCE of 12.16%. A more recent publication by Zhou et al.101 was constructed high-performance ternary all-polymer PSCs using TQ1 (Fig. 2) and PCE10 as the two polymer donors and PNDI-T10 as the non-fullerene acceptor. The ternary unit (the ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1) displayed a PCE of 4.08% after thermal annealing, leading to an improvement of 12.08% relative to the pure devices. This suggests that it is possible to produce a more effective and thermal stable all-polymer PSCs in a ternary blend using materials with excellent pristine performance together with another exhibiting increased performance under thermal annealing. In general, an efficient approach is to pick acceptable crystallinity and compatible donor materials according to the morphology of various donor–acceptor binary PSCs blend films.
1:1) displayed a PCE of 4.08% after thermal annealing, leading to an improvement of 12.08% relative to the pure devices. This suggests that it is possible to produce a more effective and thermal stable all-polymer PSCs in a ternary blend using materials with excellent pristine performance together with another exhibiting increased performance under thermal annealing. In general, an efficient approach is to pick acceptable crystallinity and compatible donor materials according to the morphology of various donor–acceptor binary PSCs blend films.
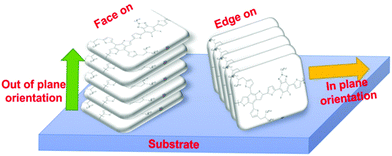
Furthermore, the molecular packing structure and molecular orientation are another aspect to be put under consideration. A proper intermolecular π–π stacking not only can construct percolate and continuous charge-transporting channels, but also obtain the best hole conductivity. In general, the active layers have two molecular orientation methods – face-on and edge-on packing (Fig. 9). When the molecules take the face-on orientation, the stacking of the π-surface will form a vertical electron transport channel, which is beneficial to charges being transported to the electrodes. When the molecules take the edge-on orientation, the charge transport through the main chains needs horizontal electron transport, which makes charge collection difficult.103,108,109 Very recently, a number of research groups have reported that a mixed face-on and edge-on orientation can enable the establishment of 3D charge pathways, which is largely responsible for the enhanced charge transport.110–112 To further improve the membrane morphology, researchers have found many effective methods, such as using additives, thermal annealing and solvent annealing. Briefly, the ternary blend film with second donor to form proper molecular packing and 3D charge pathways is the key to improving the PCE of ternary devices.
2.4 Enhancing the morphology stability of active layer
As has been widely studied, morphology degradation is one of the biggest problems for the stability of PSCs devices. An example of this is when the donor and acceptor materials intend to further aggregate to bigger domain sizes compared to the ideal domain sizes of 20 nm because of the relative molecular interaction differences. Compared with the fullerene-based devices, the non-fullerene small molecular acceptor-based devices show better molecular compatibility. However, they need further improvement to satisfy the requirements for commercialization. The strategies for improving the morphology stability of the active layer include improving the molecular compatibility, improving the Tg of film,113 and forming crosslinkable active layers.114 An emerging and effective strategy for improving the morphology strategy is the ternary structure, and it is reasonable in thermodynamics theory with a higher mixing entropy from adding the third component. Huang et al.53 utilized a nematic liquid-crystalline small molecule donor as the additive to form ternary devices. The addition of BTR is beneficial for improving the light harvesting, charge separation, transport, extraction and morphology stability of the ternary devices. Cui et al.115 reported a ternary PSCs by incorporating 20% IBC-F as the second acceptor. As a result, apart from the improved efficiency, the thermal and photoinduced stability are improved greatly over the binary devices. Ade et al.60 also obtained similar results in the PTB7-Th:IEICO-4F:PC71BM ternary devices. By systematically investigating the hypomiscibility of materials, this work demonstrates the structure–function relationships of the thermodynamics, morphology, and photovoltaic performance. This work finally carries out a rational strategy to stable morphology: the third component needs to have miscibility in the donor polymer at or above the percolation threshold, yet also needs to be partly miscible with the crystallizable acceptor (Fig. 10).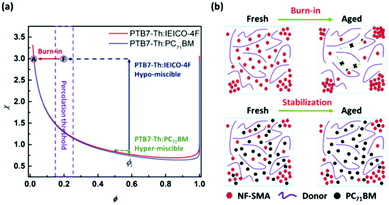 | ||
| Fig. 10 (a) Diagrams of miscible and hypermiscible χ–φ phase diagrams corresponding to PTB7-Th:IEICO-4F and PTB7-Th:PC71BM, respectively. (b) Corresponding to the aging degradation of the PTB7-Th:IEICO-4F system, and the morphological evolution of stable percolation by incorporating PC71BM.60 | ||
In addition to incorporating the small molecular material as the third component, researchers improved the morphology stability by the third polymer component. For instance, incorporating the polymer acceptor N2200 realized the enhancement of the device stability,116 and incorporating the second polymer donor enhanced the stability of the devices.117,118 Obviously, it is more difficult to regulate the film morphology with the third polymer component because of the higher requirements on the compatibility and aggregations. Conversely, it is more effective for improving the morphology stability with the third polymer component because of the stronger molecular interactions. Besides, the improvement in the working mechanism should be more deeply studied to further push the application of the ternary strategy on improving the morphology stability.
3. Fundamental mechanisms in D1:D2:A ternary PSCs
The ternary devices with two donors and binary devices have a great difference in their charge transfer and transport properties, which is not just a simple superposition of charge transfer and transport properties of the individual phases. It is highly dependent on the energy levels, the bandgap, the location in the active layer of the third component, and the microstructure of the ternary blends. There are four categories in the ternary PSCs: charge transfer,119 energy transfer,120 parallel-like,121 and alloy model.122 These four working mechanisms seem to be independent, but in many cases, they are intertwined. For example, the PTB7-Th:PBDTTS-FTAZ:PNDI-T10 ternary NF-PSCs system fabricated by Li et al.78 has two mechanisms: charge transfer and energy transfer (Fig. 6d).3.1 Charge transfer
In the charge transfer mechanism (Fig. 11a) dominated cases, the HOMO and LUMO energy levels of the third component should form the cascade energy level alignment. In general, both donors can directly contribute to generating free charge carriers upon interfacing with the acceptor. The electrons in the host donor may transfer to the acceptor or the second donor, while all holes in the second donor will likely transfer to the host donor with a higher HOMO level before extraction. The Voc of the charge transfer mechanism is pinned by the HOMO level of the two donor materials. Thus, the Voc of such systems is higher than those of the binary blend devices. The third component molecule should be located at the interface between that of the host donor and the acceptor since it depends on the host D:A system to form the percolating pathways for the charge transfer and transport. The holes or electrons generated in the third component can only be effectively captured by the corresponding electrode through the dominant charge carrier transport channels when the third component is located at the interface between the donor and the acceptor.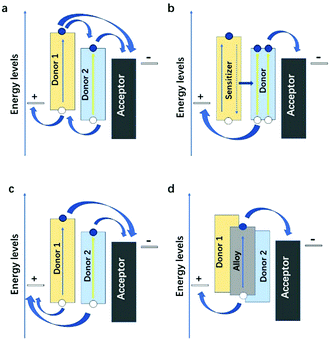 | ||
| Fig. 11 Four fundamentally different mechanisms in ternary PSCs: charge transfer, energy transfer, parallel-linkage, and alloy. | ||
The calculation of the photoluminescence (PL) is a convenient method for measuring the charge transfer or energy transfer between various materials. Generally, if there is energy transfer between two bandgap donors with similar quantum yields of different donors, the emission intensity of the donor with the lower bandgap increases, while the emission intensity of the other donor decreases. In addition, if a charge transfer occurs between two donors, the emission intensity of one donor will be quenched without increasing the emission intensity of the other donor.
One ternary PSCs of this mechanism was reported by Xu and co-workers,123 used P3HT:PCBTDPP:PC61BM fullerene system. They found the electron in P3HT can be either directly transferred to PC61BM or transferred from P3HT to PCBTDPP via FRET. In addition Yi et al.124 reported two ternary NF-PSCs, among them the system used P3HT as the second donor via charge transfer achieved 6.92% PCE. By introduced 5 wt% P3HT into PBDB-T:ITIC binary blends constructed a cascade energy levels alignment, and charge transfer occurs between P3HT and PBDB-T. Fig. 12c is PL spectra of PBDB-T:P3HT blended films. The 636 nm and 688 nm are PL emission peak of PDCBT (Fig. 2) and PBDB-T respectively. Because of the slightly increases of the PL emission peak at 688 nm (P3HT) and the PL emission peak at 688 nm (PBDB-T) gradually decreases with the P3HT content in the blend, they believed between PBDB-T and P3HT should occur charge transfer.
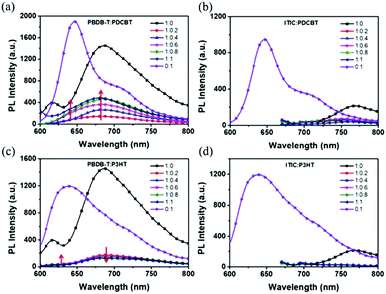 | ||
| Fig. 12 PL spectra of (a) PBDB-T:PDCBT, (b) ITIC:PDCBT, (c) PBDB-T:P3HT and (d) ITIC:P3HT blends.124 | ||
3.2 Energy transfer
For situations where the process of energy transfer (Fig. 11b) dominates, the excited second donor does not specifically produce free, but passes energy by Dexter or Förster energy transfer (FRET) pathways to the host donor or acceptor. This requires the emission of the sensitizer, and the absorption of the donor or acceptor must substantially overlap. Notably, the process of charging and transmitting energy typically happens in the same ternary devices, which can lead to further improved Jsc absorption and more effective charging. In the ternary PSCs, the “energy donor” or the “energy accepter” may be the third component. When the third component only became the “energy donor”, all holes are only formed only in the host donor, and the third component operates just as a solar absorber.125 Certainly, the energy transfer can also occur between two acceptors.Another ternary PSCs reported by Yi et al.124 was PBDB-T:PDCBT:ITIC. Energy transfer emerges between PBDB-T and PDCBT, and the energy donor (PDCBT) absorbs more photons and produces more excitons. Fig. 12a shows the PL spectra of their blended films. The 647 nm and 688 nm features are the PL emission peaks of PDCBT and PBDB-T, respectively. As the PDCBT doping ratio increases slowly, the PL emission peak at 647 nm (PDCBT) and 688 nm (PBDB-T) gradually increase. This finding suggests that the PBDB-T absorbed the PL emission of PDCBT, so there is a transition of Dexter energy from PDCBT to PBDB-T. Additionally, in the PSCs blends, the transition of the charge and energy reflect the opposing processes.
3.3 Parallel-linkage model
In a parallel-linkage model (Fig. 11c), the third component forms its own independent hole-transport network. After the third component has been inserted, an independent charging transfer network is created between the two donors or acceptors. Each donor polymer generates excitons independently, and migrates to the respective polymer/acceptor interface, and then dissociates into free electrons and holes. This model often appears in ternary PSCs containing two polymer donors or acceptors due to the weak compatibility of the two polymers. In this particular case, the photocurrent is equal to the sum of those of the individual sub-blends. A ternary solar cell's Voc value is between the Voc values of the two different binary cells, and is not constant, varying with the ternary mixture composition. The benefit of the parallel-like cells is that solar cells with different band gaps can use either two or more polymers, regardless of their HOMO or LUMO energy levels. This parallel linkage mechanism is very similar to tandem PSCs. The transportation ability of both polymer donors is a performance-influencing factor.In 2012, Yang et al.121 first reported two parallel-linkage ternary PSCs: TAZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTBT
DTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (0.5
PCBM (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and DTffBT
1) and DTffBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTPyT
DTPyT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (0.5
PCBM (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As shown in Fig. 13a and b, the absorption spectra of these parallel-linkage PSCs are the linear summation of the spectra of their two binary blend devices. In addition, the EQE spectra are shown in Fig. 13c and d and the characteristic J–V curves are shown in Fig. 13e and f. The EQE spectra of the ternary devices are exactly the sum of the measurements of the binary devices, and the short-wavelength spectrum and the Jsc of the ternary devices are nearly equal to the average of those for the two PSCs. The ternary devices’ Voc value is between the two binary systems, and the Jsc and PEC are nearly doubled.
1). As shown in Fig. 13a and b, the absorption spectra of these parallel-linkage PSCs are the linear summation of the spectra of their two binary blend devices. In addition, the EQE spectra are shown in Fig. 13c and d and the characteristic J–V curves are shown in Fig. 13e and f. The EQE spectra of the ternary devices are exactly the sum of the measurements of the binary devices, and the short-wavelength spectrum and the Jsc of the ternary devices are nearly equal to the average of those for the two PSCs. The ternary devices’ Voc value is between the two binary systems, and the Jsc and PEC are nearly doubled.
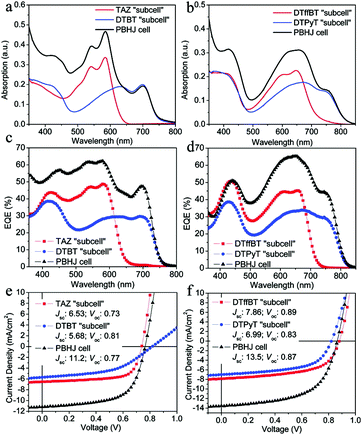 | ||
| Fig. 13 (a and b) Absorption spectra of the ternary and binary devices based on TAZ/DTBT and DTffBT/DTPyT. (c and d) EQEs of the ternary and binary devices based on TAZ/DTBT and DTffBT/DTPyT. (e and f) Characteristic J–V curves of the ternary and binary devices based on TAZ/DTBT and DTffBT/DTPyT.121 | ||
In 2015, Zhang et al.73 fabricated a series of ternary PSCs with PBDT-TS1:PTB7:PC71BM as the active layers. After the study, it was found that there is very little charge transfer between PTB7 and PBDT-TS1, and the two donors work independently with PC71BM as sub-cells, forming a parallel model. The highest PCE of ternary PSCs with 80 wt% PBDT-TS1 added to the donor was 7.91%. Compared with PBDT-TS1 or PTB7 as the best binary PSCs, the PCE value improved by 12.8% or 28.2%. Additionally, Zhang and co-workers126 introduced PhI-Se (Fig. 1) as the second guest of the polymer donor to the PM6:Y6 host blend films to enhance the overall absorption of solar light by 300–600 nm, and concurrently increase the output of the parallel ternary PSCs with Jsc and Voc. The weak compatibility of the two polymer donors determines that the ternary PSCs form a parallel structure. Fig. 14 demonstrates the PM6, Y6, and PhI-Se phases of the aggregates and networks in the binary and ternary blend films. On the PM6:Y6 binary mixture, electrons are transferred from PM6 to Y6, and the holes are transferred from Y6 to PM6. In the parallel model formed by PM6:PhI-Se:Y6, charge separation will occur between PM6 and Y6 and between PhI-Se and Y6, respectively. The ternary NF-PSCs with 15 wt% PhI-Se achieves an ultra-high PCE of 17.2% with a Voc of 0.848 V, a Jsc of 24.8 mA cm−2 and an FF of 72.1%.
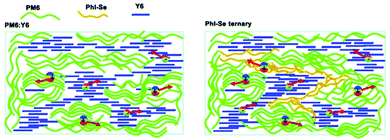 | ||
| Fig. 14 Aggregates and networks of the PM6, Y6, and PhI-Se phases in the binary and ternary blend films.126 | ||
3.4 Alloy model
In some parallel-linkage situations, the active layers can have internal electron and hole recombination between the two donors, resulting in energy loss attributable to emissions. The alloy model, where two donors or two acceptors form an electronic alloy with the average highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels, can also describe this working mechanism. If the two donors have good compatibility, the two donors will enter a new charge transfer state (CT state) through electronic coupling (Fig. 11d). The alloy system's CT state energy is a feature of the blend structure, and follows the Voc change. Two alloy donors maintain their respective molecular properties, where the amounts of HOMO and LUMO are dependent on the composition of the third component in the ternary blends.87 In addition, energy transfer also possibly occurs in the acceptor alloy due to the good compatibility between the two materials with a good spectral overlapping, which complicates the mechanisms of the photovoltaic processes.In 2015, Zhang et al.127 first reported the ternary PSCs of the alloy model with PTB7-Th and p-DTS(FBTTH2)2 (Fig. 13) as the donors, and PC71BM (Fig. 3) as the fullerene acceptor. The two-dimensional GIWAXS patterns of the active layer are shown in Fig. 15. Where the p-DTS (FBTTH2) 2 weight ratio is more than 15 wt%, there are strong lamellar (100), (200) and even (300) diffraction peaks. However, at a ratio of less than 15 wt%, there is no (100) peak, which indicates that p-DTS(FBTTH2)2 can be mixed into the host polymer donor. In addition, it is clear from the Differential Calorimetry Scanning (DSC) results (Fig. 15f) that there is no endothermic peak of p-DTS(FBTTH2)2 with a weight ratio of 15% p-DTS(FBTTH2)2. When adding 20% p-DTS(FBTTH2)2 weight ratios, the blend films occur the p-DTS(FBTTH2)2 endothermic peak. These results mean the two donors exist in alloy form.
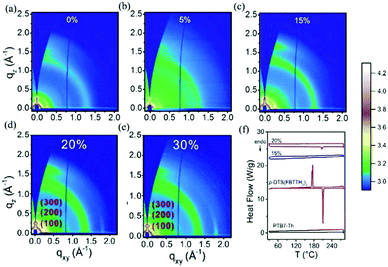 | ||
| Fig. 15 (a–e) Two-dimensional GIWAXS patterns of the ternary blend films with different weight ratios of p-DTS(FBTTH2)2. (f) From top to bottom are PTB7-Th, p-DTS (FBTTH2)2, PTB7-Th: p-TS (FBTTH2)2, and PTB7-Th:p-DTS (FBTTH2)2 DSC curve.127 | ||
Guo et al.122 found an alloy mechanism NF-PSCs PBDB-T:PBDTTPD:ITIC (Fig. 2 and 3). The two donors form an alloy phase because of the similarities between the lamellar structure of both PBDB-T and PBDTTPD. The formation of the alloy phase changed the ternary energy state of the blends, resulting in a monotonous change of Voc. The ternary blend films have been shown to exhibit a synergistic enhancement of exciton generation/dissociation levels, and an increase in electron and hole mobility. When adding 10 wt% PBDTTPD in the PBDB-T:ITIC blends, the ternary PSCs achieved a high PCE of 9.36% with a Jsc of 15.75 mA cm−2, a Voc of 0.80 V, and a FF of 66.03%.
4. Different types of D1:D2:A NF-PSCs
We reviewed the PSCs of the D1:D2:A system in the past five years, found that most ternary NF-PSCs adopt a combination of two polymer donors and small molecule non-fullerene acceptor, and also have a lot of all-polymer PSCs and a small amount of other systems containing small molecule donors. Details of the NF-PSCs parameters of the D1:D2:A systems from the last five years are summarized in Table 1. The chemical structure of the polymer donors, small molecular donors and non-fullerene acceptors are shown in Fig. 1, 2 and 3, respectively. Based on the types of electron donors and electron acceptors, the ternary NF-PSCs may be classified into five types, including polymer donor/polymer donor/polymer acceptor blend (PD1/PD2/PA or all-polymer), polymer donor/polymer donor/small molecular acceptor blend (PD1/PD2/SMA), polymer donor/small molecular donor/small molecular acceptor blend (PD1/SMD2/SMA), small molecular donor/small molecular donor/polymer acceptor blend (SMD1/SMD2/PA), and small molecular donor/small molecular donor/small molecular acceptor blend (SMD1/SMD2/SMA or all-SM).| Types | Binary blend (donor:acceptor) | The second donor | Weight ratio | J sc [mA cm−2] | V oc [V] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| PD1/PD2/SMA | FTAZ:IT-M | PBDB-T | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.1 | 0.95 | 73.6 | 13.2 | 117 |
| PSEHTT:DBFI-EDOT | PBDTT-FTTE | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:2 1:2 |
15.67 | 0.91 | 60.0 | 8.52 | 89 | |
| PM6:Br-ITIC | J71 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.39 | 0.93 | 78.4 | 14.13 | 128 | |
| J51:ITIC | PDBD-T | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.47 | 0.89 | 64.0 | 8.75 | 97 | |
| J51:ITIC | PTB7-Th | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.75 | 0.81 | 67.82 | 9.7 | 104 | |
| J51:BT-IC | PTB7-Th | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.55 | 0.80 | 67.0 | 10.32 | 129 | |
| PBDB-T:IEICO-4F | PTB7-Th | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24.14 | 0.74 | 65.03 | 11.62 | 96 | |
| J71:IT-2F | PBT1-C | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.44 | 0.88 | 75.53 | 12.26 | 139 | |
| PBDT-ST:Y6-T | PNDT-ST | 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
24.04 | 0.91 | 68.30 | 16.57 | 140 | |
| PTB-EDOTS:ITIC-Th | J71 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.02 | 0.9 | 75.60 | 12.26 | 138 | |
| PBDB-T:ITIC | PDTfBO-TT | 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.98 | 0.9 | 77.33 | 12.70 | 143 | |
| PBDB-T:IT-M | PDTfBO-TT | 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.42 | 0.94 | 76.86 | 13.52 | 143 | |
| PBDB-T:ITIC | PBDTTPD | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.75 | 0.90 | 66.03 | 9.36 | 122 | |
| PBDB-T:ITIC | PBDB-Th | 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.95 | 0.92 | 68.40 | 10.68 | 144 | |
| PBDB-T:ITIC | PDCBT | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.38 | 0.90 | 66.27 | 10.97 | 124 | |
| PBDB-T:ITIC | P3HT | 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.11 | 0.82 | 56.12 | 6.92 | 124 | |
| PBDB-T:IT-M | PDCBT | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.97 | 0.93 | 66.7 | 11.2 | 142 | |
| PBDB-T:SFBRCN | PTB7-Th | 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
17.86 | 0.93 | 73.9 | 12.27 | 141 | |
| PBDB-TS:ITIC | PBDB-BT | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
15.92 | 0.94 | 68.3 | 10.26 | 146 | |
| PTO2:IT-4F | PBDB-TF | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22.0 | 0.91 | 0.74 | 14.8 | 145 | |
| PBDTPS-FTAZ:ITIC | P(p-FDBND-2T) | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.35 1.35 |
18.78 | 0.91 | 0.67 | 11.46 | 100 | |
| PBDB-T-SF:IT-4F | PDBT(E)BTz-p | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05 |
19.34 | 0.86 | 72.5 | 12.5 | 147 | |
| PBDB-T-SF:IT-4F | PDBT(E)BTz-d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05 |
19.46 | 0.86 | 74.9 | 13.4 | 147 | |
| PTB7-Th:ITIC | PCDTBT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
16.71 | 0.80 | 55.91 | 7.75 | 130 | |
| PTB7-Th:ITIC | PffBT4T-2OD | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
15.36 | 0.84 | 62.62 | 8.22 | 98 | |
| PTB7-Th:ITIC | P8TTT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.93 | 0.88 | 67.0 | 10.1 | 131 | |
| PTB7-Th:ITIC | P8TT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.01 | 0.88 | 63.1 | 9.45 | 131 | |
| PTB7-Th:4TIC | Ppor-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
18.52 | 0.78 | 62.40 | 9.01 | 132 | |
| PTB7-Th:ITIC | Ppor-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
15.34 | 0.82 | 57.35 | 7.21 | 132 | |
| PTB7-Th:IEICO-4F | J52 | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
24.1 | 0.73 | 58.9 | 109 | 133 | |
| PTB7-Th:IEICO-4F | P2FBTT-Br | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20.4 | 0.71 | 67.5 | 9.8 | 134 | |
| PTB7-Th:IEICO-4F | P2FBTT-H | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21.1 | 0.71 | 69.8 | 10.5 | 134 | |
| PTB7-Th:IEICO-4F | P1 | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
25.11 | 0.74 | 65.18 | 12.11 | 135 | |
| PTB7-Th:FOIC | PBDB-T | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25.04 | 0.73 | 65.78 | 12.02 | 136 | |
| PTB7-Th:FOIC | PBDTm-T1 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
23.07 | 0.76 | 73.5 | 13.8 | 137 | |
| PTB7-Th:O-IDTBR | PBDB-T | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
17.07 | 1.02 | 66.52 | 11.58 | 118 | |
| PM6:Y6 | J71 | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
25.55 | 0.85 | 76.0 | 16.5 | 150 | |
| PM6:Y6 | S3 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
25.86 | 0.86 | 79.17 | 17.53 | 152 | |
| PM6:Y6 | PBDB-T | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
24.87 | 0.811 | 71.0 | 14.6 | 151 | |
| PM6:Y6 | PDHP-Th | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
26.60 | 0.850 | 71.7 | 16.8 | 151 | |
| PM6:Y6 | PDHP-Ph | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
26.58 | 0.823 | 68.2 | 15.4 | 151 | |
| PF2:Y6 | J71 | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
24.97 | 0.75 | 64.7 | 12.12 | 149 | |
| PBDTTT-EF-T:ITIC | PCDTBT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
17.77 | 0.80 | 66.9 | 9.5 | 148 | |
| PBDTTT-EF-T:IEICO | PCDTBT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
24.03 | 0.72 | 70.4 | 12.2 | 148 | |
| All-polymer | PBDTTT-EF-T:N2200 | PCDTBT | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.7 | 0.79 | 58.3 | 6.65 | 161 |
| PTB7-Th:PNDI-T10 | PBDTTS-FTAZ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.6 | 0.84 | 73.0 | 9.0 | 78 | |
| PTB7-Th:NDP-V-C7 | PBCIT | 0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.77 | 0.78 | 68.07 | 9.03 | 165 | |
| PBTA-PEG-2%:N2200 | PTB7-Th | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.89 | 0.83 | 66.13 | 9.27 | 163 | |
| PTzBI-Si:N2200 | PBTA-Si | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 1:1 |
14.89 | 0.85 | 75.65 | 9.56 | 162 | |
| PBTA-BO:N2200 | PNTB | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.77 | 0.84 | 74.98 | 9.87 | 99 | |
| PTzBi:N2200 | PBTA-BO | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.26 | 0.84 | 78.33 | 10.12 | 120 | |
| PBDB-T:PNDI-2T-TR(5) | J71 | 1.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.63 | 0.89 | 71.02 | 9.12 | 166 | |
| J51:N2200 | PTB7-Th | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.17 | 0.81 | 66.3 | 9.60 | 164 | |
| TQ1:PNDI-T10 | PTB7-Th | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 1:1 |
9.86 | 0.82 | 50.0 | 4.08 | 101 | |
| PD1/SMD2/SMA | P3HT:DPP(E-BODIPY)2-HD | p-DTS(FBTTH2)2 | 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.76 | 0.59 | 41.0 | 1.62 | 172 |
| PTB7-Th:DPP(E-BODIPY)2-HD | p-DTS(FBTTH2)2 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.025 | 0.76 | 53.0 | 2.84 | 172 | |
| PBDB-T:ITIC | SM-X | 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.86 | 0.92 | 68.15 | 11.96 | 119 | |
| PBDB-T:ITIC | SM-Y | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.22 | 0.92 | 67.61 | 11.48 | 119 | |
| PBDB-T:ITIC | SM-XY | 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.86 | 0.90 | 64.15 | 10.21 | 119 | |
| PTB7-Th:ITIC | C7 | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25 |
18.51 | 0.81 | 67.81 | 10.16 | 173 | |
| PTB7-Th:ITIC | C30 | 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25 |
15.85 | 0.80 | 63.48 | 8.04 | 173 | |
| PTB7-Th:FOIC | TR | 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1.5, |
25.13 | 0.734 | 70.9 | 13.1 | 174 | |
| PTB7-Th:IEICO-4F | BIT-4F-T | 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
27.3 | 0.72 | 70.90 | 14.0 | 175 | |
| All-SM | DCAO3TBDTT:IDIC | DR3TBDTT-S-E | 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.37 | 0.91 | 67.4 | 10.4 | 110 |
| SM:IDIC | SM-Cl | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.05 | 0.92 | 69.58 | 10.29 | 177 | |
| SMD1/SMD2/PA | DR3TBDTT:P-BNBP-fBT | BTR | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.39 | 1.18 | 55.6 | 4.85 | 176 |
4.1 PD1/PD2/SMA NF-PSCs
Among the different D1:D2:A systems, the utilization of two polymer donor and small molecule non-fullerene acceptor is continuing to receive intensive interest. This is the fastest growing and most manufactured system. In 2016, Hwang et al.89 reported a new non-fullerene electron acceptor DBFI-EDOT (Fig. 2), with a highly twisted, nonplanar 3D conformation. It was the first non-fullerene acceptor that had 8.1% and 6.7% PCE when paired with two different polymer donors, thiazolothiazoledithienosilole copolymer PSEHTT and PBDTT-FTTE (Fig. 1), respectively. Notably, when combining DBFI-EDOT with these two donors in a ternary blend, an enhanced PCE of 8.52% was observed in the ternary blend devices with a composition of PSEHTT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBDTT-FTTE
PBDTT-FTTE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBFI-EDOT = 2
DBFI-EDOT = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 (wt/wt). The enhanced PCE of the ternary blend is largely due to the much broader near IR (NIR) light harvesting, and the increased maximum Jsc of 15.67 mA cm−2.
0.1 (wt/wt). The enhanced PCE of the ternary blend is largely due to the much broader near IR (NIR) light harvesting, and the increased maximum Jsc of 15.67 mA cm−2.
In 2018, Hu et al.117 employed a low-bandgap polymer donor PBDB-T (Fig. 2) as a third component in the FTAZ:IT-M (Fig. 2 and 3) ternary blend system. The FTAZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBDB-T
PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IT-M (0.8
IT-M (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) ternary devices have an amazing highest PCE of 13.2%, with a Voc of 0.95 V, a Jsc of 18.1 mA cm−2 and an FF of 73.6%, which is significantly higher than the corresponding two binary PSCs. The weak absorption of FTAZ (Fig. 1) in the wavelength range between 600 and 660 nm was compensated by PDBT-T. Furthermore, the ability of the IT-M to crystallize has been suppressed because of the rigid polymer PBDB-T, which contributes to a more advanced morphology of the ternary blend films. The addition of a rigid polymer with less ductility and a marginally higher HOMO than the host may be a good design option, they suggested.
1 weight ratio) ternary devices have an amazing highest PCE of 13.2%, with a Voc of 0.95 V, a Jsc of 18.1 mA cm−2 and an FF of 73.6%, which is significantly higher than the corresponding two binary PSCs. The weak absorption of FTAZ (Fig. 1) in the wavelength range between 600 and 660 nm was compensated by PDBT-T. Furthermore, the ability of the IT-M to crystallize has been suppressed because of the rigid polymer PBDB-T, which contributes to a more advanced morphology of the ternary blend films. The addition of a rigid polymer with less ductility and a marginally higher HOMO than the host may be a good design option, they suggested.
A ternary device with J51, PBDB-T (Fig. 1) and ITIC as the donor and acceptor materials was made by Yu et al.97 The blend film morphology of the ternary device was optimized by incorporating 0.75 vol% of DIO, which delivered a PCE of 8.75%, a Jsc of 15.47 mA cm−2, a Voc of 0.89 V, and an FF of 0.64%. From the results of many tests, they concluded that because of the complementary solar light harvesting, cascade energy alignment, and optimized blend film morphology, the ternary device obviously showed more improvement than two copies of the binary PSCs. In 2019, An et al.128 first introduced the J71 to the PM6 (Fig. 1) and Br-ITIC (Fig. 3) binary blend to make a non-fullerene ternary PSCs with amazing performance, a PCE of 14.13%, a Jsc of 19.39 mA cm−2, a Voc of 0.93 V, and an FF of 78.4%, which is primarily beneficial from the used materials having complementary absorption spectra, good compatibility and appropriate energy levels. The photoluminescence (PL) spectra and time-resolved photoluminescence (TRPL) spectra of neat and blend films indicate the work mechanisms is energy transfer from J71 to PM6. Furthermore, due to the good compatibility of two polymer donors, there formed a alloy donor which improved efficiency of photogenerated holes transmission.
A low-bandgap polymer PTB7-Th was successfully utilized in several non-fullerene ternary PSCs as an additional donor portion. For instance, J51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC104 (0.8
ITIC104 (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), J51
1), J51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT-IC128 (0.5
BT-IC128 (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PBDB-T
1) and PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IEICO-4F96 (0.8
IEICO-4F96 (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed PCE values of 9.70%, 10.32% and 11.62%, respectively. In 2018, Zhong et al.104 introduced the PTB7-Th to the J51:ITIC binary blend devices. They obtained a high PCE of 9.7% with a higher Jsc of 17.75 mA cm−2, a Voc of 0.81 V and an FF of 67.82% at the optimal weight ratio (20%) of PTB7-Th. They reported that the high performance was largely attributable to the improved Jsc, which was influenced by the synergistic effects of the increased light absorption, greater controlled hole/electron mobilities and improved morphology. The following year, Zhong et al.128 manufactured better-performing ternary NF-PSCs adopting the same donors, but a different non-fullerene accepter (BT-IC, Fig. 3), with a PCE of 10.32%. The BT-IC demonstrated high absorption in the wavelength range of 500–900 nm compared to ITIC, which has a comparatively short wavelength absorption range of 650–750 nm. The PSCs based on J51:PTB7-Th:BT-IC (without using an additive) demonstrated a higher Jsc of 19.55 mA cm−2, which is mainly benefitted from the strong absorbance of BT-IC in the NIR. Furthermore, the second polymer donor has good compatibility with the host material without causing a larger phase separation, which also promotes the realization of larger FF to some extent. Similarly, Ma and co-workers96 chose PTB7-Th as the second donor-constructed ternary PSCs with the host materials, PBDB-T and IEICO-4F. As a result, the introduction of the very compatible donor PTB7-Th can effectively improve the photon capture ability of the ternary blend films. The “3D texture” structure formed by the mixed edge-on and face-on orientations provide more charge transport channels in the ternary active layer, which can further improve the FF of the ternary solar cells. Therefore, all of these factors have led to a high PCE of 11.62% with a Jsc of 24.14 mA cm−2, a Voc of 0.74 V, and a high FF of 65.03%. PTB7 and its benzodithiophene (BDT) derivatives related to the fused thienothiophene (TT) rings are widely used as donors in binary and ternary PSCs. Listed below are several ternary PSCs with PTB7-Th and ITIC as the host donor–acceptor blend system. To improve the Jsc of the non-fullerene ternary PSCs based on the PTB7-Th:ITIC system, Bi et al.129 introduced a polymer PCDTBT into the binary system with an energy transfer model. When 20 wt% PCDTBT (Fig. 1) was added in the host blend film, the PCE of the ternary devices increased to 7.75%. The AFM of the binary ternary blend film shows Rq1 = 14.6 nm (mean-square surface roughness) and Rq2 = 11.1 nm. It means that the ternary blend of the film surface is much smoother after 20 wt% PCDTBT has been added. Liu et al.98 employed PffBT4T-2OD (Fig. 1) to PTB7-Th:ITIC to construct a non-fullerene PSCs with a high PCE of 8.22%. The high-crystallinity PffBT4T-2OD as the third component can finely ameliorate the blend morphology and form interpenetrating network structures with an appropriate phase-separated domain size, which will be able to suppress the charge recombination and improve the charge transport properties of the devices. In 2018, Tsai et al.130 synthesized two wide-bandgap polymers, P8TT and P8TTT (Fig. 1), with absorption wavelengths below 650 nm. After optimization, the PEC of the P8TT-based devices was 9.45% and P8TTT was 10.01%. The ternary system obtained a higher Voc value after applying the deeper-lying HOMO amounts of the donor of 8T-based polymers, 0.883 V for the P8TTT-based ternary PSCs and 0.882 V for the P8TT-based ternary PSCs. It is worth noting that its packing orientation is slightly improved after blending with the 8T-based polymer, which may improve the transfer of charge in the ternary blends. Li and co-workers131 fabricated ternary ITIC-based or 4TIC-based NF-PSCs using PTB7-Th as the donor and porphyrin function-conjugated polymer PPor-1 as the blue light-absorbing additive. The third component of PPor-1 increases the Jsc and optimizes the surface morphology, and obtained two PSCs with PCE of 7.21% and 9.01%, respectively. In comparison, the binary PSCs (PTB7-Th:ITIC, PTB7-Th:4TIC, the structure is shown in Fig. 1–3) only had PEC of 6.70% and 8.10%, respectively.
1) showed PCE values of 9.70%, 10.32% and 11.62%, respectively. In 2018, Zhong et al.104 introduced the PTB7-Th to the J51:ITIC binary blend devices. They obtained a high PCE of 9.7% with a higher Jsc of 17.75 mA cm−2, a Voc of 0.81 V and an FF of 67.82% at the optimal weight ratio (20%) of PTB7-Th. They reported that the high performance was largely attributable to the improved Jsc, which was influenced by the synergistic effects of the increased light absorption, greater controlled hole/electron mobilities and improved morphology. The following year, Zhong et al.128 manufactured better-performing ternary NF-PSCs adopting the same donors, but a different non-fullerene accepter (BT-IC, Fig. 3), with a PCE of 10.32%. The BT-IC demonstrated high absorption in the wavelength range of 500–900 nm compared to ITIC, which has a comparatively short wavelength absorption range of 650–750 nm. The PSCs based on J51:PTB7-Th:BT-IC (without using an additive) demonstrated a higher Jsc of 19.55 mA cm−2, which is mainly benefitted from the strong absorbance of BT-IC in the NIR. Furthermore, the second polymer donor has good compatibility with the host material without causing a larger phase separation, which also promotes the realization of larger FF to some extent. Similarly, Ma and co-workers96 chose PTB7-Th as the second donor-constructed ternary PSCs with the host materials, PBDB-T and IEICO-4F. As a result, the introduction of the very compatible donor PTB7-Th can effectively improve the photon capture ability of the ternary blend films. The “3D texture” structure formed by the mixed edge-on and face-on orientations provide more charge transport channels in the ternary active layer, which can further improve the FF of the ternary solar cells. Therefore, all of these factors have led to a high PCE of 11.62% with a Jsc of 24.14 mA cm−2, a Voc of 0.74 V, and a high FF of 65.03%. PTB7 and its benzodithiophene (BDT) derivatives related to the fused thienothiophene (TT) rings are widely used as donors in binary and ternary PSCs. Listed below are several ternary PSCs with PTB7-Th and ITIC as the host donor–acceptor blend system. To improve the Jsc of the non-fullerene ternary PSCs based on the PTB7-Th:ITIC system, Bi et al.129 introduced a polymer PCDTBT into the binary system with an energy transfer model. When 20 wt% PCDTBT (Fig. 1) was added in the host blend film, the PCE of the ternary devices increased to 7.75%. The AFM of the binary ternary blend film shows Rq1 = 14.6 nm (mean-square surface roughness) and Rq2 = 11.1 nm. It means that the ternary blend of the film surface is much smoother after 20 wt% PCDTBT has been added. Liu et al.98 employed PffBT4T-2OD (Fig. 1) to PTB7-Th:ITIC to construct a non-fullerene PSCs with a high PCE of 8.22%. The high-crystallinity PffBT4T-2OD as the third component can finely ameliorate the blend morphology and form interpenetrating network structures with an appropriate phase-separated domain size, which will be able to suppress the charge recombination and improve the charge transport properties of the devices. In 2018, Tsai et al.130 synthesized two wide-bandgap polymers, P8TT and P8TTT (Fig. 1), with absorption wavelengths below 650 nm. After optimization, the PEC of the P8TT-based devices was 9.45% and P8TTT was 10.01%. The ternary system obtained a higher Voc value after applying the deeper-lying HOMO amounts of the donor of 8T-based polymers, 0.883 V for the P8TTT-based ternary PSCs and 0.882 V for the P8TT-based ternary PSCs. It is worth noting that its packing orientation is slightly improved after blending with the 8T-based polymer, which may improve the transfer of charge in the ternary blends. Li and co-workers131 fabricated ternary ITIC-based or 4TIC-based NF-PSCs using PTB7-Th as the donor and porphyrin function-conjugated polymer PPor-1 as the blue light-absorbing additive. The third component of PPor-1 increases the Jsc and optimizes the surface morphology, and obtained two PSCs with PCE of 7.21% and 9.01%, respectively. In comparison, the binary PSCs (PTB7-Th:ITIC, PTB7-Th:4TIC, the structure is shown in Fig. 1–3) only had PEC of 6.70% and 8.10%, respectively.
Additionally, the PTB7-Th and IEICO-4F binary blend PSCs achieved a high PCE of 10.0%. There were a small number of D1:D2:A PSCs with different polymer donors introduced to the PTB7-Th:IEICO-4F system. For instance, in 2017, Yao et al.132 used J52 (Fig. 2), PBDTTT-EFT (known as PTB7-Th) and IEICO-4F (weight ratio was 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) to make a non-fullerene PSCs with a high PCE of 10.9%. Subsequently, Lee and co-workers133 studied the burn-in loss in the ternary blended OPVs by using UV-crosslinkable P2FBTT-Br and P2FBTT-H (Fig. 1) polymers and a PTB7-Th:IEICO-4F binary blend film in an environmentally friendly solvent. The ternary PSCs had a high PCE of 9.8% and 10.5% with a better stability. In 2019, another ternary PSCs based on the PTB7-Th:IEICO-4F system appeared. Tang et al.134 reported a series of new wide bandgap donor polymers with the P1–P3 design, synthesis and application based on a novel electron-withdrawing acceptor unit called FE-T. As a result, the wide bandgap polymer P1 (Fig. 1) integrated PTB7-Th and IEICO-4F into ternary cells and obtained outstanding PSCs with improved Jsc to 25.18 mA cm−2 and PCE to 12.11%.
1.5) to make a non-fullerene PSCs with a high PCE of 10.9%. Subsequently, Lee and co-workers133 studied the burn-in loss in the ternary blended OPVs by using UV-crosslinkable P2FBTT-Br and P2FBTT-H (Fig. 1) polymers and a PTB7-Th:IEICO-4F binary blend film in an environmentally friendly solvent. The ternary PSCs had a high PCE of 9.8% and 10.5% with a better stability. In 2019, another ternary PSCs based on the PTB7-Th:IEICO-4F system appeared. Tang et al.134 reported a series of new wide bandgap donor polymers with the P1–P3 design, synthesis and application based on a novel electron-withdrawing acceptor unit called FE-T. As a result, the wide bandgap polymer P1 (Fig. 1) integrated PTB7-Th and IEICO-4F into ternary cells and obtained outstanding PSCs with improved Jsc to 25.18 mA cm−2 and PCE to 12.11%.
In 2018, Zhang and co-workers135 constructed a non-fullerene PSCs with two polymer donors PTB7-Th, PBDB-T and a ultranarrow bandgap non-fullerene acceptor FOIC by combining a blade-coating (Fig. 16a), which can increase the degree of alignment in the neat polymer thin film. The optimal blade-coated devices based on PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOIC (0.5
FOIC (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blends present the highest PCE of 12.02% with a high FF of 65.78%. The crystallization coherence length (CCL) (Fig. 16b) is a value that can quantify the crystallinity. Fig. 16b shows that all of the scattering peaks of the blade-coated films become stronger and sharper than those of the spin-coated films, meaning the blade-coating can induce the crystallization of the ternary blend films accompanied by increased carrier mobility. One year later, Xie et al.136 fabricated PTB7-Th
1) blends present the highest PCE of 12.02% with a high FF of 65.78%. The crystallization coherence length (CCL) (Fig. 16b) is a value that can quantify the crystallinity. Fig. 16b shows that all of the scattering peaks of the blade-coated films become stronger and sharper than those of the spin-coated films, meaning the blade-coating can induce the crystallization of the ternary blend films accompanied by increased carrier mobility. One year later, Xie et al.136 fabricated PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBDTm-T1
PBDTm-T1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOIC (0.8
FOIC (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) ternary PSCs, which had a high PCE of 13.8%. The introduction of the third factor resulted in load recombination and the lowering of more controlled load stability, without significant morphological changes in the ternary units. In 2019, Wang and co-workers118 fabricated a device based on PTB7-Th:PBDB-T:O-IDTBR (Fig. 2 and 3) blends at a PTB7-Th content of 30% presenting a highest PCE of 11.58% with a Jsc of 17.09 mA cm−2, a high Voc of 1.02 V, and a FF of 66.52%. The increased performance with ternary PSCs was attributed to the slightly elevated Jsc and the maximum electron and hole mobility, as well as the desirable morphology.
1.5) ternary PSCs, which had a high PCE of 13.8%. The introduction of the third factor resulted in load recombination and the lowering of more controlled load stability, without significant morphological changes in the ternary units. In 2019, Wang and co-workers118 fabricated a device based on PTB7-Th:PBDB-T:O-IDTBR (Fig. 2 and 3) blends at a PTB7-Th content of 30% presenting a highest PCE of 11.58% with a Jsc of 17.09 mA cm−2, a high Voc of 1.02 V, and a FF of 66.52%. The increased performance with ternary PSCs was attributed to the slightly elevated Jsc and the maximum electron and hole mobility, as well as the desirable morphology.
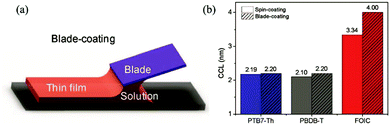 | ||
| Fig. 16 (a) The diagrammatic drawing of blade-coating. (b) The calculated CCL values of the (010) peak and 2D GIWAXS patterns.135 | ||
Liao et al.137 reported on ternary PSCs based on the DBT-TT copolymer PTB-EDOTS (Fig. 1) and large-bandgap copolymer J71, combined with the small molecular acceptor ITIC-Th. In green solvents such as tetrahydrofuran (THF), the EDOT side chains attached to the BDT skeleton significantly improved the solubility of the resulting copolymers. Notably, the ternary blend film treated with CB/CN had a smooth surface, but the insufficient phase separation impedes the diffusion of charges in the active blend. Yet the film treated with the MeTHF solvent had larger domains, which would be beneficial for the transfer of charges. When 20 wt% of J71 was added, the ternary blend devices with green solvent treatment had a high PCE of 12.26% with a high Jsc of 18.02 mA cm−2, a Voc of 0.90 V and an FF of 75.6%. The factors that improve the performance are promoted by charge transport and energy transfer via an electron cascade effect, a “hole back” phenomenon, a FRET process of the second donor J71 and an improved blend films morphology. Xu and co-workers138 prepared a series of alloy model ternary PSCs with one acceptor IT-2F (Fig. 3) and two donors PBT1-C and J71 (Fig. 2). The donors were elaborately selected with similar chemical structure and complementary photon harvesting range, and achieved a higher PCE of 12.26%. In addition, the well optimized phase separation with PBT1-C as morphology regulator achieved a high FF of 75.53%. In comparison, the binary system has only 10.45%. Owing to the good compatibility and similar HOMO levels of J71 and PBT1-C, J71 and PBT1-C formed a good alloy state.
In 2019, Xu et al.139 applied PNDT-ST, PBDT-ST and Y6-T (Fig. 1–3) to ternary PSCs of the alloy model. By replacing the 2FIC ending group with CPTCN, the energy levels of Y6-T were much higher than that of Y6. Using the novel wind-bandgap polymer donors PNDT-ST and PBDB-T as the polymer donors, and Y6-T as the non-fullerene acceptor, the ternary blend devices delivered a champion PCE of 16.57%. This is almost the best record for ternary PSCs with multiple donors in recent years. There are several studies on the morphology of pristine and blend films, GIWAXS patterns of the pristine and blend films, AFM height images and phase images. PNDT-ST showed that it possessed higher ordered lamellar stacking and π–π stacking than PNDT-ST, and Y6-T displayed preferential face-on packing. The PBDT-ST:Y6-T binary blend film showed higher ordered structures than the PNDT-ST:Y6-T binary blend film. In addition, the diffraction profiles of the ternary blend film demonstrate a good compatibility of PBDT-ST and PNDT-ST, which form an alloy model. The AFM images show that the PBDT-ST:Y6-T blend film has a comparatively weak phase separation. In contrast, the PNDT-ST:Y6-T blend film has a large scaled phase separation due to the higher crystallinity of PNDT-ST. The ternary blend film exhibited a tuned crystallinity and morphology, promoting exciton dissociation and charging transport by the well-distributed nanofibrillar textures. Thus, the ternary devices achieved a high PCE with a high FF of 75.8%, a Jsc of 24.04 mA cm−2 and a high Voc of 0.909 V.
A commercially available middle-bandgap polymer named PBDB-T is a very popular and efficient polymer donor, such that there were many ternary NF-PSCs using PBDB-T as the host donor. For example, in 2017, Xu et al.140 reported a ternary NF-SOC with the middle-bandgap polymer donor PBDB-T, low-bandgap polymer donor PTB7-Th and the non-fullerene acceptor SFBRCN (Fig. 3). Benefitting from the complimentary absorption, the hole-back transfer from PTB7-Th to PBDB-T, multiple electron charge transfer, and nonradiative FRET between the acceptor and the donor materials, the ternary devices had a high PCE of 12.27% and a Jsc of 17.86 mA cm−2, a Voc of 0.93 V, and a high FF of 73.9%. After adding the second donor PTB7-Th, the ternary blend films possessed a preferential face-on packing behaviour that obtained a larger charge mobility in NF-PSCs. Additionally, the ternary blend with 0.7 wt% of PTB7-Th exhibited the highest domain purity, which caused the highest Jsc and FF values. In 2018, Wang and co-workers141 introduced the wide-bandgap polymer donor PDCBT to PBDB-T and IT-M. The second donor added expands the absorption spectrum of the ternary NF-PSCs to the near-infrared zone, and the second polymer evidently did not improve the phase separation of the ternary film. Finally, the optimized ternary blend devices (PBDB-T:PDCBT:IT-M, 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) delivered a champion PCE of 11.2% with a Jsc of 17.97 mA cm−2, a Voc of 0.93 V, and an FF of 66.7%.
1) delivered a champion PCE of 11.2% with a Jsc of 17.97 mA cm−2, a Voc of 0.93 V, and an FF of 66.7%.
The PBDB-T:ITIC binary blend system is one of the most successful systems, and many researches on ternary PSCs are based on this system. In 2018, Guo and co-workers122 introduced PBDTTPD (Fig. 2) to this binary blend film, and achieved a 9.36% PCE. The two donors formed an alloy state, and made a monotonic change of Voc against the ternary compositions. Furthermore, in an appropriate PBDTTPD doping concentration, the ternary blended blend films achieved a synergistic enhancement in the exciton generation/dissociation rate, and improvement in the electron and hole mobilities. In the same year, Nian et al.142 used the two non-fullerene acceptors ITIC and IT-M, and the strongly aggregating polymer PDTfBO-TT with hole mobility as the additional donor, to construct PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDTfBO-TT
PDTfBO-TT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC (0.95
ITIC (0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PBDB-T
1) and PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDTfBO-TT
PDTfBO-TT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IT-M (0.95
IT-M (0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ternary NF-PSCs. The energy transfer process from P1 to PBDB-T contributes to the exciton generation and dissociation. When adding 10 wt% PDTfBO-TT in the PBDB-T:ITIC and PBDB-T:IT-M blend films, the molecular orientations were optimized, which achieved the enhancement in the hole and electron mobilities. After carefully being optimized, the ITIC-based and IT-M-based NF-PSCs got a high PEC of 12.70% and 13.52%, respectively. In 2019, Zhao and co-workers143 found that the ternary devices with PBDB-Th (Fig. 2) as a second donor had a PCE of 10.68%. They studied the spin-dependent recombination and dissociation of the electron–hole in the binary and ternary NF-PSCs based on ITIC. Finally, it came to such a conclusion that both spin-dependent dissociations of the polar and field-dependent exciton-charge reaction could lead to the magneto-Jsc.
1) ternary NF-PSCs. The energy transfer process from P1 to PBDB-T contributes to the exciton generation and dissociation. When adding 10 wt% PDTfBO-TT in the PBDB-T:ITIC and PBDB-T:IT-M blend films, the molecular orientations were optimized, which achieved the enhancement in the hole and electron mobilities. After carefully being optimized, the ITIC-based and IT-M-based NF-PSCs got a high PEC of 12.70% and 13.52%, respectively. In 2019, Zhao and co-workers143 found that the ternary devices with PBDB-Th (Fig. 2) as a second donor had a PCE of 10.68%. They studied the spin-dependent recombination and dissociation of the electron–hole in the binary and ternary NF-PSCs based on ITIC. Finally, it came to such a conclusion that both spin-dependent dissociations of the polar and field-dependent exciton-charge reaction could lead to the magneto-Jsc.
Yi et al.124 selected PBDB-T:ITIC as the host blend, and added two different polymer donors PDCBT and P3HT (Fig. 2) as the third component. When the weight ratio was 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.95
1 and 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the two ternary PSCs had PEC of 10.97% and 6.92%, respectively. When blending PDCBT (or P3HT) with PBDB-T, the crystallization of PDCBT (P3HT) is promoted, and PDCBT (or P3HT) formed bimolecular crystals with PBDB-T, which can be proved by the GIWAXS and AFM imagines. An obvious phase separation is noticed in the PDCBT:ITIC film due to the poor miscibility between PDCBT and ITIC. The domain sizes also reach about 50 nm. After the incorporation of PDCBT, the increase in the phase separation between donor and acceptor leads to improvements in the hole and electron mobilities, and eventually has a good photoelectric performance. On the contrary, good compatibility and strong interaction lead to a homogeneous morphology between P3HT and ITIC that is less clear in the separation of the PBDB-T:P3HT:ITIC phase. Although the complementary absorption between P3HT and PBBD-T promotes the efficient use of sunlight, the decreased phase separation and the integrity of the phase domain contribute to a decrease in the charge mobility. That is why the PCE of the PBDB-T:P3HT:ITIC ternary devices is below that of the PBDB-T:ITIC binary devices. It means that the complementary absorption and enhanced morphology of the blend film are of considerable significance.
1, the two ternary PSCs had PEC of 10.97% and 6.92%, respectively. When blending PDCBT (or P3HT) with PBDB-T, the crystallization of PDCBT (P3HT) is promoted, and PDCBT (or P3HT) formed bimolecular crystals with PBDB-T, which can be proved by the GIWAXS and AFM imagines. An obvious phase separation is noticed in the PDCBT:ITIC film due to the poor miscibility between PDCBT and ITIC. The domain sizes also reach about 50 nm. After the incorporation of PDCBT, the increase in the phase separation between donor and acceptor leads to improvements in the hole and electron mobilities, and eventually has a good photoelectric performance. On the contrary, good compatibility and strong interaction lead to a homogeneous morphology between P3HT and ITIC that is less clear in the separation of the PBDB-T:P3HT:ITIC phase. Although the complementary absorption between P3HT and PBBD-T promotes the efficient use of sunlight, the decreased phase separation and the integrity of the phase domain contribute to a decrease in the charge mobility. That is why the PCE of the PBDB-T:P3HT:ITIC ternary devices is below that of the PBDB-T:ITIC binary devices. It means that the complementary absorption and enhanced morphology of the blend film are of considerable significance.
In 2018, Cui and co-workers144 used two polymer donor PTO2 and PBDB-TF (Fig. 2) made ternary PSCs with a surprising PCE of 14.8%. After optimization, the best weight ratio of PTO2:PBDB-TF:IT-4F was 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Due to the additional donor material optimized film morphology, the ternary PSCs got an increased FF of 74%. In addition, they also designed and synthesized a new copolymer donor T1, and combined it with IT-4F to fabricate a binary NF-PSCs. By adding the EST package, the new polymer donor will expand its absorption range and change its HOMO amount, resulting in the simultaneously increased Jsc and Voc in the system. Surprisingly, the T1:IT-4F binary blend system treated with CB has a high PCE of 15.1%, even the devices processed by THF also yields a high PCE of 14.2% due to the good solubility of the copolymer.
1. Due to the additional donor material optimized film morphology, the ternary PSCs got an increased FF of 74%. In addition, they also designed and synthesized a new copolymer donor T1, and combined it with IT-4F to fabricate a binary NF-PSCs. By adding the EST package, the new polymer donor will expand its absorption range and change its HOMO amount, resulting in the simultaneously increased Jsc and Voc in the system. Surprisingly, the T1:IT-4F binary blend system treated with CB has a high PCE of 15.1%, even the devices processed by THF also yields a high PCE of 14.2% due to the good solubility of the copolymer.
Wu et al.145 synthesized two conjugated polymer donors with similar structure (PBDB-TS, PBDB-BT shown in Fig. 2), and applied them to ternary devices with an enhanced PCE of 10.26% compared to the PBDB-TS:ITIC-based host binary devices (PCE = 9.81%). PBDB-BT has a complementary absorption spectrum with PBDB-TS, and a slightly lower HOMO than PBDB-TS. The PL spectra of the pristine and blend films are shown in Fig. 17. Compared to the neat PBDB-TS and PBDB-BT film, the PBSB-TS:PBDB-BT blend film presents higher PL intensity excited at 560 nm or 620 nm. It means that there is no energy transfer between the two polymer donors. Furthermore, the insufficient overlap between the emission spectrum of PBDB-BT and the absorption spectrum of PBDB-TS indicate that there is no FRET in the two polymer donors’ film. Therefore, it can be confirmed that an alloy state is formed between the two donors. In addition, the third component (PBDB-BT) effectively reduces the bimolecular recombination of the ternary devices. The addition of the third component contributes to the well-developed morphology of the ternary blend film, which increases excitons separation and transport, and leads to a high FF value of the ternary devices. The results demonstrated that constructing a ternary blend film via incorporation of a structurally similar polymer donor is a promising approach to further improve the efficiency of the ternary NF-PSCs.
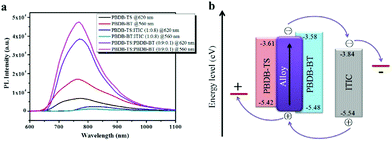 | ||
| Fig. 17 (a) PL spectra of the pristine and blend films excited at 560 and 620 nm, respectively. (b) Schematic of the alloy charge transfer process in the PBDB-TS:PBDB-BT:ITIC ternary devices.145 | ||
In 2019, Liu and co-workers146 engineered and synthesized two wide-bandgap regioisomeric polymers based on 2,20-bithiazole and 5,50-bithiazole, respectively, namely PDBT(E)BTz-p and PDBT(E)BTz-d (Fig. 1), with a deeper capacity for ionization. Beginning with the host binary scheme PBDB-T-SF:IT-4F, PDBT(E)BTz-p and PDBT(E)BTz-d were added as the second donor, respectively. The device efficiencies were shown to rise from 12.1% for the binary control devices to 12.5% and 13.4% for the ternary devices after the addition of 5 wt% PDBT(E)BTz-p and 5 wt% PDBT(E)BTz-d compared to PBDB-T-SF (Fig. 2), respectively. They demonstrated that the third component does not disturb the molecular packing, but the FRET between PDBT(E)BTz-d and PBDB-T-SF improves the Jsc and Voc of the devices, and thus results in a high PCE.
A highly efficient wide bandgap polymer P(p-FDBND-2T) was employed as a third component in the PBDTPS-FTAZ:ITIC devices.100 While the energy level alignment of the PBDTPS-FTAZ:P(p-FDBND-2T):ITIC ternary devices is unfavourable for exciton dissociation, the devices still display an intense resonant energy transfer (FRET) process for Förster (Fig. 11c). Because of the good miscibility between PBDTPS-FTAZ and P(p-FDBND-2T), the ternary blend film forms a continuous interpenetrating network and favorable phase separation, which is beneficial to obtain a high Jsc of 18.78 mA cm−2 and FF of 66.7%. That is how the ternary devices had 11.46% improved PCE. The PBDTPS-FTAZ:ITIC binary devices, on the other hand, had only 8.53% PCE.
Xiao et al.147 created a series of highly-efficient inverted ternary PSCs consisting of a host binary blend device of PBDTTT-EF-T:ITIC, which were fabricated by applying a wide-bandgap donor, PCDTBT, as the second donor. Through the precise regulation of the content of the two polymer donors, the PBDTTT-EF-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCDTBT
PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC (1
ITIC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3) and PBDTTT-EF-T
1.3) and PBDTTT-EF-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCDTBT
PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IEICO-4F (1
IEICO-4F (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) ternary PSCs had a PCE of 7.9% and 10.6%, respectively. It turns out that FRET plays a key role in enhancing the ternary PSCs performance, along with enhanced broad photon harvesting over the entire absorption wavelength range, causing an enhanced Jsc. Furthermore, the ternary NF-PSCs observed an improvement in FF due to the optimized phase separation and balanced charge transport.
1.5) ternary PSCs had a PCE of 7.9% and 10.6%, respectively. It turns out that FRET plays a key role in enhancing the ternary PSCs performance, along with enhanced broad photon harvesting over the entire absorption wavelength range, causing an enhanced Jsc. Furthermore, the ternary NF-PSCs observed an improvement in FF due to the optimized phase separation and balanced charge transport.
In 2020, Ma and co-workers148 used a novel moderate bandgap polymer PF2 and the wide bandgap polymer J71 as two donors, and the low bandgap small molecular material Y6 as the accepter, to fabricated a type of ternary NF-PSCs. The PCEs of the PF2 and J71-based binary PSCs reached 10.26% and 9.56%, respectively. The PCE of ternary PSCs, by incorporating 30 wt% J71 into donors, achieved an increased PCE of 12.12% with a simultaneously improved Jsc of 24.97 mA cm−2, a Voc of 0.75 V and an FF of 64.70%. There are some reasons for this increase. The complementary absorption spectra of PF2 and J71 improved the Jsc. Meanwhile, the optimized phase separation of the ternary blend films caused efficient exciton dissociation and charge transport. In the optimized ternary PSCs, bimolecular and trap-assisted recombination can be synchronously suppressed, which resulted in a relatively high FF of the optimized ternary PSCs. In the same year, Xie et al.149 also chose PM6, J71 and Y6 as the two donors and acceptor to fabricate a ternary PSCs. Fig. 18a shows the TEM images of the three blend films. The J71:Y6 film observes relatively large white and dark domains, while the PM6:Y6 films have no characteristics, and the 10 wt% J71 ternary blend film shows a more visible light and dark alternation in donors than the PM6:Y6 binary blend film. This indicates that by adding the correct amount of J71, the 2D-GIWAXS images (Fig. 18b) may also validate this phenomenon. The molecular structure and the phase separation in the ternary films can also further improve. The in-plane and out-of-plane line-cut profiles of the 2D-GIWAXS data (Fig. 18c) show that the ternary blend film has a more ordered face-on orientation, which can enhance the efficient charge transport in the ternary films. Benefitting from the optimized molecular arrangement and phase separation degree, the ternary devices with 10 wt% J71 in donor achieved an astonishing PCE of 16.5% with enhanced Jsc of 25.55 mA cm−2, Voc of 0.85 V, and FF of 76.0%.
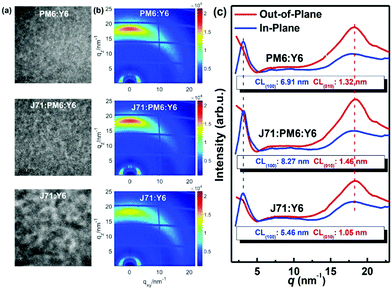 | ||
| Fig. 18 (a) TEM images of the binary and ternary blend films. (b) 2D-GIWAXS images of the binary and ternary blend films. (c) In-plane and out-of-plane line-cut profiles of the 2D-GIWAXS data.149 | ||
Bao and co-works150 also used PM6:Y6 as the binary host, and chose three polymer donor materials (PBDB-T, PDHP-Th, PDHP-Ph) as the second donor to make a series of high-efficiency ternary PSCs. It should be noted that PDHP-Th and PDHP-Ph have a thiophene arm and benzene arm, respectively. The unique stiff and twisted backbones of PDHP-Th and PDHP-Ph prohibit the over aggregation behaviour of Y6. These features allow the ternary PSCs based on these two different donors to obtain high PCEs of 16.8% and 15.4%, respectively. In contrast, the PCE of its counterpart polymer guest symmetrical PBDB-T-based PSCs is relatively low (14.6%). Most importantly, this asymmetric polymer molecule provides a molecular design strategy toward the efficient polymer guest.
Recently, Zhang et al.151 fabricated a new ternary PSCs with an amazingly high PCE of 17.53%, which should be the highest reported values for the D1:D2:A ternary devices currently known. They synthesized a new polymer donor S3, which has a blue shift of about 10 nm in the absorption spectrum comparison to PM6. Meanwhile, the two donors prefer to form an alloy-like state due to their similar structure, and have good compatibility. Thanks to the excellent film morphology, complementary absorption and low energy loss, the ternary device with 20 wt% S3 shows a PCE of 17.53% with a Jsc of 25.86 mA cm−2, FF of 79.17% and Voc of 0.856 V.
With more PD1/PD2/SMA non-fullerene PSCs research articles published, it is believed that the high photovoltaic performances can be achieved by carefully selecting the two donors according to the roles of complementary light absorption, good compatibility, matching energy levels, and working well with the same SMA. Apart from occupying the great potential in achieving high photovoltaic performances, the PD1/PD2/SMA-type devices show additional advantages in improving the morphology stability, when incorporating higher Tg or longer chains of the second polymer donor to increase the morphology stability.
4.2 PD1/PD2/PA (all-polymer) ternary NF-PSCs
All-polymer solar cells (all-PSCs), consisting of polymer donor and polymer acceptor materials, have received widespread attention in recent years due to some unique advantages: (i) the semiconducting polymers have high absorption coefficients in the visible spectral region; (ii) more effective tuning of energy levels, so the low LUMO of the polymer acceptors can result in a more efficient photoinduced charge separation; (iii) exhibit outstanding thermal and mechanical stability, as well as simple large-area fabrication due to the favorable solution viscosity.152–156 However, the ternary all-PSCs were not widely applied due to the fact that such ternary blend systems require not only complementary absorption bands, but also sufficient free carrier generation and efficient transport charges. Futhermore, some factors limit the efficiency of the all-PSCs, such as undesired blend morphology in the active films and lower electron mobility of polymer acceptors than that of fullerenes.157–159 An ideal phase-separated, nanoscale D/A interpenetrating network could facilitate exciton dissociation and charge carrier transport.The NDI-based polymer accepter N2200 (Fig. 3) is one of the most successful polymer acceptors that has been widely used. In 2016, Benten et al.160 first designed the ternary blend all-polymer solar cells, in which a wide-bandgap polymer PCDTBT was used as a multiple donor in low-bandgap PBDTTT-EF-T:N2200 blend. The PCDTBT has an absorption band in the visible region that is complementary to the PBDTTT-EF-T and N2200. With the addition of PCDTBT, the absorbance of the visible light region at 400 nm and 450–650 nm increases. Additionally, they demonstrated that the PCDTBT excitons can be transported directly to both PBDTTT-EF-T and N2200 through long-range resonant Förster energy transfer. After a series of optimizations, the PBDTTT-EF-T:PCDTBT:N2200 ternary all-PSC achieved a high PCE of 6.65% with a Jsc of 14.7 mA cm−2, a Voc of 0.79 V, and a FF of 58.3%. In 2017, Fan et al.161 designed and synthesized the BTA-based conjugated copolymers PBTA-Si (Fig. 1), and introduced them to the PTzBI-Si:N2200 (Fig. 1 and 3) binary BHJ blend. Due to the lower HOMO energy level of PTzBI-Si, the ternary devices with the added PtzBI-Si showed a high Voc. The PL measurements indicate a Förster resonance energy transfer from PBTA-Si to PTzBI-Si. The high compatibility of the two donor polymers leads to an improved crystallinity, which improves ternary blend film surface morphology, and increases the mobility of electrons and the weak recombination behaviour of ternary PSCs. Therefore, the PTzBI-Si:PBTA-Si:N2200 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1) devices with 150 nm thick film achieved a PCE of 9.56% with a Jsc of 14.65 mA cm−2, a Voc of 0.85 V and a high FF of 75.65%, in contrast to the PBTA-Si
1:1) devices with 150 nm thick film achieved a PCE of 9.56% with a Jsc of 14.65 mA cm−2, a Voc of 0.85 V and a high FF of 75.65%, in contrast to the PBTA-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2200 (2
N2200 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PTzBI-Si
1) and PTzBI-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2200 (2
N2200 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary devices only having a PEC of 6.97% and 7.24%, respectively.
1) binary devices only having a PEC of 6.97% and 7.24%, respectively.
Li and co-workers162 designed and synthesized a PEG-modified ternary copolymer PBTA-PEG-2% (Fig. 2). The binary devices based on PBTA-PEG-2%:N2200 exhibited a PEC of 5.98%, and it has a weak band from 650 to 800 nm. However, the PTB7-Th:N2200 binary films exhibit absorption peaks between 560 and 780 nm. The PTB7-Th-based binary films are complementary with the absorption profiles of the PTB7-Th:N2200 binary films. The ternary devices got a higher Jsc due to the extended absorption of the photoactive layer. In the study of the blend film morphology, they found that the binary blend films based on PBTA-PEG-2%:N2200 exhibited relatively rough surfaces a relatively high root mean square (RMS) value of 1.30 nm. However, the ternary films show a smoother film morphology (PMS = 0.9 nm) after adding 30% PTB7-Th. In general, the ternary devices based on PBTA-PEG-2%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2200 (1.4
N2200 (1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) have an increased PCE of 9.27% due to the complementary absorption, improved charge carrier transportation, and favourable film morphology and phase separation.
1) have an increased PCE of 9.27% due to the complementary absorption, improved charge carrier transportation, and favourable film morphology and phase separation.
In 2018 Li and co-workers99 introduced a novel PNTB (Fig. 1) into the PBTA-BO:N2200 BHJ layer as a third component. The complementary consumption, effective energy transmission profiles, enhanced charging stability, better morphology and sufficient compatibility of PNTB energy rates with those of PBTA-BO and N2200 culminated in a slightly better PCE of 10.09% in the ternary devices. In contrast, the binary devices based on PBTA-BO:N2200 exhibited a lower PCE of 7.24%. In the same year, Li et al.120 systematically investigated an all-PSCs ternary systems comprising PTzBI:PBTA-BO donors and N2200 as an acceptor. The intimate mixing properties of the two donors enabled the formation of an alloy-like blend in the present ternary systems. The root mean square (RMS) indicates that adding a quantity of PBTA-BO could optimize the film morphology to support charging transport in the ternary blend films. Finally, adding 30 wt% PBTA-BO into the PtzBi:N2200 binary blend films fabricated a ternary all-PSCs device that had a higher PCE of 10.12% with a Jsc of 15.64 mA cm−2, a Voc of 0.836 V and an amazing high FF of 78.33%.
Also using N2200 as the polymer acceptor, Zhang et al.163 constructed an all-PSCs with two polymer materials J51 and PTB7-Th as donors, which had a high PCE of 9.60% with a high Jsc of 17.17 mA cm−2, a Voc of 0.84 V and an FF of 66.3%. The application of a J51 not only improves light absorption, but also strengthens the ternary blend film orientation and provides an increased morphology. The topography and phase images of the J51, PTB7-Th and blend films have no detail difference, indicating the good compatibility between the polymer J51 and PTB7-T. As the AFM height images show, all films show a small root-mean-square roughness in the range of 0.70–0.79 nm, indicating that the incorporation of PTB7-Th did not increase the phase-separated ternary blend films. Additionally, the fibre structures were observed in the AFM images, depending on the high crystallinity of N2200.
Li et al.78 demonstrated the high-performance ternary all-PSCs by incorporating a high-bandgap PBDTTS-FTAZ (Fig. 1) as the second donor in a blend of PTB7-Th and PNDI-T10 (Fig. 3). PBDTTS-FTAZ, serving as a second donor, broadens the absorption and generates more free charges by simultaneous charge and energy transfer. By optimizing the weight load of PBDTTS-FTAZ, the Jsc of the ternary full PSCs is as high as 14.4 mA cm−2. Unprecedented FF is 0.74%, while PCE is as high as 9.0% in both traditional and inverted devices.
Zhou and co-workers101 also used PNDI-T10 as the sole polymer acceptor to form a ternary all-PSCs with two polymer donors (TQ1 and PNDI-T10). The ternary devices achieved a maximum PCE of 4.08% obtained in a TQ1/PCE10/PNDI-T10 (1/1/1) ternary system after thermal annealing. They studied the effect of thermal annealing and morphology on the PSCs performance. After thermal annealing, the favourable charge separation and reduced geminate recombination improved the PCE of all-PSCs. Research on the blend film morphology found that the phase separation trend between TQ1 and PNDI-T10 is weak, but there is a strong phase separation between the PCE10 and PNDI-T10 molecules, therefore TQ1 probably acts as a compatibilizer between PCE10 and the PNDI-T10. After thermal annealing, the ternary devices achieved a maximum PCE due to the improving of molecular packing structure between TQ1 and PCE10.
In 2018, Chen et al.164 fabricated an efficient ternary all-PSCs of using two analogous donors (PTB7-Th and PBCIT, Fig. 2) and a polymer acceptor NDP-V-C7 (Fig. 3). The structure of the Cascade energy levels was formed after the second donor was added, which resulted in the ternary all-PSCs being benefited by charge transfer for electrons and holes. When 15 wt% PBCIT was added to the PTB7-Th:NDP-V-C7 host blend, the RMS of the ternary blend film was between the PTB7-Th:NDP-V-C7 and PBCIT:NDP-V-C7 blend films. A suitable rough surface may provide a sufficient phase separation to enable exciton dissociation, whereas a smooth interface encourages the transportation of the load. The PBCIT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTB7-Th
PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDP-V-C7 devices with 0.85
NDP-V-C7 devices with 0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15
0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio had a champion PCE of 9.03% with a Jsc of 16.77 mA cm−2, a Voc of 0.78 V and a PCE of 68.07%.
1 weight ratio had a champion PCE of 9.03% with a Jsc of 16.77 mA cm−2, a Voc of 0.78 V and a PCE of 68.07%.
In 2019, Liu et al.165 introduced the J71 to the PDBD-T:PNDI-2T-TR(5) host donor–acceptor blend. The absorption of J71 is complementary to the absorption gap of PBDB-T and PNDI-2T-TR(5), showing a cascade energy-level alignment and Förster resonance energy transfer between PBDB-T and J71. Because of the disparity in miscibility, the second donor J71 not only optimizes the morphology in the horizontal direction, but also causes the donor and acceptor to be vertically dispersed. The best morphology eventually led to a best PCE of 9.12% with Jsc of 14.63 mA cm−2, Voc of 0.88 V and FF of 71.02%.
The biggest challenge for all-PSCs is optimizing the morphology of the active layer. The ternary strategy provides an effective way to regulate the morphology, for instance, tuning the molecular packing, phase separation and domain size. Therefore, there is much more effort that needs to be paid on the PD1/PD2/PA ternary devices to improve the efficiency, while maintaining the high morphology stability.
4.3 Other systems containing small molecule donors
In the ternary PSCs based on two polymer donor components (PD1/PD2/SMA), the introduction of the second donor polymer usually results in disrupting the crystallization of the main donor component.127 The use of a small molecule donor as the third component in non-fullerene ternary NF-PSCs consisting of the PD1/SMD2/SMA system will facilitate the phase separation into the purer phase, and induce the host donor's higher crystallinity.166,167 Polymer solar cells have always been the focus of attention, whereas there is a noticeable gap between the SM solar cells and polymer solar cells, no matter the performance or research scale. Small donors of molecules, however, also have a clear chemical composition, exceptionally high purity and outstanding reproducibility, which are special advantages over the donors of polymers.168–170In 2017, Liu and co-workers171 selected PTB7-Th:p-DTS(FBTTh2)2 and P3HT:p-DTS(FBTTh2)2 (Fig. 2 and 3) as two kinds of combinations for the co-donor materials. The two ternary systems (PTB7-Th:p-DTS(FBTTh2)2:DPP(E-BODIPY)2-HD and P3HT:p-DTS(FBTTh2)2:DPP(E-BODIPY)2-HD) gave a champion PCE of 2.84% and 1.62%, respectively. Kong et al.172 employ a general strategy to increase the Jsc and FF of PSCs by introducing intermolecular hydrogen bonds. They introduced to a PTB7-Th:ITIC binary system two coumarin derivatives with similar absorption spectrum, coumarin 7 (C7, Fig. 19), and coumarin 30 (C30, Fig. 19), as an additional donor to build ternary PSCs. The FT-IR spectra indicate that the hydrogen bonds occurred between C7 and ITIC, which enhanced the movement of electrons, enabled the quick and effective transport of charges, and thus increased Jsc. However, the C30 did not form any hydrogen bond between C30 and ITIC. Furthermore, the hydrogen bond interactions improved the ternary blend film morphology. The AFM shows the ternary blend films containing 5, 10, and 20 wt% C7. The lower RMS roughnesses indicate the HOMO generous surface morphology. Taken together, all of these factors decided the PTB7-Th:C7:ITIC ternary devices with a weight ratio 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 had an outstanding PCE of 10.16%, yet other devices had a lower PCE of 8.04%.
1.25 had an outstanding PCE of 10.16%, yet other devices had a lower PCE of 8.04%.
Dai et al.173 reported a non-fullerene PSCs using PTB7-Th:FOIC as the host binary blend, and the SMD TR (Fig. 19) as the third component. When the TR weight ratio is 25% with respect to the donor component, the devices yield the best PCE of 13.1%, which is higher than the reference binary PSCs (12.1%). The PTB7-Th:FOIC binary blends show a weaker photo response below 450 nm, but the ternary blends show enhanced EQE in 300–550 nm. Thus, adding TR increases the ternary devices absorption. In terms of the effect of TR on the ternary blend film morphology, two donors could totally be miscible together. Thanks to TR's strong crystallinity, the stacking of PTB7-Th results in improved hole mobility, which is useful for higher FF.
In 2019, Song and co-workers174 chose a SMD consisting of an IDT core coupled with thiophene and difluorobenzothiadiazole, BIT-4F-T (Fig. 19), as the third component. Adding 10 wt% BIT-4F-T into the PTB7-Th:IEICO-4F binary BHJ film not only facilitated more efficient exciton separation and extraction, but also reduced both the geminate and non-geminate recombination. The addition of the second donor promotes the dissociation and extraction of the charge, thereby increasing the FF and Jsc of the ternary solar cell. In addition, the optimized surface morphology improved the exciton separation and transport. All of these improvements yield a PCE of 14.0% with a Jsc of 27.3 mA cm−2, a Voc of 0.72 V, and an FF of 70.9%, in the best devices.
Recently, Lee119 synthesized three SMD based on a fused benzo[1,2-b:4,5-b′]dithiophene (BDT) core and 3-ethylrhodanine (RD) terminal groups with structurally different π-conjugation systems (SM-axis series), where SM-X, SM-Y, and SM-XY (Fig. 19) are horizontal-, vertical-, and cross-type structures, respectively. After a series of experiments, the ternary NF-PSCs based on SM-X and SM-Y were found to act as an alloy model, with the transfer of energy for the former and transfer of charge for the latter. Yet another NF-PSCs dependent on SM-XY only forms energy transfer because of its strong crystalline properties. The SM-X-based NF-PSCs had a champion PCE of 11.96%, the SM-Y-based NF-PSCs had a PCE of 11.48%, and the SM-XY-based devices had a PCE of 10.21%.
There are two types of NF-PSCs, SMD1/SMD2/PA and all-SM, that are far behind in development with the other three types. Only a few of these two types of batteries have been designed and manufactured in the past few years. In 2018, Zhang and co-workers175 made a NF-PSCs based on the SMD1/SMD2/PA model. They used two SM materials, DR3TBDTT and BTR (Fig. 19), as the co-donor and the P-BNBP-fBT (Fig. 3) as PA. GIWAXS showed that the molecular donors adopt a face-on orientation in the ternary blend, which is favorable for charge transporting in the PSCs devices. Compared with the DR3TBDTT:P-BNBP-fBT blend, the domain sizes of the ternary blend decreased to 65 nm, and the reduced domain sizes increased the donor/acceptor interface area, thereby higher Jsc was obtained in the ternary blend. Finally, the ternary devices got a higher PCE of 4.82% with a low Jsc of 7.39 mA cm−2, but a high Voc of 1.18 V and an FF of 55.6%.
In 2019, Chang et al.110 carefully designed and synthesized a medium bandgap small-molecule donor, DR3TBDTT-S-E (Fig. 19), as the third component for two all-SM ternary PSCs (DR3TBDTT:DR3TBDTT-S-E:PC71BM, DCAO3TBDTT:DR3TBDTT-S-E:IDIC). The second donor expands the absorption range. When adding the 5 wt% DR3TBDTT-S-E into DR3TBDTT:PC71BM binary blends, the light harvesting in 300–600 nm wavelength was improved, and a significant improvement in the light harvesting in the wavelength region from 450 to 750 nm was achieved in the DCAO3TBDTT:DR3TBDTT-S-E:IDIC non-fullerene system. Compared to the binary process, adding a large amount of second donor to both fullerene and non-fullerene structures offers comparatively higher interface morphology and a more modest phase separation. Compared to the DCAO3TBDTT:ITIC binary blend, the PCE of fullerene-based and nonfullerene-based ternary devices had increased from 9.49% to 10.38% and 10.04%, respectively.
Another all-SM NF-PSCs (SM:SM-Cl:IDIC) was constructed by Deng et al. in 2019.176 Two small molecule materials (SM, SM-Cl, Fig. 19) with similar structures were used as donors to build a ternary PSCs device. An analysis of the PL spectra (Fig. 20b) shows a better charge transfer between donors and acceptors. The α value (denotes a factor related to bimolecular recombination, shown in Fig. 20c) of the ternary, SM-based, and SM-Cl-based binary devices were 0.993, 0.978 and 0.961, respectively. The highest α value in the ternary cell represents the weakest bimolecular recombination, which improved the Jsc and FF of the ternary PSCs. The Jph/Jsat of the optimized ternary, SM-based and SM-Cl based devices were 0.971, 0.963 and 0.941, respectively. A biggest Jph/Jsat value of ternary PSCs indicated a best exciton dissociation and charge extraction, which also improved Jsc and FF. The decreased crystallinity of the mixed-donor improved the phase-separated morphology. Thus, the ternary devices doped with 10% SM-Cl exhibited distinctly improved PCE of 10.29% with Jsc of 16.05 mA cm−2, high Voc of 0.921 V and FF of 69.58%.
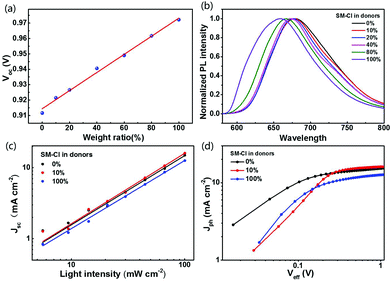 | ||
| Fig. 20 (a) Voc as a function of the SM-Cl weight ratio in the blend films. (b) Photoluminescence spectra of the binary and ternary blend films. (c) Dependence of Jsc on the light intensity for the binary and ternary blend films. (d) Photocurrent density (Jph) as a function of the effective voltage (Veff) for the binary and ternary blend films.176 | ||
5. Summary and outlook
Ternary PSCs based non-fullerene accepters have demonstrated an effective strategy to overcome the limitations of binary PSCs. Compared to ternary PSCs with two NFAs, the ternary PSCs with two donors (especially two polymer donors) exhibit great potential in obtaining stable morphology of active layer, thus leading to higher devices stability. However, the D1:D2:A NF-PSCs have been developed more slowly, mainly because of the limited entropy of polymers, resulting in a strong phase separation between two polymers. In addition, NFAs are very picky about donor materials with matched energy levels and compatible morphology. This review outlines the functions of the third components and the four fundamental mechanisms, and then summarizes the development of D1:D2:A NF-PSCs in recent years by different combination of donors and acceptors. The outlook of the development of D1:D2:A NF-PSCs are summarized as follows:(1) Guidelines for selecting a suitable second donor should be constructed by studying the relationships between molecular structure, energy level, miscibility, and aggregation of materials. Generally, the photovoltaic performances of the D1:D2:A ternary devices are much lower than that of the best photovoltaic performances of binary PSCs. This is because of the difficulty in obtaining ideal phase separation domains. In the state of the art, the selection of second donor is still processed by trial and error. Therefore, a clear guideline for selecting the suitable second donor by distinguishing the molecular structure, energy level, miscibility, and aggregation should be studied in detail.
(2) The biggest advantage for D1:D2:A NF-PSCs is the morphology stability of the devices. To integrate the photovoltaic performance and device stability performances, different types of D1:D2:A NF-PSCs should be further studied, especially with the all-polymer donor and acceptor system, for instance, incorporating a relatively more flexible donor material to the all-polymer system to destroy the over self-aggregations, which will retain good morphology stability and high photovoltaic efficiency at the same time. In addition, the mechanism for the stable morphology should be studied in detail to promote the development of PSCs commercialization. Besides, to achieve the high photovoltaic and stability performance, careful morphology regulation and phase separation (including the construction of the phase diagram) study should be complemented.
(3) The energy loss of the binary devices has been widely studied. However, the energy loss of the ternary devices, especially for D1:D2:A NF-PSCs, have rarely been studied. This is because of the more complicated material energy levels and the absent effective study object. However, this must be an important research issue for ternary devices, which concerns deeply understanding the energy transfer process, minimizing the energy losses and further improving the photovoltaic performance.
(4) To satisfy different application requirements for PSCs (thick active layer, semi-transparent, colorful, flexible), PSCs devices are the future development directions of PSCs. Based on these property characteristics, there are different requirements for donor and acceptor materials in D1:D2:A NF-PSCs. As for the thick active layer devices, the higher crystallinity and orientation are greatly needed for the efficient charge transport in the thick active layer. Therefore, the incorporation of the third component should increase or at least keep the aggregation properties of the host materials to ensure the high charge transport efficiency and reduce the charge recombination rate.
In conclusion, developing D1:D2:A NF-PSCs benefits fabricating high photovoltaic performance and morphology stable devices without additional processes, which satisfies the future commercialization requirements of an easy process and low cost manufacturing, and the related morphology stabilization mechanism studies will promote the commercialization of PSCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
(1) The Natural Scientific Foundation of China (Grant No. 51273096 and 51473082), (2) State Key Project of International Cooperation Research (2017YFE0108300 and 2016YFE0110800), (3) Shandong Double-Hundred Project, (4) the Program for Introducing Talents of Discipline to Universities (“111” plan), (5) Qingdao Basic Research on Application of Science and Technology Plan (19-6-2-82-cg), (6) The National One-Thousand Foreign Expert Program (Grant No. WQ20123700111), (7) The 1st Level Discipline Program of Shandong Province of China, and (8) Research Start-Up Funds of Qingdao University.Notes and references
- S. J. Jeon, Y. W. Han and D. K. Moon, Small, 2019, 15, 1902598 CrossRef CAS.
- M.-A. Pan, T.-K. Lau, Y. Tang, Y.-C. Wu, T. Liu, K. Li, M.-C. Chen, X. Lu, W. Ma and C. Zhan, J. Mater. Chem. C, 2019, 7, 20713–20722 RSC.
- T. Yan, W. Song, J. Huang, R. Peng, L. Huang and Z. Ge, Adv. Mater., 2019, 31, 1902210 CrossRef.
- S. Pang, R. Zhang, C. Duan, S. Zhang, X. Gu, X. Liu, F. Huang and Y. Cao, Adv. Eng. Mater., 2019, 9, 1901740 Search PubMed.
- W. Shen, G. Zhao, X. Zhang, F. Bu, J. Yun and J. Tang, Nanomaterials, 2020, 10, 944 CrossRef CAS.
- M. Grätzel, Nature, 2001, 6861, 338–344 CrossRef.
- S. Pang, L. Liu, X. Sun, S. Dong, Z. Wang, R. Zhang, Y. Guo, W. Li, N. Zheng, C. Duan, F. Huang and Y. Cao, Macromol. Rapid Commun., 2019, 40, 1900120 CrossRef.
- T. M. Razykov, C. S. Ferekides, D. Morel, E. Stefanakos, H. S. Ullal and H. M. Upadhyaya, Sol. Energy, 2011, 85, 1580–1608 CrossRef CAS.
- K. L. Chopra, P. D. Paulson and V. Dutta, Prog. Photovoltaics, 2004, 12, 69–92 CAS.
- L. Y. Lu, M. A. Kelly, W. You and L. P. Yu, Nat. Photonics, 2015, 9, 491–500 CrossRef CAS.
- G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 16, 2198–2200 RSC.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS.
- W. R. Mateker, J. D. Douglas, C. Cabanetos, I. T. Sachs-Quintana, J. A. Bartelt, E. T. Hoke, A. El Labban, P. M. Beaujuge, J. M. J. Fréchet and M. D. McGehee, Energy Environ. Sci., 2013, 6, 2529–2537 RSC.
- F. J. M. Colberts, M. M. Wienk, R. Heuvel, W. Li, V. M. Le Corre, L. J. A. Koster and R. A. J. Janssen, Adv. Energy Mater., 2018, 8, 1802197 CrossRef.
- H. Li, T. Earmme, S. Subramaniyan and S. A. Jenekhe, Adv. Energy Mater., 2015, 5, 1402041 CrossRef.
- L. Cattin, Z. El Jouad, L. Arzel, G. Neculqueo, M. Morsli, F. Martinez, M. Addou and J. C. Bernède, Sol. Energy, 2018, 171, 621–628 CrossRef CAS.
- Z. S. Wang, X. Ren, X. Xu, Q. Peng, W. E. I. Sha and W. C. H. Choy, Nano Energy, 2018, 51, 206–215 CrossRef CAS.
- B. C. Thompson and J. M. Frechet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS.
- F. Oba and Y. Kumagai, Appl. Phys. Express, 2018, 11, 060101 CrossRef.
- Q. Zafar and Z. Ahmad, J. Mater. Sci.: Mater. Electron., 2018, 29, 11144–11150 CrossRef CAS.
- E. Tatsi, M. Spanos, A. Katsouras, B. M. Squeo, O. A. Ibraikulov, N. Zimmermann, T. Heiser, P. Lévêque, V. G. Gregoriou, A. Avgeropoulos, N. Leclerc and C. L. Chochos, Macromol. Chem. Phys., 2019, 220, 1800418 CrossRef.
- M. Wright, R. Lin, M. J. Y. Tayebjee and G. Conibeer, Solar RRL, 2017, 1, 1700035 CrossRef.
- Z. Xiao, K. Sun, J. Subbiah, T. Qin, S. Lu, B. Purushothaman, D. J. Jones, A. B. Holmes and W. W. H. Wong, Polym. Chem., 2015, 6, 2312–2318 RSC.
- J. Munshi, U. F. Ghumman, A. Iyer, R. Dulal, W. Chen, T. Chien and G. Balasubramanian, J. Polym. Sci., Part B: Polym. Phys., 2019, 57, 895–903 CrossRef CAS.
- Y. Zhang, X. Xu, J. Lu and S. Zhang, RSC Adv., 2019, 9, 14657–14661 RSC.
- W. Shen, M. Xiao, J. Tang, X. Wang, W. Chen, R. Yang, X. Bao, Y. Wang, J. Jiao, L. Huang, J. Liu, W. Wang and L. A. Belfiore, RSC Adv., 2015, 5, 47451–47457 RSC.
- V. Vohra, K. Kawashima, T. Kakara, T. Koganezawa, I. Osaka, K. Takimiya and H. Murata, Nat. Photonics, 2015, 9, 403–408 CrossRef CAS.
- M. B. Upama, M. A. Mahmud, G. Conibeer and A. Uddin, Solar RRL, 2019, 4, 1900342 CrossRef.
- Z. Lin, L. Zhang, S. Tu, W. Wang and Q. Ling, Sol. Energy, 2020, 201, 489–498 CrossRef CAS.
- W. Feng, Z. Lin, J. Cui, W. Lv, W. Wang and Q. Ling, Sol. Energy Mater. Sol. Cells, 2019, 200, 109982 CrossRef CAS.
- H. Jiang, X. Li, Z. Liang, G. Huang, W. Chen, N. Zheng and R. Yang, J. Mater. Chem. A, 2019, 7, 7760–7765 RSC.
- J. Yuan, T. Huang, P. Cheng, Y. Zou, H. Zhang, J. L. Yang, S. Y. Chang, Z. Zhang, W. Huang, R. Wang, D. Meng, F. Gao and Y. Yang, Nat. Commun., 2019, 10, 570 CrossRef CAS.
- H. Yin, K. L. Chiu, P. Bi, G. Li, C. Yan, H. Tang, C. Zhang, Y. Xiao, H. Zhang, W. Yu, H. Hu, X. Lu, X. Hao and S. K. So, Adv. Electron. Mater., 2019, 5, 1900497 CrossRef CAS.
- K. Midori, T. Fukuhara, Y. Tamai, H. Do Kim and H. Ohkita, ChemPhysChem, 2019, 20, 2683–2688 CrossRef CAS.
- C.-H. Chen, Y.-J. Lu, Y.-W. Su, Y.-C. Lin, H.-K. Lin, H.-C. Chen, H.-C. Wang, J.-X. Li, K.-H. Wu and K.-H. Wei, Org. Electron., 2019, 71, 185–193 CrossRef CAS.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Tang, B. Lin, K. Xian, B. Gao, C. An, P. Bi, W. Ma and J. Hou, Natl. Sci. Rev., 2020, 7, 1239–1246 CrossRef.
- G. Dennler, M. C. Scharber, T. Ameri, P. Denk, K. Forberich, C. Waldauf and C. J. Brabec, Adv. Mater., 2008, 20, 579–583 CrossRef CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T. Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222–225 CrossRef CAS.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding and R. Xia, Ence, 2018, 361, 1094–1098 CrossRef CAS.
- X. Du, B. Liu, L. Li, X. Kong, C. Zheng, H. Lin, Q. Tong, S. Tao and X. Zhang, J. Mater. Chem. A, 2018, 6, 23840–23855 RSC.
- A. D. Rao, M. G. Murali, A. V. Kesavan and P. C. Ramamurthy, Sol. Energy, 2018, 174, 1078–1084 CrossRef CAS.
- N. Gasparini, S. Kahmann, M. Salvador, J. D. Perea, A. Sperlich, A. Baumann, N. Li, S. Rechberger, E. Spiecker, V. Dyakonov, G. Portale, M. A. Loi, C. J. Brabec and T. Ameri, Adv. Energy Mater., 2019, 9, 1803394 CrossRef.
- B. Qi and J. Wang, Phys. Chem. Chem. Phys., 2013, 15, 8972–8982 RSC.
- X. Zhang, D. Zhang, Q. Zhou, R. Wang, J. Zhou, J. Wang, H. Zhou and Y. Zhang, Nano Energy, 2019, 56, 494–501 CrossRef CAS.
- D. Liu, P. Fan, D. Zhang, X. Zhang and J. Yu, Sol. Energy, 2019, 189, 186–193 CrossRef CAS.
- A. A. Mohapatra, V. Kim, B. Puttaraju, A. Sadhanala, X. Jiao, C. R. McNeill, R. H. Friend and S. Patil, ACS Appl. Energy Mater., 2018, 1, 4874–4882 CrossRef CAS.
- R. Datt, R. Sharma, S. Bishnoi and V. Gupta, Mater. Lett., 2019, 251, 122–125 CrossRef CAS.
- R. Po, A. Bernardi, A. Calabrese, C. Carbonera, G. Corso and A. Pellegrino, Energy Environ. Sci., 2014, 7, 925–943 RSC.
- T. Kumari, S. M. Lee, K. C. Lee, Y. Cho and C. Yang, Adv. Energy Mater., 2018, 8, 1800616 CrossRef.
- Z. Li, D. Tang, Z. Ji, W. Zhang, X. Xu, K. Feng, Y. Li and Q. Peng, J. Mater. Chem. C, 2018, 6, 9119–9129 RSC.
- M. Koppe, H.-J. Egelhaaf, G. Dennler, M. C. Scharber, C. J. Brabec, P. Schilinsky and C. N. Hoth, Adv. Funct. Mater., 2010, 20, 338–346 CrossRef CAS.
- Y. Xie, L. Huo, B. Fan, H. Fu, Y. Cai, L. Zhang, Z. Li, Y. Wang, W. Ma, Y. Chen and Y. Sun, Adv. Funct. Mater., 2018, 28, 1800627 CrossRef.
- M. Xiao, K. Zhang, Y. Jin, Q. Yin, W. Zhong, F. Huang and Y. Cao, Nano Energy, 2018, 48, 53–62 CrossRef CAS.
- K.-N. Zhang, X.-Y. Yang, M.-S. Niu, Z.-C. Wen, Z.-H. Chen, L. Feng, X.-J. Feng and X.-T. Hao, Org. Electron., 2019, 66, 13–23 CrossRef CAS.
- E. A. A. Arbab, B. Gebremichael, A. Kumar and G. T. Mola, J. Mod. Opt., 2018, 66, 399–406 CrossRef.
- W. Wang, M. Du, M. Zhang, J. Miao, Y. Fang and F. Zhang, Adv. Opt. Mater., 2018, 6, 1800249 CrossRef.
- M. Vartanian, P. de la Cruz, S. Biswas, G. D. Sharma and F. Langa, Nanoscale, 2018, 10, 12100–12108 RSC.
- V. Piradi, X. Xu, Z. Wang, J. Ali, Q. Peng, F. Liu and X. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 6283–6291 CrossRef CAS.
- Y. Chen, L. Yang, J. Wu, G. Wang, W. Huang, F. S. Melkonyan, Z. Lu, Y. Huang, T. J. Marks and A. Facchetti, Chem. Mater., 2018, 30, 6810–6820 CrossRef CAS.
- Y. Zhu, A. Gadisa, Z. Peng, M. Ghasemi, L. Ye, Z. Xu, S. Zhao and H. Ade, Adv. Energy Mater., 2019, 9, 1900376 CrossRef.
- L. Duan, Y. Zhang, H. Yi, F. Haque, R. Deng, H. Guan, Y. Zou and A. Uddin, Energy Technol., 2019, 8, 1900924 CrossRef.
- Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470–488 RSC.
- A. Karki, J. Vollbrecht, A. L. Dixon, N. Schopp, M. Schrock, G. N. M. Reddy and T. Q. Nguyen, Adv. Mater., 2019, 31, 1903868 CrossRef CAS.
- Y. Zhong, M. T. Trinh, R. Chen, W. Wang, P. P. Khlyabich, B. Kumar, Q. Xu, C. Y. Nam, M. Y. Sfeir, C. Black, M. L. Steigerwald, Y. L. Loo, S. Xiao, F. Ng, X. Y. Zhu and C. Nuckolls, J. Am. Chem. Soc., 2014, 136, 15215–15221 CrossRef CAS.
- Y. Zhong, M. T. Trinh, R. Chen, G. E. Purdum, P. P. Khlyabich, M. Sezen, S. Oh, H. Zhu, B. Fowler, B. Zhang, W. Wang, C. Y. Nam, M. Y. Sfeir, C. T. Black, M. L. Steigerwald, Y. L. Loo, F. Ng, X. Y. Zhu and C. Nuckolls, Nat. Commun., 2015, 6, 8242 CrossRef CAS.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955–4961 CrossRef CAS.
- Y. Lin, Z.-G. Zhang, H. Bai, J. Wang, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610–616 RSC.
- M. Chen, W. Fu, M. Shi, X. Hu, J. Pan, J. Ling, H. Li and H. Chen, J. Mater. Chem. A, 2013, 1, 105–111 RSC.
- J. W. Jung and W. H. Jo, Chem. Mater., 2015, 27, 6038–6043 CrossRef CAS.
- X. Ma, J. Wang, J. Gao, Z. Hu, C. Xu, X. Zhang and F. Zhang, Adv. Energy Mater., 2020, 10, 2001404 CrossRef CAS.
- H. Jiang, C. Han, Y. Li, F. Bi, N. Zheng, J. Han, W. Shen, S. Wen, C. Yang and R. Yang, Adv. Funct. Mater., 2020, 2007088, DOI:10.1002/adfm.202007088.
- S. Liu, P. You, J. Li, J. Li, C.-S. Lee, B. S. Ong, C. Surya and F. Yan, Energy Environ. Sci., 2015, 8, 1463–1470 RSC.
- M. Zhang, F. Zhang, J. Wang, Q. An and Q. Sun, J. Mater. Chem. C, 2015, 3, 11930–11936 RSC.
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562–1564 CrossRef CAS.
- W. Jiang, R. Yu, Z. Liu, R. Peng, D. Mi, L. Hong, Q. Wei, J. Hou, Y. Kuang and Z. Ge, Adv. Mater., 2018, 30, 1703005 CrossRef.
- R. Yu, H. Yao, L. Hong, Y. Qin, J. Zhu, Y. Cui, S. Li and J. Hou, Nat. Commun., 2018, 9, 4645 CrossRef.
- W. Yang, Z. Luo, R. Sun, J. Guo, T. Wang, Y. Wu, W. Wang, J. Guo, Q. Wu, M. Shi, H. Li, C. Yang and J. Min, Nat. Commun., 2020, 11, 1218 CrossRef CAS.
- Z. Li, X. Xu, W. Zhang, X. Meng, Z. Genene, W. Ma, W. Mammo, A. Yartsev, M. R. Andersson, R. A. J. Janssen and E. Wang, Energy Environ. Sci., 2017, 10, 2212–2221 RSC.
- H. Li, T. Earmme, G. Ren, A. Saeki, S. Yoshikawa, N. M. Murari, S. Subramaniyan, M. J. Crane, S. Seki and S. A. Jenekhe, J. Am. Chem. Soc., 2014, 136, 14589–14597 CrossRef CAS.
- M. Nam, J. h. Kang, J. Shin, J. Na, Y. Park, J. Cho, B. Kim, H. H. Lee, R. Chang and D. H. Ko, Adv. Energy Mater., 2019, 9, 1901856 CrossRef.
- Y. Wang, T. Wang, J. Chen, H. D. Kim, P. Gao, B. Wang, R. Iriguchi and H. Ohkita, Dyes Pigm., 2018, 158, 213–218 CrossRef CAS.
- L. Li, H. Lin, X. Kong, X. Du, X. Chen, L. Zhou, S. Tao, C. Zheng and X. Zhang, Nanoscale, 2018, 10, 9971–9980 RSC.
- L. Cattin, C. Cabanetos, A. El Mahlali, L. Arzel, M. Morsli, P. Blanchard and J. C. Bernède, Org. Electron., 2020, 76, 105463 CrossRef CAS.
- X. Du, H. Lin, X. Chen, S. Tao, C. Zheng and X. Zhang, Nanoscale, 2018, 10, 16455–16467 RSC.
- J. Lee, S. M. Lee, S. Chen, T. Kumari, S. H. Kang, Y. Cho and C. Yang, Adv. Mater., 2019, 31, 1804762 CrossRef.
- T. Ameri, P. Khoram, J. Min and C. J. Brabec, Adv. Mater., 2013, 25, 4245–4266 CrossRef CAS.
- Q. S. An, F. J. Zhang, J. Zhang, W. H. Tang, Z. B. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 RSC.
- H. Lu, X. J. Xu and Z. S. Bo, Sci. China Mater., 2016, 59, 444–458 CrossRef CAS.
- Y. J. Hwang, H. Li, B. A. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124–131 CrossRef CAS.
- T. Liu, Z. Luo, Y. Chen, T. Yang, Y. Xiao, G. Zhang, R. Ma, X. Lu, C. Zhan, M. Zhang, C. Yang, Y. Li, J. Yao and H. Yan, Energy Environ. Sci., 2019, 12, 2529–2536 RSC.
- C. Liu, C. Yi, K. Wang, Y. Yang, R. S. Bhatta, M. Tsige, S. Xiao and X. Gong, ACS Appl. Mater. Interfaces, 2015, 7, 4928–4935 CrossRef CAS.
- D. C. Watters, H. Yi, A. J. Pearson, J. Kingsley, A. Iraqi and D. Lidzey, Macromol. Rapid Commun., 2013, 34, 1157–1162 CrossRef CAS.
- X. Xu, Y. Wu, J. Fang, Z. Li, Z. Wang, Y. Li and Q. Peng, Chemistry, 2014, 20, 13259–13271 CrossRef CAS.
- L. Duan, Y. Zhang, R. Deng, H. Yi and A. Uddin, ACS Appl. Energy Mater., 2020, 3, 5792–5803 CrossRef CAS.
- X. Y. Liu, Y. J. Yan, Y. Yao and Z. Q. Liang, Adv. Funct. Mater., 2018, 28, 1802004 CrossRef.
- X. Ma, Y. Mi, F. Zhang, Q. An, M. Zhang, Z. Hu, X. Liu, J. Zhang and W. Tang, Adv. Energy Mater., 2018, 8, 1702854 CrossRef.
- Y.-Y. Yu, T.-W. Tsai and C.-P. Chen, J. Phys. Chem. C, 2018, 122, 24585–24591 CrossRef CAS.
- X. Liu, J. Wang, J. Peng and Z. Liang, Macromolecules, 2017, 50, 6954–6960 CrossRef CAS.
- Z. Li, R. Xie, W. Zhong, B. Fan, J. Ali, L. Ying, F. Liu, N. Li, F. Huang and Y. Cao, Solar RRL, 2018, 2, 1800196 CrossRef.
- W. Chen, H. Jiang, G. Huang, J. Zhang, M. Cai, X. Wan and R. Yang, Solar RRL, 2018, 2, 1800101 CrossRef.
- K. Zhou, X. Zhou, X. Xu, C. Musumeci, C. Wang, W. Xu, X. Meng, W. Ma and O. Inganas, Polymers, 2019, 11, 1665 CrossRef CAS.
- Q. Liang, J. Han, C. Song, X. Yu, D.-M. Smilgies, K. Zhao, J. Liu and Y. Han, J. Mater. Chem. A, 2018, 6, 15610–15620 RSC.
- H. Kang, W. Lee, J. Oh, T. Kim, C. Lee and B. J. Kim, Acc. Chem. Res., 2016, 49, 2424–2434 CrossRef CAS.
- L. Zhong, L. Gao, H. Bin, Q. Hu, Z.-G. Zhang, F. Liu, T. P. Russell, Z. Zhang and Y. Li, Adv. Energy Mater., 2017, 7, 1602215 CrossRef.
- H. Fan, H. Shang, Y. Li and X. Zhan, Appl. Phys. Lett., 2010, 97, 133302 CrossRef.
- Y. Sun, C. Cui, H. Wang and Y. Li, Adv. Energy Mater., 2011, 1, 1058–1061 CrossRef CAS.
- M. A. Adil, J. Zhang, Y. Wang, J. Yu, C. Yang, G. Lu and Z. Wei, Nano Energy, 2020, 68, 104271 CrossRef CAS.
- C.-H. Wu, C.-C. Chueh, Y.-Y. Xi, H.-L. Zhong, G.-P. Gao, Z.-H. Wang, L. D. Pozzo, T.-C. Wen and A. K. Y. Jen, Adv. Funct. Mater., 2015, 25, 5326–5332 CrossRef CAS.
- S. Li, W. Liu, C. Z. Li, M. Shi and H. Chen, Small, 2017, 13, 1701120 CrossRef.
- Y. Chang, Y. Chang, X. Zhu, X. Zhou, C. Yang, J. Zhang, K. Lu, X. Sun and Z. Wei, Adv. Energy Mater., 2019, 9, 1900190 CrossRef.
- T. Kumari, S. M. Lee, S.-H. Kang, S. Chen and C. Yang, Energy Environ. Sci., 2017, 10, 258–265 RSC.
- M. Zhang, X. Guo, W. Ma, H. Ade and J. Hou, Adv. Mater., 2014, 26, 5880–5885 CrossRef CAS.
- A. D. de Zerio and C. Müller, Adv. Energy Mater., 2018, 8, 1702741 CrossRef.
- P. Cheng, C. Yan, T. K. Lau, J. Mai, X. Lu and X. Zhan, Adv. Mater., 2016, 28, 5822–5829 CrossRef CAS.
- Y. Dong, Y. Zou, J. Yuan, H. Yang, Y. Wu, C. Cui and Y. Li, Adv. Mater., 2019, 31, 1904601 CrossRef CAS.
- Q. An, F. Zhang, W. Gao, Q. Sun, M. Zhang, C. Yang and J. Zhang, Nano Energy, 2018, 45, 177–183 CrossRef CAS.
- H. Hu, L. Ye, M. Ghasemi, N. Balar, J. J. Rech, S. J. Stuard, W. You, B. T. O’Connor and H. Ade, Adv. Mater., 2019, 31, 1808279 CrossRef.
- Z. Wang, Y. Nian, H. Jiang, F. Pan, Z. Hu, L. Zhang, Y. Cao and J. Chen, Org. Electron., 2019, 69, 174–180 CrossRef CAS.
- S. M. Lee, T. Kumari, B. Lee, Y. Cho, J. Lee, J. Oh, M. Jeong, S. Jung and C. Yang, Small, 2020, 1905309 CrossRef CAS.
- Z. Li, L. Ying, R. Xie, P. Zhu, N. Li, W. Zhong, F. Huang and Y. Cao, Nano Energy, 2018, 51, 434–441 CrossRef CAS.
- L. Yang, H. Zhou, S. C. Price and W. You, J. Am. Chem. Soc., 2012, 134, 5432–5435 CrossRef CAS.
- Z. Guo, Z. He, M. Sun, H. Zhang, Y. Xu, X. Li, C. Liang and X. Jing, Polymer, 2018, 153, 398–407 CrossRef CAS.
- B. Xu, G. Saianand, V. A. L. Roy, Q. Qiao, K. M. Reza and S. W. Kang, Polymers, 2019, 11, 1423 CrossRef CAS.
- N. Yi, Q. Ai, W. Zhou, L. Huang, L. Zhang, Z. Xing, X. Li, J. Zeng and Y. Chen, Chem. Mater., 2019, 31, 10211–10224 CrossRef CAS.
- L. Yang, L. Yan and W. You, J. Phys. Chem. Lett., 2013, 4, 1802–1810 CrossRef CAS.
- W. Zhang, J. Huang, J. Xu, M. Han, D. Su, N. Wu, C. Zhang, A. Xu and C. Zhan, Adv. Energy Mater., 2020, 16, 2001436 CrossRef.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS.
- Q. An, J. Wang and F. Zhang, Nano Energy, 2019, 60, 768–774 CrossRef CAS.
- L. Zhong, Y. Li, H. Bin, M. Zhang, H. Huang, Q. Hu, Z.-Q. Jiang, F. Liu, T. P. Russell, Z.-G. Zhang, Z. Zhang and Y. Li, Org. Electron., 2018, 62, 89–94 CrossRef CAS.
- P. Bi, F. Zheng, X. Yang, M. Niu, L. Feng, W. Qin and X. Hao, J. Mater. Chem. A, 2017, 5, 12120–12130 RSC.
- C. Tsai, Y. Su, P.-C. Lin, C.-C. Shih, H.-C. Wu, W.-C. Chen and C.-C. Chueh, J. Mater. Chem. C, 2018, 6, 6920–6928 RSC.
- C. H. Li, C. C. Chang, Y. H. Hsiao, S. H. Peng, Y. J. Su, S. W. Heo, K. Tajima, M. C. Tsai, C. Y. Lin and C. S. Hsu, ACS Appl. Mater. Interfaces, 2019, 11, 1156–1162 CrossRef CAS.
- H. Yao, Y. Cui, R. Yu, B. Gao, H. Zhang and J. Hou, Angew. Chem., Int. Ed., 2017, 56, 3045–3049 CrossRef CAS.
- J. Lee, J. W. Kim, S. A. Park, S. Y. Son, K. Choi, W. Lee, M. Kim, J. Y. Kim and T. Park, Adv. Energy Mater., 2019, 9, 1901829 CrossRef.
- Y. Tang, H. Sun, Z. Wu, Y. Zhang, G. Zhang, M. Su, X. Zhou, X. Wu, W. Sun, X. Zhang, B. Liu, W. Chen, Q. Liao, H. Y. Woo and X. Guo, Adv. Sci., 2019, 6, 1901773 CrossRef CAS.
- L. Zhang, X. Xu, B. Lin, H. Zhao, T. Li, J. Xin, Z. Bi, G. Qiu, S. Guo, K. Zhou, X. Zhan and W. Ma, Adv. Mater., 2018, 30, 1805041 CrossRef.
- Y. Xie, T. Li, J. Guo, P. Bi, X. Xue, H. S. Ryu, Y. Cai, J. Min, L. Huo, X. Hao, H. Y. Woo, X. Zhan and Y. Sun, ACS Energy Lett., 2019, 4, 1196–1203 CrossRef CAS.
- C. Liao, M. Zhang, X. Xu, F. Liu, Y. Li and Q. Peng, J. Mater. Chem. A, 2019, 7, 716–726 RSC.
- C. Xu, J. Wang, Q. An, L. Huo and F. Zhang, Org. Electron., 2019, 71, 272–278 CrossRef CAS.
- X. Xu, K. Feng, Y. W. Lee, H. Y. Woo, G. Zhang and Q. Peng, Adv. Funct. Mater., 2020, 30, 1907570 CrossRef CAS.
- X. Xu, Z. Bi, W. Ma, Z. Wang, W. C. H. Choy, W. Wu, G. Zhang, Y. Li and Q. Peng, Adv. Mater., 2017, 29, 1704271 CrossRef.
- Y. Wang, H. D. Kim, B. Wang and H. Ohkita, J. Photopolym. Sci. Technol., 2018, 31, 177–181 CrossRef CAS.
- L. Nian, Y. Kan, H. Wang, K. Gao, B. Xu, Q. Rong, R. Wang, J. Wang, F. Liu, J. Chen, G. Zhou, T. P. Russell and A. K. Y. Jen, Energy Environ. Sci., 2018, 11, 3392–3399 RSC.
- F. Zhao, K. Wang, J. Duan, X. Zhu, K. Lu, C. Zhao, C. Zhang, H. Yu and B. Hu, Solar RRL, 2019, 3, 1900063 CrossRef.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Xu, K. Xian, B. Gao, J. Qin, J. Zhang, Z. Wei and J. Hou, Adv. Mater., 2019, 31, 1808356 CrossRef.
- Y. Wu, H. Yang, Y. Zou, Y. Dong, C. Cui and Y. Li, Solar RRL, 2018, 2, 1800060 CrossRef.
- D. Liu, Y. Zhang, L. Zhan, T.-K. Lau, H. Yin, P. W. K. Fong, S. K. So, S. Zhang, X. Lu, J. Hou, H. Chen, W.-Y. Wong and G. Li, J. Mater. Chem. A, 2019, 7, 14153–14162 RSC.
- M. Xiao, K. Zhang, S. Dong, Q. Yin, Z. Liu, L. Liu, F. Huang and Y. Cao, ACS Appl. Mater. Interfaces, 2018, 10, 25594–25603 CrossRef CAS.
- X. Ma, Q. An, O. A. Ibraikulov, P. Lévêque, T. Heiser, N. Leclerc, X. Zhang and F. Zhang, J. Mater. Chem. A, 2020, 8, 1265–1272 RSC.
- G. Xie, Z. Zhang, Z. Su, X. Zhang and J. Zhang, Nano Energy, 2020, 69, 104447 CrossRef CAS.
- J. Han, X. Wang, D. Huang, C. Yang, R. Yang and X. Bao, Macromolecules, 2020, 53, 6619–6629 CrossRef CAS.
- Q. An, J. Wang, X. Ma, J. Gao, Z. Hu, B. Liu, H. Sun, X. Guo, X. L. Zhang and F. Zhang, Energy Environ. Sci., 2020 10.1039/d0ee02516j.
- A. Facchetti, Mater. Today, 2013, 16, 123–132 CrossRef CAS.
- T. Kim, J. H. Kim, T. E. Kang, C. Lee, H. Kang, M. Shin, C. Wang, B. Ma, U. Jeong, T. S. Kim and B. J. Kim, Nat. Commun., 2015, 6, 8547 CrossRef CAS.
- S. Shi, J. Yuan, G. Ding, M. Ford, K. Lu, G. Shi, J. Sun, X. Ling, Y. Li and W. Ma, Adv. Funct. Mater., 2016, 26, 5669–5678 CrossRef CAS.
- Y. Zhou, K. L. Gu, X. Gu, T. Kurosawa, H. Yan, Y. Guo, G. I. Koleilat, D. Zhao, M. F. Toney and Z. Bao, Chem. Mater., 2016, 28, 5037–5042 CrossRef CAS.
- X. Zhan, Z. a. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246–7247 CrossRef CAS.
- L. A. Perez, K. W. Chou, J. A. Love, T. S. van der Poll, D. M. Smilgies, T. Q. Nguyen, E. J. Kramer, A. Amassian and G. C. Bazan, Adv. Mater., 2013, 25, 6380–6384 CrossRef CAS.
- B. R. Gautam, C. Lee, R. Younts, W. Lee, E. Danilov, B. J. Kim and K. Gundogdu, ACS Appl. Mater. Interfaces, 2015, 7, 27586–27591 CrossRef CAS.
- R. Zhao, N. Wang, Y. Yu and J. Liu, Chem. Mater., 2020, 32, 1308–1314 CrossRef CAS.
- H. Benten, T. Nishida, D. Mori, H. Xu, H. Ohkita and S. Ito, Energy Environ. Sci., 2016, 9, 135–140 RSC.
- B. Fan, P. Zhu, J. Xin, N. Li, L. Ying, W. Zhong, Z. Li, W. Ma, F. Huang and Y. Cao, Adv. Energy Mater., 2018, 8, 1703085 CrossRef.
- Z. Li, B. Fan, B. He, L. Ying, W. Zhong, F. Liu, F. Huang and Y. Cao, Sci. China: Chem., 2018, 61, 427–436 Search PubMed.
- Q. Zhang, Z. Chen, W. Ma, Z. Xie, J. Liu, X. Yu and Y. Han, ACS Appl. Mater. Interfaces, 2019, 11, 32200–32208 CrossRef CAS.
- H. Chen, Y. Guo, P. Chao, L. Liu, W. Chen, D. Zhao and F. He, Sci. China: Chem., 2018, 62, 238–244 CrossRef.
- S. Liu, D. Chen, W. Zhou, Z. Yu, L. Chen, F. Liu and Y. Chen, Macromolecules, 2019, 52, 4359–4369 CrossRef CAS.
- J. Peet, A. B. Tamayo, X. D. Dang, J. H. Seo and T. Q. Nguyen, Appl. Phys. Lett., 2008, 93, 163306 CrossRef.
- W. Huang, P. Cheng, Y. M. Yang, G. Li and Y. Yang, Adv. Mater., 2018, 30, 1705706 CrossRef.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS.
- D. Deng, Y. Zhang, J. Zhang, Z. Wang, L. Zhu, J. Fang, B. Xia, Z. Wang, K. Lu, W. Ma and Z. Wei, Nat. Commun., 2016, 7, 13740 CrossRef CAS.
- W. Liu, J. Yao and C. Zhan, Chin. J. Chem., 2017, 35, 1813–1823 CrossRef CAS.
- X. Kong, H. Lin, X. Du, L. Li, X. Li, X. Chen, C. Zheng, D. Wang and S. Tao, J. Mater. Chem. C, 2018, 6, 9691–9702 RSC.
- S. Dai, S. Chandrabose, J. Xin, T. Li, K. Chen, P. Xue, K. Liu, K. Zhou, W. Ma, J. M. Hodgkiss and X. Zhan, J. Mater. Chem. A, 2019, 7, 2268–2274 RSC.
- X. Song, N. Gasparini, M. M. Nahid, S. H. K. Paleti, J.-L. Wang, H. Ade and D. Baran, Joule, 2019, 3, 846–857 CrossRef CAS.
- Z. Zhang, Z. Ding, D. J. Jones, W. W. H. Wong, B. Kan, Z. Bi, X. Wan, W. Ma, Y. Chen, X. Long, C. Dou, J. Liu and L. Wang, Sci. China: Chem., 2018, 61, 1025–1033 CrossRef CAS.
- J. Ge, Q. Wei, R. Peng, E. Zhou, T. Yan, W. Song, W. Zhang, X. Zhang, S. Jiang and Z. Ge, ACS Appl. Mater. Interfaces, 2019, 11, 44528–44535 CrossRef CAS.
Footnote |
| † These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |