Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ferroelectricity promoted by cation/anion divacancies in SrMnO3†
Chiara
Ricca
ab,
Danielle
Berkowitz‡
a and
Ulrich
Aschauer
 *ab
*ab
aDepartment of Chemistry, Biochemistry and Pharmaceutical Science, University of Bern, Freiestrasse 3, CH-3012 Bern, Switzerland. E-mail: ulrich.aschauer@dcb.unibe.ch
bNational Centre for Computational Design and Discovery of Novel Materials (MARVEL), Switzerland
First published on 2nd September 2021
Abstract
We investigate the effect of polar Sr–O vacancy pairs on the electric polarization of SrMnO3 (SMO) thin films using density functional theory (DFT) calculations. This is motivated by indications that ferroelectricity in complex oxides can be engineered by epitaxial strain but also via the defect chemistry. Our results suggest that intrinsic doping by cation and anion divacancies can induce a local polarization in unstrained non-polar SMO thin films and that a ferroelectric state can be stabilized below the critical strain of the stoichiometric material. This polarity is promoted by the electric dipole associated with the defect pair and its coupling to the atomic relaxations upon defect formation that polarize a region around the defect. This suggests that polar defect pairs affect the strain-dependent ferroelectricity in semiconducting antiferromagnetic SMO. For metallic ferromagnetic SMO we find a much weaker coupling between the defect dipole and the polarization due to much stronger electronic screening. Coupling of defect-pair dipoles at high enough concentrations along with their switchable orientation thus makes them a promising route to affect the ferroelectric transition in complex transition metal oxide thin films.
1 Introduction
Ferroelectricity in complex perovskite oxides has attracted great interest due to potential applications of ferroelectric thin films for various information storage technologies, such as non volatile random access memories and high-density data storage devices.1–3 Point defects are promising to tailor the functional properties of oxides.4–14 In particular, they can affect the polarization response in ferroelectrics by controlling the local polarization and the mechanism and kinetics of polarization switching.15,16 Defect pairs such as cation–anion divacancies or vacancies coupled with substitutional atoms were shown to play an essential role in determining polarization properties.17 For example, FeTi–VO defects are able to align in the direction of the lattice polarization in ferroelectric PbTiO3.8 VPb–VO divacancies are an important source of local polarization in Pb-containing perovskite oxides such as PbTiO3, where a VPb–VO concentration of 1.7% can induce a reduction of the ferroelectric transition temperature by about 35 K.18,19 Finally, defect pairs can also promote ferroelectricity in paraelectric materials: off-centered antisite-like defects consisting of a Sr vacancy and an interstitial Ti atom or by one Ti/Sr antisite defect coupled to an oxygen vacancy or even by Sr–O–O trivacancies are believed to play a pivotal role for emerging room-temperature ferroelectricity in SrTiO3 thin films.20–23Emerging ferroelectricity in nominally non-polar stoichiometric transition-metal oxides is the result of a complex interplay between structural, electronic, and magnetic degrees of freedom.7,24 Biaxial strain, imposed by lattice matching with a substrate during coherent epitaxial thin-film growth can, for instance, stabilize the perovskite phase of SrMnO3 (SMO, space group Pnma, see Fig. 1) which has a G-type antiferromagnetic (AFM) order.25,26 Moreover, sufficiently large strain can induce a polar distortion in SMO, which involves off-centering of the Mn ions within their oxygen octahedra and leads to ferroelectric behavior.11,27 Theory predicts the in-plane polar modes to soften for tensile strain larger than about +2.5%, the polar distortion increasing for larger tensile strain where a transition towards the ferromagnetic (FM) phase is predicted.27 Compressive strain larger than 5% induces, instead, ferroelectricity in the direction perpendicular to the strain plane.12 As discussed above, the defect chemistry is an additional parameter to consider when designing and controlling ferroelectricity in complex oxides and its coupling or competition with the other degrees of freedom requires careful investigation.7 As such, while tensile strain in SMO promotes ferroelectricity, it also favors oxygen-vacancy formation, the presence of which, in turn, suppresses ferroelectricity.12 Going beyond single point defects, the formation of Sr and O divacancies (VSr–VO) in SMO with an associated defect dipole could be a viable approach to reverse this suppression without recurring to extrinsic doping. Sr deficient SrMnO3−δ thin films can be grown by pulsed laser deposition, even if crystallinity is reduced compared to cation balanced or Mn-deficient films.28
While the effect of polar defect pairs and strain are thus established separately, their interplay has not been previously considered. In the present work, we investigate – via density functional theory (DFT) calculations – the microscopic origin of polarization induced by cation–anion divacancies and gain a deep understanding of the interplay between these polar defect pairs and strain, polarization, electronic properties, structure, and magnetism in SMO thin films. Our results show that the electric dipole pointing from the positively charged VSr to the negatively charged VO indeed induces a lattice polarization around the defect pair. This defect-pair dipole can couple with applied epitaxial strain and induce ferroelectricity for strains below those predicted to stabilize the polar structure in stoichiometric SMO, especially in the G-AFM phase. This interplay strongly depends on the magnetic and electronic properties of the film, the larger electronic screening in the metallic FM phase hindering the coupling between defect-pair dipoles and the polarization in the surrounding crystal.
2 Methods
DFT calculations were performed with the Quantum ESPRESSO package29,30 using PBEsol31 as exchange–correlation functional and ultrasoft pseudopotentials32 with Sr(4s, 4p, 5s), Mn(3p, 4s, 3d), and O(2s, 2p) valence states.§ Wavefunctions were expanded in plane waves with a kinetic-energy cut-off of 70 Ry and a cut-off of 840 Ry for the augmented density. A Gaussian smearing with a broadening parameter of 0.01 Ry was used in all cases. A Hubbard correction33–35 was applied on the Mn-3d orbitals within the rotationally invariant formulation of Dudarev35 with U values computed self-consistently for the stoichiometric G-AFM and FM SMO phases.36 Since the strain dependence of U is small,36 bulk U values were used for all strains.Biaxial epitaxial strain in the ac-plane imposed by a cubic substrate was accounted for in the strained-bulk setup as described in ref. 37. We consider a single relative orientation of the substrate and film, corresponding to strain in the pseudocubic SMO ac plane. Lattice instabilities were calculated at the Γ point of a 2 × 2 × 2 supercell of the 5-atom primitive Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m cell using the frozen phonon approach38 and analyzed using the PHONOPY interface.39 A shifted 6 × 6 × 6 Monkhorst–Pack k-point mesh was used for reciprocal space integration in this case. Defect pairs were calculated in 320-atom Pnma supercells (4 × 4 × 4 supercell with respect to the 5-atom unit cell) with Γ-point sampling of the Brillouin zone. We note that, despite the coarser k-mesh compared to the 2 × 2 × 2 supercell, a good qualitative description of properties and general trends is retained, while making the computation of these large cells tractable. Before creating defect pairs, all atoms in the 320-atom supercell were displaced along the polar-mode eigenvectors of the stoichiometric structure, the resulting structure being designated as “prepolarized” in the following. Defect pairs were created by simultaneously removing one oxygen atom (VO, concentration 0.5%) and one strontium atom (VSr, concentration 1.6%) from this supercell. Different relative arrangements of the two vacancies were taken into account (see Section 3.2). Since in fully or partially ionic compounds it is generally favorable for vacancies to be charge balanced by other defects, only the charge neutral Schottky defect pair was taken into account
m cell using the frozen phonon approach38 and analyzed using the PHONOPY interface.39 A shifted 6 × 6 × 6 Monkhorst–Pack k-point mesh was used for reciprocal space integration in this case. Defect pairs were calculated in 320-atom Pnma supercells (4 × 4 × 4 supercell with respect to the 5-atom unit cell) with Γ-point sampling of the Brillouin zone. We note that, despite the coarser k-mesh compared to the 2 × 2 × 2 supercell, a good qualitative description of properties and general trends is retained, while making the computation of these large cells tractable. Before creating defect pairs, all atoms in the 320-atom supercell were displaced along the polar-mode eigenvectors of the stoichiometric structure, the resulting structure being designated as “prepolarized” in the following. Defect pairs were created by simultaneously removing one oxygen atom (VO, concentration 0.5%) and one strontium atom (VSr, concentration 1.6%) from this supercell. Different relative arrangements of the two vacancies were taken into account (see Section 3.2). Since in fully or partially ionic compounds it is generally favorable for vacancies to be charge balanced by other defects, only the charge neutral Schottky defect pair was taken into account 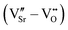 in Kröger–Vink notation,40 where the prime and dot symbols indicate, respectively, a charge of −1 and +1 relative to the respective lattice site. For simplicity, we will refer to these defect pairs as VSr–VO. For defective cells, only atomic positions were relaxed with the lattice vectors fixed at the optimized values of the corresponding stoichiometric cell. Convergence thresholds of 1.4 × 10−5 eV for the energy and 5 × 10−2 eV Å−1 for the forces are used for all relaxations.
in Kröger–Vink notation,40 where the prime and dot symbols indicate, respectively, a charge of −1 and +1 relative to the respective lattice site. For simplicity, we will refer to these defect pairs as VSr–VO. For defective cells, only atomic positions were relaxed with the lattice vectors fixed at the optimized values of the corresponding stoichiometric cell. Convergence thresholds of 1.4 × 10−5 eV for the energy and 5 × 10−2 eV Å−1 for the forces are used for all relaxations.
The 0 K strain-dependent VSr–VO formation energy (Ef) was computed according to ref. 41:
| Ef(ε,μO,μSr) = Edef(ε) − Estoi(ε) + μO + μSr, | (1) |
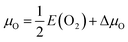 with E(O2) being the energy of an oxygen molecule and ΔμO = −1.39 eV. The Sr chemical potential (μSr = ESr + ΔμSr) was derived as function of μO, ESr being the total energy of metallic Sr and ΔμSr = −4.28 eV under O-poor conditions. The above limit to the O chemical potential was derived considering the stability of the system (ΔμSr + ΔμMn + 3ΔμO = ΔHf(SMO) = −10.22 eV) against decomposition to elemental Sr (ΔμSr ≤ 0) and Mn (ΔμMn ≤ 0) and against SrO (ΔμSr + ΔμO ≤ ΔHf(SrO) = −5.67 eV) and MnO (ΔμMn + ΔμO ≤ ΔHf(MnO) = −3.16 eV) formation, where ΔHf indicates the computed heats of formation. ΔHf for transition-metal oxides were corrected according to ref. 42 to account for mixing of DFT and DFT+U total energies.
with E(O2) being the energy of an oxygen molecule and ΔμO = −1.39 eV. The Sr chemical potential (μSr = ESr + ΔμSr) was derived as function of μO, ESr being the total energy of metallic Sr and ΔμSr = −4.28 eV under O-poor conditions. The above limit to the O chemical potential was derived considering the stability of the system (ΔμSr + ΔμMn + 3ΔμO = ΔHf(SMO) = −10.22 eV) against decomposition to elemental Sr (ΔμSr ≤ 0) and Mn (ΔμMn ≤ 0) and against SrO (ΔμSr + ΔμO ≤ ΔHf(SrO) = −5.67 eV) and MnO (ΔμMn + ΔμO ≤ ΔHf(MnO) = −3.16 eV) formation, where ΔHf indicates the computed heats of formation. ΔHf for transition-metal oxides were corrected according to ref. 42 to account for mixing of DFT and DFT+U total energies.
The polarization ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) was estimated using a point-charge model:
was estimated using a point-charge model:
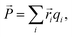 | (2) |
![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) i is the position of atom i and qi is its formal charge: +2 for Sr, −2 for O, and +4 for Mn. The polarization, being a multivalued quantity,43 has been corrected by an integer number of polarization quanta
i is the position of atom i and qi is its formal charge: +2 for Sr, −2 for O, and +4 for Mn. The polarization, being a multivalued quantity,43 has been corrected by an integer number of polarization quanta ![[Q with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0051_20d1.gif) , computed as:
, computed as: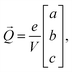 | (3) |
 to the negatively charged
to the negatively charged  , but neglects the electronic contribution to the ferroelectric polarization compared to other approaches such as the Berry phase formalism.44,45 It was nonetheless adopted both to reduce the computational cost and to allow comparison of the behavior of the G-AFM and FM phases, the Berry phase method not being applicable to metallic systems such as FM SMO.
, but neglects the electronic contribution to the ferroelectric polarization compared to other approaches such as the Berry phase formalism.44,45 It was nonetheless adopted both to reduce the computational cost and to allow comparison of the behavior of the G-AFM and FM phases, the Berry phase method not being applicable to metallic systems such as FM SMO.
Barriers for polarization switching were calculated using the climbing-image nudged elastic band (CI-NEB) method.46 Minimum energy pathways were relaxed until forces on each image converged below 1 × 10−3 eV Å−1.
3 Results and discussion
3.1 Interplay of strain, magnetism, and ferroelectricity in stoichiometric SMO
Before turning to polar VSr–VO defect pairs, we investigate the interplay between strain, magnetism, and ferroelectricity in stoichiometric SMO, which will be fundamental to understand the interplay between strain and the defect-induced properties. Strain-dependent ferroelectricity in stoichiometric G-AFM SMO thin films was previously reported by Marthinsen et al.12 Here, we evaluate polar instabilities also for the FM phase but note that its metallicity will preclude ferroelectric switching, an unstable polar mode indicating merely a polar metal state.47,48Fig. 2 shows the evolution of SMO polar-mode frequencies as a function of strain. In unstrained SMO the G-AFM phase is dynamically stable (see Fig. 2a). The double-degenerate in-plane (IP) polar modes, associated with the displacement of Mn atoms from the center of their oxygen octahedra in the ac-plane, become unstable at about 2% tensile strain, while the out-of plane (OP) mode softens between 4 and 6% compressive strain. These results are in excellent agreement with ref. 12, differences in the critical strain being attributed to different Hubbard U values.49 The FM phase exhibits a different strain-dependence: not only are the modes generally softer than in the AFM phase, but, more importantly, the IP modes become unstable only for large tensile strain of about 6%, while the OP mode softens already at −2%. As we will show in the following, this different behavior will affect the effect of polar defect pairs on the defect formation and ferroelectric behavior of SMO thin films.
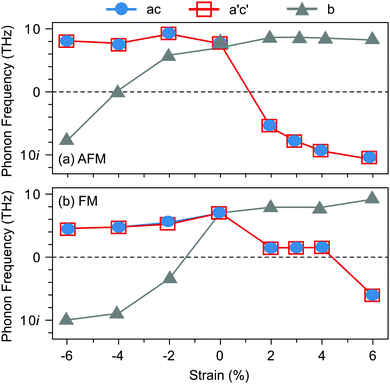 | ||
| Fig. 2 Evolution of the polar-mode phonon frequencies as a function of biaxial epitaxial strain in the Pnma SMO structure for (a) G-AFM and (b) FM order. | ||
3.2 VSr–VO formation energy and relative stability
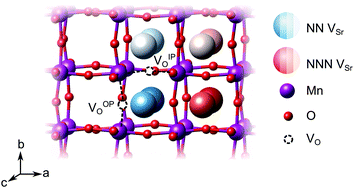
We have studied VSr–VO defects in a large 320-atom 4 × 4 × 4 SMO supercell to allow isolating the effect of individual defect dipoles. In smaller cells, the interaction of the defect and image dipoles overestimates relaxation energies relative to an isolated defect.19 As can be seen in Fig. 3, there are two symmetry-distinct oxygen-vacancy positions: an in-plane (IP) and out-of-plane (OP) O atom with the broken Mn–O–Mn bond respectively in the biaxial strain (ac) plane and perpendicular to it. For each VO, we tested inequivalent sites for a VSr in nearest-neighbor (NN) or next-nearest neighbor (NNN) positions to the oxygen vacancy. When a VIPO with the broken Mn–O–Mn bond along the a-axis is created, the four VNNSr positions indicated in different shades of blue in Fig. 3 correspond to all possible orientations of the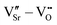 dipole in the bc plane. Instead, for the VNNNSr indicated by different shades of red in Fig. 3, one could also identify four additional equivalent configurations with VNNNSr located at negative a coordinates with respect to the VO. Similar arguments apply for VSr in NN and NNN positions with respect to VOPO. For simplicity and to reduce the computational cost, we considered only the 16 VSr–VO configurations indicated in Fig. 3, which cover all symmetry inequivalent possibilities. We also note here that defect-triplet formation is significantly less likely than defect-pair formation by more than 3 eV for both VSr–VO–VSr and VO–VSr–VO triplets.
dipole in the bc plane. Instead, for the VNNNSr indicated by different shades of red in Fig. 3, one could also identify four additional equivalent configurations with VNNNSr located at negative a coordinates with respect to the VO. Similar arguments apply for VSr in NN and NNN positions with respect to VOPO. For simplicity and to reduce the computational cost, we considered only the 16 VSr–VO configurations indicated in Fig. 3, which cover all symmetry inequivalent possibilities. We also note here that defect-triplet formation is significantly less likely than defect-pair formation by more than 3 eV for both VSr–VO–VSr and VO–VSr–VO triplets.
We first investigate the relative stability of the different VSr–VO configurations in unstrained SMO characterised by their formation energy in the O-poor limit in Fig. 4. Divacancies with a VIPO (circles) are usually more stable than those with a VOPO (squares). Generally, VSr prefer to be close to the VO: the most stable defect pairs are VNNSr–VIPO, followed by configurations with a VNNNSr at about 4.9 Å from the oxygen vacancy that have slightly (0.1 eV) larger formation energies. VNNNSr–VOPO defects, where the two vacancies are separated by as much as 6–7 Å and interact less, are the least likely to form. Compared to the semiconducting AFM phase, the metallic nature of the FM order results in smaller energetic differences between the configurations, as well as generally in lower formation energies.
 | ||
| Fig. 4 Formation energy (Ef) for VSr–VO defect pairs in unstrained (a) AFM and (b) FM SMO as a function of the distance between VSr and VO. Circle and square symbols refer to VIPO and VOPO, respectively. See Fig. 3 for the color code. | ||
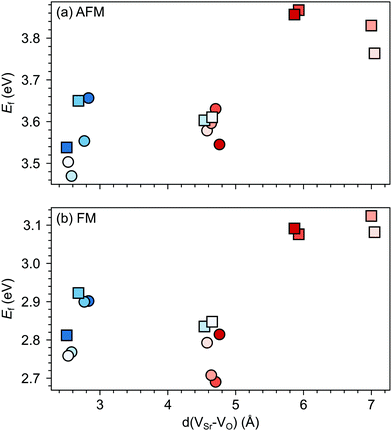
Changes in SMO structural and electronic properties induced by epitaxial strain were shown to influence the VO formation and ordering at inequivalent sites.12,50,51 Therefore, it is important to understand the interplay between defect chemistry, strain and magnetism also for VSr–VO defect pairs. In the AFM phase, the defect formation energy (Ef, see Fig. 5a) exhibits a non-monotonic strain dependence with changes in the sign of the slope close to the critical strains for the ferroelectric instability (Fig. 2a). In particular, Ef increases going from 0% to −4% or to +2% strain, where no polar instability exists. For larger tensile strain, when the IP polar modes become unstable, Ef increases or stays constant for defect pairs with a VOPO, but decreases especially for VNNSr–VIPO, which allows for strain-controlled defect ordering. Similarly, at 6% compressive strain, the formation energy is slightly reduced but a mixture of VIPO and VOPO defects is formed. In the FM phase, instead, Ef strongly increases going from the unstrained structure to the ±2% strained geometries and then decreases for larger strain, tensile and compressive strain strongly favoring defect pairs with VIPO and VOPO, respectively.
 | ||
| Fig. 5 Formation energy (Ef) for VSr–VO defects in (a) AFM and (b) FM SMO as a function of biaxial strain. The shaded grey areas indicate strain ranges with unstable polar modes in stoichiometric SMO. Circle and square symbols refer to VIPO and VOPO, respectively. See Fig. 3 for the color code. | ||
As we will further verify in the following sections, these results suggest a stronger coupling between the polar defect pair and the ferroelectric degrees of freedom in the AFM phase, compared to the metallic FM phase, where a stronger electronic screening of the defect dipole occurs.
3.3 Magnetic order
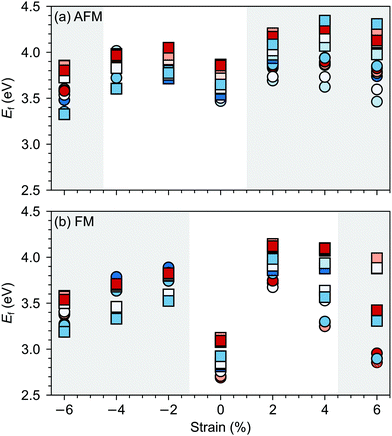
G-AFM is the ground state for stoichiometric bulk SMO but strain and/or defects can induce transition towards the FM phase. For example, in our previous work,36 using the same computational setup, we observed that 2% tensile strain can stabilize the FM order in stoichiometric SMO, while a concentration of 4.2% of oxygen vacancies leads to a FM ground state already for unstrained SMO. For oxygen vacancies, the magnetic transition is generally rationalized by Mn4+–Mn3+ double exchange due to reduced Mn3+ sites upon VO formation.Fig. 6 suggests that, in the considered strain range, VSr–VO defect pairs very slightly favor the FM phase, the preference for the ferromagnetic order increasing under tensile strain. The strong stabilization of the FM phase for −6% strain can be explained by the strong band-gap reduction in the AFM phase for such large compressive strain (cf. ESI,† Fig. S1). Nevertheless the preference for the FM order is difficult to rationalize since charge compensated neutral VSr–VO defect pairs should not lead to reduced Mn3+, which is responsible for the emergence of FM order. However, for VSr–VO defect pairs in the AFM phase we observed one or two partially reduced Mn sites (Mn(3+δ)+), as can be seen from the density of states reported for one VIPO and one VOPO in ESI,† Fig. S2. We believe these Mn(3+δ)+, and in turn the predicted stabilization of the FM order, to be a consequence of the interplay between structural relaxations taking place upon VO formation and the established underestimation of SMO band-gap within DFT+U: the elongation of Mn–O bonds upon VO formation results in the stabilization of the corresponding eg orbitals, the energy of which, due to the underestimation of the band gap, is lowered to just below the Fermi energy. This results in a partial occupation of this state, the observed Mn(3+δ)+ and the preference for the FM order. For this reason, we caution against the conclusion that VSr–VO defect pairs favor FM and also compute the polarization in defective SMO in the following section by assuming that no Mn reduction takes place upon VSr–VO formation.
 | ||
| Fig. 6 Total energy difference EFM–EAFM per formula unit between defective cells with FM and AFM magnetic order as a function of the applied epitaxial strain. AFM is more stable for positive and FM for negative differences. Circle and square symbols refer to VIPO and VOPO, respectively. See Fig. 3 for the color code. | ||
3.4 Polarization
![[D with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0044_20d1.gif) ) from the negatively charged
) from the negatively charged  to the positively charged
to the positively charged  , which results in the charge center being offset from the geometric center of the cell.52 Within a very simple ionic model, the polarization induced by the vacancy pair can be estimated as:
, which results in the charge center being offset from the geometric center of the cell.52 Within a very simple ionic model, the polarization induced by the vacancy pair can be estimated as:![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def = 2e def = 2e![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) VSr–VO/V, VSr–VO/V, | (4) |
![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) VSr–VO is the separation vector between the VSr and VO sites.19 The polarization (
VSr–VO is the separation vector between the VSr and VO sites.19 The polarization (![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def) predicted with this simple model increases linearly with the distance between the two vacancies as shown in Fig. 7a. However, when considering the lattice polarization viaeqn (2), for both AFM and FM phases (Fig. 7b and c respectively) VNNNSr–VOPO configurations, characterized by the largest VSr–VO separation, have a polarization much smaller than
def) predicted with this simple model increases linearly with the distance between the two vacancies as shown in Fig. 7a. However, when considering the lattice polarization viaeqn (2), for both AFM and FM phases (Fig. 7b and c respectively) VNNNSr–VOPO configurations, characterized by the largest VSr–VO separation, have a polarization much smaller than ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def. This suggests that, when VSr and VO are separated by more than 5 Å, defect–defect interactions are screened, which explains the formation energies in Fig. 4. Similar magnitudes of
def. This suggests that, when VSr and VO are separated by more than 5 Å, defect–defect interactions are screened, which explains the formation energies in Fig. 4. Similar magnitudes of ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) and
and ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def are obtained for the remaining VNNNSr–VO configurations, with polarizations larger than
def are obtained for the remaining VNNNSr–VO configurations, with polarizations larger than ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def when the two defects are in NN positions. This is especially the case for the most stable VNNSr–VIPO defects in the AFM phase, where a polarization almost twice as large as
def when the two defects are in NN positions. This is especially the case for the most stable VNNSr–VIPO defects in the AFM phase, where a polarization almost twice as large as ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) def is obtained (cf.Fig. 7a and b).
def is obtained (cf.Fig. 7a and b).
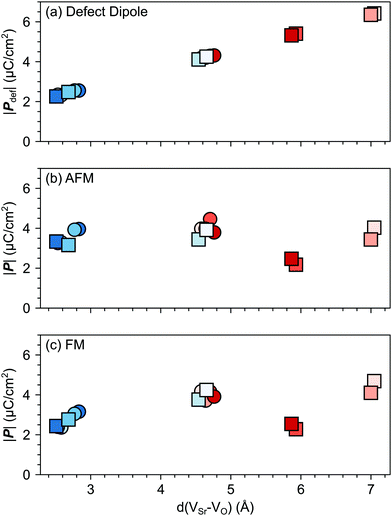 | ||
| Fig. 7 Magnitude of the total polarization vector as a function of the VSr–VO distance computed for different VSr–VO configurations considering (a) only the contribution of the defect pair (eqn (4)) or (b) and (c) also lattice contributions (eqn (2)) for the AFM and FM phases respectively. Circle and square symbols refer to data obtained for VIPO and VOPO, respectively. See Fig. 3 for the color code. | ||
The larger coupling between polar defect pairs and the polarization in the AFM phase is reflected by the angles between the polarization vector ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) and the defect-dipole vector
and the defect-dipole vector ![[D with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0044_20d1.gif) , which are quite small (lower than 30°) in the AFM phase for VSr–VO separated by less than 5 Å (see Fig. 8). This suggests an alignment of the polarization with the defect dipole in these cases. Larger angles are observed for cells containing VNNNSr–VOPO at larger separation. In the FM phase (see Fig. 8b) the angles are also larger due to enhanced electronic screening in this metallic phase that prevents the strong coupling between the defect dipole and polar displacements like in the semiconducting AFM phase.
, which are quite small (lower than 30°) in the AFM phase for VSr–VO separated by less than 5 Å (see Fig. 8). This suggests an alignment of the polarization with the defect dipole in these cases. Larger angles are observed for cells containing VNNNSr–VOPO at larger separation. In the FM phase (see Fig. 8b) the angles are also larger due to enhanced electronic screening in this metallic phase that prevents the strong coupling between the defect dipole and polar displacements like in the semiconducting AFM phase.
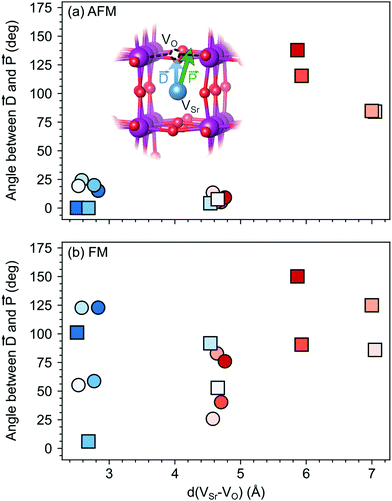 | ||
Fig. 8 Angle between the defect dipole (![[D with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0044_20d1.gif) ) and the polarization ( ) and the polarization (![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) ) computed as a function of the VSr–VO separation for unstrained (a) AFM and (b) FM SMO. Circle and square symbols refer to VIPO and VOPO, respectively. See Fig. 3 for the color code. ) computed as a function of the VSr–VO separation for unstrained (a) AFM and (b) FM SMO. Circle and square symbols refer to VIPO and VOPO, respectively. See Fig. 3 for the color code. | ||
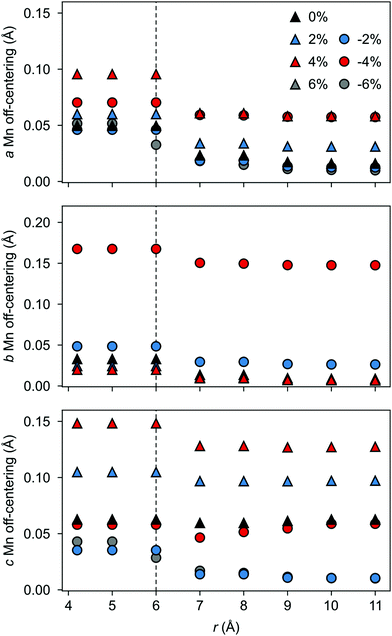
The polarization in the defective AFM cell can, indeed, be explained by the atomic displacements upon VSr–VO defect-pair formation. Large displacements from the high-symmetry positions and mainly for Mn atoms in the neighborhood of the VSr take place in the AFM phase. These Mn atoms move towards the cation vacancy, except for sites adjacent to the VO that are more strongly affected by Mn–O–Mn bond breaking (see ESI,† Fig. S3a and d). This suggests that the larger polarization arises due to the defect pair inducing polar distortions in the surrounding octahedra. We initially focus on the unstrained structure shown by black triangles in Fig. 9 and will discuss the strain dependence in the next subsection. As shown in Fig. 9 a larger Mn off-centering is observed in a sphere of about 6 Å around VSr compared to Mn ions further from the cation vacancy. When the two defects are separated by more than 6 Å and do not interact, as for VNNNSr–VOPO defects, the lattice contraction around the cation vacancy dominates and explains the smaller coupling between the defect and the lattice polarization (see ESI,† Fig. S3b and c). Finally, structural relaxations can also explain why, even when strongly interacting, ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) is not perfectly aligned with
is not perfectly aligned with ![[D with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0044_20d1.gif) : the small angle between the two vectors stems from displacements of the Mn atoms in NNN positions to VO along the axis of the broken Mn–O–Mn (see ESI,† Section S3 for more details).
: the small angle between the two vectors stems from displacements of the Mn atoms in NNN positions to VO along the axis of the broken Mn–O–Mn (see ESI,† Section S3 for more details).
In the AFM phase, the components of the polarization in the strained ac plane (![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) a and
a and ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) c in Fig. 10) increase steadily with tensile strain, reaching about 20 μC cm−2 at 6% strain, in line with the softening of the IP polar modes in the stoichiometric structure (cf.Fig. 2). We note here that this polarization is of similar magnitude as in conventional ferroelectrics such as BaTiO3 (22 μC cm−2).53,54 This increased polarization is accompanied by an average increase of the Mn off-centering up to about 0.3 Å (see Fig. 11). The larger Mn displacements computed for the defective case with respect to the stoichiometric case (white triangles in Fig. 11), confirm the ability of defect pairs to enhance the polarization. Conversely, compressive strain results in an OP polarization (
c in Fig. 10) increase steadily with tensile strain, reaching about 20 μC cm−2 at 6% strain, in line with the softening of the IP polar modes in the stoichiometric structure (cf.Fig. 2). We note here that this polarization is of similar magnitude as in conventional ferroelectrics such as BaTiO3 (22 μC cm−2).53,54 This increased polarization is accompanied by an average increase of the Mn off-centering up to about 0.3 Å (see Fig. 11). The larger Mn displacements computed for the defective case with respect to the stoichiometric case (white triangles in Fig. 11), confirm the ability of defect pairs to enhance the polarization. Conversely, compressive strain results in an OP polarization (![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) b) and increased Mn off-centering along the b-axis already for about −4% strain, which is below the critical strain to induce ferroelectricity in stoichiometric SMO (indicated by the gray background shade). The polar defect pairs can hence trigger the ferroelectric phase transition at lower strains and enhance the polarization and Mn off-centering up to 90 μC cm−2 and 0.6 Å at −6%, respectively.
b) and increased Mn off-centering along the b-axis already for about −4% strain, which is below the critical strain to induce ferroelectricity in stoichiometric SMO (indicated by the gray background shade). The polar defect pairs can hence trigger the ferroelectric phase transition at lower strains and enhance the polarization and Mn off-centering up to 90 μC cm−2 and 0.6 Å at −6%, respectively.
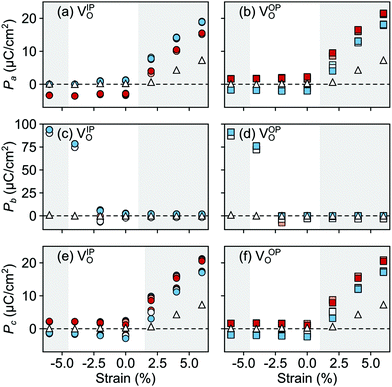 | ||
| Fig. 10 Strain dependence of the polarization component along the (a and b) a-, (c and d) b-, and (e and f) c-axis for the different defect-pair configurations in AFM SMO. (a), (c), and (e) for VSr–VIPO and (b), (d), and (f) for VSr–VOPO defects. See Fig. 3 for the color code. The shaded grey areas indicate strain ranges with unstable polar modes in stoichiometric SMO and the white triangles correspond to the polarization in stoichiometric SMO. | ||
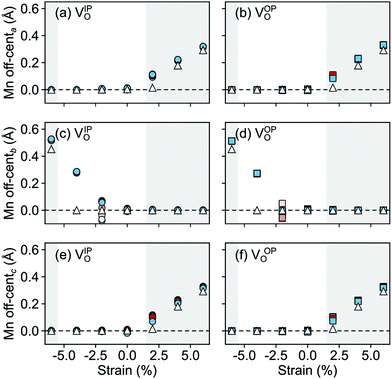 | ||
| Fig. 11 Strain dependence of the average Mn off-centerings along the (a and b) a-, (c and d) b-, and (e and f) c-axis for the different defect-pair configurations in AFM SMO. (a), (c), and (e) for VSr–VIPO and (b), (d), and (f) for VSr–VOPO defects. Off-centerings were computed excluding the Mn adjacent to VO to avoid artifacts due to the relaxations of undercoordinated sites. See Fig. 3 for the color code. The shaded grey areas indicate strain ranges with unstable polar modes in stoichiometric SMO and the white triangles correspond to the Mn off-centerings in stoichiometric SMO. | ||
Interestingly, the FM phase shows a different behavior, the computed polarization for all defect configurations being almost constant and close to the polarization in the unstrained structure (see ESI,† Fig. S4). Only for large compressive strain, when the OP polar modes becomes unstable, an increase of ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) b is observed. The different behavior of this magnetic order can be explained considering both the strain-dependence of the polar modes and the larger electronic screening in this metallic phase.
b is observed. The different behavior of this magnetic order can be explained considering both the strain-dependence of the polar modes and the larger electronic screening in this metallic phase.
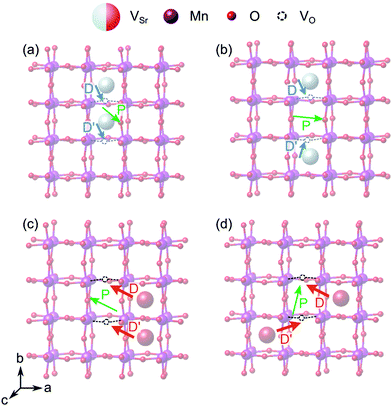
When two of the most stable VNNSr–VIPO vacancy pairs are created in unstrained SMO (Fig. 12a and b), the parallel arrangement of their defect dipoles is energetically favored by about 0.13 eV compared to the anti-parallel arrangement. For VNNNSr–VIPO defect pairs (Fig. 12c and d) with larger separation the parallel arrangement is still favored by 0.03 eV. This suggests that coupling of nearby defect-pair dipoles is possible, even at room temperature. The polarization induced by the two defect pairs can be rationalized from the orientation of the defect dipoles: for example, for two parallel VNNSr–VIPO pairs, the polarization is enhanced mainly along −b and +c compared to a single defect pair, while the anti-parallel arrangement results in an enhancement of ![[P with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0050_20d1.gif) along c, but in quenching of the polarization along b, in line with the opposite orientation of the two dipoles along this axis (see Fig. 12a and b).
along c, but in quenching of the polarization along b, in line with the opposite orientation of the two dipoles along this axis (see Fig. 12a and b).
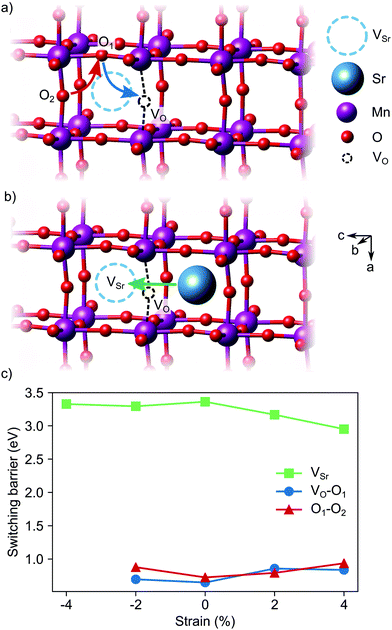
Switching of the defect-pair dipoles is the second requirement for defect-induced ferroelectricity in SMO. In presence of VSr–VO defect pairs, switching could take place via diffusion of oxygen or strontium vacancies. In the first case, a concerted double jump would move atom O1 in Fig. 13a into the initial vacancy site, while atom O2 fills the now vacant O1 site. The resulting position of the VO at location O2 inverts the orientation of the defect dipole compared to the initial VO position. For polarization switching via Sr diffusion the Sr would follow an approximately linear path between two neighboring Sr sites, as shown in Fig. 13b. In unstrained SMO, the switching barriers are about 0.65–0.72 eV and 3.33 eV for the O and Sr migration, respectively (see Fig. 13c), in good agreement with previous theoretical results in similar perovskite oxides.23,55,56 Even though tensile strain enhances Sr mobility, likely via opening the diffusion pathway along the a direction,57,58 O diffusion remains the main pathway for switching the defect dipole. While the barriers for this latter process are larger than the double-well barriers of 0.1 eV in ferroelectric PbTiO3,59 they are still low enough for polarization reversal via electric fields.
4 Conclusions
In the present work we studied the formation of VSr–VO defect pairs and their impact on the ferroelectricity of SrMnO3 thin films using DFT+U. Our results suggest that polar defect pairs made by Sr cation and O anion divacancies induce defect-pair dipoles from the negatively charged VSr to the positively charged VO, which are an important source of local polarization in non-polar SMO. Electronic screening in the metallic FM phase suppresses significant coupling of these defect-pair dipoles with the lattice polarization. In the semiconducting AFM phase we predict an alignment of the lattice polarization with the defect-pair dipole and, within a sphere of radius 6 Å round the vacancy pairs, an enhanced off-centering of the Mn ions from their high symmetry position in the oxygen octahedra. Divacancies couple with epitaxial strain, which affects their formation energy, allowing for defect ordering, as well as enhancing the polarization in thin films where strain alone could not stabilize a ferroelectric phase. In particular under compressive strain, out-of-plane polarization emerges at significantly lower critical strain in presence of VSr–VO than in the stoichiometric material. Since the direction of these defect-pair dipoles is switchable by an applied electric field and given the tendency of defect-pair dipoles to couple at sufficiently high concentrations, our findings motivate the exploration of intrinsic doping as a parameter to control the ferroelectric transition in complex transition metal oxides.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the NCCR MARVEL, funded by the Swiss National Science Foundation. Computational resources were provided by the University of Bern (on the HPC cluster UBELIX, http://www.id.unibe.ch/hpc) and by the Swiss National Supercomputing Center (CSCS) under project ID mr26.Notes and references
- J. F. Scott, Science, 2007, 315, 954–959 CrossRef CAS PubMed.
- H. Lu, X. Liu, J. D. Burton, C.-W. Bark, Y. Wang, Y. Zhang, D. J. Kim, A. Stamm, P. Lukashev, D. A. Felker, C. M. Folkman, P. Gao, M. S. Rzchowski, X. Q. Pan, C.-B. Eom, E. Y. Tsymbal and A. Gruverman, Adv. Mater., 2012, 24, 1209–1216 CrossRef CAS PubMed.
- X. Liu, D. Liu, W. Zhang, J. He, L. Zhao, S. He, D. Mou, F. Li, C. Tang and Z. Li, et al. , Nat. Commun., 2014, 5, 1–10 Search PubMed.
- K. Fuchigami, Z. Gai, T. Z. Ward, L. F. Yin, P. C. Snijders, E. W. Plummer and J. Shen, Phys. Rev. Lett., 2009, 102, 066104 CrossRef CAS PubMed.
- H. L. Tuller and S. R. Bishop, Annu. Rev. Mater. Res., 2011, 41, 369–398 CrossRef CAS.
- S. V. Kalinin, A. Borisevich and D. Fong, ACS Nano, 2012, 6, 10423–10437 CrossRef CAS PubMed.
- S. V. Kalinin and N. A. Spaldin, Science, 2013, 341, 858–859 CrossRef CAS PubMed.
- A. Chandrasekaran, D. Damjanovic, N. Setter and N. Marzari, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 214116 CrossRef.
- N. Biškup, J. Salafranca, V. Mehta, M. P. Oxley, Y. Suzuki, S. J. Pennycook, S. T. Pantelides and M. Varela, Phys. Rev. Lett., 2014, 112, 087202 CrossRef.
- A. Bhattacharya and S. J. May, Annu. Rev. Mater. Res., 2014, 44, 65–90 CrossRef CAS.
- C. Becher, L. Maurel, U. Aschauer, M. Lilienblum, C. Magén, D. Meier, E. Langenberg, M. Trassin, J. Blasco and I. P. Krug, et al. , Nat. Nanotechnol., 2015, 10, 661 CrossRef CAS PubMed.
- A. Marthinsen, C. Faber, U. Aschauer, N. A. Spaldin and S. M. Selbach, MRS Commun., 2016, 6, 182–191 CrossRef CAS.
- S. M. Griffin, M. Reidulff, S. M. Selbach and N. A. Spaldin, Chem. Mater., 2017, 29, 2425–2434 CrossRef CAS.
- T. Rojac and D. Damjanovic, Jpn. J. Appl. Phys., 2017, 56, 10PA01 CrossRef.
- M.-W. Chu, I. Szafraniak, D. Hesse, M. Alexe and U. Gösele, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 174112 CrossRef.
- S. V. Kalinin, B. J. Rodriguez, A. Y. Borisevich, A. P. Baddorf, N. Balke, H. J. Chang, L.-Q. Chen, S. Choudhury, S. Jesse, P. Maksymovych, M. P. Nikiforov and S. J. Pennycook, Adv. Mater., 2010, 22, 314–322 CrossRef CAS PubMed.
- Q. Yang, J. X. Cao, Y. Ma and Y. C. Zhou, AIP Adv., 2013, 3, 052134 CrossRef.
- F. Chu, I. M. Reaney and N. Setter, J. Appl. Phys., 1995, 77, 1671–1676 CrossRef CAS.
- E. Cockayne and B. P. Burton, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 144116 CrossRef.
- M. Choi, F. Oba and I. Tanaka, Phys. Rev. Lett., 2009, 103, 185502 CrossRef PubMed.
- Y. S. Kim, J. Kim, S. J. Moon, W. S. Choi, Y. J. Chang, J.-G. Yoon, J. Yu, J.-S. Chung and T. W. Noh, Appl. Phys. Lett., 2009, 94, 202906 CrossRef.
- F. Yang, Q. Zhang, Z. Yang, J. Gu, Y. Liang, W. Li, W. Wang, K. Jin, L. Gu and J. Guo, Appl. Phys. Lett., 2015, 107, 082904 CrossRef.
- K. Klyukin and V. Alexandrov, Phys. Rev. B, 2017, 95, 035301 CrossRef.
- N. A. Spaldin and M. Fiebig, Science, 2005, 309, 391–392 CrossRef CAS PubMed.
- O. Chmaissem, B. Dabrowski, S. Kolesnik, J. Mais, D. E. Brown, R. Kruk, P. Prior, B. Pyles and J. D. Jorgensen, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 134412 CrossRef.
- S. Kobayashi, Y. Tokuda, T. Ohnishi, T. Mizoguchi, N. Shibata, Y. Sato, Y. Ikuhara and T. Yamamoto, J. Mater. Sci., 2010, 46, 4354–4360 CrossRef.
- J. H. Lee and K. M. Rabe, Phys. Rev. Lett., 2010, 104, 207204 CrossRef PubMed.
- S. Kobayashi, Y. Tokuda, T. Ohnishi, T. Mizoguchi, N. Shibata, Y. Sato, Y. Ikuhara and T. Yamamoto, J. Mater. Sci., 2011, 46, 4354–4360 CrossRef CAS.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni and I. Dabo, et al. , J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. Buongiorno Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. Dal Corso, S. De Gironcoli, P. Delugas, R. A. Distasio, A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H. Y. Ko, A. Kokalj, E. Kücükbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H. V. Nguyen, A. Otero-De-La-Roza, L. Paulatto, S. Poncé, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, J. Phys.: Condens. Matter, 2017, 29, 465901 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892 CrossRef.
- V. I. Anisimov, J. Zaanen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 943 CrossRef CAS PubMed.
- V. Anisimov, A. Poteryaev, M. Korotin, A. Anokhin and G. Kotliar, J. Phys.: Condens. Matter, 1997, 9, 7359 CrossRef CAS.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505 CrossRef CAS.
- C. Ricca, I. Timrov, M. Cococcioni, N. Marzari and U. Aschauer, Phys. Rev. B, 2019, 99, 094102 CrossRef.
- J. M. Rondinelli and N. A. Spaldin, Adv. Mater., 2011, 23, 3363–3381 CrossRef CAS.
- K. Kunc and R. M. Martin, Phys. Rev. Lett., 1982, 48, 406–409 CrossRef CAS.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
- F. A. Kröger and H. J. Vink, Solid State Phys., 1956, 3, 307–435 Search PubMed.
- C. Freysoldt, B. Grabowski, T. Hickel, J. Neugebauer, G. Kresse, A. Janotti and C. G. Van de Walle, Rev. Mod. Phys., 2014, 86, 253 CrossRef.
- A. Jain, G. Hautier, S. P. Ong, C. J. Moore, C. C. Fischer, K. A. Persson and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 045115 CrossRef.
- N. A. Spaldin, J. Solid State Chem., 2012, 195, 2–10 CrossRef CAS.
- R. D. King-Smith and D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 1651–1654 CrossRef CAS PubMed.
- D. Vanderbilt and R. D. King-Smith, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 4442–4455 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- W. X. Zhou and A. Ariando, Jpn. J. Appl. Phys., 2020, 59, SI0802 CrossRef CAS.
- N. A. Benedek and T. Birol, J. Mater. Chem. C, 2016, 4, 4000–4015 RSC.
- J. Hong, A. Stroppa, J. Iñiguez, S. Picozzi and D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 054417 CrossRef.
- U. Aschauer, R. Pfenninger, S. M. Selbach, T. Grande and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 054111 CrossRef.
- U. Aschauer and N. A. Spaldin, Appl. Phys. Lett., 2016, 109, 031901 CrossRef.
- Y.-G. Wang, X.-G. Tang, Q.-X. Liu, Y.-P. Jiang and L.-L. Jiang, Nanomaterials, 2017, 7, 264 CrossRef PubMed.
- C. Ricca, N. Niederhauser and U. Aschauer, Phys. Rev. Res., 2020, 2, 042040 CrossRef.
- C. Ederer and N. A. Spaldin, Phys. Rev. Lett., 2005, 95, 257601 CrossRef PubMed.
- D. D. Cuong, B. Lee, K. M. Choi, H.-S. Ahn, S. Han and J. Lee, Phys. Rev. Lett., 2007, 98, 115503 CrossRef PubMed.
- A. Walsh, C. R. A. Catlow, A. G. H. Smith, A. A. Sokol and S. M. Woodley, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 220301 CrossRef.
- A. Kushima and B. Yildiz, J. Mater. Chem., 2010, 20, 4809 RSC.
- B. Yildiz, MRS Bull., 2014, 39, 147–156 CrossRef CAS.
- R. E. Cohen, Nature, 1992, 358, 136–138 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional electronic structure information, structural relaxation and FM polarization data. See DOI: 10.1039/d1tc02317a |
| ‡ Present address: Department of Materials Science and Engineering, Rensselaer Polytechnic Institute, 110 8th St., Troy, NY 12180, USA. |
| § Ultrasoft pseudopotentials from the PSLibrary were taken from www.materialscloud.org: Sr.pbesol-spn-rrkjus_psl.1.0.0.UPF, Mn.pbesol-spn-rrkjus_psl.0.3.1.UPF, and O.pbesol-n-rrkjus_psl.1.0.0.UPF. |
| This journal is © The Royal Society of Chemistry 2021 |