Solid phase extraction on reverse phase chromatographic media subjected to stresses expected for extraterrestrial implementation†
Jerome P.
Ferrance

J2F Engineering, 113 Lupine Lane, Charlottesville, VA 22911, USA. E-mail: jerome@j2f-engineering.com; Tel: +1 434-987-2036
First published on 29th June 2022
Abstract
Sample preparation techniques, such as solid phase extraction, will likely be required for in situ analysis of liquid samples collected from bodies in our Solar System that contain liquid, to concentration and desalt analytes of interest from the expected brines on these Ocean Worlds. Media to be used for these extraction procedures will have to survive the stresses of the long spaceflight required to reach these bodies, and remain functional once at that location. This work utilized tryptophan as an initial representative analyte to evaluate capture and desalting efficiencies in silica and polymeric reverse phase media, to determine how these solid phases might withstand stresses they could experience during deployment, including vacuum exposure, freezing, and heating/sonication treatments. Further experimentation on irradiation and long term freezing of media with an expanded array of analytes evaluated the utility of reverse phase media for this application. Kromasil® C-18 silica particles performed well, showing no loss in capture or desalting efficiency for the initial stress treatments or irradiation, but long term freezing after irradiation caused issues with this media. Oasis® HLB polymeric particles performed better, with 100% capture efficiency and 90% recovery of the tryptophan analyte for all treated and the untreated media. Onyx C-18 guard cartridges, a reverse phase C-18 modified silica monolithic media, exhibiting 100% capture efficiency and >90% recovery of tryptophan for both untreated and treated monoliths but also had issues after irradiation and long term frozen storage. Chromolith® RP-18e silica monolithic guard cartridges showed issues with consistency and reproducibility. In expanding the list of analytes, the Oasis® HLB media showed the best performance, capturing more of the analytes tested and remaining fully functional through both irradiation and long term storage treatments. Other media with additional reverse phase capture characteristics were also evaluated but none performed as well on the selected analytes as the Oasis® HLB media.
1. Introduction
The development of analytical methods for in situ analysis of the atmospheres and materials from other planetary and transitory bodies in our Solar System has been an international effort for a number of years. These efforts have resulted in missions to Mars1,2 and Venus,3,4 the Galileo mission detailing the Jovian atmosphere, and the Cassini–Huygens effort that explored the rings of Saturn and the atmosphere and reactions taking place above the surface of Titan.5 The Mars Science Laboratory mission provided the basis for direct surface sampling for in situ analysis through the Sample Analysis at Mars (SAM) instrument suite.6 The Cassini mission performed in situ analysis of organic compounds escaping the surface of Enceladus on emitted ice grains,7,8 while the Rossetta mission to Comet 67 P/Churyumov-Gerasimenko allowed for in situ analysis of cometary material. A number of recent missions to comets, asteroids, and Mars, are collecting samples that are to be returned to earth, where the full suite of analytical methods available to scientists can be employed to analyze and evaluate the materials collected. For in situ analysis, however, the constraints on space instrumentation (including mass, size, mechanical strength, automation and energy consumption), and the limitations on the amounts and types of reagents that can be transported severely limit the kinds of analyses that can be performed.Spectroscopic instruments provide some direct information on the molecules and minerals present in either the atmosphere or the surface layer. Landing instruments on Mars has allowed subsurface molecules to be detected through drilling or surface ablation. Direct crashes into the surface of various bodies have also provided plumes or ejection of subsurface material that could be analyzed spectroscopically in situ. Unfortunately, many of the molecules of interest for detection of current or past life on these bodies are not spectroscopically active, and thus are not going to be detected in this way. Mass spectrometry (MS), has also been a workhorse detector for extraterrestrial analytical missions, providing data on the masses of the molecular ions or ionized fragments generated from a sample. While these materials were often gas samples, laser ablation of solids or pyrolysis has provided information on volatile molecules in rock and soil samples as well.9 Since both analytes of interest and unknown/unknowable interferents are likely present in any given sample, a separation method is often also employed before the MS analysis. By breaking a single sample into multiple, time resolved fractions entering the MS detector, a separation step allows for easier interpretation of the mass data to determine what compounds may be present.
The separation technique most widely selected for deployment thus far has been gas chromatography (GC), the columns for which have had to go through flight stress testing before deployment. Irradiation tests, vibrational tests and short term thermocycling-vacuum tests were performed on all capillary columns and their liquid stationary phases for the Cometary Sampling and Composition (COSAC) experiments on the Rosetta mission to simulate launch and space conditions before being selected for the mission.10 Comparing the analytical performances of the columns, before and after the environmental tests, demonstrated that the analytical properties of the columns having either a porous layer or chiral stationary phase were preserved. While no damage was reported from these tests, identical spare GC columns were further exposed to space vacuum conditions for eight years during the mission travel phase, to determine as they were being employed in situ, if the long term exposure had affected the stationary phases of these columns.11 In addition, Szopa et al.12 evaluated the entire GC-MS system performance under pressure and temperature conditions representative of Titan's atmosphere where the system would be employed.
Many of the future missions currently being proposed for in situ sample analysis are targeting moons of other planets in our Solar System that are icy bodies with expected subsurface liquids – so called “Ocean Worlds”, such as Callisto, Enceladus, Europa, Ganymede, Titan, and Ceres.13 Liquid samples bring additional issues, in that the analytes of interest are often very dilute, and liquid samples collected from these bodies will likely have high concentrations of salt, the composition of which is still uncertain,14,15 and the pH of which is also unknown. Modeling of Europa from spectroscopic data collected during the Galileo mission by Kargel et al.16 point to an ocean likely enriched in sulfates that could be either high pH (sodium carbonate) or low pH (sulfuric acid). Salt ions in a liquid sample would interfere with MS detection and must be removed before these samples could be analyzed by this technique.
Solid phase extraction (SPE) is one sample preparation method for extracting and concentrating analytes from liquid samples that could be remotely employed with these Ocean World samples. In SPE, a liquid sample is passed through a solid chromatographic phase, which captures analytes of interest, while contaminating substances can be washed away. The captured analytes are then eluted from the column often in a purified and more concentrated form which can increase both sensitivity and specificity. SPE often utilizes the same kinds of chromatographic media that is employed in liquid chromatography (LC) separations, thus evaluation of SPE media for use in extraterrestrial applications can also provide a basis for eventual implementation of LC separation methods for remote in situ analysis. There has as yet been no testing of chromatographic media suitable for either LC or SPE for stability and performance for space based applications to ensure that it will work properly at the site of utilization. This includes functioning under conditions present at the site, as well as surviving the stresses presented by the trip to reach the destination. These include solar radiation – both photons and energetic particles, as well as space vacuum, vibrations and temperature swings and extended frozen conditions.
This study presents work on the evaluation of space flight stresses on reverse phase media that can be used for solid phase extraction applications. The work reported here specifically focused on reverse phase media, in which the surface of the solid phase is hydrophobic and can capture small molecule analytes while allowing salt ions to be washed away. To fully evaluate the technique, multiple types of media, with different forms and functionalities were employed in a SPE method for desalting of liquid samples. Aliquots of media were frozen and or irradiated to simulate storage on long term space flights, heated and sonicated to simulate the heating/shaking experienced during take-off and exposed to vacuum to simulate the lower pressure atmospheric conditions on these moons. While tryptophan was employed for the initial stress testing, a wide variety of analytes were eventually tested for selection of the media that represents the best choice for future deployment. Reports on the use and testing of media utilizing other capture modes for in situ desalting and concentration of analytes will be presented in subsequent work.
2. Materials and methods
2.1. Materials
Tryptophan, tyrosine, glycine, histidine, glutamic acid, valine, methionine, leucine, phenylalanine, γ-aminobutyric acid, 6-aminocaproic acid, isobutylamine, propylamine, sodium octanoate, malic acid, fumaric acid, benzoic acid, pyridine-2,6-dicarboxylic acid, adenine, guanine, guanosine, and guanosine 5′ monophosphate, were obtained from Millipore Sigma (St Louis, MO) and used as received. The peptides: AlaGly, GlyGlyGly, MetAlaSer, and GlyArgGlyAspSer, as well as a random hexamer DNA oligonucleotide were also purchased from Millipore Sigma. The analytical reagent grade citric acid was obtained from Mallinckrodt (Paris, KY). Stock solutions (normally 1 mM) were prepared in High Purity water (Cen-Med Enterprises, New Brunswick, NJ) then diluted to the working concentration in a simulated ocean water solution which had been adjusted to pH 6.1 for extractions. The simulated ocean water was prepared using Instant Ocean (Spectrum Brands, Blacksburg, VA), a salt mixture designed to recreate the composition of monovalent, divalent and trace mineral ions found in natural ocean water for salt water aquariums.17 The ocean water simulant was prepared using 35.0 g of the Instant Ocean salt mixture in 1 L of water – the approximate salt concentration of the Atlantic Ocean – which was vacuum filtered through a 0.2 μM filter. The as prepared solution had a density (measured by refractive index) of 1.0222 g cm−3 and a pH of ∼8.8; 1 M HCl was used to adjust the pH of the simulated ocean water to 6.1 for the sample solutions. Acetonitrile was purchased from Millipore Sigma (St Louis, MO) and used as received or diluted in High Purity water.The particle media tested in this work included Kromasil® 300 Å C18 media (16 μm), obtained from Nouryon (Amsterdam, Netherlands), Oasis® HLB media obtained from Waters (Milford, MA), Sorbtech C-18 spherical Silica Gel, Premium Rf media (70 Å; 15 μm; Endcapped, Carbon load 18–22%) purchased from ThermoFisher (Waltham, MA), and Strata® Phenyl (70 Å; 55 μm) and Strata® C8 (70 Å; 55 μm) media purchased from Phenomenex (Torrance, CA). The monoliths evaluated were Onyx Monolithic C-18 guard cartridges from Phenomenex® (Torrance, CA) and Chromolith® RP-18e guard cartridges from EMD Millipore (Billerica, MA). The monolith media were utilized in the holders for these guard cartridges purchased from the column suppliers. The particle media were tested in PEEK cartridges (200 μL volume) fabricated in house, encased in threaded stainless steel housings and capped with 2.0 μM PEEK filter frits purchased from IDEX (Oak Harbor, WA), or in stainless steel columns (600 μL volume) that utilized 2.0 μM PEEK frits and end cap compression fittings also from IDEX. To fill the cartridges/columns, particle media was weighed out and conditioned with 100% acetonitrile then packed by application of a vacuum through the bottom filter frit, then the top frit installed once the cartridge/column was fully packed. The same direction of flow was maintained for packing and for all of the extraction experiments.
2.2. Extraction experiments
To perform an extraction in the cartridge format, the inlet was connected through 200 μm I.D. tubing to a syringe mounted in a syringe pump. Sequential flow of 20% acetonitrile in water (conditioning step), water (equilibration step), simulated ocean water with or without analyte (load step), water (wash step), and 20% acetonitrile in water (elution step) was performed by replacing the syringe for each step. All steps were performed at 200 μL min−1 for 2 minutes to give a total volume of 400 μL for each step. The flow through from the load, wash, and elution steps were collected and analyzed. For multiple solid phase extractions performed on a single cartridge, the entire set of steps (conditioning-elution) was repeated after the elution step of the previous run. Before the initial extraction with packed cartridges, the conditioning step was performed twice to remove the 100% acetonitrile used in packing the media. These same conditions were utilized with the monolithic media tested in these experiments.Column experiments were performed in a similar manner, but because of the larger volume of media present, larger volumes were used. In addition, multiple load, wash and elution fractions could be collected depending upon the experiment being performed. Elution steps with higher concentrations of acetonitrile (40%) were also employed in some experiments.
2.3. Stress treatments
For stress testing, aliquots of media, or single guard cartridges were placed into microcentrifuge tubes for each treatment. Particles were packed into cartridges after treatments, except for irradiation treatments, which were performed on packed cartridges. As a simulant for heating of the spacecraft along with shaking due to engine thrust and vibrations during take-off or decent to an Ocean World body, media samples were heated to 60 °C and sonicated at a frequency of 36 kHz for 30 minutes in an iSonic® P4830 digital ultrasonic bath (iSonic Inc, Chicago, IL) with an additional hold at 60 °C for 30 minutes. These conditions are a much greater vibrational frequency than should be encountered in actual flight, and for a longer period than expected, but were used to maximize any changes which might be observed in the media. As the manufacturer of the Chromolith® monoliths indicated that the columns should not be utilized at temperatures above 45 °C, simple heating of a single column of both types of monoliths to 60 °C for 1 hour was performed and the monoliths tested, before the heating/sonication procedure was applied to additional monoliths. Frozen storage of the media was performed at −80 °C for a minimum of 25 days, even though temperature on the spacecraft may not get that low due to the need to protect the analytical instruments aboard the vessel. For pressure testing, all of the chose media is designed for high pressures, so only low pressure was tested. Low pressure testing included 2 hours of storage at less than 100 mTorr, with a minimum pressure of <40 mTorr for most of the hold time. Irradiation treatments were performed at the Penn State Radiation Science and Engineering Center on packed, sealed cartridges or guard column monoliths sealed in their holders; the samples were irradiated with gamma rays to 300 krad(Si) which correlates to an “air exposure” of 346.6 kR. This exposure was based on exposures values tested by Freissinet et al.18 investigating gamma radiation degradation effects on chemicals that might potentially be sent to Europa for use with in situ analysis methods. Following irradiation, some of the treated cartridges/monoliths were placed into a −80 °C freezer and remained there for 10 months before being tested.2.4. Fraction analysis
The collected load, wash, and elution fractions were analyzed using UV spectroscopy in either a Shimadzu UV-260 (Kyoto, Japan) or Thermo Scientific Evolution 201 (Waltham, MA) spectrometer. Measurements were taken at 278 nm for tryptophan, or at the indicated wavelengths, for the additional analytes listed in Table 1. The Evolution 201 instrument allowed for collection of spectral scans, with absorbance at ≥300 nm used to evaluate scattering if present. Fluorescence measurements were made using a PerkinElmer LS50B luminescence spectrometer (Shelton, CT) modified to include a shortpass filter in the excitation side to reduce background signal, and with a cuvette designed for use with 500 μL microcentrifuge tubes to allow for detection in small sample volumes. Fluorescence labeling of amines utilized either fluorescamine19 or o-phthalaldehyde (OPA)20,21 procedures as modified and reported in the ESI.† Specific gravity measurements were performed using a Palm Abby™ Digital refractometer (MISCO, Solon, OH) calibrated to provide sea water density values. The refractive index measurements were used to determine the salt concentration in load and wash samples, and the acetonitrile concentrations in elution samples. For each set of experiments, aliquots of the simulated ocean water elution solutions and analyte load solutions were analyzed to determine the amounts of analytes in each fraction. The percent captured was determined as 100% minus the amounts of analyte found in the load and wash fractions. The percent eluted is calculated from the amount found in the elution fraction, and the recovery is calculated from the amount found in all three fractions analyzed. All of these were relative to the measurements of blank tests, in which the load solution consisted of only the pH adjusted simulated ocean water.| Analytes | Reverse phase media | |||||
|---|---|---|---|---|---|---|
| Detection | Oasis HLB | Kromasil C-18 | Onyx C-18 | Strata Phenyl | Strata C8 | |
| a Fluorescence detection following labeling. Y/W: All captured but came out in wash. Y/W/E: All captured, some came out in wash, some in elution. S/W: Some captured but came out in wash. S/W/E: Some captured, some came out in wash, some in elution. | ||||||
| Tryptophan | Fla/278 nm | Yes | Yes | Yes | Yes | Yes |
| Tyrosine | Fl | Y/W/E | S/W | S/W | No | S/W |
| Glycine | Fl | No | ||||
| Histidine | Fl | S/W | No | No | No | No |
| Glutamic acid | Fl | No | No | No | No | No |
| Valine | Fl | No | No | No | No | S/W |
| Methionine | Fl | S/W/E | No | No | S/W | S/W |
| Adenine | 260 nm | Yes | Y/W/E | Yes | Yes | Yes |
| Guanine | 243 nm | Yes | S/W | Y/W | Y/W/E | Yes |
| Leucine | Fl | S/W | S/W | S/W | No | S/W |
| Phenylalanine | Fl | Yes | S/W/E | Y/W/E | Y/W/E | Y/W/E |
| γ-Aminobutyric acid | Fl | No | No | |||
| Fumaric acid | 227 nm | No | No | |||
| Benzoic acid | 227 nm | Y/W | No | Y/W | Y/W/E | Y/W |
| Pyridine-2,6-dicarboxylic acid | 273 nm | No | No | No | No | No |
| Guanosine | 252 nm | Yes | S/W/E | Y/W/E | Y/W/E | Yes |
| Guanosine 5′ monophosphate | 252 nm | Little | No | No | Little | Yes |
| 6-Amino caproic acid | Fl | No | No | No | S/W | S/W |
| AlaGly | Fl | No | No | No | No | No |
| GlyGlyGly | Fl | No | No | |||
| MetAlaSer | Fl | Little | Little | No | No | |
| GlyArgGlyAspSer | Fl | Little | Little | No | No | |
| Random hexamer DNA oligonucleotide | 260 nm | Yes | Yes | Yes | Y/W/E | Y/W/E |
3. Results and discussion
3.1. Stress testing
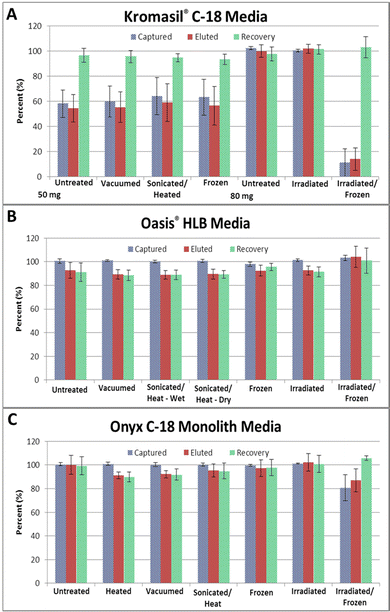
The kinds of small polar organic molecules expected to be found in Ocean World liquid samples include those which have been found on samples of meteorites. These include organic acids,22 organic amines,23 amino acids and nucleobases.24–26 It is also hoped that nucleosides, as well as oligonucleotides and peptides will also be present, to suggest the presence of past or current life in these oceans. The initial stress testing effort focused on tryptophan as a representative molecule since it should bind to the reverse phase matrices and is easily detected by UV absorbance. Without optimizing volumes or flow conditions, a fixed set of conditions was selected and used for the evaluation of all of the media before and after stress conditions. Because neutral molecules are more likely to bind to a hydrophobic phase than charged molecules, it was decided to decrease the pH of the Instant Ocean load solution from the as made pH of 8.8 to a pH of 6.1 for all experiments, as this pH is close to the isoelectric point for tryptophan (pI 5.88) and a number of other amino acids. This kind of pH adjustment has been suggested for in situ analysis through dilution of samples with a buffer solution at a specific pH,27 so is not expected to be an issue for potential use of this method on Ocean World samples. The pH of the condition, equilibration, wash, and elution solutions were not adjusted. For all reverse phase media tested, the goal was to capture analytes from the load solution and retain them as the salt ions pass through, with any remaining salt being washed from the column without loss of the analyte in a wash step. The analyte would then be removed from the column using a hydrophobic elution solution and collected in the elution fraction. The media tested included a C-18 modified silica particle media – Kromasil® C-18, a polymer based media – Oasis® HLB, and a C18 modified silica monolith – Onyx C-18.The 50 mg of untreated Kromasil® C-18 captured 58% of the tryptophan on average, but there was more variability than expected in the results. This could be due to the fact that the 50 mg of media did not completely fill the cartridges, thus solutions had to be pushed out of the system between steps to collect all of the solution for each step. Since these are percentages, small differences in the fraction volumes collected in each step can have a larger effect on the results. Interestingly, even though the amount captured, and amount eluted had higher variability, the amount recovered in each experiment had low variability, showing that what was captured in each experiment was normally recovered. As the amount captured was less than 60%, it was initially unclear if this was a concern, or simply if the amount of analyte was overloading the 50 mg of particles. Experiments were thus performed using 80 mg of the untreated Kromasil® C-18, which more completely filled the cartridges. The variability was significantly better on these experiments, and capture of almost 100% of the tryptophan was observed; this indicated that overloading of the particles could also contribute to the observed variability in the results with the 50 mg tests.
Not utilizing sufficient media to capture all of the tryptophan did allow the stress conditions of freezing, heat/sonication, and vacuum to be evaluated for both improvements in the amount captured or decreases in the amount captured. For stress testing, the media was treated then weighed and packed into the cartridges. The Kromasil® C-18 particle results show no significant change in the amount captured for any of the stress conditions evaluated. While slight increases in the average amount captured were observed for all of the stressed media, the variability also increased, making it hard to say that the increases were real. For all stress conditions tested, the recovery remained near 95%. While not technically 100% recovery, blank extractions performed after two analyte extractions on media from all treatment conditions gave no indication of analyte in any of the collected fractions, showing that there is no carryover, and repeated extractions on a single packed column are possible.
For irradiation testing, 80 mg of the Kromasil® C-18 media was first packed into the cartridges, as the stainless steel outer shell is expected to help mediate any effects of the radiation on the media. Like the 80 mg tests of untreated media, the irradiated cartridges showed 100% capture and recovery of the tryptophan used in these tests. Interestingly, the results from the 3 cartridges irradiated then stored frozen for 10 months are far worse. Because these cartridges were also used to evaluate the stressed media's ability to capture other analytes, only a single tryptophan capture experiment was performed with each cartridge. Because only 3 extractions were performed, the variability is very large, but it is still obvious that this media did not hold up to these combined stresses. While most of the tryptophan was removed from the load fraction, over 85% of the tryptophan ended up in the wash fractions with these cartridges. At this point, it is not clear what happened to the media during the irradiation/freezing treatment, but the recovery remained at 100%, showing that the extraction process itself was not an issue. Loss of the C-18 modification from the silica surface is a possibility but was not tested.
Sorbtech C18 Spherical Silica Gel, another silica based reverse phase media, was also tested in packed cartridges (100 mg) using the same extraction conditions. The Sorbtech media showed tryptophan capture of only about 10% of the tryptophan, with little tryptophan recovered in the elution fraction. It is not clear why there is such a difference between these two C18 reverse phase silica media, but it is possible that the presence of the salt ions in the load solution prevents binding of the analyte to the Sorbtech C-18 particles. Because of the low capture efficiency of the Sorbtech media, stress testing of this media was not performed.
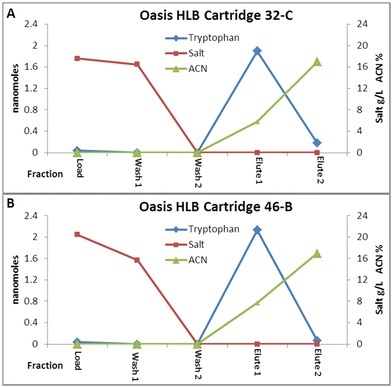
As can be seen from Fig. 1B (data in Table S1†), the capture results for the untreated Oasis® HLB media, and media treated to the different flight stresses were very consistent, showing basically complete capture of the tryptophan in all cases. What was observed with this media was a decreased recovery which averaged only about 89% compared to the 95% recovery of the silica media. Although all of the analyte was captured, it appeared that not all of it was eluted from the HLB media. Additional experiments with these cartridges later in the study, however, showed that the solution that remained in the cartridge likely contained the remaining analyte and was flushed out during reconditioning, as no analyte was observed in any post tryptophan extraction blank runs. The results from the irradiated and frozen cartridges show that 100% recovery from these cartridges is observed, as these experiments utilized a second elution step.
These later experiments also utilized a second wash step to fully define the fate of the salt from the load solution. Fig. 2 shows the graphs from both an irradiated/frozen (Cartridge 46) and an untreated (cartridge 32) Oasis® HLB cartridge. All of the tryptophan is captured from the load fraction, while much of the salt comes through. The first wash step fraction contains the remaining salt, with the specific gravity decreased to 1 by the second wash step, showing no more salt coming through the cartridge by this point. The refractive index measurements on the elution fractions show that there is still significant water from the wash step in the cartridge as the first elution fraction is collected, as the ACN concentration is only about 7% rather than the 20% being loaded into the cartridge. Most of the tryptophan does come out in this first elution fraction, but a little also shows up in the second elution fraction.
Onyx monolithic C-18 guard cartridges were 4.6 mm in diameter, and 10 mm in length, which corresponds to the internal size of the PEEK cartridges used with the particle media (4.5 mm × 12 mm). As shown in Fig. 1C (data in Table S1†), the monoliths captured 100% of the tryptophan analyte from the Instant Ocean solution as received, and following all stress treatments. The amount eluted varied some, but recoveries were all over 90%, and based on the number of extractions performed, there appears to be no effect of any of the treatments on the performance of the Onyx C-18 monoliths, except for the combined irradiation and long term frozen storage treatment. Capture of tryptophan after the combined irradiation/freezing treatment decreased to about 80%, with full recovery. As with the Kromasil® C-18 media, there was capture during the loading step, but again tryptophan was observed in the second wash fractions. Because of the limited number of experiments performed, no conclusions were drawn at this point and these treated cartridges were further employed for extraction of other analytes as detailed below.
The other silica monoliths investigated in this work were Chromolith® RP-18e guard cartridges, which measured 4.6 mm × 5 mm; this is approximately the volume of the PEEK cartridge filled with 50 mg of Kromasil® C-18 particles. Following conditioning, these monoliths captured 100% of the tryptophan from solution, with about 89% recovery. Again, the less than 100% recovery is likely due to slight fraction volume differences and some material stuck to the monolith which was not removed in the single elution step. Unfortunately, these monoliths were not consistent, as subsequent unstressed monoliths showed capture of only 52% of the tryptophan. Again, however, fractions from these monoliths show no tryptophan in the load fraction, but the tryptophan coming off in the wash solution. A similar trend was seen with the stressed Chromolith® RP-18e monoliths. Monoliths which were simply heated or frozen showed 100% capture and hold through the wash steps, but those which were sonicated and heated, or vacuum treated also showed capture, but loss of tryptophan during the wash step. It is not clear why this was happening, with tryptophan being capture from the high salt solution, but not being retained on some of the silica phases after treatment during the water wash step.
3.2. Additional analytes
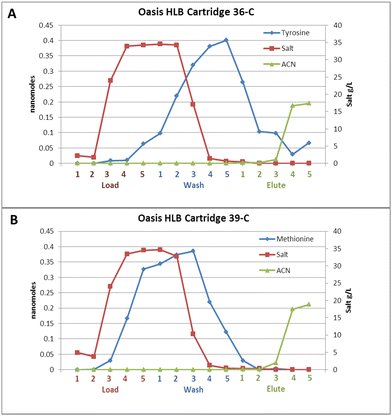
Tryptophan represented a good, easily captured analyte for performing the stress testing, but the desalting method developed in this project should be able to capture additional analytes of interest for exploring the full extent of organic molecules present in liquid oceans on other bodies in our Solar System. To further evaluate these reverse phase media, a full array of different small molecule analytes were tested as shown in Table 1, along with the method used for detection. These analytes represent amino acids, organic acids, organic amines, nucleobases, nucleosides, peptides, and an oligonucleotide mixture, covering a broad range of functionalities and molecules that would be of interest in the search for current or past life on these bodies. Introduction of fluorescence based detection allowed decreasing the concentrations of the amino acids to 5 μM, and 10 μM for the peptides being tested. Initially fluorescamine was used for amino acid labeling, but the fluorescence was affected by the amount and types of salt ions present. Labeling with o-phthalaldehyde proved to be more robust, but was still affected by ions such as sulfate, ammonia, and magnesium. Because the fluorescence of the amine labeled product is normally more stable than the product from reaction with the ions, simply delaying measurement of the fluorescence of OPA labeled samples for 30–60 minutes after the reaction, greatly reduced the background interference from contaminants.Table 1 also shows how well the various analytes were extracted with the different media. In addition to simple analyte capture from the load solution and elution in the elution solution, some of the analytes showed other possibilities. Tyrosine for example, appeared to be completely removed from the load solution when passed through the Oasis® HLB cartridges, but then appeared in both the wash and elution fraction. For the Kromasil® C-18 cartridges and Onyx monoliths, some of the tyrosine was removed from the load solution, but not all of it, but none remained bound to either of these phases after the wash step. For this project, however, the main goal of this sample preparation method is salt removal, not necessary keeping the analyte bound until the elution step. If the analyte is released as the salt concentration is decreased, this could be sufficient for use as an initial clean-up step before in situ MS analysis, simply monitoring the conductivity due to the presence of salt ions then directing the output into the MS once the conductance drops below a set value.
To look closer, and determine if this would work, extractions were performed on Oasis® HLB cartridges, but instead of collecting a single load, wash and elution fraction, 5 fractions of 80 μL (the minimum amount required for RI based salt detection) were collected for each step. Fig. 3 shows the results from a tyrosine (A) and a methionine (B) extraction. As indicated in Table 1, almost all of the tyrosine is captured from the load fractions by the Oasis® HLB media. It is easy to see in Fig. 3A that the tyrosine coming off of the column is shifted with respect to the salt passing through the column, and sufficient tyrosine exits the column after the salt concentration has dropped to zero, that detection should be possible. For the methionine graph in Fig. 3B, not all of the analyte is removed from the load fractions, and although the analyte curve is delayed relative to the salt curve, it is not clear that there is enough of a delay to make this media useful for desalting of methionine.
The results in Table 1 were not completely anticipated. The Oasis® HLB media showed slightly better performance over the range of analytes tested, capturing and holding on to phenylalanine, adenine, and guanine, through the wash step and allowing them to be collected in the elution fraction. Tyrosine (pI 5.66) and phenylalanine (pI 5.48), which are both aromatic and should be effectively neutral at pH 6, did not remain bound to the Kromasil® C-18 and Onyx monoliths once the salt concentration dropped as would be expected. While benzoic acid and guanosine 5′ monophosphate are both charged in the load solution, the benzoic acid bound to both the HLB and Onyx phases but the 5′GMP did not bind well to either, with Kromasil® C-18 cartridges capturing neither. At the same time, the random hexamer oligonucleotides should be highly charged at this pH, but bound in the high salt solution and had to be eluted off of all three media. The peptides, however, which should have a lower charge to mass ratio did not bind well.
Although not subjected to stress testing, two other silica based reverse phases with different hydrophobic functionalities, Strata® Phenyl and Strata® C8, were also tested to see if they provided capture of any different analytes. Results for these two media are also included in Table 1. Cartridges packed with 100 mg of each of these media showed excellent capture and recovery of tryptophan from the pH 6.1 ocean water simulant. While neither media clearly outperformed the Oasis® HLB phase, the C8 phase did capture some valine and 6-amino caproic acid, along with complete capture of the 5′GMP, for which the HLB media showed only limited affinity. Additional stress testing of this phase may be warranted under further studies.
3.3. Multianalyte extractions
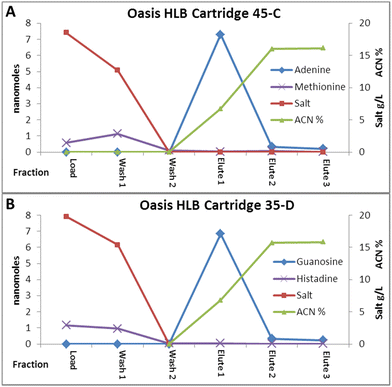
Extractions of individual analytes suggest which analytes might be investigated using the reverse phase media, but an ocean sample from a Solar System body is unlikely to have a single analyte present. Using one analyte that could be detected using fluorescence and one analyte that could be detected using absorption, dual analyte extractions were also performed on some of these media. Fig. 4 shows examples of dual extractions on Oasis® HLB packed cartridges which had been subjected to the irradiation/10 month freeze treatment – control untreated cartridges gave similar results. These extractions show complete capture of adenine or guanine with only some capture of either methionine or histidine but release in the wash fractions. These results were in line with the individual analyte extractions, except that no methionine remained bound until the elution fraction. These results also show that the irradiation/freezing treatment did not affect capture of other analytes besides tryptophan.Mixed analyte extractions were also performed on a 14 component mixture (Table S2†) that encompassed many of the individual analytes, with some additional analytes added, all at a 5 μM concentration in the Instant Ocean solution at pH 6.1. For detection of the individual analytes in these mixtures, collected fractions were sent to Signature Science (Austin, TX) for HPLC-MS analysis. The elution fractions from extractions on Oasis® HLB packed cartridges (50 mg) showed tryptophan and adenine as expected, with only small amounts of guanine, and a trace of isobutylamine (the low molecular weight and high volatility of propylamine made detection of this compound difficult). Because not all of the guanine was observed, because the other reverse phases did not bind guanine as well as adenine, and because a larger volume of load solution was employed (1200 μL) it was hypothesized that additional binding capacity was likely required to capture guanine in the presence of the more strongly bound analytes. Two longer columns were paced with ∼180 mg of the Oasis® HLB media, and used for a second set of extractions on the mixed analyte sample. With the larger volume columns, fractions were shifted by 200 μL to account for the larger dead volume in the columns. In this experiment, there were 2 elution steps, first using 20% acetonitrile in water, then 40% ACN in water, with 2 elution fractions of 500 μL each collected. These extractions showed recovery of more tryptophan, adenine and guanine, as well as small amounts of tyrosine and again traces of isobutylamine. In addition, small amounts of the sodium octanoate also showed up in the second elution fraction. Since the sodium octanoate could not be detected by either UV or fluorescence detection, at this point, it is not clear if more sodium octanoate is capture but not eluted, or if this compound is not fully captured.
To compare multianalyte extractions on the three different phases, a larger column was also prepared with the Kromasil® C-18 media (250 mg), and a longer Onyx monolith (25 mm) was purchased.
A second wash fraction (the salt concentration was near zero), and elution fractions were collected and sent for analysis. The second wash fraction from the Oasis® HLB column showed both citric and glutamic acid along with some methionine and tyrosine. As with the previous extractions, adenine, guanine, and tryptophan were well represented, with traces of tyrosine and isobutylamine. It is not clear why, but a larger amount of sodium octanoate was recovered this time along with traces of methionine and even histidine in the elution fractions. The wash fraction from the Kromasil® C-18 column showed small amounts of glutamic acid, adenine, guanine, histidine, isobutylamine, methionine, and valine. The elution fractions showed recovery of the tryptophan and adenine as expected, with a small amount of glutamic acid and isobutylamine also present. The Onyx monolith showed some guanine, histidine, isobutylamine and tryptophan in the wash fraction, with elution of the adenine, tryptophan and small amounts of guanine, methionine and isobutylamine.
Overall, the mixed analyte extractions showed that the results of the individual analyte extractions were informative, with more strongly retained compounds preferentially binding from mixed solutions. Providing sufficient binding capacity is necessary to allow for binding of more weakly retained analytes. For many of the analytes of interest, the concentrations in Ocean World samples are not likely to be as high as those used in these experiments, which means less tightly bound analytes might also bind. The problem, however, is that additional hydrophobic molecules which are not of interest in the search for life, such as PAH compounds that are still soluble in the salt oceans, are also likely to be present in these samples, and may preferentially bind to the reverse phase media.
3.4. Low concentration extractions
The concentrations of analytes used in this testing are higher than what would be expected for real samples, but are used for easy performance evaluation of the various treated media. The solid phase extraction procedure, however, also provides the possibility for concentrating analytes if they can be bound and released in a smaller volume of solution. While this may not be possible for analytes that are only bound until the wash step, it should be possible for analytes that remain bound until the elution solution releases them from the media. At the same time, it is also important to show that partition coefficients are high enough that even lower concentrations of analyte in solution are captured. Because it proved to be the best media for extractions of the selected analytes, low concentration extractions were performed on columns filled with the Oasis® HLB media.Based on sensitivity measurements with the o-phthalaldehyde fluorescent labeling reagent, the limit of detection for some amino acids could be lower than 25 nM concentration in water by increasing the amount of sample to 100 μL and eliminating the water in the reaction. There was always background fluorescence due to the ions in the Instant Ocean salt solution (Mg+2, NH4+, SO4−2), thus extractions were performed from 35 g L−1 NaCl solution to provide better sensitivity. At the same time, while the load solution remained at 1200 μL, the elution fractions were reduced to 150 μL to try to concentrate the eluted analyte in a smaller volume and increase the concentration for detection. Fig. 5 shows the results from one extraction of a 50 nM solution of phenylalanine in 35 g L−1 NaCl. It is easy to see that all of the phenylalanine was captured from the load solution and subsequently eluted in almost a single elution fraction. Additional experiments at 25 nM concentration, along with extractions of tryptophan solutions at 25 nm and 50 nm concentrations all showed similar results. To take the dilution further, tryptophan solution at 5 nM in 35 g L−1 NaCl was loaded onto columns. A total of 6 mL of solution was loaded, and again eluted mostly in a single elution fraction, with good recovery of all the loaded analyte. Lower concentrations were not tested, as the 6 mL of 5 nM solution extracted took 30 minutes to load, so lower concentration solutions would have further increased the total time needed to load sufficient analyte to detect in the elution fractions. It is also interesting to note that these low concentration extractions were the 15th extraction on one column, and the 13th extraction on another column. This shows that these columns are reusable, and can be flushed and reconditioned then utilized for extraction of subsequent samples even under the most stringent conditions.
 | ||
| Fig. 5 Extraction of low concentration (50 nM) Phenylalanine using multiple small elution fractions to show concentration of analyte in the extraction process. | ||
3.5. High salt extractions
As the actual salt concentration in any sample collected from an ocean on another Solar System body is not known, this work used the salt concentration in Earth's ocean for the initial experiments. Because the salt passes right through the column, the salt concentration should not ultimately matter for analytes which bind sufficiently to the reverse phase media. To show this, the salt concentration in the load solution was increased to 50 g L−1 of the Instant Ocean salt mixture, and extractions were again performed on the Oasis® HLB columns. Phenylalanine (2 μM) was extracted on multiple columns, with complete capture from 1200 μL of load solution. Decreasing the phenylalanine concentration to 0.5 μM in the high salt solution and increasing the amount loaded to 4 mL, did not affect the capture and release of the analyte from the Oasis® HLB media. This shows that the concentration of salt in the samples collected in situ from these bodies should not affect the performance of the reverse phase desalting process.4. Conclusions
Samples which have been analyzed in situ on or around remote bodies outside of Earth, have been solid or gaseous samples. Our desire to explore and understand bodies in our Solar System that contain liquids, however, is driven by the potential for finding new forms of life on these worlds. Bringing samples back to Earth for analysis is not realistic, thus sample preparation methods used for removal of salts and contaminants, and analytical methods for detecting specific compounds in liquids on Earth, must be transported to these worlds. This study begins experimental work to determine what kinds of media for a solid phase extraction method would be functional and useful once transported to an Ocean World. Based on the results, it was clear that the polymeric media was more stable to the tested flight stresses and more useful than either the silica particle or monolithic forms of reverse phase media tested. Additional media have been identified that should undergo similar stress testing, but at this point, the Oasis® HLB media provided the optimum stability and overall utility for extraction of some analytes of interest from these samples. Experiments with analytes not detectable by absorbance or fluorescence will be required to further evaluate these media, and to determine how the presence of PAH and other strongly hydrophobic compounds will affect capture of analytes of interest. It is also clear, that reverse phase capture will only be useful for a subset of the kinds of analytes that will need to be analyzed in sample from these Ocean Worlds, thus additional modalities for capture have also been investigated using similar stress testing, individual, and multianalyte experiments and are reported in a subsequent publication.29 It would also be of great interest to directly couple the output from these reverse phase packed columns into a MS detector, to begin to evaluate how all of the components will fit together and work together in an extraterrestrial deployment situation.Conflicts of interest
There are no conflicts of interest regarding the author or work in this manuscript.Acknowledgements
The author wishes to acknowledge Waters Corporation, who provided some of the Oasis® HLB media used in these experiments, and Nouryon Pulp & Performance Chemicals, LLC, who provided the Kromasil® C-18 media used in this work. Thank you to Killian O'Connell, Tiffany Layne, and the Landers’ laboratory at the University of Virginia for frozen storage of materials. Funding for this work was provided by NASA under contracts #80NSSC19C0172 and #80NSSC18P2053.References
- F. S. Brown, H. E. Adelson, M. C. Chapman, O. W. Clausen, A. J. Cole, J. T. Cragin, R. J. Day, C. H. Debenham, R. E. Fortney, R. I. Gilje, D. W. Harvey, J. L. Kropp, S. J. Loer, L. J. Logan Jr., W. D. Potter and G. T. Rosiak, Rev. Sci. Instrum., 1978, 49, 139 CrossRef CAS PubMed.
- V. I. Oyama and B. J. Berdhal, J. Geophys. Res., 1977, 82, 4669–4676 CrossRef CAS.
- V. I. Oyama, G. C. Carle, F. Woeller, J. B. Pollack, R. T. Reynolds and R. A. Craig, J. Geophys. Res.: Space Phys., 1980, 85, 7891–7902 CrossRef.
- H. R. Anderson, J. Geophys. Res., 1964, 69, 2651–2657 CrossRef.
- S. O. Akapo, J. M. D. Dimandja, M. T. Matyska and J. J. Pesek, Chromatographia, 1996, 42, 141–146 CrossRef CAS.
- P. R. Mahaffy, C. R. Webster, M. Cabane, P. G. Conrad, P. Coll, S. K. Atreya, R. Arvey, M. Barciniak, M. Benna, L. Bleacher, W. B. Brinckerhoff, J. L. Eigenbrode, D. Carignan, M. Cascia, R. A. Chalmers, J. P. Dworkin, T. Errigo, P. Everson, H. Franz, R. Farley, S. Feng, G. Frazier, C. Freissinet, D. P. Glavin, D. N. Harpold, D. Hawk, V. Holmes, C. S. Johnson, A. Jones, P. Jordan, J. Kellogg, J. Lewis, E. Lyness, C. A. Malespin, D. K. Martin, J. Maurer, A. C. McAdam, D. McLennan, T. J. Nolan, M. Noriega, A. A. Pavlov, B. Prats, E. Raaen, O. Sheinman, D. Sheppard, J. Smith, J. C. Stern, F. Tan, M. Trainer, D. S. W. Ming, R. V. Morris, J. Jones, C. Gundersen, A. Steele, J. Wray, O. Botta, L. A. Leshin, T. Owen, S. Battel, B. M. Jakosky, H. Manning, S. Squyres, R. Navarro-González, C. P. McKay, F. Raulin, R. Sternberg, A. Buch, P. Sorensen, R. Kline-Schoder, D. Coscia, C. Szopa, S. Teinturier, C. Baffes, J. Feldman, G. Flesch, S. Forouhar, R. Garcia, D. Keymeulen, S. Woodward, B. P. Block, K. Arnett, R. Miller, C. Edmonson, S. Gorevan and E. Mumm, Space Sci. Rev., 2012, 170, 401–478 CrossRef.
- F. Postberg, N. Khawaja, B. Abel, G. Choblet, C. R. Glein, M. S. Gudipati, B. L. Henderson, H.-W. Hsu, S. Kempf, F. Klenner, G. Moragas-Klostermeyer, B. Magee, L. Nölle, M. Perry, R. Reviol, J. Schmidt, R. Srama, F. Stolz, G. Tobie, M. Trieloff and J. H. Waite, Nature, 2018, 558, 564–568 CrossRef CAS PubMed.
- J. Perry, J. Spitale, A. Brahic, J. A. Burns, A. D. DelGenio, L. Dones, C. D. Murray and S. Squyres, Science, 2006, 311, 1393–1401 CrossRef PubMed.
- J. L. Eigenbrode, R. E. Summons, A. Steele, C. Freissinet, M. Millan, R. Navarro-González, B. Sutter, A. C. McAdam, H. B. Franz, D. P. Glavin, P. D. Archer, P. R. Mahaffy, P. G. Conrad, J. A. Hurowitz, J. P. Grotzinger, S. Gupta, D. W. Ming, D. Y. Sumner, C. Szopa, C. Malespin, A. Buch and P. Coll, Science, 2018, 360, 1096–1101 CrossRef CAS PubMed.
- C. Szopa, U. J. Meierhenrich, D. Coscia, L. Janin, F. Goesmann, A. Sternberg, J. F. Brun, G. Israel, M. Cabane, R. Roll, F. Raulin, W. Thiemann, C. Vidal-Madjar and H. Rosenbauer, J. Chromatogr., A, 2002, 982, 303–312 CrossRef CAS.
- U. J. Meierhenrich, J. R. L. Cason, C. Szopa, R. Sternberg, F. Raulin, W. H.-P. Thiemann and F. Goesmann, Adv. Space Res., 2013, 52, 2080–2084 CrossRef CAS.
- C. Szopa, G. Freguglia, R. Sternberg, M. J. Nguyen, P. Coll, F. Raulin, C. Pietrogrande and H. Niemann, J. Chromatogr., A, 2006, 1131, 215–226 CrossRef CAS.
- A. R. Hendrix, T. A. Hurford, L. M. Barge, M. T. Bland, J. S. Bowman, W. Brinckerhoff, B. J. Buratti, M. L. Cable, J. Castillo-Rogez, G. C. Collins, S. Diniega, C. R. German, A. G. Hayes, T. Hoehler, S. Hosseini, C. J. A. Howett, A. S. McEwen, C. D. Neish, M. Neveu, T. A. Nordheim, G. W. Patterson, D. A. Patthoff, C. Phillips, A. Rhoden, B. E. Schmidt, K. N. Singer, J. M. Soderblom and S. D. Vance, Astrobiology, 2019, 19, 1–27 CrossRef PubMed.
- J. B. Dalton III, J. H. Shirley and L. W. Kamp, J. Geophys. Res., 2012, 117, E03003 Search PubMed.
- P. D. Fischer, M. E. Brown and K. P. Hand, Astron. J., 2015, 150, 164 CrossRef.
- J. S. Kargel, J. Z. Kaye, J. W. Head III, G. M. Marion, R. Sassen, J. K. Crowley, O. P. Ballesteros, S. A. Grant and D. L. Hogenboom, Icarus, 2000, 148, 226–265 CrossRef CAS.
- M. J. Atkinson and C. Bingman, J. Aquaric. Aquat. Sci., 1995, 8, 39–43 Search PubMed.
- C. Freissinet, M. Millan, D. P. Glavin, X. Li, A. Grubisic, J. E. Eigenbrode, J. C. Stern, J. P. Dworkin, A. Buch, C. Szopa, M. A. Guzman, M. A. Carts, S. A. Getty and W. B. Brinckerhoff, Planet. Space Sci., 2019, 175, 1–12 CrossRef CAS.
- S. Stein, P. Böhlen, J. Stone, W. Dairman and S. Udenfriend, Arch. Biochem. Biophys., 1973, 155, 203–212 CrossRef CAS.
- M. Roth, Anal. Chem., 1971, 43, 880–882 CrossRef CAS PubMed.
- J. R. Benson and P. E. Hare, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 619–622 CrossRef CAS PubMed.
- J. C. Aponte, H. K. Woodward, N. M. Abreu, J. E. Elsila and J. P. Dworkin, Meteorit. Planet. Sci., 2019, 54, 415–430 CrossRef CAS PubMed.
- J. C. Aponte, J. P. Dworkin and J. E. Elsila, Meteorit. Planet. Sci., 2015, 50, 1733–1749 CrossRef CAS.
- A. S. Burton, J. C. Stern, J. E. Elsila, D. P. Glavin and J. P. Dworkin, Chem. Soc. Rev., 2012, 41, 5459–5472 RSC.
- A. S. Burton, J. E. Elsila, M. P. Callahan, M. G. Martin, D. P. Glavin, N. M. Johnson and J. P. Dworkin, Meteorit. Planet. Sci., 2012, 47, 374–386 CrossRef CAS.
- M. P. Callahan, A. S. Burton, J. E. Elsila, E. M. Baker, K. E. Smith, D. P. Glavin and J. P. Dworkin, Meteorit. Planet. Sci., 2013, 48, 786–795 CrossRef CAS.
- J. S. Creamer, M. F. Mora and P. A. Willis, Anal. Chem., 2017, 89, 1329–1337 CrossRef CAS PubMed.
- D. J. Marchiarullo, J. Y. Lim, Z. Vaksman, J. P. Ferrance, L. Putcha and J. P. Landers, J. Chromatogr., A, 2008, 1200, 198–203 CrossRef CAS PubMed.
- J. P. Ferrance, Testing of ion exchange chromatography media for extraterrestrial in situ sample preparation on liquid samples, Analyst Search PubMed , submitted.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2an00768a |
| This journal is © The Royal Society of Chemistry 2022 |