Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Degradation of amyloid peptide aggregates by targeted singlet oxygen delivery from a benzothiazole functionalized naphthalene endoperoxide†
Hao
Wu
,
Ziang
Liu
,
Yujie
Shao
,
Guangzhe
Li
 ,
Yue
Pan
,
Lei
Wang
* and
Engin U.
Akkaya
,
Yue
Pan
,
Lei
Wang
* and
Engin U.
Akkaya
 *
*
State Key Laboratory of Fine Chemicals, and Department of Pharmaceutical Science, Dalian University of Technology, 2 Linggong Road, 116024 Dalian, China. E-mail: eua@dlut.edu.cn; leiwang@dlut.edu.cn
First published on 24th January 2022
Abstract
Aggregate structures formed by amyloid-β (Aβ) are correlated with the progression of pathogenesis in Alzheimer's disease. Previous works have shown that photodynamic photosensitizers were effective in oxidatively degrading amyloid-β aggregates and thus decreasing their cytotoxicity under various conditions. In this work, we designed and synthesized a benzothiazole–naphthalene conjugate, with high level of structural analogy to Thioflavin T which is known to have high affinities for the amyloid peptide aggregates. The endoperoxide form (BZTN-O2) of this compound, which releases singlet oxygen with a half-life of 77 minutes at 37 °C, successfully inhibited and/or reversed amyloid aggregation. The endoperoxide is capable of singlet oxygen release without any need for light, and its charge-neutral form could allow blood–brain barrier (BBB) permeability. The therapeutic potential of such endoperoxide compounds with amyloid binding affinity is exciting.
Alzheimer's disease (AD) is the most common form of dementia.1 The discovery of amyloid beta (Aβ) plaques in the extracellular space of the brains of AD patients in 1984, led to the “amyloid hypothesis”.2 This hypothesis posits that Aβ monomers assemble into toxic oligomers and fibrous aggregates, causing neuronal damage. Resulting clinical symptoms are cognitive disfunction and memory loss. In addition to Aβ plaques, neurofibrillary tangles (NFT) composed of tau protein are correlated with cognitive disfunction.3 In one model of AD initiation/progression, Aβ is the trigger and NFT are the bullets for neurotoxicity.4
While AD has been linked to oxidative stress and reactive oxygen species (ROS),5 the process requires finer assessment of the roles of individual ROS and/or reactive nitrogen species (RNS) entities, and not appropriate for painting with a broad brush. In fact, a number of articles appeared recently,5 clearly demonstrating the fact that the oxygenation of amyloid peptides under the conditions of photodynamic action reduced their neurotoxicity. Furthermore, using Drosophila AD models two different photosensitizers resulted in full recovery of the locomotion defects.5f,g The photosensitized reaction is highly specific, and the most important change is the oxidation of a methionine residue at the position 35 (M35) of the Aβ42 peptide.5b This particular amino acid is critical for holding the conformation needed for aggregation of Aβ peptides into toxic oligomers and fibril structures, and its oxidation to methionine sulfoxide inhibits the rate of aggregation.6 The reactivity and the mode of generation suggest that singlet oxygen is involved in the oxidation reactions.7 In fact, it is known that singlet oxygen effectively reacts with methionine residues to yield sulfoxide.7
Photodynamic generation of singlet oxygen suffers from two inherent limitations, light cannot be transmitted through tissues effectively,8 especially through the skull,9 in the case of brain tissues. Also, photodynamic singlet oxygen generation depletes local oxygen resources. Based on our previous experience with on target, and on demand delivery of singlet oxygen, we set out to design and synthesize an Aβ binding compound with endoperoxide moieties which could thermally release singlet oxygen to disrupt Aβ oligomerization and aggregation. To that end, we noted that Thioflavin T (Fig. 1) is known to be a selective fluorescent stain for Aβ fibril structures. On binding, the fluorescence emission near 480 nm is significantly enhanced. Well-known 11C PET tracer “Pittsburgh compound B” (a.k.a. PiB, or BTA-1) is structurally related to ThT, and it is highly selective for amyloid β deposits in brain.
 | ||
| Fig. 1 Structures of Thioflavin T and the 11C-labeled PET imaging agent Pittsburgh compound B. In this work, the compounds BZTN and BZTN-O2 were synthesized. | ||
The simple naphthalene derivative BZTN and its endoperoxide form BZTN-O2 were designed in an attempt to keep maximum structural similarity to ThT and PiB while introducing an endoperoxide module. The synthesis of the target compounds (Fig. 2) starts with 1,4-dimethylnaphthalene (1). Functionalization at the 6 positions was done by following a useful regioselective borylation reported by the Suginome group.10 Deprotection of the compound 2 was carried out according to literature, followed by conversion of the boronic acid functionality to a formyl group by a modified Petasis reaction.11 Benzothiazole core was then generated by the reaction of the aldehyde with p-toluidine, thiocyanate and iodine in DMSO. BZTN obtained in this way can conveniently be transformed into endoperoxide BZTN-O2, by the reaction of singlet oxygen obtained by photosensitization.
The cycloreversion reaction rate of the endoperoxide BZTN-O2 was determined following the changes in the 1H NMR of the compound in CDCl3. The first order reaction rate and the half-life of the endoperoxide can be obtained by following the changes in the integral ratios of the various peaks, such as essentially unchanged singlet methyl peak of the benzothiazole core near 2.45 ppm versus the two new singlet peaks appearing near 2.7 ppm (Fig. 3).
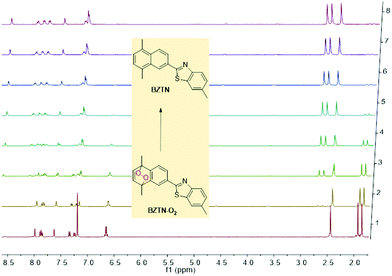 | ||
| Fig. 3 Temporal evolution of BZTN-O21H NMR in CDCl3 at 37 °C. It is noteworthy that conversion is back to BZTN in a clean reaction without any by-products. | ||
The half-life at 37 °C was determined to be 77 minutes which is in the expected range for most naphthalene endoperoxides.12 We then proceeded to establish that the other product is indeed singlet oxygen by carrying out the cycloreversion reaction of the endoperoxide BZTN-O2 (Fig. 4) in the presence of singlet oxygen trap 1,3-diphenylisobenzofuran (DPBF).
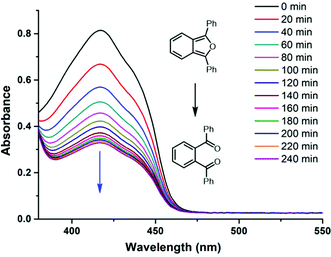 | ||
| Fig. 4 The decrease in the absorption peak of DPBF (50 μM) in the presence of BZTN-O2 (500 μM) in DMSO in dark at 37 °C. | ||
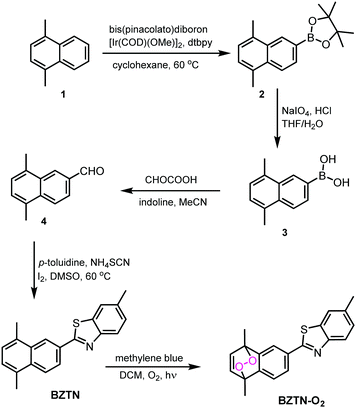
In order to assess the utility of the BZTN-O2 in degradation of the Aβ aggregates we have carried out following experiments. First, a solution of monomeric Aβ1–42 was prepared in hexafluoro-2-propanol (HFIP) and kept at room temperature overnight. The solution was divided into aliquots desiccated to afford film-like lyophilized Aβ1–42. For the ThT binding assay (ESI) Aβ1–42 film was re-dissolved in ammonium hydroxide solution, then diluted with phosphate buffer. A mixture of the peptide (50 μM, final concentration) with or without BZTN or BZTN-O2 (100 μM) was incubated at 37 °C for 48 h. After incubation, ThT (5 μM) was added. Then the fluorescence intensities were recorded after five minutes. ThT specific for β-sheet aggregate structures and our data shows that BZTN-O2 very effectively inhibits formation of these structures (Fig. 5a) as evidenced by the decrease of emission intensity. The inhibitory effect of the BZTN itself is also expected as it is capable of binding the β-sheet rich aggregates.
Another assay commonly used to assess oxidative transformation of peptides is the chromogenic 2,4-dinitrophenylhydrazine (DNPH) assay. Aβ1–42 in solution was precipitated with a trichloroacetic acid (TCA, 20% final concentration) solution at 0 °C, the peptide was then collected by centrifugation. DNPH was added (10 mM DNPH in 2.0 M of HCl) followed by 1 h incubation at room temperature. The samples processed further and finally absorption spectra were collected (Fig. 5b). As expected, +BZTN-O2 results in large hydrazone peak unlike +BZTN or Aβ alone, which is clearly due to singlet oxygen mediated side chain oxidation. The process is selective, under the same conditions, BSA is oxidized only to a small extent (ESI,† Fig. S2).
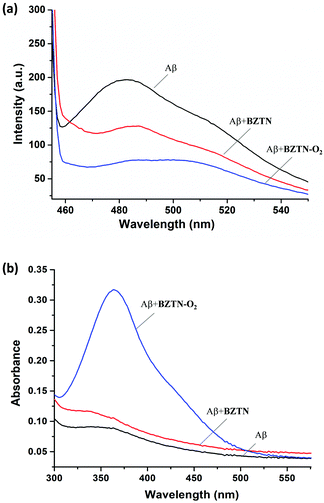
Finally, we wanted observe the changes in the amyloid fibrils and aggregate structures microscopically (TEM) compared various control cases, including curcumin, well-known13 disruptor of amyloid aggregation (ESI†). To that end, Aβ1–42 peptide stock was diluted with phosphate buffer (pH 7.4) to 100 μM before use. For the inhibition of Aβ1–42 aggregation, monomeric Aβ1–42 was incubated with BZTN-O2, BZTN or curcumin at 37 °C for 48 h, respectively. Monomeric Aβ1–42 and aggregated Aβ1–42 were used as control groups. The concentration of Aβ1–42 and the test compounds were set as 25 μM (final concentration). Aliquots of the samples were placed on a carbon coated copper/rhodium grid and each grid was stained with 0.5% phosphotungstate solution for 2 min. The excess staining solution was removed and the specimen was transferred for imaging with transmission electron microscopy. Images acquired (Fig. 6) show that 37 °C incubation results in in large aggregate structures (Fig. 6b) whereas curcumin only leads to small globular structures (Fig. 6c). Compound BZTN is less effective (Fig. 6d) than curcumin, but BZTN-O2 (Fig. 6e) inhibits aggregate structures altogether.
Similar experiments where BZTN-O2 is added after the aggregation, also showed effective degradation of the pre-formed aggregate structures (ESI,† Fig. S7) as evidenced by TEM images.
AFM images were also acquired (Fig. 7) for samples of Aβ1–42 exposed to BZTN-O2. Large aggregate structures were successfully degraded in the presence of 25 μM.
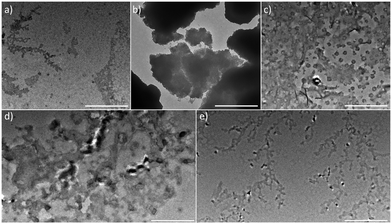
Finally, toxicity of the compounds was assessed using two normal cell cultures. We are pleased to report (Fig. 8) that within the concentration range of our study, both BZTN and BZTN-O2 are not cytotoxic.
In conclusion, we were able to demonstrate that an endoperoxide with an affinity to amyloid beta clusters, is capable of inhibiting aggregation via oxidative transformation. The therapeutic potential of this, and conceptually related compounds are currently under study in our laboratories.
The authors acknowledge financial support from LiaoNing Revitalization Talents Program (E. U. A.: XLYC1902001, L. W.: XLYC1907021) and the National Science Foundation of China (L. W.: 22007008).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Burns and S. Iliffe, BMJ, 2009, 338, 405–409 Search PubMed.
- (a) D. J. Selkoe, Neuron, 1991, 6, 487–489 CrossRef CAS PubMed; (b) J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184–185 CrossRef CAS PubMed.
- (a) H. Braak and E. Braak, Acta Neuropathol., 1991, 82, 239–259 CrossRef CAS PubMed; (b) L. M. Ittner and J. Götz, Nat. Rev. Neurosci., 2011, 12, 67–72 CrossRef PubMed.
- G. S. Bloom, JAMA Neurol., 2014, 71, 505–508 CrossRef PubMed.
- (a) H. Yagi, D. Ozawa, K. Sakurai, T. Kawakami, H. Kuyama, O. Nishimura, T. Shimanouchi, R. Kuboi, H. Naiki and Y. Goto, J. Biol. Chem., 2010, 285, 19660–19667 CrossRef CAS PubMed; (b) A. Taniguchi, Y. Shimizu, K. Oisaki, Y. Sohma and M. Kanai, Nat. Chem., 2016, 8, 974–982 CrossRef CAS PubMed; (c) A. Taniguchi, D. Sasaki, A. Shiohara, T. Iwatsubo, T. Tomita, Y. Sohma and M. Kanai, Angew. Chem., Int. Ed., 2014, 53, 1382–1385 CrossRef CAS PubMed; (d) J. S. Lee, B. I. Lee and C. B. Park, Biomaterials, 2015, 38, 43–49 CrossRef CAS PubMed; (e) A. Hirabayashi, Y. Shindo, K. Oka, D. Takahashi and K. Toshima, Chem. Commun., 2014, 50, 9543–9546 RSC; (f) B. I. Lee, S. Lee, Y. S. Suh, J. S. Lee, A.-K. Kim, O. Y. Kwon, K. Yu and C. B. Park, Angew. Chem., Int. Ed., 2015, 54, 11472–11476 CrossRef CAS PubMed; (g) B. I. Lee, Y. S. Suh, Y. J. Chung, K. Yu and C. B. Park, Sci. Rep., 2017, 7, 752 CrossRef PubMed; (h) Y.-J. Hsieh, K.-Y. Chien, I.-F. Yang, I.-N. Lee, C.-C. Wu, T.-Y. Huang and J.-S. Yu, Sci. Rep., 2017, 7, 1370 CrossRef PubMed; (i) M. W. Beck, J. S. Derrick, R. A. Kerr, S. B. Oh, W. J. Cho, S. J. C. Lee, Y. Ji, J. Han, Z. A. Tehrani, N. Suh, S. Kim, S. D. Larsen, K. S. Kim, J.-Y. Lee, B. T. Ruotolo and M. H. Lim, Nat. Commun., 2016, 7, 13115 CrossRef CAS PubMed.
- (a) L. Hou, I. Kang, R. E. Marchant and M. G. Zagorski, J. Biol. Chem., 2002, 277, 40173–40176 CrossRef CAS PubMed; (b) L. Hou, H.-G. Lee, F. Han, J. M. Tedesco, G. Perry, M. A. Smith and M. G. Zagorski, J. Alzheimer's Dis., 2013, 37, 9–18 CAS.
- (a) Y. Yan, S. A. McCallum and C. Wang, J. Am. Chem. Soc., 2008, 130, 5394–5395 CrossRef CAS PubMed; (b) M. Liu, E. Ucar, Z. Liu, L. Wang, L. Yang, J. Xu and E. U. Akkaya, RSC Adv., 2021, 11, 14513–14516 RSC; (c) F. Liu, W. Lu, X. Yin and J. Liu, J. Am. Soc. Mass Spectrom., 2016, 27, 59–72 CrossRef CAS PubMed.
- S. Stolik, J. A. Delgado, A. Perez and L. Anasagasti, J. Photochem. Photobiol., B, 2000, 57, 90–93 CrossRef CAS.
- (a) P. A. Lapchak, P. D. Boitano, P. V. Butte, D. J. Fisher, T. Hölscher, E. J. Ley, M. Nuño, A. H. Voie and P. S. Rajput, PLoS One, 2015, 10, e0127580 CrossRef PubMed; (b) F. Salehpour, P. Cassano, N. Rouhi, M. R. Hamblin, L. De Taboada, F. Farajdokht and J. Mahmoudi, Photobiomodulation, Photomed., Laser Surg., 2019, 37, 581–595 CrossRef PubMed.
- T. Yamamoto, A. Ishibashi, M. Koyanagi, H. Ihara, N. Eichenauer and M. Suginome, Bull. Chem. Soc. Jpn., 2017, 90, 604–606 CrossRef CAS.
- H. Huang, C. Yu, X. Li, Y. Zhang, Y. Zhang, X. Chen, P. S. Mariano, H. Xie and W. Wang, Angew. Chem., Int. Ed., 2017, 56, 8201–8205 CrossRef CAS PubMed.
- (a) M. Klapper and T. Linker, Chem. – Eur. J., 2015, 21, 8569–8577 CrossRef PubMed; (b) E. Ucar, D. Xi, O. Seven, C. Kaya, X. J. Peng, W. Sun and E. U. Akkaya, Chem. Commun., 2019, 55, 13808–13811 RSC; (c) M. Qu, N. Wu, W. Q. Jiang, L. Wang, M. S. Akkaya and E. U. Akkaya, RSC Adv., 2021, 11, 19083–19087 RSC.
- F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280, 5892–5901 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section outlining synthetic procedures, characterization data, kinetic studies and cell studies as well as supplementary figures. See DOI: 10.1039/d1cc07133e |
| This journal is © The Royal Society of Chemistry 2022 |