Open Access Article
Open Access ArticleSynthesis, properties and chemical modification of a persistent triisopropylsilylethynyl substituted tri(9-anthryl)methyl radical†
Tomohiko
Nishiuchi
 *ab,
Daisuke
Ishii
a,
Seito
Aibara
a,
Hiroyasu
Sato
c and
Takashi
Kubo
*ab,
Daisuke
Ishii
a,
Seito
Aibara
a,
Hiroyasu
Sato
c and
Takashi
Kubo
 *ab
*ab
aDepartment of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: nishiuchit13@chem.sci.osaka-u.ac.jp
bInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives, (ICS-OTRI), Osaka University, Suita, Osaka 565-0871, Japan
cRigaku Corporation, 3-9-12 Matsubara, Akishima, Tokyo 196-8666, Japan
First published on 11th February 2022
Abstract
In studies aimed at developing new organic spin materials, we prepared a triisopropylsilylethynyl substituted tri(9-anthryl)methyl (TAntM) radical. The TIPS-ethynyl group in this radical effectively suppresses its reactivity, resulting in extremely high stability in air for at least one month. Chemical modification of the radical using [4+2] Diels–Alder reaction proceeds even at room temperature. Because harsh conditions and metal-catalyzed reactions are not required, this post-modification strategy should be highly versatile for use in constructing unique spin-labelled molecules.
Highly persistent neutral organic radicals have attracted great attention recently. Various radicals of this type have been synthesized to explore their physical and photophysical properties and utilization as functional materials in organic magnets,1 batteries,2 magnetic resonance imaging systems,3 and doublet emission-based light emitting diodes4 and in dynamic nuclear polarization (DNP) studies.5 To enable them to be highly persistent, organic radicals usually contain nitrogen or oxygen atoms to provide thermodynamic stability.6,7 Alternatively, the stabilities of purely hydrocarbon radicals can be enhanced by incorporating bulky substituents at positions bearing high spin densities to inhibit their reactivities8,9 (Fig. 1). For example, perchlorotriphenylmethyl (PTM)10 and tetrathiatriarylmethyl (TAM)11 radicals are well known persistent aromatic hydrocarbon radicals that gain stability from kinetic protection of their spin-localized central sp2 carbons by halogen or sulfur atoms. Owing to the presence of embedded heteroatoms at high spin density locations, PTM and TAM radicals have relatively large g-anisotropies. However, persistent non-heteroatom-embedded hydrocarbon radicals with small g-anisotropies are the most ideal candidates for use in various spintronic materials.12 Unfortunately, the library of stable hydrocarbon radicals not containing heteroatoms remains small because of structural limitations and synthetic issues.
We recently prepared the purely hydrocarbon mesityl-substituted tri(9-anthryl)methyl (TAntM) radical,8e in which the central sp2 carbon is sterically protected by three 9-anthryl groups. In addition, we demonstrated that the TAntM radical displays extremely high persistence, undergoing only slight decay after more than a month in an air-saturated solution (Fig. 2). Because no heteroatoms are present, the TAntM radical is expected to have small g-anisotropy and, therefore, its functionalized derivatives should be attractive targets in efforts aimed at developing and applying new organic spin materials.
 | ||
| Fig. 2 The structures of the TAntM radical (left) and functionalized TAntM radicals 1 and 2 (right). | ||
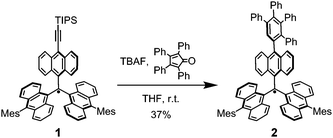
In the investigation described below, whose overall goal is to increase the scope and versatility of hydrocarbon radicals for organic-based materials, we prepared the TAntM radical 1 containing a triisopropylsilyl (TIPS) ethynyl group because the TIPS ethynyl group enhances the stability of radical species,13 and assessed its properties and chemical reactions. The results show that the TIPS-ethynyl group in 1 effectively enhances its stability in air. Moreover, we demonstrated that modification of 1 by a Diels–Alder reaction can be utilized to produce the related tetraphenylphenyl substituted TAntM radical 2.
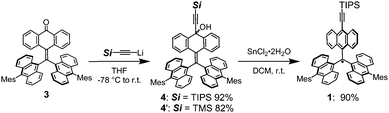
The route employed to synthesize 1, shown in Scheme 1, begins with the 10-alkylidene-9-anthrone derivative 3, which was prepared using a reported procedure.8e Reaction of 3 with TIPS-ethynyl lithium generated the corresponding alkynyl–carbinol 4 in high yield (92%). Treatment of 4 with tin(ii) chloride dihydrate then efficiently (90%) produced the target radical 1. Although the steric hindrance provided by the TIPS-ethynyl unit should be less than that by the mesityl group, it is worth noting that the radical 1 has a stability that matches that of the TAntM radical, even to the extent of allowing purification by silica gel chromatography in air.
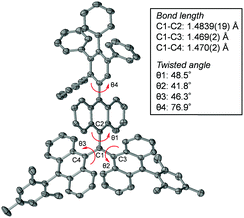
The structure of radical 1 was unambiguously confirmed by using X-ray crystallographic analysis (Fig. 3 and Fig. S1, ESI†). Analysis of the X-ray data shows that radical 1 has a C–C bond distance between the central sp2 carbon C1 and the anthryl carbon C2 (C1–C2) of 1.452(3) Å, which is slightly shorter than the C1–C3 (1.478(2) Å) and C1–C4 (1.473(2) Å) distances. Also, the anthryl unit twist angle θ1 (37.4°) is smaller than θ2 (48.5°) and θ3 (47.5°), indicating that the distribution of unpaired electrons on the TIPS ethynyl-substituted anthryl unit is slightly larger than that on the other mesityl-substituted anthryl units.
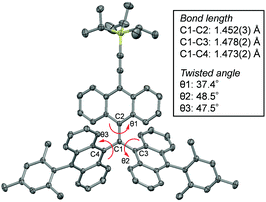 | ||
| Fig. 3 Plot of single crystal X-ray data of 1 and its structural factors (bond lengths and twist angles). Protons are omitted for clarity. | ||
Radical 1 has a C2-like structure in the crystal state. Quantum chemical calculations on its TMS-analog 1′ show that it has only a slight spin distribution on the ethynyl unit (Fig. 4a and Fig. S3, ESI†). Yet, the ESR spectrum of 1 contains a narrow peak (Fig. 4b) that has a width that is identical to that of the peak in the spectrum of the TAntM radical, which has D3 symmetry. Thus, the spin distribution of 1 is similar to that of the TAntM radical because the anthryl units can undergo ready rotation in a solution.
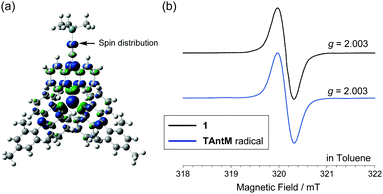 | ||
| Fig. 4 (a) Spin density map of 1′ (UBLYP/6-31G**//ωB97XD/6-31G**). (b) ESR spectra of 1 (black line) and the TAntM radical (blue line). | ||
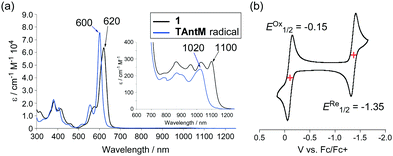
The UV-vis-NIR spectrum of 1 is similar to that of the TAntM radical (Fig. 5a). The differences consist of a slight red-shift of the maxima from those of the TAntM radical in the visible region (600–> 620 nm) and near-infrared region (1020–> 1100 nm) caused by the presence of the TIPS-ethynyl unit. These effects are also reflected in the results of TD-DFT calculations (Fig. S4, ESI†). The effect of TIPS-ethynyl substitution is also seen in the redox properties of radical 1. The cyclic voltammogram (CV) of 1 contains reversible oxidation and reduction waves at EOx1/2 = −0.15 V and ERe1/2 = −1.35 V, respectively (Fig. 5b). In contrast, CV waves of the TAntM radical (EOx1/2 = −0.19 V and ERe1/2 = −1.48 V, Fig. S5, ESI†) show that the oxidation and reduction potentials are slightly positively shifted by the TIPS-ethynyl unit.
The stability of radical 1 in an air-saturated solution was assessed by monitoring the decay of the absorption maximum at 620 nm. Surprisingly, no significant decomposition was observed to take place for at least one month (Fig. S6, ESI†).
Although the steric protection provided by its TIPS-ethynyl unit is less than that of a mesityl group, 1 has a stability that is comparable with that of the TAntM radical. To ascertain reason(s) for this persistence, the related radical 1′ containing a smaller TMS-ethynyl substituent was prepared. The solution of formed 1′ has a green color initially, but the color slowly changes to red along with formation of an orange precipitate. Quantitative analyses such as 1H- and 13C NMR, MS, IR, and single crystal X-ray measurements revealed that in solution, 1′ efficiently reacts to generate an allene-type tail-to-tail 1′ dimer in 75% yield (Scheme 2a and Fig. S7–S10, ESI†). Quantum chemical calculations indicate that the σ-dimer is energetically highly favorable (−25.5 kcal mol−1, Fig. S11, ESI†). For comparison purposes, the TMS-ethynyl substituted fluorenyl radical reacts to only form the asymmetric head-to-tail σ-dimer (allene–ethynyl) (Scheme 2b)14 probably because the fluorenyl moiety is sterically less hindered than the TAntM unit. The combined results indicate that the TIPS-ethynyl unit in 1 stabilizes the radical both through a steric effect and electronically by delocalization of the unpaired electron.
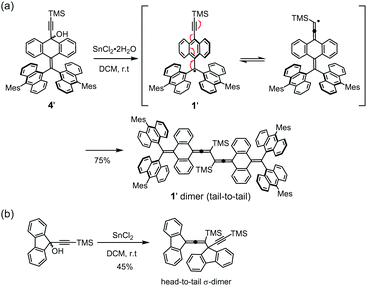 | ||
| Scheme 2 (a) Reaction of 1′ to form 1′ dimer. (b) Reaction of the TMS-ethynyl substituted fluorenyl radical.15 | ||
Finally, the chemical reactivity of radical 1 was probed to evaluate its versatility in the preparation of new organic spin materials. Direct introduction of a radical group in a molecular skeleton is an attractive strategy for creation of spin-based materials. The use of a nitronyl nitroxide radical15 exemplifies this approach. However, to use trityl-based persistent hydrocarbon radicals in this strategy, a functional group would need to be introduced before generating the radical (pre-modification) for avoiding rapid decomposition,16 and the post-modification of the persistent radicals is still limited.17 To explore this strategy, we examined reactions of radical 1 involving in situ removal of the silyl group using TBAF and subsequent [4+2] Diels–Alder reaction using tetraphenylcyclopentadienone (tetracyclone) (Scheme 3). We observed that this process leads to formation of the tetraphenylphenyl substituted TAntM radical 2 in 37% yield. Surprisingly, although Diels–Alder reactions usually require relatively high temperatures (>100 °C), the one involved in generation of 2 takes place at room temperature.
Significantly, tetraphenylphenyl substituted radical 2 has almost the same properties as the TAntM radical. X-Ray crystallographic analysis of 2 shows that its C1–C2 bond length is longer and the θ1 twist angle is larger than that of 1 (Fig. 6 and Fig. S2, ESI†), making it have close to the D3 propeller structure of the TAntM radical. In fact, the UV-vis and ESR spectra, and CV of 2 are almost identical to those of the TAntM radical (Fig. S12–S14, ESI†). Due to steric bulkiness provided by the tetraphenyphenyl (TPB) unit, the twist angle θ4 between the anthryl and TPB units is 76.9°. As a result, the stability of 2 is also comparable to that of TAntM radical and 1 (Fig. S15, ESI†). This result indicates that the radical 2 retains the properties of the TAntM radical.
 | ||
| Fig. 6 Plot of single crystal X-ray data of 2 and its structural factors (bond lengths and twist angles). Protons are omitted for clarity. | ||
In summary, in the study described above, we synthesized TIPS-ethynyl substituted TAntM radical 1 and evaluated its properties. The results of studies with the analogous TMS-ethynyl substituted TAntM radical 1′ gave important information that has led to an understanding of the stability/reactivity of the ethynyl moiety. Post-modification of radical 1 employing [4+2] Diels–Alder reaction with tetracyclone was found to occur effectively even at room temperature. In contrast to approaches often utilized to introduce radical groups into other molecules, the strategy developed in this effort avoids harsh reaction conditions and metal-catalyzed reactions that lead to decomposition and contamination with residual metals. In addition, tetracyclone, a model reactant in this study, can be readily modified such as by attaching emissive or amphiphilic components, utilized for unique NIR-emissive dyes, DNP, or spin-embedded self-assembled materials. Therefore, the facile post-modification process involving a room-temperature Diels–Alder reaction could become a highly versatile method to introduce TAntM radicals into substances used as organic spin-labelled materials. Further development of this overall strategy is ongoing.
This study was supported by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Number JP20K05475, T. N.) and Transformative Research Areas (A) (Grant Number JP20H05865, T. K.). Quantum chemical calculations were performed at the Research Center for Computational Science, Okazaki, Japan. T. N. thank Prof. Shuichi Suzuki (Graduate School of Engineering Science, Osaka University) for acquisition of HR-MS spectra.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Kinoshita, P. Truck, M. Tamura, K. Nozawa, D. Shiomi, Y. Nakazawa, M. Ishikawa, M. Takahashi, K. Awaga, T. Inabe and Y. Murayama, Chem. Lett., 1991, 1225 CrossRef CAS; (b) Y. Nakazawa, M. Tamura, N. Shirakawa, D. Shiomi, M. Takahashi, M. Kinoshita and M. Ishikawa, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 8906 CrossRef CAS PubMed; (c) T. Sugawara, M. M. Matsushita, A. Izuoka, N. Wada, N. Takeda and M. Ishikawa, J. Chem. Soc., Chem. Commun., 1994, 1723 RSC.
- (a) K. Nakahara, S. Iwasa, M. Satoh, Y. Morioka, J. Iriyama, M. Suguro and E. Hasegawa, Chem. Phys. Lett., 2002, 359, 351 CrossRef CAS; (b) H. Nishide and K. Oyazu, Science, 2008, 319, 737 CrossRef CAS PubMed; (c) Y. Morita, S. Nishida, T. Murata, M. Moriguchi, A. Ueda, K. Arifuku, M. Satoh, K. Sato and T. Takui, Nat. Mater., 2011, 10, 947 CrossRef CAS PubMed.
- (a) J. M. Backer, V. G. Budker, S. I. Eremenko and Y. N. Molin, Biochim. Biophys. Acta, 1977, 460, 152 CrossRef CAS PubMed; (b) C. S. Lai, L. E. Hopwood, J. S. Hyde and S. Lukiewicz, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 1166 CrossRef CAS PubMed; (c) D. J. Lurie, D. M. Bussell, L. H. Bell and J. R. Mallard, J. Magn. Reson., 1988, 76, 366 CAS.
- (a) Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed., 2014, 53, 11845 CrossRef CAS PubMed; (b) X. Ai, E. W. Evans, S. Dong, A. J. Gillett, H. Guo, Y. Chen, T. J. H. Hele, R. H. Friend and F. Li, Nature, 2018, 563, 536 CrossRef CAS PubMed; (c) M. Chen, Y. Liao, Y. Lin, T. Xu, W. Lan, B. Wei, Y. Yuan, D. Li and X. Zhang, J. Mater. Chem. C, 2020, 8, 14665 RSC; (d) L. Ji, J. Shi, J. Wei, T. Yu and W. Huang, Adv. Mater., 2020, 32, 1908015 CrossRef CAS PubMed; (e) J. M. Hudson, T. J. H. Hele and E. W. Evans, J. Appl. Phys., 2021, 129, 180901 CrossRef CAS.
- (a) T. R. Carver and C. P. Slichter, Phys. Rev., 1953, 92, 212 CrossRef CAS; (b) A. Abragam and M. Goldman, Rep. Prog. Phys., 1978, 41, 395 CrossRef CAS; (c) Q. Z. Ni, E. Daviso, T. V. Can, E. Markhasin, S. K. Jawla, T. M. Swager, R. J. Temkin, J. Herzfeld and R. G. Griffin, Acc. Chem. Res., 2013, 46, 1933 CrossRef CAS PubMed; (d) Y. Matsuki, T. Idehara, J. Fukazawa and T. Fujiwara, J. Magn. Reson., 2016, 264, 107 CrossRef CAS PubMed.
- For book and reviews of stable neutral radicals, see; (a) E. R. Altwicker, Chem. Rev., 1967, 67, 475 CrossRef CAS; (b) R. G. Hicks, Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, John Wiley & Sons, Inc, 2010 CrossRef; (c) K. Kato and A. Osuka, Angew. Chem., Int. Ed., 2019, 58, 8978 CrossRef CAS PubMed; (d) D. Sakamaki, S. Ghosh and S. Seki, Mater. Chem. Front., 2019, 3, 2270 RSC.
- For typical examples of stable neutral radicals with heteroatoms, see; (a) O. A. Lebedev and S. N. Kayanovskii, Zh. Obshch. Khim., 1960, 30, 1631 CAS; (b) H. M. Blatter and H. Lukaszewski, Tetrahedron Lett., 1968, 9, 2701 CrossRef; (c) E. F. Ullman, J. H. Osiecki, D. G. B. Boocock and R. Darcy, J. Am. Chem. Soc., 1972, 94, 7049 CrossRef CAS.
- For several examples of stable neutral radicals with bulky substituents, see; (a) C. F. Koelsch, J. Am. Chem. Soc., 1957, 79, 4439 CrossRef CAS; (b) Z. Zeng, Y. M. Sung, N. Bao, D. Tan, R. Lee, J. L. Zafra, B. S. Lee, M. Ishida, J. Ding, J. T. L. Navarrete, Y. Li, W. Zeng, D. Kim, K.-W. Huang, R. D. Webster, J. Casado and J. Wu, J. Am. Chem. Soc., 2012, 134, 14513 CrossRef CAS PubMed; (c) Y. Tian, K. Uchida, H. Kurata, Y. Hirao, T. Nishiuchi and T. Kubo, J. Am. Chem. Soc., 2014, 136, 12784 CrossRef CAS PubMed; (d) T. Nishiuchi, R. Ito, A. Takada, Y. Yasuda, T. Nagata, E. Stratmann and T. Kubo, Chem. – Asian J., 2019, 14, 1830 CrossRef CAS PubMed; (e) T. Nishiuchi, S. Aibara and T. Kubo, Angew. Chem., Int. Ed., 2018, 57, 16516 CrossRef CAS PubMed.
- For an exceptional example of a stable aromatic radical not containing a heteroatom or bulky substituent, achieved by using π-extension, see; T. Kubo, Y. Katada, A. Shimizu, Y. Hirao, K. Sato, T. Takui, M. Uruichi, K. Yakushi and R. C. Haddon, J. Am. Chem. Soc., 2011, 133, 14240 CrossRef CAS PubMed.
- M. Ballester, Acc. Chem. Res., 1985, 18, 380 CrossRef CAS.
- (a) S. Andersson, F. Radner, A. Rydbeek, R. Servin and L.-G. Wistrand, US Patent, 5530140, 1996 Search PubMed; (b) T. J. Reddy, T. Iwama, H. J. Halpern and V. H. Rawal, J. Org. Chem., 2002, 67, 4635 CrossRef CAS PubMed.
- P. D. Drouhard, H. Y. V. Ching, C. Decroos, R. Guillot, Y. Li, L. C. Tabares, C. Policar, H. C. Bertrand and S. Un, Phys. Chem. Chem. Phys., 2020, 22, 20792 RSC.
- For several TIPS-ethynyl substituted aromatic hydrocarbon radicals allowing them to be investigated as stable monomers, see; (a) H. Hintz, A. Vanas, D. Klose, G. Jeschke and A. Godt, J. Org. Chem., 2019, 84, 3304 CrossRef CAS PubMed; (b) Q. Xiang, J. Guo, J. Xu, S. Ding, Z. Li, G. Li, H. Phan, Y. Gu, Y. Dang, Z. Xu, Z. Gong, W. Hu, Z. Zeng, J. Wu and Z. Sun, J. Am. Chem. Soc., 2020, 142, 11022 CrossRef CAS PubMed.
- S. Qiu, Y. Zhang, X. Huang, L. Bao, Y. Hong, Z. Zeng and J. Wu, Org. Lett., 2016, 18, 6018 CrossRef CAS PubMed.
- (a) R. Tanimoto, S. Suzuki, M. Kozaki and K. Okada, Chem. Lett., 2014, 43, 678 CrossRef CAS; (b) K. Yamada, X. Zhang, R. Tanimoto, S. Suzuki, M. Kozaki, R. Tanaka and K. Okada, Bull. Chem. Soc. Jpn., 2018, 91, 1150 CrossRef CAS.
- S. Wu, M. Li, H. Phan, D. Wang, T. S. Herng, J. Ding, Z. Lu and J. Wu, Angew. Chem., Int. Ed., 2018, 57, 8007 CrossRef CAS PubMed.
- M. V. Edeleva, S. R. A. Marque, O. Y. Rogozhnikova, V. M. Tormyshev, T. I. Troitskaya and E. G. Bagryanskaya, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2656 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2123624, 2123625 and 2149932. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2cc00548d |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M