Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogen sulphide-triggered theranostic prodrugs based on the dynamic chemistry of tetrazines†
Marcelle D.
Perretti‡
ab,
Yaiza
Pérez-Pérez‡
a,
Kevin
Soler-Carracedo
c,
Endika
Martín-Encinas
d,
Concepción
Alonso
 *d,
Jimena
Scoccia
*d,
Jimena
Scoccia
 *b and
Romen
Carrillo
*b and
Romen
Carrillo
 *ab
*ab
aInstituto de Productos Naturales y Agrobiología (IPNA-CSIC), Avenida Astrofísico Francisco Sánchez 3, 38206, La Laguna, Spain. E-mail: rcarrillo@ipna.csic.es
bInstituto Universitario de Bio-Orgánica “Antonio González”, Universidad de La Laguna, Avenida Astrofísico Francisco Sánchez 2, P.O. Box 456, 38200, La Laguna, Spain. E-mail: jscoccia@ull.es
cDepartamento de Física, Universidad de La Laguna, Apdo. 456, E-38200, San Cristóbal de La Laguna, Santa Cruz de Tenerife, Spain
dDepartment of Organic Chemistry I, Faculty of Pharmacy and Lascaray Research Center, University of Basque Country (UPV/EHU), 01006 Vitoria-Gasteiz, Spain. E-mail: concepcion.alonso@ehu.eus
First published on 8th April 2022
Abstract
Dynamic nucleophilic aromatic substitution of tetrazines (SNTz) has been employed to build theranostic prodrugs that are activated by hydrogen sulfide. H2S is typically found in high concentrations in some kinds of cancer cells and it is able to trigger the disassembly of tetrazine prodrugs. In such a way, a dual release of drugs and/or fluorescent compounds can be selectively triggered.
A targeted delivery approach is a long sought aim of drug delivery.1 Its classic goals are to avoid unfavourable side effects and also to increase the efficiency of the drug. Therefore, it is particularly interesting in cancer treatments,2 in which traditional chemotherapy has remained basically the same for decades and it lacks any intrinsic selectivity inducing systemic toxicity to the patient. In order to achieve efficient targeted drug delivery, two main features are required: a distinctive hallmark in the tumoral cell, and a chemical mechanism designed to release the active drug selectively in the presence of such hallmark.3 Regarding the latter, dynamic covalent chemistry (DCvC) might be considerably helpful. DCvC deals with reversible reactions that allow the system to reach thermodynamic equilibrium and also to respond to changes in the environment.4–7 Thus, dynamic chemical systems are intrinsically responsive to a specific stimulus. Obviously, any dynamic system aimed at releasing drugs should be inherently stable in aqueous conditions.
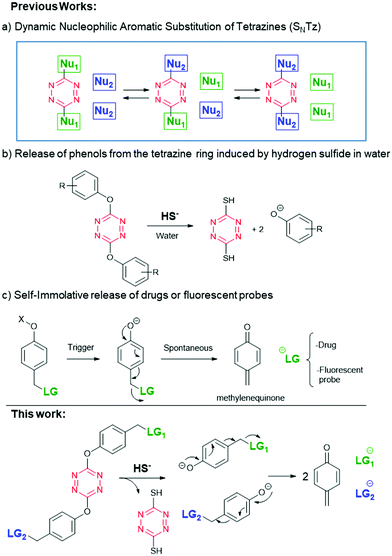
In this regard, our group has recently developed a novel dynamic reaction: the nucleophilic aromatic substitution of tetrazines (SNTz, Fig. 1a).8–10 This reaction quickly exchanges phenols and thiols in positions 3 and 6 of the tetrazine ring, at room temperature and it is compatible with aqueous environments.
We discovered that thiols efficiently release any phenol quantitatively,8 although usually a base is required to initiate the process, unless the thiol is acidic enough. Indeed, hydrogen sulfide (pKa1 6.9) is able to displace phenols from the tetrazine ring in water with no base required (Fig. 1b).9 This fact allowed a hydrogen sulfide triggered release event to be envisioned. Although H2S has been considered traditionally as an irritating gas with a smell of rotten eggs, recently its relevance in several physiological events has turned it into an interesting biomarker to detect or into a trigger to induce the activation of therapeutic systems.11–17 It is worth mentioning that some kinds of cancer cells, such as colon cancer cells, display a characteristic high intracellular concentration of H2S due to the overexpression of the enzyme cystathionine beta synthase (CBS).18 Therefore, tetrazine prodrugs could be useful to selectively release molecules in colon cancer cells.
The released compounds do not have to display a phenol necessarily, but the tetrazine release process can be combined with a self-immolative spacer.19–21 Indeed, self-immolation has been extensively used as an efficient drug-releasing method.22 One of the most common self-immolative linkers is a phenol bearing a reasonably good leaving group (LG) on a methylene in the ortho or para position (Fig. 1c). Therefore, a self-immolative phenol, bearing a drug or a fluorophore, could be attached to a tetrazine (Fig. 1, bottom). Such a system would be selectively disassembled in physiological conditions by H2S. Interestingly, as the tetrazine has two reactive points, a dual release is possible, and therefore a theranostic compound can be designed by incorporating an active drug and a fluorescent probe.
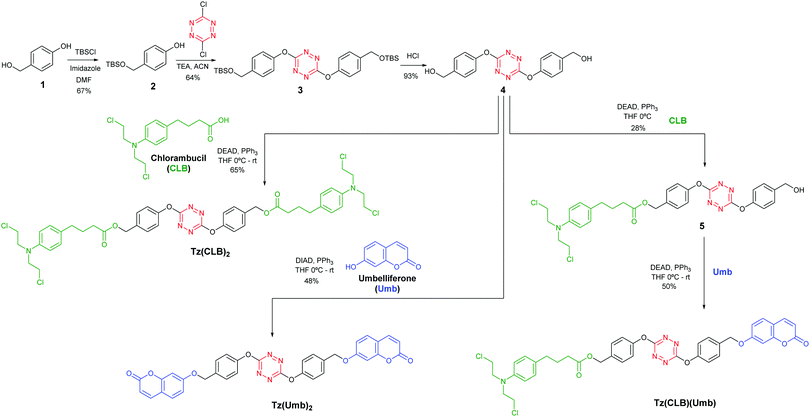
As a proof of concept, we have chosen chlorambucil (CLB) as the active drug to be released, a well-known FDA-approved anticancer drug limited by adverse effects due to its lack of selectivity;23 on the other hand, umbelliferone was chosen as a fluorescent probe, as it is known to be virtually non-fluorescent as long as it is attached by the hydroxyl group, but it turns highly luminescent once it is released.24
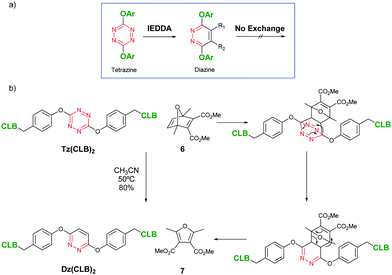
The synthesis of the prodrugs starts by selectively protecting the primary alcohol in 1 and then attaching phenol 2 to the dichlorotetrazine (Scheme 1). The resulting product (3) yields tetrazine-diol 4 by deprotection by acid hydrolysis. 4 is the starting compound for any other product: two chlorambucil or umbelliferone molecules were linked through Mitsunobu reactions, giving Tz(CLB)2 and Tz(Umb)2, respectively. The theranostic compound Tz(CLB)(Umb) was synthesized by performing a sequential Mitsunobu reaction, attaching chlorambucil first (5), followed by the umbelliferone. Finally, as a control compound, an Inverse Electron Demand Diels Alder (IEDDA) reaction was carried out on compound Tz(CLB)2 (Scheme 2). Indeed, it has been proved that IEDDA reaction cancels the exchange, as the resulting diazine ring is electron richer.8,9 Therefore, the prodrug should be deactivated by such Diels Alder reaction, and no release is expected in the analogous diazine compound. In order to keep the size and geometry as similar as possible, IEDDA reaction was performed with strained alkene 6, which after elimination of nitrogen, followed by a retro-Diels Alder reaction, yields diazine Dz(CLB)2 where the only difference with respect to Tz(CLB)2 is a substitution of two nitrogen atoms for two C–H groups.
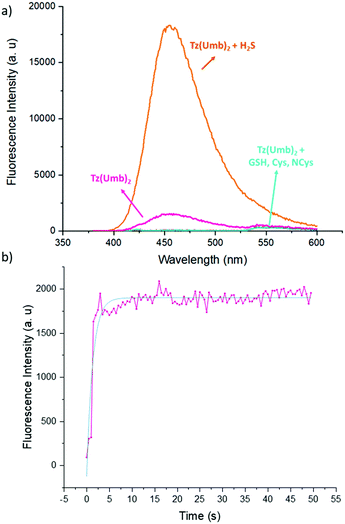
Then, we tested the H2S induced release with Tz(Umb)2, as it could be followed by the increasing fluorescence of the reaction mixture due to the released umbelliferone. We employed a solution of Na2S in water, as it is a commonly used surrogate of H2S. As expected, while the original compound shows very little fluorescence, an aqueous solution of Na2S induces a ten-fold increase in the fluorescence (Fig. 2a), in a very fast way (Fig. 2b).
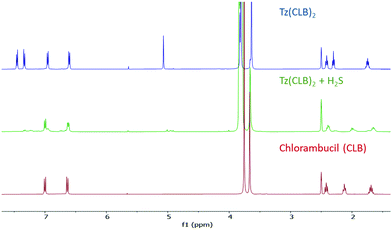
Fortunately, any other biothiol tested in the same conditions (cysteine, N-acetylcysteine and glutathione) causes no remarkable increase in the fluorescence of Tz(Umb)2 (Fig. 2a).15 The release could also be followed by NMR with compound Tz(CLB)2: upon addition of a solution of Na2S in D2O, free chlorambucil is clearly seen in the spectrum (Fig. 3).
Finally, the cytotoxicity of all the compounds was investigated in vitro by testing the antiproliferative activities against three human cell lines: on one hand HCT-116, a human colorectal carcinoma cell line in which enzyme CBS is overexpressed and therefore there is a high intracellular concentration of H2S;18 on the other hand, MRC-5 (non-malignant lung fibroblasts-like) that displays normal levels of hydrogen sulfide, and can also be considered as a test for selective toxicity between cancer and normal cells; and finally SK-OV3 (human ovarian carcinoma).25–27 As expected, (Table 1, entry 1), compound Tz(CLB)2 is only active against HCT-116 (IC50 = 3.72 μM) while it shows no cytotoxicity with the other two cell lines (IC50 > 50 μM).28 This selective performance is also evident under microscope observation (see Fig. S19 in the ESI†). Furthermore, compound Dz(CLB)2, bearing a diazine instead of a tetrazine, was not active against any of the cell lines, not even HCT-116 (Table 1, entry 2). Therefore, it has been also proved that the dynamic behaviour of the tetrazine is essential for a proper bioactivity and, as a consequence, IEDDA reaction prevents drug-release. Compounds bearing coumarins were again only active against HCT-116 although to a lesser extent than Tz(CLB)2 (Table 1, entries 3 and 4). Curiously, Tz(Umb)2 is more cytotoxic than Tz(CLB)(Umb), even when the latter contains one molecule of chlorambucil. In this regard, it is worth mentioning that some coumarins have been reported to show cytotoxicity against HCT-116 cells.29 It also has to be considered that the release of the attached compounds concomitantly delivers a methylenquinone (Fig. 1c), that has been reported to deplete glutathione levels in the cell,30 and therefore it probably contributes to the cytotoxicity of the tetrazine prodrugs.31 Further studies will be required to better understand the reasons underlying cytotoxicity.
| Cell line, IC50 (μM) | ||||
|---|---|---|---|---|
| Entry | Compound | SK-OV3 | HCT-116 | MRC-5 |
| a For a detailed experimental procedure of the antiproliferative assays, please see the ESI. | ||||
| 1 | Tz(CLB)2 | >50 | 3.72 ± 1.90 | >50 |
| 2 | Dz(CLB)2 | >50 | >50 | >50 |
| 3 | Tz(Umb)2 | >50 | 19.45 ± 2.18 | >50 |
| 4 | Tz(CLB)(Umb) | >50 | 36.50 ± 2.61 | >50 |
In conclusion, a novel method for a controlled drug release based on the dynamic chemistry of tetrazines has been described herein. It is selectively triggered by H2S and consequently very relevant for colon cancer and any other disease where there is a high concentration of H2S. Fluorescent compounds can also be released and consequently this method shows great potential for the development of H2S sensors, chemiluminescent detection,32,33 and theranostic compounds. Obviously, this strategy is modular, and therefore other cytotoxic compounds and luminescent probes could be incorporated. Tetrazine responsive systems can also easily grow in complexity and in sophistication and therefore they could probably be very helpful in the development of innovative sensing and therapeutic solutions.
This work was financially supported by Ministerio de Ciencia e Innovación (PGC2018-094503-B-C21). J.S. thanks Cabildo de Tenerife for the Agustín de Betancourt programme. M.D.P thanks the ACIISI and the European Social Fund (ESF) for a predoctoral grant.
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. T. Wong and S. K. Choi, Chem. Rev., 2015, 115, 3388 CrossRef CAS PubMed; M. Srinivasarao and P. S. Low, Chem. Rev., 2017, 117, 12133 CrossRef PubMed; E. A. Ashley, Nat. Rev. Genet., 2016, 17, 507 CrossRef PubMed.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20 CrossRef CAS PubMed.
- G. Kroemer and J. Pouyssegur, Cancer Cell, 2008, 13, 472 CrossRef CAS PubMed; W. C. Hahn and R. A. Weinberg, Nat. Rev. Cancer, 2002, 2, 331 CrossRef PubMed.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef PubMed.
- Dynamic Covalent Chemistry: Principles, Reactions, and Applications, ed. W. Zhang and Y. Jin, Wiley, Hoboken, 2018 Search PubMed.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634 RSC.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J. L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652 CrossRef CAS PubMed.
- T. Santos, D. S. Rivero, Y. Pérez-Pérez, E. Martín-Encinas, J. Pasán, A. H. Daranas and R. Carrillo, Angew. Chem., Int. Ed., 2021, 60, 18783 CrossRef CAS PubMed.
- D. S. Rivero, R. E. Paiva-Feener, T. Santos, E. Martín-Encinas and R. Carrillo, Macromolecules, 2021, 54, 10428 CrossRef CAS.
- It has not been proved yet, although it is very likely that mono- substituted tetrazines also display a dynamic behaviour. See: S. D. Schnell, L. V. Hoff, A. Panchagnula, M. H. H. Wurzenberger, T. M. Klapötke, S. Sieber, A. Linden and K. Gademann, Chem. Sci., 2020, 11, 3042 RSC; W. Mao, W. Chi, X. He, C. Wang, X. Wang, H. Yang, X. Liu and H. Wu, Angew. Chem., Int. Ed., 2022, 61, e202117386 Search PubMed.
- M. D. Hartle and M. D. Pluth, Chem. Soc. Rev., 2016, 45, 6108 RSC.
- A. K. Steiger, S. Pardue, C. G. Kevil and M. D. Pluth, J. Am. Chem. Soc., 2016, 138, 7256 CrossRef CAS PubMed.
- E. J. Mitchell, A. J. Beecroft, J. Martin, S. Thompson, I. Marques, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2021, 60, 24048 CrossRef CAS PubMed.
- X. Wang, L. An, Q. Tian and K. Cui, RSC Adv., 2019, 9, 33578 RSC.
- I. Ismail, Z. Chen, L. Sun, X. Ji, H. Ye, X. Kang, H. Huang, H. Song, S. G. Bolton, Z. Xi, M. D. Pluth and L. Yi, Chem. Sci., 2020, 11, 7823 RSC; K. Zhang, J. Zhang, Z. Xi, L. Y. Li, X. Gu, Q. Z. Zhang and L. Yi, Chem. Sci., 2017, 8, 2776 RSC.
- N. Singh, S. Sharma, R. Singh, S. Rajput, N. Chattopadhyay, D. Tewari, K. B. Joshi and S. Verma, Chem. Sci., 2021, 12, 16085 RSC.
- N. Singh, R. Singh, M. Shukla, G. Kaul, S. Chopra, K. B. Joshi and S. Verma, ACS Infect. Dis., 2020, 6, 2441 CrossRef CAS PubMed.
- C. Szabo, C. Coletta, C. Chao, K. Módis, B. Szczesny, A. Papapetropoulos and M. R. Hellmich, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12474 CrossRef CAS PubMed; M. R. Hellmich, C. Coletta, C. Chao and C. Szabo, Antioxid. Redox Signaling, 2015, 22, 424 CrossRef PubMed.
- M. Fernández, A. Shamsabadi and V. Chudasama, Chem. Commun., 2020, 56, 1125 RSC.
- Y. Yang, T. Zhou, M. Jin, K. Zhou, D. Liu, X. Li, F. Huo, W. Li and C. Yin, J. Am. Chem. Soc., 2020, 142, 1614 CrossRef CAS PubMed.
- A. Dal Corso, V. Borlandelli, C. Corno, P. Perego, L. Belvisi, L. Pignataro and C. Gennari, Angew. Chem., Int. Ed., 2020, 59, 4176 CrossRef CAS PubMed.
- M. E. Roth, O. Green, S. Gnaim and D. Shabat, Chem. Rev., 2016, 116, 1309 CrossRef CAS PubMed.
- R. T. Dorr and W. L. Fritz, Cancer Chemotherapy Handbook, Elsevier Science, New York, 1982 Search PubMed.
- R. Weinstain, E. Segal, R. Satchi-Fainaro and D. Shabat, Chem. Commun., 2010, 46, 553 RSC.
- R. C. Bast Jr., B. Hennessy and G. B. Mills, Nat. Rev. Cancer, 2009, 9, 415 CrossRef PubMed.
- X. Yang and M. Pagé, Oncol. Res., 1995, 7, 619 CAS.
- S. Bhattacharyya, S. Saha, K. Giri, I. R. Lanza, K. S. Nair, N. B. Jennings, C. Rodriguez-Aguayo, G. López-Berestein, E. Basal, A. L. Weaver, D. W. Visscher, W. Cliby, A. K. Sood, R. Bhattacharya and P. Mukherjee, PLoS One, 2013, 8, e79167 CrossRef PubMed.
- CLB shows no cytotoxicity with any of the cell lines tested, as it is seen in Table S1 (ESI†). Indeed, CLB is usually only active when an improved internalization takes place. See: P. Huang, D. Wang, Y. Su, W. Huang, Y. Zhou, D. Cui, X. Zhu and D. Yan, J. Am. Chem. Soc., 2014, 136, 11748 CrossRef CAS PubMed.
- N. E. B. Saidu, S. Valente, E. Bana, G. Kirsch, D. Bagrel and M. Montenarh, Bioorg. Med. Chem., 2012, 20, 1584 CrossRef CAS PubMed.
- H. Hagen, P. Marzenell, E. Jentzsch, F. Wenz, M. R. Veldwijk and A. Mokhir, J. Med. Chem., 2012, 55, 924 CrossRef CAS PubMed; J. Noh, B. Kwon, E. Han, M. Park, W. Yang, W. Cho, W. Yoo, G. Khang and D. Lee, Nat. Commun., 2015, 6, 6907 CrossRef PubMed.
- A dimercaptotetrazine is also released, that might enhance the global effect of the prodrug, only when activated.
- S. P. Gholap, C. Yao, O. Green, M. Babjak, P. Jakubec, T. Malatinský, J. Ihssen, L. Wick, U. Spitz and D. Shabat, Bioconjugate Chem., 2021, 32, 991 CrossRef CAS PubMed; T. S. Bailey and M. D. Pluth, J. Am. Chem. Soc., 2013, 135, 16697 CrossRef PubMed.
- Y. Chen, R. Zhao, C. Tang, C. Zhang, W. Xu, L. Wu, Y. Wang, D. Ye and Y. Liang, Angew. Chem., Int. Ed., 2022, 61, e202112734 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterization of the compounds, cell cultures and in vitro screenings. See DOI: https://doi.org/10.1039/d2cc01170k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: