Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A graceful break-up: serendipitous self-assembly of a ferromagnetically coupled [NiII14] wheel†
Eleftheria
Agapaki
 a,
Mukesh K.
Singh
a,
Mukesh K.
Singh
 a,
Angelos B.
Canaj
a,
Angelos B.
Canaj
 a,
Gary S.
Nichol
a,
Gary S.
Nichol
 a,
Jürgen
Schnack
a,
Jürgen
Schnack
 *b and
Euan K.
Brechin
*b and
Euan K.
Brechin
 *a
*a
aEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, Scotland, UK. E-mail: ebrechin@ed.ac.uk
bUniversität Bielefeld, Postfach 100131, D-33501, Bielefeld, Germany. E-mail: jschnack@uni-bielefeld.de
First published on 5th July 2022
Abstract
The complex [NiII14(HL2)12(HCOO)14Cl14(MeOH)(H2O)] describes an aesthetically pleasing wheel displaying ferromagnetic nearest neighbour exchange.
Interest in the magnetic properties of polymetallic clusters of NiII began with the development of magneto-structural correlations of [Ni2] dimers1 and [Ni4] cubanes2 that revealed a dependence of the sign and magnitude of the exchange interaction on both the Ni–X–Ni bridging angle and the anisotropy of the NiII ion.3 The large axial zero-field splitting displayed by the latter in certain geometries also lends itself to the construction of both single-molecule magnets (SMMs)4 and single-ion magnets (SIMs)5 displaying slow relaxation of the magnetisation. Indeed, recent studies of NiII SIMs at both ambient and high pressure6 have revealed how magnetic anisotropy is extremely susceptible to even small structural distortions, in turn highlighting target geometries7 and directing the synthetic methodologies required to engineer molecules possessing giant magneto-anisotropies.8
Flexible N,O-bridging ligands have proved particularly successful in the construction of polymetallic clusters of NiII displaying a variety of topologies and nuclearities, including supertetrahedra,9 wheels,10 planar discs11 and icosahedra.12 The pro-ligand (3,5-dimethyl-1H-pyrazol-1-yl)methanol (HL1) belongs in this family, having been employed to make both mono- and tetranuclear clusters of NiII.13 Here, we expand this chemistry to include the synthesis, structure and characterisation of [Ni14(HL2)12(HCOO)14Cl14(MeOH)(H2O)]·4Me2CO (1·4Me2CO, HL2 = 3,5-dimethylpyrazole), an aesthetically pleasing wheel formed serendipitously via the in situ transformation of HL1 to HL2.
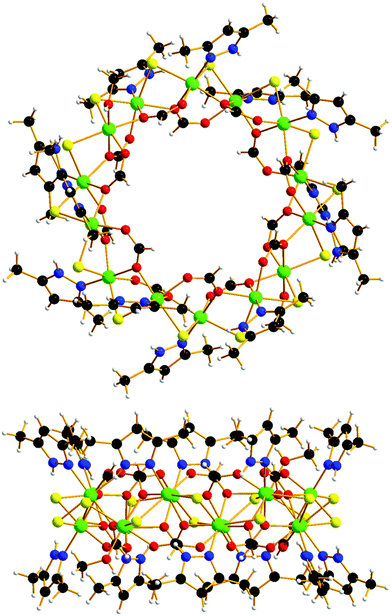
The reaction of NiCl2·6H2O and HL1 in a basic MeOH solution heated at 65 °C for 40 minutes affords compound 1 (Fig. 1) upon diffusion of acetone into the cooled mother liquor (see the ESI† for full experimental details). Crystals of 1 are in a tetragonal cell and structure solution was performed in the space group P42/n (see the ESI† for full crystallographic details, Table S1 and Fig. S1). The asymmetric unit contains half the formula unit.
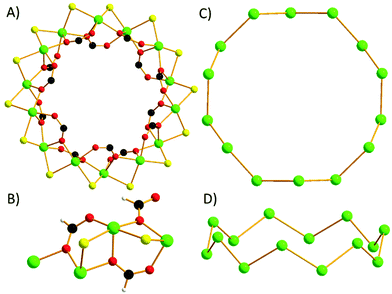
The metallic skeleton of 1 is a single stranded [NiII14] wheel (Fig. 2A). The bridging between each pair of NiII ions is the same around the entire wheel and consists of one μ-Cl ion (Ni–Cl–Ni, ∼82.0–85.1°), one μ-O atom (Ni–O–Ni, ∼102.8–103.2°) and one μ-carboxylate which both derive from the syn, syn, anti-bridging formate (Fig. 2B). The six-coordinate NiII ions are all in distorted octahedral geometries with their {NiO3Cl2N} coordination spheres completed by a terminally bonded HL2 ligand. The only exception to this is Ni5, {NiO4Cl2}, in which there resides a disordered MeOH/H2O molecule in place of the HL2 ligand. The HL2 ligands and the formate ions originate from the in situ reaction of HL1.14
The wheel is non-planar with nearest neighbour NiII ions being above and below the plane running through the middle of the fourteen metal ions, i.e., they form a zigzag/sinusoidal “up-down-up-down” motif as the wheel is circumnavigated (Fig. 2C and D). The approximate dimensions of the wheel are, Ni1⋯Ni1′, ∼12.7 Å.
The terminally bonded MeOH/H2O molecules are H-bonded to acetone molecules of crystallisation (O(H)⋯O, ∼2.72 Å). Closest inter-cluster interactions are between neighbouring HL2 ligands and between HL2 ligands and the Cl ions (C/N⋯C/N/Cl > 3.6 Å). In the extended structure the wheels pack in eclipsed columns down the c-axis, with the remaining acetone molecules of crystallisation lying in a head-tail fashion in the voids between the wheels (O⋯C, ∼3.6 Å; Fig. S2, ESI†).
A subsequent investigation of reaction conditions does not reveal any simple relationship between reaction time, temperature and ligand degradation, but did reveal that 1 can be made directly from NiCl2·6H2O, formic acid (or sodium formate) and HL2, and, perhaps unsurprisingly, in larger yields (see the ESI† for full details).
A search of the Cambridge Structural Database reveals that there are approximately thirty Ni wheels reported, ranging in nuclearity from [Ni5] to [Ni24].15 By far the most common are [Ni6] and [Ni12] wheels,16 with complex 1 being just the second example of a [Ni14] wheel.17 The first example is a large (∼2 nm diameter) oval-shaped wheel in which neighbouring Ni ions are connected by artificial tripeptides (Ni⋯Ni, ∼8 Å). Compound 1 also represents the first NiII wheel built with 3,5-dimethylpyrazole (or 1H-pyrazole) and indeed is the largest nuclearity Ni cluster known with either ligand.
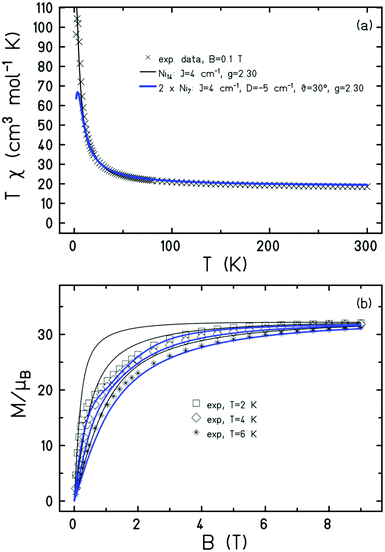
Dc magnetic susceptibility (χ) and magnetisation (M) measurements of 1 were taken in the T = 300–1.80 K, B = 0.1 T and T = 2.0–10 K and B = 0.5–9.0 T temperature and field ranges, respectively. These are plotted as the χT product versus T, and M versus B in Fig. 3 (and Fig. S3, ESI†). The T = 300 K value of χT = 18.5 cm3 K mol−1 is equal to the value expected for fourteen non-interacting NiII ions with g = 2.30. Upon cooling the χT value remains relatively constant, increasing only very slowly to ∼24 cm3 K mol−1 at 50 K before rising sharply to a maximum of ∼104 cm3 K mol−1 at T = 3 K. The value then drops to ∼96 cm3 K mol−1 at 2 K. The M vs. B data increases rapidly with increasing field, saturating at a value of M = 32.1 μB at T = 2 K and B = 9 T. The susceptibility and magnetisation data are therefore indicative of weak ferromagnetic nearest neighbour exchange and the stabilisation of an S = 14 ground state.
The magnetic susceptibility data can be simulated using exact diagonalisation18 and an isotropic spin-Hamiltonian 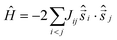 with a coupling scheme that assumes just one independent exchange interaction between nearest neighbours, J = +4 cm−1 with g = 2.30 (Fig. 3, black curve). The addition of a next nearest neighbour interaction makes no difference to the quality of the simulation. Given that this interaction is computed to be very weak and ferromagnetic by DFT (vide infra) this is to be expected. This simple isotropic model, however, does not explain the low temperature magnetisation data, which requires inclusion of the single ion anisotropy of the Ni ions, D(Ni),
with a coupling scheme that assumes just one independent exchange interaction between nearest neighbours, J = +4 cm−1 with g = 2.30 (Fig. 3, black curve). The addition of a next nearest neighbour interaction makes no difference to the quality of the simulation. Given that this interaction is computed to be very weak and ferromagnetic by DFT (vide infra) this is to be expected. This simple isotropic model, however, does not explain the low temperature magnetisation data, which requires inclusion of the single ion anisotropy of the Ni ions, D(Ni), 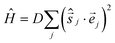 , where
, where ![[e with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0065_20d1.gif) j =
j = ![[e with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0065_20d1.gif) j(θj, φj) is the direction of the local easy axis. Computational limitations direct us toward employing a [Ni7] wheel, with the results multiplied by two to mimic the [Ni14] wheel in order to assess the impact of anisotropy. The magnetisation data is simulated nicely with J = +4 cm−1 and D(Ni) = −5 cm−1 with the anisotropy axes tilted from the axis of the wheel by θ = 30°, φj = 2πj/7, in agreement with ab initio NEVPT2 calculations (Fig. 3, blue curves). The magenta curves in Fig. S3 (ESI†) demonstrate for the isotropic case that the substituted [Ni7] model system is close to the original except for low temperatures where the S = 14 ground state obviously cannot be reproduced.
j(θj, φj) is the direction of the local easy axis. Computational limitations direct us toward employing a [Ni7] wheel, with the results multiplied by two to mimic the [Ni14] wheel in order to assess the impact of anisotropy. The magnetisation data is simulated nicely with J = +4 cm−1 and D(Ni) = −5 cm−1 with the anisotropy axes tilted from the axis of the wheel by θ = 30°, φj = 2πj/7, in agreement with ab initio NEVPT2 calculations (Fig. 3, blue curves). The magenta curves in Fig. S3 (ESI†) demonstrate for the isotropic case that the substituted [Ni7] model system is close to the original except for low temperatures where the S = 14 ground state obviously cannot be reproduced.
The ferromagnetic exchange in 1 is consistent with magneto-structural correlations developed for halide-bridged NiII dimers where the sign and magnitude of the interaction is dictated by the Ni–Cl–Ni angle – with a switch from antiferromagnetic to ferromagnetic occurring at approximately ≤102° and increasing with decreasing angle.19 Note the Ni–Cl–Ni angles in 1 are ∼82–85°. Alternating current (ac) susceptibility measurements between 50–3000 Hz under zero external dc field and an oscillating ac field of Bac = 5 Oe were performed to investigate the relaxation dynamics of 1. A plot of 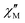 versus frequency yielded temperature dependent maxima in the range 1.8–2.5 K (Fig. S4, ESI†).
versus frequency yielded temperature dependent maxima in the range 1.8–2.5 K (Fig. S4, ESI†).
To further understand the origin and sign of the magnetic coupling constants we have performed DFT calculations on models created from 1 (1A–C, Fig. S5 and Tables S2, S3, ESI†). All seven unique exchange interactions are in the range +1.7 ≤ J ≤ +3.9 cm−1, consistent with the experimental values (Table S4, ESI†). The DFT calculated values for the seven crystallographically unique interactions also simulate the susceptibility well if they are scaled by a factor of 1.4 (ESI,† Fig. S3, red curve). The narrow range of values obtained can be attributed to the presence of similar structural parameters for each metal ion, with the relatively small Ni−μ-O/Cl–Ni angles resulting in ferromagnetic exchange (Table S4, ESI†).19 To further explore the origin of the sign and magnitude of these interactions we have performed overlap integral calculations20–22 between the singly occupied molecular orbitals (SOMOs) of the NiII ions in a bimetallic model (1D) created from 1 (Fig. S6, ESI†). These calculations suggest competition between one moderate interaction [〈Ni(α)dx2−y2||Ni(β)dz2〉] and three weak interactions [〈Ni(α)dx2−y2||Ni(β)dx2−y2〉], [〈Ni(α)dz2||Ni(β)dx2−y2〉], [〈Ni(α)dz2||Ni(β)dz2〉]. The former contributes to the antiferromagnetic and the latter to the ferromagnetic part of the exchange. In this case, the three weak interactions dominate and the overall result is the observation of a weak ferromagnetic interaction. Spin density analysis suggests a strong spin delocalisation mechanism, with the spin densities on the NiII ions being between 1.668–1.682. Of the three different bridging moieties, the Cl ion has the largest spin density (0.122–0.141; Fig. S7, ESI†). To further investigate the contribution from the μ-Cl ion to the total magnetic exchange, we have replaced it with a point charge in model 1D. This results in an antiferromagnetic interaction, changing from +2.3 cm−1 to −6.2 cm−1, clearly suggesting the major ferromagnetic contribution to the exchange comes from the μ-Cl ion. Bearing in mind the connectivity of next-nearest neighbour NiII centres through a formate group, we have also estimated the next-nearest neighbour magnetic exchange interaction using model 1E (Fig. S8, ESI†). This is estimated to be very small and ferromagnetic, J = +0.5 cm−1. All the NiII ions in 1 possess slightly distorted octahedral geometries (Table S5, ESI†) and are therefore expected to have axial zero-field splitting parameters of the order D ≤ −10 cm−1. Ab initio NEVPT2 calculations performed using ORCA23 reveal values in the range −1.9 ≤ D ≤ −6.5 cm−1, with the major contribution arising from the dxy → dx2−y2 electronic transition (Fig. S9 and Tables S6–S12, ESI†).24 The D(Ni) axes are oriented approximately along the N(pyrazole)–Ni–O and O(MeOH)–Ni–O vectors, tilted at angles of θ = ∼32–37° from the axis of the wheel. This non-collinear nature of the easy axes explains why 1 is a relatively poor single-molecule magnet.
In summary, the in situ transformation of HL1 to HL2 and concurrent formation of formate anions results in the self-assembly of an aesthetically pleasing [Ni14] wheel, with subsequent examination of reaction conditions leading to a more ‘rational’ synthetic procedure. Magnetic measurements reveal weak, ferromagnetic exchange interactions, with the susceptibility data simulated with a single exchange constant, J = +4 cm−1. The DFT computed values also simulate the data well, albeit they need scaled by a factor of 1.4. The simulation of the magnetisation data requires inclusion of D(Ni) = −5 cm−1 tilted at an angle θ = 30° with respect to the axis of the wheel. Theoretical calculations are in agreement with experimental observations, revealing the major contribution to the ferromagnetic exchange is mediated through the bridging Cl ions. Attempts to make analogues of compound 1 containing different MII ions and other bridging halides, pseudohalides and carboxylates are underway.
This work was supported by The Leverhulme Trust (RPG-2021-176) and the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 832488. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. P. Ginsberg, R. L. Martin and R. C. Sherwood, Inorg. Chem., 1968, 7, 932–936 CrossRef CAS; (b) A. P. Ginsberg, Inorg. Chim. Acta, Rev., 1971, 5, 45–68 CrossRef CAS.
- M. A. Halcrow, J.-S. Sun, J. C. Huffman and G. Christou, Inorg. Chem., 1995, 34, 4167–4177 CrossRef CAS.
- (a) A. Wilson, J. Lawrence, E.-C. Yang, M. Nakano, D. N. Hendrickson and S. Hill, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 140403 CrossRef; (b) J. P. S. Walsh, S. Sproules, N. F. Chilton, A. L. Barra, G. A. Timco, D. Collison, E. J. L. McInnes and R. E. P. Winpenny, Inorg. Chem., 2014, 53, 8464–8472 CrossRef CAS PubMed.
- J. Miklovič, D. Valigura, R. Boča and J. Titiš, Dalton Trans., 2015, 44, 12484–12487 RSC.
- G. Aromí, S. Parsons, W. Wernsdorfer, E. K. Brechin and E. J. L. McInnes, Chem. Commun., 2005, 5038–5040 RSC.
- R. Ruamps, R. Maurice, L. Batchelor, M. Boggio-Pasqua, R. Guillot, A. L. Barra, J. Liu, E.-E. Bendeif, S. Pillet, S. Hill, T. Mallah and N. Guihéry, J. Am. Chem. Soc., 2013, 135, 3017–3026 CrossRef CAS PubMed.
- K. E. R. Marriott, L. Bhaskaran, C. Wilson, M. Medarde, S. T. Ochsenbein, S. Hill and M. Murrie, Chem. Sci., 2015, 6, 6823–6828 RSC.
- G. A. Craig, A. Sarkar, C. H. Woodall, M. A. Hay, K. E. R. Marriott, K. V. Kamenev, S. A. Moggach, E. K. Brechin, S. Parsons, G. Rajaraman and M. Murrie, Chem. Sci., 2018, 9, 1551–1559 RSC.
- R. Shaw, I. S. Tidmarsh, R. H. Laye, B. Breeze, M. Helliwell, E. K. Brechin, S. L. Heath, M. Murrie, S. Ochsenbein, H.-U. Güdel and E. J. L. McInnes, Chem. Commun., 2004, 1418–1419 RSC.
- A. J. Blake, C. M. Grant, S. Parsons, J. M. Rawson and R. E. P. Winpenny, J. Chem. Soc., Chem. Commun., 1994, 2363–2364 RSC.
- S. T. Meally, G. Karotsis, E. K. Brechin, G. S. Papaefstathiou, P. W. Dunne, P. McArdle and L. F. Jones, CrystEngComm, 2010, 12, 59–63 RSC.
- D. Geng, X. Han, Y. Bi, Y. Qin, Q. Li, L. Huang, K. Zhou, L. Song and Z. Zheng, Chem. Sci., 2018, 9, 8535–8541 RSC.
- (a) V. M. Leovac, R. Petković, A. Kovács, G. Pokol and K. M. Szécsény, J. Therm. Anal. Calorim., 2007, 89, 267 CrossRef CAS; (b) R. Touzani, M. Haibach, A. J. Nawara-Hultzsch, S. El Kadiri, T. J. Emge and A. S. Goldman, Polyhedron, 2011, 30, 2530 CrossRef CAS; (c) J. Lim, G. Kim, K. Do, S. Lee, S. Ryu, D. Yoshioka and M. Mikuriya, X-Ray Struct. Anal. Online, 2015, 31, 49 CrossRef CAS; (d) A. Mar, S. J. Retting, A. Storr and J. Trott, Can. J. Chem., 1988, 66, 101 CrossRef CAS; (e) F. Paap, E. Bouwman, W. L. Driessen, R. A. G. de Graaff and J. Reedijk, J. Chem. Soc., Dalton Trans., 1985, 737 RSC.
- W. L. Driessen, Recl. Trav. Chim. Pays-Bas, 1982, 101, 441–443 CrossRef CAS.
- See for example: (a) A. H. Mahmoudkhani and V. Langer, Inorg. Chim. Acta, 1999, 294, 83–86 CrossRef CAS; (b) A. L. Dearden, S. Parsons and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2001, 40, 151–154 CrossRef CAS.
- See for example: (a) G. E. Lewis and C. S. Kraihanzel, Inorg. Chem., 1983, 22, 2895–2899 CrossRef CAS; (b) A. J. Blake, C. M. Grant, S. Parsons, J. M. Rawson and R. E. P. Winpenny, J. Chem. Soc., Chem. Commun., 1994, 2363–2364 RSC.
- R. Miyake, A. Ando, M. Ueno and T. Muraoka, J. Am. Chem. Soc., 2019, 141, 8675–8679 CrossRef CAS PubMed.
- (a) K. Bärwinkel, H.-J. Schmidt and J. Schnack, J. Magn. Magn. Mater., 2000, 212, 240–250 CrossRef; (b) R. Schnalle and J. Schnack, Int. Rev. Phys. Chem., 2010, 29, 403–452 Search PubMed; (c) T. Glaser, V. Hoeke, K. Gieb, J. Schnack, C. Schröder and P. Müller, Coord. Chem. Rev., 2015, 289–290, 261–278 CrossRef CAS.
- A. Tamayo, L. Escriche, C. Lodeiro, J. Ribas-Ariño, J. Ribas, B. Covelo and J. Casabó, Inorg. Chem., 2006, 45, 7621–7627 CrossRef CAS PubMed.
- M. K. Singh, Dalton Trans., 2020, 49, 4539–4548 RSC.
- M. K. Singh, A. Etcheverry-Berríos, J. Vallejo, S. Sanz, J. Martínez-Lillo, G. S. Nichol, P. J. Lusby and E. K. Brechin, Dalton Trans., 2022, 51, 8377–8381 RSC.
- (a) C. Desplanches, E. Ruiz, A. Rodríguez-Fortea and S. Alvarez, J. Am. Chem. Soc., 2002, 124, 5197–5205 CrossRef CAS PubMed; (b) M. K. Singh, N. Yadav and G. Rajaraman, Chem. Commun., 2015, 51, 17732–17735 RSC.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- A. Sarkar, S. Dey and G. Rajaraman, Chem. – Eur. J., 2020, 26, 14036–14058 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterisation details, computational methodology. CCDC 2169986. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03445j |
| This journal is © The Royal Society of Chemistry 2022 |